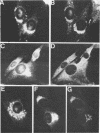
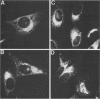
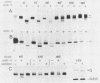
Abstract
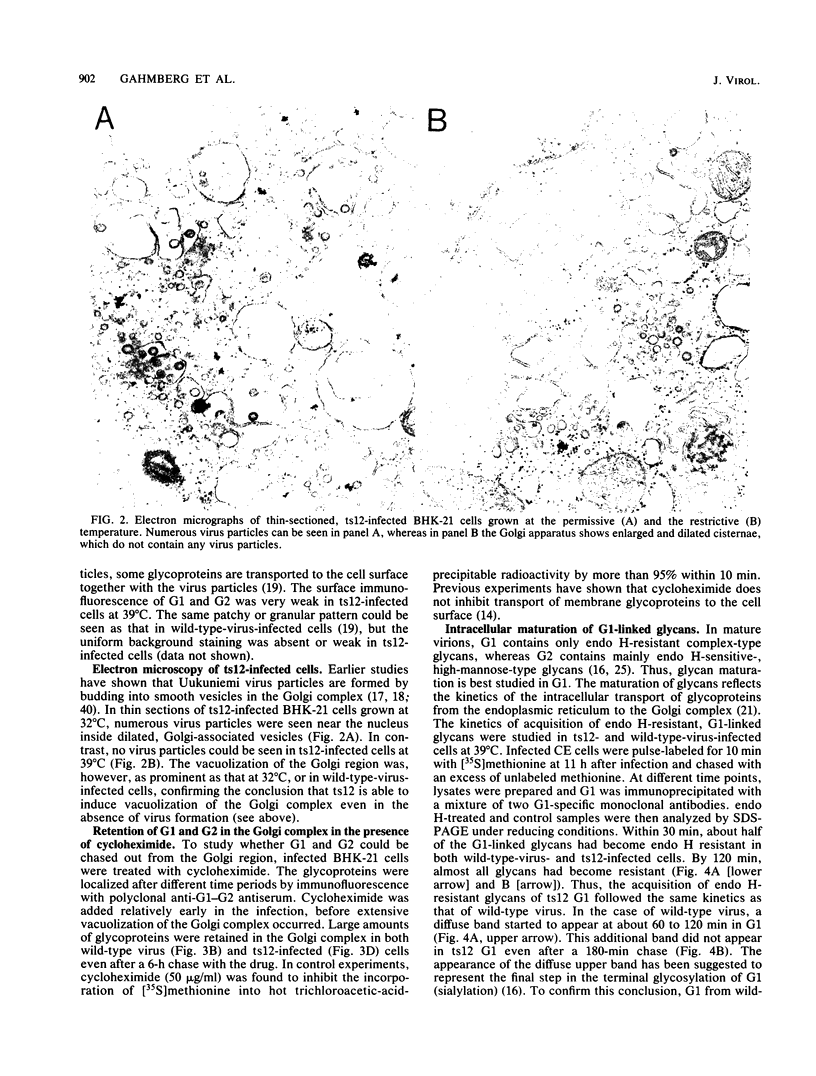
We have studied the transport of the Uukuniemi virus membrane glycoproteins in baby hamster kidney and chick embryo cells by using a temperature-sensitive mutant (ts12). Uukuniemi virus assembles in the Golgi complex, where both glycoproteins G1 and G2 and nucleocapsid protein N accumulate (E. Kuismanen, B. Bång, M. Hurme, and R. F. Pettersson, J. Virol. 51:137-146, 1984). At the restrictive temperature (39 degrees C), the glycoproteins of ts12 were transported to the Golgi complex as in wild-type, virus-infected cells, whereas the nucleocapsid protein failed to accumulate there. Pulse-chase labeling followed by immunoprecipitation and treatment with endo-beta-N-acetylglucosaminidase H showed that G1 synthesized at 39 degrees C in ts12-infected cells had an altered mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis, suggesting a lack of terminal glycosylation. The typical Uukuniemi virus-induced vacuolization and expansion of the Golgi complex could be seen also in ts12-infected cells at 39 degrees C, although no virus particles were formed. This suggests that the morphological changes were induced by the Uukuniemi virus glycoproteins. In wild-type virus- or ts12-infected cells, G1 and G2 could not be chased out from the Golgi complex even after 6 h of treatment with cycloheximide. The glycoproteins were thus retained in the Golgi even under conditions when no virus maturation took place and when nucleocapsids did not accumulate in the Golgi region. Accordingly, the glycoproteins of Uukuniemi virus were found to have properties resembling those of Golgi-specific proteins. This virus model system may be useful in studying the synthesis and transport of membrane proteins that are transported to and retained in the Golgi.
Full text
PDF

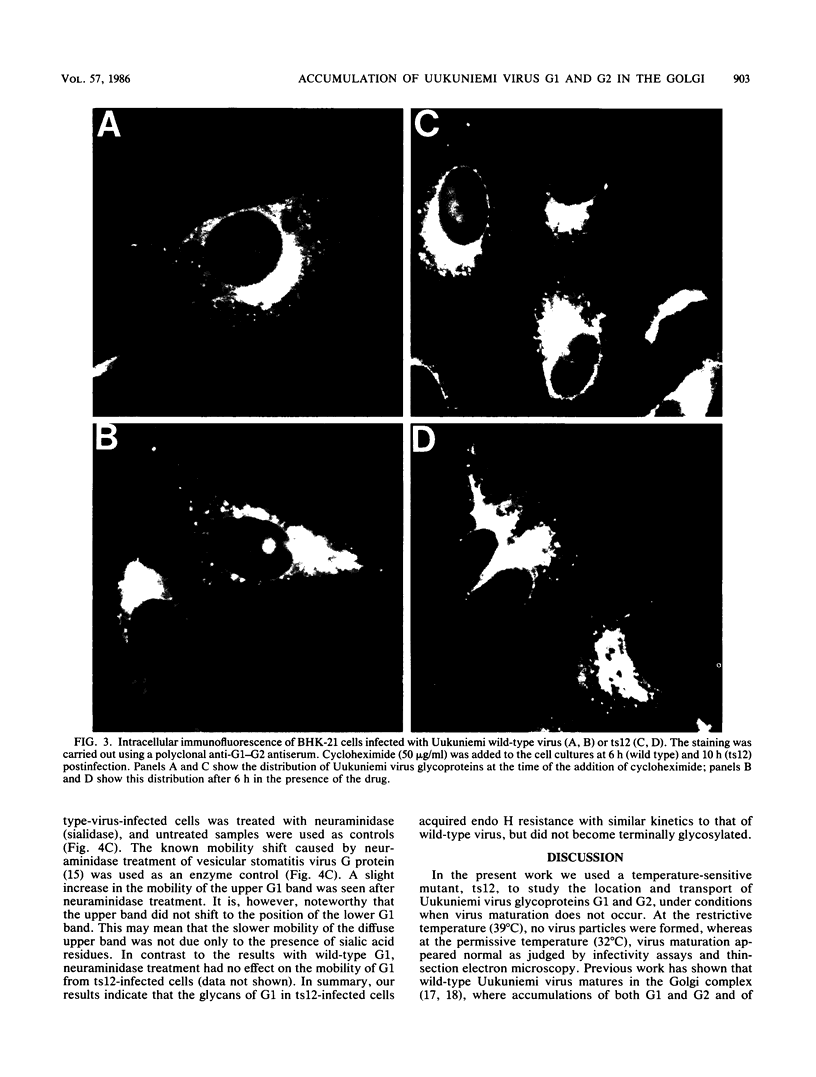


Images in this article
Selected References
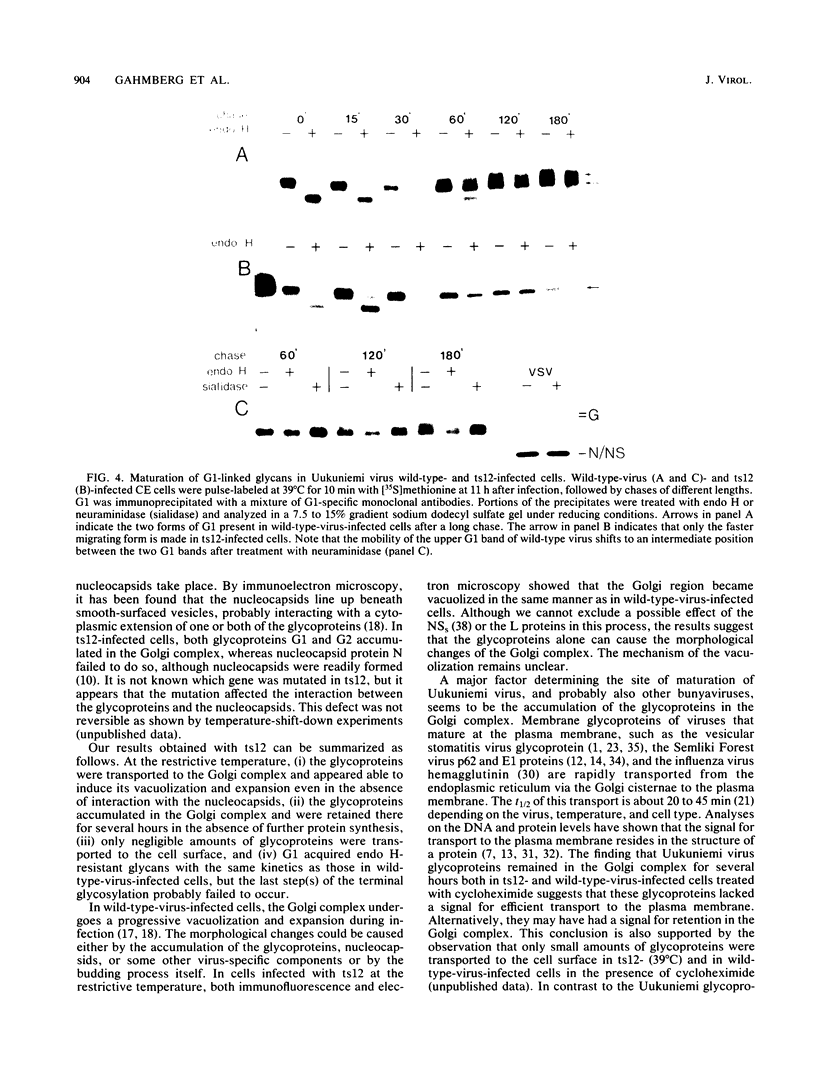
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann J. E., Tokuyasu K. T., Singer S. J. Passage of an integral membrane protein, the vesicular stomatitis virus glycoprotein, through the Golgi apparatus en route to the plasma membrane. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1746–1750. doi: 10.1073/pnas.78.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Calisher C. H., Casals J., Chumakov M. P., Gaidamovich S. Y., Hannoun C., Lvov D. K., Marshall I. D., Oker-Blom N., Pettersson R. F. Bunyaviridae. Intervirology. 1980;14(3-4):125–143. doi: 10.1159/000149174. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burke B., Griffiths G., Reggio H., Louvard D., Warren G. A monoclonal antibody against a 135-K Golgi membrane protein. EMBO J. 1982;1(12):1621–1628. doi: 10.1002/j.1460-2075.1982.tb01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham R. D., Morré D. J., Pannek C., Friend D. S. Isolation of a Golgi apparatus-rich fraction from rat liver. IV. Thiamine pyrophosphatase. J Cell Biol. 1971 Jun;49(3):899–905. doi: 10.1083/jcb.49.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle C., Roth M. G., Sambrook J., Gething M. J. Mutations in the cytoplasmic domain of the influenza virus hemagglutinin affect different stages of intracellular transport. J Cell Biol. 1985 Mar;100(3):704–714. doi: 10.1083/jcb.100.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Brands R., Rothman J. E. Attachment of terminal N-acetylglucosamine to asparagine-linked oligosaccharides occurs in central cisternae of the Golgi stack. Cell. 1985 Feb;40(2):463–472. doi: 10.1016/0092-8674(85)90161-8. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Rothman J. E. Compartmental organization of the Golgi stack. Cell. 1985 Aug;42(1):13–21. doi: 10.1016/s0092-8674(85)80097-0. [DOI] [PubMed] [Google Scholar]
- Gahmberg N. Characterization of two recombination-complementation groups of Uukuniemi virus temperature-sensitive mutants. J Gen Virol. 1984 Jun;65(Pt 6):1079–1090. doi: 10.1099/0022-1317-65-6-1079. [DOI] [PubMed] [Google Scholar]
- Goldfischer S. The internal reticular apparatus of Camillo Golgi: a complex, heterogeneous organelle, enriched in acid, neutral, and alkaline phosphatases, and involved in glycosylation, secretion, membrane flow, lysosome formation, and intracellular digestion. J Histochem Cytochem. 1982 Jul;30(7):717–733. doi: 10.1177/30.7.6286754. [DOI] [PubMed] [Google Scholar]
- Green J., Griffiths G., Louvard D., Quinn P., Warren G. Passage of viral membrane proteins through the Golgi complex. J Mol Biol. 1981 Nov 15;152(4):663–698. doi: 10.1016/0022-2836(81)90122-4. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Rose J. K. Conversion of a secretory protein into a transmembrane protein results in its transport to the Golgi complex but not to the cell surface. Cell. 1984 Jul;37(3):779–787. doi: 10.1016/0092-8674(84)90413-6. [DOI] [PubMed] [Google Scholar]
- Knipe D. M., Lodish H. F., Baltimore D. Localization of two cellular forms of the vesicular stomatitis viral glycoprotein. J Virol. 1977 Mar;21(3):1121–1127. doi: 10.1128/jvi.21.3.1121-1127.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuismanen E., Bång B., Hurme M., Pettersson R. F. Uukuniemi virus maturation: immunofluorescence microscopy with monoclonal glycoprotein-specific antibodies. J Virol. 1984 Jul;51(1):137–146. doi: 10.1128/jvi.51.1.137-146.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuismanen E., Hedman K., Saraste J., Pettersson R. F. Uukuniemi virus maturation: accumulation of virus particles and viral antigens in the Golgi complex. Mol Cell Biol. 1982 Nov;2(11):1444–1458. doi: 10.1128/mcb.2.11.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuismanen E. Posttranslational processing of Uukuniemi virus glycoproteins G1 and G2. J Virol. 1984 Sep;51(3):806–812. doi: 10.1128/jvi.51.3.806-812.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuismanen E., Saraste J., Pettersson R. F. Effect of monensin on the assembly of Uukuniemi virus in the Golgi complex. J Virol. 1985 Sep;55(3):813–822. doi: 10.1128/jvi.55.3.813-822.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käriäinen L., Hashimoto K., Saraste J., Virtanen I., Penttinen K. Monensin and FCCP inhibit the intracellular transport of alphavirus membrane glycoproteins. J Cell Biol. 1980 Dec;87(3 Pt 1):783–791. doi: 10.1083/jcb.87.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Braell W. A., Schwartz A. L., Strous G. J., Zilberstein A. Synthesis and assembly of membrane and organelle proteins. Int Rev Cytol Suppl. 1981;12:247–307. doi: 10.1016/b978-0-12-364373-5.50016-0. [DOI] [PubMed] [Google Scholar]
- Madoff D. H., Lenard J. A membrane glycoprotein that accumulates intracellularly: cellular processing of the large glycoprotein of LaCrosse virus. Cell. 1982 Apr;28(4):821–829. doi: 10.1016/0092-8674(82)90061-7. [DOI] [PubMed] [Google Scholar]
- Morrison T. G., Ward L. J. Intracellular processing of the vesicular stomatitis virus glycoprotein and the Newcastle disease virus hemagglutinin-neuraminidase glycoprotein. Virus Res. 1984;1(3):225–239. doi: 10.1016/0168-1702(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Whitfield S. G. Bunyaviridae: morphologic and morphogenetic similarities of Bunyamwera serologic supergroup viruses and several other arthropod-borne viruses. Intervirology. 1973;1(4):297–316. doi: 10.1159/000148858. [DOI] [PubMed] [Google Scholar]
- Pesonen M., Kuismanen E., Pettersson R. F. Monosaccharide sequence of protein-bound glycans of Uukuniemi virus. J Virol. 1982 Feb;41(2):390–400. doi: 10.1128/jvi.41.2.390-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson R. F., Hewlett M. J., Baltimore D., Coffin J. M. The genome of Uukuniemi virus consists of three unique RNA segments. Cell. 1977 May;11(1):51–63. doi: 10.1016/0092-8674(77)90316-6. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F., von Bonsdorff C. H. Ribonucleoproteins of Uukuniemi virus are circular. J Virol. 1975 Feb;15(2):386–392. doi: 10.1128/jvi.15.2.386-392.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson R., Käriäinen L. The ribonucleic acids of Uukuniemi virus, a noncubical tick-borne arbovirus. Virology. 1973 Dec;56(2):608–619. doi: 10.1016/0042-6822(73)90062-7. [DOI] [PubMed] [Google Scholar]
- Pettersson R., Käriäinen L., von Bonsdorff C. H., Oker-Blom N. Structural components of Uukuniemi virus, a noncubical tick-borne arbovirus. Virology. 1971 Dec;46(3):721–729. doi: 10.1016/0042-6822(71)90074-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Paskiet K. T., Salas P. J., Bard E. Intracellular transport of influenza virus hemagglutinin to the apical surface of Madin-Darby canine kidney cells. J Cell Biol. 1984 Jan;98(1):308–319. doi: 10.1083/jcb.98.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Altered cytoplasmic domains affect intracellular transport of the vesicular stomatitis virus glycoprotein. Cell. 1983 Sep;34(2):513–524. doi: 10.1016/0092-8674(83)90384-7. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Expression from cloned cDNA of cell-surface secreted forms of the glycoprotein of vesicular stomatitis virus in eucaryotic cells. Cell. 1982 Oct;30(3):753–762. doi: 10.1016/0092-8674(82)90280-x. [DOI] [PubMed] [Google Scholar]
- Roth J., Berger E. G. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol. 1982 Apr;93(1):223–229. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., von Bonsdorff C. H., Hashimoto K., Käriäinen L., Keränen S. Semliki forest virus mutants with temperature-sensitive transport defect of envelope proteins. Virology. 1980 Jan 30;100(2):229–245. doi: 10.1016/0042-6822(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Van Kerkhof P., Willemsen R., Geuze H. J., Berger E. G. Transport and topology of galactosyltransferase in endomembranes of HeLa cells. J Cell Biol. 1983 Sep;97(3):723–727. doi: 10.1083/jcb.97.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Ulmanen I., Seppälä P., Pettersson R. F. In vitro translation of Uukuniemi virus-specific RNAs: identification of a nonstructural protein and a precursor to the membrane glycoproteins. J Virol. 1981 Jan;37(1):72–79. doi: 10.1128/jvi.37.1.72-79.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bonsdorff C. H., Saikku P., Oker-Blom N. Electron microscope study on the development of Uukuniemi virus. Acta Virol. 1970 Mar;14(2):109–114. [PubMed] [Google Scholar]
- von Bonsdorff C. H., Pettersson R. Surface structure of Uukuniemi virus. J Virol. 1975 Nov;16(5):1296–1307. doi: 10.1128/jvi.16.5.1296-1307.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]