Abstract
Chronic obstructive pulmonary disease (COPD) is an incurable, progressive illness that is the fourth commonest cause of death worldwide. Death tends to occur after a prolonged functional decline associated with uncontrolled symptoms, emotional distress and social isolation. There is increasing evidence that the end of life needs of those with advanced COPD are not being met by existing services. Many barriers hinder the provision of good end of life care in COPD, including the inherent difficulties in determining prognosis. This review provides an evidence-based approach to overcoming these barriers, summarising current evidence and highlighting areas for future research. Topics include end of life needs, symptom control, advance care planning, and service development to improve the quality of end of life care.
Keywords: chronic obstructive pulmonary disease (MeSH), palliative care (MeSH), dyspnoea (MeSH), advance care planning (MeSH)
Introduction
Provision of good quality end of life care has become a healthcare priority over the last decade. Medical advances and demographic trends mean that the proportion of people living with serious chronic conditions into old age is increasing rapidly. In the USA in 1997, the Institute of Medicine report ‘Approaching Death: Improving Care at the End of Life’ concluded that “the timing appears right to press for a vigorous societal commitment to improve care at the end of life” (Field and Cassel 1997).
End of life care aims to optimise the quality of life of patients with advanced, incurable disease (Figure 1). Its principles are the same as those of palliative care, which developed in the UK in the 1960s in response to the needs of terminally ill cancer patients. With increasing recognition that those with incurable non-malignant disease also have extensive palliative care needs, specialist palliative care services are attempting to provide needs-based rather than diagnosis-based care (Addington-Hall 1998). In practice, limited resources mean that only those with the most complex needs receive specialist palliative care input. In the UK, approximately 20% of those that die have contact with specialist palliative care services, of which over 90% have malignant disease (NCPC 2006).
Figure 1.

Definitions (NHS Executive 1996; Department of Health 2007).
Care for those approaching the end of life therefore cannot be, and indeed should not be, simply the domain of palliative care specialists. All healthcare professionals should be able to provide good quality generalist palliative care for their patients (NHS Executive 1996). The relative neglect of end of life care until recently may be due, in part, to death being viewed as a failure in medical care, rather than inevitable (Middlewood et al 2001). It is a duty and privilege to be able to provide compassionate and effective care from diagnosis to death.
The challenge for providers of generalist palliative care is particularly great when caring for those with advanced chronic obstructive pulmonary disease (COPD). COPD is the fourth leading cause of mortality worldwide, and compared to chronic cardiovascular and cerebrovascular disease, mortality from COPD continues to rise (NIH 2003; WHO 2003). Its sufferers have extensive end of life needs. Death tends to occur after a prolonged functional decline associated with a heavy symptom load, emotional distress and social isolation. The quality of life of COPD patients appears to be at least as poor, and indeed may be worse than that of patients with lung cancer (Gore et al 2000; Edmonds et al 2001). There is a growing body of evidence that existing service provision is unable to meet these needs (Heffner et al 1996; Claessens et al 2000; Jones et al 2004; Elkington et al 2005; Au et al 2006). The emphasis appears to be on reactive crisis intervention at the time of acute exacerbations, rather than continual supportive care (Skilbeck et al 1998).
Over the last few years, there have been increasing calls for improvements in end of life care for those with advanced COPD (Claessens et al 2000; Gore et al 2000; Edmonds et al 2001; Elkington et al 2001; Guthrie et al 2001; O’Donnell et al 2003; Healthcare Commission 2006; Varkey 2006). In a recent statement, the American College of Chest Physicians support the position that good quality palliative and end of life care should become an integral part of cardiopulmonary medicine (Selecky et al 2005). Barriers to the provision of good quality palliative care have been identified (Varkey 2006). Some steps have been made to overcome these barriers and develop services. End of life programmes and other political initiatives are in the process of being developed in many countries (Byock et al 2006; NHS 2006).
The aim of this review is to provide readers with an appraisal of recent developments in end of life care for COPD patients. An evidence-based approach will be given to overcoming many of the barriers that currently hinder the practice of good quality EOL care. This review is aimed at respiratory specialists, who have a key role in improving the quality of generalist palliative care received by patients with COPD.
The review is based on a detailed search of work published since 1980 in the databases Medline and Embase using Medical Subject Heading (MeSH) terms that included Pulmonary disease chronic obstructive, Palliative care, Needs assessment, Anxiety, Depression, Pain, Fatigue, Communication, Advance directives, Terminal Care, and the textwords Dyspnoea and Breathlessness. The search was extended by hand-searching recent journals and reference lists, and using internet search engines to help identify other pertinent literature.
End of life needs in COPD
Unmet palliative care needs
Over the last decade, several studies have attempted to identify the palliative care needs of patients with advanced COPD (Claessens et al 2000; Edmonds et al 2001; Seamark et al 2004; Elkington et al 2005). Despite heterogeneity in terms of study populations and outcome measures, the consistent conclusion of all these studies is that patients with advanced COPD experience a poor quality of life.
Skilbeck et al (1998) interviewed 63 patients who had been admitted to hospital with an exacerbation of COPD in the last six months. On a numerical rating scale of 0–100, with 0 meaning poor and 100 meaning excellent, the mean quality of life score was 33. Poor quality of life correlated particularly strongly with a low level of social functioning. In a large retrospective analysis of a prospective cohort in the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment (SUPPORT), out of 416 patients who died within one year of an index hospitalisation, 75% described their quality of life as fair or poor (Lynn et al 2000).
Gore et al (2000) studied 50 patients with an FEV <0.75l and at least one previous admission with hypercapnic respiratory failure, and also found evidence of a low quality of life. Interestingly, the study also included 50 patients with inoperable non-small cell lung cancer and found, using a generic quality of life measure, a significantly worse quality of life in the COPD patients than in those with cancer. These data should, however, be interpreted with a degree of caution as the two study populations were atypical in sex distribution and disease severity (Hill and Muers 2000).
There are many factors contributing to the poor quality of life suffered by patient with COPD. The symptom burden is considerable. Studies based on post-bereavement interviews show that over 90% of patients are breathless in the last year of life, and in nearly half of these the breathlessness is unrelieved by treatment (Edmonds et al 2001). Skilbeck et al (1998) found that 95% of a cohort of patients that had been admitted with an exacerbation of COPD in the preceding 6 months were experiencing severe breathlessness, defined as ‘very much’ on a four point intensity scale ranging from ‘not at all’ to ‘very much.’ Other symptoms were strikingly prevalent, including pain (68%), fatigue (68%) and insomnia (55%). Psychological morbidity is also high in COPD. Gore et al (2000) found that 90% of patients had clinically relevant anxiety or depression. Overall, there can be no doubt that the physical and psychological symptom burden from advanced COPD is at least as severe as that from other incurable diseases. Indeed, in a recent systematic review, Solano et al (2006) found that breathlessness, fatigue and anxiety occur more commonly in COPD than in advanced cancer, heart disease or renal disease (Table 1).
Table 1.
Symptom prevalence in advanced COPD (Solano et al 2006)
| Symptom | Prevalence |
|---|---|
| Breathlessness | 60%–88% |
| Fatigue | 68%–80% |
| Anxiety | 51%–75% |
| Pain | 34%–77% |
| Depression | 37%–71% |
| Insomnia | 55%–65% |
| Anorexia | 35%–67% |
| Constipation | 27%–44% |
Qualitative studies have an important role in identifying unmet social, education and communication needs. Patients in the late stages of COPD are often housebound, but receive little support from community services (Gore et al 2000; Elkington et al 2004, 2005). They experience a high degree of social isolation (Skilbeck et al 1998; Elofsson and Ohlén 2004). Care-givers also suffer and have great demands placed on them (Guthrie et al 2001; Jones et al 2004; Seamark et al 2004). There is evidence that patients with COPD have greater information needs than in those with other advanced disease, such as cancer (Curtis et al 2002). Patients’ information requirements are not matched by what they receive. Heffner et al (1996) found that amongst 105 patients enrolled in a pulmonary rehabilitation programme, although 99% wished to discuss end of life issues such as prognosis and advance directives, less than 20% had such discussions.
Patients with COPD tend to experience significant morbidity for longer than patients with lung cancer. In addition, studies of existing services show they are more likely to be admitted to admitted to acute hospital, in particular to intensive care units, they are less likely to know that they are dying, and they are less likely to receive medication for symptom control (Lynn et al 2000; Edmonds et al 2001; McKinley et al 2004; Au et al 2006). This occurs despite the fact that most COPD patients prefer treatment focussed on comfort rather than on prolonging life, and COPD patients are equally as likely as lung cancer patients to prefer not to be intubated or receive cardiopulmonary resuscitation (Claessens et al 2000).
Barriers to good end of life care in COPD
Why, then, are there such considerable unmet palliative care needs in patients with advanced COPD? In order to answer this question and focus future service development appropriately, it is important to establish the causes of the current suboptimal quality of care at the end of life.
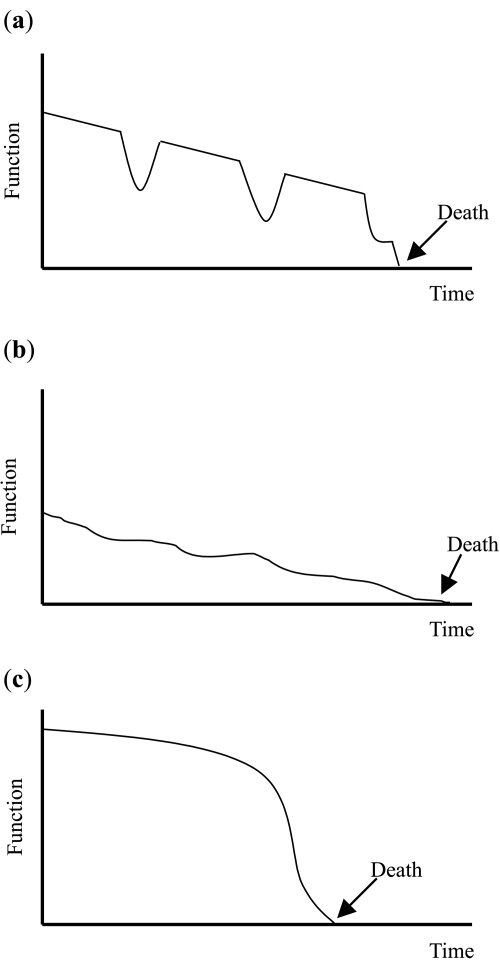
Arguably the greatest challenge to provision of EOL care in COPD is the unpredictable disease trajectory (Figure 2). The disease trajectory in COPD is typically that of a slow decline, punctuated by dramatic exacerbations that often end in unexpected death (Lunney et al 2003; Murray et al 2005). This contrasts with that of cancer, where there is often maintenance of good function until a short period of relatively predictable decline in the last weeks or months of life. COPD is particularly unpredictable as it progresses at a highly variable rate. Exacerbations causing respiratory failure occur suddenly and unpredictably, and the outcome of those exacerbations is often determined by last-minute decisions regarding life support.
Figure 2.
Typical disease trajectories for progressive chronic illness. (a) Long-term limitation with intermittent acute episodes eg COPD. (b) Prolonged dwindling eg dementia. (c) Short period of decline eg cancer. (Adapted from Murray et al with permission from BMJ Publishing Group Ltd.).
This disease trajectory has two important implications. First, it causes significant prognostication difficulties. Only dementia has been found to have a more uncertain prognosis than COPD (Hansen-Flaschen 2004). How can good EOL care be provided when it is not clear that the end of life is approaching? Until recently there has been little evidence-based information available to help determine the prognosis in advanced COPD. Commonly used prognostic criteria, including measures of air-flow limitation such as FEV1, degree of hypoxia, complications such as cor-pulmonale and recent hospitalisation requiring ventilation, have all been found to be unreliable (Fox et al 1999; Domingo-Salvany et al 2002; Nishimura et al 2002; Coventry et al 2005).
Second, unexpected death at the time of an acute exacerbation generates significant communication challenges. It is usually only possible to discover patients’ end of life preferences if these have been established in advance. Such advance care planning is often inadequate, making it harder to conform to patients’ wishes and provide appropriate EOL care.
Another important barrier to end of life care is that misconceptions about both COPD and palliative care abound. It is sobering that patients and caregivers generally fail to appreciate that COPD is a life-threatening disease that results in an inexorable decline in health status and function (Lynn et al 2000). Good EOL care is impossible without recognition that death may occur prematurely. The appreciation amongst patients that COPD is often a self-inflicted disease lead some to believe that they are not eligible or deserving of measures to improve quality of life. The practice of palliative care is also misunderstood. In our death-denying culture, embarking on a palliative approach can be misconstrued as evidence of failure or ‘giving up’ (Croft 2005). Patients and professionals often still expect a dichotomous model of care moving from disease modification to palliation, when this has long been superseded by a mixed model of care combining both approaches.
Resource limitations are a significant impediment to the delivery of effective EOL care (Traue and Ross 2005). Even for cancer patients, the historical focus of palliative care, palliative care provision is not uniform, and patients’ needs are not always being met. Improving the palliation of COPD has significant implications for funding, manpower and education. Palliative care specialists are not always well informed about the management of non-malignant disease and respiratory specialists require training in the skills of palliation.
Finally, it is increasingly recognised that a lack of research evidence on end of life care is hindering service development (Field and Cassel 1997). Research in this vulnerable patient group is beset by significant ethical and methodological challenges (Lawton 2001; Addington-Hall 2002; Kendall et al 2007). These include difficulties in gaining informed consent, poor recruitment rates and high attrition rates.
Clearly, the route to improving the quality of EOL provision for COPD patients lies in overcoming these barriers. Challenges caused by the unpredictable disease trajectory can be overcome with improved HCP prognostication and communication skills. Resource limitations can be compensated for by careful evidence-based service development, using existing resources to provide cost-effective interventions. Generalist palliative care education, advance care planning and service development must each be improved in order to enhance the quality of EOL care received by those with COPD. The remainder of this review will provide an evidence-based analysis of aspects of each of these important topics.
Key points
The end of life needs of patients with COPD are at least as great as, if not greater than, those suffering from advanced lung cancer.
Patients experience a prolonged deterioration with low quality of life, uncontrolled symptoms, psychological morbidity, social isolation, and unmet communication and information needs.
Prognostication difficulties, resource limitations and inadequate research evidence to support service development are the principle barriers to the provision of good end of life care.
Symptom control at the end of life
In landmark research, Steinhauser et al (2000a, 2000b) used qualitative methods to generate descriptors of the components of end of life care considered most important by patients, carers and healthcare professionals, and subsequently tested these for generalizability in a national survey. The greatest concern of patients and carers was to avoid dying in distress with uncontrolled symptoms.
Why do patients with advanced COPD suffer from uncontrolled symptoms? Healthcare professionals may be hesitant about proactively managing symptoms, in part, due to concerns about adverse effects of pharmacological interventions, such as respiratory depression from opioids or anxiolytics. There may be an element of therapeutic nihilism, believing that symptoms such as fatigue cannot be ameliorated. Good generalist palliative care education, providing an evidence-based approach to symptom management, is the key.
Dyspnea
Dyspnea is an almost universal symptom in advanced COPD, and it is one of the most significant contributors to the poor quality of life experienced by these patients (Lynn et al 1997; Skilbeck et al 1998; Edmonds et al 2001). The “neuromechanical dissociation theory” suggests that dyspnea is a consequence of mismatch between central respiratory motor activity and incoming afferent information from receptors in the airways, lungs and chest wall (Schwartzstein et al 1989). It can therefore be a consequence either of increased ventilatory demand, or impairment of the mechanical process of ventilation. Cognitive, emotional and behavioral factors contribute to the central perception of dyspnea.
The multidimensional pathophysiology of dyspnea gives scope to a wide range of potential palliative interventions. Interventions that reduce ventilatory demand include oxygen therapy and exercise training (Swinburn et al 1984). Impairment of ventilation may be helped by breathing techniques that improve respiratory muscle function, and oxygen therapy which can reduce dynamic hyperinflation (Somfay et al 2001). The central perception of dyspnea can be modified by pharmacological interventions such as opioids and anxiolytics, and by non-pharmacological measures such as education, psychological support and behavioral interventions. Which of these approaches are of use in the last weeks and months of life?
Oxygen therapy
The use of long-term oxygen therapy (LTOT), involving continuous use of oxygen for 15 or more hours each day is well established in severely hypoxic patients with COPD (PaO2 less than 7.3 kPa), as two landmark trials have shown that oxygen for 15 or more hours per day improves survival (NOTT group 1980; MRC Working Party 1981). As well as giving benefit in terms of survival, LTOT appears to enhance quality of life in severely hypoxic patients. Eaton et al (2004) have examined the effect of LTOT on health-related quality of life (HRQL) and found significantly improvements in HRQL and dyspnoea scores at 2 months and 6 months relative to a comparison group that did not qualify for LTOT. The two patient groups were well matched in all variables other than the greater degree of hypoxia in the LTOT group.
We have previously systematically reviewed the role of short-burst oxygen therapy (SBOT) in palliating breathlessness in patients with COPD (Booth et al 2004). From examination of all studies included in the original review and three studies published subsequently (Lewis et al 2003; Nandi et al 2003; Stevenson and Calverley 2004), it is clear that there are little data to support the use of SBOT at rest, including before or after exercise. SBOT during exercise does, however, appear to be of greater value. Out of nineteen controlled single assessment studies that recorded dyspnoea scores at ‘isotime,’ all but one revealed significant relief of dyspnoea when exercising with oxygen.
It cannot, of course, be assumed that acute responders who gain benefit from oxygen in experimental conditions will also benefit when oxygen is used over a longer time period at home. Just four controlled studies have examined the effect of SBOT on breathlessness when used at home during activities of daily living. Two studies, both included in our original review, examined quality of life in patients randomized to receive either home oxygen or air over a 12 week period. Eaton et al (2002), using a study population with significant exercise desaturation and providing light oxygen cylinders, did find an improvement in quality of life in patients who had been receiving oxygen. Interestingly, 41% of patients who had had a response to oxygen declined to use it after the study, citing poor acceptability or tolerability. McDonald et al (1995) recruited patients who desaturated less on exercise and supplied heavier oxygen cylinders; they found no improvement in quality of life. In a more recent study, Eaton et al (2006) randomized 78 patients discharged after an acute exacerbation to cylinder oxygen, cylinder air or usual care. There were no significant differences between the groups in quality of life, breathlessness or acute healthcare utilization. Nonoyama et al (2007), in an interesting study incorporating multiple N-of-1 RCTs for each of 27 patients, also found no evidence to support the use of long-term ambulatory oxygen therapy for patients who do not qualify for LTOT.
Importantly, no studies have been able to find factors that can predict which patients are likely to experience symptom relief from supplemental oxygen. Furthermore, Eaton et al (2002) found that an acute response to oxygen did not predict those that would benefit when using longer-term oxygen. Response to oxygen does not correlate with the extent of dyspnea at rest, level of hypoxemia at rest, degree of desaturation on exercise, or any tests of lung function. The response to oxygen is extremely variable between individuals, although more reproducible on an individual level (Waterhouse and Howard 1983). The only way to select the patients who will benefit from oxygen therapy is to undertake an individual clinical assessment. At its most rigorous, the undertaking an N-of-1 RCT has been described and recommended (Bruera et al 1992; Uronis et al 2006).
The burdens of oxygen therapy are considerable (Spathis et al 2006). A degree of psychological dependence is inevitable, and some patients become acutely anxious during even a short interruption in oxygen supply. Cumbersome, heavy equipment may restrict movement and activities within the home and limit excursions outside. Use of an oxygen mask may impair communication between a patient and family. Some patients feel a sense of social stigma and embarrassment, which may further hinder interaction and lead to isolation. Other issues include its combustibility, its considerable cost being, and the uncomfortable drying of airways including the nasal mucosa.
Overall, therefore, there is little evidence to support the use of oxygen in palliating dyspnea. Although single assessment studies appear to show benefit from oxygen during exercise, these findings are not reproduced when oxygen is used longer-term during activities of daily living. Despite this lack of evidence of benefit, breathless patients approaching the end of life are often commenced on oxygen. Abernethy et al (2005) found in an email survey that 58% of surveyed palliative care and respiratory physicians believe that patients benefit from palliative oxygen. Given the negative aspects of oxygen therapy, it is likely that during end of life care, the burdens of oxygen therapy may well outweigh the benefits in a significant proportion of patients.
Withdrawal of oxygen therapy is, of course, not easy. It can cause distress, feelings of abandonment and fear that life may be shortened. Through careful and sensitive communication, patients may be helped to understand the lack of evidence for benefit from SOBT, the inevitable development of psychological dependence, the burdens of oxygen therapy and the lack of selection criteria to determine who may benefit. With individual clinical assessment, the few individuals who may benefit from SBOT can be identified.
Non-pharmacological approaches
There is good evidence that exercise is one of the most successful non-pharmacological approaches to managing breathlessness. Reconditioning is central to the benefit provided by pulmonary rehabilitation programs (Lacasse et al 2006). In the end of life phase capacity for exercise reduces, and the four most common approaches are use of a fan, energy conserving measures, breathing techniques and relaxation strategies.
The flow of cool air through the nose, mouth or over the cheek can reduce the perception of dyspnea (Schwartzstein et al 1987; Liss and Grant 1988). It is believed that stimulation of nasal or pharyngeal mucosal receptors or facial receptors in the region of the trigeminal nerve leads to afferent information being projected to the sensory cortex where it alters the central perception of dyspnea. There is extensive anecdotal evidence that patients find benefit from use of a fan, or from standing by an open window. Even with end-stage disease, patients find a small hand-held fan easy to use. It is a cheap piece of equipment that, unlike use of oxygen does not draw untoward attention to its user, and has no adverse effects. Interestingly, Booth et al (1996), in a study of cancer patients who were breathless at rest, found that both cylinder air and cylinder oxygen improve breathlessness, without there being a statistically significant difference between them. It may be that some of the benefit that patients perceive from oxygen may simply be due to it being a flow of cool gas. There is, to date, only a small amount of research evidence examining the use of a fan (Booth et al 2006); further studies are known to be underway.
Energy conservation techniques reduce dyspnea by reducing demand for ventilation. Strategies include pacing activities, avoiding unnecessary activities, and rest periods or ‘breathing stations’ during prolonged tasks (Carrieri and Janson 1986). A variety of recommendations have been published that help patients to complete activities of daily living with less effort (Carrieri-Kohlman and Stulbarg 2002). Possible techniques include sitting where possible, avoiding bending by arranging equipment closely and sliding or pushing items instead of lifting. There are no controlled studies evaluating such techniques; observational studies have described patient experiences (Brown et al 1986).
Breathing techniques improve the mechanical efficiency of respiration. They also lower the demand for ventilation by reducing dynamic hyperinflation, which in turn increases tidal volume and improves carbon dioxide elimination (Belman et al 1996; O’Donnell et al 2001). The aim of these techniques is to reduce respiratory rate and prolong expiration, while using a gently leaning forward posture that improves the mechanical efficiency of the diaphragm. Several studies confirm the benefit of pursed-lips breathing on dyspnea (Tiep et al 1986; Breslin 1992). Several step-by-step exercises to alter breathing rhythm have been published (Gallo-Silver and Pollack 2000). Counting during the respiratory cycle has been advocated, such as a count of 4 during inhalation, of 7 during exhalation and a count to 2 before recommencing inhalation. Such breathing patterns need practice until they become unconscious and automatic. It is hard to learn such techniques close to the end of life, and is therefore important to teach patients early.
Relaxation techniques and training in anxiety reduction are particularly important with advanced disease. Anxiety increases breathlessness, which in turn contributes to the anxiety, leading to a deteriorating vicious circle (Bailey 2004). Various methods have been described, and techniques should be tailored and adapted for each individual. Methods include progressive muscular relaxation with systematic tensing and relaxing of all muscle groups, visualization and guided imagery, self-hypnosis and distraction by music (Walker 2004; Carrieri-Kohlman 2006). Renfroe (1988) found that taught relaxation techniques in ten COPD patients reduced anxiety more than in a control group that were told to relax without specific instructions. However, benefits were not maintained after the study, a finding confirmed in other studies (Gift et al 1992). Again this supports the view that such techniques should be continually practiced and should be taught well before the end of life phase in order to be of use when need arises.
Pharmacological approaches
The use of pharmacological measures to palliate breathlessness is important in the final weeks and months of life, in conjunction with continued use of the non-pharmacological techniques described above. Their use is, however, often hindered by controversy, mainly in relation to safety concerns. A recent and commendable review in this journal has examined this topic in detail with reference to COPD patients (Uronis et al 2006). Therefore, only a summary of the most relevant evidence will be given here, with focus on issues of safety.
There is consistent evidence that opioids reduce the sensation of breathlessness. Jennings et al (2002) undertook a meta-analysis of all data prior to 2000 and found a highly statistically significant effect of oral and parenteral opioids on breathlessness and a trend towards improved exercise tolerance. There was no evidence of benefit from nebulized opioids. Thirteen of the eighteen included studies involved patients with COPD. There was more nausea, vomiting, dizziness, drowsiness and constipation in patients taking opioids compare to placebo. No deaths in any of the studies were attributed to opioids. Out of four studies that measured arterial blood gas tensions, in one study there was a statistically significant rise in pCO2 in patients on dihydrocodeine. However, in no instance did the PaCO2 rise above 5.3kPa, or the PaO2 fall significantly. Amongst the nine studies that measured oxygen saturation, none reported a significant change in patients on opioids.
Abernethy et al (2003) have subsequently undertaken a good quality, adequately powered, randomized, double-blind, crossover study of oral morphine (20mg/24hrs) in a study population where 32 of 38 patients had dyspnea caused by COPD. Dyspnea improved by 15%–22%. Despite all patients being opioid-naïve, the only adverse effect significantly more prevalent in those taking morphine was constipation.
There is no evidence to date that the doses of opioids used to palliate breathlessness causes clinically detectable respiratory depression or increased mortality (Mazzocato et al 1999; Jennings et al 2002). Why, then, is this beneficial therapy so commonly withheld on grounds of safety? The answer lies in the form of a deep societal misconception. The idea that opioids may kill when used for symptom control entered UK society in 1957 when Dr Bodkin Adams used the doctrine of double effect (DDE) as defense when accused of murdering an elderly lady from whose will he was to benefit. Since then this defense has been reused to the extent that unintended death from opioids used for symptom control has now become the classic example of DDE in clinical practice (George and Regnard 2007). This misconception seems to be entrenched, even in the face of lack of evidence. Interestingly, when opioids are given after withdrawing ventilator support in intensive care patients they not only do not hasten death, but may even enable breathing to continue longer after ventilator withdrawal (Chan et al 2004).
Public and healthcare professional suspicion of opioids will continue until there is good evidence for lack of harm, not simply lack of evidence of harm. A safety study using parenteral opioids for pain in cancer patients has been recently published, showing no evidence for respiratory depression (Estfan et al 2007). A large safety study in COPD patients is urgently needed as, to date, no studies have been sufficiently powered to detect rare but serious adverse effects (Currow et al 2003). In the meantime a pragmatic approach is needed. Opioids should be started at a low dose and titrated up carefully, with appropriate monitoring (Mashford et al 2001; National Institute of Clinical Excellence 2004; Selecky et al 2005). Education of healthcare professionals would facilitate the appropriate use of morphine; for example, it would help prescribers to know that 1.25mg oral morphine four hourly is equivalent to just 75mg codeine over 24 hours (Twycross et al 1999). Clear protocols need to be developed so that patients are not denied this potentially important treatment.
There is almost no evidence to support the use of any other non-specific pharmacological agents in the palliation of dyspnea. Other than opioids, the most commonly used drugs are anxiolytics. Only one controlled study has examined the effect of benzodiazepines on breathlessness in COPD; there was no significant benefit from alprazolam, and of concern, there was a trend for deterioration in blood gases in the active arm (Man et al 1986). The anxiolytic, buspirone is of potential interest as it is a respiratory stimulant. However, two RCTs exploring its role in moderate to severe COPD have failed to find evidence of significant benefit (Argyropoulou et al 1993; Singh et al 1993).
Fatigue
Fatigue is an important and common symptom in COPD. It is a subjective and multidimensional symptom that ‘incorporates total body feelings ranging from tiredness to exhaustion, creating an unrelenting overall condition which interferes with individuals’ ability to function’ (Ream and Richardson 1997). Its prevalence varies from 45-80% in COPD, with the variation due to inconsistent definitions of fatigue and study heterogeneity in parameters such as study population and methods of symptom assessment (Lynn et al 1997; Skilbeck et al 1998; Theander and Unosson 2004). Overall, however, there is consistent evidence that fatigue is the second most prevalent symptom in COPD after breathlessness. There is also little doubt that it has a profoundly negative impact on quality of life (Small and Lamb 1999; Theander and Unosson 2004; Katsura et al 2005).
Fatigue in other chronic, progressive diseases, such as cancer and multiple sclerosis has been the subject of increasing interest in recent years with extensive research into its prevalence, etiology and management (Vogelzang et al 1997; Ahlberg et al 2003; NCCN 2005). This is in marked contrast to the situation in COPD. Despite its significant prevalence and negative impact on quality of life, the topic of fatigue in COPD has been relatively neglected.
There have, to date, been very few studies investigating interventions that may help COPD-related fatigue. The only intervention that has been found to give any benefit is pulmonary rehabilitation. A recently updated meta-analysis of 31 RCTs found that rehabilitation, defined as exercise training for at least four weeks with or without education and/or psychological support, lead to a clinically significant reduction in fatigue, as well as improving dyspnea, emotional function and patients’ sense of control (Lacasse et al 2006).
Exercise and reconditioning are rarely feasible options in very advanced disease. The key to improving fatigue in end of care is to improve as much as is possible the underlying causes of the symptom. Fatigue is inextricably linked to three other common symptoms in COPD, dyspnea, depression and insomnia (Woo 2000; Reishtein 2005). Although there is no research evidence to support this, it is intuitively likely that managing these symptoms may improve fatigue. In particular, non-pharmacological measures such as energy conservation and relaxation techniques may have an important role (Theander and Unosson 2004).
Future research examining fatigue in COPD is urgently needed. It is important that the profile of this hitherto neglected symptom is raised. There is some evidence that central nervous system stimulants, such as methylphenidate and modafinil, may have a role in ameliorating fatigue related to cancer and chronic neurological conditions (Rammohan et al 2002; Bruera et al 2006). It may be worth investigating the role of such drugs in advanced COPD where exercise and rehabilitation are no longer an option.
Pain
Pain is another common but neglected symptom in COPD. Several studies have examined the prevalence of pain in advanced COPD. Edmonds et al (2001) conducted post-bereavement structured interviews of the carers of lung cancer and COPD patients and found a prevalence of ‘very distressing’ pain of 56% in both disease groups, despite anecdotal evidence that cancer patients suffer more pain than those dying of other conditions. The SUPPORT study found that 21% of patients admitted to hospital with acute exacerbation of COPD had severe pain, a value not dissimilar to the 28% of lung cancer patients with severe pain (Claessens et al 2000). Skilbeck et al (1998) found that 68% of COPD patients with a recent hospital admission for an exacerbation had pain. Despite the wide range of documented prevalence caused by considerable study heterogeneity, there can be no doubt that pain is a significant problem in patients with advanced COPD.
Surprisingly, there have been no studies investigating the management of pain in COPD. Pain is usually felt in the chest, and may have a musculoskeletal or pleuropulmonary origin (Leach 2005). Causes of pain in COPD include subcostal pain due to diaphragmatic and intercostal muscle fatigue, rib fractures relating to coughing and/or corticosteroid-induced osteoporosis, and pleural inflammation caused by infection.
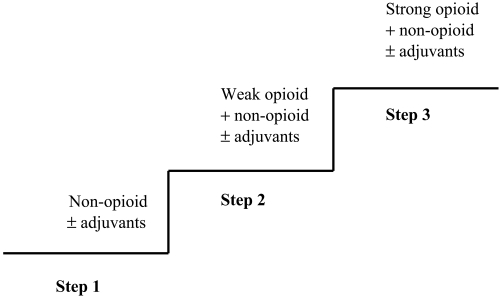
Why is pain such a problem in COPD? In a large retrospective cohort study, Au et al (2006) found that patients who died within six months from COPD were significantly less likely to be given strong opioids for symptom control than patients with lung cancer. A strong possibility is that analgesics are withheld because of concerns about adverse effects, in particular respiratory depression and bronchospasm due to opioids and NSAIDs respectively. The presence of pain should be taken as seriously in COPD as in any other condition. The World Health Organization guidelines for the use of analgesic drugs (Figure 3) provide a useful framework for all chronic pain not just for cancer pain (WHO 1996; Leach 2005).
Figure 3.
World Health Organisation three step analgesic ladder (WHO 1996). Non opioids include paracetamol and NSAIDs; weak opioids include codeine (approx. 1/10th potency of oral morphine) and tramadol (approx. 1/5th potency of oral morphine); strong opioids include morphine, oxycodone and fentanyl; adjuvants are additional drugs that can be used as part of pain management, such as secondary analgesics (eg. gabapentin for neuropathic pain) and drugs to control analgesic adverse effects.
As discussed above, there is no evidence that clinically significant respiratory depression occurs with use of opioids for symptom control. Care should of course be taken, commencing on low doses with judicious upward titration. NSAIDs are often avoided in COPD because of the perceived risk of precipitating bronchospasm. McKeever et al (2005) in a recently published study that analyzed data from over 13,000 individuals from the Third National Health and Nutrition Examination Survey in the USA, found a significant dose-response relationship between paracetamol and the prevalence of COPD, which was not found with aspirin and ibuprofen. Furthermore, paracetamol use was inversely associated with FEV1, whereas regular ibuprofen use was associated with a non-linear improvement in FEV1. It is hypothesized that regular paracetamol use depletes levels of the antioxidant, glutathione, in lung tissue, leading increased lung tissue damage. Ibuprofen may improve lung function through its anti-inflammatory effect. Even in asthma, ibuprofen does not appear to cause harm in population terms (Lesko 2003). In a large study, children with asthma were randomly assigned to receive paracetamol or ibuprofen for a febrile illness (Lesko et al 2002). Those on ibuprofen had significantly less asthma morbidity. These interesting findings are consistent with those of several other studies and deserve further investigation (Konstan et al 1995; Shaheen et al 2000; Shaheen et al 2002).
Overall, existing evidence suggests that paracetamol and NSAIDs should be used with equal care in COPD patients, but that for use in pain at the end of life, benefit from both ibuprofen and paracetamol is likely to outweigh any harm. Ibuprofen may be particularly useful because of its effectiveness for pain of musculoskeletal or pleural origin. A pragmatic approach in COPD may be to use ibuprofen in preference to other NSAIDs, avoid its use individuals with a previous adverse reaction to NSAIDs, and monitor carefully when commencing NSAID-naïve individuals on these drugs. In those that cannot tolerate or gain little benefit from systemic analgesics, intercostal nerve blocks or a thoracic epidural may have a role (Luchette et al 1994).
Anxiety and depression
Advanced COPD is associated with considerable psychological morbidity. Depressive symptoms are not surprising given the associated poor quality of life, limited functioning and social isolation (Skilbeck et al 1998). Anxiety is well known to be both caused by and exacerbated by dyspnea (Bailey 2004). Prevalence data is again highly variable due to inconsistent definitions, heterogenous study populations, and the difficulty in separating symptoms of an affective disorder, such as anorexia, weight loss and sleep disturbance, from that of the disease itself. Overall the majority of studies in patients with moderate to severe COPD give prevalence rates of anxiety and depression each between 20% and 50%, with up to 90% of patients experiencing either or both disorders (Claessens et al 2000; Gore et al 2000; Brenes 2003; Kunik et al 2005; Norwood 2006).
Both affective disorders have a significant negative impact on outcomes in COPD, in particular by reducing perceived quality of life (Aydin and Ulusahin 2001; Felker et al 2001). Comorbid anxiety increases dyspnea and is a significant predictor of the frequency of hospital admission for acute exacerbations of COPD (Gift and Cahill 1990; Yohannes et al 2000; Gudmundsson et al 2005) and both disorders have been linked with poorer outcomes during exacerbations (Dahlén and Janson 2002).
Despite the high prevalence of depression and anxiety, there is evidence that only approximately a third of patients with such disorders are being treated (Kunik et al 2005). This is unfortunate particularly given that, unlike other symptoms of advanced COPD, there is some evidence that simple interventions can provide significant benefit.
Antidepressants have been studied in a number of randomized controlled trials. Both tricyclic antidepressants and selective serotonin reuptake inhibitors (SSRIs) have been found to improve anxiety and depression in patients with COPD. Borson et al (1992) compared the efficacy of nortriptyline with placebo in 30 patients with comorbid depression, of whom 86% had anxiety symptoms, and found that both symptoms of depression and anxiety were improved. Several RCTs have shown benefit from paroxetine and sertraline in both disorders (Papp et al 1995; Smoller et al 1998; Lacasse et al 2004). However, positive outcomes have not always reached statistical significance, and the quality of evidence is limited by small study sizes (Eiser et al 2005).
Pulmonary rehabilitation has been shown to improve anxiety and depression, though this intervention become less feasible in very advanced disease (Paz et al 2007). Two other non-pharmacological approaches that have been studied in those with COPD are cognitive behavioral therapy (CBT) and progressive muscular relaxation (PMR). Evidence in favor of CBT is mixed and PMR may be the most useful non-pharmacological intervention in advanced disease (Renfroe 1988; Lisansky and Clough 1996; Kunik et al 2001). These exercises need to be practiced, and patients will derive most benefit if these techniques are taught early, preferably before the end of life phase.
Key points
There is little evidence to support the commencement of oxygen therapy to palliate dyspnea in those patients who do not qualify for LTOT. Individual clinical assessments can identify those who may benefit from oxygen.
Although there is no evidence that judicious use of opioids for dyspnea leads to clinically significant respiratory depression, there is an urgent need for adequately powered safety studies in COPD patients.
Benefit from both ibuprofen and paracetamol for pain is likely to outweigh harm. There is increasing evidence that, in population terms, ibuprofen may improve lung function, whereas paracetamol may worsen it; further research is needed.
Communication issues in advanced COPD
Inadequate communication is one of the greatest barriers to the provision of good end of life care (Curtis et al 2005). Participants in a large qualitative study identified six major components of a ‘good death,’ of which five depend heavily on the quality of communication between patients and healthcare professionals (Steinhauser et al 2000b) (Table 2). Clear decision-making about treatment preferences empowers patients and leads to a sense of control. Patients often wish to prepare for death by knowing what to expect during the course of their illness and planning for events that would follow their deaths. Completion is a process of individual life review, resolving conflicts, spending time with family and friends and saying goodbye.
Table 2.
Major components of a good death (Steinhauser et al 2000)
| Pain and symptom management |
| Clear decision making |
| Preparation for death |
| Completion |
| Contributing to others |
| Affirmation of the whole person |
Advance care planning, acknowledging the approach of the end of life and discussing the issues that relate to it, is a vital component of end of life care. There is evidence that clinicians’ estimates of patient preferences are influenced by their estimates of patients’ quality of life (Uhlmann and Pearlman 1991). Not only are healthcare professionals a poor judge of patients’ quality of life (Wilson et al 2000), but Stapleton et al (2005) have shown that that patients preferences are, in fact, not determined by their quality of life anyway. It is, therefore, impossible for healthcare professionals to predict patients’ preferences without advance care planning discussions.
Advance care planning
There is good evidence that patients benefit from advance care planning. In COPD patients, advance care planning increases patient satisfaction and sense of control, and reduces fear, anxiety and emotional distress (Smucker et al 1993; Heaven and Maguire 1997; Tierney et al 2001; Curtis et al 2004). The vast majority of COPD patients believe advance care planning is important, and wish to undertake these discussions (Heffner et al 1996; Guthrie et al 2001; Curtis et al 2002). Heffner et al surveyed 105 participants in pulmonary rehabilitation programs and found that over 98% wanted to take part in advance care planning. Surprisingly, given the overwhelming desire to discuss these issues, only 19% had had the opportunity to undertake it. Unmet information needs in COPD patients have been confirmed in subsequent studies (Gore et al 2000). Curtis et al (2004) examined patients’ perceptions of communication quality, and found that patients rated physicians poorly in the areas of discussing prognosis, the process of dying and spirituality. Over half of complaints received by the Healthcare Commission (2007) in the UK relate to end of life care, and the majority of these involve communication issues.
Health care professionals have insight into this discrepancy between need and receipt of advance care planning. Elkington et al (2001) found in a questionnaire survey of UK General Practitioners (GPs) that the majority of GPs felt that discussions of prognosis are necessary and that they have an important role in facilitating these, but a minority actually undertook such discussions. Half of the GPs surveyed believed that patients who wished to discuss prognosis did not get the chance to do this.
Why, then, is advance care planning inadequate? Several barriers to communication about end of life issues have been identified (Curtis et al 2000; Knauft et al 2005; Davison and Simpson 2006) (Table 3). There is a general consensus in the literature that the greatest impediment to good advance care planning is the difficulty of prognostication in COPD, and the consequent difficulty in timing such discussions (Curtis et al 2005). This should not, however, be such a significant concern. There is evidence that patients need information much earlier than is perceived by physicians (Heffner et al 1996; Sullivan et al 1996; Pfeifer et al 2003; Davison and Simpson 2006). With sensitive communication it is unlikely that these discussions will occur too early.
Table 3.
Barriers to communication about end of life issues in COPD
| Healthcare professional barriers |
| Difficulty in timing discussions because of uncertain prognosis |
| Lack of time during consultations |
| Concern about taking away patients’ hope |
| Belief that patients are not ready to discuss end of life issues |
| Patient barriers |
| Expectation that healthcare professionals will initiate discussions |
| Societal taboos with regard to discussing death |
| Uncertainty about which professionals will be involved during end of life phase |
| Lack of certainty about the type of care that would be wanted when less well |
Another significant barrier is that healthcare professionals are reluctant to engage in end of life discussions because of fear of destroying patients’ hope and also patients’ trust in them (Selecky et al 2005). Although understandable, such concerns are unfounded. Indeed, there is evidence that the converse is true. Patients’ ability to hope appears to be sustained by open communication that relieves fears, empowers, enhances relationships with professionals and family, and allows them to see future possibilities consistent with their values (Davison and Simpson 2006). Fears that are not discussed are often far worse than reality.
Strategies to improve communication
The first step in communicating about end of life issues must be to openly acknowledge progressive and irreversible disease with a limited prognosis. Once this has occurred, advance care planning can take place. Issues that should be covered include discussion of patients’ preferred place of care and preferred providers of care during the terminal phase. Future treatment preferences during an acute exacerbation should be established, including views about the future use of antibiotics, mechanical ventilation and advanced life support (Hansen-Flaschen 2004).
Healthcare professionals can develop triggers that lead them to initiate advance care planning discussions. It has been suggested that clinicians can target such discussions by asking themselves, “Would I be surprised if this patient died within the next six months?” (Lynn and Goldstein 2003). Severity of dyspnea is a useful marker of poor prognosis. Nishimura et al (2002) found that the level of dyspnea significantly correlated with survival, and that dyspnea severity was more discriminatory that FEV1 in assessment of prognosis. Admission to hospital with an acute exacerbation of disease could be a trigger for end of life discussions, as there is evidence that over 40% of patients die within one year of an index admission (Lynn et al 2000).
A profile of COPD patients at risk of dying within a year is emerging and includes significant dyspnea, best FEV1 less than 30% predicted, declining performance status with increasing dependence on others, uninterrupted walking distance limited to a few yards, more than one urgent hospitalization within the last year, left heart or other chronic co-morbid disease, older age and depression (Hansen-Flaschen 2004; Yohannes et al 2006). Interestingly, this profile is very similar to that of COPD patients who chose not to have advanced life support following cardiopulmonary arrest (Pang et al 2004).
Good communication skills are a prerequisite in these sensitive discussions. A well-established technique is to take the dual approach of encouraging and sharing hope whilst also preparing for death, in other words “to hope for the best and prepare for the worst” (Back et al 2003). Open questions can be valuable such as, “Have you any concerns about the future?” and “What are you hoping that we can achieve?” Unhelpful phrases that should be avoided include ‘nothing more than can be done,’ ‘stopping treatment’ and ‘withdrawing care’ (Back et al 2003; Hansen-Flaschen 2004; Braun et al 2007). Patients should be reassured that limiting life-sustaining treatment in no way equates to limiting care (Sulmasy and Lynn 1997). It is important that patients are given specific information about outcomes with different treatment options. For example, detailed information about chances of survival after cardiopulmonary resuscitation has been shown to strongly predict treatment preferences (Fried et al 2002).
Advance directives or ‘living wills’ are legally binding documents that can be used to record future treatment preferences. Their usefulness is limited because it is rarely feasible to give precise instructions for all potential eventualities. A non-specific statement such as ‘no life-sustaining treatment if death is certain’ is of no value (Teno et al 1997; Goodman et al 1998). Patients’ views may change over time and there is significant concern about the validity of decisions made when a patient is relatively healthy, or decisions made while suffering from depression (Tonelli 1996; Teno et al 1997; Stapleton et al 2005). Experts in end of life care are increasingly calling for professionals to guard against advance care planning being document-based and focused around the completion of an advance directive (Ditto et al 2001; Hansen-Flaschen 2004; Barnes et al 2007). Clinicians working with patients to plan future care can be supported by, but should not be driven by, legal concerns.
Key points
Advance care planning is of particular importance in COPD patients because of the unpredictable timing of death. Difficulties in determining prognosis should not lead to communication paralysis.
The obligation is on healthcare professionals to initiate discussions about end of life care, and should take place as early as possible in the disease trajectory.
Honest and compassionate advance care planning involves a dual approach of optimism and realism, “hoping for the best and preparing for the worst.”
It must be made clear to patients that limiting life-sustaining treatment does not equate to limiting care.
Service development in end of life care
Current healthcare services for patients with COPD tend to be focused on the management of acute exacerbations and prolongation of life (Au et al 2006). Patients, who are often housebound with advanced disease, seem to remain ‘socially invisible’ until they enter an acute phase, involving either contact with a primary care clinician or admission to an acute hospital (Skilbeck et al 1998). This focus on crisis intervention has led to a fragmented, reactive service rather than a supportive, preventative one (Brumley 2002). This is a major cause of the existing inadequacies in care for patients with advanced COPD.
Principles of service development
The traditional model of care of patients with advanced disease, including COPD, is based on a dichotomy involving an abrupt transition from active, life-sustaining care to palliative care. This model does not work for patients with COPD for several reasons. First, as previously discussed, the timing of death is unpredictable, and it will always be hard to judge when the transition should be made. Second, patients with far advanced disease should not necessarily be denied active interventions such as ventilatory support during an acute exacerbation. Finally, COPD patients often live with a heavy burden of uncontrolled symptoms and psychosocial needs for a relatively long time, compared to patients with other diseases such as cancer; it would be unethical to deny such patients good palliation because they are not yet in the end of life phase.
The mixed management model of care is more appropriate for COPD, and involves combining the principles of good end of life care with active treatment (Glare and Virik 2001). Rather than making an abrupt move from disease-modifying therapy and life prolonging interventions to symptom control and death preparation, all these modalities are combined throughout the disease trajectory (Claessens et al 2000; Braun et al 2007). The key to improving healthcare in COPD is to develop services that reflect the principles of the mixed management model by combining the attributes of both aspects of the traditional dichotomous model.
Patient-centered care is central to the philosophy of palliative care. A disease-orientated approach is inextricably linked with perceptions of failure as patients deteriorate, whereas a patient-centered approach aims for success in optimizing patients’ quality of life. Patient-centered care includes user involvement, and incorporates the views of patients into service design (Blackler et al 2004). Patients with advanced COPD, often isolated by immobility, require community-based care with continuity of care during hospitalisation (Elkington et al 2005). This can only be achieved with a coordinated, collaborative, multidisciplinary service (Brumley 2002). Service development must reflect the trajectory of advanced COPD, integrating rapid intervention during acute exacerbations with education on self-care and planning for unexpected death (Dy and Lynn 2007).
Practicalities of service development
In the last few years, several national initiatives have aimed to drive forward the quality of provision of end of life care. In the USA, the ‘Promoting Excellence in End of Life Care Strategy’ was set up in 1999 as a national program to provide $15 million to support research and fund innovative service developments (Byock et al 2006). In 2004, a national ‘End of Life Care Programme’ was developed in the UK (NHS 2006). £12 million funding over 3 years has been used to disseminate the principles of end of life care for cancer patients to other patient groups, and provide increased patient choice and access to high quality end of life care. As preparation for a forthcoming End of Life Strategy, an ongoing review of end of life services is comparing current service provision with population needs and identifying areas where service improvement is required (Department of Health 2007).
These and other national strategies have lead to a plethora of recent community-based service developments. The PhoenixCare program was a service of home-based palliative care case management for patients with COPD or cardiac failure with a life-expectancy of less than two years (Aiken et al 2006). Services were provided by registered nurse case-managers, in addition to usual active disease treatment provided by managed care organizations. The intervention included education on self-management, preparation for the end of life, and intensive case-management to improve physical and mental functioning. The service was evaluated by randomizing 192 patients into two groups, an intervention group receiving the PhoenixCare program as well as ‘usual care,’ and a control group. Patients who received the intervention reported significantly lower symptom distress, greater vitality and higher self-rated health than controls. Those with COPD showed stronger responsiveness to the intervention than those with cardiac failure.
Two other studies, in patients with a range of advanced conditions including COPD, have evaluated the impact of mixed management service models, combining multidisciplinary palliative care input with active disease management in the community (Brumley 2002; Edes et al 2006). Although uncontrolled and less rigorous in design, both studies showed significant cost-savings, caused by reduced hospitalization.
The End of Life Programme in the UK has promoted the use of three tools to improve the quality of end of life care, the Liverpool Care Pathway (LCP), the Gold Standards Framework (GSF) and the Preferred Place of Care (PPC) document. These tools aim to increase the quality of palliative care provided by generalists. The LCP is an integrated care pathway that prompts, provides guidance on, and documents appropriate care in the terminal phase, for patients with all advanced diseases in all settings (Ellershaw and Ward 2003). It incorporates advice on the use of drugs to control symptoms in the last few days of life. The GSF is a primary care-based system that improves the quality of care by empowering generalists to provide evidence-based best practice including good communication, symptom control, out-of-hours care and carer support. In the UK, over a third of the population are cared for by general practices that have adopted the framework (Munday and Dale 2007; Thomas and Noble 2007). The PPC document can trigger and facilitate advance care planning conversations. Although there is a growing body of evidence to support the use of these tools, including evidence that healthcare practitioners believe that they improve the quality of care (King et al 2005; Veerbeek et al 2006), there is as yet no rigorous controlled trial evidence that these tools improve patient outcomes (Shah 2005; NHS 2006).
Which healthcare professionals are best place to co-ordinate and provide the necessary end of life care for COPD patients? There is an increasing consensus that respiratory nurse specialists should have a central role in this process (Skilbeck et al 1998; Gore et al 2000; Guthrie et al 2001; Elkington and White 2002; Robinson 2005; Shuttleworth 2005; Mulligan 2006). In the UK, such nurse specialists have responsibility for delivery of care and continuity of care across different settings, including the community. They assess and manage patients’ physical, psychosocial and practical needs, and facilitate access to multiprofessional help. Respiratory nurse specialist input appears to increase patient satisfaction, but there is conflicting evidence as to whether they improve quality of life or reduce admission to acute hospital (Skwarska et al 2000; Poole et al 2001; Hermiz et al 2002).
Primary care doctors, such as GPs in the UK, have a significant role in community-based provision of end of life care, and in the recent NHS contract for GPs, incentive payments for COPD management are being used to encourage them to provide this care (Elkington et al 2001, 2005; Lehman 2004; Freeman and Price 2006). Chest physicians also have a vital role. In a recent position statement, the American College of Chest Physicians strongly urges chest physicians to take the lead in focusing on and developing services to provide good quality end of life care (Selecky et al 2005).
As discussed earlier, service development is hindered by an inadequate evidence base. The key to improving end of life care in COPD patients is to undertake good quality research in this patient group, as it cannot be assumed that research outcomes from other study populations are valid in those with COPD (Field and Cassel 1997; Selecky et al 2005). There is increasing evidence that patients facing the end of life actually benefit from taking part in research (Madsen et al 2002; Kendall et al 2007). A recent NIH State-of-the Science conference statement (2004) has outlined future research directions for improving end of life care, with an emphasis on evaluating models of care in terms of patients and family outcomes, and in terms of resource utilization.
Research findings can be used to develop evidence-based education. Incorporating the skills of end of life care into the training all healthcare professionals is a vital step in service development (Hallenbeck 2006). A US national report on the status of medical education in end of life care has revealed that current educational practices are inadequate (Sullivan et al 2003). Palliative care curricula at both undergraduate and postgraduate levels are being developed, incorporating integrated and systematic teaching about end of life care (Simpson et al 1999; Simpson 2000; Wee et al 2001). However, major efforts at an institutional and cultural level are still needed to improve the status and quality of end of life education.
Key points
Good end of life care does not preclude life-sustaining interventions. The COPD disease trajectory means that a mixed management model is vital.
Community-based, co-ordinated, multidisciplinary care is needed, with chest physicians and respiratory nurse specialists having a central role in service development and provision.
End of life care has recently become highly topical, with many national initiatives funding evidence-based developments in services and generalist palliative care education.
Conclusion
COPD is the fourth commonest cause of death worldwide. There is strong evidence that these patients have a particularly poor quality of life in the end of life phase, due to uncontrolled symptoms, psychological morbidity, social isolation and unmet information and communication needs. Traditionally, the palliative care approach has focused on the needs of cancer patients. All patients, however, deserve good quality end of life care, irrespective of diagnosis. Mixed management models where active disease-modifying treatments are combined with palliative care interventions show great promise. Chest physicians and respiratory nurse specialists have a vital role in ensuring that the care of patients dying from COPD improves to the level of the best. As stated by Hansen-Flaschen (2004), “Death is not a failure, but an opportunity to practice a form of professional care that is as old as medicine, and as gratifying as any other services we offer.”
References
- Abernethy A, Currow D, Frith P, et al. Prescribing palliative oxygen: a clinician survey of expected benefit and patterns of use. Palliat Med. 2005;19:168–70. doi: 10.1177/026921630501900219. [DOI] [PubMed] [Google Scholar]
- Abernethy A, Currow D, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327:523–8. doi: 10.1136/bmj.327.7414.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington-Hall J. Reaching out: specialist palliative care for adults with non-malignant disease, Occasional Paper 14. London: National Council for Hospices and Specialist Palliative Care Services; 1998. [Google Scholar]
- Addington-Hall J. Research sensitivities to palliative care patients. Eur J Cancer Care Engl. 2002;11:220–4. doi: 10.1046/j.1365-2354.2002.00343.x. [DOI] [PubMed] [Google Scholar]
- Ahlberg K, Ekman T, Gaston J, et al. Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362:640–50. doi: 10.1016/S0140-6736(03)14186-4. [DOI] [PubMed] [Google Scholar]
- Aiken L, Butner J, Lockhart C, et al. Outcome evaluation of a randomized trial of the PhoenixCare intervention: program of case management and coordinated care for the seriously chronically ill. J Palliat Med. 2006;9:111–26. doi: 10.1089/jpm.2006.9.111. [DOI] [PubMed] [Google Scholar]
- Argyropoulou P, Patakas D, Koukou A, et al. Buspirone effect on breathlessness and exercise performance in patients with chronic obstructive pulmonary disease. Respiration. 1993;60:216–20. doi: 10.1159/000196202. [DOI] [PubMed] [Google Scholar]
- Au D, Udris E, Fihn S, et al. Differences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancer. Arch Intern Med. 2006;166:326–31. doi: 10.1001/archinte.166.3.326. [DOI] [PubMed] [Google Scholar]
- Aydin IO, Ulusahin A. Depression, anxiety comorbidity, and disability in tuberculosis and chronic obstructive pulmonary disease patients: applicability of GHQ-12. 2001 doi: 10.1016/s0163-8343(01)00116-5. [DOI] [PubMed] [Google Scholar]
- Back A, Arnold R, Quill T. Hope for the best, and prepare for the worst. Ann Intern Med. 2003;138:439–43. doi: 10.7326/0003-4819-138-5-200303040-00028. [DOI] [PubMed] [Google Scholar]
- Bailey P. The dyspnea-anxiety-dyspnea cycle: COPD patients’ stories of breathlessness. Qual Health Res. 2004;14:760–78. doi: 10.1177/1049732304265973. [DOI] [PubMed] [Google Scholar]
- Barnes K, Jones L, Tookman A, et al. Acceptability of an advance care planning interview schedule: a focus group study. Palliat Med. 2007;21:23–8. doi: 10.1177/0269216306073638. [DOI] [PubMed] [Google Scholar]
- Belman MJ, Botnick WC, Shin JW. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153:967–75. doi: 10.1164/ajrccm.153.3.8630581. [DOI] [PubMed] [Google Scholar]
- Blackler L, Mooney C, Jones C. Palliative care in the management of chronic obstructive pulmonary disease. Br J Nurs. 2004;13:518–21. doi: 10.12968/bjon.2004.13.9.12960. [DOI] [PubMed] [Google Scholar]
- Booth S, Farquhar M, Gysels M, et al. The impact of a breathlessness intervention service BIS on the lives of patients with intractable dyspnea: a qualitative phase 1 study. Palliat Support Care. 2006;4:287–93. doi: 10.1017/s1478951506060366. [DOI] [PubMed] [Google Scholar]
- Booth S, Kelly MJ, Cox NP, et al. Does oxygen help dyspnea in patients with cancer? Am J Respir Crit Care Med. 1996;153:1515–8. doi: 10.1164/ajrccm.153.5.8630595. [DOI] [PubMed] [Google Scholar]
- Booth S, Wade R, Johnson M, et al. The use of oxygen in the palliation of breathlessness A report of the expert working group of the Scientific Committee of the Association of Palliative Medicine. Respir Med. 2004;98:66–77. doi: 10.1016/j.rmed.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. 1992 doi: 10.1016/S0033-3182(92)71995-1. [DOI] [PubMed] [Google Scholar]
- Braun U, Beyth R, Ford M, et al. Defining limits in care of terminally ill patients. BMJ. 2007;334:239–41. doi: 10.1136/bmj.39048.475046.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenes G. Anxiety and chronic obstructive pulmonary disease: prevalence, impact, and treatment. Psychosom Med. 2003;65:963–70. doi: 10.1097/01.psy.0000097339.75789.81. [DOI] [PubMed] [Google Scholar]
- Breslin EH. The pattern of respiratory muscle recruitment during pursed-lip breathing. Chest. 1992;101:75–8. doi: 10.1378/chest.101.1.75. [DOI] [PubMed] [Google Scholar]
- Brown ML, Carrieri V, Janson B, et al. Lung cancer and dyspnea: the patient’s perception. Oncol Nurs Forum. 1986;13:19–24. [PubMed] [Google Scholar]
- Bruera E, Schoeller T, MacEachern T. Symptomatic benefit of supplemental oxygen in hypoxemic patients with terminal cancer: the use of the N of 1 randomized controlled trial. J Pain Symptom Manage. 1992;7:365–8. doi: 10.1016/0885-3924(92)90091-u. [DOI] [PubMed] [Google Scholar]
- Bruera E, Valero V, Driver L, et al. Patient-controlled methylphenidate for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2006;24:2073–8. doi: 10.1200/JCO.2005.02.8506. [DOI] [PubMed] [Google Scholar]
- Brumley R. Future of end-of-life care: the managed care organization perspective. J Palliat Med. 2002;5:263–70. doi: 10.1089/109662102753641250. [DOI] [PubMed] [Google Scholar]
- Byock I, Twohig J, Merriman M, et al. Promoting excellence in end-of-life care: a report on innovative models of palliative care. J Palliat Med. 2006;9:137–51. doi: 10.1089/jpm.2006.9.137. [DOI] [PubMed] [Google Scholar]
- Carrieri VK, Janson B. Strategies patients use to manage the sensation of dyspnea. West J Nurs Res. 1986;8:284–305. doi: 10.1177/019394598600800303. [DOI] [PubMed] [Google Scholar]
- Carrieri-Kohlman V. Non-pharmacological approaches. In: Booth S, Dudgeon D, editors. Dyspnoea in Advanced Disease. Oxford: Oxford University Press; 2006. pp. 171–203. [Google Scholar]
- Carrieri-Kohlman V, Stulbarg MS. Dyspnea: assessment and management. In: Hodgkin JE, Celli B, Connors G, editors. Pulmonary Rehabilitation. New York: Lippincot Williams and Wilkins; 2002. pp. 57–90. [Google Scholar]
- Chan J, Treece P, Engelberg R, et al. Narcotic and benzodiazepine use after withdrawal of life support: association with time to death? Chest. 2004;126:286–93. doi: 10.1016/S0012-3692(15)32925-1. [DOI] [PubMed] [Google Scholar]
- Claessens MT, Lynn J, Zhong Z, et al. Dying with lung cancer or chronic obstructive pulmonary disease: insights from SUPPORT Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. J Am Geriatr Soc. 2000;48:S146–53. doi: 10.1111/j.1532-5415.2000.tb03124.x. [DOI] [PubMed] [Google Scholar]
- Coventry P, Grande G, Richards D, et al. Prediction of appropriate timing of palliative care for older adults with non-malignant life-threatening disease: a systematic review. Age Ageing. 2005;34:218–27. doi: 10.1093/ageing/afi054. [DOI] [PubMed] [Google Scholar]
- Croft M. Palliative care in end-stage COPD. Br J Gen Pract. 2005;55:234. [PMC free article] [PubMed] [Google Scholar]
- Currow DC, Abernethy AP, Peter F. Morphine for the management of refractory dyspnoea: authors’ reply. BMJ. 2003;327:1288–1289. doi: 10.1136/bmj.327.7426.1288-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J, Engelberg R, Wenrich M, et al. Communication about palliative care for patients with chronic obstructive pulmonary disease. J Palliat Care. 2005;21:157–64. [PubMed] [Google Scholar]
- Curtis J, Wenrich M, Carline J, et al. Patients’ perspectives on physician skill in end-of-life care: differences between patients with COPD, cancer, and AIDS. Chest. 2002;122:356–62. doi: 10.1378/chest.122.1.356. [DOI] [PubMed] [Google Scholar]
- Curtis JR, Engelberg RA, Nielsen EL, et al. Patient-physician communication about end-of-life care for patients with severe COPD. Eur Respir J. 2004;24:200–5. doi: 10.1183/09031936.04.00010104. [DOI] [PubMed] [Google Scholar]
- Curtis JR, Patrick DL, Caldwell ES, et al. Why don’t patients and physicians talk about end-of-life care? Barriers to communication for patients with acquired immunodeficiency syndrome and their primary care clinicians. Arch Intern Med. 2000;160:1690–6. doi: 10.1001/archinte.160.11.1690. [DOI] [PubMed] [Google Scholar]
- Dahlén I, Janson C. Anxiety and depression are related to the outcome of emergency treatment in patients with obstructive pulmonary disease. Chest. 2002;122:1633–7. doi: 10.1378/chest.122.5.1633. [DOI] [PubMed] [Google Scholar]
- Davison S, Simpson C. Hope and advance care planning in patients with end stage renal disease: qualitative interview study. BMJ. 2006;333:886–889. doi: 10.1136/bmj.38965.626250.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health. 2007. Operating framework 2007/8: PCT baseline review of services for end of life care, Department of Health.
- Ditto PH, Danks JH, Smucker WD, et al. Advance directives as acts of communication: a randomized controlled trial. Arch Intern Med. 2001;161:421–30. doi: 10.1001/archinte.161.3.421. [DOI] [PubMed] [Google Scholar]
- Domingo-Salvany A, Lamarca R, Ferrer M, et al. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:680–5. doi: 10.1164/rccm.2112043. [DOI] [PubMed] [Google Scholar]
- Dy S, Lynn J. Getting services right for those sick enough to die. BMJ. 2007;334:511–3. doi: 10.1136/bmj.39127.653704.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton T, Fergusson W, Kolbe J, et al. Short-burst oxygen therapy for COPD patients: a 6-month randomised, controlled study. Eur Respir J. 2006;27:697–704. doi: 10.1183/09031936.06.00098805. [DOI] [PubMed] [Google Scholar]
- Eaton T, Garrett JE, Young P, et al. Ambulatory oxygen improves quality of life of COPD patients: a randomised controlled study. Eur Respir J. 2002;20:306–12. doi: 10.1183/09031936.02.00301002. [DOI] [PubMed] [Google Scholar]
- Eaton T, Lewis C, Young P, et al. Long-term oxygen therapy improves health-related quality of life. Respir Med. 2004;98:285–93. doi: 10.1016/j.rmed.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Edes T, Lindbloom E, Deal J, et al. Improving care at lower cost for end-stage heart and lung disease: integrating end of life planning with home care. Mo Med. 2006;103:146–51. [PubMed] [Google Scholar]
- Edmonds P, Karlsen S, Khan S, et al. A comparison of the palliative care needs of patients dying from chronic respiratory diseases and lung cancer. Palliat Med. 2001;15:287–95. doi: 10.1191/026921601678320278. [DOI] [PubMed] [Google Scholar]
- Eiser N, Harte R, Spiros K, et al. Effect of treating depression on quality-of-life and exercise tolerance in severe COPD. COPD. 2005;2:233–41. [PubMed] [Google Scholar]
- Elkington H, White P. Chronic obstructive pulmonary disease and primary care. Br J Gen Pract. 2002;52:532–4. [PMC free article] [PubMed] [Google Scholar]
- Elkington H, White P, Addington-Hall J, et al. The healthcare needs of chronic obstructive pulmonary disease patients in the last year of life. Palliat Med. 2005;19:485–91. doi: 10.1191/0269216305pm1056oa. [DOI] [PubMed] [Google Scholar]
- Elkington H, White P, Addington-Hall J, et al. The last year of life of COPD: a qualitative study of symptoms and services. Respir Med. 2004;98:439–45. doi: 10.1016/j.rmed.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Elkington H, White P, Higgs R, et al. GPs’ views of discussions of prognosis in severe COPD. Fam Pract. 2001;18:440–4. doi: 10.1093/fampra/18.4.440. [DOI] [PubMed] [Google Scholar]
- Ellershaw J, Ward C. Care of the dying patient: the last hours or days of life. BMJ. 2003;326:30–34. [PMC free article] [PubMed] [Google Scholar]
- Elofsson L, Carling, Ohlén J. Meanings of being old and living with chronic obstructive pulmonary disease. Palliat Med. 2004;18:611–8. doi: 10.1191/0269216304pm922oa. [DOI] [PubMed] [Google Scholar]
- Estfan B, Mahmoud F, Shaheen P, et al. Respiratory function during parenteral opioid titration for cancer pain. Palliat Med. 2007;21:81–6. doi: 10.1177/0269216307077328. [DOI] [PubMed] [Google Scholar]
- Felker B, Katon W, Hedrick SC, et al. The association between depressive symptoms and health status in patients with chronic pulmonary disease. 2001 doi: 10.1016/s0163-8343(01)00127-x. [DOI] [PubMed] [Google Scholar]
- Field M, Cassel K, editors. Approaching death: improving care at the end of life. Washington: Institute of Medicine; 1997. [Google Scholar]
- Fox E, Landrum M, Zhong Z, et al. Evaluation of prognostic criteria for determining hospice eligibility in patients with advanced lung, heart, or liver disease SUPPORT Investigators Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. JAMA. 1999;282:1638–45. doi: 10.1001/jama.282.17.1638. [DOI] [PubMed] [Google Scholar]
- Freeman D, Price D. ABC of chronic obstructive pulmonary disease Primary care and palliative care. BMJ. 2006;333:188–90. doi: 10.1136/bmj.333.7560.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried T, Bradley E, Towle V, et al. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061–6. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- Gallo-Silver L, Pollack B. Behavioral interventions for lung cancer-related breathlessness. Cancer Pract. 2000;8:268–73. doi: 10.1046/j.1523-5394.2000.86005.x. [DOI] [PubMed] [Google Scholar]
- George R, Regnard C. Lethal opioids or dangerous prescribers? Palliat Med. 2007;21:77–80. doi: 10.1177/0269216307076714. [DOI] [PubMed] [Google Scholar]
- Gift AG, Cahill CA. Psychophysiologic aspects of dyspnea in chronic obstructive pulmonary disease: a pilot study. Heart Lung. 1990;19:252–7. [PubMed] [Google Scholar]
- Gift AG, Moore T, Soeken K. Relaxation to reduce dyspnea and anxiety in COPD patients. Nurs Res. 1992;41:242–6. [PubMed] [Google Scholar]
- Glare PA, Virik K. Can we do better in end-of-life care? The mixed management model and palliative care. Med J Aust. 2001;175:530–3. doi: 10.5694/j.1326-5377.2001.tb143711.x. [DOI] [PubMed] [Google Scholar]
- Goodman MD, Tarnoff M, Slotman GJ. Effect of advance directives on the management of elderly critically ill patients. Crit Care Med. 1998;26:701–4. doi: 10.1097/00003246-199804000-00018. [DOI] [PubMed] [Google Scholar]
- Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease COPD? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55:1000–6. doi: 10.1136/thorax.55.12.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson G, Gislason T, Janson C, et al. Risk factors for rehospitalisation in COPD: role of health status, anxiety and depression. Eur Respir J. 2005;26:414–9. doi: 10.1183/09031936.05.00078504. [DOI] [PubMed] [Google Scholar]
- Guthrie SJ, Hill KM, Muers ME. Living with severe COPD: a qualitative exploration of the experience of patients in Leeds. Respir Med. 2001;95:196–204. doi: 10.1053/rmed.2000.1021. [DOI] [PubMed] [Google Scholar]
- Hallenbeck J. Palliative care training for the generalist a luxury or a necessity? J Gen Intern Med. 2006;21:1005–6. doi: 10.1111/j.1525-1497.2006.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen-Flaschen J. Chronic obstructive pulmonary disease: the last year of life. Respir Care. 2004;49:90–7. [PubMed] [Google Scholar]
- Healthcare Commission. 2006. Clearing the air: a national study of chronic obstructive pulmonary disease, Commission for Healthcare Audit and Inspection.
- Healthcare Commission. 2007. Spotlight on complaints: a report on second stage complaints about the NHS in England, Commission for Healthcare, Audit and Inspection.
- Heaven CM, Maguire P. Disclosure of concerns by hospice patients and their identification by nurses. Palliat Med. 1997;11:283–90. doi: 10.1177/026921639701100404. [DOI] [PubMed] [Google Scholar]
- Heffner JE, Fahy B, Hilling L, et al. Attitudes regarding advance directives among patients in pulmonary rehabilitation. Am J Respir Crit Care Med. 1996;154:1735–40. doi: 10.1164/ajrccm.154.6.8970363. [DOI] [PubMed] [Google Scholar]
- Hermiz O, Comino E, Marks G, et al. Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ. 2002;325:938. doi: 10.1136/bmj.325.7370.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KM, Muers MF. Palliative care for patients with non-malignant end stage respiratory disease. Thorax. 2000;55:979–81. doi: 10.1136/thorax.55.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings AL, Davies AN, Higgins JPT, et al. A systematic review of the use of opioids in the management of dyspnoea. Thorax. 2002;57:939–44. doi: 10.1136/thorax.57.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones I, Kirby A, Ormiston P, et al. The needs of patients dying of chronic obstructive pulmonary disease in the community. Fam Pract. 2004;21:310–3. doi: 10.1093/fampra/cmh317. [DOI] [PubMed] [Google Scholar]
- Katsura H, Yamada K, Wakabayashi R, et al. The impact of dyspnoea and leg fatigue during exercise on health- related quality of life in patients with COPD. Respirology. 2005;10:485–90. doi: 10.1111/j.1440-1843.2005.00729.x. [DOI] [PubMed] [Google Scholar]
- Kendall M, Harris F, Boyd K, et al. Key challenges and ways forward in researching the good death: qualitative in-depth interview and focus group study. BMJ. 2007;334:521. doi: 10.1136/bmj.39097.582639.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N, Thomas K, Martin N, et al. ‘Now nobody falls through the net’: practitioners’ perspectives on the Gold Standards Framework for community palliative care. Palliat Med. 2005;19:619–27. doi: 10.1191/0269216305pm1084oa. [DOI] [PubMed] [Google Scholar]
- Knauft E, Nielsen E, Engelberg R, et al. Barriers and facilitators to end-of-life care communication for patients with COPD. Chest. 2005;127:2188–96. doi: 10.1378/chest.127.6.2188. [DOI] [PubMed] [Google Scholar]
- Konstan MW, Byard PJ, Hoppel CL, et al. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med. 1995;332:848–54. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- Kunik M, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127:1205–11. doi: 10.1378/chest.127.4.1205. [DOI] [PubMed] [Google Scholar]
- Kunik ME, Braun U, Stanley MA, et al. One session cognitive behavioural therapy for elderly patients with chronic obstructive pulmonary disease. Psychol Med. 2001;31:717–23. doi: 10.1017/s0033291701003890. [DOI] [PubMed] [Google Scholar]
- Lacasse Y, Beaudoin L, Rousseau L, et al. Randomized trial of paroxetine in end-stage COPD. Monaldi Arch Chest Dis. 2004;61:140–7. doi: 10.4081/monaldi.2004.692. [DOI] [PubMed] [Google Scholar]
- Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006:CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- Lawton J. Gaining and maintaining consent: ethical concerns raised in a study of dying patients. Qual Health Res. 2001;11:693–705. doi: 10.1177/104973201129119389. [DOI] [PubMed] [Google Scholar]
- Leach R. Palliative medicine and non-malignant, end-stage respiratory disease. In: Doyle D, Hanks G, Cherny N, Calman K, editors. Oxford Textbook of Palliative Medicine. Oxford: Oxford University Press; 2005. pp. 894–916. [Google Scholar]
- Lehman R. How long can I go on like this? Dying from cardiorespiratory disease. Br J Gen Pract. 2004;54:892–3. [PMC free article] [PubMed] [Google Scholar]
- Lesko SM. The safety of ibuprofen suspension in children. Int J Clin Pract Suppl. 2003:50–3. [PubMed] [Google Scholar]
- Lesko SM, Louik C, Vezina RM, et al. Asthma morbidity after the short-term use of ibuprofen in children. Pediatrics. 2002;109:E20. doi: 10.1542/peds.109.2.e20. [DOI] [PubMed] [Google Scholar]
- Lewis CA, Eaton TE, Young P, et al. Short-burst oxygen immediately before and after exercise is ineffective in nonhypoxic COPD patients. Eur Respir J. 2003;22:584–8. doi: 10.1183/09031936.03.00027603a. [DOI] [PubMed] [Google Scholar]
- Lisansky DP, Clough DH. A cognitive-behavioral self-help educational program for patients with COPD: a pilot study. Psychother Psychosom. 1996;65:97–101. doi: 10.1159/000289054. [DOI] [PubMed] [Google Scholar]
- Liss HP, Grant BJ. The effect of nasal flow on breathlessness in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1988;137:1285–8. doi: 10.1164/ajrccm/137.6.1285. [DOI] [PubMed] [Google Scholar]
- Luchette FA, Radafshar SM, Kaiser R, et al. Prospective evaluation of epidural versus intrapleural catheters for analgesia in chest wall trauma. 1994 doi: 10.1097/00005373-199406000-00018. [DOI] [PubMed] [Google Scholar]
- Lunney J, Lynn J, Foley D, et al. Patterns of functional decline at the end of life. JAMA. 2003;289:2387–92. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- Lynn J, Ely EW, Zhong Z, et al. Living and dying with chronic obstructive pulmonary disease. J Am Geriatr Soc. 2000;48:S91–100. doi: 10.1111/j.1532-5415.2000.tb03147.x. [DOI] [PubMed] [Google Scholar]
- Lynn J, Goldstein N. Advance care planning for fatal chronic illness: avoiding commonplace errors and unwarranted suffering. Ann Intern Med. 2003;138:812–8. doi: 10.7326/0003-4819-138-10-200305200-00009. [DOI] [PubMed] [Google Scholar]
- Lynn J, Teno JM, Phillips RS, et al. Perceptions by family members of the dying experience of older and seriously ill patients SUPPORT Investigators Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med. 1997;126:97–106. doi: 10.7326/0003-4819-126-2-199701150-00001. [DOI] [PubMed] [Google Scholar]
- Madsen SM, Mirza MR, Holm S, et al. Attitudes towards clinical research amongst participants and nonparticipants. J Intern Med. 2002;251:156–68. doi: 10.1046/j.1365-2796.2002.00949.x. [DOI] [PubMed] [Google Scholar]
- Man G, Hsu K, Sproule B. Effect of alprazolam on exercise and dyspnea in patients with chronic obstructive pulmonary disease. Chest. 1986;90:832–836. doi: 10.1378/chest.90.6.832. [DOI] [PubMed] [Google Scholar]
- Mashford M, Aranda S, Ashby M, et al. Therapeutic guidelines in palliative care. North Melbourne, Australia: Therapeutic Guidelines Limited; 2001. [Google Scholar]
- Mazzocato C, Buclin T, Rapin CH. The effects of morphine on dyspnea and ventilatory function in elderly patients with advanced cancer: a randomized double-blind controlled trial. Ann Oncol. 1999;10:1511–4. doi: 10.1023/a:1008337624200. [DOI] [PubMed] [Google Scholar]
- McDonald CF, Blyth CM, Lazarus MD, et al. Exertional oxygen of limited benefit in patients with chronic obstructive pulmonary disease and mild hypoxemia. Am J Respir Crit Care Med. 1995;152:1616–9. doi: 10.1164/ajrccm.152.5.7582304. [DOI] [PubMed] [Google Scholar]
- McKeever TM, Lewis SA, Smit HA, et al. The association of acetaminophen, aspirin, and ibuprofen with respiratory disease and lung function. American journal of respiratory and critical care medicine. 2005;171:966–71. doi: 10.1164/rccm.200409-1269OC. [DOI] [PubMed] [Google Scholar]
- McKinley R, Stokes T, Exley C, et al. Care of people dying with malignant and cardiorespiratory disease in general practice. Br J Gen Pract. 2004;54:909–13. [PMC free article] [PubMed] [Google Scholar]
- Middlewood S, Gardner G, Gardner A. Dying in hospital: medical failure or natural outcome? J Pain Symptom Manage. 2001;22:1035–41. doi: 10.1016/s0885-3924(01)00362-1. [DOI] [PubMed] [Google Scholar]
- MRC Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;1:681–686. [PubMed] [Google Scholar]
- Mulligan J. Who will champion COPD patients? Nurs Times. 2006;102:45. [PubMed] [Google Scholar]
- Munday D, Dale J. Palliative care in the community. BMJ. 2007;334:809–10. doi: 10.1136/bmj.39174.605081.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray S, Kendall M, Boyd K, et al. Illness trajectories and palliative care. BMJ. 2005;330:1007–11. doi: 10.1136/bmj.330.7498.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi K, Smith AA, Crawford A, et al. Oxygen supplementation before or after submaximal exercise in patients with chronic obstructive pulmonary disease. Thorax. 2003;58:670–3. doi: 10.1136/thorax.58.8.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Clinical Excellence. Chronic obstructive pulmonary disease. Thorax. 2004;59:1–232. [Google Scholar]
- NCCN. Cancer-related fatigue: clinical practice guidelines in oncology, National Comprehensive Cancer Network. 2005 doi: 10.6004/jnccn.2003.0029. [DOI] [PubMed] [Google Scholar]
- NCPC. Minimum dataset report 2005/6, National Council for Palliative Care. 2006 [Google Scholar]
- NHS. End of life care programme: a progress report [online] 2006 URL: http://www.endoflifecare.nhs.uk.
- NHS Executive. 1996. A policy framework for commissioning cancer services: palliative care services, EL(96)85: NHS Executive.
- NIH. 2003. Data Fact Sheet: Chronic Obstructive Pulmonary Disease, 30–5229: National Heart, Blood and Lung Institute.
- NIH. 2004. National Institutes of Health State-of-the-Science Conference Statement on Improving End-of-Life Care. NIH Consensus Development Program.
- Nishimura K, Izumi T, Tsukino M, et al. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121:1434–40. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]
- Nonoyama M, Brooks D, Guyatt G, et al. Effect of Oxygen on Health Quality of Life in COPD Patients with Transient Exertional Hypoxemia. Am J Respir Crit Care Med. 2007 doi: 10.1164/rccm.200702-308OC. [DOI] [PubMed] [Google Scholar]
- Norwood R. Prevalence and impact of depression in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2006;12:113–7. doi: 10.1097/01.mcp.0000208450.50231.c6. [DOI] [PubMed] [Google Scholar]
- NOTT group. Continuous or nocturnal oxygen therapy in hypoxaemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;93:391–398. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- O’Donnell D, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2003. Can Respir J. 2003;10:11A–65A. doi: 10.1155/2003/567598. [DOI] [PubMed] [Google Scholar]
- O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:770–7. doi: 10.1164/ajrccm.164.5.2012122. [DOI] [PubMed] [Google Scholar]
- Pang SC, Tse C, Chan K, et al. An empirical analysis of the decision-making of limiting life- sustaining treatment for patients with advanced chronic obstructive pulmonary disease in Hong Kong, China. J Crit Care. 2004;19:135–44. doi: 10.1016/j.jcrc.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Papp LA, Weiss JR, Greenberg HE, et al. Sertraline for chronic obstructive pulmonary disease and comorbid anxiety and mood disorders. 1995 doi: 10.1176/ajp.152.10.1531a. [DOI] [PubMed] [Google Scholar]
- Paz D, de Montes O, López J, et al. Pulmonary rehabilitation improves depression, anxiety, dyspnea and health status in patients with COPD. Am J Phys Med Rehabil. 2007;86:30–6. doi: 10.1097/phm.0b013e31802b8eca. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Mitchell C, Chamberlain L. The value of disease severity in predicting patient readiness to address end-of-life issues. Arch Intern Med. 2003;163:609–12. doi: 10.1001/archinte.163.5.609. [DOI] [PubMed] [Google Scholar]
- Poole PJ, Chase B, Frankel A, et al. Case management may reduce length of hospital stay in patients with recurrent admissions for chronic obstructive pulmonary disease. Respirology. 2001;6:37–42. doi: 10.1046/j.1440-1843.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- Rammohan KW, Rosenberg JH, Lynn DJ, et al. Efficacy and safety of modafinil Provigil for the treatment of fatigue in multiple sclerosis: a two centre phase 2 study. J Neurol Neurosurg Psychiatr. 2002;72:179–83. doi: 10.1136/jnnp.72.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ream E, Richardson A. Fatigue in patients with cancer and chronic obstructive airways disease: a phenomenological enquiry. Int J Nurs Stud. 1997;34:44–53. doi: 10.1016/s0020-7489(96)00032-6. [DOI] [PubMed] [Google Scholar]
- Reishtein J. Relationship between symptoms and functional performance in COPD. Res Nurs Health. 2005;28:39–47. doi: 10.1002/nur.20054. [DOI] [PubMed] [Google Scholar]
- Renfroe KL. Effect of progressive relaxation on dyspnea and state anxiety in patients with chronic obstructive pulmonary disease. 1988 [PubMed] [Google Scholar]
- Robinson T. Living with severe hypoxic COPD: the patients’ experience. Nurs Times. 2005;101:38–42. [PubMed] [Google Scholar]
- Schwartzstein RM, Lahive K, Pope A, et al. Cold facial stimulation reduces breathlessness induced in normal subjects. Am Rev Respir Dis. 1987;136:58–61. doi: 10.1164/ajrccm/136.1.58. [DOI] [PubMed] [Google Scholar]
- Schwartzstein RM, Simon PM, Weiss JW, et al. Breathlessness induced by dissociation between ventilation and chemical drive. Am Rev Respir Dis. 1989;139:1231–7. doi: 10.1164/ajrccm/139.5.1231. [DOI] [PubMed] [Google Scholar]
- Seamark DA, Blake SD, Seamark CJ, et al. Living with severe chronic obstructive pulmonary disease COPD: perceptions of patients and their carers An interpretative phenomenological analysis. Palliat Med. 2004;18:619–25. doi: 10.1191/0269216304pm928oa. [DOI] [PubMed] [Google Scholar]
- Selecky P, Eliasson C, Hall R, et al. Palliative and end-of-life care for patients with cardiopulmonary diseases: American College of Chest Physicians position statement. Chest. 2005;128:3599–610. doi: 10.1378/chest.128.5.3599. [DOI] [PubMed] [Google Scholar]
- Shah S. The Liverpool Care Pathway: its impact on improving the care of the dying. Age Ageing. 2005;34:197–8. doi: 10.1093/ageing/afi040. [DOI] [PubMed] [Google Scholar]
- Shaheen SO, Newson RB, Sherriff A, et al. Paracetamol use in pregnancy and wheezing in early childhood. Thorax. 2002;57:958–63. doi: 10.1136/thorax.57.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen SO, Sterne JA, Songhurst CE, et al. Frequent paracetamol use and asthma in adults. Thorax. 2000;55:266–70. doi: 10.1136/thorax.55.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth A. Palliative care for people with end-stage non-malignant lung disease. Nurs Times. 2005;101:48–9. [PubMed] [Google Scholar]
- Simpson DE. Introduction to the National Consensus Conference on Medical Education for Care Near the End of Life: executive summary. J Palliat Med. 2000;3:87–92. doi: 10.1089/jpm.2000.3.87. [DOI] [PubMed] [Google Scholar]
- Simpson DE, Rehm J, Biernat K, et al. Advancing educational scholarship through the End of Life Physician Education Resource Center EPERC. J Palliat Med. 1999;2:421–4. doi: 10.1089/jpm.1999.2.421. [DOI] [PubMed] [Google Scholar]
- Singh NP, Despars JA, Stansbury DW, et al. Effects of buspirone on anxiety levels and exercise tolerance in patients with chronic airflow obstruction and mild anxiety. Chest. 1993;103:800–4. doi: 10.1378/chest.103.3.800. [DOI] [PubMed] [Google Scholar]
- Skilbeck J, Mott L, Page H, et al. Palliative care in chronic obstructive airways disease: a needs assessment. Palliat Med. 1998;12:245–54. doi: 10.1191/026921698677124622. [DOI] [PubMed] [Google Scholar]
- Skwarska E, Cohen G, Skwarski KM, et al. Randomized controlled trial of supported discharge in patients with exacerbations of chronic obstructive pulmonary disease. Thorax. 2000;55:907–12. doi: 10.1136/thorax.55.11.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small S, Lamb M. Fatigue in chronic illness: the experience of individuals with chronic obstructive pulmonary disease and with asthma. J Adv Nurs. 1999;30:469–78. doi: 10.1046/j.1365-2648.1999.01102.x. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Pollack MH, Systrom D, et al. Sertraline effects on dyspnea in patients with obstructive airways disease. 1998 doi: 10.1016/S0033-3182(98)71377-5. [DOI] [PubMed] [Google Scholar]
- Smucker WD, Ditto PH, Moore KA, et al. Elderly outpatients respond favorably to a physician-initiated advance directive discussion. J Am Board Fam Pract. 1993;6:473–82. [PubMed] [Google Scholar]
- Solano J, Gomes B, Higginson I. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31:58–69. doi: 10.1016/j.jpainsymman.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Somfay A, Porszasz J, Lee SM, et al. Dose-response effect of oxygen on hyperinflation and exercise endurance in nonhypoxaemic COPD patients. Eur Respir J. 2001;18:77–84. doi: 10.1183/09031936.01.00082201. [DOI] [PubMed] [Google Scholar]
- Spathis A, Wade R, Booth S. Oxygen in the palliation of breathlessness. In: Booth S, Dudgeon D, editors. Dyspnoea in advanced disease. Oxford: Oxford University Press; 2006. [Google Scholar]
- Stapleton R, Nielsen E, Engelberg R, et al. Association of depression and life-sustaining treatment preferences in patients with COPD. Chest. 2005;127:328–334. doi: 10.1378/chest.127.1.328. [DOI] [PubMed] [Google Scholar]
- Steinhauser KE, Christakis NA, Clipp EC, et al. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000a;284:2476–82. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- Steinhauser KE, Clipp EC, McNeilly M, et al. In search of a good death: observations of patients, families, and providers. Ann Intern Med. 2000b;132:825–32. doi: 10.7326/0003-4819-132-10-200005160-00011. [DOI] [PubMed] [Google Scholar]
- Stevenson NJ, Calverley PMA. Effect of oxygen on recovery from maximal exercise in patients with chronic obstructive pulmonary disease. Thorax. 2004;59:668–72. doi: 10.1136/thx.2003.014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan A, Lakoma M, Block S. The status of medical education in end-of-life care: a national report. J Gen Intern Med. 2003;18:685–95. doi: 10.1046/j.1525-1497.2003.21215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KE, Hébert PC, Logan J, et al. What do physicians tell patients with end-stage COPD about intubation and mechanical ventilation? Chest. 1996;109:258–64. doi: 10.1378/chest.109.1.258. [DOI] [PubMed] [Google Scholar]
- Sulmasy DP, Lynn J. End-of-life care. JAMA. 1997;277:1854–1855. [PubMed] [Google Scholar]
- Swinburn CR, Wakefield JM, Jones PW. Relationship between ventilation and breathlessness during exercise in chronic obstructive airways disease is not altered by prevention of hypoxaemia. Clin Sci. 1984;67:515–9. doi: 10.1042/cs0670515. [DOI] [PubMed] [Google Scholar]
- Teno JM, Licks S, Lynn J, et al. Do advance directives provide instructions that direct care? SUPPORT Investigators Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. J Am Geriatr Soc. 1997;45:508–512. doi: 10.1111/j.1532-5415.1997.tb05179.x. [DOI] [PubMed] [Google Scholar]
- Theander K, Unosson M. Fatigue in patients with chronic obstructive pulmonary disease. J Adv Nurs. 2004;45:172–7. doi: 10.1046/j.1365-2648.2003.02878.x. [DOI] [PubMed] [Google Scholar]
- Thomas K, Noble B. Improving the delivery of palliative care in general practice: an evaluation of the first phase of the Gold Standards Framework. Palliat Med. 2007;21:49–53. doi: 10.1177/0269216306072501. [DOI] [PubMed] [Google Scholar]
- Tiep BL, Burns M, Kao D, et al. Pursed lips breathing training using ear oximetry. Chest. 1986;90:218–21. doi: 10.1378/chest.90.2.218. [DOI] [PubMed] [Google Scholar]
- Tierney WM, Dexter PR, Gramelspacher GP, et al. The effect of discussions about advance directives on patients’ satisfaction with primary care. J Gen Intern Med. 2001;16:32–40. doi: 10.1111/j.1525-1497.2001.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli MR. Pulling the plug on living wills A critical analysis of advance directives. Chest. 1996;110:816–22. doi: 10.1378/chest.110.3.816. [DOI] [PubMed] [Google Scholar]
- Traue DC, Ross JR. Palliative care in non-malignant diseases. J R Soc Med. 2005;98:503–6. doi: 10.1258/jrsm.98.11.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twycross R, Wilcock A, Thorp S. Palliative Care Formulary. Abingdon: Radcliffe Medical Press; 1999. [Google Scholar]
- Uhlmann RF, Pearlman RA. Perceived quality of life and preferences for life-sustaining treatment in older adults. Arch Intern Med. 1991;151:495–7. [PubMed] [Google Scholar]
- Uronis HE, Currow DC, Abernethy AP. Palliative management of refractory dyspnoea in COPD. Int J COPD. 2006;1:289–304. doi: 10.2147/copd.2006.1.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varkey B. Unfulfilled palliative care needs of chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2006;12:103–5. doi: 10.1097/01.mcp.0000208448.65478.8f. [DOI] [PubMed] [Google Scholar]
- Veerbeek L, van Zuylen L, Gambles M, et al. Audit of the Liverpool Care Pathway for the Dying Patient in a Dutch cancer hospital. J Palliat Care. 2006;22:305–308. [PubMed] [Google Scholar]
- Vogelzang NJ, Breitbart W, Cella D, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey The Fatigue Coalition. Semin Hematol. 1997;34:4–12. [PubMed] [Google Scholar]
- Walker L. Hypnotherapeutic insights and interventions: a cancer odyssey. Contemp Hypnosis. 2004;21:35–45. [Google Scholar]
- Waterhouse JC, Howard P. Breathlessness and portable oxygen in chronic obstructive airways disease. Thorax. 1983;38:302–6. doi: 10.1136/thx.38.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee B, Hillier R, Coles C, et al. Palliative care: a suitable setting for undergraduate interprofessional education. Palliat Med. 2001;15:487–92. doi: 10.1191/026921601682553978. [DOI] [PubMed] [Google Scholar]
- WHO. Cancer Pain Relief. Geneva: World Health Organisation; 1996. [Google Scholar]
- WHO. World Health Report. Geneva: World Health Organisation; 2003. URL: http://who.int/whr/2003/en/ [Google Scholar]
- Wilson KA, Dowling AJ, Abdolell M, et al. Perception of quality of life by patients, partners and treating physicians. Qual Life Res. 2000;9:1041–52. doi: 10.1023/a:1016647407161. [DOI] [PubMed] [Google Scholar]
- Woo K. Physical activity as a mediator between dyspnea and fatigue in patients with chronic obstructive pulmonary disease. Can J Nurs Res. 2000;32:85–98. [PubMed] [Google Scholar]
- Yohannes A, Baldwin RC, Connolly MJ. Depression and anxiety in elderly patients with chronic obstructive pulmonary disease. Age Ageing. 2006;35:457–9. doi: 10.1093/ageing/afl011. [DOI] [PubMed] [Google Scholar]
- Yohannes AM, Baldwin RC, Connolly MJ. Depression and anxiety in elderly outpatients with chronic obstructive pulmonary disease: prevalence, and validation of the BASDEC screening questionnaire. Int J Geriatr Psychiatry. 2000;15:1090–6. doi: 10.1002/1099-1166(200012)15:12<1090::aid-gps249>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]