Abstract
COPD exerts a substantial burden on health and health care systems globally and will continue to do so for the foreseeable future. Treatment however can be costly and health care providers are interested in both whether treatments can offer improvements in disease burden and whether they represent value for money. Economic evaluations seek to resolve this issue by producing results that can be used to inform and assist the decision maker in allocating scarce health care resources. In this paper we introduce economic evaluation and then use these themes to review and critically appraise the existing COPD economic evaluations, in order to assess quality in light of today’s standards. The use of existing economic evaluations in informing the decision maker is then discussed. Ten out of the fifteen studies were clinical trial or observational study based, and the remaining five on a decision analytic model. Study design, interventions, outcome measures and the use of uncertainty varied considerably; consequentially the results are difficult to compare in any consistent manner. Efforts for future studies to harmonize study design and methodology, particularly towards adopting a modeling framework, using current treatment as comparator and adopting a common effectiveness measure, such as the QALY, should be made in order to produce results that are comparable and useful to a decision maker.
Keywords: COPD, burden of disease, economic evaluation, cost effectiveness, pharmacoeconomic
Introduction
COPD is defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) as:
“…a preventable and treatable disease…characterised by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and associated with an abnormal inflammatory response of the lung to noxious particles or gases.” (GOLD 2006)
COPD is a major cause of morbidity and mortality worldwide and is the only major cause of morbidity that is increasing. In the US, the death rate for COPD doubled between 1970 and 2002 whilst that for all other major causes either decreased or stagnated (Jemal et al 2005). A substantial increase in the global burden of COPD is therefore expected for the future.
Against this backdrop, there is increasing interest in potential treatments for COPD, in particular the use of pharmacotherapy. The aim of current pharmacotherapy, in the absence of a disease cure, is to prevent and control symptoms, reduce the frequency and severity of exacerbations, improve health status and improve exercise tolerance (GOLD 2006). There are numerous treatments available for COPD and these are illustrated in Table 1 below together with current treatment guidelines for the management of the disease. Treatment is dependent upon disease severity; classified here in terms of the GOLD guidelines, with four states from mild through to very severe. Disease management is additive; as the disease progresses through the stages, more treatments are added as shown in Table 1 and because COPD is progressive, treatment must be continuous throughout the lifetime of the patient.
Table 1.
Treatment for COPD
| I) Mild | II) Moderate | III) Severe | IV) Very severe | |
|---|---|---|---|---|
| Classification | FEV1/FVC <0.70 FEV1 ≥80% predicted | FEV1/FVC <0.70 50%≤ FEV1 <80% predicted | FEV1/FVC <0.70 30%≤FEV1 <50% predicted | FEV1/FVC <0.70 FEV1<30% predicted or FEV1 <50% predicted plus chronic respiratory failure |
| Treatment | Influenza vaccination Short acting broncholdilator added when necessary: 1) Short acting β2 agonists: Fenoterol, Salbutamol, Terbutaline. 2) Short acting anticholinergic: Ipratropium. |
|||
| Add rehabilitation. Add regular treatment with one or more long acting broncholdilators if needed: 1) Long acting β2 agonists: Formoterol, Salmeterol. 2) Long acting anticholinergic: Tiotropium. |
||||
| Add inhaled corticosteroids if exacerbations are repeated: Fluticasone, Beclomethasone, Triamcinolone, Budesonide. | ||||
| Add long term oxygen if chronic respiratory failure. Consider surgical treatments: Lung volume reduction surgery; Lung transplantation; Bullectomy. | ||||
Note: This table has been adapted from the GOLD guidelines (GOLD 2006).
New treatments can be costly, however, and health care providers are increasingly interested not only in whether treatments can offer improvements in disease burden, but also whether they offer value for money. Economic evaluation aims to establish the value of a new treatment by bringing together available evidence on the efficacy, effectiveness and costs of that drug compared to an alternative management strategy, such as an existing treatment.
In order to set the scene for the pharmacoeconomics in COPD, the first section describes the burden of illness and financial costs associated with COPD. The second section presents a review and critical appraisal of the published pharmacoeconomic literature in COPD. Finally, the use of existing economic evaluations in informing and assisting the decision maker to allocate scarce health care resources is discussed.
Burden of COPD
This section reviews the health burden of COPD – mortality, morbidity, the effects of age, gender and smoking – and the financial burden, with particular emphasis on the key drivers of cost; disease severity and exacerbation frequency and severity.
Health burden
Morbidity and mortality
Current estimates of the prevalence of COPD in the general population in Europe are within the range of 4%–10% (European Respiratory Society and European Lung Foundation 2003). However actual rates of diagnosis are much lower, approximately 1% in the UK (Calverley and Bellamy 2000), and there is a wealth of evidence that suggests that COPD is heavily under diagnosed worldwide (Calverley and Bellamy 2000; Fukuchi et al 2004; Zielinski et al 2006). It is likely that a substantial number of people are unaware of having COPD (especially so in mild to moderate stages), and who, rather than seek help, may attribute problems such as breathlessness and fatigue to old age rather than on any underlying cause.
The World Health Organization (2000) found global COPD deaths to be the fifth largest killer, accounting for 4.5% of deaths worldwide (Murray Christopher et al 2001). The proportion of deaths for COPD varies between regions of the world: particularly concerning is the Western Pacific region (including China, Malaysia, the Philippines and Vietnam) where COPD accounts for 13.8% of all deaths and where COPD is ranked as the second leading cause of death, as shown in Table 2.
Table 2.
The burden of COPD – mortality
| WHO region | Ranka | %b |
|---|---|---|
| African | 15 | 1.1 |
| Americas | 6 | 3.5 |
| Eastern Mediterranean | 15 | 1.4 |
| European | 5 | 2.8 |
| South East Asia | 9 | 2.2 |
| Western Pacific | 2 | 13.8 |
Ranking for COPD deaths within region, compared to all other diseases/illnesses.
Percentage of total deaths attributable to COPD in each region.
Age and gender
COPD increases with age and is a disease of the elderly. Data from both the US and the UK show that the prevalence of COPD is comparatively small among the under 45s but increases markedly throughout later years (US Department of Health and Human Services 2004). In the UK, about 1% of the general population is diagnosed with COPD increasing with age to around 5% of men between 65 and 74 and rising to 10% in men over 75 (Calverley and Bellamy 2000).
Gender specific mortality and prevalence for COPD seems to be country specific. In Canada and in Northern Europe, there is little difference between death rates by sex (European Respiratory Society and European Lung Foundation 2003). In other countries there are differences: mortality rates for Europe as a whole, suggest that two to three times as many men as women die from COPD (European Respiratory Society and European Lung Foundation 2003): many more men than women die of COPD in Eastern and Southern European countries (Zielinski et al 2006). In Singapore, female hospitalization and mortality from COPD is significantly less than for male counterparts (Zielinski et al 2006). This difference is most probably attributable to historical reasons where smoking rates amongst men have been higher than for women. In recent years and in some countries, women now smoke as much as their male counterparts. Where smoking prevalence rates are equal—and have been for some time—we would expect to see similar mortality rates for COPD.
Smoking
COPD causes an accelerated depreciation of lung function over time compared with the average or predicted level for a healthy person, and is further accentuated by smoking. Calverley suggests that around 20% of smokers are susceptible to some form of progressive lung disease (Calverley and Bellamy 2000), but it may be larger than this; Lokke reports that the absolute risk of developing COPD in smokers is at least 25% (Lokke et al 2006). In China where COPD represents a major public health problem, there is a huge population of smokers: 67% of men smoke (approximately 300 million) (Mackay and Eriksen 2002). It is estimated that 15% of people in China, who have ever smoked may have COPD (5% of never smokers) (Zielinski et al 2006).
As yet no pharmacological treatments have been found to alter this decline: only smoking cessation has been shown to effectively slow the deterioration in FEV1 and return the trajectory of lung function to one consistent with that of a non-smoker (Fletcher and Peto 1977). The Lung Health study, at five years, found significantly lower all-cause mortality rates in a ‘special intervention’ group where smoking cessation was actively encouraged, compared to the nonintervention group (Anthonisen et al 2005).
Financial burden
In the UK, total costs to the NHS for COPD have been estimated somewhere between £486 million (€ = 719 million) (Calverley and Sondhi 1998; Britton 2003) and £848 million (€ = 1255 million) (Guest 1999; Sullivan et al 2000) per year. Additionally, including some societal costs, most notably productivity costs (costs arising from loss of income through inability or absence from work), pushes total costs for COPD up to £982 million (€ = 1453 million) per year (Guest 1999; Britton 2003). Translating to a per patient cost of between £781 (€ = 1156) (Calverley and Sondhi 1998)and £1154 (€ = 1708) per year (£1639 (€ = 2425) when including societal costs). The major drivers of this burden are disease severity and exacerbations. These are discussed below.
Drivers of cost
Disease severity
Costs increase substantially as disease severity moves from moderate to severe (with a much smaller increase between mild and moderate) (Britton 2003; Chapman et al 2003; Dal Negro et al 2003; Halpern et al 2003; Izquierdo 2003; Piperno et al 2003; Wouters 2003). Britton estimates that the direct costs of the three groups to be mild, €232 moderate, €477 and severe, €2026. These values almost double with the inclusion of productivity costs (Britton 2003). As FEV1 deteriorates, a general shift from outpatient care to hospitalization, an increase in the use of oxygen therapy and a subsequent increase in total costs, especially in the most advanced stages of the disease has been shown to occur (Jansson et al 2002).
Exacerbations
Exacerbations are the leading driver of cost in COPD. A serious exacerbation will lead to hospitalization; indeed an exacerbation is the main reason why a COPD patient would attend hospital. COPD has been found to be the cause of approximately 90,000 hospital admissions within the UK alone and around 1 million hospital bed days per year (Lung and Asthma Information Agency 2003). The cost of exacerbations has been found to increase in line with the severity of exacerbations; a Swedish study reports: SEK 120 (€ = 13) for a mild, SEK 354 (€ = 38) for mild/moderate, SEK 2111 (€ = 225) moderate and SEK 21,852 (€ = 2326) for a severe exacerbation. Exacerbations account for between 35%–40% of total health care costs (Andersson et al 2002). Hospitalizations as a proportion of total costs in other countries are also high; in the USA: 70.3% (Halpern et al 2003), Italy: 76.3% (Dal Negro et al 2003) and Spain: 83.62% (Izquierdo 2003).
In the UK, the excess cost arising from acute exacerbations has been estimated to be £45 million at 1994 prices (€ = 67 million) for a COPD population of 233,000 (McGuire et al 2001). Treatment which acts to reduce or prevent disease progression and or an exacerbation (particularly severe exacerbations) will have a direct effect on the total cost for COPD: in England and Wales, McGuire calculated that for every exacerbation related hospital admission avoided a total saving of approximately £1200 (€ = 1776) would be made (McGuire et al 2001).
Productivity costs
Productivity costs are generally regarded as the cost of time off work due to illness. Productivity costs for COPD represent a significant burden on society as COPD is a major cause of absenteeism from work (Britton 2003; Halpin 2006). People with COPD have a “substantially shortened’ work life compared to the population average (Yelin et al 2006). Between 1994 and 1995, it was estimated that 24 million lost work days were attributable to COPD within the UK alone (Calverley and Sondhi 1998). Within the 15 ‘original’ EU member states, COPD is estimated to account for 41 300 lost work days per 100 000 people and productivity losses of around €28.5 billion per year. In Central and Eastern Europe, the number of lost work days is just 10% of this value; 4300 per 100 000 people (European Respiratory Society and European Lung Foundation 2003).
Within the UK, 44% of COPD patients were found to be below retirement age: because of the condition, 24% were completely prevented from working. 5% of patients’ carers missed work. Around 12 days were missed from work, per patient per year. Productivity costs were found to be almost equivalent in size to direct costs; imposing an additional £820 (€ = 1213) per patient per year upon the economy (Britton 2003).
Whilst productivity costs can represent a considerable burden on both the individual and on society, their use in cost effectiveness analyses is an area of controversy. This is partly due to methodological uncertainty concerning the appropriate method of measuring productivity losses, especially where significant unemployment is evident (Sculpher 2001). The US Panel on cost-effectiveness analysis recommends a societal perspective which can include productivity losses (Gold et al 1996). By contrast, the National Institute for Health and Clinical Excellence in England and Wales limits its perspective to the health service and personal social services perspective and explicitly excludes the use of productivity costs in its evaluations.
Introduction to economic evaluation
Economic evaluation methodology has developed significantly over recent years. In this section, we present some of the key concepts within economic evaluation. First, we provide an overview of economic evaluation and the various types that are often used. Second, we cover the evaluative process and describe important characteristics that should be conceptualized within each study: the perspective of the study and costs, the timeframe of the analysis, the use of appropriate comparators and outcome measures, the importance of accounting for uncertainty and issues surrounding industrial sponsorship.
Economic evaluation: an overview
Economic evaluations are used to assist decision makers in allocating scarce resources. Within health care, the overall objective is often considered to be to maximize the health of a given population, subject to the budget constraint for health care.
Many countries around the world now include a role for the incorporation of economic evidence into the decision making process for health, including many European countries, Australia and Canada (ISPOR 2007). In England and Wales, the relevant decision making agency is the National Institute for Health and Clinical Excellence (NICE) who are an organization independent from Government, responsible for providing guidance on the use of health technologies and the implementation of public health programs.
In the process of developing guidance, NICE brings together all the available clinical and economic evidence in order to decide whether the adoption of the technology (drug or treatment) represents good value for the NHS (National Institute of Health and Clinical Excellence 2005).
Types of economic evaluation
An economic evaluation can either be a cost effectiveness, cost utility analysis, cost minimisation or cost benefit analysis.
A cost benefit analysis measures the value of an intervention by a monetary value such as the dollar, or the Euro, and compares it to the costs of providing the treatment. The decision criteria as to whether or not to adopt the technology is based upon whether the benefits are greater than the costs. Where the alternatives under consideration have equal benefit or effect, then it is possible to choose the optimal treatment based only upon their cost; this method is known as cost minimization. However, this scenario occurs infrequently because more often than not, different treatments will cause different effect and unless this is the case, cost minimization is inappropriate (Briggs and O’Brien 2001). More useful are cost effectiveness and cost utility analyses, which allow a full economic evaluation to take place where both the costs and outcomes are analyzed. The main difference between them lies in the outcome measure. Cost effectiveness studies present results in terms of natural units such as the cost per exacerbation avoided (Oostenbrink et al 2005), and improvement in health status (Jones and Wilson 2003). A cost utility evaluation identifies the cost per unit of utility (usually the Quality Adjusted Life Year [QALY]). Utilities and the QALY concept are described in detail below.
The decision making framework
The outcome measure(s) and corresponding type of economic evaluation, should be based upon the aim and intended use of the evaluation. Because of the outcome measures used within a cost effectiveness analysis, these studies can but inform the efficient use of resources within COPD care. In order to inform the choice of whether to allocate more health care resources to the treatment of COPD compared to other disease areas, a generic outcome – a cost utility analysis – is required as this enables a comparison both within and across disease areas to be made. Indeed both NICE and the US Panel book recommend cost-utility analyses as the appropriate way to make comparative assessments of value for money within a health system (Gold et al 1996; Jones and Wilson 2003; National Institute for Clinical Excellence 2004).
Utilities and QALYs
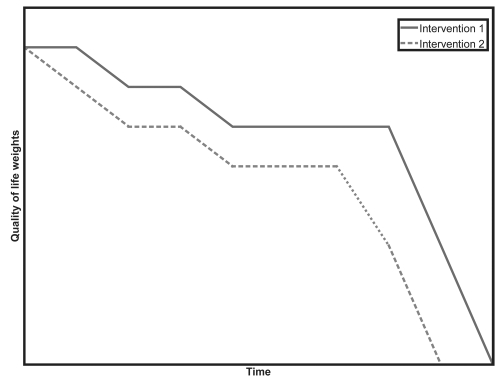
A utility measure is a measure of the relative satisfaction – or quality of life – gained. In essence, a new treatment that leads to improved quality of life over a time period, compared to existing treatment, will generate greater utility than if the patient remained taking the existing treatment. The QALY quantifies changes in utility over the life of the patient. The QALY has two components; quality and quantity of life.
In Figure 1 we can see that intervention 1 leads to higher quality of life (the line is above the other) and, greater life expectancy (quantity) than for intervention 2. The area between these lines represents the difference in the number of QALYs between the two treatments.
Figure 1.
QALY’s.
For example, a treatment may improve quality of life over the remainder of the patient’s life by 0.03, from 0.6 to 0.63 and extend the life of the patient from 10 years to 11. This will give:
| New treatment | Existing treatment |
| 10 years at 0.63 = 6.3 | 10 years at 0.6 = 6 |
| Plus one extra year of life = 0.63 | |
| QALYs = 6.93 | QALYs = 6 |
The new treatment will add a total of 0.93 QALYs compared with the existing treatment. The QALY is by far the most accepted health-related utility measure and is the preferred outcome measure in many countries including Canada, New Zealand, Sweden, England and Wales, the Netherlands, and the US (ISPOR 2007).
ICERs
Using QALYs within an economic evaluation can allow treatments to be compared with one another across and within disease areas using a cost per QALY ratio, usually an Incremental Cost Effectiveness Ratio (ICER). ICERs allow comparison of the incremental costs (C) and incremental benefits (B) of a new therapy (x) compared to the main comparator (y). The ICER is worked out using the simple formula:
Using the previous example of a gain of 0.93 QALYs following treatment with the new drug and comparing this to the cost of the new treatment; £15,000 over the remainder of the patient’s life, compared to the cost of the existing treatment; £1000 gives an ICER of:
The resulting ICER describes the additional cost of an extra QALY by taking the new treatment rather than the existing one: the new treatment can provide one extra QALY for a cost of £15,054.
Threshold
Because the reimbursement of one treatment will displace monies spent from other treatments within the health system, it is important to ensure that the treatment reimbursed provides sufficient value for money: we need a ‘guide price’ or a threshold from which we can decide whether or not any one treatment should be reimbursed by the health care system. In the UK, NICE’s documented threshold is said to be between £20,000 and £30,000 per QALY, but this varies. It can be said that below £20,000 per QALY there is a high probability of the technology being accepted and that above £30,000 per QALY there is less chance of the technology being accepted. A more detailed discussion of relevant thresholds, including why a single threshold is not an appropriate concept, has been dealt with elsewhere (Culyer et al 2007).
Study-specific characteristics
Characteristics of health economic evaluations are outlined in this section in order to provide a foundation for the critical appraisal of studies.
Perspective and costs
The choice of perspective depends upon the target audience for the study (Philips et al 2006). There are several perspectives: a specific provider/provider institution (ie, the National Health Service [NHS]), the patient/patient group, a third party payer (ie, an insurer) or the perspective of society (Drummond et al 2005). The perspective used will determine the costs employed and these must be appropriate and as comprehensive as required. A societal perspective would typically include productivity loss due to absence from/inability to work: a study from the perspective of a third party payer would not. NICE prefer the perspective of the NHS in their decision making process. It is often argued however, that economic studies for COPD should include all relevant costs associated with the illness (Sullivan 2003) and several countries – including Sweden and the Netherlands – suggest a societal perspective.
Study design
A study based upon a single source generally has patient level data and relies solely upon the results generated from that source to provide values for relative treatment effects, resource use, baseline characteristics, health outcomes and costs. Conversely, model-based evaluations usually incorporate data from a wide range of sources including: clinical and observational trials, burden of disease studies, epidemiologic and natural history studies.
Patient group
An economic evaluation can use efficacy results from either, the whole population of interest or of different subgroups (ie, disease severity, age) within this population. It is important to consider any subgroups in order to permit, where evidence allows, the identification of any specific group of patients to whom the technology is particularly cost-effective.
Timeframe of the analysis
Economic evaluations attached to clinical trials are naturally constrained by the timeframe of the trial to which they are connected. Decision analytic models have an advantage in that through applying adequate and transparent assumptions, the results can be extrapolated into the future, up to a lifetime timeframe. The definition of the timeframe of a study is crucial because the full benefit of treatment may not occur within the period of the trial: the timeframe of the model should extend far enough into the future so that the key differences between the comparators in the analysis can be established (Philips et al 2006).
“There is no natural interpretation for life-years gained during a finite period of time, and the CE ratios that result from using different time horizons, such as one year and five years, cannot be compared in any meaningful way … researchers who truncate their analyses have made, perhaps unwittingly, the implausible alternative assumption that study subjects experience neither the costs nor the benefits of living beyond the period of study” (Garber 2000 p191–2).
Comparators
Drummond defines economic evaluation as “the comparative analysis of alternative courses of action in terms of both their costs and consequences” (Drummond et al 2005 p 9). The comparative nature of economic analysis is all important since the use of an inappropriate comparator can bias an analysis and render it worthless for decision making. Most published guidelines for economic evaluations assert that the comparator of interest is current treatment (Drummond and Sculpher 2005). However, as the primary endpoint of RCTs which feed the economic evaluation with the clinical effectiveness data is drug registration, trials often provide the relative treatment effect compared to placebo only and this has been highlighted as a potentially fundamental problem of conducting economic analyses alongside clinical trials (Sculpher et al 2006).
Outcome measures and presentation of results
Whilst outcome measures should represent the aim of treatment; to prevent and control symptoms, reduce the frequency and severity of exacerbations, improve health status and improve exercise tolerance (GOLD 2006), in order to make resource allocation decisions across disease areas, a generic form of outcome measure such as the QALY is required. Presenting cost-effectiveness analysis in natural units, such as cost per exacerbation avoided, will potentially limit the scope for decision making on the efficient allocation of resources within COPD.
Handling of uncertainty
Uncertainty exists around all economic evaluations: there are uncertainties about the inputs into the study, and additionally, for decision analytic models, around the design of the study and any extrapolation of the results into the future. Where studies are undertaken within patient level data, a statistical analysis of the data will be possible allowing the use of confidence intervals around cost-effectiveness results to summarize uncertainty. If the study uses a decision analytical modeling framework, sensitivity analysis, ideally probabilistic should be employed (Claxton et al 2005); this allows the combined uncertainty of all the parameters in the model to be included (Briggs 2002), by using the full probability distributions of each input into the model, rather than just the point estimates. A number of best practice guidelines (eg, the BMJ, NICE and US Panel) state that the uncertainty surrounding estimates of cost effectiveness needs to be explored when presenting economic evaluation results (National Institute for Clinical Excellence 2004). Probabilistic sensitivity analysis is now a formal requirement for cost effectiveness models submitted to NICE (National Institute for Clinical Excellence 2004).
Sponsorship
Industry sponsored studies represent a major source of funding for economic evaluations; however, concerns have been raised regarding potential biases arising as a result of this alliance. Studies have found an association between pharmaceutical sponsorship and the probability of a favorable result (Friedman 1999; Baker et al 2003; Bell et al 2006).
Pharmacoeconomics of COPD treatments
This section reviews the published literature on pharmacoeconomic assessments of treatments for COPD and uses the characteristics of studies identified in the previous section to structure the review. The section begins with the search strategy and then we summarize the economic evaluations found from the search, categorized naturally into two: studies based on clinical trial or observational data alone and those based on a decision analytic model.
Study identification
Search strategy
Four databases were used to search the literature: MEDLINE, EMBASE, the Centre for Reviews and Dissemination (CRD) and the Health Economics Evaluations Database (HEED) and restricted from 1990 until 2007. The search strategy was designed for MEDLINE and also applied to EMBASE. Included terms were: “Lung diseases, obstructive/ or bronchitis/ or pulmonary disease, chronic obstructive” or “COPD”, and “costs and cost analysis”/ or “cost benefit analysis”/ or “cost of illness”/ or “health care costs”/ or “health expenditures” and “quality adjusted life years/ or cost utility” or “health adj4 utilization” or “economic$ or economics, Pharmaceutical.”
For CRD and HEED—where this type of search strategy is not possible to apply—“cost” or “effectiveness” and “chronic” or “COPD” and the names of the individual drugs were used: “Fluticasone”, “Salbutamol”, “Ipratropium”, “Formoterol”, “Tiotropium” etc. MEDLINE gave 732 hits; EMBASE = 159; CRD = 235 and HEED = 53. Removing duplicates, a total of 918 papers were found (March 2007). Reference lists from key papers were also searched for studies and papers: relevant papers were selected by first reviewing the abstracts and then if deemed appropriate, the papers were obtained. Papers satisfying the inclusion and exclusion criteria were selected. Inclusion criteria were: economic evaluations of pharmacotherapy for COPD and in the English language. Exclusion criteria included papers where the focus was upon: cost alone, antibiotics or nonpharmacotherapy treatments. Fifteen pharmacoeconomic evaluations of COPD were identified.
Table 3 provides a concise summary of the features of the economic evaluations, including the type of study, the main outcomes measures used and the design of the study.
Table 3.
Summary of COPD economic evaluations
| Type | Study design | Outcome measures | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors | CUA | CEA | Clinical trial | Observational | Markov model | FEV1a | Exacerbationsa | SGRQa | Survivala | QALY |
| Ayres (2003) |  |
 |
 |
 |
||||||
| Briggs (2006) |  |
 |
 |
 |
||||||
| Friedman (1999) |  |
 |
 |
|||||||
| Gagnon (2005) |  |
 |
 |
|||||||
| Hogan (2003) |  |
 |
 |
 |
||||||
| Jones (2003) |  |
 |
 |
 |
||||||
| Jubran (1993) |  |
 |
||||||||
| Lofdahl (2005) |  |
 |
 |
|||||||
| Maniadakis (2006) |  |
 |
||||||||
| Oostenbrink (2004) |  |
 |
 |
 |
 |
|||||
| Oostenbrink (2005) |  |
 |
 |
 |
||||||
| Rutten-van Molken (1995) |  |
 |
 |
 |
||||||
| Sin (2004) |  |
 |
 |
 |
||||||
| Spencer (2005) |  |
 |
 |
 |
||||||
| Van den Boom (2001) |  |
 |
 |
 |
||||||
Note: Referring to a clinically meaningful change in each of these parameters, eg, some authors use a four point change on the SGRQ to mean a clinically significant change.
The majority of studies identified were conducted alongside clinical trials (9/15); one used an observational study and five a decision analytic model. Evaluations were either cost utility analyses (5/15) or cost effectiveness analyses (10/15). All the cost utility analysis studies used the QALY as an outcome measure. Along with the QALY, the main outcome measures were survival, reduction in exacerbations, improvement in SGRQ and improvement in FEV1.
Review of the clinical trial papers and the observational study
The key results from the nine randomized controlled trial studies and observational study are displayed in Table 4 and are described more fully within this section.
Table 4.
Summary of trial and observational study based economic evaluations
| Perspective | Countrya | Cost year (currency) | Duration (mths) | Patient groupb | Interventionsc (n = numbers in each arm of the study) | Outcome measure | Outcome | Positive outcomed | Uncertainty | Sponsor | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical trial based | |||||||||||
| Ayres (2003) | NHS Societal | UK | 1998(£) | 6 | I,II,III | Placebo n = 139 Fluticasone n = 142 |
Improvement in FEV1 (≥10%) Proportion of patients remaining exacerbation free (moderate/ severe and mild) after 6 months. | Per patient per day: Cost per relevant improvement in FEV1 = £0.25 (NHS perspective) = -£3.39 (societal). Cost per relevant proportion of patients remaining free of moderate and severe exacerbations = £0.25 (NHS) and £-3.28 (Societal). | ✓ | Univariate | GSK |
| Briggs (2006) | NHS | UK | 1998(£) | 36 | II, III | Placebo n = 370 Fluticasone n = 372 |
Quality adjusted life expectancy over three years. Life expectancy. | Cost per additional life year gained = £17,700. Cost per QALY gained £9500. Gain in Life expectancy = 23 days | ✓ | PSA | GSK |
| Friedman (1999) | – | USA | 1998($) | ≈3 | II, III | Ipratropium n = 362 Albuterol n = 347 Ipratropium and Albuterol n = 358 |
Peak change in FEV1. Area under the FEV1 response time curve from time 0 to 4 hrs (FEV1 AUC0-4). | ICER not calculated: Combination product striclty dominated albuterol. | ✓ | None | BI |
| Hogan (2003) | Payer | USA | 2002($) | ≈3 | II, III | Placebo n = 200 Ipratropium n = 194 Formoterol 12 μg n = 194 Formoterol 24 μg n = 192* |
Change in FEV1. Change in Quality of life as assessed via the St Georges Respiratory Questionnaire. | Cost per relevant improvement in FEV1: Pl vs Ip = $273.03. Ip vs Fo 12μg = $1611.32. Cost per relevant improvement in QoL: pl vs Fo 12μg = $25.20. | ✓ | Univariate | Partly by Novartis |
| Jones (2003) | Payer | UK | /(£) | ≈4 | II | Placebo n = 227 Salmeterol 50 mcg n = 229 Salmeterol 100 mcg n = 218 |
Improvement in FEV1(≥15%). % of symptom free nights. % of days with a daytime symptom card <2. Improvement in health status as measured by the SGRQ (≥(−)4 points). | Per patient per day: Cost per relevant improvement in FEV1 = £4.62. Cost per symptom free night = £5.67. Cost per daytime symptom card of <2 = £12.33. Cost per relevant increase in health status = £4.44. | ✓ | None | One author working for GSK |
| Lofdahl (2005) | Payer | Sweden | 2001(€) | 12 | III, IV | Placebo n = 228 Budesonide n = 243 Formoterol n = 235 Budesonide and Formoterol n = 245 |
Avoidance of an exacerbation requiring medical intervention. | Comb is cost effective vs placebo if a decision maker is willing to pay about €2 per day per avoided exacerbation. | ✓ | PSA | AZ |
| Oostenbrink (2004) | Societal | NL BE | 2001(€) | 12 | II, III |
Tiotropium n = 344 Ipratropium n = 175 |
Reduction in exacerbations. Improvement in health status as measured by the SGRQ (≥(−)4 points). Improvement in trough FEV1(≥12%). Improvement in the transitional dyspnoea index (TDI) (≥1 unit). | Per patient per year: Cost per: exacerbation avoided = €667; improvement in health status = €1084; improvement in dyspnea = €1259; relevant improvement in FEV1 = €796. At a threshold of €2000, probability of being cost effective to avoid one exacerbation is 80%. | ✓ | PSA | BI |
| Rutten-van Molken (1995) | Societal | NL | 1989($) | 30 | II, III |
β2 agonist and corticosteroid n = 91 β2 agonist and anticholiner gic n = 92 β2 agonist and placebo n = 91 |
Improvement in FEV1(≥10%). Hyper-responsiveness. Restricted activity days. Symptom free days. |
Cost per relevant improvement in FEV1: Co vs Pl = $200 and $5.35 per symptom free day. | ✓ | None | Partly by GSK |
| Van den Boom (2001) | – | NL | 1999($) | 12 | I, II, III, IV | Placebo n = 41 Fluticasone 250 μg (×2 daily) n = 33 |
Improvement in FEV1. Quality adjusted life years. | Basecase: Cost per QALY = $13,016. Early detection and treatment: Cost per QALY = $33,921 (direct costs) $14,031 (direct and productivity costs). | ✓ | None | Various |
| Observational study based | |||||||||||
| Gagnon (2005) | Payer | USA | 2001($) | 36 | I, II, III, IV | No ICS, no LABA (placebo) n = 274 Inhaled corticosteroid (ICS) n = 538 B2 agonists (LABA) n = 130 LABA and ICS n = 212 |
Life expectancy | 3 yr: ICER: LABA vs ICS/LABA = $91,430. Lifetime: ICER: placebo vs LABA = $6110. ICER: LABA vs comb = $27 570. Discounted gain in life expectancy in days (within study/lifetime respectively) No ICS and no LABA = 2.41/3.88, LABA and ICS = 2.70/6.14. | ✓ | PSA | Partly by GSK |
Notes: NL stands for the Netherlands and BE for Belguim.
Patient groupings within each study, standardised in line with the GOLD guidelines.
Bold type corresponds to the primary drug of interest.
Positive outcome for the primary drug of interest.
Perspective
Three different perspectives were used: the provider (ie, the NHS) (Ayres et al 2003; Briggs et al 2006) , societal (Ayres et al 2003; Oostenbrink et al 2004) and the payer (Hogan et al 2003; Jones and Wilson 2003; Gagnon et al 2005; Lofdahl et al 2005). In two cases no reference to perspective was made (Friedman 1999; van den Boom 2001).
Patient group
In order to compare patient severity for COPD, we converted the information given into groups in line with the GOLD classifications. However, often scarce reporting within some papers around inclusion and exclusion criteria as well as heterogeneous classifications made it difficult to convert accurately the disease severities into the GOLD classifications. All studies seemed to include severe COPD patients: most focusing upon moderate to severe (Rutten-van Molken et al 1995; Friedman 1999; Ayres et al 2003; Oostenbrink et al 2004; Briggs et al 2006). The observational study had COPD patients in each severity category as did Van den Boom’s. No reference to patient severity was made in Hogan’s paper and so the original trial publication had to be searched for this information (Dahl et al 2001).
Patient numbers and duration of the study
Patient numbers in each of the studies varied substantially from 74 participants (van den Boom 2001) to 1067 (Friedman 1999). Most tended to have upwards of 500 participant, usually equally distributed between the treatment arms.
The shortest study durations were 3 months each (Friedman 1999; Hogan et al 2003), three were 12 months and the longest reported studies were 36 months (Gagnon et al 2005; Briggs et al 2006).
Comparators
Perhaps due to the very nature of the RCT and the complexities that doing so would demand, none of these studies used the preferred comparator of ‘current treatment’. Placebo was a comparator in seven out of the ten studies. In four out of the ten studies, the main point of interest was the cost effectiveness of a combined drug compared to its component parts and sometimes placebo. (Friedman 1999; Gagnon et al 2005; Lofdahl et al 2005; Rutten-van Molken et al 1995). For example, Lofdahl investigated the cost effectiveness of placebo, Budesonide, Formoterol and the combination Budesonide and Formoterol. Oostenbrink measured the effects of replacing the short acting anticholinergic, Ipratropium with the long acting equivalent, Tiotropium. Where two different dosages were examined, the dominated treatment was dropped from further study (Hogan et al 2003; Jones and Wilson 2003).
For the purposes of drug efficacy, these trials are perfectly acceptable. A problem arises when establishing cost effectiveness: where, as previously described, we use incremental effects and benefits arising from treatment compared to the alternative. However, if the comparator is not a real life existing/usual treatment or mix of treatments, the results have little value for the decision maker to base a decision.
Outcome and outcome measures
A wide range of outcome measures are used within the ten studies. As previously described, some of the most common measures were QALYs, survival, change in SGRQ, and reduction in number of exacerbations and improvement in FEV1. In addition to this there were others, including: proportion of patients remaining free of exacerbations after six months (Ayres et al 2003), number of symptom free nights (Jones and Wilson 2003), a daytime symptom card of less than 2 (Jones and Wilson 2003), avoided exacerbation (Lofdahl et al 2005; Oostenbrink et al 2004) and improvement in dyspnea (Oostenbrink et al 2004). Only two of the studies used QALYs (one derived QALYs through the SF-36 [Briggs et al 2006] and the other through the HUI [van den Boom 2001]); an important measure from the point of view of the decision maker in resource allocation decisions across disease areas.
Results
All of these studies report a favorable outcome compared to the comparator(s): six found the study drug cost effective compared with the comparator(s) (van den Boom 2001; Ayres et al 2003; Oostenbrink et al 2004; Gagnon et al 2005; Lofdahl et al 2005; Briggs et al 2006). The remaining four reported improvements in outcome associated with the study drug compared to the comparator(s) (Friedman 1999; Hogan et al 2003; Jones and Wilson 2003; Rutten-van Molken et al 1995). We believe that whilst the conclusions to come out of these studies may be valid within the context of the study, because of methodological gaps identified above in all of these studies, whether that be outcome measures employed, perspective, interventions considered, patient group etc, their use within a decision making context – in which their primary purpose lies – is strictly limited.
Handling of uncertainty
Four of the studies used probabilistic sensitivity analysis (Oostenbrink et al 2004; Gagnon et al 2005; Lofdahl et al 2005; Briggs et al 2006). Two studies used univariate sensitivity analysis around the underlying assumptions such as adjusting the value for the cost per day (Ayres et al 2003) and inflating/deflating the cost of treatment drugs and rescue medication by 50% (Hogan et al 2003). Four made no mention of the uncertainty surrounding the economic evaluation (Rutten-van Molken et al 1995; Friedman 1999; van den Boom 2001; Jones and Wilson 2003). Uncertainty into the inputs into the model inherently exists, ie costs and effects. In order to account for this, a sensitivity analysis should be conducted in order to ensure that this uncertainty is captured.
Sponsorship
Most of the papers were sponsored by industry: five fully sponsored (Friedman 1999; Ayres 2003; Oostenbrink et al 2004; Lofdahl et al 2005; Briggs et al 2006). The paper by Jones did not state any information on financial support, though one of the authors was working for GlaxoSmithKline (GSK). Three papers were partially supported by the industry (Rutten-van Molken et al 1995; Hogan et al 2003; Gagnon et al 2005). GSK supported most of the studies (5/10), Boehringer Ingelheim (BI; 2/10) and AstraZeneca and Novartis sponsored one each.
Review of the decision analytic models
In this section, we examine the five studies that used a decision analytic model framework for the economic evaluation: we take each model individually and inspect the methodology/characteristics employed and some of the inputs used. The studies are summarized in Table 5.
Table 5.
Summary of decision analytic based economic evaluations
| Perspective | Country | Cost year (currency) | Duration (mths)a | Patient groupb | Interventions | Outcome measure | Outcome | Extrapolation | Uncertainty | Sponsor | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Decision analytic model based | |||||||||||
| Jubran (1993) | Payer | USA | /($) | 7.1 5.9 |
II,III | Theophylline n = 311 Ipratropium n = 289 |
Complication free therapy months. | Ipratropium was found to result in 11.30 complication free therapy months compared to Theophylline’s 10.68. No ICER was generated: Ipratropium was found to strictly dominate Theophylline. | 1 yr | Univariate | BI |
| Sin (2004) | Societal | CA | 1999($CA) | 36 | I,II, III,IV | No patients treated with inhaled corticosteroids (ICS). All patients treated with ICS. ICS to patients in stage 2 or 3. ICS to stage 3 patients only. (n:various sources) |
Patient health related quality of life. All cause mortality. | Lifetime: Cost per QALY with a mortality effect: $4600 (All patients). $2900 (stage 2/3), $2000 (stage 3). Cost per QALY with no mortality effect: $26,200 (All patients), $21,200 (stage 2/3), $15,000 (stage 3). | Lifetime | PSA | GSK/Institute of Health Economics |
| Oostenbrink (2005) | – | NL CA |
2001(€) | 12 6 12 |
II, III | Tiotropium n = 1296 Salmeterol n = 405 Ipratropium n = 175 |
Number of exacerbations. Quality adjusted life months. | NL: The prob tio is cost effective (CE) for: QALY’s is almost indpendent of threshold, and for exacerbation avoided is 60% at €500. CA: The prob of tio being CE for: QALY’s is highest from a threshold of ≥€120, and for exacerbation avoided is highest from a threshold of ≥€160. | 1 yr | PSA | BI |
| Spencer (2005) | Payer | CA | 2002($CA) | 12 | II,III,IV | Usual care. Salmeterol/fluticasone. (n:various sources) | Exacerbations. Mortality. Patient health status. | 25 years: Cost per QALY is $74,997 (basecase), $11,125 (survival effect) and $49,928 (delayed progression of disease) | 25 yr | PSA | GSK |
| Maniadakis (2006) | NHS | Greece | 2005(€) | 12 | II, III,IV | Tiotropium 18 mcg Salmeterol (n: various sources) | Number of Exacerbations. Quality adjusted life months. | The probability Tiotropium is cost effective is 77% at €1000 and 95% at €20,000. | No | PSA | BI |
Duration of efficacy data.
The patient group according to that from the effects data, standardised in line with the GOLD guidelines.
Jubran (1993)
The Jubran paper (1993) is the earliest example of an economic model applied to COPD. The study compares the costs and cost effectiveness of Theophylline vs Ipratropium from a societal perspective; based on three observational data sets, totaling 600 people with a diagnosis of moderate to severe COPD in three US sites. Resource use was extracted from these data sets to include: the number and type of visits made, drug treatments, lab tests, consultations and toxic events. From this data, estimates for labor, nonlabor and overhead costs were made. The datasets were uneven in duration (Theophylline: 7.1 months and Ipratropium: 5.9 months) but the results were extrapolated to one year.
The model consists of 7 Markov states; Stable, Clinic visit, Consult, ER Visit, Hospital, Major toxicity, Minor toxicity. The model assumes that the patient is in one of these states at any one time and transitions between these states take place at the end of the one month cycle. There are 12 cycles in total.
No ICER was calculated since Ipratropium was found to be both less costly and more cost effective than Theophylline. Univariate sensitivity analysis was used to represent the uncertainty around costs and probabilities into the model. The study was sponsored by BI.
Sin (2004)
A societal perspective was adopted by Sin and colleagues (2004) for his Markov Model which examined the effects of adding inhaled corticosteroids to treatment for three groups; all COPD patients; patients with stage 2 or 3 disease and stage 3 disease. The model was three years in duration with twelve cycles; each cycle being three months in length. The model was split into three states according to disease severity, 1 being least severe and 3 most severe. A final position of death was not stated as an explicit state, though was inferred.
Data from the third National Health and Nutritional Examination survey were used to estimate the proportion of patients in each state. All cause mortality was estimated from published data; risk increased subject to disease severity and varied between those treated with ICS vs those who were not.
Assumptions used in the model:
Lung function declines over time (11.75 ml per cycle), regardless of severity group. From these values, the probability of progressing to the next stage was calculated and applied to all groups.
Health status declines with severity: QALY values applied were: 1.00 for stage 1, 0.92 for stage 2 and 0.84 for stage 3. From baseline, a reduction of 0.32 QALYs per exacerbation was applied to each stage and the estimated duration of effect was 1 week (mild), 2 weeks (moderate) and 4 weeks (severe).
The rate and severity of exacerbations increases according to COPD stage.
Taking corticosteroids reduces the risk of all types of exacerbations by 30%.
Whilst the viewpoint was that of society, only direct marginal costs were included within the analysis and an estimation of the productivity costs associated with work loss during exacerbations for those 65 years or younger was only conducted during a secondary analysis.
Sin found that treatment was cost effective when given to patients with stage 2 or 3 disease. Probabilistic sensitivity analysis was performed to account for the uncertainty around the inputs of the model.
Oostenbrink (2005)
Oostenbrink et al (2005) designed this one year Markov model around disease severity states (moderate, severe and very severe) and exacerbations (severe) in order to examine the effects of substituting Ipratropium with Tiotropium in the Netherlands and in Canada. The model did not include a mild state or a death state.
Effectiveness data for the Tiotropium arm was based on data from six randomized controlled trials and for the Salmeterol and Ipratropium arms, from the relative difference to Tiotropium seen during the individual trials. In total, clinical trial data from 1876 people was used within the analysis. For the Netherlands, costs were mainly derived from an economic evaluation on resource use that was piggybacked onto the Ipratropium trials. For Canada, costs were estimated from an observational study.
Assumptions used in the modeling:
The length of the first cycle was 8 days and all subsequent cycles were one month. Only one exacerbation was allowed during any one cycle. Transitions between states were assumed to take place halfway through the model.
Treatment was assumed to affect the transition probabilities; the average Ipratropium patient was found to have a probability of 2.7 times more than the average Tiotropium for movement from a moderate to severe state (in the Sin model, the treatment was not assumed to affect these probabilities, compared to no treatment).
Mean EQ-5D index scores used were: moderate = 0.755; severe = 0.748 and very severe = 0.549. During a cycle in which an exacerbation occurs, utility is assumed to decrease (for the whole cycle) by 15% for a nonsevere exacerbation and 50% for a severe exacerbation.
In the Netherlands and in Canada, Tiotropium was found to be associated with maximum expected net benefit for plausible values of the ceiling ratio.
In order to account for the uncertainties in the evaluation, Oostenbrink used probabilistic sensitivity analysis to test for the robustness of the result to changes in the baseline values of the model eg utility values and the baseline distribution of patients assigned to each state. The study was sponsored by BI.
Spencer (2005)
Spencer and colleagues’ model (Spencer et al 2005) employ a Markov model in order to compare the cost effectiveness of the combination drug Salmeterol/Fluticasone, to usual care. The model has four mutually exclusive states: mild, moderate, severe and death, (which are equivalent to the GOLD stages: mild/moderate, severe and very severe). The cycles of the model were 3 months in duration and a maximum time horizon of 25 years applied. Baseline values for the model were sourced from GSK clinical trial data (TRISTAN), published medical literature and from expert opinion. It was not transparent where the cost data came from, though references to published medical literature, clinical trial data and expert opinion were made.
Assumptions used in the modelling:
Transition probabilities between the states were calculated from a published formula. Smokers and ex-smokers were found to have increased FEV1 rates of decline: 62 ml and 31ml respectively.
Estimates of health status by disease stage were mild: 0.81, moderate: 0.72 and severe: 0.67.
Exacerbations were split into major and minor, (major being those requiring hospital treatment)and the estimated frequency of each was calculated, for every health state. Using the EQ-5D score, estimates for health status during an exacerbation were obtained from 27 respiratory physicians who completed the questionnaire from the perspective of their patients. Utility weights were as follows: For a minor exacerbation health status dropped to 0.61 (mild), 0.61 (moderate) and 0.05 (severe). For a major exacerbation, health status fell to −0.26 regardless of disease severity.
The study assumes a nonlinear recovery from the ‘low point’ to a position of 0.03 utility points below those of others in the study that have not had an exacerbation.
Treatment was assumed to affect the risk of an exacerbation, the risk of disease progression, risk of mortality and the patients’ health status.
The paper concludes that by adding a long acting B2 agonist to an inhaled corticosteroid, this may represent a cost effective treatment in those patients who have a history of frequent exacerbations and poorly reversible COPD. A probabilistic sensitivity analysis was performed around the discount rate, exacerbation rate and the mortality benefit. The study was sponsored by GSK.
Maniadakis (2006)
This model, adapted from Oostenbrink’s model to a Greek setting, sought to establish the cost effectiveness of Tiotropium compared with Salmeterol, from the perspective of the Greek NHS (Maniadakis et al 2006). Whilst Tiotropium was concluded to be cost effective, there was actually no statistically significant difference found between the treatments. Probabilistic sensitivity analysis was performed around the baseline values. As with Oostenbrink’s paper, this too was financially supported by BI.
Summary
From the descriptions of the decision analytic models, it is clear, excluding Jubran, that there are many similarities in the approach adopted. The four papers all used a utility measure in their analysis. The disease has been modeled using stages of the disease and at each stage; an exacerbation causes a drop in health related quality of life for the modeled patient. However, at the same time, there are differences concerning inputs into the model, some of which have been described elsewhere (Rutten-van Molken et al 2006). Table 6 illustrates the differences between the papers by Sin, Oostenbrink, and Spencer. The patients were grouped according to severity in line with those reported: FEV1 > 50% (GOLD moderate); 35% < FEV1 ≤50% (approx GOLD severe); and FEV1 < 35% (approx GOLD very severe).
Table 6.
Health status according to disease severity and impact of an exacerbation (utilities)
| Moderate | Severe | Very Severe | ||
|---|---|---|---|---|
| Oostenbrink (2005) | Baseline | 0.76 | 0.75 | 0.55 |
| ↓ to (minor exac) | 0.64 | 0.64 | 0.47 | |
| ↓ to (major exac) | 0.38 | 0.37 | 0.27 | |
| Duration of effect | 1 month | 1 month | 1 month | |
| Sin (2004) | Baseline | 1.00 | 0.92 | 0.84 |
| ↓ to (exac) | 0.68 | 0.60 | 0.52 | |
| Duration of effect | 1 week | 2 weeks | 4 weeks | |
| Spencer (2005) | Baseline | 0.81 | 0.72 | 0.67 |
| ↓ to (minor exac) | 0.61 | 0.61 | 0.05 | |
| ↓ to (major exac) | −0.26 | −0.26 | −0.26 | |
| Duration of effect | Recovery over six months | |||
Differences between the values given for health state at each disease status differ dramatically between the papers eg, very severe: 0.55 (Oostenbrink) 0.84 (Sin) and 0.67 (Spencer). In the same way, health state and duration of effect assigned to an exacerbation also differ: the reduction in health following a major exacerbation for a very severe patient was assessed to be 0.27 (one month) Oostenbrink, 0.52 (one month) Sin and Spencer −0.26 (six months recovery time).
In order to compare and utilise economic models in the future for COPD, work must be done it ensure that the correct methodology is applied and that appropriate and valid inputs are used to populate the model. Disparities in utility values and the effect upon health related quality of life both during and following an exacerbation need to be ironed out, and a common consensus sought through further research.
Discussion
The output of an economic evaluation is to inform and assist the decision maker in allocating scarce health care resources, but how far does the existing literature go in fulfilling this role?
Fundamental is the design of the study; RCT, observational study based or employing a decision analytic model: this decision is all-important. For an economic evaluation to provide us with results suitable for applying to a wider population, the study should be internally and externally valid. Internal validity refers to the results of the study being true for the population under study (randomized controlled trials are regarded as the gold standard for efficacy and effectiveness data because of the way in which the trials are conducted results in a high level of internal validity, producing unbiased estimates of treatment effects (Cook et al 2004)). External validity on the other hand refers to the results of the study being generalizable to a wider population. Economic evaluations based entirely upon randomized controlled trials (RCTs: with tight inclusion and exclusion criteria) may have limited generalizabilty to a wider population which may “seriously restrict their relevance for policy making” (Baltussen et al 1999 p 450). The validity of observational studies depends upon the extent to which the component study populations are equivalent, the conclusions of such studies may need supporting evidence from RCTs. It is further suggested that modeling and the addition of observational data can enhance the external validity of the cost effectiveness study based on RCTs (Baltussen et al 1999). In addition, within an RCT based study, efficacy data is confined to the length of the trial. To be of most value to clinicians and health care funding agencies, the costs and benefits should be considered over a period that reflects the longevity of the effects of the intervention. (Halpin 2006). This may be much longer than the trial itself; consequently, a cost effectiveness ratio based solely upon the duration of the trial may fail to capture the longer-term effects of treatment, such as extended life of study patients. Indeed there is current debate around the impact on survival of some treatments for COPD: recent evidence from trials such as ISOLDE, TRISTAN, and TORCH has suggested a survival effect of therapy in COPD patients following treatment with the combination product Salmeterol and Fluticasone (TORCH). Incorporation of this effect into economic evaluations by using decision-analytic models to extrapolate from trial evidence may result in dramatic reductions of the resulting incremental cost effectiveness ratios (Sin et al 2004; Spencer et al 2005).
We see a clear and continuing role for the use of decision analytic models in economic evaluation of COPD therapies. A modeling framework can produce externally valid studies (based on internally valid evidence of treatment effects), capturing the long-term effects of treatment, thereby being useful in assisting the decision maker in allocating resources. Economic models in all their forms need to be methodologically sound, have relevant and valid inputs and to be well described and explained. In addition, sensitivity analysis needs to be executed with care, distributions around the inputs explained and reasoned, and extrapolation needs to be adopted and presented with caution. As we have seen in this paper, within pharmacoeconomics for COPD, there are twice as many economic evaluations alongside clinical trials or observational studies (10) compared to those based on decision analytic modeling (5). Economic evaluations in the future should ideally be based on modeling or on sufficiently long study durations so as to capture the relevant costs and effects to enable the analysis to deliver results that can be used by decision makers.
Results of economic evaluations are likely to vary according to the perspective employed. Perspective should be clearly stated within the paper and the results presented should be based upon the adopted perspective. Three evaluations did not mention perspective (Friedman 1999; van den Boom 2001; Oostenbrink et al 2005).
Most economic evaluations used, as comparators, one or two drugs that are currently available for treatment of COPD and 7/15 included placebo as one of the comparators. Only two studies (Sin et al 2004; Spencer et al 2005) include a range of relevant alternatives, including existing treatment. Decision makers need to know the full impact of the introduction of a new therapy to a disease or treatment area and this can best be achieved by using usual care as a comparator. “Decisions on cost effectiveness should be based on the comparison of a new intervention with current practice, rather than with a placebo” (Claxton et al 2002 p 711).
A wide range of outcome measures have been used in economic evaluations of COPD. Although it may be the case that “…it is neither known nor generally agreed which outcomes are most relevant” (Gross 2004 p 41); a range of outcome measures causes a problem for the decision maker, who is faced with the problem of making a judgment based on disparate results that are not directly comparable. For example, to what extent is the avoidance of a certain number of exacerbations equivalent to an average improvement in FEV1? The economists’ solution is the QALY (described above), which combines the effects of treatment into a single measure which can be compared both within and across disease areas. Within the England and Wales, NICE have stated “the QALY is considered to be the most appropriate generic measure of health benefit that reflects both mortality and HRQL” (National Institute for Clinical Excellence 2004 p 22). The QALY is a particularly useful outcome measure for economic evaluations of interventions in COPD, as treatment for which may result in both survival and quality of life gains.
The strength of cost utility analyses based on QALYs depends upon the robustness of the derivation of the utility values. In each of the three modeling studies that used QALYs, the utility weights applied to different COPD states and to the impact of exacerbations varied quite considerably as previously shown in Table 6. The most likely reason behind these differences in utility values is the different methods of elicitation (and the population surveyed). NICE suggests utilities should be derived using a choice based method such as the time trade off or the standard gamble, applied to a representative sample of the public. The decrement in utility (from baseline) associated with each exacerbation and length of time to which this decrease is applied (duration of exacerbation), differs considerably between studies. It would be valuable to undertake further research into the derivation of utility values for COPD patients, and in particular, the recovery time and drop in health related quality of life, both during the exacerbation and recovery following an exacerbation. Further research needs to be conducted within this area in order to harmonize utility values.
Four out of the fifteen studies did not consider uncertainty within their analyses, three used one way, eight used probabilistic sensitivity analysis. An assessment of uncertainty should be included within an economic evaluation, to reflect uncertainty in the composition of the study population and in the cost and health outcomes results obtained from the study. In the case of modeling studies, there are further uncertainties, such as in the design of the model itself and the extrapolation of study data to a time horizon that extends the life of the trial. Probabilistic sensitivity analysis is preferred for assessing uncertainty in modeled economic evaluations because it allows the combined uncertainty surrounding all of the parameters within the model to be assessed (Briggs 2002).
All, bar one of the 15 studies have been sponsored to some extent by the pharmaceutical industry and the study drugs in each of these papers were reported to have a favorable cost-effectiveness result. Concerns about the outcomes of these studies, because of issues around: selection of study design, patient population and the potential for bias in the outcome and in the publication, are often raised. Nevertheless, the industry is an important provider of cost effectiveness data, especially to support submission for reimbursement in particular countries. In addition, the industry, because of tight regulating standards, may pay closer attention to quality control than academic institutes. Whatever the pro’s and con’s are, industry financed studies will continue to be a valuable source of data, however there is a gap for nonindustry sponsored evaluations in COPD and efforts should be made to provide the resources necessary in order to support nonindustry bodies in producing such studies.
Conclusion
This paper has provided an overview of the burden of illness and costs associated with COPD, explained economic evaluation, reviewed and critically appraised the published economic evaluation of therapies in COPD and has discussed the use of existing economic evaluations in informing and assisting the decision maker to allocate scarce health care resources.
It has been suggested that consistency between evaluations is necessary in order for comparisons to be made between different treatments over time (National Institute for Clinical Excellence 2004). Whilst all the fifteen economic evaluations report benefits of the main study drug; either that is cost effective, or that, compared to the alternative under consideration, the treatment represented significant improvements in the measured outcome. As previously reported in relation to early economic evaluations for COPD (Ruchlin and Dasbach 2001), there is little consistency between the studies methodologically. Differences in study design, comparators, interventions, outcome measures and the analysis of uncertainty make meaningful comparison between the studies very difficult. Indeed direct comparisons between treatments for COPD are not available, precisely because of this issue.
However, for the decision maker and for the clinician, it is of utmost importance that interventions are directly comparable. Decisions must be made as to the most suitable treatment; informed decisions, based upon and supported by all available knowledge and evidence of substitute or alternative treatments are most likely to be appropriate.
Efforts should be made for future clinical trials and economic studies to harmonize study design and methods, particularly towards adopting a universal modeling framework, using current treatment as comparator and adopting an effectiveness measure such as the QALY in order to produce results that are comparable across interventions and disease areas, and that are useful to a decision maker.
References
- Andersson F, Borg S, Jansson SA, et al. The costs of exacerbations in chronic obstructive pulmonary disease (COPD) Respiratory Medicine. 2002;96:700–8. doi: 10.1053/rmed.2002.1334. [DOI] [PubMed] [Google Scholar]
- Anthonisen N, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5 year mortality. Annals of Internal Medicine. 2005;142:233–9. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- Ayres JG, Price MJ, Efthimiou J. Cost-effectiveness of fluticasone propionate in the treatment of chronic obstructive pulmonary disease: a double-blind randomized, placebo-controlled trial. Respiratory Medicine. 2003;97:212–20. doi: 10.1053/rmed.2003.1441. [DOI] [PubMed] [Google Scholar]
- Baker CB, Johnsrud MT, Lynn Crismon JM, et al. Quantitative analysis of sponsorship bias in economic studies of antidepressants. British Journal of Psychiatry. 2003;183:498–506. doi: 10.1192/bjp.183.6.498. [DOI] [PubMed] [Google Scholar]
- Baltussen R, Leidl R, Ament A. Real world designs in economic evaluation: brigding the gap between clinical research and policy-making. Pharmacoeconomics. 1999;16:449–58. doi: 10.2165/00019053-199916050-00003. [DOI] [PubMed] [Google Scholar]
- Bell CM, Urbach DR, Ray JG, et al. Bias in published cost effectiveness studies: systematic review. BMJ. 2006;332:699–703. doi: 10.1136/bmj.38737.607558.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs AH. Probabilistic analysis of cost-effectiveness odels: choosing between treatment strategies for gastroesophageal reflux disease. Medical Decision Making. 2002;22:290–308. doi: 10.1177/0272989X0202200408. [DOI] [PubMed] [Google Scholar]
- Briggs AH, Lozano-Ortega G, Spencer S, et al. Estimating the cost-effectiveness of fluticasone propionate for treating chronic obstructive pulmonary disease in the presence of missing data. Value in Health. 2006;9:227–35. doi: 10.1111/j.1524-4733.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Briggs AH, O’Brien BJ. The death of cost-minimisation analysis? Health Economics. 2001;10:179–84. doi: 10.1002/hec.584. [DOI] [PubMed] [Google Scholar]
- Britton M. The burden of COPD in the U.K.: results from the Confronting COPD survey. Respiratory Medicine. 2003;97(Suppl C):S71–9. doi: 10.1016/s0954-6111(03)80027-6. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Bellamy D. The challenge of providing better care for patients with chronic obstructive pulmonary disease: the poor relation of airways obstruction? Thorax. 2000;55:78–82. doi: 10.1136/thorax.55.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley PM, Sondhi S. The burden of obstructive lung disease in the UK - COPD and asthma. Thorax. 1998;53(Supp 4):A83. [Google Scholar]
- Chapman KR, Bourbeau J, Rance L, et al. The burden of COPD in Canada: results from the Confronting COPD survey. Respiratory Medicine. 2003;97(Suppl C):S23–31. doi: 10.1016/s0954-6111(03)80022-7. [DOI] [PubMed] [Google Scholar]
- Claxton K, Sculpher M, Drummond M. A rational framework for decision making by the National Institute for Clinical Excellence (NICE) The Lancet. 2002;360:711–15. doi: 10.1016/S0140-6736(02)09832-X. [DOI] [PubMed] [Google Scholar]
- Claxton K, Sculpher M, McCabe C, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Economics. 2005;14:339–47. doi: 10.1002/hec.985. [DOI] [PubMed] [Google Scholar]
- Cook J, Drummond M, Heyes J. Economic endpoints in clinical trials. Statistical Methods in Medical Research. 2004;13:157–76. doi: 10.1191/0962280204sm359ra. [DOI] [PubMed] [Google Scholar]
- Culyer A, McCabe C, Briggs A, et al. Searching for a threshold, not setting one: the role of the National Institute for Health and Clinical Excellence. Journal of Health Services Research and Policy. 2007;12:56–8. doi: 10.1258/135581907779497567. [DOI] [PubMed] [Google Scholar]
- Dahl R, Greefhorst LA, Nowak D. Inhaled formoterol dry powder versus ipratroipumbromide in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2001;164:778–84. doi: 10.1164/ajrccm.164.5.2007006. [DOI] [PubMed] [Google Scholar]
- Dal Negro R, Rossi A, Cerveri I, et al. The burden of COPD in Italy: results from the confronting COPD survey. Respiratory Medicine. 2003;97(Suppl C):S43–50. doi: 10.1016/s0954-6111(03)80024-0. [DOI] [PubMed] [Google Scholar]
- Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. 3. Oxford University Press; 2005. [Google Scholar]
- Drummond M, Sculpher M. Common methodological flaws in economic evaluations. Medical Care. 2005;43(7 Suppl):II5–14. doi: 10.1097/01.mlr.0000170001.10393.b7. [DOI] [PubMed] [Google Scholar]
- European Respiratory Society and European Lung Foundation. European lung white book. Huddersfield: ERSJ; 2003. Chronic obstructive pulmonary disease. [Google Scholar]
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. British Medical Journal. 1977;1:1645–8. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MS., C.M. Pharmacoeconomic evaluation of a combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone in COPD. Chest. 1999;115:635–41. doi: 10.1378/chest.115.3.635. [DOI] [PubMed] [Google Scholar]
- Fukuchi Y, Nishimura M, Ichinose M, et al. COPD in Japan: The Nippon COPD Epidemiology study. Respirology. 2004;9:4. doi: 10.1111/j.1440-1843.2004.00637.x. [DOI] [PubMed] [Google Scholar]
- Gagnon YM, Levy AR, Spencer MD, et al. Economic evaluation of treating chronic obstructive pulmonary disease with inhaled corticosteroids and long-acting beta2-agonists in a health maintenance organization. Respiratory Medicine. 2005;99:1534–45. doi: 10.1016/j.rmed.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Garber AM. Advances in cost-effectiveness analysis of health interventions. In: Culyer A, Newhouse J, editors. Handbook of health economics. 1. Amsterdam: Elsevier BV; 2000. pp. 181–221. [Google Scholar]
- GOLD. Global initiative for chronic obstructive lung disease. 2006 [Google Scholar]
- Gold M, Siegel JE, Russel LB, et al. Cost-effectiveness in health and medicine. New York: Oxford Universty Press; 1996. [Google Scholar]
- Gross N. Outcome measures for COPD treatments: a critical evaluation. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2004;1:41–57. doi: 10.1081/COPD-120030413. [DOI] [PubMed] [Google Scholar]
- Guest JF. The annual cost of chronic obstructive pulmonary disease to the UK’s National Health Service. Disease Management and Health Outcomes. 1999;5:2. [Google Scholar]
- Halpern MT, Stanford RH, Borker R, et al. The burden of COPD in the U.S.A.: results from the Confronting COPD survey. Respiratory Medicine. 2003;97(Suppl C):S81–9. doi: 10.1016/s0954-6111(03)80028-8. [DOI] [PubMed] [Google Scholar]
- Halpin DM. Health economics of chronic obstructive pulmonary disease. [Review] [70 refs] Proceedings of the American Thoracic Society. 2006;3:227–33. doi: 10.1513/pats.200507-072SF. [DOI] [PubMed] [Google Scholar]
- Hogan TJ, Geddes R, Gonzalez E. An economic assessment of inhaled formoterol dry powder versus ipratropium bromide pressurized metered dose inhaler in the treatment of chronic obstructive pulmonary disease. Clinical Therapeutics. 2003;25:285–97. doi: 10.1016/s0149-2918(03)90039-7. [DOI] [PubMed] [Google Scholar]
- ISPOR. Pharmacoeconomic guidelines around the world [online] 2007 URL: http://www.ispor.org/PEguidelines/index.asp.
- Izquierdo JL. The burden of COPD in Spain: results from the Confronting COPD survey. Respiratory Medicine. 2003;97(Suppl C):S61–9. doi: 10.1016/s0954-6111(03)80026-4. [DOI] [PubMed] [Google Scholar]
- Jansson SA, Andersson F, Borg S, et al. Costs of COPD in Sweden according to disease severity. Chest. 2002;122:1994–2002. doi: 10.1378/chest.122.6.1994. [DOI] [PubMed] [Google Scholar]
- Jemal A, Ward E, Hao Y, et al. Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294:14. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- Jones PW, Wilson KS. Cost-effectiveness of salmeterol in patients with chronic obstructive pulmonary disease: an economic evaluation. Respiratory Medicine. 2003;97:20–6. doi: 10.1053/rmed.2002.1425. [DOI] [PubMed] [Google Scholar]
- Jubran A, Gross N, Ramsdell J, et al. Comparative cost-effectiveness analysis of theophylline and ipratropium bromide in chronic obstructive pulmonary disease. A three-centre study. Chest. 1993;103:678–84. doi: 10.1378/chest.103.3.678. [DOI] [PubMed] [Google Scholar]
- Lofdahl CG, Ericsson A, Svensson K, et al. Cost effectiveness of budesonide/formoterol in a single inhaler for COPD compared with each monocomponent used alone. Pharmacoeconomics. 2005;23:365–75. doi: 10.2165/00019053-200523040-00006. [DOI] [PubMed] [Google Scholar]
- Lokke A, Lange P, Scharling H, et al. Developing COPD: a 25 year follow up study of the general population. Thorax. 2006;61:935–9. doi: 10.1136/thx.2006.062802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung and Asthma Information Agency. Trends in COPD [pamphlet] 2003 [Google Scholar]
- Mackay J, Eriksen M. The tobacco atlas. World Health Organization; 2002. [Google Scholar]
- Maniadakis N, Tzanakis N, Fragoulakis V, et al. Economic evaluation of tiotropium and salmeterol in the treatment of chronic obstructive pulmonary disease (COPD) in Greece. Current Medical Research and Opinion. 2006;22:8. doi: 10.1185/030079906X112778. [DOI] [PubMed] [Google Scholar]
- McGuire A, Irwin DE, Fenn P, et al. The excess cost of acute exacerbations of chronic bronchitis in patients aged 45 and older in England and Wales. Value in Health. 2001;4:5. doi: 10.1046/j.1524-4733.2001.45049.x. [DOI] [PubMed] [Google Scholar]
- Murray Christopher JL, Lopez AD, Mathers CD, et al. The Global Burden of Disease 2000 project: aims, methods and data sources. World Health Organization; 2001. [Google Scholar]
- National Institute for Clinical Excellence. Guide to the methods of technology appraisal. London: National Institute for Clinical Excellence; 2004. [PubMed] [Google Scholar]
- National Institute of Health and Clinical Excellence. A guide to NICE [pamphlet] 2005 [Google Scholar]
- Oostenbrink JB, Rutten-van Molken MP, Monz BU, et al. Probabilistic Markov model to assess the cost-effectiveness of bronchodilator therapy in COPD patients in different countries [see comment] Value in Health. 2005;8:32–46. doi: 10.1111/j.1524-4733.2005.03086.x. [DOI] [PubMed] [Google Scholar]
- Oostenbrink JB, Rutten-van Molken MP, et al. One-year cost-effectiveness of tiotropium versus ipratopium to treat chronic obstructive pulmonary disease. European Respiratory Journal. 2004;23(2) doi: 10.1183/09031936.03.00083703. [DOI] [PubMed] [Google Scholar]
- Philips Z, Bojke L, Sculpher M, et al. Good practice guidelines for decision-analytic modelling in Health Technology Assessment: A review and consolidation of quality assessment. Pharmacoeconomics. 2006;24:355–71. doi: 10.2165/00019053-200624040-00006. [DOI] [PubMed] [Google Scholar]
- Piperno D, Huchon G, Pribil C, et al. The burden of COPD in France: results from the Confronting COPD survey. Respiratory Medicine. 2003;97(Suppl C):S33–42. doi: 10.1016/s0954-6111(03)80023-9. [DOI] [PubMed] [Google Scholar]
- Ruchlin HS, Dasbach EJ. An economic overview of chronic obstructive pulmonary disease. Pharmacoeconomics. 2001;19(6) doi: 10.2165/00019053-200119060-00002. [DOI] [PubMed] [Google Scholar]
- Rutten-van Molken M, Lee TA, Authors FN, et al. Economic modeling in chronic obstructive pulmonary disease. [Review] [15 refs] Proceedings of the American Thoracic Society. 2006;3:630–4. doi: 10.1513/pats.200603-095SS. [DOI] [PubMed] [Google Scholar]
- Rutten-van Molken M, Van Doorslaer EK, Jansen MC, et al. Costs and effects of inhaled corticosteroids and bronchodilators in asthma and chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1995;151:975–82. doi: 10.1164/ajrccm.151.4.7697275. [DOI] [PubMed] [Google Scholar]
- Sculpher M, Claxton K, Drummond M, et al. Whither trial-based economic evaluation for health care decision making? Health Economics. 2006;15:677–87. doi: 10.1002/hec.1093. [DOI] [PubMed] [Google Scholar]
- Sculpher MJ. The role and estimation of productivity costs in economic evaluation. In: Drummond MF, McGuire A, editors. Theory and practice of economic evaluation in health. Oxford: Oxford University Press; 2001. [Google Scholar]
- Sin DD, Golmohammadi K, Jacobs P. Cost-effectiveness of inhaled corticosteroids for chronic obstructive pulmonary disease according to disease severity. American Journal of Medicine. 2004;116:325–31. doi: 10.1016/j.amjmed.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Spencer M, Briggs AH, Grossman RF, et al. Development of an economic model to assess the cost-effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics. 2005;23:619–37. doi: 10.2165/00019053-200523060-00008. [DOI] [PubMed] [Google Scholar]
- Sullivan SD. The burden of illness and economic evaluation for COPD. European Respiratory Journal. 2003;22(Suppl 43):7S–8S. doi: 10.1183/09031936.03.00078203. [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2000;117(2 Suppl S):5–9. doi: 10.1378/chest.117.2_suppl.5s. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Summary health statistics for us adults: National Health Interview Survey, 2004. 2004 [Google Scholar]
- van den Boom GR. The cost effectiveness of early treatment with fluticasone propionate 250 microg twice a day in subjects with obstructive airway disease. Results of the DIMCA program. American Journal of Respiratory and Critical Care Medicine. 2001;164:2057–66. doi: 10.1164/ajrccm.164.11.2003151. [DOI] [PubMed] [Google Scholar]
- Wouters EF. The burden of COPD in The Netherlands: results from the Confronting COPD survey. Respiratory Medicine. 2003;97(Suppl C):S51–9. doi: 10.1016/s0954-6111(03)80025-2. [DOI] [PubMed] [Google Scholar]
- Yelin E, Katz P, Balmes J. Work life of persons with asthma, rhinits, and COPD: A study using a national, population based sample. Journal of Occupational Medicine and Toxicology. 2006;1(2) doi: 10.1186/1745-6673-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski J, Bednarek M, Gorecka D, et al. Increasing COPD awareness. European Respiratory Journal. 2006;27:833–52. doi: 10.1183/09031936.06.00025905. [DOI] [PubMed] [Google Scholar]