Abstract
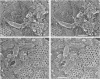
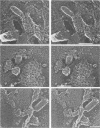
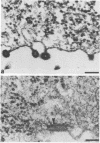
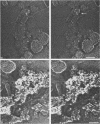
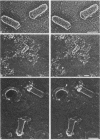
Viral assembly was studied by viewing platinum replicas of cytoplasmic and outer plasma membrane surfaces of baby hamster kidney cells infected with vesicular stomatitis virus. Replicas of the cytoplasmic surface of the basilar plasma membrane revealed nucleocapsids forming bullet-shaped tight helical coils. The apex of each viral nose cone was anchored to the membrane and was free of uncoiled nucleocapsid, whereas tortuous nucleocapsid was attached to the base of tightly coiled structures. Using immunoelectron microscopy, we identified the nucleocapsid (N) viral protein as a component of both the tight-coil and tortuous nucleocapsids, whereas the matrix (M) protein was found only on tortuous nucleocapsids. The M protein was not found on the membrane. Using immunoreagents specific for the viral glycoprotein (G protein), we found that the amount of G protein per virion varied. The G protein was consistently localized at the apex of viral buds, whereas the density of G protein on the shaft was equivalent to that in the surrounding membrane. These observations suggest that G-protein interaction with the nucleocapsid via its cytoplasmic domain may be necessary for the initiation of viral assembly. Once contact is established, nucleocapsid coiling proceeds with nose cone formation followed by formation of the helical cylinder. M protein may function to induce a nucleocapsid conformation favorable for coiling or may cross-link adjacent turns in the tight coil or both.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggeler J., Werb Z. Initial events during phagocytosis by macrophages viewed from outside and inside the cell: membrane-particle interactions and clathrin. J Cell Biol. 1982 Sep;94(3):613–623. doi: 10.1083/jcb.94.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnheiter H., Dubois-Dalcq M., Lazzarini R. A. Direct visualization of protein transport and processing in the living cell by microinjection of specific antibodies. Cell. 1984 Nov;39(1):99–109. doi: 10.1016/0092-8674(84)90195-8. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Roy P. Dissociation of vesicular stomatitis virus and relation of the virion proteins to the viral transcriptase. J Virol. 1972 Aug;10(2):234–243. doi: 10.1128/jvi.10.2.234-243.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Roy P. Kinetics of RNA synthesis by vesicular stomatitis virus particles. J Mol Biol. 1971 May 14;57(3):513–527. doi: 10.1016/0022-2836(71)90106-9. [DOI] [PubMed] [Google Scholar]
- Büechi M., Bächi T. Microscopy of internal structures of Sendai virus associated with the cytoplasmic surface of host membranes. Virology. 1982 Jul 30;120(2):349–359. doi: 10.1016/0042-6822(82)90036-8. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Smale C. J., Brown F., Hull R. Model for vesicular stomatitis virus. J Virol. 1972 Aug;10(2):256–260. doi: 10.1128/jvi.10.2.256-260.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Talbot P., Brown F. The proteins of biologically active sub-units of vesicular stomatitis virus. J Gen Virol. 1970 Jun;7(3):267–272. doi: 10.1099/0022-1317-7-3-267. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Little S. P., Hagen F. S., Huang A. S. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978 Dec;15(4):1455–1462. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- De B. P., Thornton G. B., Luk D., Banerjee A. K. Purified matrix protein of vesicular stomatitis virus blocks viral transcription in vitro. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7137–7141. doi: 10.1073/pnas.79.23.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M., Hooghe-Peters E. L., Lazzarini R. A. Antibody-induced modulation of rhabdovirus infection of neurons in vitro. J Neuropathol Exp Neurol. 1980 Sep;39(5):507–522. doi: 10.1097/00005072-198009000-00001. [DOI] [PubMed] [Google Scholar]
- Dubovi E. J., Wagner R. R. Spatial relationships of the proteins of vesicular stomatitis virus: induction of reversible oligomers by cleavable protein cross-linkers and oxidation. J Virol. 1977 May;22(2):500–509. doi: 10.1128/jvi.22.2.500-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner G., Dubois-Dalcq M., Hooghe-Peters E., McFarland H. F., Lazzarini R. A. Defective interfering particles modulate VSV infection of dissociated neuron cultures. Cell. 1979 Aug;17(4):979–991. doi: 10.1016/0092-8674(79)90337-4. [DOI] [PubMed] [Google Scholar]
- Hecht T. T., Summers D. F. Interactions of vesicular stomatitis virus with murine cell surface antigens. J Virol. 1976 Sep;19(3):833–845. doi: 10.1128/jvi.19.3.833-845.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Kirschner M. W. Filament organization revealed in platinum replicas of freeze-dried cytoskeletons. J Cell Biol. 1980 Jul;86(1):212–234. doi: 10.1083/jcb.86.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. Three-dimensional visualization of coated vesicle formation in fibroblasts. J Cell Biol. 1980 Mar;84(3):560–583. doi: 10.1083/jcb.84.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Glicksman M. A., Willard M. B. Organization of mammalian neurofilament polypeptides within the neuronal cytoskeleton. J Cell Biol. 1984 Apr;98(4):1523–1536. doi: 10.1083/jcb.98.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Heuser J. The inside and outside of gap-junction membranes visualized by deep etching. Cell. 1982 Sep;30(2):395–406. doi: 10.1016/0092-8674(82)90237-9. [DOI] [PubMed] [Google Scholar]
- Howatson A. F. Vesicular stomatitis and related viruses. Adv Virus Res. 1970;16:195–256. doi: 10.1016/s0065-3527(08)60024-x. [DOI] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Maturation of viral proteins in cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1149–1158. doi: 10.1128/jvi.21.3.1149-1158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Separate pathways of maturation of the major structural proteins of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1128–1139. doi: 10.1128/jvi.21.3.1128-1139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Lodish H. F., Baltimore D. Analysis of the defects of temperature-sensitive mutants of vesicular stomatitis virus: intracellular degradation of specific viral proteins. J Virol. 1977 Mar;21(3):1140–1148. doi: 10.1128/jvi.21.3.1140-1148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobler R. L., Dubois-Dalcq M., Haspel M. V., Claysmith A. P., Lampert P. W., Oldstone M. B. Selective localization of wild type and mutant mouse hepatitis virus (JHM strain) antigens in CNS tissue by fluorescence, light and electron microscopy. J Neuroimmunol. 1981 Mar;1(1):81–92. doi: 10.1016/0165-5728(81)90010-2. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Keene J. D., Schubert M. The origins of defective interfering particles of the negative-strand RNA viruses. Cell. 1981 Oct;26(2 Pt 2):145–154. doi: 10.1016/0092-8674(81)90298-1. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Porter M. Heterogeneity of vesicular stomatitis virus particles: implications for virion assembly. J Virol. 1980 Jan;33(1):52–58. doi: 10.1128/jvi.33.1.52-58.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Porter M. Specific incorporation of host cell surface proteins into budding vesicular stomatitis virus particles. Cell. 1980 Jan;19(1):161–169. doi: 10.1016/0092-8674(80)90397-9. [DOI] [PubMed] [Google Scholar]
- Lombardi T., Montesano R., Wohlwend A., Amherdt M., Vassalli J. D., Orci L. Evidence for polarization of plasma membrane domains in pancreatic endocrine cells. Nature. 1985 Feb 21;313(6004):694–696. doi: 10.1038/313694a0. [DOI] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Role of the vesicular stomatitis virus matrix protein in maintaining the viral nucleocapsid in the condensed form found in native virions. J Virol. 1981 Jul;39(1):295–299. doi: 10.1128/jvi.39.1.295-299.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Tobin G. J., McGowan J. J., Brown J. C. In vitro reassembly of vesicular stomatitis virus skeletons. J Virol. 1982 Mar;41(3):1055–1062. doi: 10.1128/jvi.41.3.1055-1062.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein J., Johnson L., Shelton E., Lazzarini R. A. The shape of vesicular stomatitis virus. Virology. 1976 May;71(1):291–301. doi: 10.1016/0042-6822(76)90113-6. [DOI] [PubMed] [Google Scholar]
- Orenstein J., Shelton E., Lazzarini R. A. Association of ribosomes with intracellular vesicular stomatitis virus particles. J Virol. 1975 Aug;16(2):447–452. doi: 10.1128/jvi.16.2.447-452.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R. The genetics of vesiculoviruses. Arch Virol. 1982;72(1-2):1–34. doi: 10.1007/BF01314447. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Pendergast M. Polarized distribution of viral envelope proteins in the plasma membrane of infected epithelial cells. Cell. 1980 May;20(1):45–54. doi: 10.1016/0092-8674(80)90233-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Repik P., Hefti E., Bishop D. H. Complementary RNA species isolated from vesicular stomatitis (HR strain) defective virions. J Virol. 1973 Jun;11(6):915–925. doi: 10.1128/jvi.11.6.915-925.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer T. J., Dickson C., Weiss R. A. Morphological and biochemical characterization of viral particles produced by the tsO45 mutant of vesicular stomatitis virus at restrictive temperature. J Virol. 1979 Jan;29(1):185–195. doi: 10.1128/jvi.29.1.185-195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer T. J., Lodish H. F. Noninfectious vesicular stomatitis virus particles deficient in the viral nucleocapsid. J Virol. 1979 Feb;29(2):443–447. doi: 10.1128/jvi.29.2.443-447.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Newcomb W. W., Brown J. C., Wall J. S., Hainfeld J. F., Trus B. L., Steven A. C. Mass and molecular composition of vesicular stomatitis virus: a scanning transmission electron microscopy analysis. J Virol. 1985 May;54(2):598–607. doi: 10.1128/jvi.54.2.598-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakowski J. J., Petri W. A., Jr, Wagner R. R. Role of matrix protein in assembling the membrane of vesicular stomatitis virus: reconstitution of matrix protein with negatively charged phospholipid vesicles. Biochemistry. 1981 Jun 23;20(13):3902–3907. doi: 10.1021/bi00516a037. [DOI] [PubMed] [Google Scholar]
- Zakowski J. J., Wagner R. R. Localization of membrane-associated proteins in vesicular stomatitis virus by use of hydrophobic membrane probes and cross-linking reagents. J Virol. 1980 Oct;36(1):93–102. doi: 10.1128/jvi.36.1.93-102.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]