Abstract
Addition of two equivalents of lithium pyrrolide to Mo(NR)(CHCMe2R')(OTf)2(DME) (OTf = OSO2CF3; R = 2,6-i-Pr2C6H3, 1-adamantyl, or 2,6-Br2-4-MeC6H2; R' = Me or Ph) produces Mo(NR)(CHCMe2R')(NC4H4)2 complexes in good yield. All compounds can be recrystallized readily from toluene or mixtures of pentane and ether and are sensitive to air and moisture. An X-ray structure of a 2,6-diisopropylphenylimido species shows it to be an unsymmetric dimer, {Mo(NAr)(syn-CHCMe2Ph)(η5-NC4H4)(η1-NC4H4)}{Mo(NAr)(syn-CHCMe2Ph)(η1-NC4H4)2}, in which the nitrogen in the η5-pyrrolyl bound to one Mo behaves as a donor to the other Mo. All complexes are fluxional on the NMR time scale at room temperature, with one symmetric species being observed on the NMR time scale at 50 °C in toluene-d8. The dimers react with PMe3 (at Mo) or B(C6F5)3 (at a η5-NC4H4 nitrogen) to give monomeric products in high yield. They also react rapidly with two equivalents of monoalcohols (e.g., Me3COH or (CF3)2MeCOH) or one equivalent of a biphenol or binaphthol to give two equivalents of pyrrole and bisalkoxide or diolate complexes in ~100% yield.
We have been searching for methods of synthesizing Mo(NR)(CHCMe2R')(OR")2 (R' = Me or Ph) species (or species that contain enantiomerically pure biphenolate or binaphtholate ligands1) in situ by treating an appropriate Mo(NR)(CHCMe2R')X2 species with a monoalcohol or diol. The main reason is that an increasing number of applications (e.g., asymmetric olefin metathesis1) require that many catalysts having different combinations of imido and alkoxide ligands be evaluated for a given metathesis transformation, and therefore that many catalysts be synthesized, isolated, stored, and manipulated. In the long run the synthesis and isolation of many catalysts will be impractical. Of course the synthesis of Mo(NR)(CHCMe2R')(OR")2 species from Mo(NR)(CHCMe2R')X2 species requires that both X groups be replaced readily with OR, that the HX product of this reaction not interfere to any significant degree with subsequent reactions that involve Mo(NR)(CHCMe2R')(OR")2, and that the HX product not react with any organic species in the reaction. We found that when X = CH2CMe3 only one equivalent of alcohol reacts readily to yield Mo(NAr)(CH-t-Bu)(CH2-t-Bu)(OR) or Mo(NAr)(CH2-t-Bu)3(OR) species.2,3 A second approach in which X = NPh2 allows both X groups to be replaced, but often slowly and incompletely, and not at all when NR = NAr (Ar = 2,6-diisopropylphenyl) and the diol is the bulky H2[Biphen] (H2[Biphen] = 3,3′-Di-t-butyl-5,5′,6,6′-tetramethyl-1,1′-Biphenyl-2,2′-diol).4 Syntheses of Mo(NR)(CHCMe2R')(NPh2)2 species from Mo(NR)(CHCMe2R')(OTf)2(dimethoxyethane) species5 are also plagued by poor yields as a consequence of competitive deprotonation of the alkylidene. We have now found that a variety of dipyrrolyl complexes, Mo(NR)(CHCMe2R')(NC4H4)2, can be prepared in good yield from bistriflate precursors and that they react rapidly, even with H2[Biphen] when NR = NAr, to yield two equivalents of pyrrole and bisalkoxide or biphenolate or binaphtholate species.
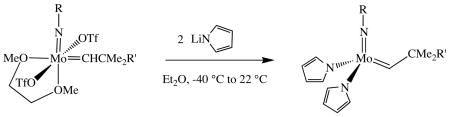
Addition of two equivalents of lithium pyrrolide to a stirred diethyl ether suspension of Mo(NR)(CHCMe2R')(OTf)2(DME) (OTf = OSO2CF3; R = 2,6-i-P r2C6H3 or 1-adamantyl) produces yellow to orange Mo(NR)(CHCMe2R')(NC4H4)2 complexes in ~75% yield (equation 1). An analogous reaction when R = 2,6-Br2-4-MeC6H2 is successful when the solvent is a mixture of diethyl ether and dichloromethane. Little or no competitive deprotonation of the alkylidene to give an alkylidyne complex6,7 has been observed in any case. All compounds are sensitive to air and moisture and can be recrystallized readily from toluene or mixtures of pentane and ether.
(1).
All dipyrrolyl complexes are fluxional on the proton NMR time scale. At 22 °C the spectra contain broad resonances, as shown, for example, for Mo(NAr)(CHCMe2Ph)(NC4H4)2 in toluene-d8 (at 500 MHz) in Figure 1. At high temperature one alkylidene resonance at ~13.3 ppm and two pyrrolyl resonances at ~6.1 and ~6.3 ppm are observed. At low temperatures two alkylidene resonances at ~13.2 and ~13.6 ppm are observed in a 1:1 ratio and the pyrrolyl proton resonances are resolved into an obscured set of resonances downfield of 6.3 ppm, along with a pattern of four sharp resonances near 5 ppm.8 No fluoride resonance is observed in the 19F NMR spectrum, and no solvent resonances are observed in the 1H NMR spectrum upon addition of trimethylphosphine, which yields a base adduct (vide infra). A 13C NMR spectrum of Mo(NAr)(CHCMe2Ph)(NC4H4)2 at −50 °C in methylene chloride-d2 reveals resonances at 313.9 ppm (JCH = 122.8 Hz) and 293.9 ppm (JCH = 121.3 Hz) characteristic of syn alkylidene species.9
An X-ray structural study of Mo(N-2,6-i-Pr2C6H3)(CHCMe2Ph)(NC4H4)2 shows it to be an unsymmetric dimer, {Mo(NAr)(syn-CHCMe2Ph)(η5-NC4H4)(η1-NC4H4)}{Mo(NAr)(syn-CHCMe2Ph)(η1-NC4H4)2}, in which the nitrogen in the η5-pyrrolyl behaves as a donor to the other Mo (Figure 2). The electron count in the Mo(NAr)(syn-CHCMe2Ph)(η5-NC4H4)(η1-NC4H4) half is 18, and in the Mo(NAr)(syn-CHCMe2Ph)(η1-NC4H4)2(donor) half is 16. The Mo(NAr)(syn-CHCMe2Ph)(η1-NC4H4)2(donor) fragment is approximately a square pyramid with the alkylidene in the apical position. Bond distances and angles are unexceptional. (See Figure caption for selected values.) This dimeric structure is consistent with the NMR spectra at low temperature, i.e., one half (containing Mo(2)) has no symmetry, while the second (containing Mo(1)) effectively has Cs symmetric. (The asymmetry that is present at Mo(2) apparently cannot be detected at Mo(1), at least under the NMR conditions employed so far.) The four sharp resonances near 5 ppm are assigned to the four protons in the η5-NC4H4 that is bound to a chiral metal center. η5-Pyrrolyl complexes (most of them di- or tetrasubstituted pyrroles10) have been prepared and studied for many years, the main driving force being the analogy between η5 NC4H4 and η5-C5H5.11 To the best of our knowledge, only one other molybdenum pyrrolyl complex, Mo(Tp*)(NO)(η1-NC4H4)2 (Tp* = HB(3,5-Me2C3N2H)3−), has been structurally characterized.12
The NMR spectra at high-temperatures are consistent with a Cs symmetric Mo(NR)(CHCMe2R')(η1-NC4H4)2 species on the NMR time scale in which the pyrrolyl ligands are η1 (on average) and rotate rapidly about the Mo-N bonds. Variable temperature spectra are identical at different concentrations, a result that does not reveal whether a small fraction of the dimer breaks up into monomers in which interconversion of η1-NC4H4 and η5-NC4H4 ligands is facile, or whether the equilibration process takes place entirely within the dimer. We favor the former in view of the high reactivity of the {Mo(NR)(CHCMe2R')(NC4H4)2}2 species toward alcohols and a Lewis acid or base (vide infra).
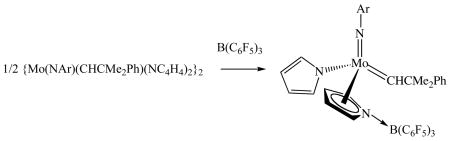
Addition of one equivalent of trimethylphosphine to Mo(NAd)(CHCMe2Ph)(NC4H4)2 results in immediate formation of syn-Mo(NAd)(CHCMe2Ph)(η1-NC4H4)2(PMe3), in which the alkylidene proton resonance is found at 12.49 ppm with JHP = 5 Hz. An X-ray structural study13 shows that trimethylphosphine binds to one of the CNimidoNpyrrolyl faces of the pseudotetrahedral species, which is the face analogous to the CNO face where trimethylphosphine is observed to bind in bisalkoxide species.9 The Lewis acid B(C6F5)3 also reacts immediately with {Mo(NAr)(CHCMe2Ph)(NC4H4)2}2 to yield a mixture of what we propose are syn and anti alkylidenes of the adduct shown in equation 2. The four η5-pyrrolyl protons in the major (syn) isomer are found at 7.7, 7.2, 5.7, and 5.4 ppm in benzene-d6.
(2).
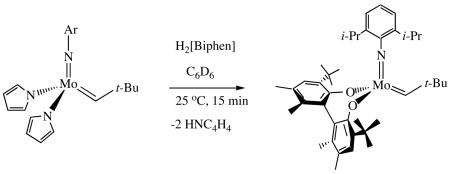
Addition of two equivalents of monoalcohols (e.g., Me3COH or (CF3)2MeCOH) or one equivalent of a biphenol or binaphthol to ~10 mM solutions of the Mo(NR)(CHCMe2R')(NC4H4)2 (NR = NAd or NAr) species described above results in rapid formation of two equivalents of pyrrole and previously characterized bisalkoxide or diolate complexes. The reaction is rapid and ~100% yield in all combinations screened thus far, including the combination of what we consider to be sterically the most challenging, a 2,6-diisopropylphenylimido precursor reacting with H2[Biphen] (H2[Biphen] = 3,3′-Di-t-butyl-5,5′,6,6′-tetramethyl-1,1′-Biphenyl-2,2′-diol (equation 3). In the case of 3,3′-bis(2,4,6-triisopropylphenyl)-2,2′-binaphthol1 the resulting binaphtholate appears to bind one equivalent of pyrrole weakly, but the known THF adduct is generated immediately upon addition of one or more equivalents of THF. Catalysts that have been isolated only as THF adducts, or that have proven to be too unstable to isolate, are likely to be preparable from dipyrrolyl complexes. One
(3).
example is Mo(N-2,6-Br2-4-MeC6H2)(CHCMe3)[Biphen]. Previous attempts to prepare this species through addition of K2[Biphen] to Mo(N-2,6-Br2-4-MeC6H2)(CHCMe3)(OTf)2(DME) failed to produce the desired species in pure form and in a practical yield.14 We find that Mo(N-2,6-Br2-4-MeC6H2)(CHCMe3)(NC4H4)2 reacts with rac-H2[Biphen] in benzene rapidly to yield the previously unknown Mo(N-2,6-Br2-4-MeC6H2)(CHCMe3)[rac-Biphen] species in high yield. The alkylidene proton in Mo(N-2,6-Br2-4-MeC6H2)(CHCMe3)[rac-Biphen] is found at 11.3 ppm with a JCH coupling constant of 132.6 Hz, consistent with a syn alkylidene isomer. The catalytic activity of in situ prepared Mo(N-2,6-Br2-4-MeC6H2)(CHCMe3)[rac-Biphen] was confirmed through the ring-closing metathesis of ~80 equivalents of diallyl ether to dihydrofuran in 15 minutes at room temperature in C6D6.
In conclusion we have found that dimeric dipyrrolyl complexes, {Mo(NR)(CHCMe2R')(NC4H4)2}2, can be prepared readily and in good yield from Mo(NR)(CHCMe2R')(OTf)2(DME) species. All {Mo(NR)(CHCMe2R')(NC4H4)2}2 species react rapidly and completely with monoalcohols and diols to yield known, and in one case, an unknown catalyst, even those that contain sterically the most challenging combination of imido, neopentylidene or neophylidene, and diolate ligands. On the basis of these results we expect to be able to prepare catalysts in situ and use them for a wide variety of reactions. We expect that in some cases we can generate relatively unstable catalysts that could not be isolated, but that still may be useful for catalytic purposes. The possibilities for rapid screening of known and new catalysts we believe to be significant. We also are exploring the fundamental organometallic chemistry of dipyrrolyl alkylidene complexes and their derivatives.
Supplementary Material
Experimental details for the synthesis of all compounds. Crystal data and structure refinement, atomic coordinates and equivalent isotropic displacement parameters, bond lengths and angles, anisotropic displacement parameters, hydrogen coordinates, and isotropic displacement parameters for {Mo(NAd)(CHCMe2Ph)(η1-NC4H4)2}2. Supporting Information is available free of charge via the Internet at http://pubs.acs.org. Data for the structure (06172) are also available to the public at http://www.reciprocalnet.org/.
Acknowledgments
This research was funded by the NIH (GM-59426 to A.H.H. and R.R.S.) and the National Science Foundation (CHE-0138495 and CHE-0554734 to R.R.S.). We thank Dr. Peter Müller for assistance with the X-ray structural solution.
References
- 1.Schrock RR, Hoveyda AH. Angew Chem Int Ed. 2003;42:4592. doi: 10.1002/anie.200300576. [DOI] [PubMed] [Google Scholar]
- 2.Sinha A, Lopez LPH, Schrock RR, Hock AS, Müller P. Organometallics. 2006;25:1412. doi: 10.1021/om060430j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanc F, Baudouin A, Copéret C, Thivolle-Cazat J, Basset JM, Lesage A, Emsley L, Sinha A, Schrock RR. Angew Chem Int Ed. 2006;45:1216. doi: 10.1002/anie.200503205. [DOI] [PubMed] [Google Scholar]
- 4.Sinha A, Schrock RR, Müller P, Hoveyda AH. Organometallics. 2006;25:4621–4626. doi: 10.1021/om060430j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrock RR, Murdzek JS, Bazan GC, Robbins J, DiMare M, O'Regan M. J Am Chem Soc. 1990;112:3875. [Google Scholar]
- 6.Schrock RR, Jamieson JY, Araujo JP, Bonitatebus PJJ, Sinha A, Lopez LPH. J Organometal Chem. 2003;684:56. [Google Scholar]
- 7.Tonzetich ZJ, Schrock RR, Müller P. Organometallics. 2006;25:4301. doi: 10.1021/om060430j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.In structurally characterized η5-pyrrolyl complexes in which the pyrrolyl is disubstituted an upfield shift is observed compared to η1-pyrrolyl proton resonances. For example the pyrrole hydrogens in Ti(η5-2,5-Me2C4H2N)(NMe2)2Cl are found at 5.93 ppm; Duarte MT, Ferreira A, Dias AR, Salema MM, da Silva JF. Acta Cryst C. 2005;C61:m104. doi: 10.1107/S0108270104031695.
- 9.(a) Schrock RR. Chem Rev. 2002;102:145–180. doi: 10.1021/cr0103726. [DOI] [PubMed] [Google Scholar]; (b) Feldman J, Schrock RR. Prog Inorg Chem. 1991;39:1. [Google Scholar]
- 10.(a) Dias AR, Galvão AM, Galvão AC, Salema MS. J Chem Soc, Dalton Trans. 1997:1055. [Google Scholar]; (b) Ascenso JR, Dias AR, Ferreira AP, Galvão AC, Salema MS, Veiros LF. Inorg Chim Acta. 2003;356:249. [Google Scholar]
- 11.Kershner DL, Basolo F. Coord Chem Rev. 1987;79:279. [Google Scholar]
- 12.Al Obaidi N, Brown KP, Edwards AJ, Hollins SA, Jones CJ, McCleverty JA, Neaves BD. J Chem Soc, Chem Commun. 1984:690. [Google Scholar]
- 13.Details will be provided in a full paper in due course.
- 14.Jamieson JY. PhD thesis. Massachusetts Institute of Technology; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental details for the synthesis of all compounds. Crystal data and structure refinement, atomic coordinates and equivalent isotropic displacement parameters, bond lengths and angles, anisotropic displacement parameters, hydrogen coordinates, and isotropic displacement parameters for {Mo(NAd)(CHCMe2Ph)(η1-NC4H4)2}2. Supporting Information is available free of charge via the Internet at http://pubs.acs.org. Data for the structure (06172) are also available to the public at http://www.reciprocalnet.org/.


