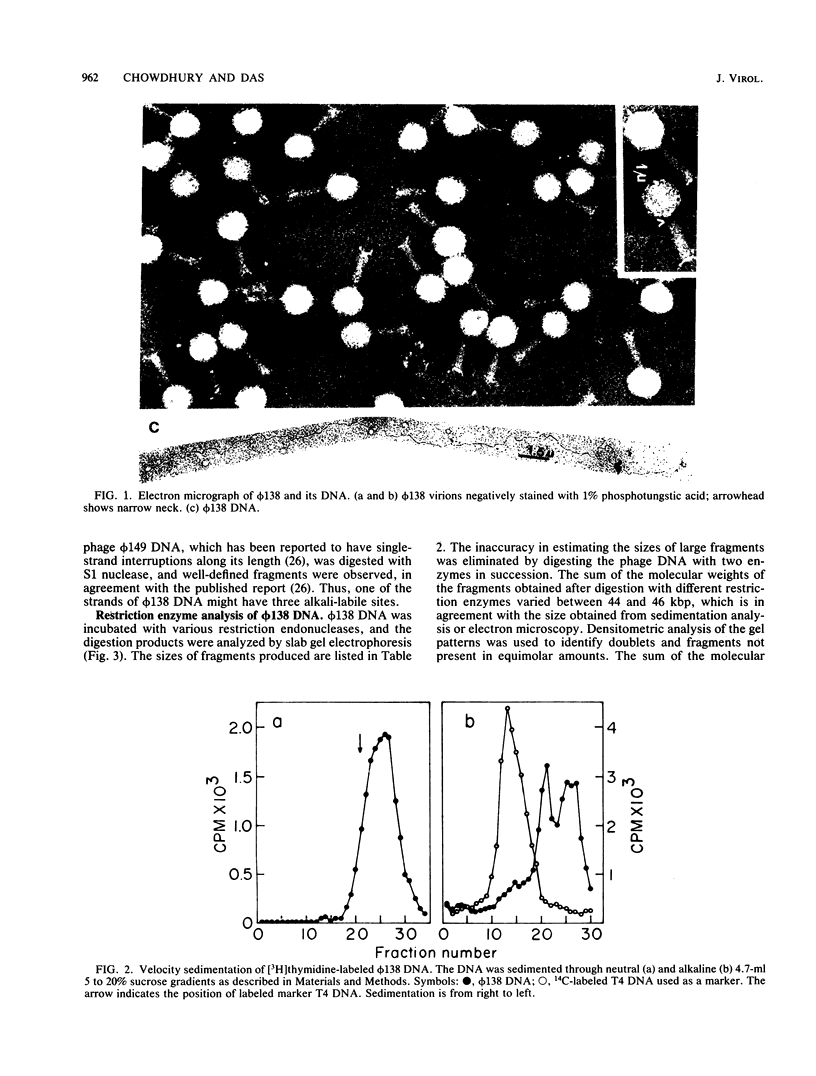
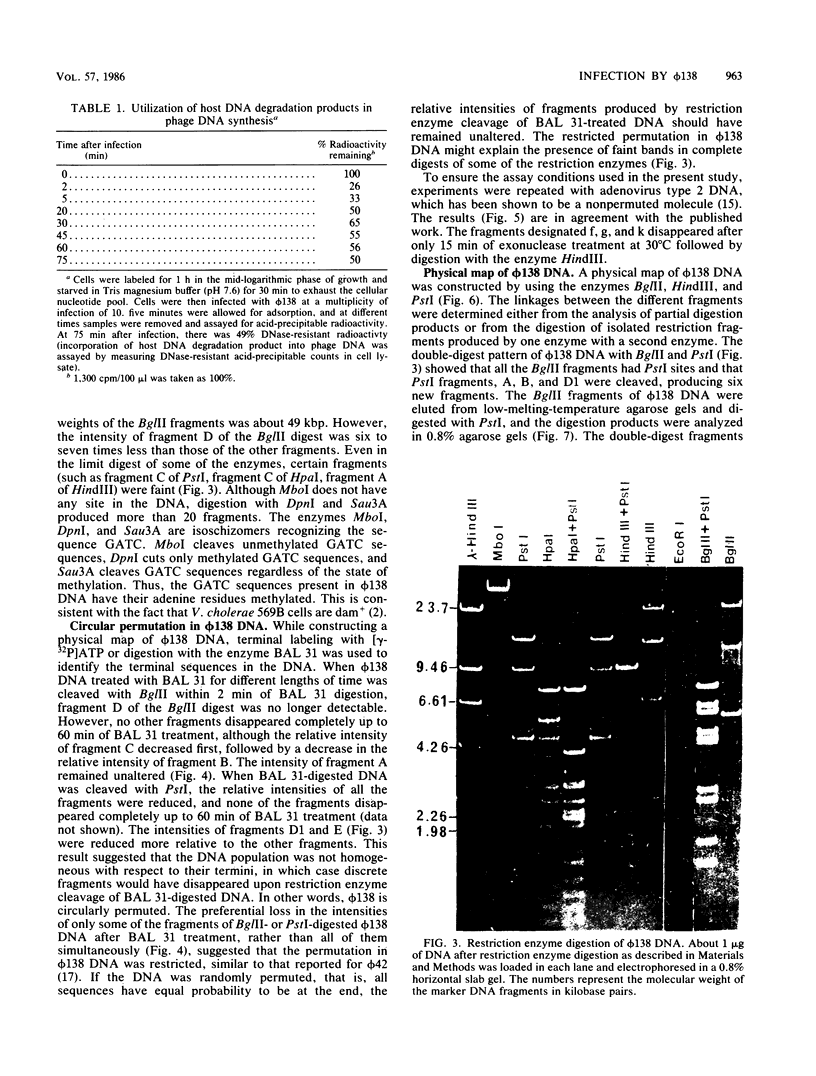
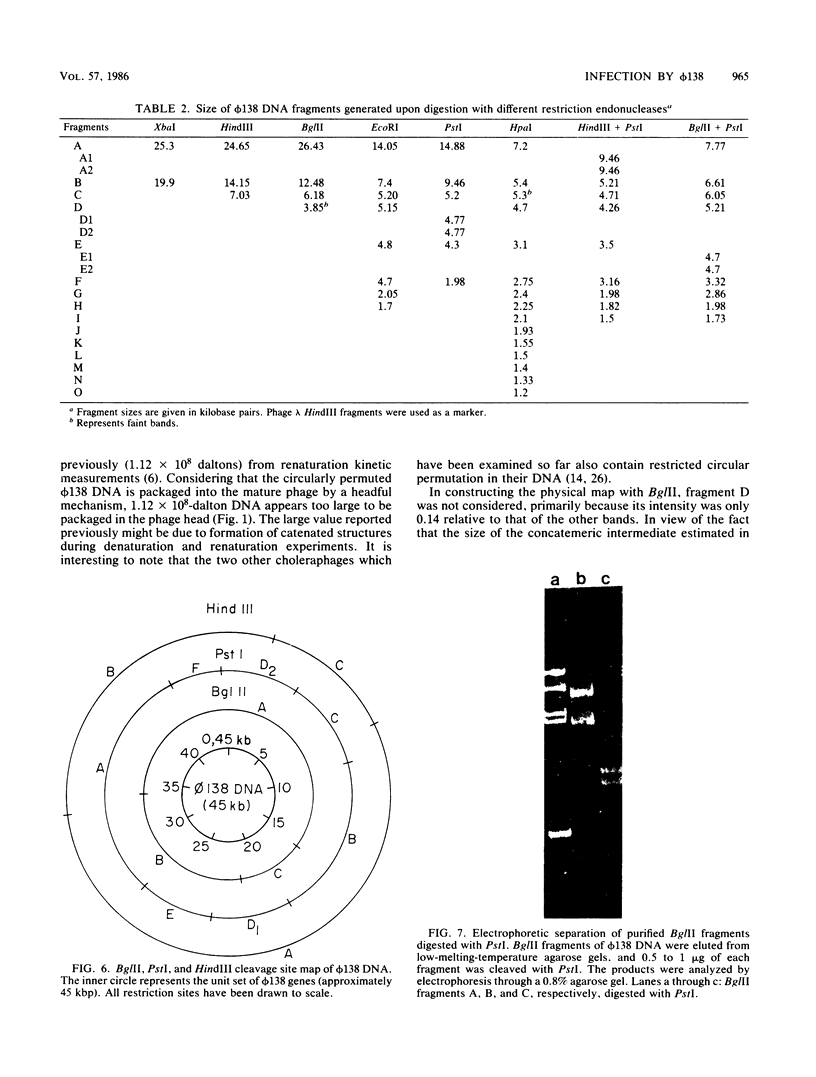
Abstract
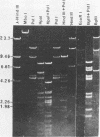
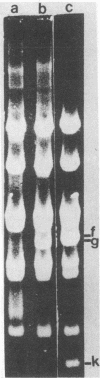
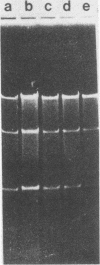
Choleraphage phi 138 contains a linear, double-stranded, circularly permuted DNA molecule of 30 X 10(6) daltons or 45 kilobase pairs. Upon infection, the host DNA is degraded, and synthesis of phage-specific DNA is detectable 20 min after infection. The phage utilizes primarily the host DNA degradation products for its own DNA synthesis. A physical map of phi 138 DNA was constructed with the restriction endonucleases Bg/II, HindIII, and PstI. A concatemeric replicative DNA intermediate equivalent to eight mature genome lengths was identified. The concatemer was shown to be the precursor for the synthesis of mature bacteriophage DNA which is subsequently packaged by a headful mechanism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balganesh M., Das J. Transfection of Vibrio cholerae by bacteriophage phi 149 DNA. Biochem Biophys Res Commun. 1979 Oct 12;90(3):726–733. doi: 10.1016/0006-291x(79)91888-6. [DOI] [PubMed] [Google Scholar]
- Barbeyron T., Kean K., Forterre P. DNA adenine methylation of GATC sequences appeared recently in the Escherichia coli lineage. J Bacteriol. 1984 Nov;160(2):586–590. doi: 10.1128/jb.160.2.586-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein C. Deoxyribonucleic acid repair in bacteriophage. Microbiol Rev. 1981 Mar;45(1):72–98. doi: 10.1128/mr.45.1.72-98.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran K., Sinha V. B., Iyer S. S. Chromosome mobilization in Vibrio cholerae (biotype eltor) mediated by sex factor P. J Gen Microbiol. 1973 Sep;78(1):119–124. doi: 10.1099/00221287-78-1-119. [DOI] [PubMed] [Google Scholar]
- Chatterjee S. N., Das J., Barua D. Electron microscopy of cholera phages. Indian J Med Res. 1965 Oct;53(10):934–937. [PubMed] [Google Scholar]
- Chaudhuri K., Maiti M. Genome & structural protein characterization of Vibrio phage phi 2. Indian J Biochem Biophys. 1980 Jun;17(3):207–212. [PubMed] [Google Scholar]
- Chiang T., Harm W. On the lack of host-cell reactivation of UV-irradiated phage T5. I. Interference of T5 infection with the host-cell reactivation of phage T1. Mutat Res. 1976 Aug;36(2):121–134. doi: 10.1016/0027-5107(76)90001-4. [DOI] [PubMed] [Google Scholar]
- Citarella R. V., Colwell R. R. Polyphasic taxonomy of the genus Vibrio: polynucleotide sequence relationships among selected Vibrio species. J Bacteriol. 1970 Oct;104(1):434–442. doi: 10.1128/jb.104.1.434-442.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G., Sil K., Das J. Repair of ultraviolet-light-induced DNA damage in vibrio cholerae. Biochim Biophys Acta. 1981 Oct 27;655(3):413–420. doi: 10.1016/0005-2787(81)90053-8. [DOI] [PubMed] [Google Scholar]
- Das J., Nowak J. A., Maniloff J. Host cell and ultraviolet reactivation of ultraviolet-irradiated Mycoplasmaviruses. J Bacteriol. 1977 Mar;129(3):1424–1427. doi: 10.1128/jb.129.3.1424-1427.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEINER R. R., HILL R. F. EFFECT OF BASIC DYES ON HOST-CELL REACTIVATION OF ULTRA-VIOLETIRRADIATED PHAGE. Nature. 1963 Oct 19;200:291–293. doi: 10.1038/200291a0. [DOI] [PubMed] [Google Scholar]
- Guidolin A., Morelli G., Kamke M., Manning P. A. Vibrio cholerae bacteriophage CP-T1: characterization of bacteriophage DNA and restriction analysis. J Virol. 1984 Jul;51(1):163–169. doi: 10.1128/jvi.51.1.163-169.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larive A., Pourcel C., Tiollais P. Localization of Streptomyces stanfordii endonuclease I (SstI) cleavage sites on genomes of human adenovirus types two and five. Gene. 1979 Jan;5(1):77–83. doi: 10.1016/0378-1119(79)90093-3. [DOI] [PubMed] [Google Scholar]
- Lohia A., Majumdar S., Chatterjee A. N., Das J. Effect of changes in the osmolarity of the growth medium on Vibrio cholerae cells. J Bacteriol. 1985 Sep;163(3):1158–1166. doi: 10.1128/jb.163.3.1158-1166.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynet D. J., DeFilippes F. M. Characterization of bacteriophage phi 42 DNA. Virology. 1982 Mar;117(2):475–484. doi: 10.1016/0042-6822(82)90485-8. [DOI] [PubMed] [Google Scholar]
- Ogg J. E., Timme T. L., Alemohammad M. M. General Transduction in Vibrio cholerae. Infect Immun. 1981 Feb;31(2):737–741. doi: 10.1128/iai.31.2.737-741.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palit B. N., Das G., Das J. Repair of ultraviolet light-induced DNA damage in cholera bacteriophages. J Gen Virol. 1983 Aug;64(Pt 8):1749–1755. doi: 10.1099/0022-1317-64-8-1749. [DOI] [PubMed] [Google Scholar]
- Parker C., Gauthier D., Tate A., Richardson K., Romig W. R. Expanded linkage map of Vibrio cholerae. Genetics. 1979 Feb;91(2):191–214. doi: 10.1093/genetics/91.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P., Sengupta A., Das J. Phosphate repression of phage protein synthesis during infection by choleraphage phi 149. Virology. 1984 Jul 15;136(1):110–124. doi: 10.1016/0042-6822(84)90252-6. [DOI] [PubMed] [Google Scholar]
- Roy N. K., Das G., Balganesh T. S., Dey S. N., Ghosh R. K., Das J. Enterotoxin production, DNA repair and alkaline phosphatase of Vibrio cholerae before and after animal passage. J Gen Microbiol. 1982 Sep;128(9):1927–1932. doi: 10.1099/00221287-128-9-1927. [DOI] [PubMed] [Google Scholar]
- Roy N. K., Ghosh R. K., Das J. Repression of the alkaline phosphatase of Vibrio cholerae. J Gen Microbiol. 1982 Feb;128(2):349–353. doi: 10.1099/00221287-128-2-349. [DOI] [PubMed] [Google Scholar]
- Sengupta A., Ray P., Das J. Characterization and physical map of choleraphage phi 149 DNA. Virology. 1985 Jan 30;140(2):217–229. doi: 10.1016/0042-6822(85)90360-5. [DOI] [PubMed] [Google Scholar]
- Shishido K., Ando T. Site-specific fragmentation of bacteriophage T5 DNA by single-strand-specific S1 endonuclease. Biochim Biophys Acta. 1975 Apr 16;390(1):125–132. doi: 10.1016/0005-2787(75)90015-5. [DOI] [PubMed] [Google Scholar]
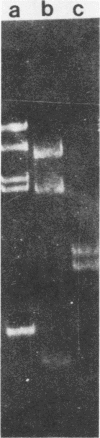
- Wieslander L. A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem. 1979 Oct 1;98(2):305–309. doi: 10.1016/0003-2697(79)90145-3. [DOI] [PubMed] [Google Scholar]