Abstract
Trypanosoma cruzi is a protozoan parasite that belongs to an early branch in evolution. Although it lacks several features of the pathway of protein N-glycosylation and oligosaccharide processing present in the endoplasmic reticulum of higher eukaryotes, it displays UDP-Glc:glycoprotein glucosyltransferase and glucosidase II activities. It is herewith reported that this protozoan also expresses a calreticulin-like molecule, the third component of the quality control of glycoprotein folding. No calnexin-encoding gene was detected. Recombinant T. cruzi calreticulin specifically recognized free monoglucosylated high-mannose-type oligosaccharides. Addition of anti-calreticulin serum to extracts obtained from cells pulse–chased with [35S]Met plus [35S]Cys immunoprecipitated two proteins that were identified as calreticulin and the lysosomal proteinase cruzipain (a major soluble glycoprotein). The latter but not the former protein disappeared from immunoprecipitates upon chasing cells. Contrary to what happens in mammalian cells, addition of the glucosidase II inhibitor 1-deoxynojirimycin promoted calreticulin–cruzipain interaction. This result is consistent with the known pathway of protein N-glycosylation and oligosaccharide processing occurring in T. cruzi. A treatment of the calreticulin-cruzipain complexes with endo-β-N-acetylglucosaminidase H either before or after addition of anti-calreticulin serum completely disrupted calreticulin–cruzipain interaction. In addition, mature monoglucosylated but not unglucosylated cruzipain isolated from lysosomes was found to interact with recombinant calreticulin. It was concluded that the quality control of glycoprotein folding appeared early in evolution, and that T. cruzi calreticulin binds monoglucosylated oligosaccharides but not the protein moiety of cruzipain. Furthermore, evidence is presented indicating that glucosyltransferase glucosylated cruzipain at its last folding stages.
INTRODUCTION
N-Glycoproteins are first glycosylated upon transfer of an oligosaccharide (Glc3Man9GlcNAc2 in most cells, see below) from a dolichol-P-P derivative to Asn residues in the lumen of the endoplasmic reticulum (ER). Protein-linked oligosaccharides are then processed in the same subcellular location by glucosidase I (GI), which removes the more external glucose unit, and glucosidase II (GII), which excises the two remaining glucoses. Specific mannosidases may remove up to two mannose units in the mammalian cell ER (Kornfeld and Kornfeld, 1985). An additional ER processing reaction is that catalyzed by the UDP-Glc:glycoprotein glucosyltransferase (GT). This enzyme adds a single glucose unit to glucose-free, high-mannose-type oligosaccharides in glycoproteins not displaying their properly folded tertiary structures. GT behaves, therefore, as a sensor of glycoprotein conformations (Parodi et al., 1983b; Sousa et al., 1992). GII deglucosylates not only the monoglucosylated oligosaccharides generated by partial deglucosylation of the transferred oligosaccharides but also those formed by GT, because they have identical structures (Trombetta et al., 1989).
Glycoproteins acquire their proper tertiary structure in the ER. This process requires participation of chaperones and other folding–assisting proteins. Glycoproteins that fail to properly fold are retained in the ER and further transported to the cytosol where they are degraded in the proteasomes. On the other hand, properly folded species may continue their transit through the secretory pathway to their final destinations. Two unconventional chaperones (calnexin and calreticulin), which recognize monoglucosylated high-mannose-type oligosaccharides, have been described in the ER lumen of mammalian cells (for review, see Helenius et al., 1997). Membrane-bound calnexin and soluble calreticulin display a 35% similarity in their amino acid sequence. It has been shown that interaction of calnexin and calreticulin with monoglucosylated glycoproteins generated either by partial deglucosylation of the transferred oligosaccharide or by reglucosylation of glucose-free compounds by GT facilitates glycoprotein folding in mammalian cells by preventing aggregation and suppressing formation of nonnative disulfide bonds (Hebert et al., 1996). Moreover, the above-mentioned interaction provides one of the mechanisms by which cells retain misfolded glycoproteins in the ER (Zhang et al., 1997). The calnexin and calreticulin interaction with monoglucosylated glycoproteins has been considered, therefore, a quality control of glycoprotein folding. Although the bulk of evidence supporting the model of quality control as proposed comes from experiments performed with mammalian cell systems, indirect evidence indicates that the same features of the model occur in the yeast Schizosaccharomyces pombe and in plants (Parodi et al., 1984; Trombetta et al., 1989; Fernández et al., 1994, 1996; Jannatipour and Rokeach, 1995; Parlati et al., 1995; Lupattelli et al., 1997).
There is a controversy on whether calnexin and calreticulin behave as lectins that exclusively recognize the above-mentioned oligosaccharides or whether, alternatively, such recognition is the first and necessary step for an interaction between misfolded glycoprotein protein moieties and calnexin and calreticulin. According to this last interpretation, once the protein–protein interaction is established, recognition of monoglucosylated oligosaccharides would be irrelevant for the stability of the complex. Liberation of glycoproteins from the complex would result from a conformational change in the substrate polypeptides. On the other hand, according to the first interpretation release of glycoproteins from the complex would exclusively occur through the action of GII. In both cases, properly folded species would not be able to be reglucosylated by GT, and thus no further interaction with calnexin and calreticulin would occur (Helenius et al., 1997). Evidence supporting both models has certain drawbacks that will be discussed further below.
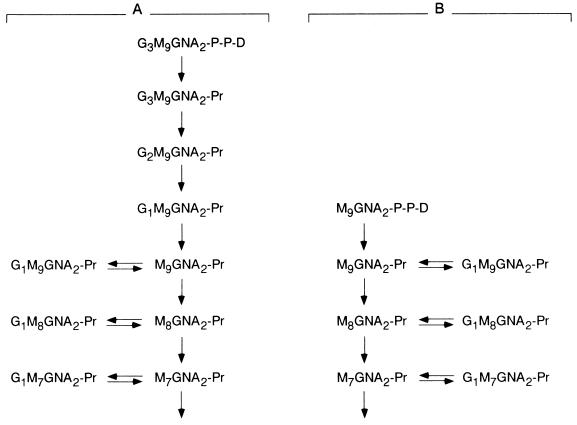
Trypanosomatid protozoa are microorganisms that according to most commonly used criteria (rRNA and several protein sequences) belong to a an early branch of evolution (Baldauf and Palmer, 1993; Solgin, 1997). From an evolutionary point of view they are much more distant from mammals than fungi. Several features of the pathway leading to the formation of N-glycoproteins in trypanosomatids reveal significant differences with those present in mammalian cells: 1) the dolichol moiety has, in trypanosomatid protozoa, 11 or 12 isoprene residues, the same as polyprenols involved in bacterial cell wall synthesis (Parodi and Quesada-Allué, 1982; Quesada-Allué and Parodi, 1983; Low et al., 1991); trypanosomatid dolichols are, therefore, significantly shorter than those found in fungal and mammalian cells (16–18 and 19–21 isoprenes, respectively); 2) trypanosomatid protozoa are the only wild-type eukaryotic cells known so far to be unable to synthesize dolichol-P-Glc (de la Canal and Parodi, 1987); 3) Man6GlcNAc2, Man7GlcNAc2, or Man9GlcNAc2 (depending on the species), instead of Glc3Man9GlcNAc2, is transferred in vivo in trypanosomatid cells (Parodi et al., 1981; Parodi and Quesada-Allué, 1982; Mendelzon et al., 1986); and 4) the oligosaccharyltransferase from trypanosomatids transfers, in cell-free assays, Man6–9GlcNAc2 and Glc1–3Man9GlcNAc2 at the same rate (Bosch et al., 1988). Its specificity significantly varies from the fungal and mammalian enzymes that transfer the triglucosylated compound 10- to 25-fold faster than the other oligosaccharides. GT and GII activities have been detected both in intact trypanosomatid cells and in cell-free assays (Parodi et al., 1983a; Bosch et al., 1988; Trombetta et al., 1989). As expected, no GI activity was found. This result is consistent with the fact that no triglucosylated compounds occur in these parasites. Glycosylation and oligosaccharide-processing reactions occurring in the ER of mammalian and Trypanosoma cruzi (the causative agent of Chagas’ disease) cells are depicted in Figure 1. It is worth stressing the fact that in this parasite, the same as in all other trypanosomatids, monoglucosylated compounds are exclusively formed through GT-dependent glucosylation.
Figure 1.
Mammalian and T. cruzi ER glycoprotein processing reactions. Initial glycoprotein-processing reactions occurring in mammalian (A) and T. cruzi (B) cells are shown. G, glucose; M, mannose; GNA, N-acetylglucosamine; D, dolichol; Pr, protein.
Results presented here show that the quality control of glycoprotein folding appeared early in evolution, before completion of the pathway of protein N-glycosylation and oligosaccharide processing occurring in the ER of higher eukaryotes. Moreover, evidence is presented showing that calreticulin exclusively behaves as a lectin in trypanosomatid protozoa.
MATERIALS AND METHODS
Cells and Culture Media
T. cruzi Tulahuen 2 epimastigotes were grown as described before (Cazzulo et al., 1985). Escherichia coli NovaBlue (Novagen, Madison, WI) was used for cloning experiments. E. coli strain Y1090 (Stratagene, La Jolla, CA) was used for screening the T. cruzi genomic library constructed in λgt11 phages. For expression of the recombinant protein the strain was E. coli BL26 (DE3; Novagen). Bacteria were grown in Luria–Bertani medium, 0.5% NaCl, 1% tryptone (Difco, Detroit, MI), 0.5% yeast extract (Difco), and 100 μg/ml ampicillin if necessary or in the same medium supplemented with 0.2% maltose and 10 mM MgSO4.
Other Procedures
Extraction of RNA and Northern blots were performed as described before (Ausubel et al., 1994). mRNA was purified through an oligo-dT-cellulose column. Western blots were carried out as described previously (Sambrook et al., 1989). T. cruzi genomic DNA was prepared as already described (Borst et al., 1980). The T. cruzi genomic DNA library in phage λgt11 was that described before (Ibáñez et al., 1987). Subcellular fractionation was performed as described previously (Bontempi et al., 1989).
PCR Reactions
Standard procedures were used for PCR. Primers used for synthesis of the 250-, 320-, and 375-bp fragments were ATHATGTTYGGNCCNGAYAARTG (sense) and TCCCARTCYTCNGGYTT (antisense). For synthesis of the entire calreticulin-encoding gene the primers were CATGCCCATGGGCATGCGTGCAGCAATTTTTTTCTGTGCAC (sense) and TAGTCCTCGAGCAAATCCTCCTTATCAC (antisense).
Cloning and Sequencing of the T. cruzi Calreticulin-encoding Gene
Degenerate oligonucleotides (see above) were designed according to conserved sequences of calnexins and calreticulins from different species (Jannatipour and Rokeach, 1995; Parlati et al., 1995). PCR reactions that used those primers and T. cruzi genomic DNA as template yielded fragments having ∼250, 320, and 375 bp. Both larger fragments were cloned and sequenced. Two 320-bp fragments from independent clones gave identical sequences. A single 375-bp cloned fragment was sequenced and found to contain the 320-bp fragment. The 250-bp fragment was not sequenced. The 375-bp fragment was used as probe for screening a genomic DNA library in λgt11. A phage containing a 3200-bp insert was thus isolated. The insert was cloned in the EcoRI of pBluescript vector (Stratagene) and sequenced. It contained the 320- and 375-bp fragments and the entire gene as well. The T. cruzi calreticulin-encoding gene GenBank accession number is AF107115.
Expression of Calreticulin
The entire calreticulin-encoding gene was synthesized by PCR amplification using primers indicated above that are complementary to the 5′ and 3′ termini and introduce NcoI and XhoI restriction sites, Pfu DNA polymerase (Stratagene), and the pBluescript vector that contained the entire gene as template. The fragment synthesized, previously treated with NcoI and XhoI, was ligated to pET22B+ (Novagen), also treated with the same enzymes. The resulting plasmid was amplified in E. coli NovaBlue and used for expressing calreticulin in BL26 (DE3) E. coli cells. Synthesis of calreticulin (a 46.7-kDa protein) after isopropylthiogalactoside induction of ampicillin-resistant cells was monitored by breaking cells by lysozyme treatment (100 μg/ml), followed by centrifugation at 12,000 × g for 5 min. Supernatant and precipitate fractions were submitted to 10% SDS-PAGE. Calreticulin was found in inclusion bodies.
Purification and Renaturation of Calreticulin
Cells from a 20-ml culture were resuspended in 20 mM Tris-HCl buffer, pH 7.9, 1 mM PMSF (Sigma, St. Louis, MO), 1% Triton X-100, 20 μg/ml DNase, 10 mM MgCl2, and lysozyme (Sigma, 100 μg/ml) and centrifuged. The pellet was resuspended in 4 ml of binding buffer (20 mM Tris-HCl, pH 7.9, 0.5 M NaCl, and 5 mM imidazole). The suspension was centrifuged, and the supernatant was discarded. The pellet was resuspended in binding buffer containing 6 M urea. After 1 h at 0°C, the suspension was centrifuged for 40 min at 19,000 × g. The supernatant was submitted to Ni2+ affinity chromatography using 2.5 ml of an iminodiacetic acid-agarose column (Sigma) equilibrated with 6 M urea in binding buffer. The column was washed three times with 2 vol of washing buffer (20 mM Tris-HCl buffer, pH 7.9, and 6 M urea) containing increasing imidazole concentrations (10, 15, and 20 mM) in each washing. Calreticulin was eluted with 6 vol of 300 mM imidazole, 0.25 M NaCl, 10 mM Tris-HCl buffer, pH 7.9, and 6 M urea and successively dialyzed for 12 h at 4°C each time against 10 mM Tris-HCl buffer, pH 7.9, and 10 mM CaCl2 containing 4 or 2 M or no urea. This preparation was used for biochemical assays and for generating antibodies because it was homogeneous, as revealed by 10% SDS-PAGE stained with Coomassie Brilliant Blue.
Oligosaccharide Binding to Calreticulin
[14C]Glc1–3Man9GlcNAc2 and [14C]Man9GlcNAc2 were obtained upon mild acid hydrolysis of dolichol-P-P-oligosaccharides isolated from hen oviduct slices incubated with [14C]glucose (250 Ci/mol; New England Nuclear, Boston, MA) for 3 h, as described before (Parodi et al., 1981). Oligosaccharide binding to calreticulin was performed as previously described (Ware et al., 1995) with slight modifications: 20 μg of hexahistidine-tagged calreticulin was mixed with 40 μl of Ni2+-iminodiacetic acid-agarose in binding buffer (10 mM Tris-HCl buffer, pH 7.6, 100 mM NaCl, and 10 mM CaCl2) and 2000 cpm of above-mentioned oligosaccharides in 100 μl of binding buffer. The mixture was incubated in an orbital shaker at room temperature for 1 h, after which the sample was centrifuged for 1 min at 2600 × g. The supernatant was collected, and the beads were washed with 100 μl of binding buffer and centrifuged as above. The supernatant was discarded. The agarose beads were then incubated for 1 h with 100 μl of binding buffer containing 10 mM α-methylglucoside plus 10 mM α-methylmannoside and centrifuged. The supernatant was collected. All samples were desalted with a Dowex 50 W (H+ form) resin (Dow Chemical, Midland, MI), freeze dried, and spotted on Whatman (Clifton, NJ) 1 papers. Chromatographies were developed with 1-propanol:nitromethane:water (5:2:4).
Generation of Anti-Calreticulin Serum
Calreticulin purified as described above (200 μg) was intradermically injected to a rabbit together with complete Freund’s adjuvant. Two successive subcutaneous injections of 200 μg of calreticulin in incomplete adjuvant were performed with 15-d intervals. The animal was bled 15 d after the last booster. The serum was preadsorbed with a crude E. coli BL26 (DE3) extract as already described (Sambrook et al., 1989).
Pulse–Chase Labeling of T. cruzi Cells
Cells in the exponential phase (4.0 × 107 cells/ml) were harvested, and 2.5 g of them were washed twice with Ham’s F-12 (Met, Pro, and Gly free; Biochrom KG, Berlin, Germany) medium (10.65 mg/ml) supplemented with 34.5 mg/l of Pro, 7.5 mg/l of Gly, and 1.2 g/l of NaHC03. The parasites were resuspended in 9 ml of the above-mentioned medium. The suspension was divided in halves. 1-Deoxynojirimycin (DNJ, Sigma) was added to one of them up to a 6 mM final concentration. Both halves were preincubated for 20 min at 28°C. [35S]Met plus [35S]Cys (2 mCi, >1000 Ci/mmol; EasyTag protein labeling mix, New England Nuclear) were added, and both halves were then incubated for 2 min at 28°C. The suspensions were submitted to low-speed centrifugations, and the pellets were washed with 6 ml of T. cruzi normal growth medium supplemented with 3 mM Met plus 3 mM Cys. DNJ (6 mM) was added to the medium used for washing cells previously incubated with the drug. Pellets were resuspended in 6 ml of the respective washing media, and 1-ml aliquots were withdrawn after 0, 5, 10, 30, 60, and 120 min at 28°C. The suspensions were centrifuged, and the pellets were lysed in 1 ml of buffer A (50 mM HEPES buffer, pH 7.5, 0.2 M NaCl, and 1% Nonidet P-40) containing 0.3 M iodoacetamide, 1 mM PMSF, and 100 μM trans-epoxysuccinyl-1-leucylamido(4-guanidino)butane (E-64, Sigma; this compound irreversibly inhibits cruzipain proteinase activity). After 1 h at 0°C, cell lysates were precleared by centrifugation. Where indicated 10 mU of endo-β-N-acetylglucosaminidase H (Endo H, Sigma) per 200 μl of solution were added. The supernatants were then maintained for 1 h at 28°C and submitted to immunoprecipitation.
Immunoprecipitations
For nondenaturing conditions, supernatants (200 μl) were incubated with 1:50 diluted anti-calreticulin serum for 2 h at 4°C in a shaker incubator. Protein A-Sepharose (40 μl, Sigma) was added, and the suspensions were further incubated overnight. Beads were then twice washed with 400 μl of buffer A. Where indicated 10 mU of Endo H/200 μl of suspension were added. The suspensions were then maintained for 1 h at 28°C; the beads washed twice with 200 μl of buffer A, resuspended in cracking buffer, and submitted to 10% SDS-PAGE. For denaturing conditions, SDS (0.5% final concentration) was added to supernatants (200 μl). Solutions were first heated at 95°C for 5 min and then diluted twofold with buffer A. Immunoprecipitations were further performed as above. Sequential immunoprecipitations were first performed under nondenaturing conditions, and washed protein A-Sepharose beads were resuspended in 50 μl of buffer A containing 0.5% SDS and heated for 5 min at 95°C. Suspensions were threefold diluted with buffer A, and supernatants obtained upon centrifugation were submitted to immunoprecipitation with anti-cruzipain serum (Campetella et al., 1990).
Isolation of Labeled Mature Cruzipain
T. cruzi cells were harvested and labeled as above, but labeling was prolonged for 20 min and performed in the presence and absence of 6 mM DNJ. Cells were then chased for 450 min, also in the presence and absence of the drug, and mature cruzipain was isolated from lysosomes as already described (Labriola et al., 1995). Cruzipain was purified up to the concanavalin A affinity chromatography step.
RESULTS
Cloning and Sequencing of T. cruzi Calreticulin
As mentioned above, two of the three components of the quality control of glycoprotein folding (GT and GII) have been described previously in T. cruzi. To test whether the third component (calnexin–calreticulin) is also present in this parasite, T. cruzi genomic DNA was used as template in PCR reactions together with degenerate primers designed according to amino acid sequences conserved among several fungal and mammalian calnexins and calreticulins (IMFGPDKC and the KPEDWDE repeat motif for the sense and antisense primers, respectively). Three bands of ∼250, 320, and 375 bp were obtained. Both larger fragments were cloned and sequenced. Two 320-bp fragments from independent clones gave identical sequences. A single 375-bp cloned fragment was sequenced and found to contain the 320-bp fragment. The 250-bp fragment was not sequenced. The 375-bp fragment was used as probe for screening a genomic DNA library in λgt11. A phage containing a 3200-bp insert was thus isolated and sequenced. It contained the 320- and 375-bp fragments and the entire gene as well. As with all trypanosomatid genes sequenced so far, it contained no introns. A conceptual translation of the ORF is depicted in Figure 2. The gene codes for a 46.7-kDa protein that is 40% identical and 64% similar to human calreticulin. It has the characteristic acidic domain at the C terminus as well as an ER retrieval sequence (KEDL) that resembles that present in grp78, a T. cruzi ER protein that ends in MDDL (Tibbetts et al., 1994). The parasite calreticulin has three consensus Ca2+ binding motifs, the same as its human counterpart (KPEDWDE or its conserved variations), and also both Cys residues present in conserved positions in other calreticulins. Synthesis of the 250-, 320-, and 375-bp fragments as PCR products was probably due to the recognition of the three consensus Ca2+ binding motifs by the antisense primer.
Figure 2.
Comparison of human (A), L. donovani (B), and T. cruzi (C) calreticulin amino acid sequences. Black boxes indicate identical residues. Spaces introduced for sequence alignment are represented by dashes.
Calreticulin has also been sequenced from another trypanosomatid protozoan, Leishmania donovani (Figure 2) (Josi et al., 1996). Calreticulin from this parasite is 39 and 42% identical to its human and T. cruzi counterparts, respectively. L. donovani calreticulin has two consensus N-glycosylation sites, at least one of which was occupied when the corresponding gene was transcribed and translated in the presence of dog pancreas microsomes. No N-glycosylation sites occur in T. cruzi calreticulin. The L. donovani protein had, the same as its human and simian homologues, the capacity of binding RNA. The possible physiological role of this property remains obscure (see below).
We have been unable to detect a calnexin-encoding gene in T. cruzi. As mentioned above, although primers were designed according to sequences conserved in both calreticulins and calnexins, the PCR fragments synthesized corresponded to a calreticulin-like protein. When the same primers were used with genomic S. pombe DNA as template in PCR reactions, the fragment formed had the size expected for all calnexin-encoding genes sequenced so far (450 bp). No fragment having this size was formed with T. cruzi DNA as template. The 450-bp fragment was sequenced, and because it corresponded to the highly conserved region of calnexins, it was used as probe for screening T. cruzi genomic DNA at low-stringency hybridization conditions (50°C, 2× SSC). No positive signal was obtained. It would appear that T. cruzi is the opposite of S. pombe, a microorganism whose genome codes for a calnexin- but not for a calreticulin-like protein (Jannatipour and Rokeach, 1995; Parlati et al., 1995).
Expression and Subcellular Localization of T. cruzi Calreticulin
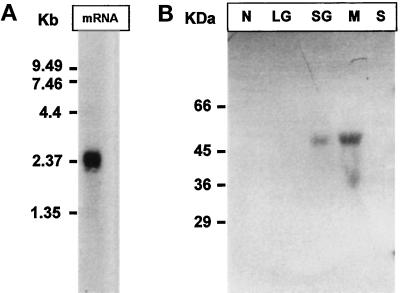
Northern blotting analysis of total T. cruzi epimastigote mRNA yielded a single 2300-bp positive signal when the 375-bp fragment was used as probe (Figure 3A). This confirmed that the calreticulin-encoding gene was expressed in T. cruzi.
Figure 3.
Expression and subcellular localization of T. cruzi calreticulin. (A) T. cruzi mRNA was submitted to Northern blotting analysis using a 375-bp T. cruzi calreticulin-encoding gene fragment as probe. (B) T. cruzi epimastigote cells were submitted to subcellular fractionation followed by Western blotting analysis using anti-calreticulin serum as probe. Subcellular fractions: N, nuclear; LG, large granule; SG, small granule; M, microsomes; S, soluble.
The entire gene was cloned in pET22B+ (which introduces a His tag at the C terminus of the protein) and expressed in E. coli BL26. Cells were lysed after isopropylthiogalactoside induction and centrifuged. SDS-PAGE analysis of soluble and insoluble material showed that practically all of an induced 48-kDa protein was in the last fraction. Insoluble material was solubilized in 6 M urea and purified by Ni2+ affinity chromatography. Calreticulin was renatured by successive dialysis against solutions containing decreasing urea concentrations. This preparation was used for studying calreticulin binding properties and for generating antibodies because it was homogeneous as judged by SDS-PAGE.
T. cruzi epimastigote cells were submitted to a subcellular fractionation by differential centrifugation. Similar protein amounts from each fraction (soluble, nuclear, small granule, large granule, and microsomal) were submitted to SDS-PAGE and further analyzed by Western blotting analysis using anti-calreticulin serum. A single positive signal corresponding to a 47-kDa protein was detected in the microsomal fraction (Figure 3B). A much weaker signal of the same size was observed in the small granule fraction. These results are compatible with the presence of an ER retrieval sequence in T. cruzi calreticulin. The presence of the protein in the small granule fraction might be due to a cross-contamination of fractions. The absence of calreticulin from the soluble and nuclear fractions strongly argues against the possibility that the capacity of RNA binding shown by L. donovani calreticulin in cell-free assays (see above) could have a physiological role.
T. cruzi Calreticulin Specifically Binds Free Monoglucosylated Oligosaccharides
A mixture of [14C]Glc1–3Man9GlcNAc2 and [14C]Man9GlcNAc2 was mixed with recombinant T. cruzi calreticulin-Ni2+-iminodiacetic acid-agarose. Bound material was eluted with a mixture of 10 mM α-methylglucoside plus 10 mM α-methylmannoside. Comparison of patterns of applied, unbound, and retained material showed that T. cruzi calreticulin, the same as mammalian calnexin and calreticulin, specifically binds monoglucosylated oligosaccharides (Figure 4, A–C) (Ware et al., 1995; Spiro et al., 1996).
Figure 4.
T. cruzi calreticulin binds monoglucosylated oligosaccharides. A mixture of 14C-labeled oligosaccharides were mixed with calreticulin-iminodiacetic acid-agarose beads. Applied (A), unbound (B), and bound (C; but liberated by α-methylglucoside and α-methylmannoside) compounds were run on paper chromatography. Standards: 3, Glc3Man9GlcNAc; 2, Glc2Man9GlcNAc; 1, Glc1Man9GlcNAc; 9, Man9GlcNAc.
DNJ Promotes Binding of Glycoproteins to Calreticulin
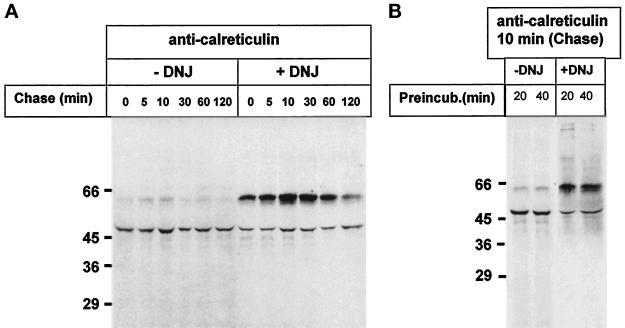
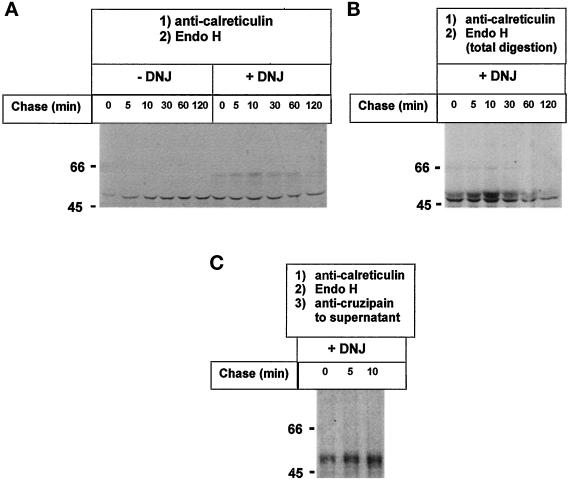
It has been firmly established that addition of GI and GII inhibitors as castanospermine and DNJ to mammalian cells inhibits binding of glycoproteins to calnexin and calreticulin, because those compounds promote accumulation of di- and triglucosylated oligosaccharides (Figure 1A) (Ou et al., 1993; Hammond et al., 1994; Kearse et al., 1994; Hebert et al., 1995; Nauseef et al., 1995; Peterson et al., 1995). According to the known glycosylation and oligosaccharide processing pathway occurring in the ER of T. cruzi cells (Figure 1B), addition of GI and GII inhibitors should have the opposite effect if a quality control of glycoprotein folding similar to that described for mammalian cells is operative in this parasite. T. cruzi cells were incubated for 2 min with [35S]Met plus [35S]Cys both in the absence and in the presence of 6 mM DNJ and chased for different periods. Cells were lysed upon addition of detergent and iodoacetamide, and anti-calreticulin serum was added. The immunoprecipitates obtained under nondenaturing conditions were submitted to reducing SDS-PAGE. As depicted in Figure 5A, autoradiography of gels showed two main bands of 47 and 60 kDa. The intensities of the bands having the lower molecular mass were approximately similar in samples incubated with or without DNJ. On the contrary, the 60-kDa bands were much more intense in samples incubated in the presence of the GII inhibitor. In addition, whereas the 60-kDa band gradually disappeared upon chasing cells, the intensity of the 47-kDa band did not show a noticeable decrease.
Figure 5.
Effect of DNJ on calreticulin–glycoprotein interactions. T. cruzi cells were preincubated for 20 min, pulsed for 2 min with [35S]Met plus [35S]Cys, and chased for the indicated periods at 28°C in the absence or presence of 6 mM DNJ. (A) Cells were lysed, and material immunoprecipitated by anti-calreticulin serum under nondenaturing conditions was submitted to 10% SDS-PAGE under reducing conditions followed by autoradiography. (B) Cells were preincubated with or without DNJ for 20 or 40 min, pulsed, chased for 10 min, and further treated as above. Molecular masses (in kilodaltons) are indicated on the left.
DNJ does not penetrate instantly into the ER lumen. For this reason, inhibition of calnexin and calreticulin binding of folding glycoproteins may only be observed in mammalian cells when the drug is added some time before the labeled glycoprotein precursor. A short or null preincubation of cells with the drug might result in an apparent enhancement of lectin and glycoprotein binding because of inhibition of removal not of the outer or middle but of the innermost glucose units. In the experiment shown in Figure 5A, DNJ was added 20 min before [35S]Met plus [35S]Cys. On the other hand, we have previously observed that the percentage of glucosylated Endo H–sensitive oligosaccharides was the same in T. cruzi cells incubated with [14C]glucose and 6 mM DNJ for either 30 min or grown continuously for several generations under similar conditions (Gañán et al., 1991). This puts an upper limit of 30 min for penetration of DNJ into the T. cruzi ER lumen. To confirm that indeed DNJ-mediated enhancement of the interaction between the 47- and 60-kDa proteins was not due to incomplete glucosidase inhibition after 20 min of preincubation with the drug, parasite cells were preincubated with or without DNJ for 20 or 40 min, labeled for 2 min, chased for 10 min, and further treated as in Figure 5A. As depicted in Figure 5B, DNJ promoted calreticulin binding of the 60-kDa protein under both preincubation conditions. This result confirmed that DNJ enhancement of the interaction between the 47- and 60-kDa proteins was not due to incomplete inhibition of glucose removal but to the particular features of the glycoprotein formation and processing pathway occurring in T. cruzi as shown in Figure 1.
Identification of Proteins Precipitated by Anti-Calreticulin Serum
The 47-kDa protein was identified as calreticulin because it was the only band present when immunoprecipitation was performed under denaturing conditions (Figure 6A). This identification is consistent with the molecular mass of calreticulin. On the other hand, the 60-kDa protein was identified as cruzipain, a lysosomal cysteine proteinase (for a review on cruzipain, see Cazzulo et al., 1997). A Western blotting analysis of gel shown in Figure 5A gave a positive signal when probed with [125I]protein A–anti-cruzipain serum (Figure 6B). Moreover, a 60-kDa signal appeared when the immunocomplexes formed upon addition of anti-calreticulin serum to samples incubated both in the presence and in the absence of DNJ were dissociated, precipitated with anti-cruzipain serum, run on SDS-PAGE, and submitted to autoradiography (Figure 6C).
Figure 6.
Identification of proteins precipitated by anti-calreticulin serum. (A) Proteins from cells labeled in the presence of DNJ and chased for 10 min were submitted to immunoprecipitation with anti-calreticulin serum under denaturing conditions. (B) Proteins from cells labeled in the absence of DNJ and chased for 20 min were immunoprecipitated with anti-calreticulin serum and further submitted to Western blotting analysis using anti-cruzipain serum-[125I]protein A as probe. CP, pure cruzipain was loaded in the gel (lower molecular mass bands correspond to self-proteolysis products). (C) Proteins from cells labeled in the absence or presence of DNJ and chased for 20 min were sequentially precipitated with anti-calreticulin and anti-cruzipain sera. Molecular masses (in kilodaltons) are indicated on the left.
Cruzipain constitutes 5–8% of total soluble cellular protein (Cazzulo et al., 1989). This may explain why it was the only labeled glycoprotein that was recognized by calreticulin after a 2-min pulse. Cruzipain has at least six (and probably seven) disulfide bridges and three N-glycosylation consensus sequences, at least two of which are occupied by high-mannose-type oligosaccharides (Labriola et al., 1995; Metzner et al., 1996). Moreover, we have determined previously that 60–65% of N-oligosaccharides contain a single glucose unit in mature cruzipain molecules isolated from lysosomes of cells grown in the presence of 6 mM DNJ (Labriola et al., 1995).
Results shown in Figures 5 and 6 show, therefore, that calreticulin transiently recognized at least one glycoprotein and that, contrary to what happens in mammalian cells and consistently with the model of quality control of glycoprotein folding proposed for those cells and with the known processing pathway of oligosaccharides in T. cruzi, DNJ promotes binding of calreticulin to the glycoprotein.
Calreticulin Behaves Exclusively as a Lectin
Endo H Treatment of Calreticulin–Cruzipain Complexes.
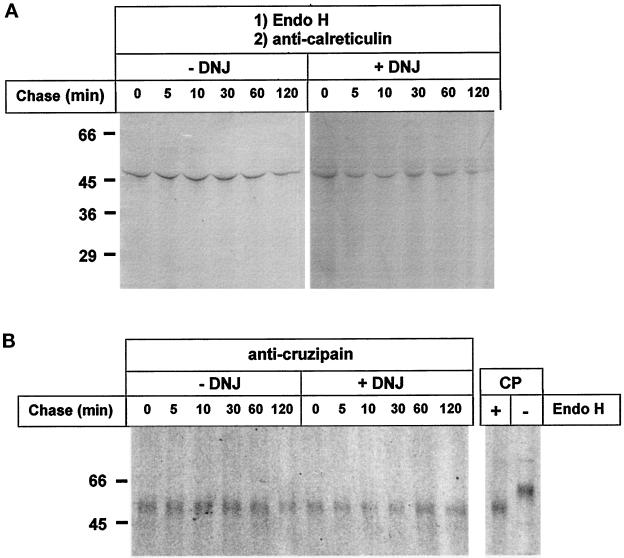
To test whether calreticulin recognized the protein moiety of cruzipain in addition to the monoglucosylated oligosaccharides, lysates from cells pulse–chased in the absence or presence of DNJ were treated with Endo H before addition of anti-calreticulin serum. Autoradiography of the SDS-PAGE of immunoprecipitates obtained upon addition of the antiserum showed that the enzymatic treatment had completely abolished the appearance of cruzipain in the immunoprecipitates in samples labeled both in the presence and in the absence of DNJ (Figure 7A, −DNJ and +DNJ; compare with Figure 5, −DNJ and +DNJ, respectively). SDS-PAGE of immunoprecipitates obtained upon addition of anti-cruzipain serum to Endo H–treated samples revealed that in all cases cruzipain had been completely deglycosylated by the enzyme (Figure 7B, −DNJ and +DNJ).
Figure 7.
Effect of Endo H treatment on cruzipain precipitation by anti-calreticulin serum. Cells were treated as in Figure 5, but samples were incubated with Endo H before addition of anti-calreticulin (A) or anti-cruzipain (B) sera. Immunoprecipitates were run on 10% SDS-PAGE. CP, migration of mature cruzipain treated or not with Endo H. Molecular masses (in kilodaltons) are indicated on the left.
Endo H Treatment of Calreticulin–Cruzipain–Anti-calreticulin Antibody Immunocomplexes.
Complete disappearance of cruzipain from the complexes was also observed when treatment with Endo H was performed not before addition of the anti-calreticulin serum but on the immunoprecipitates obtained upon addition of anti-calreticulin serum to lysates of cells labeled in the absence of DNJ (Figure 8A, −DNJ; compare with Figure 5, −DNJ). On the other hand, residual cruzipain in the complexes was observed when Endo H treatment was similarly performed on immunoprecipitates obtained from samples labeled in the presence of DNJ (Figure 8A, +DNJ; compare with Figure 5, +DNJ). This result was due not to an interaction of the protein moieties of cruzipain and calreticulin but to the fact that not all cruzipain molecules had been degraded by Endo H as cruzipain migrated in Figure 8A, +DNJ, the same as in samples not treated with the glycosidase. To check that cruzipain in Figure 8 A, +DNJ, had not been degraded by Endo H, immunoprecipitates obtained from samples incubated with DNJ were incubated with Endo H but in the presence of 0.5% SDS. The whole samples (not only bead-bound proteins) were then submitted to SDS-PAGE and autoradiography. As depicted in Figure 8B, +DNJ, cruzipain incubated with Endo H under slightly denaturing conditions migrated much closer to calreticulin than in Figure 8A, +DNJ.
Figure 8.
Effect of Endo H treatment of immunoprecipitates. (A) Cells were treated as in Figure 5, but immunoprecipitates obtained with anti-calreticulin serum were treated with Endo H. (B) Immunoprecipitates obtained from cells labeled in the presence of DNJ were treated with Endo H in the presence of 0.5% SDS, and the whole samples (not only material bound to the Sepharose beads) were run on 10% SDS-PAGE and submitted to autoradiography. (C) Anti-cruzipain serum was added to supernatants of Endo H-treated 0-, 5-, and 10-min chase (+DNJ) samples (A, +DNJ), and immunoprecipitates were run on 10% SDS-PAGE. Molecular masses (in kilodaltons) are indicated on the left.
To confirm that cruzipain was indeed liberated by Endo H from the immunocomplexes obtained from cells labeled in the presence of DNJ, supernatants obtained upon centrifugation of the glycosidase-treated 0-, 5-, and 10-min (+DNJ) chase samples (Figure 8A, +DNJ) were treated with anti-cruzipain serum, and immunoprecipitates were run on SDS-PAGE. As depicted in Figure 8C, cruzipain had been liberated from the immunocomplexes by Endo H.
The fact that no cruzipain with a migration on gels intermediate between calreticulin and untreated cruzipain was observed in Figures 7A, −DNJ and +DNJ, and 8A, −DNJ and +DNJ, indicated that no interaction between calreticulin and cruzipain protein moieties had occurred.
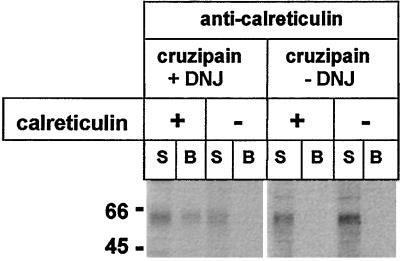
Calreticulin Binds Mature Monoglucosylated Cruzipain
To confirm that the presence of monoglucosylated oligosaccharides was a necessary and sufficient condition for calreticulin–cruzipain interaction, mature cruzipain was isolated from lysosomes of cells pulse–chase labeled with [35S]Met plus [35S]Cys in the presence or absence of 6 mM DNJ, mixed with recombinant calreticulin, and immunoprecipitated with anti-calreticulin serum. As mentioned above, we have previously shown that ∼65% of N-oligosaccharides present in mature cruzipain synthesized in the presence of the GII inhibitor are monoglucosylated. No glucosylated oligosaccharides were detected in the mature proteinase formed in the absence of DNJ (Labriola et al., 1995). SDS-PAGE of immunoprecipitates and supernatants showed that mature glucosylated but not unglucosylated cruzipain interacted with calreticulin (Figure 9). A control experiment showed that the appearance of glucosylated cruzipain in the immunoprecipitate was dependent on the addition of recombinant calreticulin.
Figure 9.
Binding of calreticulin to mature glucosylated cruzipain. Mature cruzipain isolated from lysosomes of cells pulse–chase labeled in the presence or absence of 6 mM DNJ was mixed with recombinant calreticulin and immunoprecipitated with anti-calreticulin serum. Supernatants (S) and protein A-Sepharose–bound material (B) were run on 10% SDS-PAGE. Where indicated, calreticulin was omitted from the mixtures. Molecular masses (in kilodaltons) are indicated on the left.
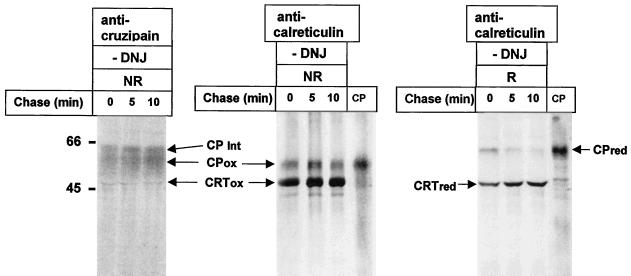
Calreticulin Recognizes Cruzipain at Its Last Folding Stages
To study the folding status of cruzipain recognized by calreticulin, cells were pulse–chase labeled as described above (absence of DNJ) and lysed in the presence of detergent and iodoacetamide, and immunoprecipitates obtained with anti-calreticulin serum were run on SDS-PAGE under reducing and nonreducing conditions. As depicted in Figure 10 (anti-calreticulin), in all samples calreticulin-bound cruzipain migrated under nonreducing conditions as the fully oxidized glycoprotein, differently from the completely reduced proteinase. Cruzipain species with intermediate migration corresponding to different oxidation stages (as mentioned above, cruzipain has six or seven disulfide bridges) were nevertheless present as they were immunoprecipitated with anti-cruzipain serum (Figure 10, anti-cruzipain). It was concluded that calreticulin and therefore also GT recognized cruzipain at its last folding stages.
Figure 10.
Calreticulin recognizes cruzipain at its last folding stages. T. cruzi cells were pulse–chase labeled as described in Figure 5 (absence of DNJ). Immunoprecipitates obtained with anti-cruzipain and anti-calreticulin sera were run under nonreducing (NR) conditions on 10% SDS-PAGE. Those obtained with the last antiserum were also run under reducing (R) conditions. In CP mature cruzipain was run under nonreducing (NR) and reducing (R) conditions. CPred, reduced cruzipain; CPox, oxidized cruzipain; CPInt, partially oxidized cruzipain; CRTred, reduced calreticulin; CRTox, oxidized calreticulin. Molecular masses (in kilodaltons) are indicated on the left.
DISCUSSION
As mentioned above, trypanosomatid protozoa are microorganisms belonging to an early branch in evolution. Trypanosomatids display certain distinctive features in the pathway of protein N-glycosylation and ER oligosaccharide processing when compared with that occurring in mammalian cells, such as a much shorter dolichol moiety, an inability to synthesize dolichol-P-Glc, the in vivo transfer of unglucosylated oligosaccharides to nascent polypeptide chains, the absence in certain species of the dolichol-P-Man–dependent mannosyltransferases responsible for the addition of the seventh, eighth, and ninth or the eighth and ninth mannosyl residues, and the presence of an oligosaccharyltransferase unable to discriminate between glucosylated and unglucosylated oligosaccharides (for review, see Parodi, 1993). Nevertheless, all 12 species tested so far were found to express an in vivo functional GT (Previato et al., 1986). Moreover, in two of them (T. cruzi and Crithidia fasciculata) the enzyme was detected in cell-free assays (Trombetta et al., 1989). In fact, it was precisely in T. cruzi, in which direct glucosylation of high-mannose-type protein-linked oligosaccharides was first described (Parodi and Cazzulo, 1982). Trypanosomatid protozoa also express a GII activity able to remove glucose units from monoglucosylated oligosaccharides created by GT (Bosch et al., 1988). Concentrations of inhibitors (DNJ and castanospermine) required for attaining 50% inhibition of trypanosomatid GII were similar (if not identical) to values reported for the mammalian enzyme, thus suggesting a high similarity between GII activities from both origins (Gañán et al., 1991; Gotz et al., 1991).
We demonstrate in this report that T. cruzi expresses a protein that is 40% identical and 64% similar to human calreticulin. The same as its mammalian counterpart, the protozoan protein has three Ca2+ binding motifs, and more important, it specifically binds monoglucosylated oligosaccharides. No gene coding for a calnexin homologue was detected in T. cruzi. Nevertheless, only completion of the currently World Health Organization–sponsored T. cruzi genome project would confirm the apparent absence of a calnexin-encoding gene in this parasite.
A single glycoprotein (cruzipain, a lysosomal proteinase) was found to interact with calreticulin in T. cruzi cells pulse–chased with [35S]Met plus [35S]Cys. Cruzipain is a major cellular protein (5–8% of total soluble protein) that has at least two N-linked high-mannose-type oligosaccharides and at least six disulfide bridges (Labriola et al., 1995; Metzner et al., 1996; Cazzulo et al., 1997). It cannot be discarded, however, that other minor glycoproteins might also interact with calreticulin. Nevertheless, the fact that only a single species was recognized by the lectin after a 2-min pulse, and that this recognition had a transient character as it disappeared upon chasing cells, shows that the cruzipain–calreticulin interaction was highly specific and that it followed the same time course already described for the interaction of calnexin and calreticulin with mammalian glycoproteins.
A crucial result that validates the mechanism proposed for the quality control of glycoprotein folding was the fact that, contrary to what has been described for mammalian cells, DNJ (a GII inhibitor) promoted, rather than inhibited, the interaction of calreticulin and cruzipain (Ou et al., 1993; Hammond et al., 1994; Kearse et al., 1994; Hebert et al., 1995; Nauseef et al., 1995; Peterson et al., 1995). This result is consistent with the known differences between mammalian and trypanosomatid ER oligosaccharide processing pathways (Figure 1). We have previously reported that addition of DNJ to T. cruzi cell cultures delayed arrival of cruzipain to lysosomes. Forced interaction of the proteinase with calreticulin was probably responsible for the observed delay (Labriola et al., 1995). Moreover, ∼60–65% of all N-linked oligosaccharides present in mature cruzipain isolated from lysosomes of cells grown in the presence of 6 mM DNJ were found to be glucosylated. The same proportion was found when the structure of a specific oligosaccharide (that closer to the C terminus of the glycoprotein) was studied (Labriola et al., 1995). This suggests that GT-mediated glucosylation is not a deterministic process but that it is restricted in vivo to glycoprotein molecules that for some reason present folding problems, probably because they have not been recognized by the proper chaperones at the proper time and at the proper place.
Interaction of cruzipain with calreticulin was solely dependent on the presence of glucosylated N-oligosaccharides in the former protein. Incubation with Endo H of the cruzipain–calreticulin complexes isolated from cells labeled in the presence or absence of DNJ completely abolished the interaction when treatment was performed before addition of the anti-calreticulin serum. Some residual cruzipain was found in the complexes when Endo H degradation was performed on the immunoprecipitates obtained from cells labeled in the presence of DNJ. However, this was not due to an interaction of calreticulin with the protein moiety of cruzipain but to a hindrance in the accessibility of Endo H to cruzipain in the immunocomplexes, because residual cruzipain had the same migration on gels as the fully glycosylated protein. In addition, it was demonstrated that the presence of monoglucosylated oligosaccharides was a necessary and sufficient condition for the interaction of mature cruzipain isolated from lysosomes with recombinant calreticulin.
A rather surprising result was the fact that only cruzipain migrating as the fully oxidized species under nonreducing conditions was recognized by calreticulin even after a 2-min pulse and 0-min chase. This result is at variance with that reported by Hebert et al. (1996). They showed that both calreticulin and calnexin recognized folding intermediates of influenza virus hemagglutinin differing in their oxidation status. Because glycoprotein recognition by both lectins only depends on the presence of glucosylated oligosaccharides and not on the folding status of the protein moieties (see below), it may be speculated that in the case described by Hebert et al. (1996), the monoglucosylated oligosaccharides recognized by the lectins in the folding intermediates were those created by deglucosylation of the transferred compound (Glc3Man9GlcNAc2). As shown in Figure 1B, those monoglucosylated derivatives are not created in T. cruzi. This indicates that GT glucosylated cruzipain at its last folding stages (i.e., when all or almost all disulfide bonds have been already formed). In fact, it has been reported that GT may quite efficiently glucosylate a glycoprotein enzyme having ∼25–35% of the enzymatic activity of the native enzyme, that is, a tertiary structure closely resembling that of the properly folded species (Sousa and Parodi, 1995).
As mentioned above, there is a controversy about whether calnexin and calreticulin behave as lectins that exclusively recognize the above-mentioned oligosaccharides or whether, alternatively, such recognition is the first and necessary step for an interaction between misfolded glycoprotein protein moieties and calnexin and calreticulin. Evidence for this last possibility was provided by experiments in which several transmembrane glycoproteins (murine major histocompatibility complex [MHC] class I heavy chain Kb and Db, human MHC class I human leukocyte antigen heavy chain, MHC class II DRα and DRβ and invariant chains, and T cell receptor α chain) were found to immunoprecipitate with anti-calnexin antibodies even after enzymatic removal of all oligosaccharide chains (Arunachalam and Cresswell, 1995; Ware et al., 1995; Zhang et al., 1995; Bennett et al., 1998). The possibility exists, however, that because both calnexin and the substrates are transmembrane species, they may remain trapped in the same detergent micelles after removal of oligosaccharides. On the other hand, a soluble glycoprotein such as α1-anti-trypsin was also found to be apparently immunoprecipitated by anti-calnexin serum after removal of oligosaccharides. In this case the above-mentioned possible colocalization of calnexin and the substrate glycoprotein in the same micelles may be ruled out (Ware et al., 1995). However, a poor solubility of the yet not properly folded α1-anti-trypsin (and also of the above-mentioned transmembrane deglycosylated glycoproteins) may explain its appearance in the immunoprecipitate.
Evidence for the role of calnexin and calreticulin as lectins that exclusively recognize monoglucosylated oligosaccharides comes from experiments in which the interaction of glycosylated bovine pancreas RNase with calnexin and calreticulin was studied either in a dog pancreas microsome–rabbit reticulocyte translation system or with isolated RNase and calnexin (Rodan et al., 1996; Zapun et al., 1997). It was concluded in both studies that removal of oligosaccharides with endoglycosidases or of the glucose units present in the oligosaccharides with GII completely abolished calnexin and calreticulin interaction with monoglucosylated RNase. The main drawback of the above-mentioned conclusion is that the model glycoprotein chosen (RNase) is practically devoid of hydrophobic domains, as revealed by a Kyte–Doolittle hydrophilicity plot. An extremely weak interaction of the protein moiety of RNase with calnexin and calreticulin would be expected if such interaction involved hydrophobic domains in the substrate glycoprotein, as has been described for folding proteins and classical chaperones.
Results presented in the present report obviate objections mentioned above for conclusions supporting either one of both models of the calnexin–calreticulin interaction. Both calreticulin and cruzipain are soluble proteins (i.e., they cannot be trapped in micelles), and the substrate glycoprotein has several hydrophobic domains interspersed along the entire molecule. Moreover, the conclusion that calreticulin behaves exclusively as a lectin that binds monoglucosylated oligosaccharides was derived from experiments performed in intact cells, using a naturally expressed glycoprotein. However, the occurrence of extremely weak protein–protein interactions between calreticulin and folding glycoproteins that might be disrupted on immunoprecipitation cannot be ruled out.
Glycoprotein folding facilitation mediated by the exclusive interaction of protein-linked monoglucosylated oligosaccharides and ER lectins is, therefore, a process that appeared early in evolution.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant GM44500 and by grants from the United Nations Development Program/World Bank/World Health Organization Special Program for Research and Training in Tropical Diseases, Scientific Research Cooperation Department of the Swedish International Development Agency, the University of Buenos Aires, and the Argentine Federal Government (Consejo Nacional de Investigaciones Científicas y Técnicas and Agencia Nacional de Promoción Científica y Tecnológica). C.L. is a doctoral fellow and J.J.C. and A.J.P. are career investigators of the National Research Council (Argentina). A.J.P. is a Howard Hughes Medical Institute international research scholar.
REFERENCES
- Arunachalam B, Cresswell P. Molecular requirements for the interaction of class II major histocompatibility complex and invariant chain with calnexin. J Biol Chem. 1995;270:2784–2790. doi: 10.1074/jbc.270.6.2784. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. K. Janson K, New York: John Wiley and Sons; 1994. Preparation and analysis of RNA; pp. 4.1.1–4.1.6. 4.9.1–4.9.6. [Google Scholar]
- Baldauf SL, Palmer JD. Animals and fungi are each other’s closest relatives: congruent evidence from multiple proteins. Proc Natl Acad Sci USA. 1993;90:11558–11562. doi: 10.1073/pnas.90.24.11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MJ, Van Leeuwen JEM, Kearse KP. Calnexin association is not sufficient to protect TCRα proteins from rapid degradation in CD4+CD8+ thymocytes. J Biol Chem. 1998;273:23674–23680. doi: 10.1074/jbc.273.37.23674. [DOI] [PubMed] [Google Scholar]
- Bontempi E, Martínez J, Cazzulo JJ. Subcellular localization of a cysteine proteinase from Trypanosoma cruzi. Mol Biochem Parasitol. 1989;33:43–48. doi: 10.1016/0166-6851(89)90040-6. [DOI] [PubMed] [Google Scholar]
- Borst P, Fase-Fowler F, Frasch ACC, Hoeijmakers JHJ, Weijers PJ. Characterization of DNA from Trypanosoma brucei and related trypanosomes by restriction endonuclease digestion. Mol Biochem Parasitol. 1980;1:221–246. [Google Scholar]
- Bosch M, Trombetta S, Engstrom U, Parodi AJ. Characterization of dolichol diphosphate oligosaccharide:protein oligosaccharyltransferase and of glycoprotein processing glucosidases occurring in trypanosomatids. J Biol Chem, 1988;263:17360–17365. [PubMed] [Google Scholar]
- Campetella O, Martínez J, Cazzulo JJ. A major cysteine proteinase is developmentally regulated in Trypanosoma cruzi. FEMS Microbiol Lett. 1990;67:145–150. doi: 10.1016/0378-1097(90)90184-r. [DOI] [PubMed] [Google Scholar]
- Cazzulo JJ, Couso R, Raimondi A, Wernstedt C, Hellman U. Further characterization and partial amino acid sequence of a cysteine proteinase from Trypanosoma cruzi. Mol Biochem Parasitol. 1989;33:33–42. doi: 10.1016/0166-6851(89)90039-x. [DOI] [PubMed] [Google Scholar]
- Cazzulo JJ, Franke de Cazzulo BM, Engel JC, Cannata JJ. End products and enzyme levels of aerobic fermentation in trypanosomatids. Mol Biochem Parasitol. 1985;16:329–343. doi: 10.1016/0166-6851(85)90074-x. [DOI] [PubMed] [Google Scholar]
- Cazzulo JJ, Stoka V, Turk V. Cruzipain, the major cysteine proteinase from the protozoan parasite Trypanosoma cruzi. Biol Chem. 1997;378:1–10. doi: 10.1515/bchm.1997.378.1.1. [DOI] [PubMed] [Google Scholar]
- de la Canal L, Parodi AJ. Synthesis of dolichol derivatives in trypanosomatids. Characterization of enzymatic patterns. J Biol Chem. 1987;262:11128–11133. [PubMed] [Google Scholar]
- Fernández F, Jannatipour M, Hellman U, Rokeach L, Parodi AJ. A new stress protein: synthesis of Schizosaccharomyces pombe UDP-Glc:glycoprotein glucosyltransferase mRNA is induced under stress conditions but the enzyme is not essential for cell viability. EMBO J. 1996;15:705–713. [PMC free article] [PubMed] [Google Scholar]
- Fernández F, Trombetta SE, Hellman U, Parodi AJ. Purification to homogeneity of UDP-Glc:glycoprotein glucosyltransferase from Schizosaccharomyces pombe and apparent absence of the enzyme from Saccharomyces cerevisiae. J Biol Chem. 1994;269:30701–30706. [PubMed] [Google Scholar]
- Gañán S, Cazzulo JJ, Parodi AJ. A major proportion of N-glycoproteins is transiently glucosylated in the endoplasmic reticulum. Biochemistry. 1991;30:3098–3104. doi: 10.1021/bi00226a017. [DOI] [PubMed] [Google Scholar]
- Gotz G, Gañán S, Parodi AJ. Glucosylation of glycoproteins in Crithidia fasciculata. Mol Biochem Parasitol. 1991;45:265–274. doi: 10.1016/0166-6851(91)90094-m. [DOI] [PubMed] [Google Scholar]
- Hammond C, Braakman I, Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci USA. 1994;91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert DN, Foellmer B, Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995;81:425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- Hebert DN, Foellmer B, Helenius A. Calnexin and calreticulin promote folding, delay oligomerization and suppress degradation of influenza hemagglutinin in microsomes. EMBO J. 1996;15:2961–2968. [PMC free article] [PubMed] [Google Scholar]
- Helenius A, Trombetta ES, Hebert DN, Simons JF. Calnexin, calreticulin and the folding of glycoproteins. Trends Cell Biol. 1997;7:193–200. doi: 10.1016/S0962-8924(97)01032-5. [DOI] [PubMed] [Google Scholar]
- Ibáñez CF, Affranchino JL, Frasch ACC. Antigenic determinants of Trypanosoma cruzi defined by cloning of parasite DNA. Mol Biochem Parasitol. 1987;25:175–184. doi: 10.1016/0166-6851(87)90006-5. [DOI] [PubMed] [Google Scholar]
- Jannatipour M, Rokeach LA. The Schizosaccharomyces pombe homologue of the chaperone calnexin is essential for viability. J Biol Chem. 1995;270:4845–4853. doi: 10.1074/jbc.270.9.4845. [DOI] [PubMed] [Google Scholar]
- Josi M, Pogue GP, Duncan RC, Lee NS, Singh NK, Atreya CD, Dwyer DM, Nakhasi HL. Isolation and characterization of Leishmania donovani calreticulin gene and its conservation of the RNA binding activity. Mol Biochem Parasitol. 1996;81:53–64. doi: 10.1016/0166-6851(96)02676-x. [DOI] [PubMed] [Google Scholar]
- Kearse KP, Williams DB, Singer A. Persistence of glucose residues on core oligosaccharides prevents association of TCRα and TCRβ proteins with calnexin and results specifically in accelerated degradation of nascent TCRα proteins within the endoplasmic reticulum. EMBO J. 1994;13:3678–3686. doi: 10.1002/j.1460-2075.1994.tb06677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. Assemby of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Labriola C, Cazzulo JJ, Parodi AJ. Retention of glucose residues added by the UDP-Glc:glycoprotein glucosyltransferase delays exit of glycoproteins from the endoplasmic reticulum. J Cell Biol. 1995;130:771–779. doi: 10.1083/jcb.130.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low P, Dallner G, Mayor S, Cohen S, Chait BT, Menon AK. The mevalonate pathway in the blood-stream form of Trypanosoma brucei. Identification of dolichols containing 11 and 12 isoprene residues. J Biol Chem. 1991;266:19250–19257. [PubMed] [Google Scholar]
- Lupattelli F, Pedrazzini E, Bollini R, Vitale A, Ceriotti A. The rate of phaseolin assembly is controlled by the glucosylation state of its N-linked oligosaccharide chains. Plant Cell. 1997;9:597–609. doi: 10.1105/tpc.9.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelzon DH, Previato JO, Parodi AJ. Characterization of protein-linked oligosaccharides in trypanosomatid flagellates. Mol Biochem Parasitol. 1986;18:355–367. doi: 10.1016/0166-6851(86)90092-7. [DOI] [PubMed] [Google Scholar]
- Metzner SI, Sousa MC, Hellman U, Cazzulo JJ, Parodi AJ. The use of UDP-Glc:glycoprotein glucosyltransferase for radiolabeling protein-linked high mannose-type oligosaccharides. Cell Mol Biol. 1996;42:631–635. [PubMed] [Google Scholar]
- Nauseef WM, McCormick SJ, Clark RA. Calreticulin functions as a molecular chaperone in the biosynthesis of myeloperoxidase. J Biol Chem. 1995;270:4741–4747. doi: 10.1074/jbc.270.9.4741. [DOI] [PubMed] [Google Scholar]
- Ou W-J, Cameron PH, Thomas DY, Bergeron JJM. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993;364:771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- Parlati F, Dignard D, Bergeron JJ, Thomas DY. The calnexin homologue cnx1+ in Schizosaccharomyces pombe is an essential gene which can be complemented by its soluble ER domain. EMBO J. 1995;14:3064–3072. doi: 10.1002/j.1460-2075.1995.tb07309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi AJ. N-Glycosylation in trypanosomatid protozoa. Glycobiology. 1993;3:193–199. doi: 10.1093/glycob/3.3.193. [DOI] [PubMed] [Google Scholar]
- Parodi AJ, Cazzulo JJ. Protein glycosylation in Trypanosoma cruzi. II. Partial characterization of protein-bound oligosaccharides labeled “in vivo.”. J Biol Chem. 1982;257:7641–7645. [PubMed] [Google Scholar]
- Parodi AJ, Lederkremer GZ, Mendelzon DH. Protein glycosylation in Trypanosoma cruzi. The mechanism of glycosylation and structure of protein-bound oligosaccharides. J Biol Chem. 1983a;258:5589–5595. [PubMed] [Google Scholar]
- Parodi AJ, Mendelzon DH, Lederkremer GZ. Transient glucosylation of protein-bound Man9GlcNAc2, Man8GlcNAc2 and Man7GlcNAc2 in calf thyroid slices: a possible recognition signal in the processing of glycoproteins. J Biol Chem. 1983b;258:8260–8265. [PubMed] [Google Scholar]
- Parodi AJ, Mendelzon DH, Lederkremer GZ, MartínBarrientos J. Evidence that transient glucosylation of protein-linked Man9GlcNAc2, Man8GlcNAc2 and Man7GlcNAc2 occurs in rat liver and Phaseolus vulgaris cells. J Biol Chem. 1984;259:6351–6357. [PubMed] [Google Scholar]
- Parodi AJ, Quesada-Allué LA. Protein glycosylation in Trypanosoma cruzi. I. Characterization of dolichol-bound monosaccharides and oligosaccharides synthesized in vivo. J Biol Chem. 1982;257:7641–7645. [PubMed] [Google Scholar]
- Parodi AJ, Quesada-Allué LA, Cazzulo JJ. Pathway of protein glycosylation in the trypanosomatid Crithidia fasciculata. Proc Natl Acad Sci USA. 1981;78:6201–6205. doi: 10.1073/pnas.78.10.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JR, Ora A, Nguyen Van P, Helenius A. Calreticulin is a lectin-like molecular chaperone for glycoproteins of the endoplasmic reticulum. Mol Biol Cell. 1995;6:1173–1184. doi: 10.1091/mbc.6.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previato JO, Mendelzon DH, Parodi AJ. Characterization of dolichol monophosphate- and dolichol diphosphate-linked saccharides in trypanosomatid flagellates. Mol Biochem Parasitol. 1986;18:343–357. doi: 10.1016/0166-6851(86)90091-5. [DOI] [PubMed] [Google Scholar]
- Quesada-Allué LA, Parodi AJ. Novel mannose carrier in the trypanosomatid Crithidia fasciculata behaving as a short α-saturated polyprenyl phosphate. Biochem J. 1983;212:123–128. doi: 10.1042/bj2120123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan AR, Simons JF, Trombetta ES, Helenius A. N-Linked oligosaccharides are necessary and sufficient for association of glycosylated forms of bovine RNase with calnexin and calreticulin. EMBO J. 1996;15:6921–6930. [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Solgin ML. History assignment: when was the mitochondrion founded? Curr Opin Genet Dev. 1997;7:792–799. doi: 10.1016/s0959-437x(97)80042-1. [DOI] [PubMed] [Google Scholar]
- Sousa M, Ferrero-García M, Parodi AJ. Recognition of the oligosaccharide and protein moieties of glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. Biochemistry. 1992;31:97–105. doi: 10.1021/bi00116a015. [DOI] [PubMed] [Google Scholar]
- Sousa M, Parodi AJ. The molecular basis for the recognition of misfolded glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. EMBO J. 1995;15:4196–4203. doi: 10.1002/j.1460-2075.1995.tb00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro RG, Zhu Q, Bhoyroo V, Söling H-D. Definition of the lectin-like properties of the molecular chaperone, calreticulin, and demonstration of its copurification with endomannosidase from rat liver Golgi. J Biol Chem. 1996;271:11588–11594. doi: 10.1074/jbc.271.19.11588. [DOI] [PubMed] [Google Scholar]
- Tibbetts RS, Kim IY, Olson CL, Barthel LM, Sullivan MA, Winquist AG, Miller SD, Engman DM. Molecular cloning and characterization of the 78-kilodalton glucose-regulated protein of Trypanosoma cruzi. Infect Immun. 1994;62:2499–2507. doi: 10.1128/iai.62.6.2499-2507.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta S, Bosch M, Parodi AJ. Glucosylation of glycoproteins by mammalian, plant, fungal and trypanosomatid protozoa microsomal proteins. Biochemistry. 1989;28:8108–8116. doi: 10.1021/bi00446a022. [DOI] [PubMed] [Google Scholar]
- Ware FE, Vassilakos A, Peterson PA, Jackson MR, Lehrman MA, Williams DB. The molecular chaperone calnexin binds Glc1Man9GlcNAc2 oligosaccharide as an initial step in recognizing unfolded glycoproteins. J Biol Chem. 1995;270:4697–4704. doi: 10.1074/jbc.270.9.4697. [DOI] [PubMed] [Google Scholar]
- Zapun A, Petrescu S, Rudd PM, Dwek RA, Thomas DY, Bergeron JJ. Conformation independent binding of monoglucosylated ribonuclease B to calnexin. Cell. 1997;88:29–38. doi: 10.1016/s0092-8674(00)81855-3. [DOI] [PubMed] [Google Scholar]
- Zhang J-X, Braakman I, Matlack KES, Helenius A. Quality control in the secretory pathway: the role of calreticulin, calnexin and BiP in the retention of glycoproteins with C-terminal truncations. Mol Biol Cell. 1997;8:1943–1954. doi: 10.1091/mbc.8.10.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Tector M, Salter RD. Calnexin recognizes carbohydrate and protein determinants of class I major histocompatibility complex molecules. J Biol Chem. 1995;270:3944–3948. doi: 10.1074/jbc.270.8.3944. [DOI] [PubMed] [Google Scholar]