Abstract
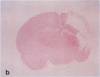
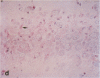
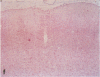
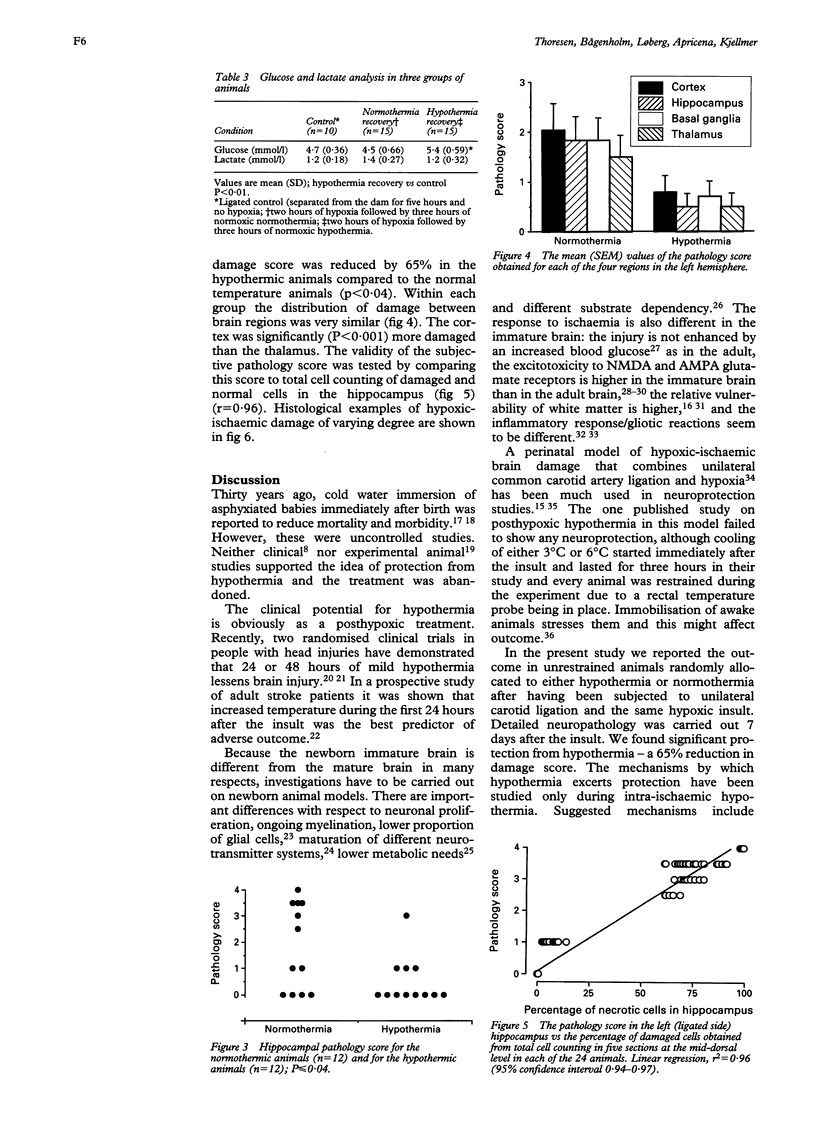
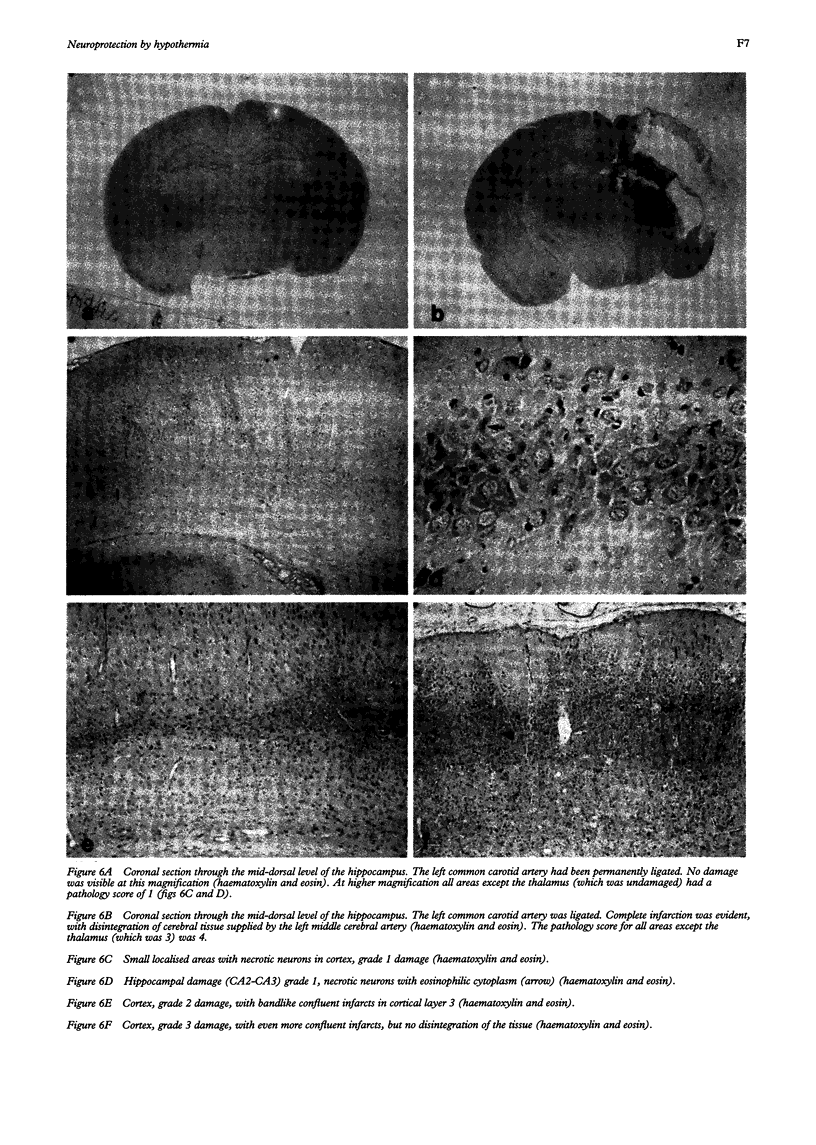
AIM: To determine whether moderate hypothermia, applied after a hypoxic-ischaemic insult in neonatal rats, reduces cerebral damage. METHOD: Unilateral hypoxic-ischaemic brain damage was induced in 7 day old rats by left carotid ligation, followed by 120 minutes of normothermic exposure to 8% O2, followed by random selection to three hours of hypothermia (rectal temperature, mean (SD), 32.5 (0.4) degrees C) or normothermia (38.3 (0.4) degrees C). One hundred and one animals were used for brain temperature or blood chemistry studies and 24 for survival studies (7 days) with neuropathology, including cell counting as outcome measures. RESULTS: Thirty sections from each brain were histologically examined with respect to distribution and pattern of damage and given a score from 0 to 4. Animals treated with hypothermia had significantly less damage than normothermic animals (score 0.5 (0.3) vs 1.8 (0.5)). CONCLUSIONS: Posthypoxic hypothermia reduces brain damage in awake, unrestrained 7 day old rats.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams R., Caton D., Clapp J., Barron D. H. Thermal and metabolic features of life in utero. Clin Obstet Gynecol. 1970 Sep;13(3):549–564. doi: 10.1097/00003081-197009000-00005. [DOI] [PubMed] [Google Scholar]
- Andiné P., Lehmann A., Ellrén K., Wennberg E., Kjellmer I., Nielsen T., Hagberg H. The excitatory amino acid antagonist kynurenic acid administered after hypoxic-ischemia in neonatal rats offers neuroprotection. Neurosci Lett. 1988 Jul 19;90(1-2):208–212. doi: 10.1016/0304-3940(88)90813-0. [DOI] [PubMed] [Google Scholar]
- Andiné P., Thordstein M., Kjellmer I., Nordborg C., Thiringer K., Wennberg E., Hagberg H. Evaluation of brain damage in a rat model of neonatal hypoxic-ischemia. J Neurosci Methods. 1990 Dec;35(3):253–260. doi: 10.1016/0165-0270(90)90131-x. [DOI] [PubMed] [Google Scholar]
- Bergstedt K., Hu B. R., Wieloch T. Postischaemic changes in protein synthesis in the rat brain: effects of hypothermia. Exp Brain Res. 1993;95(1):91–99. doi: 10.1007/BF00229658. [DOI] [PubMed] [Google Scholar]
- Busto R., Dietrich W. D., Globus M. Y., Ginsberg M. D. Postischemic moderate hypothermia inhibits CA1 hippocampal ischemic neuronal injury. Neurosci Lett. 1989 Jul 3;101(3):299–304. doi: 10.1016/0304-3940(89)90549-1. [DOI] [PubMed] [Google Scholar]
- Busto R., Dietrich W. D., Globus M. Y., Valdés I., Scheinberg P., Ginsberg M. D. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987 Dec;7(6):729–738. doi: 10.1038/jcbfm.1987.127. [DOI] [PubMed] [Google Scholar]
- Busto R., Globus M. Y., Dietrich W. D., Martinez E., Valdés I., Ginsberg M. D. Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke. 1989 Jul;20(7):904–910. doi: 10.1161/01.str.20.7.904. [DOI] [PubMed] [Google Scholar]
- CORDEY R. HYPOTHERMIA IN RESUSCITATING NEWBORNS IN WHITE ASPHYXIA; A REPORT OF 14 CASES. Obstet Gynecol. 1964 Nov;24:760–767. [PubMed] [Google Scholar]
- Carroll M., Beek O. Protection against hippocampal CA1 cell loss by post-ischemic hypothermia is dependent on delay of initiation and duration. Metab Brain Dis. 1992 Mar;7(1):45–50. doi: 10.1007/BF01000440. [DOI] [PubMed] [Google Scholar]
- Clifton G. L., Allen S., Barrodale P., Plenger P., Berry J., Koch S., Fletcher J., Hayes R. L., Choi S. C. A phase II study of moderate hypothermia in severe brain injury. J Neurotrauma. 1993 Fall;10(3):263–273. doi: 10.1089/neu.1993.10.263. [DOI] [PubMed] [Google Scholar]
- Conradi N. G., Müntzing K., Sourander P., Hamberger A. Effect of ambient temperature on rectal temperature in normal and malnourished rats during early postnatal development. Acta Physiol Scand. 1984 Jun;121(2):147–153. doi: 10.1111/j.1748-1716.1984.tb07441.x. [DOI] [PubMed] [Google Scholar]
- Dietrich W. D., Busto R., Alonso O., Globus M. Y., Ginsberg M. D. Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1993 Jul;13(4):541–549. doi: 10.1038/jcbfm.1993.71. [DOI] [PubMed] [Google Scholar]
- Duffy T. E., Nelson S. R., Lowry O. H. Cerebral carbohydrate metabolism during acute hypoxia and recovery. J Neurochem. 1972 Apr;19(4):959–977. doi: 10.1111/j.1471-4159.1972.tb01417.x. [DOI] [PubMed] [Google Scholar]
- Ginsberg M. D., Sternau L. L., Globus M. Y., Dietrich W. D., Busto R. Therapeutic modulation of brain temperature: relevance to ischemic brain injury. Cerebrovasc Brain Metab Rev. 1992 Fall;4(3):189–225. [PubMed] [Google Scholar]
- Kiyota Y., Pahlmark K., Memezawa H., Smith M. L., Siesjö B. K. Free radicals and brain damage due to transient middle cerebral artery occlusion: the effect of dimethylthiourea. Exp Brain Res. 1993;95(3):388–396. doi: 10.1007/BF00227131. [DOI] [PubMed] [Google Scholar]
- Levene M. I., Gibson N. A., Fenton A. C., Papathoma E., Barnett D. The use of a calcium-channel blocker, nicardipine, for severely asphyxiated newborn infants. Dev Med Child Neurol. 1990 Jul;32(7):567–574. doi: 10.1111/j.1469-8749.1990.tb08540.x. [DOI] [PubMed] [Google Scholar]
- Lo E. H., Steinberg G. K., Panahian N., Maidment N. T., Newcomb R. Profiles of extracellular amino acid changes in focal cerebral ischaemia: effects of mild hypothermia. Neurol Res. 1993 Aug;15(4):281–287. doi: 10.1080/01616412.1993.11740149. [DOI] [PubMed] [Google Scholar]
- Lorek A., Takei Y., Cady E. B., Wyatt J. S., Penrice J., Edwards A. D., Peebles D., Wylezinska M., Owen-Reece H., Kirkbride V. Delayed ("secondary") cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1994 Dec;36(6):699–706. doi: 10.1203/00006450-199412000-00003. [DOI] [PubMed] [Google Scholar]
- Marion D. W., Obrist W. D., Carlier P. M., Penrod L. E., Darby J. M. The use of moderate therapeutic hypothermia for patients with severe head injuries: a preliminary report. J Neurosurg. 1993 Sep;79(3):354–362. doi: 10.3171/jns.1993.79.3.0354. [DOI] [PubMed] [Google Scholar]
- McDonald J. W., Johnston M. V. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Brain Res Rev. 1990 Jan-Apr;15(1):41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- McDonald J. W., Johnston M. V. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Brain Res Rev. 1990 Jan-Apr;15(1):41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- McDonald J. W., Trescher W. H., Johnston M. V. Susceptibility of brain to AMPA induced excitotoxicity transiently peaks during early postnatal development. Brain Res. 1992 Jun 26;583(1-2):54–70. doi: 10.1016/s0006-8993(10)80009-5. [DOI] [PubMed] [Google Scholar]
- McRae A., Gilland E., Bona E., Hagberg H. Microglia activation after neonatal hypoxic-ischemia. Brain Res Dev Brain Res. 1995 Feb 16;84(2):245–252. doi: 10.1016/0165-3806(94)00177-2. [DOI] [PubMed] [Google Scholar]
- Morioka T., Streit W. J. Expression of immunomolecules on microglial cells following neonatal sciatic nerve axotomy. J Neuroimmunol. 1991 Dec;35(1-3):21–30. doi: 10.1016/0165-5728(91)90158-4. [DOI] [PubMed] [Google Scholar]
- Nehlig A., Pereira de Vasconcelos A. Glucose and ketone body utilization by the brain of neonatal rats. Prog Neurobiol. 1993 Feb;40(2):163–221. doi: 10.1016/0301-0082(93)90022-k. [DOI] [PubMed] [Google Scholar]
- Oates R. K., Harvey D. Failure of hypothermia as treatment for asphyxiated newborn rabbits. Arch Dis Child. 1976 Jul;51(7):512–516. doi: 10.1136/adc.51.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice J. E., 3rd, Vannucci R. C., Brierley J. B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981 Feb;9(2):131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- SILVERMAN W. A., FERTIG J. W., BERGER A. P. The influence of the thermal environment upon the survival of newly born premature infants. Pediatrics. 1958 Nov;22(5):876–886. [PubMed] [Google Scholar]
- Silverstein F., Buchanan K., Johnston M. V. Pathogenesis of hypoxic-ischemic brain injury in a perinatal rodent model. Neurosci Lett. 1984 Aug 31;49(3):271–277. doi: 10.1016/0304-3940(84)90301-x. [DOI] [PubMed] [Google Scholar]
- Swann J. W., Brady R. J., Martin D. L. Postnatal development of GABA-mediated synaptic inhibition in rat hippocampus. Neuroscience. 1989;28(3):551–561. doi: 10.1016/0306-4522(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Thordstein M., Bågenholm R., Thiringer K., Kjellmer I. Scavengers of free oxygen radicals in combination with magnesium ameliorate perinatal hypoxic-ischemic brain damage in the rat. Pediatr Res. 1993 Jul;34(1):23–26. doi: 10.1203/00006450-199307000-00006. [DOI] [PubMed] [Google Scholar]
- Thoresen M., Penrice J., Lorek A., Cady E. B., Wylezinska M., Kirkbride V., Cooper C. E., Brown G. C., Edwards A. D., Wyatt J. S. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res. 1995 May;37(5):667–670. doi: 10.1203/00006450-199505000-00019. [DOI] [PubMed] [Google Scholar]
- Thoresen M., Penrice J., Lorek A., Cady E. B., Wylezinska M., Kirkbride V., Cooper C. E., Brown G. C., Edwards A. D., Wyatt J. S. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res. 1995 May;37(5):667–670. doi: 10.1203/00006450-199505000-00019. [DOI] [PubMed] [Google Scholar]
- Towfighi J., Yager J. Y., Housman C., Vannucci R. C. Neuropathology of remote hypoxic-ischemic damage in the immature rat. Acta Neuropathol. 1991;81(5):578–587. doi: 10.1007/BF00310141. [DOI] [PubMed] [Google Scholar]
- Vannucci R. C., Yager J. Y. Glucose, lactic acid, and perinatal hypoxic-ischemic brain damage. Pediatr Neurol. 1992 Jan-Feb;8(1):3–12. doi: 10.1016/0887-8994(92)90045-z. [DOI] [PubMed] [Google Scholar]
- WESTIN B., MILLER J. A., Jr, NYBERG R., WEDENBERG E. Neonatal asphyxia pallida treated with hypothermia alone or with hypothermia and transfusion of oxygenated blood. Surgery. 1959 May;45(5):868–879. [PubMed] [Google Scholar]
- WESTIN B., NYBERG R., MILLER J. A., Jr, WEDENBERG E. Hypothermia and transfusion with oxygenated blood in the treatment of asphyxia neonatorum. Acta Paediatr Suppl. 1962;139:1–80. [PubMed] [Google Scholar]
- Wasterlain C. G., Adams L. M., Schwartz P. H., Hattori H., Sofia R. D., Wichmann J. K. Posthypoxic treatment with felbamate is neuroprotective in a rat model of hypoxia-ischemia. Neurology. 1993 Nov;43(11):2303–2310. doi: 10.1212/wnl.43.11.2303. [DOI] [PubMed] [Google Scholar]
- Welsh F. A., Harris V. A. Postischemic hypothermia fails to reduce ischemic injury in gerbil hippocampus. J Cereb Blood Flow Metab. 1991 Jul;11(4):617–620. doi: 10.1038/jcbfm.1991.112. [DOI] [PubMed] [Google Scholar]
- Yager J. Y., Heitjan D. F., Towfighi J., Vannucci R. C. Effect of insulin-induced and fasting hypoglycemia on perinatal hypoxic-ischemic brain damage. Pediatr Res. 1992 Feb;31(2):138–142. doi: 10.1203/00006450-199202000-00009. [DOI] [PubMed] [Google Scholar]
- Yager J., Towfighi J., Vannucci R. C. Influence of mild hypothermia on hypoxic-ischemic brain damage in the immature rat. Pediatr Res. 1993 Oct;34(4):525–529. doi: 10.1203/00006450-199310000-00029. [DOI] [PubMed] [Google Scholar]
- de Vries L. S., Eken P., Groenendaal F., van Haastert I. C., Meiners L. C. Correlation between the degree of periventricular leukomalacia diagnosed using cranial ultrasound and MRI later in infancy in children with cerebral palsy. Neuropediatrics. 1993 Oct;24(5):263–268. doi: 10.1055/s-2008-1071554. [DOI] [PubMed] [Google Scholar]