Abstract
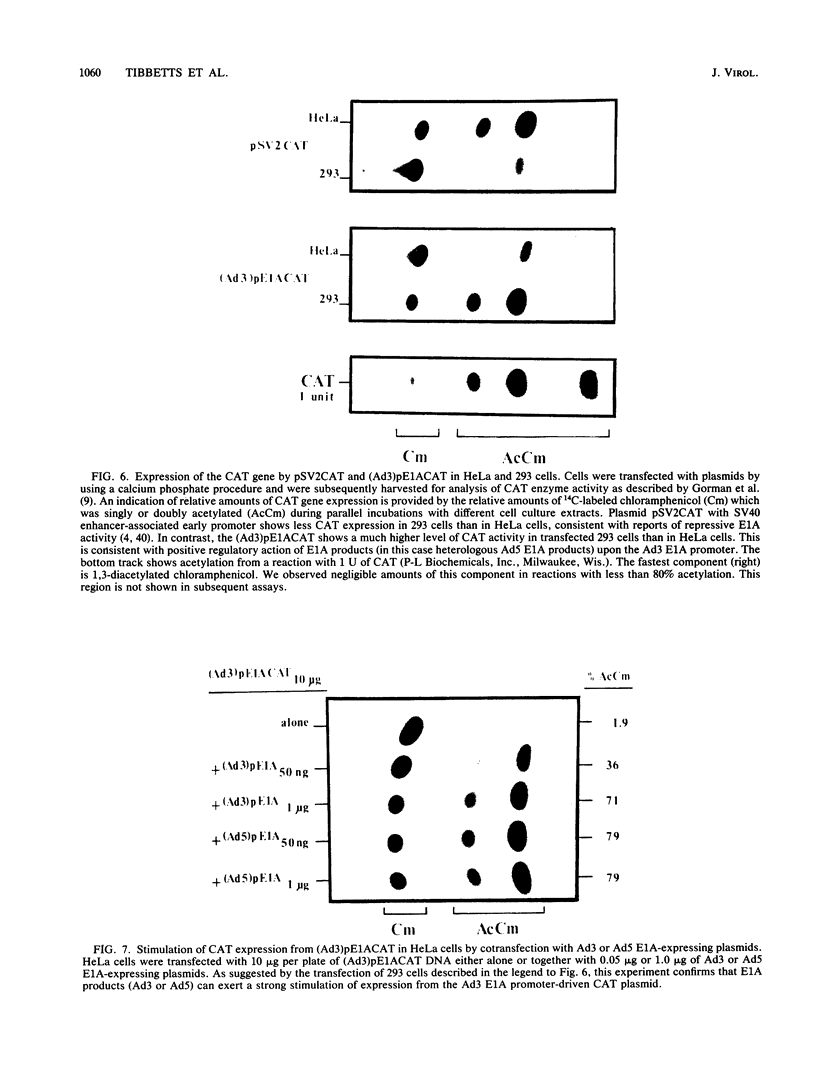
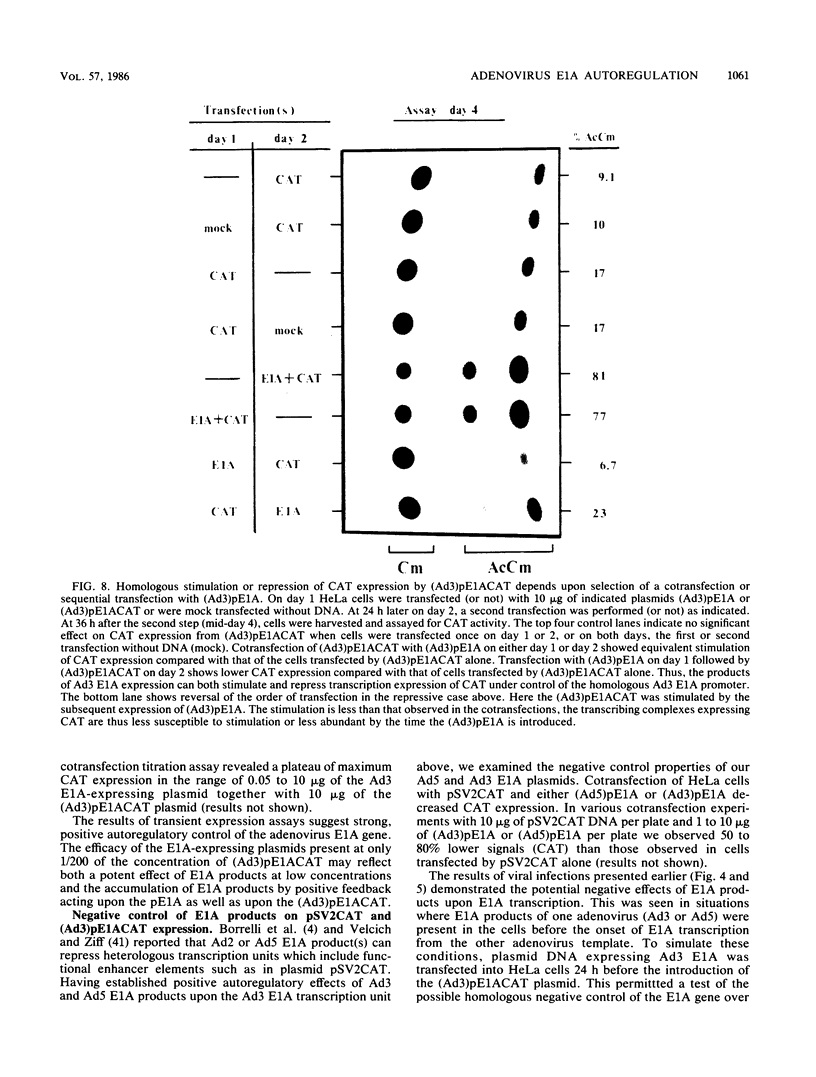
We examined E1A gene expression by two evolutionarily divergent human adenoviruses, type 5 (subgroup C) and type 3 (subgroup B). Adenovirus type 3 (Ad3)-infected A549 cells contained much larger amounts of E1A-specific RNA than adenovirus type 5 (Ad5)-infected cells, from very early (3 h) through the late stages (20 h) after infection. The appearance of such abundant Ad3 E1A transcripts was delayed after infection of Ad5 E1A-expressing 293 cells, suggesting a down regulation of the Ad3 E1A gene by Ad5 E1A gene products. In a reciprocal manner, coinfection of A549 cells led to typically early and intense Ad3 E1A transcription and strongly inhibited transcription of the Ad5 E1A gene. Transient expression assays were developed so that the autoregulation of the E1A gene could be studied apart from the more complex background of infected cells. The DNA sequence surrounding the transcription start site of the Ad3 E1A gene was placed 5' to the sequence which encodes the bacterial chloramphenicol acetyltransferase gene. Cotransfection of HeLa cells with Ad3 or Ad5 E1A-expression plasmids increased the expression of the Ad3 E1A promoter-driven chloramphenicol acetyltransferase gene. Taken together, these results suggest dual autoregulatory features of adenovirus E1A gene expression. The positive and negative effects appear to be temporally distinguished under different conditions, both in viral infection and in transient assays with plasmid-cloned genes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiello L., Guilfoyle R., Huebner K., Weinmann R. Adenovirus 5 DNA sequences present and RNA sequences transcribed in transformed human embryo kidney cells (HEK-Ad-5 or 293). Virology. 1979 Apr 30;94(2):460–469. doi: 10.1016/0042-6822(79)90476-8. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Davison B. L., Egly J. M., Mulvihill E. R., Chambon P. Formation of stable preinitiation complexes between eukaryotic class B transcription factors and promoter sequences. Nature. 1983 Feb 24;301(5902):680–686. doi: 10.1038/301680a0. [DOI] [PubMed] [Google Scholar]
- Gaynor R. B., Berk A. J. Cis-acting induction of adenovirus transcription. Cell. 1983 Jul;33(3):683–693. doi: 10.1016/0092-8674(83)90011-9. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Green M. R., Treisman R., Maniatis T. Transcriptional activation of cloned human beta-globin genes by viral immediate-early gene products. Cell. 1983 Nov;35(1):137–148. doi: 10.1016/0092-8674(83)90216-7. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R. C. Biochemical studies on adenovirus multiplication. XII. Plaquing efficiencies of purified human adenoviruses. Virology. 1967 Mar;31(3):562–565. doi: 10.1016/0042-6822(67)90241-3. [DOI] [PubMed] [Google Scholar]
- Guilfoyle R. A., Osheroff W. P., Rossini M. Two functions encoded by adenovirus early region 1A are responsible for the activation and repression of the DNA-binding protein gene. EMBO J. 1985 Mar;4(3):707–713. doi: 10.1002/j.1460-2075.1985.tb03687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., Roeder R. G. Separation and partial characterization of three functional steps in transcription initiation by human RNA polymerase II. J Biol Chem. 1985 Jul 5;260(13):8163–8172. [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983 Jul;33(3):695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Sassone-Corsi P., Chambon P. An enhancer element is located 340 base pairs upstream from the adenovirus-2 E1A capsite. Nucleic Acids Res. 1983 Dec 20;11(24):8747–8760. doi: 10.1093/nar/11.24.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperiale M. J., Feldman L. T., Nevins J. R. Activation of gene expression by adenovirus and herpesvirus regulatory genes acting in trans and by a cis-acting adenovirus enhancer element. Cell. 1983 Nov;35(1):127–136. doi: 10.1016/0092-8674(83)90215-5. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Hart R. P., Nevins J. R. An enhancer-like element in the adenovirus E2 promoter contains sequences essential for uninduced and E1A-induced transcription. Proc Natl Acad Sci U S A. 1985 Jan;82(2):381–385. doi: 10.1073/pnas.82.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston R. E., Kaufman R. J., Sharp P. A. Regulation of transcription of the adenovirus EII promoter by EIa gene products: absence of sequence specificity. Mol Cell Biol. 1984 Oct;4(10):1970–1977. doi: 10.1128/mcb.4.10.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosturko L. D., Sharnick S. V., Tibbetts C. Polar encapsidation of adenovirus DNA: cloning and DNA sequence of the left end of adenovirus type 3. J Virol. 1982 Sep;43(3):1132–1137. doi: 10.1128/jvi.43.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. L., Tibbetts C. Spontaneous reiterations of DNA sequences near the ends of adenovirus type 3 genomes. Virology. 1985 Nov;147(1):187–200. doi: 10.1016/0042-6822(85)90238-7. [DOI] [PubMed] [Google Scholar]
- Leff T., Corden J., Elkaim R., Sassone-Corsi P. Transcriptional analysis of the adenovirus-5 EIII promoter: absence of sequence specificity for stimulation by EIa gene products. Nucleic Acids Res. 1985 Feb 25;13(4):1209–1221. doi: 10.1093/nar/13.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. B., Mathews M. B. Control of adenovirus early gene expression: a class of immediate early products. Cell. 1980 Aug;21(1):303–313. doi: 10.1016/0092-8674(80)90138-5. [DOI] [PubMed] [Google Scholar]
- Montell C., Courtois G., Eng C., Berk A. Complete transformation by adenovirus 2 requires both E1A proteins. Cell. 1984 Apr;36(4):951–961. doi: 10.1016/0092-8674(84)90045-x. [DOI] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Resolving the functions of overlapping viral genes by site-specific mutagenesis at a mRNA splice site. Nature. 1982 Feb 4;295(5848):380–384. doi: 10.1038/295380a0. [DOI] [PubMed] [Google Scholar]
- Osborne T. F., Arvidson D. N., Tyau E. S., Dunsworth-Browne M., Berk A. J. Transcription control region within the protein-coding portion of adenovirus E1A genes. Mol Cell Biol. 1984 Jul;4(7):1293–1305. doi: 10.1128/mcb.4.7.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Virtanen A., Perricaudet M., Akusjärvi G. The messenger RNAs from the transforming region of human adenoviruses. Curr Top Microbiol Immunol. 1984;109:107–123. doi: 10.1007/978-3-642-69460-8_5. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robinson C. C., Tibbetts C. Polar encapsidation of adenovirus DNA: evolutionary variants reveal dispensable sequences near the left ends of Ad3 genomes. Virology. 1984 Sep;137(2):276–286. doi: 10.1016/0042-6822(84)90219-8. [DOI] [PubMed] [Google Scholar]
- Safer B., Yang L., Tolunay H. E., Anderson W. F. Isolation of stable preinitiation, initiation, and elongation complexes from RNA polymerase II-directed transcription. Proc Natl Acad Sci U S A. 1985 May;82(9):2632–2636. doi: 10.1073/pnas.82.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Gruss P. Specific interaction between enhancer-containing molecules and cellular components. Cell. 1984 Feb;36(2):403–411. doi: 10.1016/0092-8674(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Shenk T., Williams J. Genetic analysis of adenoviruses. Curr Top Microbiol Immunol. 1984;111:1–39. doi: 10.1007/978-3-642-69549-0_1. [DOI] [PubMed] [Google Scholar]
- Spector D. J., Halbert D. N., Raskas H. J. Regulation of integrated adenovirus sequences during adenovirus infection of transformed cells. J Virol. 1980 Dec;36(3):860–871. doi: 10.1128/jvi.36.3.860-871.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R., Ziff E. B. HeLa cell beta-tubulin gene transcription is stimulated by adenovirus 5 in parallel with viral early genes by an E1a-dependent mechanism. Mol Cell Biol. 1984 Dec;4(12):2792–2801. doi: 10.1128/mcb.4.12.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts C. Physical organization of subgroup B human adenovirus genomes. J Virol. 1977 Nov;24(2):564–579. doi: 10.1128/jvi.24.2.564-579.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Green M. R., Maniatis T. cis and trans activation of globin gene transcription in transient assays. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7428–7432. doi: 10.1073/pnas.80.24.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a proteins repress transcription from the SV40 early promoter. Cell. 1985 Mar;40(3):705–716. doi: 10.1016/0092-8674(85)90219-3. [DOI] [PubMed] [Google Scholar]
- Weinmann R., Ackerman S., Bunick D., Concino M., Zandomeni R. In vitro transcription of adenovirus genes. Curr Top Microbiol Immunol. 1984;109:125–145. doi: 10.1007/978-3-642-69460-8_6. [DOI] [PubMed] [Google Scholar]
- Winberg G., Shenk T. Dissection of overlapping functions within the adenovirus type 5 E1A gene. EMBO J. 1984 Aug;3(8):1907–1912. doi: 10.1002/j.1460-2075.1984.tb02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ormondt H., Maat J., Dijkema R. Comparison of nucleotide sequences of the early E1a regions for subgroups A, B and C of human adenoviruses. Gene. 1980 Dec;12(1-2):63–76. doi: 10.1016/0378-1119(80)90016-5. [DOI] [PubMed] [Google Scholar]







