Abstract
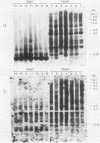
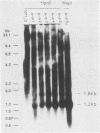
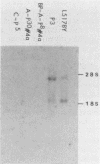
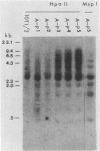
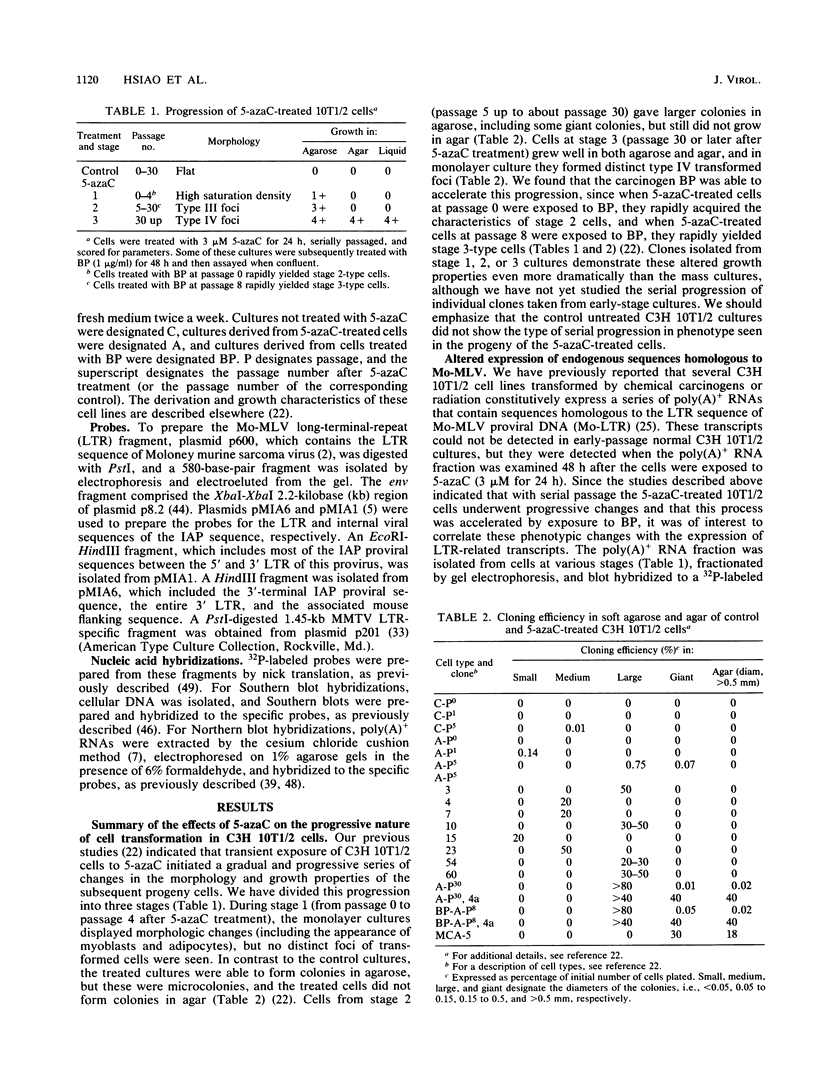
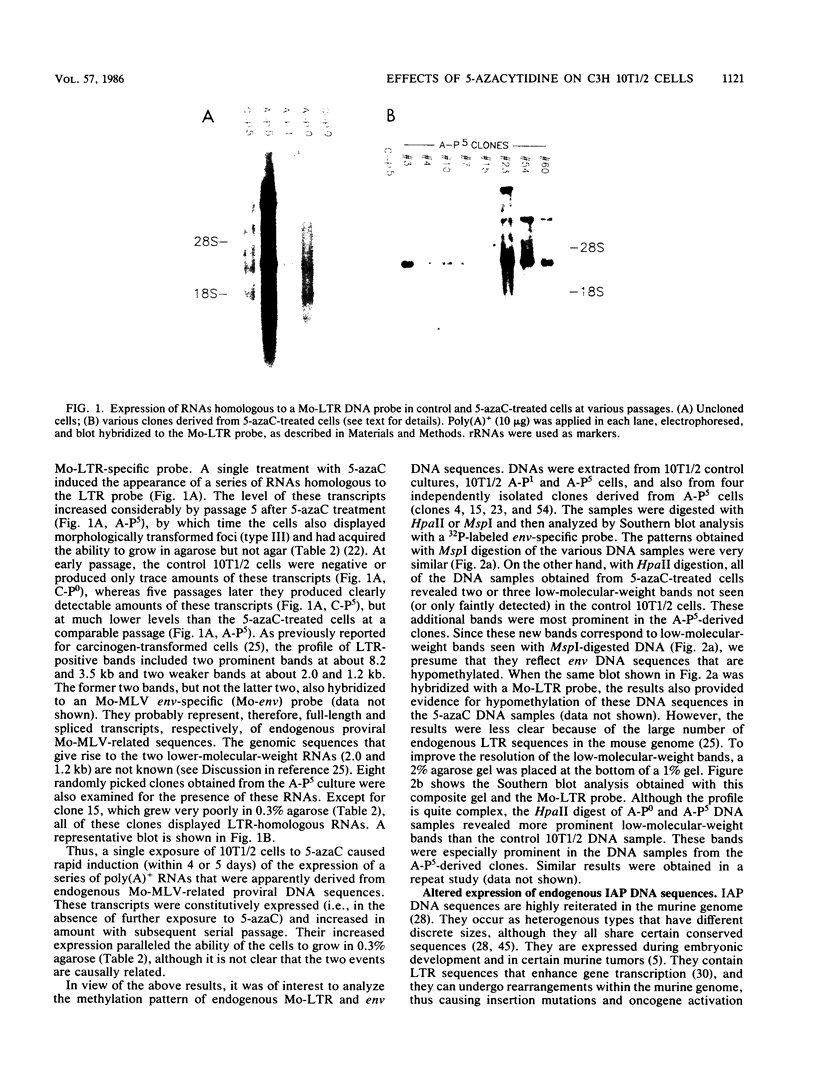
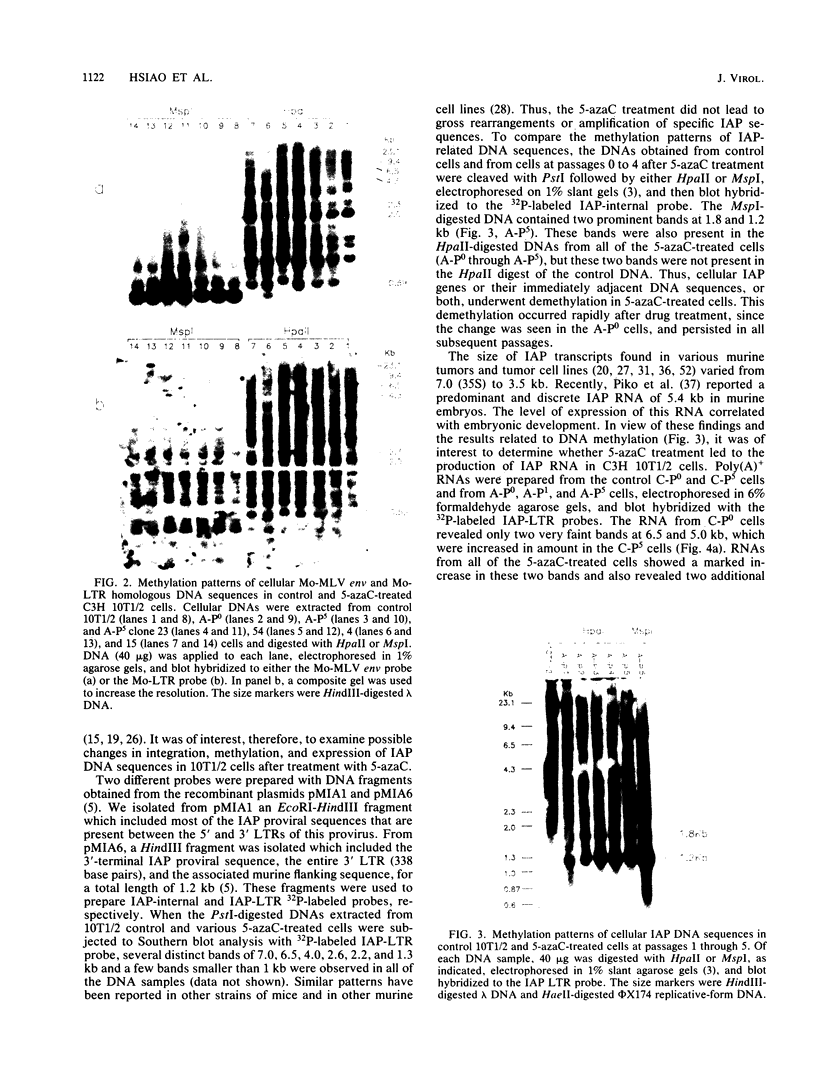
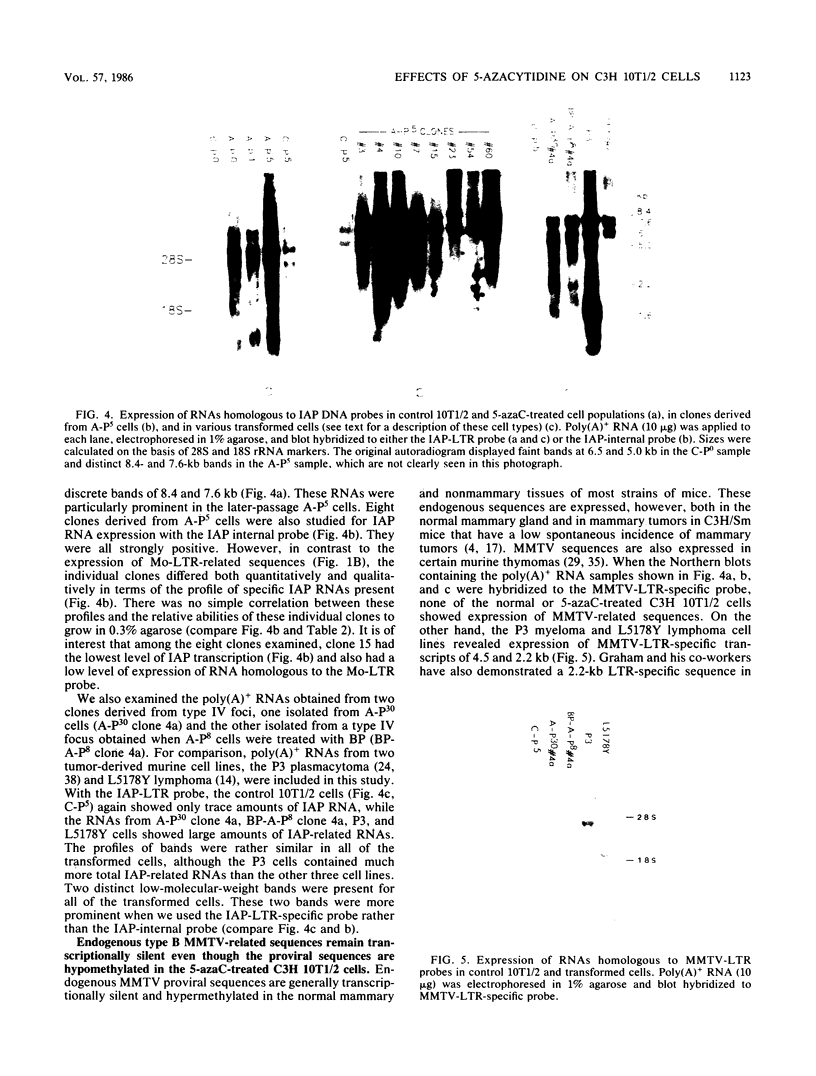
In a previous study (22) we found that transient exposure of C3H 10T1/2 mouse embryo fibroblasts to 5-azacytidine (5-azaC) induced several changes in growth properties. The treated cells showed progressive changes in morphology, saturation density, growth rate, and serum dependence. By passage 5, the cells had acquired the ability to grow in 0.3% agarose, and by passage 30, they had given rise to fully transformed foci that grew in agarose, agar, and liquid suspension. This progression was rapidly accelerated if the cultures derived from 5-azaC-treated cells were exposed for 48 h to the carcinogen benzo[a]pyrene. The present studies demonstrate that both type C and type A, but not type B, retrovirus-related sequences were expressed in the 5-azaC-treated cells. There was negligible expression of these sequences in the control 10T1/2 cells. The level of expression of the related RNAs tended to correlate with loss of anchorage dependence and other markers of an increase in the transformed phenotype. These changes were associated with hypomethylation of the corresponding cellular DNA sequences, as revealed by differential digestion with the restriction enzymes HpaII and MspI. These studies provide evidence that aberrations in DNA methylation and induction of expression of certain endogenous retroviruses may be one of a series of critical events during the course of multistage carcinogenesis, thus enhancing the evolution of malignant tumor cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedict W. F., Banerjee A., Gardner A., Jones P. A. Induction of morphological transformation in mouse C3H/10T1/2 clone 8 cells and chromosomal damage in hamster A(T1)C1-3 cells by cancer chemotherapeutic agents. Cancer Res. 1977 Jul;37(7 Pt 1):2202–2208. [PubMed] [Google Scholar]
- Boncinelli E., Simeone A., de Falco A., Fidanza V., La Volpe A. An agarose gel resolving a wide range of DNA fragment lengths. Anal Biochem. 1983 Oct 1;134(1):40–43. doi: 10.1016/0003-2697(83)90260-9. [DOI] [PubMed] [Google Scholar]
- Breznik T., Cohen J. C. Altered methylation of endogenous viral promoter sequences during mammary carcinogenesis. Nature. 1982 Jan 21;295(5846):255–257. doi: 10.1038/295255a0. [DOI] [PubMed] [Google Scholar]
- Callahan R., Kuff E. L., Lueders K. K., Birkenmeier E. Genetic relationship between the Mus cervicolor M432 retrovirus and the Mus Musculus intracisternal type A particle. J Virol. 1981 Dec;40(3):901–911. doi: 10.1128/jvi.40.3.901-911.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiff R. D. Protoneoplasia: the molecular biology of murine mammary hyperplasia. Adv Cancer Res. 1984;42:167–190. doi: 10.1016/s0065-230x(08)60458-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Courtney M. G., Schmidt L. J., Getz M. J. Organization and expression of endogenous virus-like (VL30) DNA sequences in nontransformed and chemically transformed mouse embryo cells in culture. Cancer Res. 1982 Feb;42(2):569–576. [PubMed] [Google Scholar]
- Cox R. DNA methylase inhibition in vitro by N-methyl-N'-nitro-N-nitrosoguanidine. Cancer Res. 1980 Jan;40(1):61–63. [PubMed] [Google Scholar]
- Drohan W. N., Benade L. E., Graham D. E., Smith G. H. Mouse mammary tumor virus proviral sequences congenital to C3H/Sm mice are differentially hypomethylated in chemically induced, virus-induced, and spontaneous mammary tumors. J Virol. 1982 Sep;43(3):876–884. doi: 10.1128/jvi.43.3.876-884.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmuir P., Brorein W. J., Jr, Simon M. A., Rubin G. M. Insertion of the Drosophila transposable element copia generates a 5 base pair duplication. Cell. 1980 Sep;21(2):575–579. doi: 10.1016/0092-8674(80)90495-x. [DOI] [PubMed] [Google Scholar]
- FISCHER G. A. Studies of the culture of leukemic cells in vitro. Ann N Y Acad Sci. 1958 Dec 5;76(3):673–680. doi: 10.1111/j.1749-6632.1958.tb54884.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983 Jan 6;301(5895):89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- Gama-Sosa M. A., Slagel V. A., Trewyn R. W., Oxenhandler R., Kuo K. C., Gehrke C. W., Ehrlich M. The 5-methylcytosine content of DNA from human tumors. Nucleic Acids Res. 1983 Oct 11;11(19):6883–6894. doi: 10.1093/nar/11.19.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattoni-Celli S., Hsiao W. L., Weinstein I. B. Rearranged c-mos locus in a MOPC 21 murine myeloma cell line and its persistence in hybridomas. Nature. 1983 Dec 22;306(5945):795–796. doi: 10.1038/306795a0. [DOI] [PubMed] [Google Scholar]
- Graham D. E., Medina D., Smith G. H. Increased concentration of an indigenous proviral mouse mammary tumor virus long terminal repeat-containing transcript is associated with neoplastic transformation of mammary epithelium in C3H/Sm mice. J Virol. 1984 Mar;49(3):819–827. doi: 10.1128/jvi.49.3.819-827.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Hawley R. G., Shulman M. J., Hozumi N. Transposition of two different intracisternal A particle elements into an immunoglobulin kappa-chain gene. Mol Cell Biol. 1984 Dec;4(12):2565–2572. doi: 10.1128/mcb.4.12.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojman-Montes de Oca F., Dianoux L., Peries J., Emanoil-Ravicovitch R. Intracisternal A particles: RNA expression and DNA methylation in murine teratocarcinoma cell lines. J Virol. 1983 Apr;46(1):307–310. doi: 10.1128/jvi.46.1.307-310.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao W. L., Gattoni-Celli S., Kirschmeier P., Weinstein I. B. Effects of 5-azacytidine on methylation and expression of specific DNA sequences in C3H 10T1/2 cells. Mol Cell Biol. 1984 Apr;4(4):634–641. doi: 10.1128/mcb.4.4.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao W. L., Gattoni-Celli S., Weinstein I. B. Effects of 5-azacytidine on the progressive nature of cell transformation. Mol Cell Biol. 1985 Jul;5(7):1800–1803. doi: 10.1128/mcb.5.7.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan M. B., Gowans B. J., Lieberman M. W. Methylation of deoxycytidine incorporated by excision-repair synthesis of DNA. Cell. 1982 Sep;30(2):509–516. doi: 10.1016/0092-8674(82)90248-3. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kirschmeier P., Gattoni-Celli S., Dina D., Weinstein I. B. Carcinogen- and radiation-transformed C3H 10T1/2 cells contain RNAs homologous to the long terminal repeat sequence of a murine leukemia virus. Proc Natl Acad Sci U S A. 1982 May;79(9):2773–2777. doi: 10.1073/pnas.79.9.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Rechavi G., Givol D., Canaani E. Homology between an endogenous viral LTR and sequences inserted in an activated cellular oncogene. Nature. 1983 Apr 7;302(5908):547–548. doi: 10.1038/302547a0. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Fewell J. W. Intracisternal A-particle gene expression in normal mouse thymus tissue: gene products and strain-related variability. Mol Cell Biol. 1985 Mar;5(3):474–483. doi: 10.1128/mcb.5.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Smith L. A., Lueders K. K. Intracisternal A-particle genes in Mus musculus: a conserved family of retrovirus-like elements. Mol Cell Biol. 1981 Mar;1(3):216–227. doi: 10.1128/mcb.1.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B. S., Weissman S. M. Mouse mammary tumor virus-related sequences in mouse lymphocytes are inducible by 12-O-tetradecanoyl phorbol-13-acetate. J Virol. 1984 Dec;52(3):1000–1004. doi: 10.1128/jvi.52.3.1000-1004.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Fewell J. W., Kuff E. L., Koch T. The long terminal repeat of an endogenous intracisternal A-particle gene functions as a promoter when introduced into eucaryotic cells by transfection. Mol Cell Biol. 1984 Oct;4(10):2128–2135. doi: 10.1128/mcb.4.10.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Sequences associated with intracisternal A particles are reiterated in the mouse genome. Cell. 1977 Dec;12(4):963–972. doi: 10.1016/0092-8674(77)90161-1. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Segal S., Kuff E. L. RNA sequences specifically associated with mouse intracisternal A particles. Cell. 1977 May;11(1):83–94. doi: 10.1016/0092-8674(77)90319-1. [DOI] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981 Jan 22;289(5795):253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- McKay R. Movable genes. Nature. 1980 Sep 18;287(5779):188–189. doi: 10.1038/287188a0. [DOI] [PubMed] [Google Scholar]
- Mermod J. J., Bourgeois S., Defer N., Crépin M. Demethylation and expression of murine mammary tumor proviruses in mouse thymoma cell lines. Proc Natl Acad Sci U S A. 1983 Jan;80(1):110–114. doi: 10.1073/pnas.80.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Segal S., Lueders K. K., Kuff E. L. RNA associated with murine intracisternal type A particles codes for the main particle protein. J Virol. 1978 Jul;27(1):118–126. doi: 10.1128/jvi.27.1.118-126.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikó L., Hammons M. D., Taylor K. D. Amounts, synthesis, and some properties of intracisternal A particle-related RNA in early mouse embryos. Proc Natl Acad Sci U S A. 1984 Jan;81(2):488–492. doi: 10.1073/pnas.81.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., Pumphrey J. G., Bailey D. W. Genetics of susceptibility to plasmacytoma induction. I. BALB/cAnN (C), C57BL/6N (B6), C57BL/Ka (BK), (C times B6)F1, (C times BK)F1, and C times B recombinant-inbred strains. J Natl Cancer Inst. 1975 Jun;54(6):1413–1417. doi: 10.1093/jnci/54.6.1413. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Royston M. E., Augenlicht L. H. Biotinated probe containing a long-terminal repeat hybridized to a mouse colon tumor and normal tissue. Science. 1983 Dec 23;222(4630):1339–1341. doi: 10.1126/science.6689218. [DOI] [PubMed] [Google Scholar]
- Ryo H., Shiba T., Fukunaga A., Kondo S., Gateff E. Chromosomal aberrations and retrovirus-like particles produced by in vivo transplantation in neoplastic brain cells of a Drosophila mutant strain. Gan. 1984 Jan;75(1):22–28. [PubMed] [Google Scholar]
- Schwartz S. A. Transcriptional activation of endogenous rat retrovirus with and without hypomethylation of proviral DNA. Biochem Biophys Res Commun. 1983 Apr 29;112(2):571–577. doi: 10.1016/0006-291x(83)91502-4. [DOI] [PubMed] [Google Scholar]
- Schwartzberg P., Colicelli J., Goff S. P. Deletion mutants of Moloney murine leukemia virus which lack glycosylated gag protein are replication competent. J Virol. 1983 May;46(2):538–546. doi: 10.1128/jvi.46.2.538-546.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Otten J. A., Myer F. E., Rascati R. J. Induction of retrovirus gene expression in mouse cells by some chemical mutagens. Cancer Res. 1982 Aug;42(8):3050–3055. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V. L., Jones P. A. Inhibition of DNA methylation by chemical carcinogens in vitro. Cell. 1983 Jan;32(1):239–246. doi: 10.1016/0092-8674(83)90514-7. [DOI] [PubMed] [Google Scholar]
- Wujcik K. M., Morgan R. A., Huang R. C. Transcription of intracisternal A-particle genes in mouse myeloma and Ltk- cells. J Virol. 1984 Oct;52(1):29–36. doi: 10.1128/jvi.52.1.29-36.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]