Abstract
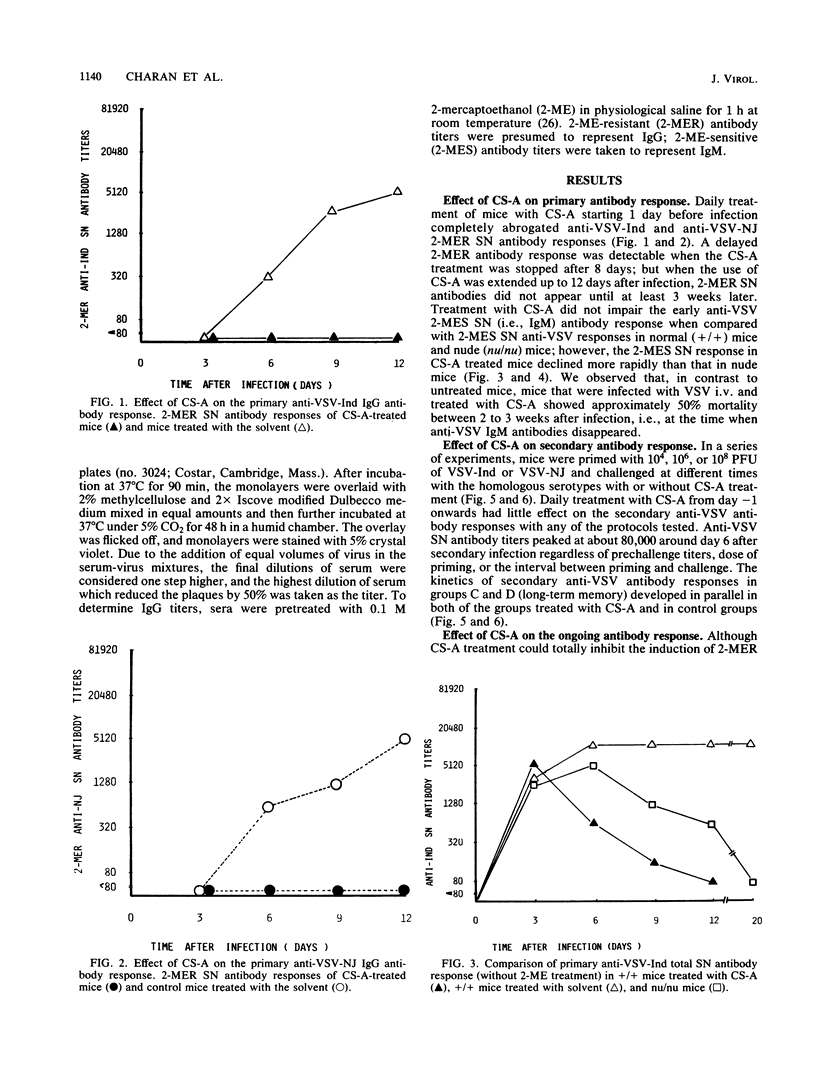
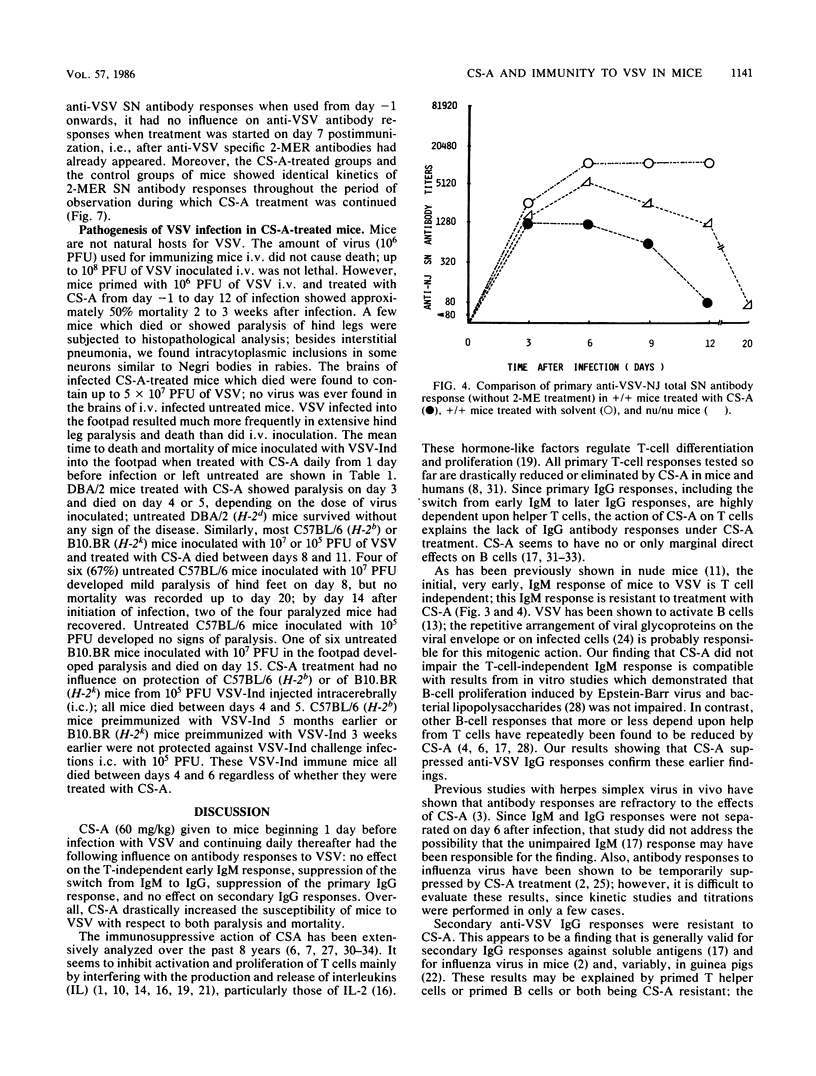
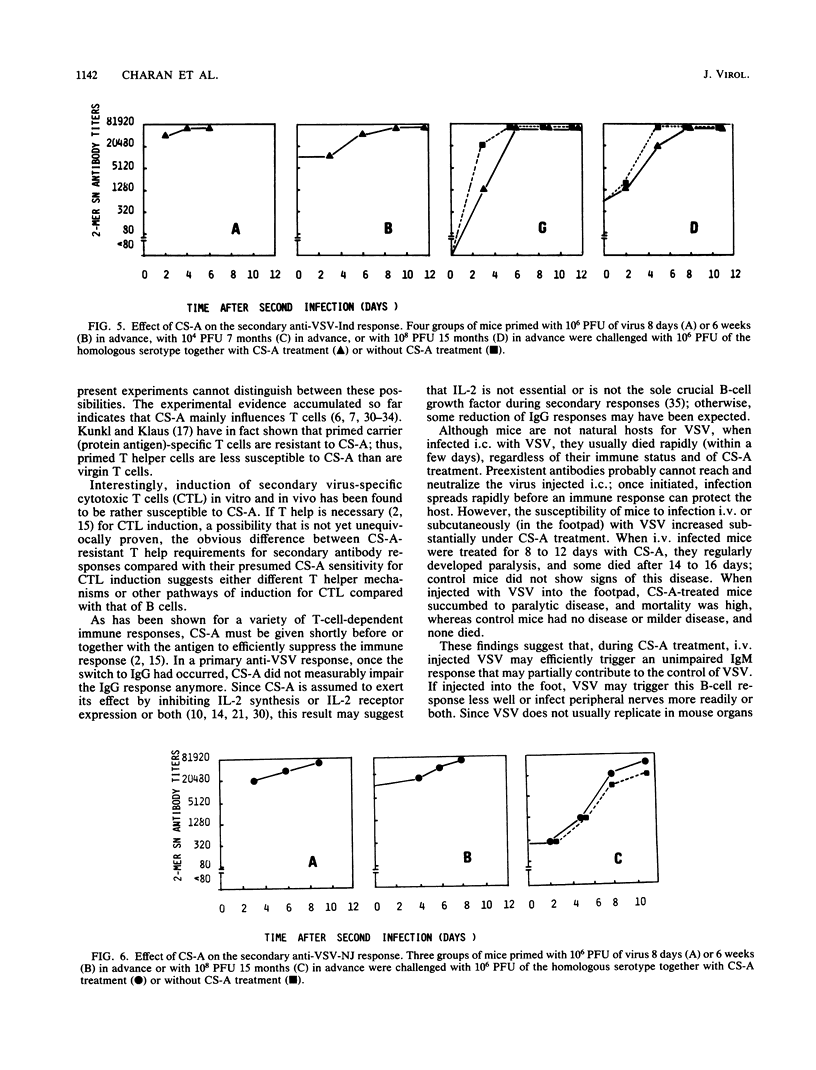
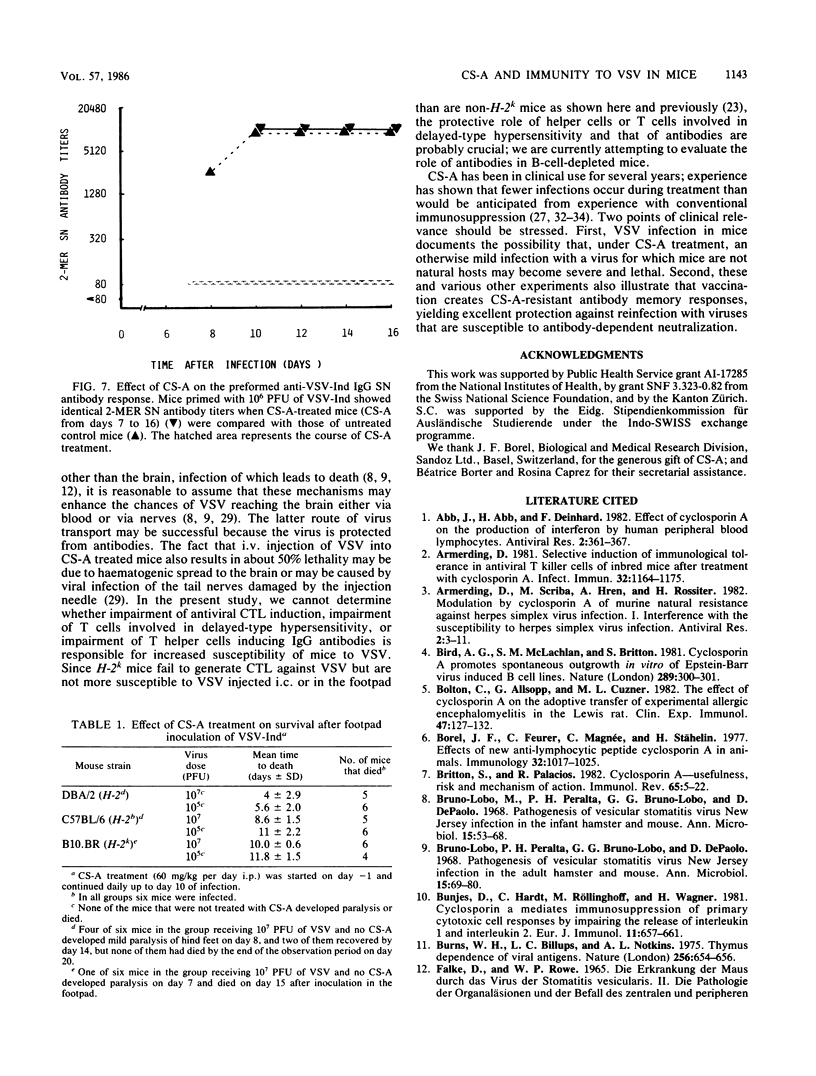
The effect of cyclosporin A (CS-A) on the antiviral humoral response was studied by using vesicular stomatitis virus (VSV); VSV provided the opportunity to simultaneously assess both T-independent and T-dependent antibody responses. The T-independent anti-VSV immunoglobulin M (IgM) response was virtually unaffected, whereas the T-dependent primary anti-VSV IgG response was suppressed by CS-A; in contrast, the secondary IgG response was highly resistant to CS-A. Moreover, once the switch from IgM to IgG had occurred, the primary response also became refractory to suppression by CS-A. We concluded that the effect of CS-A on the primary anti-VSV antibody response was mediated via impairment of a T-dependent mechanism; in contrast, memory T cells or memory B cells or both were quite resistant to the suppressive effects of CS-A. CS-A treatment rendered mice highly susceptible to VSV infection; under CS-A treatment, mortality was 100% after infection via footpads, whereas immunocompetent mice survived. Since CS-A does not impair induction of early T-independent anti-VSV IgM neutralizing antibodies, this high mortality in CS-A treated mice illustrates the crucial role of CS-A-sensitive cells in resistance against VSV.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abb J., Abb H., Deinhardt F. Effect of cyclosporin A on the production of interferon by human peripheral blood leukocytes in vitro. Antiviral Res. 1982 Dec;2(6):361–367. doi: 10.1016/0166-3542(82)90006-7. [DOI] [PubMed] [Google Scholar]
- Armerding D., Scriba M., Hren A., Rossiter H. Modulation by cyclosporin A of murine natural resistance against herpes simplex virus infection. I. Interference with the susceptibility to herpes simplex virus infection. Antiviral Res. 1982 May;2(1-2):3–11. doi: 10.1016/0166-3542(82)90022-5. [DOI] [PubMed] [Google Scholar]
- Armerding D. Selective induction of immunological tolerance in antiviral T killer cells of inbred mice after treatment with cyclosporin A. Infect Immun. 1981 Jun;32(3):1164–1175. doi: 10.1128/iai.32.3.1164-1175.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. G., McLachlan S. M., Britton S. Cyclosporin A promotes spontaneous outgrowth in vitro of Epstein-Barr virus-induced B-cell lines. Nature. 1981 Jan 22;289(5795):300–301. doi: 10.1038/289300a0. [DOI] [PubMed] [Google Scholar]
- Bolton C., Allsopp G., Cuzner M. L. The effect of cyclosporin A on the adoptive transfer of experimental allergic encephalomyelitis in the Lewis rat. Clin Exp Immunol. 1982 Jan;47(1):127–132. [PMC free article] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Britton S., Palacios R. Cyclosporin A--usefulness, risks and mechanism of action. Immunol Rev. 1982;65:5–22. doi: 10.1111/j.1600-065x.1982.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Bruno-Lobo M., Peralta P. H., Bruno-Lobo G. G., de Paola D. Pathogenesis of vesicular stomatitis virus New Jersey infection in the adult hamster and mouse. An Microbiol (Rio J) 1968;15:69–80. [PubMed] [Google Scholar]
- Bruno-Lobo M., Peralta P. H., Bruno-Lobo G. G., de Paola D. Pathogenesis of vesicular stomatitis virus infection in the infant hamster and mouse. An Microbiol (Rio J) 1968;15:53–68. [PubMed] [Google Scholar]
- Bunjes D., Hardt C., Röllinghoff M., Wagner H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol. 1981 Aug;11(8):657–661. doi: 10.1002/eji.1830110812. [DOI] [PubMed] [Google Scholar]
- Burns W., Billups L. C., Notkins A. L. Thymus dependence of viral antigens. Nature. 1975 Aug 21;256(5519):654–656. doi: 10.1038/256654a0. [DOI] [PubMed] [Google Scholar]
- Goodman-Snitkoff G. W., McSharry J. J. Activation of mouse lymphocytes by vesicular stomatitis virus. J Virol. 1980 Sep;35(3):757–765. doi: 10.1128/jvi.35.3.757-765.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Inaba K., Steinman R. M. Stimulation of lymphokine release from T lymphoblasts. Requirement for mRNA synthesis and inhibition by cyclosporin A. J Exp Med. 1984 Dec 1;160(6):1792–1802. doi: 10.1084/jem.160.6.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huegin A. W., Cerny A., Hengartner H., Zinkernagel R. M. Suppression by cyclosporin A of murine T-cell-mediated immunity against viruses in vivo and in vitro. Cell Immunol. 1985 Feb;90(2):464–473. doi: 10.1016/0008-8749(85)90211-4. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Rebar L., Keller J., Lee J. C., Hapel A. J. Interleukin 3: possible roles in the regulation of lymphocyte differentiation and growth. Immunol Rev. 1982;63:5–32. doi: 10.1111/j.1600-065x.1982.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Arya S. K., Wong-Staal F., Gallo R. C., Waldmann T. A., Greene W. C. Cyclosporin A inhibits T-cell growth factor gene expression at the level of mRNA transcription. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5214–5218. doi: 10.1073/pnas.81.16.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkl A., Klaus G. G. Selective effects of cyclosporin A on functional B cell subsets in the mouse. J Immunol. 1980 Dec;125(6):2526–2531. [PubMed] [Google Scholar]
- Like A. A., Dirodi V., Thomas S., Guberski D. L., Rossini A. A. Prevention of diabetes mellitus in the BB/W rat with Cyclosporin-A. Am J Pathol. 1984 Oct;117(1):92–97. [PMC free article] [PubMed] [Google Scholar]
- Nussenblatt R. B., Palestine A. G., Rook A. H., Scher I., Wacker W. B., Gery I. Treatment of intraocular inflammatory disease with cyclosporin A. Lancet. 1983 Jul 30;2(8344):235–238. doi: 10.1016/s0140-6736(83)90230-1. [DOI] [PubMed] [Google Scholar]
- Palacios R., Martinez-Maza O., De Ley M. Production of human immune interferon (Hu IFN-gamma) studied at the single cell level. Origin, evidence for spontaneous secretion and effect of cyclosporin A. Eur J Immunol. 1983 Mar;13(3):221–225. doi: 10.1002/eji.1830130308. [DOI] [PubMed] [Google Scholar]
- Parker D., Drössler K., Turk J. L. Kinetics of the effect of a single dose of cyclosporin-A on antibody and cell mediated immune responses in the guinea pig. Int J Immunopharmacol. 1984;6(1):67–74. doi: 10.1016/0192-0561(84)90037-7. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. L., Zinkernagel R. M. Inability of mice to generate cytotoxic T lymphocytes to vesicular stomatitis virus restricted to H-2Kk or H-2Dk. J Immunol. 1981 Feb;126(2):446–451. [PubMed] [Google Scholar]
- Rothman J. E., Fries E. Transport of newly synthesized vesicular stomatitis viral glycoprotein to purified Golgi membranes. J Cell Biol. 1981 Apr;89(1):162–168. doi: 10.1083/jcb.89.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltknecht E., Ada G. L. In vivo effects of cyclosporine on influenza A virus-infected mice. Cell Immunol. 1985 Mar;91(1):227–239. doi: 10.1016/0008-8749(85)90046-2. [DOI] [PubMed] [Google Scholar]
- Scott D. W., Gershon R. K. Determination of total and merecaptothanol-resistant antibody in the same serum sample. Clin Exp Immunol. 1970 Feb;6(2):313–316. [PMC free article] [PubMed] [Google Scholar]
- Shevach E. M. The effects of cyclosporin A on the immune system. Annu Rev Immunol. 1985;3:397–423. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]
- Shidani B., Colle J. H., Motta I., Truffa-Bachi P. Effect of cyclosporin A on the induction and activation of B memory cells by thymus-independent antigens in mice. Eur J Immunol. 1983 May;13(5):359–363. doi: 10.1002/eji.1830130503. [DOI] [PubMed] [Google Scholar]
- Smith J. S. Mouse model for abortive rabies infection of the central nervous system. Infect Immun. 1981 Jan;31(1):297–308. doi: 10.1128/iai.31.1.297-308.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. W. Immunobiology of cyclosporin A--a review. Aust J Exp Biol Med Sci. 1983 Apr;61(Pt 2):147–172. doi: 10.1038/icb.1983.14. [DOI] [PubMed] [Google Scholar]
- Thomson A. W., Whiting P. H., Simpson J. G. Cyclosporine: immunology, toxicity and pharmacology in experimental animals. Agents Actions. 1984 Oct;15(3-4):306–327. doi: 10.1007/BF01972366. [DOI] [PubMed] [Google Scholar]
- Tosato G., Pike S. E., Koski I. R., Blaese R. M. Selective inhibition of immunoregulatory cell functions by cyclosporin A. J Immunol. 1982 May;128(5):1986–1991. [PubMed] [Google Scholar]
- Weil C. Cyclosporin a: review of results in organ and bone-marrow transplantation in man. Med Res Rev. 1984 Apr-Jun;4(2):221–265. doi: 10.1002/med.2610040205. [DOI] [PubMed] [Google Scholar]
- White D. J., Calne R. Y. The use of Cyclosporin A immunosuppression in organ grafting. Immunol Rev. 1982;65:115–131. doi: 10.1111/j.1600-065x.1982.tb00430.x. [DOI] [PubMed] [Google Scholar]
- Zubler R. H., Lowenthal J. W., Erard F., Hashimoto N., Devos R., MacDonald H. R. Activated B cells express receptors for, and proliferate in response to, pure interleukin 2. J Exp Med. 1984 Oct 1;160(4):1170–1183. doi: 10.1084/jem.160.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]



