Abstract
The ability of signaling via the JNK (c-Jun NH2-terminal kinase)/stress-activated protein kinase cascade to stimulate or inhibit DNA synthesis in primary cultures of adult rat hepatocytes was examined. Treatment of hepatocytes with media containing hyperosmotic glucose (75 mM final), tumor necrosis factor α (TNFα, 1 ng/ml final), and hepatocyte growth factor (HGF, 1 ng/ml final) caused activation of JNK1. Glucose, TNFα, or HGF treatments increased phosphorylation of c-Jun at serine 63 in the transactivation domain and stimulated hepatocyte DNA synthesis. Infection of hepatocytes with poly-l-lysine–coated adenoviruses coupled to constructs to express either dominant negatives Ras N17, Rac1 N17, Cdc42 N17, SEK1−, or JNK1− blunted the abilities of glucose, TNFα, or HGF to increase JNK1 activity, to increase phosphorylation of c-Jun at serine 63, and to stimulate DNA synthesis. Furthermore, infection of hepatocytes by a recombinant adenovirus expressing a dominant-negative c-Jun mutant (TAM67) also blunted the abilities of glucose, TNFα, and HGF to stimulate DNA synthesis. These data demonstrate that multiple agonists stimulate DNA synthesis in primary cultures of hepatocytes via a Ras/Rac1/Cdc42/SEK/JNK/c-Jun pathway. Glucose and HGF treatments reduced glycogen synthase kinase 3 (GSK3) activity and increased c-Jun DNA binding. Co-infection of hepatocytes with recombinant adenoviruses to express dominant- negative forms of PI3 kinase (p110α/p110γ) increased basal GSK3 activity, blocked the abilities of glucose and HGF treatments to inhibit GSK3 activity, and reduced basal c-Jun DNA binding. However, expression of dominant-negative PI3 kinase (p110α/p110γ) neither significantly blunted the abilities of glucose and HGF treatments to increase c-Jun DNA binding, nor inhibited the ability of these agonists to stimulate DNA synthesis. These data suggest that signaling by the JNK/stress-activated protein kinase cascade, rather than by the PI3 kinase cascade, plays the pivotal role in the ability of agonists to stimulate DNA synthesis in primary cultures of rat hepatocytes.
INTRODUCTION
Data from several laboratories have suggested that signaling via the JNK (c-Jun NH2-terminal kinase)/stress-activated protein (SAP) kinase cascade plays a role in the transformation and proliferation of several types of cells, including epithelial cells such as hepatocytes (Liu et al., 1994; Rai et al., 1994; Westwick et al., 1995; Boylan et al., 1996; Bruccderi et al., 1997; Michalopoulos and DeFrances, 1997). This is in contrast to other data using transformed and established fibroblast cell lines, which suggests that signaling by the mitogen-activated protein (MAP) kinase cascade plays the dominant role in stimulating cellular proliferation (Williams and Roberts, 1994). JNK1/2 have been shown to be activated by a variety of similar stimuli, such as by hepatocyte growth factor (HGF), the proinflammatory cytokine tumor necrosis factor α (TNFα), and hyperosmotic shock (Bagrodia et al., 1995; Read et al., 1997; Rodrigues et al., 1997; Whitmarsh et al., 1997). However, despite this evidence, the direct significance of JNK1/2 activation or c-Jun function in proliferative responses of nontransformed, nonestablished epithelial cells has not been definitively tested by molecular dissection of this pathway. To determine the significance of JNK/SAP kinase cascade signaling in the control of DNA synthesis in a nontransformed, nonestablished epithelial cell, we performed experiments in primary cultures of rat hepatocytes in vitro.
Stress-related signals have been shown to lead to the activation of the Ras/Rac1/Cdc42 small molecular weight G protein family, which plays a role in the activation of MEK kinase homologues (Clark et al., 1997; Deak and Templeton, 1997). Downstream of MEK kinase is the stress-regulated equivalent of MEK1/2 in the MAP kinase pathway, SEK1 (also termed MKK4) (Yan et al., 1994). SEK1 phosphorylates and activates JNK1/2. JNK1/2 phosphorylate the transcription factor c-Jun (AP-1 complex) by phosphorylation of serines 63 and 73 in the NH2 terminus of the molecule, which positively regulates c-Jun function (Smeal et al., 1991; Sanchez et al., 1994; Karin, 1995; Kallunki et al., 1996; Santana et al., 1996; Verheij et al., 1996). Phosphorylation of c-Jun in its NH2 terminus has been shown to play an important role in the commitment to apoptosis in some transformed cells, but has also been suggested to be essential in the early proliferative response of hepatocytes after partial hepatectomy in vivo (Diehl et al., 1994, 1995; Jarvis et al., 1994; Westwick et al., 1996). The function of c-Jun can also be negatively regulated by phosphorylation in the COOH terminus of the molecule, which inhibits c-Jun DNA binding (Boyle et al., 1990). Phosphorylation of c-Jun in the COOH terminus has been proposed to be catalyzed by glycogen synthase kinase 3 (GSK3), a downstream effector of PI3 kinase and c-Akt (Cross et al., 1995; Jarvis et al., 1996). Thus, to obtain full c-Jun activation potentially requires the coordinate actions of two separate signaling cascades.
Recent data from our laboratory have demonstrated that hepatocytes from regenerating livers in vivo and proliferating hepatocytes in vitro have increased basal activity of the JNK/SAP kinase cascade (Jarvis et al., 1997a; Spector et al., 1997). Other studies in hepatocytes have demonstrated that TNFα can enhance hepatocyte proliferation and activate JNK1 and c-Jun. Furthermore, it has also been shown that inhibition of TNFα function by use of neutralizing antibodies during the proliferative response of hepatocytes after partial hepatectomy blocks liver regeneration in vivo (Diehl et al., 1994, 1995; Westwick et al., 1996; Columbano et al., 1997). Thus, evidence exists supporting the hypothesis that c-Jun function is important in the proliferation of hepatocytes in response to stress signals. We have tested whether hyperosmotic glucose treatment and the hepatocyte primary mitogens, TNFα and HGF, stimulate hepatocyte DNA synthesis via activation of the JNK/SAP kinase cascade and activation of c-Jun. The studies reported herein demonstrate that these agonists stimulate DNA synthesis in primary cultures of rat hepatocytes via a Ras/Rac1/Cdc42/SEK/JNK/c-Jun–dependent mechanism. Our data also demonstrate that the ability of cells to sense alterations in osmolarity lies at the level of the plasma membrane, above the level of the Ras proto-oncogene, suggesting that plasma membrane receptors may play a direct role in sensing changes in osmolarity.
MATERIALS AND METHODS
Materials
Male Sprague Dawley rats (200 g) had access to food and water ad libitum (Kunos et al., 1995). Anti-p42MAP kinase, Anti-JNK1, and anti-p70S6 kinase antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-GSK3α and anti-Rac1 antibodies and substrate peptide (YRRAAVPPSPSLSRHSSPHQpSEDEEE) were obtained from Upstate Biotechnologies (Lake Placid, NY). d-Glucose, HGF, and TNFα were purchased from Sigma Chemical (St. Louis, MO). AP-1 oligonucleotide for gel-shift assays (CGC TTG ATG ACT CAG CCG GAA) was purchased from Santa Cruz Biotechnology. Dominant-negative c-Jun (TAM67) was generated from wild-type c-Jun by deletion of residues 3–122 in the amino-terminal transcriptional activation domain (Jarvis et al., 1997b). Radiolabeled [γ-32P]ATP and [α-32P]ATP, free 32P-labeled inorganic phosphate, and [3H]thymidine were purchased from New England Nuclear (Boston, MA). Glutathione S-transferase (GST)-c-Jun (aa1–169) was synthesized in Escherichia coli and purified on glutathione-Sepharose. Protein preparations and purchase of other reagents were as described previously (Dent et al., 1995; Kunos et al., 1995; Jarvis et al., 1997a; Spector et al., 1997).
Methods
Recombinant Adenoviral Vectors: Generation and Infection In Vitro
Studies herein are performed using two adenoviral technologies. First, replication-defective adenovirus was conjugated to poly-l-lysine (as described in Cristiano et al., 1993; Nazareth and Weigel, 1996). Using polystyrene tubes, mixes of virus and a HEPES-buffered saline (HBS) solution were combined with 1 ng of mammalian expression plasmid (cytomegalovirus promoter) containing the gene of interest and incubated in the dark for 30 min. After 30 min, poly-l-lysine and HBS were mixed at 1.3 μg of lysine per μg DNA and incubated for another 30 min. The DNA-conjugated virus was added to hepatocytes at a multiplicity of infection (moi) of 250 and the cells were incubated for 4 h at 37°C. The cells were washed with media to remove virus. Cells expressed transduced gene products by 10–24 h after infection. Using a plasmid to express β-galactosidase under control of the cytomegalovirus promoter, we determined that 1 ng of plasmid conjugated to virus particles and infected into hepatocytes before plating at a moi of 250 gave 100% infection as judged by blue coloration after 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) incubation 24 h after infection. Second, we have generated recombinant PI3 kinase adenoviruses using a novel methodology developed by Drs. Matthias Paul Wymann and Stefano Brenz-Verca. In this procedure, the full-length recombinant adenovirus genome is cloned in a plasmid, flanked by a rare cutter (PacI) restriction site, and is generated using a recombination proficient E. coli strain (BJ5183) with the genotype recBC sbcBC (Chartier et al., 1996). Recombinant adenovirus to express dominant-negative c-Jun mutant (TAM67) was generated as described in the study by Valerie and Singhal (1995). To assess the effectiveness of recombinant adenoviral infection, we generated a recombinant adenovirus containing the gene for β-galactosidase. Primary hepatocytes were infected with this virus immediately after isolation in vitro (moi 250) and incubated at 37°C for another 24 h; cells were fixed and incubated with X-gal. A moi of 250 gave 100% infection after 24 h. To assess infection of other constructs used herein, we routinely performed Western immunoblots at 10–24 h after infection. Gene products were expressed approximately 18 h after infection.
Preparation of Hepatocytes
Rats were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg), and the lower thorax and abdomen were shaved to remove fur. A small (3-cm) vertical midline incision was made in the abdominal wall from just below the costal margin/xiphoid process. Hepatocytes were prepared by cannulation of the portal vein and collagenase perfusion of the liver. A cannula was inserted into the portal vein and two ties were tightened to occlude the inferior vena cava and secure the cannula in the portal vein. Gassed Krebs-Henseleit buffer (95% air, 5% CO2, 37°C) containing 0.3 mM EGTA (to prevent clotting) was passed through the liver (20 ml/min flow rate) to wash out erythrocytes; a second cannula was inserted into the inferior vena cava via the right atrium of the heart and also was secured with a tie, to act as a drain. Livers were washed sequentially with 200 ml of this buffer, followed by 200 ml of the same media omitting EGTA and including 0.5 mg/ml collagenase. The liver was then removed, filtered through muslin, and washed three times with DMEM. Hepatocytes were resuspended to 5.0 × 106 cells/ml in serum-free DMEM and placed into primary culture.
Primary Culture and Assay for DNA Synthesis in Hepatocytes
Hepatocytes were cultured on rat-tail collagen (Vitrogen)-coated plastic dishes (12 × 20 mm, 2 × 105 cells) in 1 μl of DMEM containing 100 nM insulin, 1 nM dexamethasone, and 1 nM thyroxine and were allowed to adhere to the dish. Cells were then infected with various adenoviruses, depending on which experiment was being performed. For cells undergoing acute assay, treatments occurred 4 h after plating. For adenoviral-infected cells, after 4 h of infection, media were replaced and hepatocytes were cultured (for DNA synthesis assays) in supplemented DMEM containing 2 μCi of [3H]thymidine in 5% (vol/vol) CO2. Hormonal treatments and/or protein kinase inhibitors were added 24 h after the media change. Cells were cultured for another 48 h, after which time cells were lysed with 0.5 M NaOH and DNA precipitated with 12.5% (wt/vol) trichloroacetic acid (final). Acid-precipitable material was transferred to glass fiber filters, washed with 5% (wt/vol) trichloroacetic acid, and [3H]thymidine incorporation into DNA as quantified by liquid scintillation spectrometry (Spector et al., 1997).
Treatment of Primary Cultures of Hepatocytes with Hormones and Cell Homogenization
Primary cultures of hepatocytes were cultured in 12-well plates or 20-mm dishes (2 × 105 cells) in DMEM as above (0.5 mg of total protein used per immunoprecipitate). d-Glucose, 2-deoxyglucose, and sorbitol (1 M stocks for each) in DMEM, TNFα, or HGF [in phosphate-buffered saline (PBS) as a 2 mg/ml stock] were added to give final specified concentrations (see text and figure legends), mixed, and incubated for the specified times at 37°C in a cell culture incubator. Cells were pretreated with protein kinase inhibitors (30 min) before hormonal additions. Twenty seconds before termination, plated cells were aspirated, followed by immediate homogenization. Cells were homogenized in 1 ml of ice-cold buffer A [25 mM HEPES, pH 7.4, at 4°C, 5 mM EDTA, 5 mM EGTA, 5 mM benzamidine, 1 mM phenylmethylsulfonyl fluoride, 1 mg/μl soybean trypsin inhibitor, 1.0 mM sodium o-vanadate, 1.0 mM sodium pyrophosphate, 0.05% (wt/vol) sodium deoxycholate, 1% (vol/vol) Triton X-100, and 0.1% (vol/vol) 2-mercaptoethanol], with trituration using a P1000 pipette to lyse the cells. Homogenates were clarified by centrifugation (14,000 × g, 4°C).
Immunoprecipitations from Homogenates
Fifty microliters of protein A-agarose (Ag) slurry (25-μl bead volume) were washed twice with 1 μl of PBS containing 0.1% (vol/vol) Tween 20 and were resuspended in 0.1 μl of the same buffer. Antibodies (2 μg, 20 μl) or serum (20 μl) were added to each tube and incubated (3 h, 4°C). Clarified hepatocyte homogenates (0.5 μl, 1 μg of total protein) were mixed with protein A-Ag–conjugated antibody in duplicate using gentle agitation (2.5 h, 4°C). Protein A-Ag was recovered by centrifugation and the supernatant was discarded and washed (10 min) sequentially with 0.5 μl of buffer A (twice), PBS, and buffer B [25 mM HEPES, pH 7.4, 15 mM MgCl2, 0.1 mM Na3VO4 0.1% (vol/vol) 2-mercaptoEtOH].
Assay of p42MAP kinase Activity
Immunoprecipitates were incubated (final vol 50 μl) with 50 μl of buffer B containing 0.2 mM [[gamma]-32P]ATP (5000 cpm/pmol), 1 mM Microcystin-LR, and 0.5 μg/μl myelin basic protein, which initiated reactions. After 20 min, 40 μl of the reaction mixtures were spotted onto a 2-cm circle of P81 paper (Whatman, Maidstone, England) and immediately placed into 180 mM phosphoric acid. Papers were washed four times (10 min each) with phosphoric acid and once with acetone, and 32P-labeled incorporation into myelin basic protein was quantified by liquid scintillation spectroscopy. Preimmune controls were performed to ensure that myelin basic protein phosphorylation was dependent on specific immunoprecipitation of p42MAP kinase (Spector et al., 1997).
Assay of JNK1 Activity
Immunoprecipitates were incubated (final vol 100 μl) with 2 μl (10 μg) of GST-c-Jun (aa1–169) and reactions were initiated with 98 μl of buffer B containing 0.2 mM [[gamma]-32P]ATP (5000 cpm/pmol) and 1 μM Microcystin-LR. After 30 min, reactions were terminated with sample buffer and prepared for SDS-PAGE (10% gel) to quantify 32P-labeled incorporation into excised, Coomassie blue-stained GST-c-Jun (aa1–169) bands by liquid scintillation spectroscopy. Preimmune control assays were performed to ensure that GST-c-Jun (aa1–169) phosphorylation was dependent on specific immunoprecipitation of JNK1 in the assay (Spector et al., 1997).
Assay for GSK3 Activity
GSK3 was immunoprecipitated and assayed versus a phosphopeptide substrate (YRRAAVPPSPSLSRHSSPHQpSEDEEE). Immunoprecipitates were incubated (final vol 50 μl) with 50 μl of buffer B containing 0.2 mM [[gamma]-32P]ATP (5000 cpm/pmol), 1 μM Microcystin-LR, and 200 μM phosphopeptide substrate, which initiated reactions. After 20 min, 40 μl of the reaction mixtures were spotted onto a 2-cm circle of P81 paper (Whatman) and immediately placed into 180 mM phosphoric acid. Papers were washed four times (10 min each) with phosphoric acid and once with acetone, and 32P-labeled incorporation into phosphopeptide substrate compared with identical samples containing enzyme but without substrate (Welsh et al., 1994).
Assay for p70S6 kinase Activity
Immunoprecipitation of p70S6 kinase was performed as above and assayed versus a peptide substrate (RRRLSSLA). Immunoprecipitates were incubated (final vol 50 μl) with 50 μl of buffer B containing 0.2 mM [[gamma]-32P]ATP (5000 cpm/pmol), 1 μM Microcystin-LR, and peptide substrate (50 μM, final). After 20 min, 40 μl of the reaction mixtures were spotted onto a 2-cm circle of P81 paper (Whatman) and immediately placed into 180 mM phosphoric acid. Papers were washed four times (10 min each) with phosphoric acid and once with acetone, and 32P-labeled incorporation into peptide substrate compared with identical samples containing enzyme but without substrate determined by liquid scintillation spectroscopy (Welsh et al., 1994).
Transcription Factor DNA-binding Assay
Nuclear extracts were prepared as described (Stravitz et al., 1996). Oligonucleotides were 32P labeled with [[gamma]-32P]ATP using polynucleotide kinase. Binding reaction mixtures (20 ml) were incubated at room temperature for 45 min in a mixture containing 1 ng of DNA probe and 5 μg of nuclear extract in 10 mM Tris/HCl (pH 7.5) at 25°C, 40 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA, 5% (vol/vol) glycerol, and 2 μg poly(dl-dC) to inhibit nonspecific binding in the extract. DNA–protein complexes were resolved by electrophoresis through a 4%/8% nondenaturing polyacrylamide gel containing 50 mM Tris, 0.38 M glycine, and 2 mM EDTA. Gels were dried and quantified in the linear range (500–15,000 dpm) and processed using a phosphorimager.
Luciferase Assay
Luciferase assays were performed as described using a kit purchased from Promega (Madison, WI). Briefly, cells were scraped into PBS, pelleted by centrifugation, lysed, incubated on ice for 5 min, and clarified by centrifugation at 4°C for 10 min. Portions of the supernatant were added to assay mixture and the luminosity of each sample was determined in triplicate in a luminometer (20-s exposure; de Wet et al., 1987).
Guanine Nucleotide-binding Assays
Determination of guanine nucleotide binding to Rac1 was determined as described in the report by Gibbs (1995). Briefly, hepatocytes (12-well plates, 2 × 105 cells) were cultured in phosphate-free DMEM and infected with plasmids to express Rac1N17, Rac1V12, Cdc42N17, and Cdc42V12. Twenty-four hours after infection, hepatocytes were incubated with 0.3 μCi of 32P-labeled Pi for 90 min at 37°C. Radioactive media were aspirated, and the cells were washed once in ice-cold PBS and lysed with trituration for 1 h at 4°C in 50 mM Tris/HCl (pH 7.5), at 4°C, 20 mM MgCl2, 150 mM NaCl, 0.5% (vol/vol) Nonidet P-40, 5 mM benzamidine, and 1.0 mM phenylmethylsulfonyl fluoride, each sample containing 4 μg of anti-Rac1 antibody. After 1 h, 0.1 ml of a bovine serum albumin-blocked 10% (wt/vol) charcoal slurry was added to the homogenate to remove unbound nucleotides; after 1 h at 4°C, samples were clarified by centrifugation. The radioactivity in a 2-μl aliquot of each sample was determined. Immunoprecipitation of Rac1 was completed by adding 50 μl of a 50% slurry of protein A-agarose (Bio-Rad, Richmond, CA) to a constant amount (0.5 × 107 cpm) of sample. Immunoprecipitates were heated to 85–90°C in 20 μl of 1 M KH2PO4 (pH 3.4), which releases coimmunoprecipitating nucleotides from Rac1. The solution was clarified and spotted onto a polyethyleneimine cellulose sheet. Chromatograms are developed with pH 3.4 1 M KH2PO4. Guanine nucleotides complexed to Rac1 were visualized by use of a phosphorimager, followed by scraping of each spot to quantify the amount of radioactivity with scintillant in a scintillation counter. A correction was made for the ratio of moles of phosphate to moles of guanosine (GTP cpm × 0.33; GDP cpm × 0.50) assuming uniform labeling of all phosphates. The data were expressed as percentage of GTP relative to the total GTP + GDP detected.
Data Analysis
Comparison of the effects of various hormone treatments was done using one-way analysis of variance and a two-tailed t test. Differences with a p < 0.05 were considered statistically significant. All bar graph fold values and means shown are ±SE from 2 to 12 independent experiments (individual liver isolations).
RESULTS
Glucose, TNFα, and HGF Treatments of Primary Cultures of Hepatocytes Stimulate JNK1 Activity
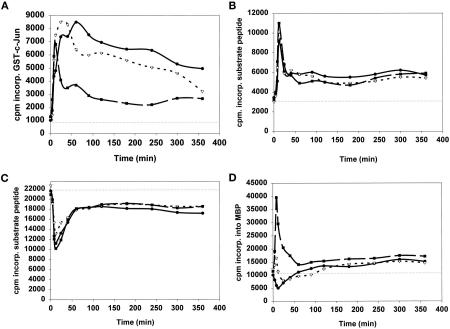
Hepatocytes cultured in DMEM were hyperosmotically shocked with 50 mM glucose 4 h after plating, and the activities of various components within signal transduction cascades were examined (Figure 1). Ten minutes after the 50 mM glucose treatment, the activities of the SAP kinase JNK1 (Figure 1A) and p70S6 kinase protein kinase (Figure 1B) were increased, whereas the activities of GSK3 (Figure 1C) and the p42 MAP kinase (Figure 1D) were reduced. Treatment of hepatocytes with TNFα (1 ng/μl) also caused activations of both JNK1 (Figure 1A) and p70S6 kinase (Figure 1B) and an inhibition of GSK3 activity (Figure 1C). TNFα caused an acute transient increase in p42MAP kinase activity followed by a decrease in activity, which rebounded to give a small activation at later time points (Figure 1D). Treatment of hepatocytes with HGF (1 ng/μl) potently increased JNK1, p70S6 kinase, and p42MAP kinase activities (Figure 1, A, B, and D) and decreased GSK3 activity (Figure 1C). Thus, all agonists tested stimulated the JNK/SAP kinase cascade (Figure 1A) and the PI3 kinase cascade (Figure 1, B and C), whereas only HGF caused significant acute and chronic increases in the activity of p42MAP kinase (Figure 1D).
Figure 1.
(A) Hyperosmotic glucose, TNFα, or HGF treatments activate JNK1. (B) Hyperosmotic glucose, TNFα, or HGF treatments activate p70S6 kinase. (C) Hyperosmotic glucose, TNFα, or HGF treatments inhibit GSK3. (D) Modulation of p42MAP kinase activity after hyperosmotic glucose, TNFα, or HGF treatments. Hepatocytes were cultured as described in MATERIALS AND METHODS. After 4 h, cells were treated for the indicated times with either media control, 50 mM glucose (closed circles), 1 ng/μl TNFα (open triangles), or 1 ng/μl HGF (closed squares) at 37°C, after which time media were aspirated and the cells frozen. Hepatocytes were lysed and subjected to immunoprecipitation followed by immune complex kinase assays for the indicated protein kinase. Activities shown are expressed as cpm incorporated into substrate versus media control treatment (5000 cpm/pmol) and are the means of triplicates from a representative of four experiments. Media control treatment did not alter the basal activities of protein kinases versus no treatment.
Glucose, TNFα, and HGF Treatments of Hepatocytes Cause Phosphorylation of c-Jun at Serine 63 in the Transactivation Domain via the Ras/Rac1/Cdc42/SEK/JNK Pathway
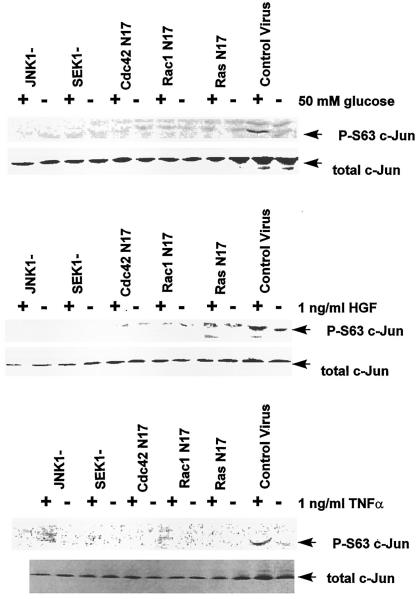
Because hyperosmotic glucose, TNFα, and HGF treatments all activated JNK1, we next investigated whether these agonists caused functional activation of the downstream effector of JNK1, the immediate early transcription factor c-Jun. To test whether JNK1 activation is modulating c-Jun phosphorylation at its NH2 terminus in our system, plasmids containing control (null plasmid), dominant-negative RasN17, dominant-negative Rac1N17, dominant-negative Cdc42N17, dominant-negative JNK1, and dominant-negative SEK1 (Verheij et al., 1996; Read et al., 1997; Rodrigues et al., 1997) were infected into hepatocytes at a high moi using a poly-l-lysine–conjugated adenovirus system. The infected hepatocytes were treated 24 h later with either 50 mM glucose, 1 ng/μl HGF, or 1 ng/μl TNFα for either 10 min (Table 1) or 20 min (Figure 2). In control-infected cells, glucose, HGF, and TNFα treatments stimulated JNK1 activity (Table 1) and phosphorylation of c-Jun at position serine 63 in the NH2-terminal transactivation domain (Figure 2). Stimulation of JNK1 activity and c-Jun serine 63 phosphorylation were blocked in cells expressing dominant-negative RasN17, dominant-negative Rac1N17, dominant-negative Cdc42N17, dominant-negative JNK1, or dominant-negative SEK1 (Table 1 and Figure 2). Thus, treatment of hepatocytes with multiple agonists increases JNK1 activity and increases phosphorylation of serine 63 in the transactivation domain of c-Jun via a Ras/Rac1/Cdc42/SEK/JNK-dependent mechanism. These data also demonstrate that both nontyrosine kinase receptor molecules (TNFα) and hyperosmotic agents (glucose) can activate the JNK/SAP kinase pathway via the Ras proto-oncogene.
Table 1.
Hyperosmotic glucose, HGF, and TNFα stimulate JNK1 activity, which is blocked by expression of dominant-negative RasN17, dominant-negative Rac1N17, dominant-negative Cdc42N17, dominant-negative SEK1, and dominant-negative JNK1, but not by expression of dominant-negative c-Jun(TAM67)
| Glucose | null | RasN17 | Rac1N17 | Cdc42N17 | SEK1− | JNK1− | c-Jun(TAM67) |
|---|---|---|---|---|---|---|---|
| Control | 3,719 ± 570 | 1,627 ± 180a | 833 ± 180a | 533 ± 90a | 1,605 ± 220a | 2,172 ± 300a | 4,438 ± 190 |
| Glucose | 10,168 ± 2,400b | 1,377 ± 330a | 929 ± 50a | 1,184 ± 320a | 2,567 ± 140a | 2,107 ± 110a | 11,313 ± 1,050b |
| HGF | 12,585 ± 1,820b | 1,711 ± 410a | 1,263 ± 290a | 1,517 ± 280a | 2,564 ± 380a | 1,873 ± 220a | 11,647 ± 1,670b |
| TNFα | 12,440 ± 1,750b | 1,710 ± 240a | 1,596 ± 300a | 1,851 ± 60a | 1,897 ± 180a | 2,559 ± 145a | 12,647 ± 770b |
Hepatocytes were infected with either control plasmid poly-l-lysine adenovirus or dominant-negative Ras N17/Rac1 N17/Cdc42 N17/SEK1−/JNK1− poly-l-lysine adenovirus (250 moi), followed by culture as described in MATERIALS AND METHODS. In a parallel experiment, hepatocytes were infected with a recombinant adenovirus (250 moi) to express a nontransactivatable dominant-negative c-Jun(TAM67). After 24 h to allow expression of dominant-negative gene products, hepatocytes were treated for 10 min with either media control, 50 mM glucose, 1 ng/μl HGF, or 1 ng/μl TNFα. After 10 min, media were aspirated and cells frozen. Hepatocytes were lysed and subjected to immunoprecipitation followed by immune complex kinase assays for JNK1. Activities shown are expressed as cpm incorporated into substrate versus media control treatment (5000 cpm/pmol) and are the means ± SE from two to five experiments, each performed in duplicate.
p < 0.05 decrease compared with corresponding value in null/control plasmid infected.
p < 0.05 increase compared with control.
Figure 2.
Hyperosmotic glucose (top panel), HGF (middle panel), and TNFα (bottom panel) stimulate phosphorylation of serine 63 in the transactivation domain of c-Jun, which is blocked by expression of dominant-negative RasN17, dominant-negative RacN17, dominant-negative Cdc42N17, dominant-negative SEK1, and dominant-negative JNK1. Hepatocytes were infected with either control plasmid poly-l-lysine adenovirus or dominant-negative Ras N17/Rac1 N17/Cdc42 N17/SEK1/JNK1 poly-l-lysine adenovirus (250 moi), followed by culture as described in MATERIALS AND METHODS. After 24 h to allow expression of dominant-negative gene products, hepatocytes were treated for 20 min with either media control, 50 mM glucose, 1 ng/μl TNFα, or 1 ng/μl HGF. After 20 min, media were aspirated and cells frozen. Hepatocytes were lysed with 100 μl of 5× SDS-polyacrylamide sample buffer, diluted to 250 μl with distilled water, and placed in a 100°C dry bath for 15 min. One hundred-microliter aliquots of each time point were subjected to SDS-PAGE on two 10% gels. Gels were transferred to nitrocellulose and Western blotting versus either anti-serine 63 c-Jun or anti–c-Jun protein (performed as in MATERIALS AND METHODS). The top panels show blotting versus serine 63 c-Jun; the bottom panels show total c-Jun protein immunoblotting.
Glucose Treatment of Hepatocytes Causes Activation of c-Jun Using an AP-1-Luciferase Reporter Assay
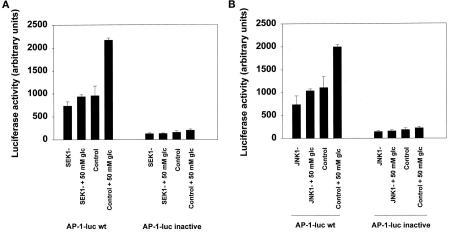
To test whether phosphorylation of c-Jun at serine 63 was functional for c-Jun transactivation in hepatocytes, cells were coinfected with poly-l-lysine–conjugated adenoviruses using constructs to express either dominant-negative SEK1 or dominant-negative JNK1 together with an AP-1–regulatable luciferase gene. Twenty-four hours later, hepatocytes were treated for 12 h with glucose and luciferase activity was measured (Figure 3). Glucose treatment of hepatocytes increased luciferase AP-1 activity, which was blocked by expression of either dominant-negative SEK1 or dominant-negative JNK1 (Figure 3). Similar data were obtained when hepatocytes were infected with a dominant negative c-Jun mutant (TAM67) construct. Thus, phosphorylation of serine 63 in hepatocyte c-Jun corresponds to a functional activation of transcription from AP-1–regulatable promoters.
Figure 3.
Hyperosmotic glucose transactivates c-Jun and stimulates luciferase activity from an AP-1-luciferase reporter construct, which is inhibited by expression of either dominant-negative SEK1 (A) or dominant-negative JNK1 (B). Hepatocytes were infected with either an AP-1-luciferase plasmid poly-l-lysine adenovirus or a mutant AP-1-luciferase poly-l-lysine adenovirus (100 moi) and coinfected in combination with either control plasmid poly-l-lysine adenovirus, dominant-negative SEK1 plasmid poly-l-lysine adenovirus, or dominant- negative JNK1 plasmid poly-l-lysine adenovirus (250 moi), followed by culture as described in MATERIALS AND METHODS. After 24 h to allow expression of dominant-negative SEK1 or dominant-negative JNK1, hepatocytes were treated for another 12 h with either media control or 50 mM glucose, after which media were aspirated and the cells frozen. Cells were prepared for assay of luciferase activity using a kit, as described in the manufacturer’s instructions [see MATERIALS AND METHODS (de Wet et al., 1987)]. Data are means ± SE from the averages of triplicates (six data points) from two experiments.
Treatment of Hepatocytes with Glucose, TNFα, or HGF Increases DNA Synthesis via a Ras/Rac1/Cdc42/SEK/JNK/c-Jun–dependent Mechanism
Further experiments were performed to test whether stimulation of the JNK/SAP kinase pathway by glucose, TNFα, or HGF was causally related to the abilities of these agonists to stimulate hepatocyte DNA synthesis. Hepatocytes were infected with poly-l-lysine–conjugated adenoviruses using constructs to express either control (null plasmid), dominant-negative RasN17, dominant-negative Rac1N17, dominant-negative Cdc42N17, dominant-negative SEK1, dominant-negative JNK1, or dominant-negative SEK1 (Tables 2 and 3). In parallel experiments, hepatocytes were also infected with a recombinant adenovirus to express dominant-negative c-Jun (TAM67). TAM67 is an NH2-deleted c-Jun molecule that is incapable of transactivation. Infected hepatocytes were treated 24 h after infection with either media control, 50 mM glucose, 1 ng/μl TNFα, or 1 ng/μl HGF, and the ability of each condition to synthesize DNA was determined 30 h later. Glucose treatment of control-infected hepatocytes stimulated DNA synthesis by ∼225%. TNFα and HGF treatments of control-infected hepatocytes also increased DNA synthesis as judged by [3H]thymidine incorporation into DNA, by ∼250 and ∼400%, respectively (Tables 2 and 3). However, expression of dominant-negative RasN17, dominant-negative Rac1N17, dominant-negative Cdc42N17, dominant-negative SEK1, dominant-negative JNK1, or dominant-negative c-Jun (TAM67) reduced basal DNA synthesis by ∼70% and completely blocked the abilities of glucose, TNFα, or HGF to stimulate DNA synthesis (Tables 2 and 3).
Table 2.
Hyperosmotic glucose treatment, TNFα treatment, or HGF treatment increases DNA synthesis in primary cultures of rat hepatocytes, which is blocked by expression of either dominant-negative SEK1, dominant-negative JNK1, or dominant-negative c-Jun (TAM67)
| Treatment | Control plasmid | Dominant-negative SEK1− | Dominant-negative JNK1− | Dominant-negative c-Jun (TAM67) |
|---|---|---|---|---|
| Media control | 3,596 ± 640 | 1,236 ± 225a | 994 ± 290a | 1,119 ± 330a |
| 50 mM glucose | 12,189 ± 990b | 2,007 ± 340a | 1,325 ± 410a | 1,600 ± 405a |
| 1 ng/μl TNFα | 12,274 ± 780b | 1,614 ± 375a | — | 1,826 ± 620a |
| 1 ng/μl HGF | 15,389 ± 1,120b | — | 1,797 ± 630a | 1,577 ± 380a |
Hepatocytes were cultured as described in MATERIALS AND METHODS. Hepatocytes were infected with either control plasmid poly-l-lysine adenovirus, dominant-negative SEK1 poly-l-lysine adenovirus, dominant-negative JNK1 poly-l-lysine adenovirus, or recombinant dominant-negative c-Jun (TAM67) adenovirus (all at 250 moi each), followed by culture as described in MATERIALS AND METHODS. After 24 h to allow expression of dominant-negative SEK1, dominant-negative JNK1, and dominant-negative c-Jun (TAM67), hepatocytes were treated with either media control for 24 h, 50 mM glucose for 6 h followed by incubation with unsupplemented media for 18 h, or with 1 ng/μl TNFα for 24 h, or with 1 ng/μl HGF for 24 h (total time in primary culture 48 h). Hepatocytes under all conditions were continually incubated with 2 μCi of [3H]thymidine throughout the final 24 h of culture, after which they were lysed and [3H]thymidine incorporation into DNA was determined as in MATERIALS AND METHODS (n = 12).
p < 0.05 decrease compared with corresponding value in control plasmid infected.
p < 0.05 increase compared with media control.
Table 3.
Hyperosmotic glucose, HGF, and TNFα stimulate hepatocyte DNA synthesis via a Ras/Rac1/Cdc42/SEK/JNK/c-Jun–dependent mechanism
| Control plasmid | Dominant-negative Ras N17 | Dominant-negative Rac N17 | Dominant-negative Cdc42 N17 | |
|---|---|---|---|---|
| Media control | 3,522 ± 400 | 1,127 ± 70a | 1,808 ± 120a | 985 ± 160a |
| 50 mM glucose | 9,763 ± 650b | 1,995 ± 120a | 1,517 ± 210a | 810 ± 145a |
| 1 ng/μl TNFα | 12,385 ± 1,080b | 868 ± 265a | 1,389 ± 110a | 1,287 ± 140a |
| 1 ng/μl HGF | 15,185 ± 870b | 1,480 ± 440a | 2,517 ± 405a | 1,496 ± 105a |
Hepatocytes were cultured as described in MATERIALS AND METHODS. Hepatocytes were infected with either control plasmid poly-l-lysine adenovirus, dominant-negative Ras N17 poly-l-lysine adenovirus, dominant-negative Rac1 N17 poly-l-lysine adenovirus, dominant-negative Cdc42 N17 poly-l-lysine adenovirus (all at 250 moi each), followed by culture as described in MATERIALS AND METHODS. After 24 h to allow expression of dominant-negative Ras N17, dominant-negative Rac1 N17, and dominant-negative Cdc42 N17, hepatocytes were treated with either media control for 24 h or with 50 mM glucose, 1 ng/μl HGF, or 1 ng/μl TNFα for 24 h (total time in primary culture 48 h). Hepatocytes under all conditions were continually incubated with 2 μCi of [3H]thymidine throughout the final 24 h of culture, after which they were lysed and [3H]thymidine incorporation into DNA was determined as in MATERIALS AND METHODS (n = 6). Under control conditions, HGF stimulated JNK1 activity 3.75 ± 0.25-fold after 8 min, which was completely blocked by expression of dominant-negatives Ras N17, Rac1 N17, and Cdc42 N17.
p < 0.05 decrease compared with corresponding value in control plasmid infected.
p < 0.05 increase compared with media contol.
In contrast to our data showing that inhibition of the JNK/SAP kinase pathway causes an ∼70% reduction in the basal level of hepatocyte DNA synthesis, when we inhibited the MAP kinase pathway by expression of dominant- negative MEK1, basal hepatocyte DNA synthesis was reduced by only 20 ± 3%. Furthermore, expression of dominant-negative MEK1 blunted the abilities of hyperosmotic glucose, TNFα, and HGF to stimulate DNA synthesis by 46 ± 5%, 41 ± 3%, and 65 ± 6%, respectively. These data suggest that signaling by the MAP kinase pathway plays a lesser role in stimulating hepatocyte DNA synthesis than does signaling by the JNK/SAP kinase pathway. The data presented in Tables 1–3 and Figures 1–3 strongly suggest that multiple stimuli increase DNA synthesis in primary cultures of hepatocytes by signaling via the Ras/Rac1/Cdc42/SEK/JNK/c-Jun pathway.
Dominant-Negative Cdc42 N17 Does Not Block the Ability of HGF to Stimulate Rac1 GTP Association
Expression of both dominant-negatives Rac1N17 and Cdc42N17 blocked the ability of HGF to stimulate JNK1 activity and to increase hepatocyte DNA synthesis. To determine whether signaling by HGF to JNK1 progressed via Rac1 to Cdc42 or vice versa, we examined the GTP:GDP ratio of Rac1 after HGF stimulation in the presence or absence of dominant-negative Cdc42N17. Under basal conditions, 11.1 ± 0.7% of Rac1 was in the GTP-associated state, which was stimulated 2 min after HGF treatment to 20.0 ± 1.3%. Expression of dominant-negative Cdc42N17 did not block the ability of HGF to increase the GTP-associated state of Rac1 (19.2 ± 1.2%), and expression of dominant-active Cdc42V12 also did not increase the GTP-associated state of Rac1 (13.5 ± 1.8%). These data suggest that HGF signaling progresses via Rac1 to Cdc42 and then to the JNK/SAP kinase cascade in hepatocytes.
Glucose and HGF Treatments Stimulate c-Jun DNA Binding in Primary Cultures of Hepatocytes Which Are Not Blocked by Dominant-Negative PI3 Kinase Mutants
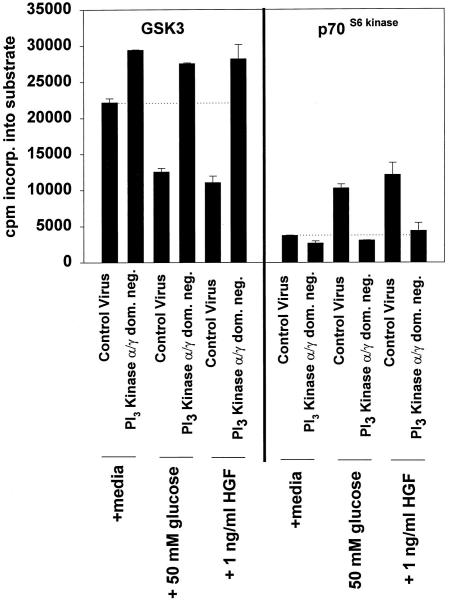
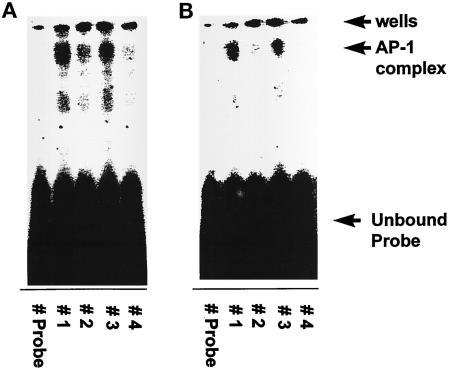
We next tested whether either glucose or HGF treatments of hepatocytes could stimulate c-Jun DNA binding, and whether this binding correlated with the abilities of these agonists to modulate GSK3 activity. Hepatocytes were infected with either control virus or recombinant adenoviruses to express dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ, and were treated 24 h later with either 50 mM glucose or 1 ng/μl HGF (Chung et al., 1992; Ferrari and Thomas, 1994; Franke et al., 1995; Vanhaesebroeck et al., 1997). Glucose and HGF treatments of control-infected hepatocytes activated p70S6 kinase and inhibited GSK3 activities, modulations that were both blocked by coexpression of dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ (Figure 4). Glucose and HGF treatments of control-infected hepatocytes also stimulated c-Jun/AP-1 DNA binding (Figure 5). However, although expression of dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ reduced the basal level c-Jun/AP-1 DNA binding by 30%, it did not block the abilities of either glucose or HGF treatments to stimulate c-Jun/AP-1 binding (Figure 5). These data suggest that inhibition of GSK3 may not be the only mechanism by which agonists can increase c-Jun/AP-1 DNA binding.
Figure 4.
Coexpression of dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ block the ability of hyperosmotic glucose treatment and HGF treatment to activate p70S6 kinase and to inhibit GSK3 activities. Hepatocytes were infected with either control recombinant adenovirus (250 moi) or recombinant adenoviruses to express dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ (125 moi each), followed by culture as described in MATERIALS AND METHODS. After 24 h to allow expression of dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ, hepatocytes were treated for 10 min at 37°C with either media control, 50 mM glucose, or 1 ng/μl HGF. After 10 min, media were aspirated and the cells frozen. Hepatocytes were lysed and subjected to immunoprecipitation, followed by immune complex kinase assays for both GSK3 and p70S6 kinase. Activities shown are expressed as cpm incorporated into substrate versus media control treatment (5000 cpm/pmol) and are the means ± SE of duplicates from four experiments. Media control treatment did not alter the basal activities of protein kinases versus no treatment.
Figure 5.
Hyperosmotic glucose (A) and HGF (B) treatments stimulate DNA binding of AP-1/c-Jun complex, which is not blocked by coexpression of dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ. Hepatocytes were infected with either control recombinant adenovirus (250 moi) or recombinant adenoviruses to express dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ (125 moi each), followed by culture as described in MATERIALS AND METHODS. After 24 h to allow expression of dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ, hepatocytes were treated for 20 min at 37°C with either media control, 50 mM glucose, or 1 ng/μl HGF. After 20 min, media were aspirated and the cells frozen. Hepatocytes were lysed, nuclear extracts prepared, and AP-1 DNA-binding experiments performed as in MATERIALS AND METHODS. Data are representative of four independent experiments. No alteration in c-Jun/AP-1 DNA binding was observed in media control samples. Lane 1, control virus + 50 mM glucose; lane 2, control virus; lane 3, dominant-negative PI3 kinase (p110α and p110γ) + 50 mM glucose; and lane 4, PI3 kinase (p110α and p110γ).
Additional experiments were performed to test whether dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ blocked the abilities of glucose, TNFα, or HGF treatments to stimulate DNA synthesis (Table 4). Coexpression of dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ alone caused a significant reduction in the ability of primary cultures of hepatocytes to synthesize DNA. However, this reduced level could be stimulated ∼250% by 50 mM glucose treatment, 1 ng/μl TNFα treatment, or 1 ng/μl HGF treatment. Thus, coexpression of dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ did not block the ability of multiple agonists to stimulate DNA synthesis in primary cultures of hepatocytes.
Table 4.
Hyperosmotic glucose increases DNA synthesis in primary cultures of rat hepatocytes, which is not blocked by inhibition of the P13 kinase/GSK3 pathway
| Treatment | Control virus | Dominant-negative PI3 kinase (p110α + γ) |
|---|---|---|
| Media control | 4,190 ± 470 | 3,149 ± 430 |
| 50 mM glucose | 14,439 ± 1,245a | 10,263 ± 1,060a |
| 1 ng/μl TNFα | 10,419 ± 640a | 9,128 ± 685a |
| 1 ng/μl HGF | 14,670 ± 910a | 11,636 ± 1,070a |
Hepatocytes were cultured as described in MATERIALS AND METHODS. Hepatocytes were infected with either control recombinant adenovirus (250 moi) or dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ recombinant adenoviruses (125 moi each), followed by culture as described in MATERIALS AND METHODS. After 24 h to allow expression of dominant-negative PI3 kinase p110α and dominant-negative PI3 kinase p110γ, hepatocytes were treated with either media control for 24 h, 50 mM glucose, 1 ng/μl TNFα, or 1 ng/μl HGF for 6 h, followed by incubation with unsupplemented media for 18 h (total time in primary culture 48 h). Hepatocytes under all conditions were continually incubated with 2 μCi of [3H]thymidine throughout the final 24 h of culture, after which they were lysed and {3H]thymidine incorporation into DNA was determined as in MATERIALS AND METHODS (n = 6-12).
p < 0.05 versus media control.
DISCUSSION
These studies were designed to test whether signaling by the JNK/SAP kinase cascade increased or decreased the ability of a nontransformed, nonestablished epithelial cell, the hepatocyte, to synthesize DNA in vitro. Treatments of hepatocytes with hyperosmotic glucose, TNFα, and HGF increased the activity of JNK1. Expression of dominant-negative RasN17, dominant-negative RacN17, dominant-negative Cdc42N17, dominant-negative SEK1, and dominant-negative JNK1 blocked both JNK1 activation and c-Jun serine 63 phosphorylation after agonist treatments. Similarly, increased transactivation of c-Jun was dependent on activation of the JNK/SAP kinase cascade, as judged by the abilities of dominant-negative SEK1 and dominant-negative JNK1 to block glucose-induced luciferase activity from the AP-1-luciferase reporter construct. Glucose, TNFα, and HGF treatments increased DNA synthesis in primary cultures of hepatocytes, which was also blocked by expression of dominant-negative RasN17, dominant-negative RacN17, dominant-negative Cdc42N17, dominant-negative SEK1, dominant-negative JNK1, and dominant-negative c-Jun (TAM67). Thus, molecular inhibition of the JNK/SAP kinase cascade results in a blunting of signals from proximal receptors to the distal transcription factor c-Jun, and these data strongly suggest that multiple agonists stimulate hepatocyte DNA synthesis via a Ras/Rac1/Cdc42/SEK/JNK/c-Jun cascade–dependent mechanism.
In a previous publication, we demonstrated that inhibition of the other known stress-activated protein kinase cascade, the p38-RK cascade, by the drug SB203580 also blunted the ability of primary cultures of hepatocytes to synthesize DNA (Spector et al., 1997). These data, along with the data presented in this article and elsewhere (Westwick et al., 1995; Bogoyevitch et al., 1996; Loyer, et al., 1996; Read et al., 1997; Whitmarsh et al., 1997) strongly suggest that the coordinate actions/activities of both the JNK/SAP kinase and p38-RK cascades play essential roles in the regulation of DNA synthesis in hepatocytes.
In contrast to the similar abilities of hyperosmotic glucose, TNFα, and HGF to activate JNK1, the abilities of these agonists to alter activity of the MAP kinase cascade were disparate. These data suggest that activation of the MAP kinase pathway plays a less important role in stimulating hepatocyte DNA synthesis than does activation of the SAP kinase pathway. This finding also correlates with previously published data demonstrating a reduction in MAP kinase basal activity when hepatocytes are proliferating and showing that an ∼90% inhibition of basal MAP kinase activity caused only a small (∼20%) decrease in DNA synthesis (Spector et al., 1997). More recent data from this laboratory have demonstrated that chronic activation of the MAP kinase cascade in hepatocytes results in the expression of the cyclin-dependent kinase inhibitor proteins p21Cip-1/WAF1 and p16INK4a and reduces DNA synthesis (Tombes et al., 1998). Presumably the balance of activities between the MAP and SAP kinase pathways determines whether a hepatocyte will proliferate or senesce (Xia et al., 1995; Gomez-Lechon et al., 1996; Bogoyevitch et al., 1997).
Hyperosmotic glucose and HGF treatments stimulated the activity of p70S6 kinase and decreased the activity of GSK3. The regulation of p70S6 kinase and GSK3 activities has been suggested to be downstream of signaling by PI3 kinase (Franke et al., 1995; Rahimi et al., 1996), and we found that coexpression of dominant-negative PI3 kinase (p110α and p110γ) inhibited the ability of these agonists to activate p70S6 kinase and to inhibit GSK3. The ability of these agonists to modulate GSK3 activity is of interest because GSK3 has been proposed to be the protein kinase responsible for phosphorylating the DNA-binding domain of c-Jun, resulting in a reduction in the ability of c-Jun to associate with DNA (Boyle et al., 1990). In agreement with this hypothesis, glucose and HGF treatments inhibited GSK3 activity and stimulated c-Jun DNA binding. In further agreement, expression of dominant-negative PI3 kinase (p110α and p110γ) increased basal GSK3 activity and reduced basal c-Jun DNA binding. However, expression of dominant-negative PI3 kinase (p110α and p110γ) did not block the abilities of glucose and HGF treatments to stimulate c-Jun DNA binding. These data suggest that inhibition of GSK3 may not be the only mechanism by which agonists can stimulate c-Jun/AP-1 DNA binding. Of note, however, is that the AP-1 transcription factor complex consists of both homodimers of c-Jun as well as heterodimers of c-Jun associated with several other JNK/SAP kinase and p38-RK effector molecules such as ATF2, JunD, and c-Fos [through Elk1 (Karin, 1995; Kallunki et al., 1996; Columbano et al., 1997)]. Thus, inhibition of GSK3-mediated c-Jun COOH-terminal phosphorylation, and thereby c-Jun/AP-1 DNA binding, may be obscured by effects mediated by these other JNK/SAP kinase– and p38-RK–regulated transcription factors. To definitively prove whether GSK3 mediates both phosphorylation of c-Jun in its COOH-terminal sites and DNA binding in vivo, additional studies will need to be performed to directly examine the stoichiometry of phosphorylation within these sites.
Expression of dominant-negative PI3 kinase (p110α and p110γ) reduced the basal ability of hepatocytes to synthesize DNA, but did not reduce the ability of agonist treatments to stimulate DNA synthesis. These data were surprising, based on the data of Rahimi et al. (1996), who demonstrated that blockade of PI3 kinase function in transformed established epithelial cells abolished the abilities of agonists to stimulate DNA synthesis. It is unclear why blockade of PI3 kinase function abolished stimulation of DNA synthesis in transformed, established epithelial cells but did not block stimulation of DNA synthesis in nontransformed, nonestablished epithelial cells. The most likely explanation for the difference between the data may be due to cellular transformation and the establishment in culture of the cells used by Rahimi et al.(1996).
It was recently demonstrated that a rapid reduction in glucose concentration also leads to a stimulation of JNK1 and p42MAP kinase activities in drug-resistant MCF-7 breast cancer cells (Gupta et al., 1997, Liu et al., 1997). Other studies, including the data presented in this article, have demonstrated that increases in osmolarity can also elevate JNK1/2 activity (Sanchez et al., 1994; Bagrodia et al., 1995; Xia et al., 1995; Rosette and Karin, 1996). This suggests that positive and negative modulations in osmolarity can cause similar changes in activity within the same signal transduction cascade. Our data also demonstrated that the ability of hyperosmotic glucose to activate JNK1 (Table 1) is dependent on signaling downstream of the Ras proto-oncogene. Rosette and Karin (1996) demonstrated that increases in osmolarity cause clustering of plasma membrane receptors in PC12 cells, and it is likely that treatment of primary cultures of hepatocytes with hyperosmotic glucose will do likewise. They proposed that receptor clustering, via increased osmolarity, leads to the activation of signal transduction cascades. The key plasma membrane receptors upstream of the Ras proto-oncogene, by which positive and negative alterations in osmolarity regulate signaling pathways in primary cultures of hepatocytes, are yet to be determined.
ACKNOWLEDGMENTS
This work was funded by start-up money from the Department of Radiation Oncology, Massey Cancer Center, Medical College of Virginia (Richmond, VA); by an institutional grant from the American Cancer Society (IN-105V); and by a fellowship from the V Foundation to P.D. P.D. thanks Dr. Ross Mikkelsen for help with Rac1 GTP-binding experiments, Dr. P. Hylemon for generous assistance with hepatocyte cell cultures, M. Spector for assistance with the c-Jun/AP-1 DNA-binding experiments, and Dr. W.D. Jarvis and Dr. L. Zon for kindly providing the dominant-negative SEK1. P.D. also thanks Dr. C.J. Marshall (Chester Beatty Research Institute, London, United Kingdom) for providing the dominant-negative MEK1 construct. This manuscript is dedicated to the Rev. Alan Farley and the members of the 19th Virginia Infantry who took part in P.D.’s recent wedding.
Footnotes
Abbreviations used: JNK, c-Jun NH2-terminal kinase; MAP, mitogen-activated protein; SAP, stress-activated protein; SEK, stress/extracellular-regulated kinase.
REFERENCES
- Bagrodia S, Derijard B, Davis RJ, Cerione R. Cdc42 and PAK-mediated signaling leads to JNK and p38 MAP kinase activation. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA, Gillespie-Brown J, Ketterman AJ, Fuller SJ, Ben-Levy R, Ashworth A, Marshall CJ, Sugden PH. Stimulation of the stress-activated protein kinase subfamilies in perfused heart. Circ Res. 1996;79:162–173. doi: 10.1161/01.res.79.2.162. [DOI] [PubMed] [Google Scholar]
- Boylan JM, Gruppuso PA. A comparative study of the hepatic mitogen activated protein kinase and JNK pathways in late gestational fetal rat. Cell Growth & Differ. 1996;7:1261–1269. [PubMed] [Google Scholar]
- Boyle WJ, Smeal T, Defize LHK, Angel P, Woodgett JR, Karin M, Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA binding ability. Cell. 1990;64:573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Bruccderi A, Gallucci R, Germolec DR, Blackshear P, Simeonova P, Thurman RG, Luster MI. Induction of early-immediate genes by TNFα contribute to liver repair following chemical induced hepatotoxicity. Hepatology. 1997;25:133–141. doi: 10.1002/hep.510250125. [DOI] [PubMed] [Google Scholar]
- Chartier C, Degryse E, Gantzer M, Dieterle A, Pavirani A, Mehtali M. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J Virol. 1996;70:4805–4810. doi: 10.1128/jvi.70.7.4805-4810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Kuo CJ, Crabtree GR, Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- Clark GJ, Westwick JK, Der CJ. p120 GAP modulates Ras activation of Jun kinases and transformation. J Biol Chem. 1997;272:1677–1681. doi: 10.1074/jbc.272.3.1677. [DOI] [PubMed] [Google Scholar]
- Columbano A, Ledda-Columbano GM, Pibiri M, Piga R, Shinozuka H, De Luca V, Cerignoli F, Tripodi M. Increased expression of c-fos, c-jun and LRF-1 is not required for in vivo priming of hepatocytes by the mitogen TCPOBOP. Oncogene. 1997;14:857–863. doi: 10.1038/sj.onc.1200891. [DOI] [PubMed] [Google Scholar]
- Cristiano RJ, Smith LC, Kay MA, Brinkley BR, Woo SLC. Hepatic gene therapy: efficient gene delivery and expression in primary hepatocytes utilizing a conjugated adenovirus-DNA complex. Proc Natl Acad Sci USA. 1993;90:11548–11552. doi: 10.1073/pnas.90.24.11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DAE, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Deak JC, Templeton DJ. Regulation of the activity of MEK kinase 1 (MEKK1) by autophosphorylation within the kinase activation domain. Biochem J. 1997;322:185–192. doi: 10.1042/bj3220185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent P, Reardon DB, Morrison DK, Sturgill TW. Regulation of Raf-1 and Raf-1 mutants by Ras-dependent and Ras-independent mechanisms in vitro. Mol Cell Biol. 1995;15:4125–4135. doi: 10.1128/mcb.15.8.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet JR, Wood KV, DeLuca M, Helinski DR, Subramani S. Firefly luciferase gene structure and expression in mammalian cells Mol. Cell Biol. 1987;7:725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl AM, Yang SQ, Yin M, Lin HZ, Nelson S, Bagby G. Tumor necrosis factor-α modulates CCAAT/enhancer binding proteins-DNA binding activities and promotes hepatocyte-specific gene expression during liver regeneration. Hepatology. 1995;22:252–261. doi: 10.1016/0270-9139(95)90379-8. [DOI] [PubMed] [Google Scholar]
- Diehl AM, Yin M, Fleckenstein J, Yang SQ, Lin HZ, Brenner D, Westwick J, Bagby G, Nelson S. Tumor necrosis factor α induces c-Jun during the regenerative response to liver injury. Am J Physiol. 1994;267:C552–561. doi: 10.1152/ajpgi.1994.267.4.G552. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Thomas G. S6 phosphorylation and the p70s6k/p85s6k. Crit Rev Biochem Mol Biol. 1994;29:385–413. doi: 10.3109/10409239409083485. [DOI] [PubMed] [Google Scholar]
- Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Gibbs JB. Determination of guanine nucleotides bound to Ras in mammalian cells. Methods Enzymol. 1995;255:118–125. doi: 10.1016/s0076-6879(95)55014-3. [DOI] [PubMed] [Google Scholar]
- Gomez-Lechon MJ, Guillen I, Ponsoda X, Fabra R, Trullenque R, Nakamura T, Castell JV. Cell cycle progression proteins (cyclins), oncogene expression, and signal transduction during the proliferative response of human hepatocytes to hepatocyte growth factor. Hepatology. 1996;23:1012–1019. doi: 10.1002/hep.510230511. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Lee YJ, Galoforo SS, Berns CM, Martinez AA, Corry PM, X-Wu Y, Guan KL. Differential effect of glucose deprivation on MAPK activation in drug sensitive human breast carcinoma MCF-7 and multidrug resistant MCF-7/ADR cells. Mol Cell Biochem. 1997;170:23–30. doi: 10.1023/a:1006890316102. [DOI] [PubMed] [Google Scholar]
- Jarvis WD, Fornari FA, Browning JL, Gewirtz DA, Kolesnik RN, Grant S. Attenuation of ceramide induced apoptosis by diglyceride in human myeloid leukemia cells. J Biol Chem. 1994;269:31685–31692. [PubMed] [Google Scholar]
- Jarvis DA, Fornari FA, Traylor RS, Martin HA, Kramer LB, Erukulla RK, Bittman R, Grant S. Induction of apoptosis and potentiation of ceramide mediated cytotoxicity by sphingoid bases in human myeloid leukemia cells. J Biol Chem. 1996;271:8275–8284. doi: 10.1074/jbc.271.14.8275. [DOI] [PubMed] [Google Scholar]
- Jarvis WD, Auer KL, Spector M, Kunos G, Hylemon P, Grant S, Dent P. Positive and negative regulation of p38 SAP kinase and p46/54 SAP kinase by protein kinase C. FEBS Lett. 1997a;412:9–14. doi: 10.1016/s0014-5793(97)00705-9. [DOI] [PubMed] [Google Scholar]
- Jarvis WD, Fornari FA, Freemerman AJ, Szabo E, Martin HA, Rao AS, Birrer MJ, Barbour SE, Dent P, Grant S. Differential requirement for c-Jun in the apoptotic actions of ceramide and sphingosine in human myeloid leukemia cells. Mol Pharmacol. 1997b;52:935–947. doi: 10.1124/mol.52.6.935. [DOI] [PubMed] [Google Scholar]
- Kallunki T, Deng T, Hibi M, Karin M. c-Jun can recruit JNK to phosphorylate dimerization partners via specific docking interactions. Cell. 1996;87:929–939. doi: 10.1016/s0092-8674(00)81999-6. [DOI] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Kunos G, Ishac EJN, Gao B, Jiang L. Inverse regulation of hepatic α1B- and β2-adrenergic receptors. Ann NY Acad Sci. 1995;757:261–270. doi: 10.1111/j.1749-6632.1995.tb17483.x. [DOI] [PubMed] [Google Scholar]
- Liu M-L, Mars WM, Zarengar R, Michalopoulos GK. Collagenase pre-treatment and mitogenic effects of hepatocyte growth factor and transforming growth factor-alpha in adult rat liver. Hepatology. 1994;19:1521–1527. [PubMed] [Google Scholar]
- Liu X, Gupta AK, Corry PM, Lee YJ. Hypoglycemia-induced c-Jun phosphorylation is mediated by c-Jun N-terminal kinase 1 and Lyn kinase in drug-resistant human breast carcinoma MCF-7/ADR cells. J Biol Chem. 1997;272:11690–11693. doi: 10.1074/jbc.272.18.11690. [DOI] [PubMed] [Google Scholar]
- Loyer P, Cariou S, Glaise D, Bilodeau M, Baffet G, Gugen-Guillouzo C. Growth factor dependence of progression through G1 and S phases of adult rat hepatocytes in vitro. J Biol Chem. 1996;271:11484–11492. doi: 10.1074/jbc.271.19.11484. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Nazareth LV, Weigel NL. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem. 1996;271:19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- Rahimi N, Tremblay E, Elliott B. Phosphatidyinositol 3-kinase activity is required for hepatocyte growth factor induced mitogenic signals in epithelial cells. J Biol Chem. 1996;271:24850–24855. doi: 10.1074/jbc.271.40.24850. [DOI] [PubMed] [Google Scholar]
- Rai RM, Yang SQ, McClain C, Karp CL, Klein AS, Diehl AM. Kupffer cell depletion by gadolinim chloride enhances liver regeneration after PHX in rats. Am J Physiol. 1994;270:909–918. doi: 10.1152/ajpgi.1996.270.6.G909. [DOI] [PubMed] [Google Scholar]
- Read MA, Whitley MZ, Gupta S, Pierce JW, Best J, Davis RJ, Collins T. Tumor necrosis factor α-induced E-selectin expression is activated by the nuclear factor-kB and c-Jun N-terminal kinase/p38 mitogen activated protein kinase pathways. J Biol Chem. 1997;272:2753–2761. doi: 10.1074/jbc.272.5.2753. [DOI] [PubMed] [Google Scholar]
- Rodrigues GA, Park M, Schlessinger J. Activation of the JNK pathway is essential for transformation by the Met oncogene. EMBO J. 1997;16:2634–2645. doi: 10.1093/emboj/16.10.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- Sanchez I, Hughes RT, Mayer BJ, Yee K, Woodgett JR, Avruch J, Kyriakis JM, Zon L. Role of SAPK/ERK kinase-1 in the stress activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman EH, Fuks Z, Kolesnick R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- Smeal T, Binetruy B, Mercola D, Birrer M, Karin M. Phosphorylation of c-Jun on serines 63 and 73 is required for oncogenic and transcriptional cooperation with Ha-Ras. Nature. 1991;354:494–496. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- Spector M, Auer KL, Jarvis WD, Ishac E, Gao B, Kunos G, Dent P. Differential regulation of the MAP and SAP kinases in quiescent and regenerating adult rat hepatocytes. Mol Cell Biol. 1997;17:3556–3565. doi: 10.1128/mcb.17.7.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stravitz RT, Rao YP, Vlahcevic R, Gurley EC, Jarvis WD, Hylemon PB. Hepatocellular protein kinase C activation by bile acids: implications for regulation of cholesterol 7a-hydroxylase. Am J Physiol. 1996;271:293–303. doi: 10.1152/ajpgi.1996.271.2.G293. [DOI] [PubMed] [Google Scholar]
- Valerie K, Singhal A. Host-cell reactivation of reporter genes introduced into cells by adenovirus as a convenient way to measure cellular DNA repair. Mutat Res. 1995;336:91–100. doi: 10.1016/0921-8777(94)00046-9. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- Verheij M, et al. Requirement for ceramide-initiated SAPK/JNK signalling in stress induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- Welsh GI, Foulstone EJ, Young SJ, Tavare JM, Proud CG. Wortmannin inhibits the effects of insulin and serum on the activities of glycogen synthase kinase 3 and the Mitogen activated protein kinase. Biochem J. 1994;303:15–20. doi: 10.1042/bj3030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwick JK, Fleckenstein J, Yin M, Yang SQ, Bradham CA, Brenner DA, Diehl AM. Differential regulation of hepatocyte DNA synthesis by cAMP in vitro and in vivo. Am J Physiol. 1996;271:780–790. doi: 10.1152/ajpgi.1996.271.5.G780. [DOI] [PubMed] [Google Scholar]
- Westwick JK, Weitzel C, Letfert LH, Brenner DA. Activation of Jun kinase is an early event in hepatic regeneration. J Clin Invest. 1995;95:803–810. doi: 10.1172/JCI117730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh AJ, S-Yang S, Su M, Sharrocks AD, Davis RJ. Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol Cell Biol. 1997;17:2360–2371. doi: 10.1128/mcb.17.5.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NG, Roberts TM. Signal transduction pathways involving the Raf proto-oncogene. Cancer Metastasis Rev. 1994;13:105–116. doi: 10.1007/BF00690421. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yan M, Dai T, Deak JC, Kyriakis JM, Zon LI, Woodgett JR, Templeton DJ. Activation of stress-activated protein kinase SEK1 by phosphorylation of its activator MEKK1. Nature. 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]