Abstract
Completion of mitotic exit and cytokinesis requires the inactivation of mitotic cyclin-dependent kinase (Cdk) activity. A key enzyme that counteracts Cdk during budding yeast mitotic exit is the Cdc14 phosphatase. Cdc14 is inactive for much of the cell cycle, sequestered by its inhibitor Net1 in the nucleolus. At anaphase onset, separase-dependent down-regulation of PP2ACdc55 allows phosphorylation of Net1 and consequent Cdc14 release. How separase causes PP2ACdc55 down-regulation is not known. Here, we show that two Cdc55-interacting proteins, Zds1 and Zds2, contribute to timely Cdc14 activation during mitotic exit. Zds1 and Zds2 are required downstream of separase to facilitate nucleolar Cdc14 release. Ectopic Zds1 expression in turn is sufficient to down-regulate PP2ACdc55 and promote Net1 phosphorylation. These findings identify Zds1 and Zds2 as new components of the mitotic exit machinery, involved in activation of the Cdc14 phosphatase at anaphase onset. Our results suggest that these proteins may act as separase-regulated PP2ACdc55 inhibitors.
Introduction
Complexes between cyclins and cyclin-dependent kinases (Cdk) govern the progression through the cell division cycle in all eukaryotes. Entry into mitosis occurs when cyclin B/Cdk (Clb2/Cdk in budding yeast) reaches a peak of kinase activity. This leads to formation of the mitotic spindle, condensation of the chromosomes, and their alignment on the spindle. Successful bipolar attachment of the chromosomes releases an inhibitory mitotic checkpoint signal, allowing the activation of the anaphase-promoting complex (APC) by its coactivator Cdc20 (APCCdc20; for review see Morgan, 2007; Peters, 2006). APCCdc20 is a ubiquitin ligase that ubiquitinates securin, an inhibitor of the protease separase. Ubiquitinated securin is targeted for degradation by the proteasome, thus freeing separase. Sister chromatid separation, and thereby anaphase onset, is now triggered when separase cleaves the Scc1 subunit of the chromosomal cohesin complex (Uhlmann et al., 2000). At this time, APCCdc20 also targets mitotic cyclins for degradation to down-regulate mitotic Cdk activity. However, APCCdc20 is not sufficient for complete cyclin destruction and Cdk inactivation. In addition, activation of the mitotic phosphatase Cdc14 is essential to promote exit from mitosis (for review see Stegmeier and Amon, 2004). Cdc14 contributes to the down-regulation of Cdk activity by dephosphorylating and thereby activating a second coactivator of the APC, Cdh1, which completes the destruction of mitotic cyclins, as well as the Cdk inhibitor Sic1. Cdc14 furthermore directly counteracts Cdk activity by dephosphorylating mitotic Cdk targets (Visintin et al., 1998).
For much of the cell cycle, Cdc14 is kept inactive in the nucleolus by binding to its nucleolar inhibitor Net1 (also called Cfi1; Shou et al., 1999; Visintin et al., 1999). During anaphase, mitotic kinase-dependent phosphorylation of Net1 releases active Cdc14. Phosphorylated Net1 shows reduced affinity for, and looses its ability to inhibit Cdc14 (Shou et al., 2002; Yoshida and Toh-e, 2002; Azzam et al., 2004). Two complementary regulatory pathways are essential for Cdc14 release from the nucleolus. During early anaphase, separase initiates Net1 phosphorylation and Cdc14 release in conjunction with a series of proteins, Slk19, Spo12, and Fob1. The action of these proteins has been summarized as the Cdc fourteen early anaphase release (FEAR) pathway (Stegmeier et al., 2002; Sullivan and Uhlmann, 2003; Azzam et al., 2004; Queralt et al., 2006). Initial Cdc14 release is promoted by Cdk-dependent Net1 phosphorylation (Azzam et al., 2004), which is counteracted in metaphase by the type 2A protein phosphatase (PP2A) in conjunction with its Cdc55 activatory subunit (PP2ACdc55; Queralt et al., 2006; Yellman and Burke, 2006). Separase-dependent PP2ACdc55 down-regulation at anaphase onset allows Net1 phosphorylation and Cdc14 release. PP2ACdc55 down-regulation, declining Cdk activity, and released Cdc14 now activate a G protein–coupled kinase signaling cascade, the mitotic exit network (MEN; Jaspersen et al., 1998; Lee et al., 2001). The MEN maintains Cdc14 active during the later stages of anaphase when mitotic Cdk activity declines (Jaspersen and Morgan, 2000; Stegmeier et al., 2002; Queralt et al., 2006). Kinases of the MEN, including Polo kinase, may act to sustain Net1 phosphorylation at this time (Lee et al., 2001; Shou et al., 2002; Yoshida and Toh-e, 2002; Visintin et al., 2008).
PP2A is a family of widely conserved protein serine/threonine phosphatases, with roles in a multitude of cellular processes (Stark, 1996). They function as a trimeric complex, a catalytic subunit (in budding yeast one of the two closely related proteins Pph21 or Pph22), a scaffold subunit (Tpd3 in budding yeast), and a regulatory subunit (one of the three Rts1, Rts3, or Cdc55). With PP2ACdc55, we refer to a complex consisting of Pph21 or Pph22, Tpd3, and Cdc55. The mechanistic basis for separase-dependent PP2ACdc55 down-regulation at anaphase onset is not known. Recently Zds1 and Zds2, two sequence paralogues in budding yeast, have been identified as putative interactors of Cdc55 by large-scale affinity purification analyses (Gavin et al., 2002; Krogan et al., 2006). Zds1 and Zds2 (zillion different screens) have also been isolated in numerous genetic screens, relating to regulators of the Cdc42 GTPase (Bi and Pringle, 1996), the RNA polymerase II mediator complex (Yu et al., 1996), sister chromatid cohesion (Heo et al., 1999), chromatin silencing (Roy and Runge, 2000), cell wall integrity signaling (Griffioen et al., 2003), cell polarity (Zanelli and Valentini, 2005), and other cellular processes. Contributions of Zds1 and Zds2 to cell cycle progression have been noted (Bi and Pringle, 1996; Ma et al., 1996; Yu et al., 1996; Mizunuma et al., 1998). Both Zds1 and separase have been found to act as high copy suppressors of the cdc28-1N mutation, suggesting a possible functional link between the two proteins, although the mechanism by which either Zds1 or separase act in cdc28-1N suppression has not yet been elucidated (Yu et al., 1996; Jones et al., 2000). The molecular function of Zds1 and Zds2 thus remains poorly understood. No sequence motifs have been detected in their primary amino acid sequence that could yield hints as to their activity. Orthologues of Zds1 and Zds2 can be found in most ascomycetes, with the strongest similarity between them confined to a C-terminal “Zds1 C” motif.
Here, we confirm a physical interaction of Zds1 and Zds2 with PP2ACdc55, and show that Zds1 and Zds2 are required for timely nucleolar release of Cdc14 in early anaphase. Separase-induced Cdc14 activation requires Zds1 and Zds2, and ectopic Zds1 expression in metaphase down-regulates PP2ACdc55 and releases Cdc14 in a Net1 phosphorylation-dependent reaction. This implies that Zds1 and Zds2 act downstream of separase to initiate Cdc14 activation at anaphase onset, and suggests that Zds1 and Zds2 may exert their biological function as PP2A regulators.
Results
Zds1 and Zds2 interact with PP2ACdc55
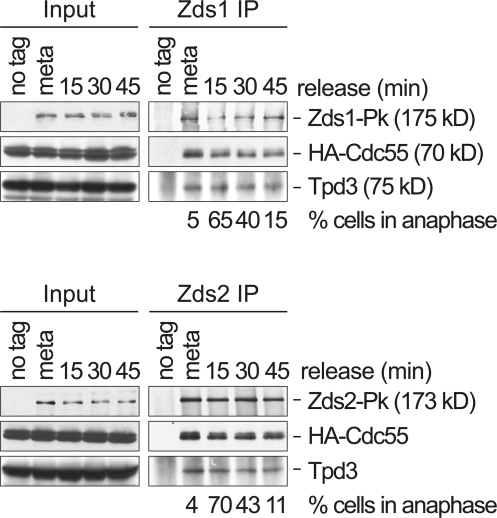
We have previously shown that separase activation at anaphase onset causes down-regulation of the PP2ACdc55 phosphatase to facilitate Net1 phosphorylation and Cdc14 release. Separase and PP2ACdc55 physically interact (Queralt et al., 2006). However, PP2ACdc55 is present in cells in stoichiometric excess over separase (Ghaemmaghami et al., 2003), making a mechanism of direct PP2ACdc55 inhibition by separase binding unlikely. This suggests that additional factors are involved downstream of separase in PP2ACdc55 regulation. High throughput affinity purification of budding yeast protein complexes has detected interactions between the subunits of the PP2ACdc55 complex and both Zds1 and Zds2 (Gavin et al., 2002; Krogan et al., 2006). This prompted us to confirm a possible interaction between Zds1, Zds2, and PP2ACdc55, and ask whether the interaction occurs in a cell cycle–regulated manner. We arrested cells in metaphase by depletion of the APC coactivator Cdc20 under the control of the galactose-inducible GAL1 promoter, and released them into synchronous anaphase progression by reintroduction of Cdc20. In immunoprecipitates of Zds1 from metaphase-arrested cells, we detected Cdc55 as well as the PP2A scaffold subunit, Tpd3 by Western blotting (Fig. 1). This suggests that Zds1 and PP2ACdc55 interact at this stage in the cell cycle. We were unable to analyze coprecipitation of the catalytic subunit Pph21/Pph22, as epitope tags at either terminus of these proteins interfere with PP2ACdc55 function (unpublished data). Tpd3 is the limiting subunit for PP2A holocomplex assembly, and Cdc55 is thought to associate with a preassembled Tpd3-Pph21/Pph22 dimer (Hombauer et al., 2007). It is therefore likely that also Pph21 or Pph22 subunits are present in the Zds1-PP2ACdc55 complex.
Figure 1.
Zds1 and Zds2 interact with PP2ACdc55. Cell extracts were prepared from strains Y2541 (MATa GAL-CDC20 HA3-CDC55), Y3131 (as Y2541, but also ZDS1-Pk9), and Y3200 (as Y2541, but also ZDS2-Pk9), which were synchronized at the metaphase to anaphase transition, and coimmunoprecipitation of subunits of the PP2ACdc55 complex with Zds1 and Zds2 was analyzed by Western blotting. The apparent molecular weights of the subunits, relative to marker proteins during gel electrophoresis, are indicated. Anaphase progression was monitored by tubulin staining.
Zds1 immunoprecipitates from cells released into synchronous anaphase contained similar amounts of Cdc55 and Tpd3 compared with immunoprecipitates in metaphase (Fig. 1). This indicates that the interaction between Zds1 and PP2ACdc55 is constitutive throughout progression through mitosis and mitotic exit. We also analyzed the interaction between Zds2 and PP2ACdc55 in similar immunoprecipitation experiments. Zds1 and Zds2 share 37.5% sequence identity, concentrated in 6 regions of homology (Bi and Pringle, 1996). The two paralogues show largely similar, but not identical, behavior in the reported genetic interactions (Roy and Runge, 2000). Again, coimmunoprecipitation demonstrated interaction of Zds2 with Cdc55 and Tpd3 in metaphase as well as during synchronous progression through anaphase and mitotic exit (Fig. 1). This suggests that both Zds1 and Zds2 interact with PP2ACdc55 throughout mitosis.
Zds1 and Zds2 are required for Cdc14 nucleolar release in early anaphase
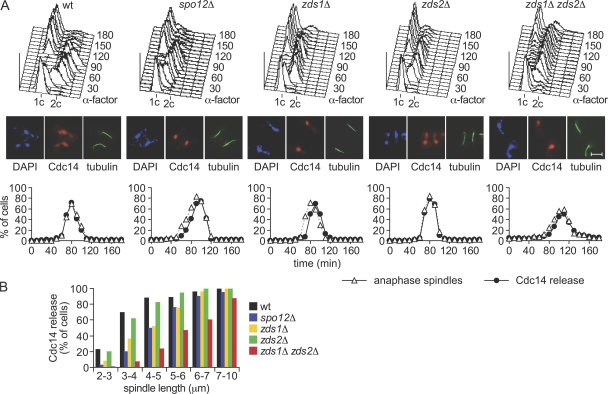
The interaction with Zds1 and Zds2 in mitosis opened the possibility that these proteins are involved in down-regulation of PP2ACdc55 phosphatase activity and nucleolar release of Cdc14. To test this possibility, we constructed single and double deletion mutants of the ZDS1 and ZDS2 genes and analyzed cell cycle progression and Cdc14 release after synchronous release of these strain from α-factor block in G1. For comparison, we included a spo12Δ strain, in which Cdc14 nucleolar release is delayed due to the Spo12 requirement for Cdc14 early anaphase release (Stegmeier et al., 2002; Fig. 2 A). zds2Δ cells showed kinetics of cell cycle progression, and Cdc14 release at the time of anaphase onset, indistinguishable from the wild-type control. In contrast, in zds1Δ cells, Cdc14 release from the nucleolus was retarded by 10 min relative to the onset of spindle elongation, similar to the delay observed in spo12Δ cells. In zds1Δ zds2Δ double-mutant cells, Cdc14 release was also delayed relative to anaphase onset. The difference in timing was more difficult to establish, as zds1Δ zds2Δ cells entered anaphase with a delay, and less synchronously, compared with the control.
Figure 2.
Delayed Cdc14 nucleolar release in the absence of Zds1 and Zds2. (A) Analysis of mitotic progression in the absence of Zds1 and Zds2. Strains Y2786 (MATa CDC14-Pk9), Y1016 (as Y2786, but spo12Δ), Y3013 (as Y2786, but zds1Δ), Y3036 (as Y2786, but zds2Δ), and Y3117 (as Y2786, but zds1Δ zds2Δ) were arrested with α-factor and released into a synchronous cell cycle. FACS analysis of DNA content was used to monitor cell cycle progression. Cdc14 nucleolar release and anaphase spindle elongation was analyzed by in situ immunofluorescence. At least 100 cells were scored at each time point. Photographs are shown of cells in early anaphase with a spindle length of 3–5 μm. Bar, 5 μm. (B) Cdc14 nucleolar release was analyzed in samples of the experiment in A as a function of spindle length.
To analyze the delay in Cdc14 activation relative to an internal marker for mitotic progression, we analyzed Cdc14 nucleolar release with respect to the anaphase spindle length. Cdc14 was half-maximally released in zds2Δ cells at a spindle length of 3 μm, similar to wild-type cells (Fig. 2 B). In contrast, zds1Δ cells released Cdc14 only when the spindle reached lengths of 4–5 μm, similar to what is observed in spo12Δ cells. More strikingly, most zds1Δ zds2Δ cells released Cdc14 only at spindle lengths of 6–7 μm and longer. These results indicate that Cdc14 activation is delayed in zds1Δ and further delayed in zds1Δ zds2Δ double-mutant cells. Thus, both Zds1 and Zds2 participate in timely Cdc14 activation in early anaphase. The contribution of Zds1 appeared to be stronger than that of Zds2, as zds1Δ but not zds2Δ single-mutant cells showed a discernible delay of Cdc14 release.
Zds1 and Zds2 promote Net1 phosphorylation
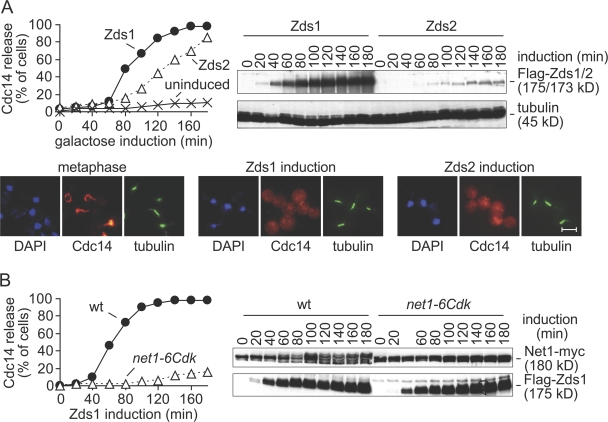
Ectopic expression of separase in metaphase is sufficient to cause Cdc14 nucleolar release (Sullivan and Uhlmann, 2003). We therefore also asked whether ectopic expression of Zds1 or Zds2 is sufficient to activate Cdc14. We arrested cells in metaphase by depletion of Cdc20, and induced ectopic Zds1 or Zds2 expression under control of the galactose-inducible GAL1 promoter (Fig. 3 A). Both Zds1 and Zds2 induction led to Cdc14 nucleolar release. Even though similar expression constructs were used for both paralogues, Zds1 accumulated more rapidly and to higher levels after induction. We do not know the reason for this difference. Correlating with its faster accumulation, Cdc14 release occurred earlier after induction of Zds1 expression, compared with Zds2 expression. At later time points, Cdc14 was efficiently released also in cells expressing Zds2. Cells expressing Zds1 or Zds2 remained arrested in mitosis despite the activation of Cdc14. This can be explained by the observation that Cdc14 activation is not sufficient to promote mitotic exit if not Cdk activity is down-regulated at the same time (Queralt et al., 2006). We conclude that ectopic expression of either Zds1 or Zds2 in metaphase-arrested cells is sufficient to cause nucleolar release of Cdc14. Because of its greater efficiency in this setting, we will use expression of Zds1 in most of the following experiments.
Figure 3.
Zds1 promotes Cdc14 nucleolar release by Net1 phosphorylation. (A) Ectopic expression of Zds1 and Zds2 promote Cdc14 nucleolar release. Strains Y3288 (MATa MET-CDC20 GAL-Flag-ZDS1 CDC14-Pk9) and Y2220 (MATa MET-CDC20 GAL-Flag-ZDS2 CDC14-Pk9) were arrested in metaphase (meta) and Zds1 or Zds2 expression induced. Part of the culture of strain Y3288 was maintained without Zds1 induction during the course of the experiment. Cdc14 nucleolar release was monitored by immunofluorescence, and Zds1 and Zds2 expression levels were analyzed by Western blotting. Tubulin served as a loading control. Photographs are shown of cells in metaphase and 120 min after Zds1 or Zds2 induction. Bar, 5 μm. (B) Cdk phosphorylation sites are required for Zds1-induced Net1 phosphorylation and Cdc14 release. Strains Y1008 (MATa MET-CDC20 GAL-Flag-ZDS1 CDC14-Pk9 NET1-myc9) and Y1013 (as Y1008, but net1-6Cdk-myc9) were arrested in metaphase by Cdc20 depletion and Zds1 expression was induced. Net1 phosphorylation was monitored by Western blotting against the myc epitope. Apparent relative molecular weights are indicated.
Cdc14 activation during early anaphase depends on Cdk-dependent phosphorylation of the Cdc14 inhibitor Net1 (Azzam et al., 2004). To analyze whether Cdc14 release by ectopic Zds1 expression involved Net1 phosphorylation, we analyzed the phosphorylation status of Net1 during the induction timecourse. At the time of Zds1 accumulation, slower migrating forms of Net1 became detectable by Western blotting, indicative of phosphorylation. To test whether indeed Net1 phosphorylation was responsible for Cdc14 release, we repeated the experiment in a strain carrying the net1-6Cdk allele (Azzam et al., 2004). In this strain, 6 Cdk consensus recognition sites within the regulatory Net1 N terminus have been made nonphosphorylatable by site-directed mutagenesis. After Zds1 expression in this strain, a Net1 mobility shift was no longer observed, and Cdc14 remained sequestered in the nucleolus (Fig. 3 B). This suggests that Net1 phosphorylation is required for Zds1-induced Cdc14 release.
The dependence of Zds1-induced Cdc14 release on 6 Cdk consensus sites in Net1 suggests that Zds1 expression facilitates Cdk-dependent Net1 phosphorylation. To directly address whether Cdk activity is required for Zds1-induced Cdc14 release, as opposed to another kinase that might recognize the Net1 Cdk consensus sites, we used cells carrying the ATP analogue (1NM-PP1)–sensitive Cdk allele cdc28-as1 (Bishop et al., 2000). We again arrested cells in metaphase by Cdc20 depletion, then 1NM-PP1 was added to inhibit Cdk activity, and Zds1 expression was induced. Cells containing wild-type Cdc28, and cdc28-as1 cells in the absence of inhibitor, released Cdc14 from the nucleolus as expected. Inhibition of Cdc28-as1 by 1NM-PP1 blocked Net1 phosphorylation and Cdc14 release (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200801054/DC1). This suggests that Zds1 expression causes Cdc14 release by promoting Cdk-dependent Net1 phosphorylation.
Cdc14 activation by Zds1 is independent of MEN and PAK kinases
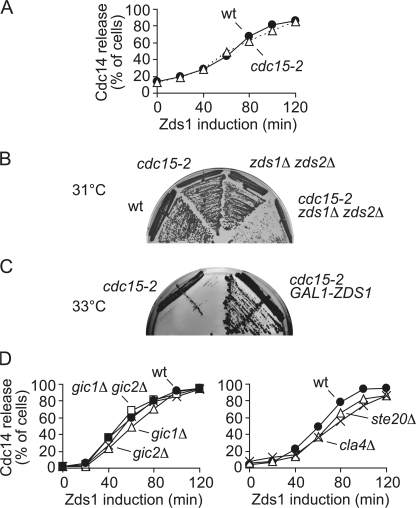
To further address the mechanism of Zds1-induced Net1 phosphorylation and Cdc14 release, we analyzed the requirement of known components of the mitotic exit machinery. The MEN is essential to maintain Cdc14 activity during mitotic exit. We therefore tested whether Zds1 is still able to cause Cdc14 nucleolar release in the absence of MEN function. We expressed Zds1 in metaphase-arrested cells carrying a temperature-sensitive allele, cdc15-2, of the essential MEN kinase Cdc15. After ectopic Zds1 expression at the restrictive temperature, Cdc14 was released from the nucleolus in a manner indistinguishable between wild-type and cdc15-2 mutant cells (Fig. 4 A). This suggests that Cdc14 activation by Zds1 is independent of MEN, and therefore that Zds1 acts in parallel or downstream of the MEN pathway.
Figure 4.
Zds1 and the MEN. (A) The essential MEN kinase Cdc15 is not required for Zds1-induced Cdc14 release. Strains Y3288 (MATa MET-CDC20 GAL-Flag-ZDS1 CDC14-Pk9) and Y1429 (as Y3288, but cdc15-2) were arrested in metaphase by Cdc20 depletion and shifted to 37°C for 60 min before Zds1 induction. (B) cdc15-2 and zds1Δ zds2Δ mutations display a synthetic growth defect. Strains Y699 (W303), Y3196 (MATa cdc15-2), Y3117 (MATa zds1Δ zds2Δ), and Y3428 (as Y3117, but cdc15-2) were streaked onto YPD plates and incubated at 31°C for 2 d. (C) Zds1 overexpression rescues growth of cdc15-2 cells at restrictive temperature. Strains Y3196 and Y3191 (MATa cdc15-2 GAL1-ZDS1) were streaked onto YP plates containing raffinose and galactose, to induce Zds1 expression, at 33°C for 2 d. (D) Cdc42 effectors and PAK-kinases are not required for Zds1-induced Cdc14 release. Strains Y3288 (MATa MET-CDC20 GAL-Flag-ZDS1 CDC14-Pk9), Y1409 (as Y3288, but ste20Δ), Y1410 (as Y3288, but cla4Δ), Y1412 (as Y3288, but gic1Δ), Y1411 (as Y3288, but gic2Δ), and Y1414 (as Y3288, but gic1Δ gic2Δ) were arrested in metaphase by Cdc20 depletion and Zds1 expression was induced. Cdc14 nucleolar release was monitored by immunofluorescence. Equal Zds1 expression levels were confirmed by Western blotting (not depicted).
To further dissect the genetic relationship between Zds1, Zds2, and MEN, we analyzed interactions between these factors in a synthetic growth defect assay. cdc15-2 mutant cells grow well at temperatures up to 31°C (Fig. 4 B). Introduction of zds1Δ or zds2Δ individually into the cdc15-2 background did not change this growth pattern (unpublished data). However, cdc15-2 cells deleted for both paralogues (cdc15-2 zds1Δ zds2Δ) showed a synthetic growth defect at 31°C. This suggests that Zds1 and Zds2 are unlikely to be downstream effectors of MEN, and are likely to act in a parallel pathway. Consistent with this possibility, GAL1-promoter driven overexpression of Zds1 rescued the growth of cdc15-2 cells at their restrictive temperature of 33°C (Fig. 4 C).
A large-scale two-hybrid screen of cell polarity proteins has reported an interaction of Zds1 and Zds2 with two Cdc42 GTPase effector proteins, Gic1 and Gic2 (Drees et al., 2001). Gic1 and Gic2, as well as two additional Cdc42 effectors, the p21-activated kinase (PAK) kinases Cla4 and Ste20, have been shown to take part in mitotic exit regulation (Höfken and Schiebel, 2002, 2004; Chiroli et al., 2003; Seshan and Amon, 2005). The mechanism by which these Cdc42 effectors promote mitotic exit is not yet understood. The reported genetic interactions with components of the mitotic exit machinery are consistent with the possibility that these proteins act in parallel to MEN, and therefore possibly in conjunction with Zds1 and Zds2. We analyzed whether Gic1, Gic2, Cla4, or Ste20 were involved in Zds1-induced Cdc14 activation. We constructed gic1Δ, gic2Δ, gic1Δ gic2Δ, ste20Δ, and cla4Δ strains that we arrested in metaphase by Cdc20 depletion. Then, ectopic Zds1 expression was induced. Cdc14 nucleolar release occurred with similar kinetics in all these mutant strains as compared with the wild-type control (Fig. 4 D). This suggests that Zds1 promotes Cdc14 release independently of the Cdc42 effectors Gic1 and Gic2 and the PAK kinases Ste20 and Cla4. These observations leave open the possibility that Zds1, or Zds2, act downstream of Gic1, Gic2, Ste20, or Cla4 to mediate their effect on mitotic exit.
Zds1 is required for separase-induced Cdc14 activation
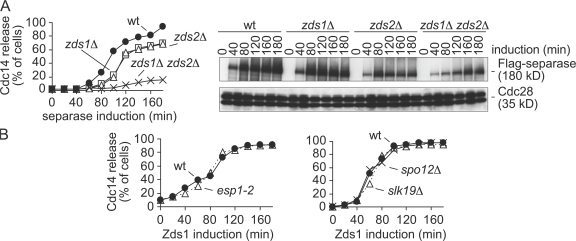
The findings that Zds1 and Zds2 are required for Cdc14 nucleolar release in early anaphase, and that they act independently of MEN, prompted us to further analyze the relationship of these proteins with separase, which is essential for Cdc14 activation at this time. Cdc14 nucleolar release triggered by ectopic separase expression depends on Slk19 and Spo12 (Sullivan and Uhlmann, 2003), and we wondered whether it also depended on Zds1 or Zds2. To analyze this, we used the microtubule poison nocodazole to arrest wild-type, zds1Δ, zds2Δ, and zds1Δ zds2Δ, cells in metaphase and induced ectopic separase expression under control of the GAL1 promoter by galactose addition to the growth medium. Separase accumulated in all strains, but zds1Δ and zds2Δ cells released Cdc14 from the nucleolus with a delay compared with wild-type cells (Fig. 5 A). Cdc14 nucleolar release was almost completely abolished in zds1Δ zds2Δ cells. This indicates that Zds1 and Zds2 are required for separase-induced Cdc14 activation.
Figure 5.
Zds1 is required for separase-induced Cdc14 early anaphase release. (A) Zds1 and Zds2 are required for separase-induced Cdc14 release. Strains Y1748 (MATa GAL-ESP1-HA CDC14-Pk9), Y1790 (as Y1748, but zds1Δ), Y1959 (as Y1748, but zds2Δ), and Y2189 (as Y1790, but zds1Δ zds2Δ) were arrested in metaphase by nocodazole treatment and separase expression induced. Separase levels were analyzed by Western blotting, using Cdc28 as loading control. (B) Separase, Slk19, and Spo12 are not required for Zds1-induced Cdc14 release. Strains Y3288 (MATa MET-CDC20 GAL-Flag-ZDS1 CDC14-Pk9) and Y1430 (as Y3288, but esp1-2) were arrested in metaphase by Cdc20 depletion at 23°C and shifted to 37°C for 60 min before Zds1 induction. Strains Y3288 (MATa MET-CDC20 GAL-Flag-ZDS1 CDC14-Pk9), Y1521 (as Y3288, but slk19Δ), and Y1646 (as Y3288, but spo12Δ) were arrested in metaphase by Cdc20 depletion at 23°C and Zds1 expression was induced.
We next investigated whether reciprocally also Zds1 depended on separase in its ability to activate Cdc14. Zds1 expression in metaphase-arrested wild-type or separase mutant esp1-2 cells led to Cdc14 nucleolar release with indistinguishable kinetics (Fig. 5 B). This suggests that although separase depends on Zds1 in its ability to promote Cdc14 activation, Zds1 can act independently of separase. Cdc55 levels are reduced in esp1-2 cells (Queralt et al., 2006), and we cannot exclude that this could have facilitated Zds1-induced Cdc14 activation and masked a possible separase requirement. Together, these findings are consistent with a role of Zds1, and probably Zds2, in mitotic exit in concert with or downstream of separase.
Separase-dependent Cdc14 nucleolar release in early anaphase requires the two proteins Slk19 and Spo12, but their mechanism of action is poorly understood. We therefore analyzed a possible dependence of Zds1 on Slk19 or Spo12 in its ability to induce Cdc14 nucleolar release. After Zds1 expression in metaphase-arrested wild-type, slk19Δ, and spo12Δ cells, Cdc14 was released from the nucleolus with similar kinetics in all strains (Fig. 5 B). This shows that Zds1 can promote Cdc14 release independently of Slk19 and Spo12. Therefore, Slk19 and Spo12 are likely to either function as mediators between separase and Zds1, or act in a pathway that promotes Cdc14 release in parallel to separase.
Zds1 regulates PP2ACdc55 activity
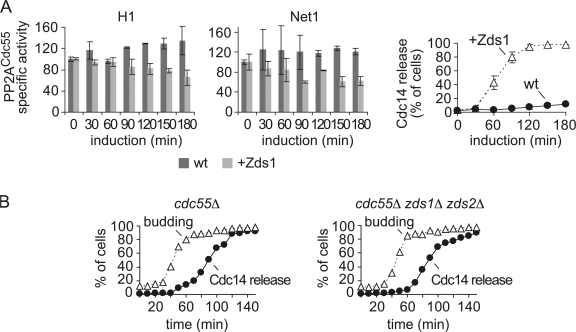
The above results suggest that Zds1 and Zds2 act together with separase to promote Cdc14 nucleolar release in early anaphase. Furthermore, ectopic Zds1 expression induces Cdk-dependent Net1 phosphorylation. Together with the physical interaction of Zds1 and Zds2 with PP2ACdc55, this opens the possibility that the two paralogues are involved in separase-dependent down-regulation of PP2ACdc55 phosphatase activity. To test this possibility, we measured the specific in vitro phosphatase activity of PP2ACdc55 isolated from cells before and after ectopic Zds1 expression. The PP2ACdc55 phosphatase-specific activity decreased to about half of its initial value in response to Zds1 induction (Fig. 6 A). This suggests that Zds1 has the ability to cause inhibition of PP2ACdc55 activity, opening the possibility that it is directly involved in PP2ACdc55 down-regulation in early anaphase. Unfortunately, we were unable to measure PP2ACdc55 activity during anaphase in the absence of Zds1 and Zds2. Cells lacking both paralogues did not tolerate GAL1 or MET3 promoter-driven CDC20 expression, which would be required to provide the necessary synchrony for PP2ACdc55 analysis during anaphase progression.
Figure 6.
Zds1 promotes PP2ACdc55 down-regulation. (A) Zds1 causes down-regulation of PP2ACdc55 phosphatase activity. Strains Y2627 (MATa MET-CDC20 HA3-CDC55 CDC14-myc18) and Y1015 (as Y2627, but GAL-Flag-ZDS1 CDC14-Pk9) were arrested in metaphase by Cdc20 depletion and galactose added to induce Zds1 expression. The specific phosphatase activity of immunopurified Cdc55 was measured as described in Materials and methods. Mean and standard deviation of the specific phosphatase activity relative to metaphase in three experiments are shown. Cdc14 nucleolar release in these experiments was analyzed in parallel. (B) Zds1 and Zds2 are dispensable for early Cdc14 nucleolar release in the absence of Cdc55. Strains Y2831 (MATa cdc55Δ CDC14-Pk9) and Y3419 (as 2831, but zds1Δ zds2Δ) were synchronized in G1 by pheromone α-factor treatment and released to progress through the cell cycle into nocodazole-imposed metaphase arrest. The budding index and Cdc14 nucleolar release were determined.
If the role of Zds1 in mitotic exit is indeed down-regulation of PP2ACdc55 activity, then deletion of Cdc55 should make Zds1 and Zds2 dispensable for timely Cdc14 release. In the absence of Cdc55, nucleolar release of Cdc14 occurs prematurely when cells enter mitosis. The increasing Cdk activity at this time phosphorylates Net1 without being counteracted by PP2ACdc55 activity, and thus without having to await PP2ACdc55 down-regulation at anaphase onset (Queralt et al., 2006). We therefore analyzed whether Cdc14 nucleolar release during mitotic entry in cdc55Δ cells depended on Zds1 and Zds2. We synchronized cdc55Δ cells in G1 by pheromone α-factor treatment, and released them to progress through S phase and into nocodazole-imposed rearrest in mitosis. As expected, Cdc14 was released from the nucleolus as cells entered mitosis. The timing of Cdc14 activation during the cell cycle was indistinguishable in a parallel culture of cdc55Δ cells lacking Zds1 and Zds2 (Fig. 6 B). Therefore, Zds1 and Zds2 no longer influence Cdc14 release from the nucleolus in the absence of Cdc55. This is consistent with the possibility that PP2ACdc55 acts as the downstream target of Zds1 and Zds2, and that the two paralogues act in its inhibition.
Mutual interactions between separase, Zds1, and Cdc55
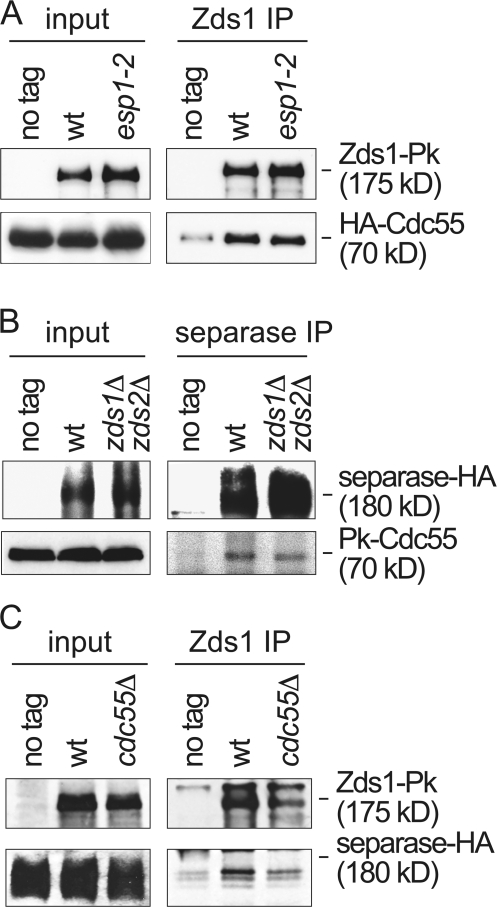
To begin to understand the mechanism by which Zds1 contributes to separase-mediated PP2ACdc55 down-regulation, we studied the interactions between these three components. We have above, and previously (Queralt et al., 2006), described interactions between Zds1 and PP2ACdc55, as well as between separase and PP2ACdc55. Zds1 might therefore interact with PP2ACdc55 directly, or the interaction might be mediated by separase. To address this, we performed coimmunoprecipitation analysis between Zds1 and Cdc55 in protein extracts obtained from both wild-type and separase mutant esp1-2 strains in which separase had been inactivated at the restrictive temperature for the esp1-2 allele. The interaction between Zds1 and Cdc55 remained unaffected by separase inactivation (Fig. 7 A), suggesting that Zds1 and Cdc55 interact with each other independently of separase. Because of the strong phenotype of the esp1-2 allele in mitotic exit we consider it less likely, but cannot exclude, that separase mediates an interaction between Zds1 and PP2ACdc55 that is maintained despite separase inactivation by the esp1-2 mutation.
Figure 7.
Interactions between separase, Zds1, and Cdc55. (A) Zds1 and Cdc55 interact independently of separase. Co-immunoprecipitation between Zds1 and Cdc55 was analyzed in protein extracts from strains Y585 (MATa Zds1-Pk6 HA3-CDC55) and Y586 (as 585, but esp1-2) that were grown at 33°C, a restrictive temperature for the separase esp1-2 allele, for 1 h before extract preparation. Protein extracts from strain Y584 (MATa HA3-CDC55) lacking a Pk epitope on Zds1 served as a control. (B) Separase and Cdc55 interact independently of Zds1 and Zds2. (B) As (A), but Cdc55 co-immuoprecipitation with separase from extracts of strains Y587 (MATa ESP1-HA6), Y3434 (as 587, but Pk3-CDC55), and Y3427 (as 3434, but zds1Δ zds2Δ) grown at 25°C was analyzed. (C) An interaction between separase and Zds1. Co-immunoprecipitation of separase with Zds1 was analyzed in extracts from strains Y587 (MATa ESP1-HA6), Y588 (as 587, but Zds1-Pk6), and Y589 (as 588, but cdc55Δ).
Next, we asked whether the interaction between separase and Cdc55 is mediated by Zds1. We compared coimmunoprecipitates of Cdc55 with separase in protein extracts obtained from wild-type and zds1Δ zds2Δ strains. The interaction of Cdc55 with separase appeared relatively weaker than that of Cdc55 with Zds1, but it remained unaffected in the zds1Δ zds2Δ strain (Fig. 7 B). Therefore separase interacts with Cdc55 independently of Zds1 and Zds2.
The interactions of separase with Cdc55, and of Cdc55 with Zds1, suggested that we should also be able to detect an interaction of separase with Zds1, and that this interaction may be Cdc55 dependent. Consistent with this expectation, we detected separase in Zds1 immunoprecipitates by Western blotting. In the absence of Cdc55, this interaction was maintained, albeit with somewhat reduced intensity (Fig. 7 C). This suggests that separase makes contact with Zds1 independently of Cdc55, but that the presence of Cdc55 may stabilize this interaction. Together, these interaction studies open the possibility that separase, Zds1, and PP2ACdc55 are found together in a protein complex formed by pairwise interactions between all three components. Molecular size analysis of separase in protein extracts from both budding and fission yeast has shown separase to exist as part of large protein complexes (Funabiki et al., 1996; and unpublished data). These results suggest that PP2ACdc55, Zds1, and likely also Zds2, form part of these complexes.
Discussion
Mitotic exit encompasses an ordered series of events, leading from chromosome segregation during anaphase to completion of cell division by cytokinesis. The budding yeast Cdc14 phosphatase is a key regulator of these events. Cdc14 is kept inactive in the nucleolus for much of the cell cycle, but is released from there during mitotic exit to contribute to Cdk down-regulation and dephosphorylation of numerous Cdk substrates. Initial models for Cdc14 activation in anaphase suggested that anaphase spindle elongation brings the spindle pole–bound MEN GTPase Tem1 in contact with its activator Lte1 at the bud cortex (Bardin et al., 2000; Pereira et al., 2000). In this way Cdc14 would be activated, and mitotic exit events initiated, once anaphase is complete. Meanwhile, it has become clear that Cdc14 is already activated at the onset of anaphase, and that release of separase from its inhibitor securin plays a crucial role in Cdc14 activation (Stegmeier et al., 2002; Sullivan and Uhlmann, 2003; Queralt et al., 2006). The early activated Cdc14 makes crucial contributions to successful anaphase progression. It leads to relocation of Fin1 and the aurora B kinase complex to the spindle midzone, stabilizes the dynamic behavior of mitotic spindle microtubules, and is required for condensation and resolution of the late-segregating rDNA locus (Pereira and Schiebel, 2003; D'Amours et al., 2004; Sullivan et al., 2004; Higuchi and Uhlmann, 2005; Woodbury and Morgan, 2007). It thus appears that the “mitotic exit” machinery orchestrates both, progression through faithful anaphase, then followed by mitotic exit and cytokinesis. How the ordering of anaphase and subsequent mitotic exit events is achieved by a common set of regulators remains an important open question.
In this study, we address the question of how separase causes Cdc14 activation at anaphase onset. We have previously shown that separase-dependent PP2ACdc55 down-regulation at anaphase onset allows Cdk-dependent phosphorylation of the nucleolar Cdc14 inhibitor Net1, thus promoting Cdc14 release (Queralt et al., 2006). The mechanistic basis for separase-dependent PP2ACdc55 down-regulation remained to be elucidated. Separase and PP2ACdc55 interact, and this interaction might be enhanced, or it may change its quality, as securin is degraded at anaphase onset. A model by which separase binding to PP2ACdc55 directly inhibits its phosphatase activity nevertheless appears unlikely, as PP2ACdc55 is present in a large stoichiometric excess over separase. Separase would thus only be able to regulate a small fraction of PP2ACdc55 directly, while overall PP2ACdc55 activity is reduced by approximately half during anaphase. This suggests that a catalytic step must be involved downstream of separase, and therefore that additional factors are likely required to convey separase-dependent down-regulation of the phosphatase. The observation of a direct interaction of Zds1 and Zds2 with PP2ACdc55 prompted us to analyze the contribution of these two proteins to mitotic exit regulation.
Our analysis revealed that Zds1 and Zds2 are required for timely Cdc14 activation during anaphase. Ectopic Zds1 expression in turn is sufficient to trigger PP2ACdc55 down-regulation, Cdk-dependent Net1 phosphorylation, and Cdc14 nucleolar release. Furthermore, we found that Zds1 physically interacts with separase. These observations open the possibility that separase acts through Zds1 and Zds2 to cause PP2ACdc55 down-regulation at anaphase onset. This notion is further supported by the similarity in requirements for Cdc14 activation by ectopic expression of either separase or Zds1. In both cases, Cdc14 nucleolar release depends on Cdk-dependent Net1 phosphorylation. Consistent with this requirement, ectopic expression of Zds1, like that of separase, leads to Cdc14 activation in mitotic cells, but not cells arrested in G1 or S phase of the cell cycle (Sullivan and Uhlmann, 2003; and unpublished data). The mechanism by which Zds1 and Zds2 impinge on PP2ACdc55 activity, and how this mechanism is in turn controlled by separase, remain important questions for future studies. It is noteworthy that, like separase, Zds1 and Zds2 are relatively low abundant proteins (Ghaemmaghami et al., 2003). Therefore, it does not appear likely that Zds1 and Zds2 act as direct inhibitory components of the PP2ACdc55 complex. Instead, they might be involved in regulating the status of posttranslational modifications or in controlling conformational changes in the PP2ACdc55 holoenzyme that impinge on its activity (Hombauer et al., 2007).
Zds1 and Zds2 have been found as high copy modulators of a large variety of cellular processes. These include cell cycle regulation, cell polarity establishment, and chromatin silencing, among others. These processes are known, or are conceivable, to be influenced by PP2A (Evans and Stark, 1997). The pleiotropic effect of Zds1 and Zds2 on cell physiology may therefore stem from the multitude of processes that are influenced by PP2A. If this possibility holds true, Zds1 and Zds2 might emerge as PP2A regulators not only during mitotic exit, but in a variety of cellular settings. Recently, fission yeast zds1 has been identified and characterised (Yakura et al., 2006). Like its budding yeast orthologue, it has been found to contribute to a variety of cellular processes, including sexual differentiation, cell wall integrity, and cell morphology. This spectrum of activities again overlaps with the physiological roles described for PP2A in this organism (Kinoshita et al., 1996). It will now be of interest to elucidate the mechanism by which separase, together with Zds1 and Zds2, regulate PP2ACdc55 activity to promote progression through anaphase and out of mitosis. This may serve as a paradigm for PP2A regulation by Zds1 and Zds2 also in other contexts. Finally, a contribution of mammalian separase to Cdk down-regulation and progression through meiotic cell division has been documented (Gorr et al., 2006; Kudo et al., 2006). Although some of the downstream effectors in mammalian cells may differ from those in budding yeast, an understanding of the nonproteolytic function of separase during mitotic exit in the yeast model may yield insight that will also help to elucidate the function of human separase in cell cycle progression.
Materials and methods
Yeast strains, plasmids, and cell cycle synchronization procedures
All strains were derivatives of W303. Epitope tagging of endogenous genes and gene deletions were performed by gene targeting using PCR products. For ectopic expression of Zds1, a GAL1-ZDS1 fragment from YEp352-GAL1-ZDS1 (Bi and Pringle, 1996) was introduced into the YIplac204 vector. Three tandem Flag epitopes were inserted in front of the open reading frame, and plasmids were integrated into the yeast genome after linearization within the TRP1 marker gene. YIplac204-GAL1-ZDS2 was created by inserting GAL1-3Flag into YIplac204, and adding a fragment containing the ZDS2 open reading frame originating from plasmid M2655 (Yu et al., 1996). For N-terminal tagging of endogenous CDC55, 500 base pairs of the CDC55 promoter, and in a second reaction the first 178 base pairs of the CDC55 open reading frame, were amplified by PCR using genomic DNA as the template. The fragments were introduced into plasmid pRS306 and three tandem HA epitopes inserted between promoter and open reading frame. The plasmid was integrated at the CDC55 locus after linearization with Msc I. Ectopic expression of separase in cells arrested in metaphase by Cdc20 depletion was as described previously (Uhlmann et al., 2000). Ectopic expression of Zds1 and Zds2 followed the same protocol. Cell synchronization using α-factor, and metaphase arrest by Cdc20 depletion and release into synchronous anaphase by Cdc20 reinduction, were described previously (Uhlmann et al., 1999).
Immunoprecipitation and PP2ACdc55 phosphatase assay
Protein extracts were prepared after spheroplast lysis as described previously (Uhlmann et al., 1999). For immunoprecipitation, the clarified extracts were precleared with protein A sepharose beads (GE Healthcare), incubated with antibody, and immunocomplexes adsorbed to magnetic protein A dynabeads (Invitrogen). Beads were washed in extraction buffer and boiled with SDS-PAGE loading buffer. For the phosphatase assays, 32P-phosphorylated histone H1 and Net1(1–600) were prepared as substrates as described previously (Queralt et al., 2006). PP2ACdc55 was immunopurified from cell extracts via N-terminally HA epitope-tagged Cdc55 as above, and the dynabeads were washed with phosphatase buffer (50 mM Tris/HCl, pH 7.5, 0.1 mM EGTA, 1 mg/ml BSA [Sigma-Aldrich], and 0.1% β-mercaptoethanol). Reaction mix (1 μg 32P-phospho-histone H1, or 0.5 μg 32P-phospho-Net1[1–600], in 50 μl phosphatase buffer) was added to the beads, followed by incubation at 30°C for 20 min. Reactions were terminated on ice, protein was precipitated with 16% trichloroacetic acid, and acid-soluble 32P-phosphate was quantified in a scintillation counter. Cdc55 recovered on the beads was quantified by Western blotting using an IRdye 800 coupled secondary antibody and the Odyssey Infrared Imaging System (LI-COR Biosciences).
Other techniques
Antibodies used for immunoprecipitation and Western blotting were α-Pk clone SV5-Pk1 (AbD Serotec), α-HA clone 12CA5 (Roche), α-myc clone 9E10, α-FLAG clone M2 (Sigma-Aldrich), α-Tpd3 (a gift from E. Ogris; Hombauer et al., 2007), anti-α-tubulin clone YOL1/34 (AbD Serotec), and α-PSTAIRE serum recognizing Cdc28 sc-53 (Santa Cruz Biotechnology, Inc.). Antibodies used for indirect immunofluorescence were α-Pk clone SV5-Pk1 and anti-α-tubulin clone YOL1/34. Secondary antibodies were Cy3 labeled goat α-mouse antibody (GE Healthcare) to visualize Pk-epitope tagged Cdc14, and affinity-purified fluorescein-conjugated donkey α-rat antibody (Millipore) to visualize tubulin. Stained cells were mounted in Fluoroguard (Bio-Rad Laboratories), containing 0.1 μg/ml DAPI. Cells were imaged at room temperature using an Axioplan 2 imaging microscope (Carl Zeiss, Inc.) equipped with a 100x (NA 1.45) Plan-Neofluar objective and an ORCA-ER camera (Hamamatsu Photonics). Anaphase spindle length measurements were conducted with the quantitative analysis tools of the Volocity software (Improvision), which was used for image acquisition. Brightness and contrast of the images were adjusted using Photoshop (Adobe).
Online supplemental material
Fig. S1 shows that Zds1-induced Cdc14 release depends on Cdk activity. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200801054/DC1.
Supplementary Material
Acknowledgments
We wish to thank E. Bi, E. Ogris, J. Pringle, and D. Stillman for their generous gifts of antibodies and constructs; and C. Bouchoux, S. Lopez-Aviles, and all other members of the laboratory for discussions and critical reading of the manuscript.
Abbreviations used in this paper: APC, anaphase-promoting complex; MEN, mitotic exit network; PAK, p21-activated kinase; PP2A, type 2A protein phosphatase.
References
- Azzam, R., S.L. Chen, W. Shou, A.S. Mah, G. Alexandru, K. Nasmyth, R.S. Annan, S.A. Carr, and R.J. Deshaies. 2004. Phosphorylation by cyclin B-Cdk underlies release of mitotic exit activator Cdc14 from the nucleolus. Science. 305:516–519. [DOI] [PubMed] [Google Scholar]
- Bardin, A.J., R. Visintin, and A. Amon. 2000. A mechanism for coupling exit from mitosis to partitioning of the nucleus. Cell. 102:21–31. [DOI] [PubMed] [Google Scholar]
- Bi, E., and J.R. Pringle. 1996. ZDS1 and ZDS2, genes whose products may regulate Cdc42p in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:5264–5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, A.C., J.A. Ubersax, D.T. Petsch, D.P. Matheos, N.S. Gray, J. Blethrow, E. Shimizu, J.Z. Tsien, P.G. Schultz, M.D. Rose, et al. 2000. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 407:395–401. [DOI] [PubMed] [Google Scholar]
- Chiroli, E., R. Fraschini, A. Beretta, M. Tonelli, G. Lucchini, and S. Piatti. 2003. Budding yeast PAK kinases regulate mitotic exit by two different mechanisms. J. Cell Biol. 160:857–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amours, D., F. Stegmeier, and A. Amon. 2004. Cdc14 and condensin control the dissolution of cohesin-independent chromosome linkages at repeated DNA. Cell. 117:455–469. [DOI] [PubMed] [Google Scholar]
- Drees, B.L., B. Sundin, E. Brazeau, J.P. Caviston, G.C. Chen, W. Guo, K.G. Kozminski, M.W. Lau, J.J. Moskow, A. Tong, et al. 2001. A protein interaction map for cell polarity development. J. Cell Biol. 154:549–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, D.R., and M.J. Stark. 1997. Mutations in the Saccharomyces cerevisiae type 2A protein phosphatase catalytic subunit reveal roles in cell wall integrity, actin cytoskeleton organization and mitosis. Genetics. 145:227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki, H., K. Kumada, and M. Yanagida. 1996. Fission yeast Cut1 and Cut2 are essential for sister chromatid separation, concentrate along the metaphase spindle and form large complexes. EMBO J. 15:6617–6628. [PMC free article] [PubMed] [Google Scholar]
- Gavin, A.C., M. Bosche, R. Krause, P. Grandi, M. Marzioch, A. Bauer, J. Schultz, J.M. Rick, A.M. Michon, C.M. Cruciat, et al. 2002. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature. 415:141–147. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami, S., W.-K. Huh, K. Bower, R.W. Howson, A. Belle, N. Dephoure, E.K. O'Shea, and J.S. Weissman. 2003. Global analysis of protein expression in yeast. Nature. 425:737–741. [DOI] [PubMed] [Google Scholar]
- Gorr, I.H., A. Reis, D. Boos, M. Wühr, S. Madgwick, K.T. Jones, and O. Stemmann. 2006. Essential CDK1-inhibitory role for separase during meiosis I in vertebrate oocytes. Nat. Cell Biol. 8:1035–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffioen, G., S. Swinnen, and J.M. Thevelein. 2003. Feedback inhibition on cell wall integrity signaling by Zds1 involves Gsk3 phosphorylation of a cAMP-dependent protein kinase regulatory subunit. J. Biol. Chem. 278:23460–23471. [DOI] [PubMed] [Google Scholar]
- Heo, S.J., K. Tatebayashi, and H. Ikeda. 1999. The budding yeast cohesin gene SCC1/MCD1/RHC21 genetically interacts with PKA, CDK and APC. Curr. Genet. 36:329–338. [DOI] [PubMed] [Google Scholar]
- Higuchi, T., and F. Uhlmann. 2005. Stabilization of microtubule dynamics at anaphase onset promotes chromosome segregation. Nature. 433:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfken, T., and E. Schiebel. 2002. A role for cell polarity proteins in mitotic exit. EMBO J. 21:4851–4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfken, T., and E. Schiebel. 2004. Novel regulation of mitotic exit by the Cdc42 effectors Gic1 and Gic2. J. Cell Biol. 164:219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombauer, H., D. Weismann, I. Mudrak, C. Stanzel, T. Fellner, D.H. Lackner, and E. Ogris. 2007. Generation of active protein phosphatase 2A is coupled to holoenzyme assembly. PLoS Biol. 5:e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspersen, S.L., and D.O. Morgan. 2000. Cdc14 activates Cdc15 to promote mitotic exit in budding yeast. Curr. Biol. 10:615–618. [DOI] [PubMed] [Google Scholar]
- Jaspersen, S.L., J.F. Charles, R.L. Tinker-Kulberg, and D.O. Morgan. 1998. A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae. Mol. Biol. Cell. 9:2803–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, A.L., B.B. Quimby, J.K. Hood, P. Ferrigno, P.H. Keshava, P.A. Silver, and A.H. Corbett. 2000. SAC3 may link nuclear protein export to cell cycle progression. Proc. Natl. Acad. Sci. USA. 97:3224–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita, K., T. Nemoto, K. Nabeshima, H. Kondoh, H. Niwa, and M. Yanagida. 1996. The regulatory subunits of fission yeast protein phosphatase 2A (PP2A) affect cell morphogenesis, cell wall synthesis and cytokinesis. Genes Cells. 1:29–45. [DOI] [PubMed] [Google Scholar]
- Krogan, N.J., G. Cagney, H. Yu, G. Zhong, X. Guo, A. Ignatchenko, J. Li, S. Pu, N. Datta, A.P. Tikuisis, et al. 2006. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 440:637–643. [DOI] [PubMed] [Google Scholar]
- Kudo, N.R., K. Wassmann, M. Anger, M. Schuh, K.G. Wirth, H. Xu, W. Helmhart, H. Kudo, M. McKay, B. Maro, et al. 2006. Resolution of chiasmata in oocytes requires separase-mediated proteolysis. Cell. 126:135–146. [DOI] [PubMed] [Google Scholar]
- Lee, S.E., L.M. Frenz, N.J. Wells, A.L. Johnson, and L.H. Johnston. 2001. Order of function of the budding-yeast mitotic exit-network proteins Tem1, Cdc15, Mob1, Dbf2, and Cdc5. Curr. Biol. 11:784–788. [DOI] [PubMed] [Google Scholar]
- Ma, X.J., Q. Lu, and M. Grunstein. 1996. A search for proteins that interact genetically with histone H3 and H4 amino termini uncovers novel regulators of the Swe1 kinase in Saccharomyces cerevisiae. Genes Dev. 10:1327–1340. [DOI] [PubMed] [Google Scholar]
- Mizunuma, M., D. Hirata, K. Miyahara, E. Tsuchiya, and T. Miyakawa. 1998. Role of calcineurin and Mpk1 in regulating the onset of mitosis in budding yeast. Nature. 392:303–306. [DOI] [PubMed] [Google Scholar]
- Morgan, D.O. 2007. The Cell Cycle: Principles of Control. New Science Press, London. 297 pp.
- Pereira, G., and E. Schiebel. 2003. Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science. 302:2120–2124. [DOI] [PubMed] [Google Scholar]
- Pereira, G., T. Hofken, J. Grindlay, C. Manson, and E. Schiebel. 2000. The Bub2p spindle checkpoint links nuclear migration with mitotic exit. Mol. Cell. 6:1–10. [PubMed] [Google Scholar]
- Peters, J.-M. 2006. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 7:644–656. [DOI] [PubMed] [Google Scholar]
- Queralt, E., C. Lehane, B. Novak, and F. Uhlmann. 2006. Downregulation of PP2ACdc55 phosphatase by separase initiates mitotic exit in budding yeast. Cell. 125:719–732. [DOI] [PubMed] [Google Scholar]
- Roy, N., and K.W. Runge. 2000. Two paralogs involved in transcriptional silencing that antagonistically control yeast life span. Curr. Biol. 10:111–114. [DOI] [PubMed] [Google Scholar]
- Seshan, A., and A. Amon. 2005. Ras and the Rho effector Cla4 collaborate to target and anchor Lte1 at the bud cortex. Cell Cycle. 4:940–946. [DOI] [PubMed] [Google Scholar]
- Shou, W., J.H. Seol, A. Shevchenko, C. Baskerville, D. Moazed, Z.W.S. Chen, J. Jang, A. Shevchenko, H. Charbonneau, and R.J. Deshaies. 1999. Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell. 97:233–244. [DOI] [PubMed] [Google Scholar]
- Shou, W., R. Azzam, S.L. Chen, M.J. Huddleston, C. Baskerville, H. Charbonneau, R.S. Annan, S.A. Carr, and R.J. Deshaies. 2002. Cdc5 influences phosphorylation of Net1 and disassembly of the RENT complex. BMC Mol. Biol. 3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark, M.J. 1996. Yeast protein serine/threonine phosphatases: multiple roles and diverse regulation. Yeast. 12:1647–1675. [DOI] [PubMed] [Google Scholar]
- Stegmeier, F., and A. Amon. 2004. Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu. Rev. Genet. 38:203–232. [DOI] [PubMed] [Google Scholar]
- Stegmeier, F., R. Visintin, and A. Amon. 2002. Separase, Polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell. 108:207–220. [DOI] [PubMed] [Google Scholar]
- Sullivan, M., and F. Uhlmann. 2003. A non-proteolytic function of separase links the onset of anaphase to mitotic exit. Nat. Cell Biol. 5:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, M., T. Higuchi, V.L. Katis, and F. Uhlmann. 2004. Cdc14 phosphatase induces rDNA condensation and resolves cohesin-independent cohesion during budding yeast anaphase. Cell. 117:471–482. [DOI] [PubMed] [Google Scholar]
- Uhlmann, F., F. Lottspeich, and K. Nasmyth. 1999. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature. 400:37–42. [DOI] [PubMed] [Google Scholar]
- Uhlmann, F., D. Wernic, M.A. Poupart, E.V. Koonin, and K. Nasmyth. 2000. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell. 103:375–386. [DOI] [PubMed] [Google Scholar]
- Visintin, R., K. Craig, E.S. Hwang, S. Prinz, M. Tyers, and A. Amon. 1998. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell. 2:709–718. [DOI] [PubMed] [Google Scholar]
- Visintin, R., E.S. Hwang, and A. Amon. 1999. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature. 398:818–823. [DOI] [PubMed] [Google Scholar]
- Visintin, C., B.N. Tomson, R. Rahal, J. Paulson, M. Cohen, J. Taunton, A. Amon, and R. Visintin. 2008. APC/C-Cdh1-mediated degradation of the Polo kinase Cdc5 promotes the return of Cdc14 into the nucleolus. Genes Dev. 22:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury, E.L., and D.O. Morgan. 2007. Cdk and APC activities limit the spindle-stabilizing function of Fin1 to anaphase. Nat. Cell Biol. 9:106–112. [DOI] [PubMed] [Google Scholar]
- Yakura, M., F. Ozoe, H. Ishida, T. Nakagawa, K. Tanaka, H. Matsuda, and M. Kawamukai. 2006. zds1, a novel gene encoding an ortholog of Zds1 and Zds2, controls sexual differentiation, cell wall integrity and cell morphology in fission yeast. Genetics. 172:811–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellman, C.M., and D.J. Burke. 2006. The role of Cdc55 in the spindle checkpoint is through regulation of mitotic exit in Saccharomyces cerevisiae. Mol. Biol. Cell. 17:658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, S., and A. Toh-e. 2002. Budding yeast Cdc5 phosphorylates Net1 and assists Cdc14 release from the nucleolus. Biochem. Biophys. Res. Commun. 294:687–691. [DOI] [PubMed] [Google Scholar]
- Yu, Y., Y.W. Jiang, R.J. Wellinger, K. Carlson, J.M. Roberts, and D.J. Stillman. 1996. Mutations in the homologous ZDS1 and ZDS2 genes affect cell cycle progression. Mol. Cell. Biol. 16:5254–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanelli, C.F., and S.R. Valentini. 2005. Pkc1 acts through Zds1 and Gic1 to suppress growth and cell polarity defects of a yeast eIF5A mutant. Genetics. 171:1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.