Abstract
IGF-II stimulates both mitogenesis and myogenesis through its binding and activation of the IGF-I receptor (IGF-IR). How this growth factor pathway promotes these two opposite cellular responses is not well understood. We investigate whether local IGF binding protein-5 (IGFBP-5) promotes the myogenic action of IGF-II. IGFBP-5 is induced before the elevation of IGF-II expression during myogenesis. Knockdown of IGFBP-5 impairs myogenesis and suppresses IGF-II gene expression. IGF-II up-regulates its own gene expression via the PI3K-Akt signaling pathway. Adding IGF-II or constitutively activating Akt rescues the IGFBP-5 knockdown-caused defects. However, an IGF analogue that binds to the IGF-IR but not IGFBP has only a limited effect. When added with low concentrations of IGF-II, IGFBP-5 restores IGF-II expression and myogenic differentiation, whereas an IGF binding–deficient IGFBP-5 mutant has no effect. These findings suggest that IGFBP-5 promotes muscle cell differentiation by binding to and switching on the IGF-II auto-regulation loop.
Introduction
Myogenesis is a highly ordered and balanced process, which includes myoblast proliferation, cell cycle withdrawal, differentiation, and cell fusion (Buckingham et al., 2003). This process is regulated by a variety of hormones and growth factors. Insulin-like growth factors (IGFs), including IGF-I and IGF-II, play critical roles in skeletal muscle differentiation and growth, as well as adult muscle regeneration and hypertrophy (Musaro et al., 2001, 2004). Mice deficient in IGF ligand or IGF-I receptor exhibit muscle hypoplasia and die shortly after birth due to impaired muscle mass to inflate their lungs (Liu et al., 1993; Powell-Braxton et al., 1993). Transgenic mice with overexpression of IGF-I in muscle have larger muscle fibers and enhanced muscle strength during aging (Coleman et al., 1995; Barton-Davis et al., 1998; Barton et al., 2002; Kaspar et al., 2003). In cultured muscle cells, IGF-II levels increase dramatically during myogenesis. IGF-II antisense oligonucleotides can abolish differentiation (Florini et al., 1991, 1993), and IGF-II overexpression accelerates myoblast differentiation (Stewart et al., 1996).
Although most growth factors stimulate myoblast proliferation and inhibit myogenic differentiation, IGFs are unique in that they not only stimulate myoblast proliferation but also promote myogenic differentiation, two mutually exclusive processes (Florini et al., 1996). These actions are mediated through the IGF-I receptor (IGF-IR), a transmembrane tyrosine kinase, and activation of the IGF-IR initiates downstream signaling cascades including the phosphatidylinositol 3-kinase (PI3K)-Akt pathway. Earlier studies have shown that there is a temporal separation between these two responses to IGFs: IGF treatment causes a proliferative response in the first 24–36 h, and this is followed by subsequent increase in myogenic differentiation. The increase in differentiation is not secondary to increased cell number after IGF treatment (Florini et al., 1996). Rosenthal and Cheng (1995) have reported a biphasic effect of IGF-I on pRb, a regulator of cell cycle progression that is present in the hyperphosphorylated state during cell proliferation and hypophosphorylated state during differentiation. They found that IGF treatment resulted in persistent Rb hyperphosphorylation for over 24 h before hypophosphorylation became the dominant form. Several laboratories have shown that activation of PI3K, Akt, and FoxO promotes myogenic differentiation and survival (Engert et al., 1996; Kaliman et al., 1996; Coolican et al., 1997; Jiang et al., 1999; Rommel et al., 1999, 2001; Lawlor and Rotwein, 2000a,b; Lawlor et al., 2000; Wilson et al., 2004; Wilson and Rotwein, 2007). Rotwein and colleagues have reported that in cultured muscle cells, secreted IGF-II stimulates the IGF-IR, PI3K, and Akt to induce the expression of the cyclin-dependent kinase inhibitor p21 and Myogenin, and through this mechanism, maintains myoblast viability during early myogenesis (Lawlor and Rotwein, 2000a,b; Lawlor et al., 2000; Wilson et al., 2004; Wilson and Rotwein, 2007). Despite these advances in various intracellular signaling mechanisms underlying IGF actions in myogenesis, it remains puzzling how the activation of the same IGF-IR by the same ligand (IGF-II) can elicit opposite biological responses.
We now understand that most, if not all IGFs in the extracellular environment are bound to IGF binding proteins (IGFBPs). IGFBPs are a family of secreted proteins that specifically bind IGFs with affinities that are equal to or greater than those of the IGF-IR. Six distinct IGFBPs, designated as IGFBP-1 to -6, have been isolated and characterized in humans and a variety of other vertebrate species (Clemmons, 2001; Duan, 2002; Firth and Baxter, 2002). IGFBP-5, the most conserved IGFBP, is the major protein secreted by skeletal muscles. IGFBP-5 is expressed in the myotomal compartments during early development in rodent and zebrafish (Green et al., 1994; Wood et al., 2005) and its expression is induced during muscle differentiation (James et al., 1993). In cultured myoblasts, IGFBP-5 expression levels increased dramatically during myogenic differentiation (Rotwein et al., 1995; Bayol et al., 2000). These findings suggest that IGFBP-5 may play a role in modulating the actions of IGF-II on muscle differentiation. The precise role(s) of IGFBP-5 in myogenesis, however, remains under debate. Knockout of the IGFBP-5 in mice had a minimal effect on muscle size and whole body size, but IGFBP-3, -4, and -5 triple knockout mice showed smaller body size and reduced quadriceps muscles (Ning et al., 2006), indicating the potential compensatory effects from other IGFBPs in the single knockout mice. When overexpressed in vivo in transgenic mice (Salih et al., 2004) and in vitro in cultured murine muscle cells under the control of a constitutive promoter (James et al., 1996; Cobb et al., 2004; Mukherjee et al., 2007), IGFBP-5 inhibits muscle differentiation. When exogenous IGFBP-5 was added to cultured rat myoblasts together with IGF-I at an appropriate ratio, it stimulated IGF-I–induced differentiation (Ewton et al., 1998). Although these gain-of-function studies, either adding large amounts of purified IGFBP-5 to or stably and constitutively overexpressing of IGFBP-5 in cultured myoblasts, are useful in demonstrating the biological capabilities of IGFBP-5, they do not necessarily provide insight into the physiological function(s) of the endogenous protein. Furthermore, interpretation of these data are not always straightforward because the IGFBP-5 expression is dramatically induced during myogenesis.
The objective of this study is to investigate the physiological role(s) of endogenous IGFBP-5 in regulating IGF-II actions in skeletal muscle differentiation. Our results suggest the induction of IGFBP-5 precedes that of IGF-II in cultured C2C12 myoblast cells and in primary skeletal muscle cells. Knockdown of IGFBP-5 impairs myogenic differentiation and reduces the expression of Myogenin and myosin heavy chain (MHC). Interestingly, knockdown of IGFBP-5 suppresses IGF-II expression and reduces IGF-IR–mediated signaling activity. Further mechanistic analyses suggest that IGFBP-5 promotes muscle cell differentiation by binding to IGF-II and enhances the IGF-II autocrine regulatory loop.
Results
Induction of IGFBP-5 precedes the induction of IGF-II during myogenesis
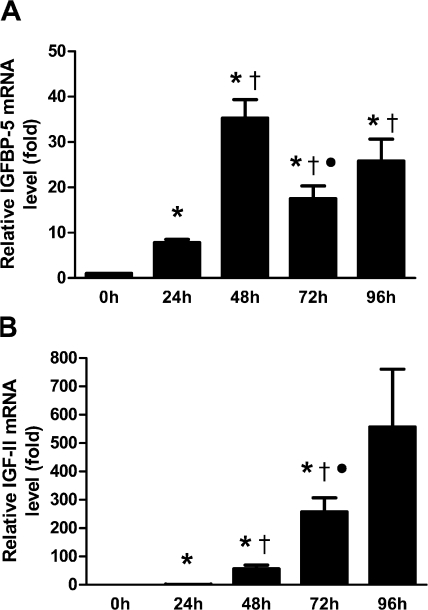
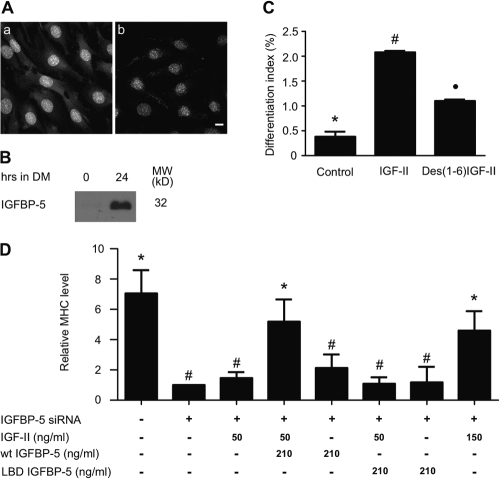
C2C12 myoblasts undergo terminal differentiation after switching to horse serum containing differentiation medium (DM). As shown in Fig. 1 A, at 24 h after the induction of differentiation, the expression of IGFBP-5 mRNA showed an approximate sevenfold increase over the 0 h control (P < 0.05). There was a further increase (approximately fivefold) at 48 h. Thereafter, the IGFBP-5 mRNA levels decreased considerably, but remained higher than 0 h. In comparison, while the absolute IGF-II mRNA level (copy number/μl) remained extremely low, the relative levels of IGF-II mRNA increased dramatically at 48 h (51-fold over 24 h, P < 0.05). There was a further approximate fourfold increase at 72 h and an additional approximate twofold increase at 96 h. RT-PCR analysis indicated that there are no significant changes in the mRNA levels of IGF-IR, IGFBP-2, and IGFBP-4 (unpublished data; also see Fig. 5 A). These results suggest that although both IGF-II and IGFBP-5 expression are induced during myogenic differentiation, the induction of IGFBP-5 expression precedes that of IGF-II.
Figure 1.
The induction of IGFBP-5 precedes that of IGF-II during myogenesis. Cultured C2C12 myoblasts were induced to differentiate by switching to differentiation medium (DM). Total RNA was extracted at different time points. IGFBP-5 mRNA (A) and IGF-II mRNA (B) levels were measured by qRT-PCR. Values are expressed as relative levels to that of 24 h for IGF-II (IGF-II mRNA levels were under the detection limit at 0 h) or to that of the 0 h group for IGFBP-5 after normalized to cyclophilin levels. Data shown are means ± SE of three independent experiments. *, P < 0.05, compared with the 0 h group; †, P < 0.05, compared with the 24-h group; •, P < 0.05, compared with the 48 h group.
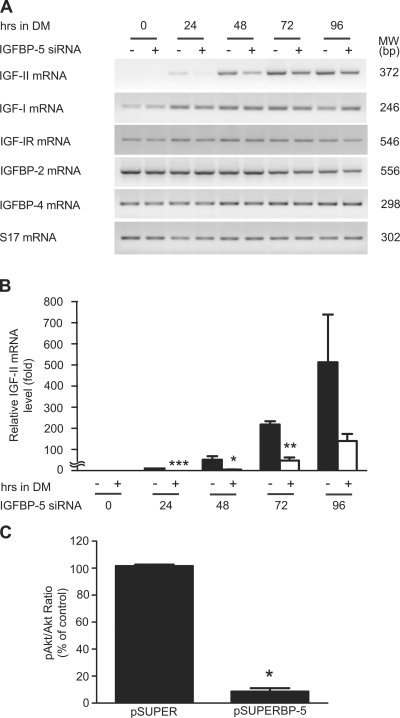
Figure 5.
Knockdown of IGFBP-5 suppresses IGF-II gene expression and decreases IGF-IR–mediated signaling activity. (A) C2C12 cells transfected with pSUPER or pSUPER-BP5 were induced to differentiate by switching to DM. RNA samples were prepared at the indicated time points and subjected to RT-PCR. (B) RNA samples described in A were analyzed by qRT-PCR. Data are expressed as relative value to those of the pSUPER transfected cells at 24 h after the induction of differentiation. Data shown are means ± SE of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with the pSUPER-transfected control groups at the same time point. (C) Cells transfected with pSUPER or pSUPER-BP5 was induced to differentiate. 48 h later, cells were lysed and analyzed by Western immunoblot. The phospho-Akt/total Akt ratio was calculated by densitometry. Values are expressed as relative levels to pSUPER group. Data shown are means ± SE; n = 4; *, P < 0.05.
Induction of IGFBP-5 expression is required for myogenic differentiation
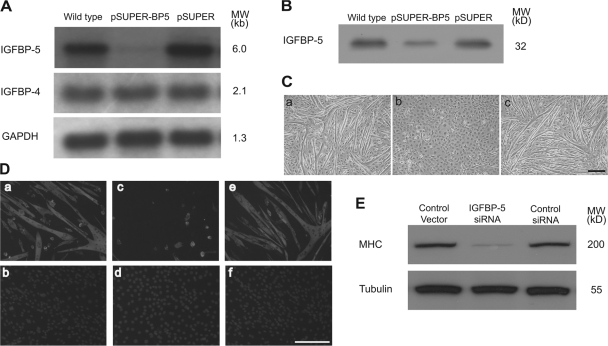
To determine the role of endogenous IGFBP-5, pSUPER-BP5, a plasmid that generates siRNA specifically targeting IGFBP-5 mRNA, was transfected to cultured C2C12 cells. The effect in silencing the IGFBP-5 gene was examined by measuring changes in IGFBP-5 mRNA, as well as changes in IGFBP-5 protein levels. Introducing pSUPER-BP5 into these cells resulted in a marked reduction of IGFBP-5 mRNA levels, whereas the empty pSUPER plasmid had no such effect (Fig. 2 A). Ligand blot analysis of the conditioned media revealed a similar decrease in IGFBP-5 protein levels (Fig. 2 B). The specificity of IGFBP-5 siRNA was confirmed by the unchanged levels of IGFBP-4 mRNA and GAPDH mRNA (Fig. 2 A). Quantification of three independent experiments showed that IGFBP-5 siRNA caused a 72.8% decrease (P < 0.05) in IGFBP-5 mRNA levels and a 71.2% decrease (P < 0.05) in protein levels.
Figure 2.
Knockdown of IGFBP-5 impairs myogenic differentiation. (A) C2C12 cells were transfected with pSUPER or pSUPER-BP5. 3 d later, total RNA was isolated and subjected to Northern blot analysis. (B) Conditioned media obtained from wild-type, pSUPER-BP5–transfected, and pSUPER-transfected cells were subjected to ligand blot analysis. (C) Knockdown of IGFBP-5 inhibits myotube formation. C2C12 cells were transfected with the pSUPER vector (a), pSUPER-BP5 (b), or control pSUPER (c). 30 h after transfection, cells were induced to differentiate for 4 d. Images were representative from three reproducible experiments. Bar, 200 μm. (D) Knockdown of IGFBP-5 reduces MHC expression. Cells described in C were analyzed by immunostaining for MHC (a, c, and e) and counterstained with DAPI (b, d, and f). Bar, 200 μm. (E) Western immunoblot analysis of cells described in C.
When switched to DM, the empty control vector–transfected myoblasts formed morphologically distinctive multinucleated myotubes, while pSUPER-BP5–transfected myoblasts remained as individual mononucleated cells (Fig. 2 C). We examined the expression of MHC by immunostaining and Western blot. Both assays showed a dramatic decrease in MHC expression in the pSUPER-BP5–transfected cells (Fig. 2, D and E). Quantification of Western blot data from four independent experiments showed that the MHC levels were decreased by 84.4% (P < 0.05). To rule out the possibility of any nonspecific interferon responses associated with siRNA, a pSUPER construct expressing siRNA with a sequence unrelated to IGFBP-5 was engineered and introduced to C2C12 cells. This construct did not affect differentiation or MHC expression (Fig. 2, C–E). Together, these results indicated that knockdown of IGFBP-5 impairs myogenic differentiation in C2C12 cells.
Knockdown of IGFBP-5 suppresses myogenin expression, but expression of Myogenin cannot “rescue” the myogenic defects caused by IGFBP-5 knockdown
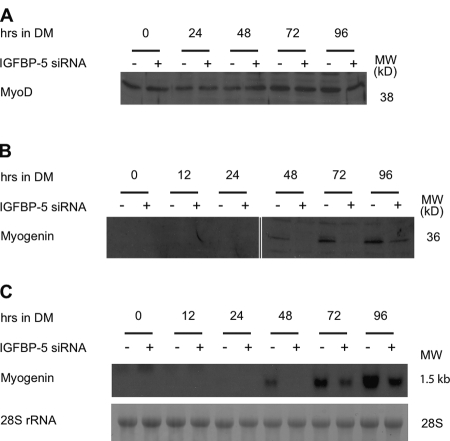
The muscle-specific myogenic regulatory factors, including MyoD, Myf5, MRF4, and Myogenin regulate myoblast specification and differentiation through their ability to activate muscle specific structural genes, such as MHC (Perry and Rudnick, 2000). We examined whether the expression of some of these critical myogenic transcription factors is altered by IGFBP-5 knockdown. There was little difference of MyoD levels through the time course of differentiation (Fig. 3 A). Myogenin was not detectable in undifferentiated myoblast cells or within the first 24 h after DM was added (Fig. 3 B). At 48 h, Myogenin was easily detectable and its levels increased dramatically at 72 and 96 h in the control group. At 48 and 72 h, Myogenin was not detected in pSUPER-BP5–transfected cells (Fig. 3 B). Its levels were markedly lower even at 96 h. Northern blot analysis revealed similar decreases in myogenin mRNA levels (Fig. 3 C).
Figure 3.
Knockdown of IGFBP-5 decreases Myogenin expression. After transfection, C2C12 myoblasts were switched to DM. Samples were collected at the indicated time. (A) Western immunoblot of MyoD. (B) Western immunoblot analysis of Myogenin. (C) Northern blot analysis of myogenin.
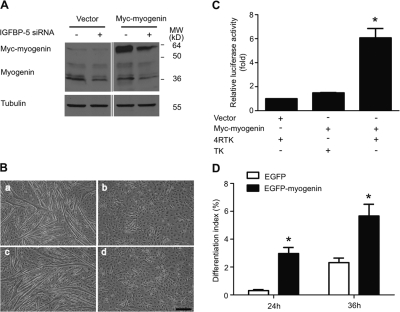
To determine whether myogenin mediates the myogenic action of IGFBP-5, we cotransfected C2C12 myoblasts with pSUPER-BP5 and a Myogenin expression plasmid. Western blot analysis verified the successful expression of the Myc-tagged Myogenin. Knockdown of IGFBP-5 suppressed the endogenous Myogenin and modestly decreased the levels of the transgene (Fig. 4 A). As shown in Fig. 4 B, cells cotransfected with Myogenin and pSUPER-BP5 were indistinguishable from those transfected with pSUPER-BP5 alone, i.e., they did not form myotubes and had minimal MHC expression when grown in DM. To confirm that the myogenin transgene is functional in this particular experimental setting, the transcriptional activity of Myc-Myogenin was examined. Myogenin forms heterodimers with other ubiquitous basic helix-loop-helix transcription factors and binds to the cis-control elements termed E-boxes often found in the promoters of muscle-specific genes (Sabourin and Rudnicki, 2000; Buckingham, 2001). 4RTK is a reporter construct that contains the firefly luciferase gene driven by a minimal thymidine kinase (TK) promoter linked with four copies of the E-box derived from the mouse muscle creatine kinase promoter. As shown in Fig. 4 C, when cotransfected with 4RTK, the Myc-Myogenin caused a sixfold increase in luciferase activity. Furthermore, when transfected to C2C12 cells, Myogenin significantly increased differentiation at 24 and 36 h after the induction of differentiation (Fig. 4 D), suggesting that overexpression of Myogenin is capable of promoting differentiation. Together, these data indicate that while knockdown of IGFBP-5 reduces myogenin gene expression, myogenin alone is insufficient to promote myogenic differentiation in the absence of IGFBP-5.
Figure 4.
Forced expression of Myogenin does not rescue the myogenic defects in IGFBP-5–deficient cells. (A) Western immunoblot analysis of C2C12 cells transfected with pSUPER + pCS2, pSUPER-BP5 + pCS2, pSUPER + Myc-tagged Myogenin, or pSUPER-BP5 + Myc-tagged Myogenin 4 d after they were induced to differentiate. (B) Phase-contrast images of cells transfected with pSUPER + pCS2 (a), pSUPER-BP5 + pCS2 (b), pSUPER + Myc-tagged Myogenin (c), and pSUPER-BP5 + Myc-tagged (d). These were representative images from four reproducible experiments. Bar, 200 μm. (C) Luciferase activity was measured 2 d after differentiation was induced in C2C12 cells transfected with an empty vector and the Myogenin reporter construct 4RTK, Myc-tagged Myogenin and the reporter construct TK, and overexpression of Myogenin and 4RTK, respectively. Data are expressed as relative value to those of the empty vector and 4RTK transfected cells. Data shown are means ± SE of three independent experiments. *, P < 0.05. (D) C2C12 cells were transfected with either EGFP or EGFP-tagged Myogenin and induced to differentiate for 24 or 36 h, and their differentiation indexes were determined. Data shown are means ± SE of two independent experiments each performed in triplicate. *, P < 0.05.
Knockdown of IGFBP-5 suppresses IGF-II gene expression and exogenous IGF-II “rescues” the myogenic defects caused by IGFBP-5 knockdown
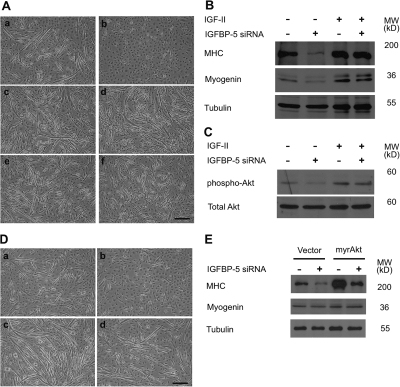
It has been reported that autocrine IGF-II is a critical regulator of myogenesis (Wilson et al., 2004). We therefore examined the impact of IGFBP-5 knockdown on IGF-II expression. No IGF-II mRNA was detected by RT-PCR in the control undifferentiated C2C12 myoblast cells (Fig. 5 A). 24 h after the addition of DM, the IGF-II mRNA levels were appreciated and showed further increases at 48 h and thereafter. IGF-II mRNA expression was undetectable at 24 h and was markedly lower at 48 h in the pSUPER-BP5–transfected cells. This effect appears to be specific to the IGF-II gene, as knockdown of IGFBP-5 did not reduce the mRNA levels of other members of the IGF signaling pathway expressed in these cells, including IGF-I, IGF-IR, and IGFBP-2 and -4 (Fig. 5 A). The effect on IGF-II gene expression was further examined by qRT-PCR. IGF-II mRNA levels were significantly lower in IGFBP-5 knocked down cells at 24, 48, and 72 h after the induction of differentiation (Fig. 5 B). Knockdown of IGFBP-5 markedly decreased phospho-Akt levels, whereas the levels of total Akt remained comparable to those of the control. When quantified and expressed as the ratio of phospho-Akt/total Akt, the levels in the IGFBP-5–deficient group showed a 94% reduction (Fig. 5 C).
Because knockdown of IGFBP-5 suppresses IGF-II expression and reduces the IGF signaling activity, we tested whether IGFBP-5 affects myogenesis via IGF-II. As shown in Fig. 6 A, while pSUPER-BP-5 transfected cells remained as mononucleated myoblasts, addition of exogenous IGF-II not only increased myotube formation in the control group, it also reversed the myogenic defects caused by IGFBP-5 knockdown. IGF-I, which signals through the same IGF-IR, had a similar effect. Western blot analysis indicated that knockdown of IGFBP-5 suppressed MHC and Myogenin expression and addition of IGF-II restored their expression (Fig. 6 B). This action of exogenous IGF-II is likely mediated by the IGF-IR and Akt signaling pathway because knockdown of IGFBP-5 reduced phospho-Akt levels and IGF-II increased phospho-Akt levels in differentiating cells (Fig. 6 C).
Figure 6.
Exogenous IGFs or expression of a constitutively active Akt “rescues” the myogenic defects caused by IGFBP-5 knockdown. (A) Effects of IGFs. C2C12 cells transfected with pSUPER (a, c, and e) or pSUPER-BP5 (b, d, and f) were induced to differentiate by switching to the DM containing 0.5% horse serum without IGF-I (a and b), or with 400 ng/ml IGF-I (c and d), or 400 ng/ml IGF-II (e and f) for 4 d. Representative images from four reproducible experiments are shown. Bar, 200 μm. (B) Western immunoblot analysis of cell lysates from the groups indicated. (C) Western immunoblot analysis of Akt in cell lysates from the groups indicated. (D) Effect of myrAkt. C2C12 cells transfected with pSUPER + pCS2 (a), pSUPER-BP5 + pCS2 (b), pSUPER + pCS2+myr-Akt (c), and pSUPER-BP5 + pCS2+myr-Akt (d) were switched to the DM containing 0.5% horse serum for 4 d. Phase-contrast images were representative images from two independent experiments. Bar, 200 μm. (E) Western immunoblot analysis of cell lysates from the groups indicated.
If IGFBP-5 indeed promotes myogenesis through the IGF-IR-PI3K-Akt signaling pathway, then activation of Akt should also “rescue” IGFBP-5 knockdown-induced differentiation defects. To test this idea, myrAkt, a constitutively active form of Akt, was introduced to the cells. As shown in Fig. 6 D, C2C12 cells cotransfected with pSUPER-BP5 and a myrAkt-expressing plasmid clearly formed myotubes when growing in DM, whereas cells transfected with pSUPER-BP5 did not. Cells transfected with the myrAkt plasmid alone showed enhanced differentiation. Similarly, expression of myrAkt restored pSUPER-BP5 caused reduction in Myogenin and MHC expression (Fig. 6 E). Together, these findings suggest that knockdown of IGFBP-5 suppresses IGF-II expression, which in turn leads to reduced signaling intensity of the IGF-IR-PI3K-Akt signaling pathway.
IGF-II up-regulates its own gene expression via the PI3K-Akt signaling pathway
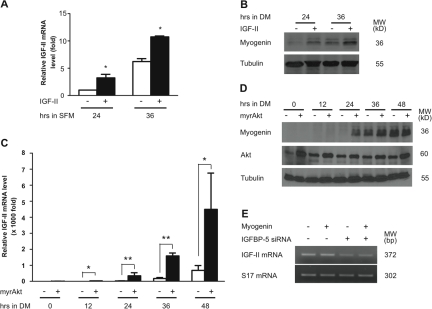
Although it has been shown that autocrine IGF-II production increases dramatically during muscle differentiation, the regulatory mechanisms of IGF-II gene expression is not well understood. Given the fact that IGF-II gene expression is continuously and dramatically elevated during myogenesis (see Fig. 1), we speculated that IGF-II might up-regulate its own gene expression through a positive auto-regulatory loop. Two independent approaches, adding exogenous IGF-II and overexpressing myrAkt, were taken to address this issue. As shown in Fig. 7 A, addition of IGF-II to wild-type C2C12 cells resulted in a significant increase in IGF-II mRNA levels. Likewise, expression of myrAkt significantly increased IGF-II mRNA levels (Fig. 7 C). These results suggest that IGF-II up-regulates its own gene expression through the PI3K and Akt signaling pathway.
Figure 7.
IGF-II up-regulates its own gene expression through the PI3K-Akt signaling pathway. (A) Wild-type C2C12 cells were switched to SFM supplemented with or without 300 ng/ml IGF-II. IGF-II mRNA levels were measured by qRT-PCR and normalized by cyclophilin mRNA levels. Data shown represent means ± SE of two independent experiments. *, P < 0.05 compared with the control group at the same time point. (B) Wild-type C2C12 cells were switched to DM (containing 0.5% horse serum) supplemented with or without 300 ng/ml IGF-II. 24 and 36 h later, cells were lysed and subjected to Western blot analysis. (C) Cells transfected with the empty pCS2 (open box) or pCS2+myr-Akt plasmid (closed box) were switched to DM. IGF-II mRNA levels were measured by qRT-PCR and normalized. The results were expressed as relative value to those of the empty vector transfected cells at 0 h. Data shown are means ± SE of three independent experiments. *, P < 0.05; **, P < 0.01 compared with the control group at the same time point. (D) C2C12 cells transfected with the empty pCS2+ or pCS2+myr-Akt plasmid were switched to the DM. Cells were lysed at the indicated time and subjected to Western blot analysis. (E) Overexpression of Myogenin does not restore the reduced IGF-II expression in IGFBP-5 knocked down cells. Cells transfected with pSUPER + pCS2, pSUPER + Myc-Myogenin, pSUPER-BP5 + pCS2, and pSUPER-BP5 + Myc-Myogenin were induced to differentiate for 4 d. RNA samples were prepared and subjected to RT-PCR analysis.
In addition to IGF-II, Myogenin expression was also increased by exogenous IGF-II or myrAkt expression (Fig. 7, B and D). These data are consistent with previous reports (Florini et al., 1993; Xu and Wu, 2000). However, expression of Myogenin did not restore the reduced IGF-II expression caused by IGFBP-5 knockdown cells (Fig. 7 E). Although the increased Myogenin plays a role in myogenesis, it does not appear to play any direct role in the expression of the IGF-II gene during myogenesis.
Endogenous IGFBP-5 is not localized in the nucleus, and forced nuclear expression of the IGFBP-5 transactivation domain does not increase IGF-II expression
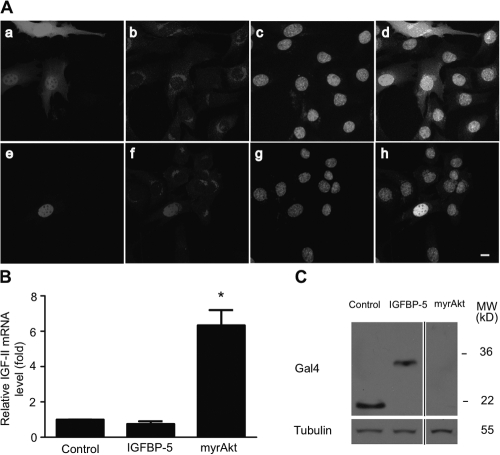
Recent studies have shown that IGFBP-5 is not only secreted, but can also enter the nucleus in certain cell types and that IGFBP-5 contains a functional transactivation domain in its conserved N-domain (Firth and Baxter, 2002; Xu et al., 2004; Zhao et al., 2006). We wondered whether IGFBP-5 could exert a direct effect on IGF-II transcription in the nucleus. To test this idea, immunocytochemical experiments were performed to determine whether endogenous IGFBP-5 is present in the nucleus in differentiating C2C12 cells. Confocal microscopy analysis showed that endogenous IGFBP-5 signal was detected in the Golgi apparatus, in the shape of a crescent moon outside of the nucleus (Fig. 8 A, a–d). This is consistent with the fact that IGFBP-5 is secreted from these cells. To show that the IGFBP-5 antibody could detect nuclear IGFBP-5 if present, we generated a plasmid that expresses a nonsecreted form of IGFBP-5 (with the signal peptide deleted) with an EGFP tag at its N terminus, and introduced it into cells by transient transfection. The nonsecreted EGFP-mIGFBP-5 was clearly localized in the nucleus as indicated by the overlap of GFP signal and IGFBP-5 immunoreactivity with DAPI signal (Fig. 8 A, e–h). These results indicate endogenous IGFBP-5 is not localized in the nucleus in myoblasts. Next, we transfected C2C12 cells with an IGFBP-5 construct that contains its functional transactivation domain (Xu et al., 2004) and examined its impact on the IGF-II gene expression. As shown in Fig. 8 B, no significant increase was detected in IGF-II mRNA expression levels in the control or IGFBP-5 transfected cells, although the IGFBP-5 fusion protein was highly expressed (Fig. 8 C). In comparison, myrAkt overexpression significantly increased IGF-II mRNA levels (Fig. 8 B). Together, these results suggest that endogenous IGFBP-5 is not localized in the nucleus and forced nuclear expression of the IGFBP-5 transactivation domain does not increase IGF-II expression in differentiating myoblast cells.
Figure 8.
IGFBP-5 does not exert direct effect on IGF-II gene expression. (A) IGFBP-5 is not localized in the nucleus. C2C12 cells were transfected with either pcDNA3.1-EGFP (a–d) or pcDNA3.1-EGFP-mIGFBP-5 (e–h). 24 h after the induction of differentiation, cells were fixed, the membrane permeabilized, and stained with an IGFBP-5 antibody (b and f) and counter-stained with DAPI (c and g). Panels a and e are GFP signal, and panels d and h are overlays. Bar, 10 μm. (B) Forced nuclear expression of the IGFBP-5 transactivation domain does not increase IGF-II expression. C2C12 cells were transfected with either pBIND vector, pBIND-BP5, or myrAkt and induced to differentiate. 36 h later, total RNA samples were isolated and IGF-II mRNA levels were determined by qRT-PCR. Values are relative to the control group after normalized by cyclophilin levels. *, P < 0.05 compared with the control group. (C) Western blot of cell lysates described in B using a Gal4 antibody.
IGFBP-5 promotes myogenic differentiation by switching on the IGF-II auto-regulation loop
How does IGFBP-5 act to increase IGF-II gene expression and to promote myogenesis? IGFBP-5 has been shown to be located on the cell surface and/or extracellular matrix in fibroblasts and muscle cells (Clemmons, 2001). Because IGF-II stimulates its own gene expression through a positive auto-regulatory loop, we hypothesized that cell surface–associated IGFBP-5 up-regulates IGF-II gene expression and promotes myogenesis by binding to IGF-II and enhances its interaction with the IGF-IR, thereafter amplifying IGF signaling activity during myogenesis. To test this hypothesis, immunocytochemical staining was performed. As shown in Fig. 9 A, IGFBP-5 signal was clearly observed on the surface of cells that were fixed in a buffer containing no detergent and therefore with the integrity of the plasma membrane intact (panel a). When the plasma membrane was permealized, however, no such signal was observed, even though intracellular IGFBP-5 was clearly visible (Fig. 9 A, panel b). We further demonstrated that intact IGFBP-5 is present on the surface of differentiating myoblasts and is capable of IGF binding by treating C2C12 cells with a high salt buffer to release its cell surface–bound IGFBP-5. As shown in Fig. 9 B, no cell surface–associated IGFBP-5 was found in myoblasts before the induction of differentiation. In contrast, a large amount of intact IGFBP-5 was detected on the surface of these cells 24 h after the induction of differentiation.
Figure 9.
IGFBP-5 is localized on the cell surface and promotes myogenic differentiation by binding to and promoting IGF-II action. (A) 24 h after the induction of differentiation, C2C12 cells were fixed without (a) or with (b) membrane permeabilization and stained with an IGFBP-5 antibody. Bar, 10 μm. (B) 0 and 24 h after induction of differentiation, C2C12 cells were incubated with a high salt buffer to strip off membrane/ECM bound IGFBP-5. The released IGFBP-5 was detected by ligand blot. (C) C2C12 cells were switched to serum-free medium containing 300 ng/ml IGF-II or Des(1-6)IGF-II. 36 h later, the cells were subjected to MHC immunostaining and DAPI staining and the differentiation index determined as described in Materials and methods. Data are means ± SE of three independent experiments with duplicates. Group *, #, and • are significantly different from each other at P < 0.05. (D) Cells transfected with pSUPER or pSUPER-BP5 were switched to DM (containing 0.5% horse serum) without or with 50 ng/ml IGF-II, 50 ng/ml IGF-II + 210 ng/ml wild–type IGFBP-5, 210 ng/ml wild-type IGFBP-5, 50 ng/ml IGF-II + 210 ng/ml LBD-IGFBP-5, 210 ng/ml LBD-IGFBP-5, or 150 ng/ml IGF-II for 4 d. MHC levels were measured by Western immunoblot and quantified by densitometry. Values are expressed as relative to the IGFBP-5 siRNA group. Data are means ± SE of 3–4 independent experiments. Group * is significantly different from group # at P < 0.05.
Next, we compared the myogenic activities of IGF-II or Des(1-6)IGF-II. Des(1-6)IGF-II binds to the IGF-IR but does not bind to IGFBPs. As shown in Fig. 9 C, compared with IGF-II, Des(1-6)IGF-II was significantly less potent in stimulating differentiation. Because IGFBP-5 is the predominant IGFBP secreted by these cells, the reduced activity of Des(1-6)IGF-II is consistent with the notion that endogenous IGFBP-5 binds to IGF-II and promotes IGF-II actions. To test this further and to determine whether the potentiation effect of IGFBP-5 is indeed due to its binding to IGF-II, IGF-II and IGFBP-5 were added individually or in combination to cells transfected with pSUPER or pSUPER-BP5. pSUPER-BP5–transfected cells did not form myotubes and had significantly reduced MHC levels (Fig. 9 D). Addition of a relatively high concentration (150 ng/ml) of IGF-II partially “rescued” these myogenic defects caused by IGFBP-5 knockdown. At a low concentration (50 ng/ml), however, IGF-II by itself had little effect. When the low concentration of IGF-II (50 ng/ml) was added in combination with an equal molar concentration of IGFBP-5 (210 ng/ml) to pSUPER-BP5–transfected cells, these cells showed comparable degree of myotube formation and MHC expression to those of the high IGF-II (150 ng/ml) group and the control pSUPER group. IGFBP-5 (210 ng/ml) alone had no rescuing effect (Fig. 9 D). These results suggest that IGFBP-5 does not act directly to affect myogenic differentiation. Rather, it works primarily by potentiating or amplifying IGF-II actions.
Next, LBD-IGFBP-5, an IGFBP-5 mutant with amino acid substations in the ligand binding domain and having 800-fold lower affinity to IGFs (Imai et al., 2000), was used to examine the mechanistic basis of IGFBP-5 action. As shown in Fig. 9 D, when added together with IGF-II (50 ng/ml) to pSUPER-BP5–transfected cells, IGFBP-5-LBD did not increase myotube formation or MHC expression, suggesting that the action of IGFBP-5 in promoting myogenic differentiation is dependent on its ability to bind to IGF-II.
The IGFBP-5 and IGF-II genes are similarly induced in primary myoblasts and IGFBP-5 knockdown inhibits primary cell differentiation
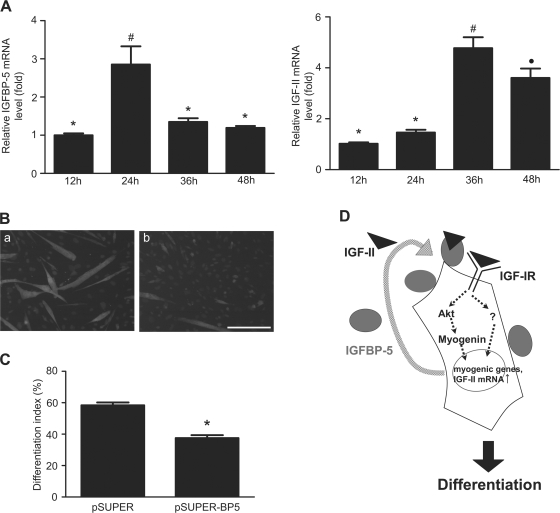
To establish the physiological relevance of the above observations, we examined the IGF-II and IGFBP-5 mRNA expression patterns in primary skeletal myoblasts prepared from neonatal mice. As shown in Fig. 10 A, 24 h after the initiation of differentiation, the expression of IGFBP-5 mRNA showed a 2.9-fold increase over the 12 h control (P < 0.05). Thereafter, the IGFBP-5 mRNA levels returned to the basal levels. In comparison, the IGF-II mRNA level remained unchanged at this early stage of differentiation. The IGF-II mRNA levels increased significantly at 36 h (4.6-fold over 12 h, P < 0.05) and remained significantly high at 48 h (3.6-fold, P < 0.05). Therefore, the overall patterns of IGF-II and IGFBP-5 induction and their temporal relationship are similar in these primary cells, although the magnitude of inductions is lower. To examine the role of IGFBP-5, primary skeletal muscle cells were transfected with pSUPER or pSUPER-BP5. When switched to DM, the vector transfected myoblasts formed morphologically distinctive multinucleated myotubes and expressed MHC (Fig. 10 B). In comparison, most pSUPER-BP5–transfected myoblasts had fewer MHC-positive cells. The differentiation index in the pSUPER-BP5–transfected group is 37.5% (Fig. 10 C), which is significantly lower than that of the control group (58.4%, P < 0.05). These findings suggest that the induction of IGFBP-5 occurs earlier than that of IGF-II and that IGFBP-5 promotes myogenic differentiation in primary skeletal muscle cells.
Figure 10.
The IGF-II and IGFBP-5 genes exhibit similar induction patterns and IGFBP-5 knockdown inhibits myogenic differentiation in primary skeletal muscle cells. (A) Total RNA was extracted from primary mouse skeletal myoblasts at the time indicated after the induction of differentiation. IGF-II mRNA and IGFBP-5 mRNA levels were measured by qRT-PCR. Values are expressed as relative levels to that of 12 h. Data shown are means ± SE of three independent experiments each performed in duplicate. Groups labeled with different symbols are different from each other at P < 0.05. (B) Neonatal mouse myoblasts were transfected with the empty pSUPER vector (a) or pSUPER-BP5 (b). 30 h after transfection, cells were induced to differentiate. Representative images from two independent groups are shown. Bar, 200 μm. (C) Quantified results of B. (D) A proposed model on how IGFBP-5 and IGF-II act in concert to stimulate myogenic differentiation. IGFBP-5 is induced in early stages of myogenesis and is located on the cell surface. Cell surface–associated IGFBP-5 binds to IGF-II and targets IGF-II to the close proximity of the IGF-IR receptor, thereby enhancing IGF-IR–mediated signaling activity, leading to a further increase in IGF-II gene expression. This, in turn, results in an accelerated increase in the IGF-IR-PI3K-Akt signaling activity, leading to increases in Myogenin expression, and promoting myogenic differentiation.
Discussion
In this study, we have explored the role(s) of endogenous IGFBP-5 in modulating the action of autocrine IGF-II in promoting myogenic differentiation. We show that knockdown of IGFBP-5 impairs myogenic differentiation. Addition of IGF-II “rescues” IGFBP-5 knockdown-induced myogenic defects, while Des(1-6)IGF-II, an IGF-II analogue with greatly reduced binding affinity for IGFBP, is significantly less effective. These data strongly argue that the endogenously secreted IGFBP-5 is a differentiation-promoting factor in skeletal muscle cells. This conclusion makes physiological sense in light of the highly induced IGFBP-5 expression before an appreciable increase in IGF-II production during early myogenesis. It is also in agreement with several previous studies. It has been reported that NFB4 cells, a mutant muscle cell line derived from C2C12 cells that are deficient in IGFBP-5 and IGF-II, do not undergo differentiation under the conditions in which C2C12 cells differentiate (Sarbassov et al., 1995). When added in an appropriate ratio to IGF-I, IGFBP-5 stimulates IGF-induced myoblast differentiation in rat muscle cells (Ewton et al., 1998). Recent loss-of-function studies in other cell types also suggest that IGFBP-5 promote bone, neuronal and epithelial cell differentiation (Yin et al., 2004; Tanno et al., 2005; Kiepe et al., 2006; Lochrie et al., 2006). More importantly, the IGFBP-5 and IGF-II genes exhibit similar expression patterns in primary skeletal muscle cells and knockdown of IGFBP-5 inhibits myogenic differentiation in primary cells. These findings suggest that IGFBP-5 promotes muscle cell differentiation and this finding is of physiological relevance.
Our finding, however, contradicts the view that IGFBP-5 is a negative factor of myogenesis. Several previous studies in C2 myoblasts have shown that stably overexpressing IGFBP-5 under a constitutive promoter impairs myogenic differentiation, presumably by binding to and sequestering IGF-II from its binding to the IGF-IR (James et al., 1996; Mukherjee et al., 2007). The different findings on the precise role IGFBP-5 in myogenesis could be due to different experimental approaches used. In the present study, we explored the role of endogenous IGFBP-5 through a loss-of-function approach followed by “rescuing” using proteins, whereas previous studies primarily relied on overexpression approaches. The different results may also be due to different cell lines used. Previous studies were conducted experiments with C2 cells (James et al., 1996; Cobb et al., 2004; Mukherjee et al., 2007). Instead, we used the C2C12 cell line, which is subcloned from the parent C2 cell line by selection for the ability to differentiate rapidly and produce extensive contracting myotubes (Silberstein et al., 1986). Moreover, we obtained similar results in primary skeletal muscle cells, strongly supporting the physiological relevance of our findings.
IGFBP-5, Myogenin, and IGF-II are all induced during differentiation (Rotwein et al., 1995; Bayol et al., 2000). The expression of IGFBP-5 correlates well with the expression of Myogenin (Bergstrom and Tapscott, 2001). Myogenin, IGF-II, and IGFBP-5 were undetectable in the mutant NFB4 cells, which cannot go through differentiation (Sarbassov et al., 1995). In cultured muscle cells, endogenously secreted IGF-II stimulates the IGF-IR, PI3K, and Akt to induce the expression of Myogenin (Wilson et al., 2004). The functional relationship between IGFBP-5, Myogenin, and IGF-II is not well understood. In this study, we found that IGFBP-5 knockdown caused marked reduction in both Myogenin and IGF-II expression, but had little effect on MyoD expression. Forced expression of functional Myogenin, however, failed to rescue the differentiation defects caused by IGFBP-5 knockdown, although it increased the differentiation index in the wild-type cells. It is noted that IGFBP-5 siRNA not only markedly decreased endogenous Myogenin expression levels but also caused a modest reduction in the expression levels of the Myogenin transgene. Importantly, forced expression of Myogenin did not cause significant changes in either IGF-II or IGFBP-5 mRNA levels. This suggests that while IGF-II and IGFBP-5 regulates Myogenin expression, Myogenin itself is not sufficient to induce myogenic differentiation in the absence of IGFBP-5 and/or IGF-II. Other factors must also be involved in the effect of IGFBP-5 on myogenesis. Future studies are needed to identify these factors.
One of the intriguing findings made in this study is that IGFBP-5 knockdown suppresses IGF-II expression. IGFBP-5 knockdown had little effect on the IGF-I and IGF-IR mRNA levels. Knockdown of IGFBP-5 did not affect the levels of other IGFBPs synthesized by C2C12 either. The specificity of this siRNA construct has been verified using a siRNA resistant form of IGFBP-5 in U2OS cells (Yin et al., 2004). In addition, the suppressed IGF-II expression was reversed by adding IGFBP-5 together with low concentrations of IGF-II or by a high concentration of IGF-II. Expression of myrAkt also restores IGF-II expression levels. We also ruled out nonspecific interferon responses using another pSUPER construct expressing siRNA with a sequence unrelated to IGFBP-5. Our data showed that myrAkt overexpression significantly increased IGF-II gene expression 12 h after differentiation was induced. It is possible that Akt also regulates IGF-II gene expression during the early phase of myogenesis. Erbay et al. (2003) showed that mTOR signaling, which is downstream of Akt, is important for IGF-II induction during early phase of myogenic differentiation (3 h after differentiation was induced). However, further studies are needed to further clarify this issue.
IGFBP-5 is not only secreted but can also enter the nucleus in certain cell type (Firth and Baxter, 2002). We have recently reported that IGFBP-5 contains a functional transactivation domain in its conserved N-domain and this activity is IGF-binding independent (Xu et al., 2004; Zhao et al., 2006). Furthermore, full-length IGFBP-5 has transcriptional repressing activity (Zhao et al., 2006). Our finding that IGFBP-5 knockdown suppresses IGF-II mRNA expression in differentiating muscle cells raised the possibility that IGFBP-5 may exert direct an effect on IGF-II gene transcription in the nucleus. Two approaches were taken to address this intriguing hypothesis. First, immunocytochemical staining experiments showed that endogenous IGFBP-5 protein was detected in the Golgi apparatus and on the cell surface but not in the nuclei in myoblasts. Second, expression of the IGFBP-5 transactivation domain did not cause any significant change in IGF-II mRNA expression levels. These results argue against a direct action of IGFBP-5 on the IGF-II gene transcription in this cell type.
It is of interest to find that IGF-II up-regulates its own gene expression in differentiating C2C12 myoblasts. Although previous studies have indicated that nutrients influence IGF-II gene expression in C2C12 cells through the PI3K-Akt-mTOR pathway (Erbay et al., 2003), to our knowledge, this is the first demonstration that IGF-II stimulates its own gene expression in skeletal muscle cells. Furthermore, forced expression of a constitutive Akt strongly increases IGF-II expression in these cells, suggesting that IGF-II auto-regulates itself through activating the IGF-IR-PI3K-Akt signaling pathway in C2C12 cells. This and other findings led us to propose a new model, depicted in Fig. 10 D, on how IGFBP-5 acts to promote myogenic differentiation. According to this model, the induction of IGFBP-5 in early stages of myogenic differentiation plays a crucial role in promoting the myogenic action of autocrine IGF-II. IGFBP-5 acts by binding to IGF-II and promoting its interaction with IGF-IR. This interaction may contribute to reaching a certain threshold that turns on the IGF-II positive auto-regulatory loop, thereby resulting in increased IGF-II gene expression. The increase in IGF-II production, in turn, results in an accelerated elevation in the IGF-IR-PI3K-Akt signaling activity, leading to increases in Myogenin, MHC expression, and myotube formation. This model is supported by several lines of evidence. First, IGFBP-5 is induced in early stages of myogenesis, before the elevation of IGF-II expression, in the C2C12 myoblast model and in primary cells. Second, knockdown of IGFBP-5 impairs myogenic differentiation. Third, knockdown of IGFBP-5 suppresses IGF-II gene expression and addition of exogenous IGF-II “rescues” IGFBP-5 knockdown-induced myogenic defects. Furthermore, we show that IGF-II up-regulates its own gene expression via the PI3K-Akt signaling pathway. Addition of native IGFBP-5 but not a ligand binding IGFBP-5 mutant, together with a low concentration of IGF-II, restores IGF-II expression and myogenic differentiation.
Although it is known that IGFBP-5 has IGF-independent actions (Clemmons, 2001; Duan, 2002; Firth and Baxter, 2002), the myogenic promotion action of IGFBP-5 is clearly IGF-dependent because: (1) immunocytochemistry revealed that endogenous IGFBP-5 is not localized to the nuclei of C2C12 cells; (2) adding high concentrations of exogenous IGF-I or IGF-II reversed IGFBP-5 knockdown caused myogenic defects; (3) Des(1-6)IGF-II has lower activity in regulating differentiation; and (4) adding native IGFBP-5, but not the LBD mutant IGFBP-5, rescued the myogenic defects in the presence of low concentrations of IGF-II. It has been shown that IGFBP-5 is not only present in the extracellular fluids, but is also localized on the cell surface and/or extracellular matrix (Clemmons, 2001). In this study, we have provided two lines of independent evidence indicating that abundant amounts of intact IGFBP-5 is indeed located on the surface of differentiating myoblasts and is capable of IGF binding. It is possible that cell-surface associated IGFBP-5 provides a means of attracting IGF-II to the close proximity of the IGF-IR receptor, thereby enhancing IGF-II signaling activity and initiating the IGF-II positive auto-regulation loop.
In summary, we have uncovered a novel mechanism by which the induction of IGFBP-5 promotes the myogenic action of autocrine IGF-II. We provide evidence suggesting that IGFBP-5 is located on the cell surface and binds to autocrine IGF-II and potentiates its interaction with IGF-IR, leading to the enhanced activation of the IGF-IR-PI3K-Akt signaling activity and a further increase in IGF-II gene expression. An accelerated activation of this auto-regulatory loop stimulates muscle cell differentiation after reaching a certain threshold. We have extended these findings to primary cultures, suggesting that this mechanism is of physiological relevance.
Materials and methods
Materials and animals
Monoclonal MHC antibody (MF20) was purchased from the Developmental Studies Hybridoma Bank (Iowa City, IA). Antibodies against MyoD (M-318) and Myogenin (M-225) were purchased from Santa Cruz Biotechnology, Inc., and antibodies to Akt and phospho-Akt (Ser473) were from Cell Signaling Technology. Monoclonal anti-tubulin antibody (T6793) was from Sigma-Aldrich. The secondary antibody conjugates were purchased from Jackson ImmunoResearch Laboratories. Recombinant human IGF-I, IGF-II, Des(1-6)IGF-II, and IGFBP-5 were purchased from GroPep. Mutant IGFBP-5 with reduced affinity for IGF was provided by Dr. David Clemmons (University of North Carolina at Chapel Hill; Imai et al., 2000). Trypsin, fetal bovine serum (FBS), horse serum (HS), DMEM, OPTI-minimum essential medium, and penicillin-streptomycin were purchased from Invitrogen. The BCA protein assay kit was purchased from Thermo Fisher Scientific. Reagents for enhanced chemifluorescence were obtained from GE Healthcare. The Dual-luciferase reporter assay kit was from Promega. TriPure Isolation Reagent was from Roche. Oligonucleotide primers for PCR were purchased from Invitrogen. iQ SYBR Green Supermix was from Bio-Rad Laboratories. All other chemicals were reagent grade and were purchased from Thermo Fisher Scientific unless otherwise noted. Mice (strains CD1) were obtained from Charles River Laboratories.
Plasmid construction
pSUPER Vector was provided by Dr. Reuven Agami (Netherlands Cancer Institute). The construction and verification of pSUPER-BP-5, which generates IGFBP-5 siRNA, was described previously (Yin et al., 2004). A control pSUPER construct expressing siRNA with a sequence (TCCTGCAGTGGATGGATGT, targeting the mouse TSC2 N-terminal sequence) unrelated to IGFBP-5 was also used as a control. Mouse IGF-II cDNA and partial mouse IGFBP-5 cDNA were amplified by RT-PCR and cloned into the pGEM-T easy vector (Promega). Myogenin plasmids (pCS2MTMyogenin and pCS2EGFP) and the Myogenin reporter construct 4RTK and control TK reporter construct (Tang et al., 2004) were provided by Dr. Daniel Goldman (University of Michigan). MyoD cDNA was a gift from Dr. Lassar Andrew (Harvard Medical School). The pCS2+myr-Akt construct, which expresses a constitutively active, membrane-localized full-length mouse Akt1 (Kohn et al., 1998), was provided by Dr. Anne Vojtek (University of Michigan). The pcDNA3.1 EGFP-mIGFBP5, which expresses a nonsecreted form of IGFBP-5 with EGFP tagged at its N terminus, was generated by inserting mature IGFBP-5 (with signal peptide deleted) into the pcDNA3.1(+) vector. All of the plasmids were verified by DNA sequencing.
Cell culture and transfection
Mouse C2C12 cells were purchased from American Type Culture Collection (Manassas, VA) and cultured in DMEM supplemented with 10% FBS in a humidified air atmosphere containing 5% CO2. For transfection, 6 × 104 cells were seeded in 6-well plates (Falcon). 2 μg of pSUPER-BP5 or pSUPER plasmid DNA was transfected into cells as previously reported (Yin et al., 2004). 30 h after transfection, the cells were washed and incubated with fresh serum-free medium (SFM) for 48 h. At the end of incubation, the conditioned media and total RNA were prepared for further analysis. 30 h after transfection, the cells were washed with SFM and then transferred to differentiation medium (DM) consisting of DMEM plus 0.5–2% horse serum. For rescue experiments, 30 h after transfection, the cells were switched to DM plus IGF-I, IGF-II, and/or IGFBP-5 with concentration designated in each experiment. Primary mouse skeletal myoblasts were isolated from 2–5-d-old mice, grown, and differentiated following a previously reported method (Tang and Goldman, 2006). In brief, the forelimbs and hindlimbs were removed from neonatal mice. Muscle mass was minced into a coarse slurry and enzymatically dissociated at 37°C for 35–45 min with frequent trituration. Isolated muscle cells were grown in DMEM supplemented with 10% FBS and 10% HS on collagen-coated plates at a density of 2–4 × 105 cells per 35-mm culture dish. FuGene6 (Roche) was used for transfection of these primary cells. Differentiation was induced by switching to DMEM medium containing 5% or 1% HS. All experiments were conducted in accordance with guidelines approved by the University Committee on the Use and Care of Animals (University of Michigan).
Northern blot
Total RNA was extracted using TriPure Reagent following the manufacturer's instructions. RNA samples were size-fractionated in 1.2% agarose gels, blotted, and fixed onto Hybond N membranes (GE Healthcare). Hybridization was performed using 32P-dCTP-labeled (MP Biomedicals) IGFBP-5 or IGFBP-4 cDNA probes as reported previously (Duan et al., 1996). Labeled GAPDH cDNA was used as a control. The band densities were quantified using Quantity One quantification software (Bio-Rad Laboratories).
Reverse transcription (RT)-PCR and quantitative real-time RT-PCR (qRT-PCR)
After treated with DNase, RNA samples were subjected to reverse-transcription using SuperScript II reverse transcription (Invitrogen). Gene specific primers for IGF-I, IGF-I receptor, IGFBP-2, IGFBP-4, and S17 were described previously (Wilson et al., 2003; Boutinaud et al., 2004). IGF-II primers for RT-PCR are 5′-GGCTTCTACTTCAGCAGGC-3′ (sense), 5′-GGTGGTAACACGATCAGGG-3′ (antisense). The linear range of product amplification was established in pilot studies for each primer pair.
qRT-PCR was performed using an iCycler iQ Multicolor real-time PCR detection system (Bio-Rad Laboratories). Primer sequences for qRT-PCR were reported by others (Boutinaud et al., 2004). 4 μl of the cDNA product (1:20 dilution) was used as a PCR template. Plasmid cDNA for cyclophilin (Hasel and Sutcliffe, 1990), a housekeeping gene, was provided by Dr. Gregor Sutcliffe (Scripps Research Institute). Serial dilutions of the plasmids ranging from 108 to 10 molecules/2 μl were used for standard curve. The number of molecules of particular gene transcript was calculated based on the standard curve and normalized to the cyclophilin mRNA level. The specificity of the PCR was verified by denaturing curve analysis and direct sequencing of the products.
Immunohistochemistry
Cells cultured on 6-well dishes were washed twice with 1x PBS before fixation in 4% paraformaldehyde for 10 min. Cells were permeabilized for 5 min in 1x PBS containing 0.2% Triton X-100 (PBST), washed twice with PBST, and incubated with primary antibodies at 4°C overnight. The cells were next washed three times with PBST before incubation with Cy3-conjugated secondary antibodies for 2 h at room temperature. For experiments that required intact membrane integrity, staining was performed in 1x PBS without detergent. Immunofluorescence or phase-contrast images were obtained at room temperature using a microscope (Eclipse E600; Nikon) with Plan Fluor 10x/0.3 or 20x/0.5 dry objectives. Images were acquired using a camera (DC50NN; Nikon) and Scion acquisition software. Images were processed with Photoshop for overlay. A Leica SP5 microscope with HCX PL APO CS 40.0×/0.85 dry objective was used for confocal imaging at room temperature. Images were acquired and processed using LAS AF software (Leica) with background subtraction for some images. Nucleus was stained with DAPI. Differentiation index (%) was quantified as the percentage of MHC-positive nuclei over total nuclei number.
Western and ligand blot analysis
Equal amounts of protein were separated by SDS-PAGE and transferred to Immobilon P membranes (Millipore) and subjected to Western blot following published procedures (Duan et al., 1996). The antibodies were used at concentrations recommended by the commercial sources. Ligand blot analysis was performed using digoxigenin-labeled IGF-I following published procedure (Yin et al., 2004). To determine cell surface–bound IGFBP-5, cultured cells were washed in 1x PBS twice and then incubated briefly in a high salt buffer (2N NaCl).
Luciferase reporter assay
The transcription activity of Myogenin was determined using the dual luciferase reporter assay kit (Promega). In brief, myoblasts were cotransfected with pCS2MTmyogenin or pCS2 plasmid, 4RTK or control minimal TK reporter, and an internal control vector encoding Renilla luciferase. Differentiation was induced 1 d after transfection. 2 d after inducing differentiation, cells were washed, and cell lysates were used to measure the firefly and Renilla luciferase activities. The result was expressed as fold change over the empty control vector and 4RTK transfected group. Transfection efficiency was normalized by Renilla luciferase activity.
Statistical analysis
Differences among groups were analyzed by t test or one-way analysis of variance followed by Tukey post test, using Prizm (GraphPad Software, Inc.). Significance was accepted at P < 0.05.
Acknowledgments
We thank Drs. D. Clemmons, R. Agami, G. Sutcliffe, D. Goldman, L. Andrew, and A. Vojtek for providing reagents for this work. We are very grateful to Dr. Huibin Tang (University of Michigan), for his advice on primary muscle cell cultures. We also thank John Allard and Justin Lenhart for proofreading this manuscript.
This study was supported by National Institutes of Health Grant 2RO1HL60679, National Science Foundation Research Grant IOB 0110864, and a fellowship from the Changjiang Scholars Program.
H. Ren and P. Yin contributed equally to this paper.
Abbreviations used in this paper: DM, differentiation medium; IGF, insulin-like growth factor; IGFBP, IGF binding protein; IGF-IR, IGF-I receptor; MHC, myosin heavy chain; PI3K, phosphatidylinositol 3-kinase; TK, thymidine kinase.
References
- Barton, E.R., L. Morris, A. Musaro, N. Rosenthal, and H.L. Sweeney. 2002. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J. Cell Biol. 157:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton-Davis, E.R., D.I. Shoturma, A. Musaro, N. Rosenthal, and H.L. Sweeney. 1998. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc. Natl. Acad. Sci. USA. 95:15603–15607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayol, S., P.T. Loughna, and C. Brownson. 2000. Phenotypic expression of IGF binding protein transcripts in muscle, in vitro and in vivo. Biochem. Biophys. Res. Commun. 273:282–286. [DOI] [PubMed] [Google Scholar]
- Bergstrom, D.A., and S.J. Tapscott. 2001. Molecular distinction between specification and differentiation in the myogenic basic helix-loop-helix transcription factor family. Mol. Cell. Biol. 21:2404–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutinaud, M., J.H. Shand, M.A. Park, K. Phillips, J. Beattie, D.J. Flint, and G.J. Allan. 2004. A quantitative RT-PCR study of the mRNA expression profile of the IGF axis during mammary gland development. J. Mol. Endocrinol. 33:195–207. [DOI] [PubMed] [Google Scholar]
- Buckingham, M. 2001. Skeletal muscle formation in vertebrates. Curr. Opin. Genet. Dev. 11:440–448. [DOI] [PubMed] [Google Scholar]
- Buckingham, M., L. Bajard, T. Chang, P. Daubas, J. Hadchouel, S. Meilhac, D. Montarras, D. Rocancourt, and F. Relaix. 2003. The formation of skeletal muscle: from somite to limb. J. Anat. 202:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons, D.R. 2001. Use of mutagenesis to probe IGF-binding protein structure/function relationships. Endocr. Rev. 22:800–817. [DOI] [PubMed] [Google Scholar]
- Cobb, L.J., D.A. Salih, I. Gonzalez, G. Tripathi, E.J. Carter, F. Lovett, C. Holding, and J.M. Pell. 2004. Partitioning of IGFBP-5 actions in myogenesis: IGF-independent anti-apoptotic function. J. Cell Sci. 117:1737–1746. [DOI] [PubMed] [Google Scholar]
- Coleman, M.E., F. DeMayo, K.C. Yin, H.M. Lee, R. Geske, C. Montgomery, and R.J. Schwartz. 1995. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J. Biol. Chem. 270:12109–12116. [DOI] [PubMed] [Google Scholar]
- Coolican, S.A., D.S. Samuel, D.Z. Ewton, F.J. McWade, and J.R. Florini. 1997. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J. Biol. Chem. 272:6653–6662. [DOI] [PubMed] [Google Scholar]
- Duan, C. 2002. Specifying the cellular responses to IGF signals: roles of IGF-binding proteins. J. Endocrinol. 175:41–54. [DOI] [PubMed] [Google Scholar]
- Duan, C., S.B. Hawes, T. Prevette, and D.R. Clemmons. 1996. Insulin-like growth factor-I (IGF-I) regulates IGF-binding protein-5 synthesis through transcriptional activation of the gene in aortic smooth muscle cells. J. Biol. Chem. 271:4280–4288. [DOI] [PubMed] [Google Scholar]
- Engert, J.C., E.B. Berglund, and N. Rosenthal. 1996. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J. Cell Biol. 135:431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbay, E., I.H. Park, P.D. Nuzzi, C.J. Schoenherr, and J. Chen. 2003. IGF-II transcription in skeletal myogenesis is controlled by mTOR and nutrients. J. Cell Biol. 163:931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewton, D.Z., S.A. Coolican, S. Mohan, S.D. Chernausek, and J.R. Florini. 1998. Modulation of insulin-like growth factor actions in L6A1 myoblasts by insulin-like growth factor binding protein (IGFBP)-4 and IGFBP-5: a dual role for IGFBP-5. J. Cell. Physiol. 177:47–57. [DOI] [PubMed] [Google Scholar]
- Firth, S.M., and R.C. Baxter. 2002. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 23:824–854. [DOI] [PubMed] [Google Scholar]
- Florini, J.R., K.A. Magri, D.Z. Ewton, P.L. James, K. Grindstaff, and P.S. Rotwein. 1991. “Spontaneous” differentiation of skeletal myoblasts is dependent upon autocrine secretion of insulin-like growth factor-II. J. Biol. Chem. 266:15917–15923. [PubMed] [Google Scholar]
- Florini, J.R., D.Z. Ewton, K.A. Magri, and F.J. Mangiacapra. 1993. IGFs and muscle differentiation. Adv. Exp. Med. Biol. 343:319–326. [DOI] [PubMed] [Google Scholar]
- Florini, J.R., D.Z. Ewton, and S.A. Coolican. 1996. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr. Rev. 17:481–517. [DOI] [PubMed] [Google Scholar]
- Green, B.N., S.B. Jones, R.D. Streck, T.L. Wood, P. Rotwein, and J.E. Pintar. 1994. Distinct expression patterns of insulin-like growth factor binding proteins 2 and 5 during fetal and postnatal development. Endocrinology. 134:954–962. [DOI] [PubMed] [Google Scholar]
- Hasel, K.W., and J.G. Sutcliffe. 1990. Nucleotide sequence of a cDNA coding for mouse cyclophilin. Nucleic Acids Res. 18:4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, Y., A. Moralez, U. Andag, J.B. Clarke, W.H. Busby Jr., and D.R. Clemmons. 2000. Substitutions for hydrophobic amino acids in the N-terminal domains of IGFBP-3 and -5 markedly reduce IGF-I binding and alter their biologic actions. J. Biol. Chem. 275:18188–18194. [DOI] [PubMed] [Google Scholar]
- James, P.L., S.B. Jones, W.H. Busby Jr., D.R. Clemmons, and P. Rotwein. 1993. A highly conserved insulin-like growth factor-binding protein (IGFBP-5) is expressed during myoblast differentiation. J. Biol. Chem. 268:22305–22312. [PubMed] [Google Scholar]
- James, P.L., C.E. Stewart, and P. Rotwein. 1996. Insulin-like growth factor binding protein-5 modulates muscle differentiation through an insulin-like growth factor-dependent mechanism. J. Cell Biol. 133:683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, B.H., M. Aoki, J.Z. Zheng, J. Li, and P.K. Vogt. 1999. Myogenic signaling of phosphatidylinositol 3-kinase requires the serine-threonine kinase Akt/protein kinase B. Proc. Natl. Acad. Sci. USA. 96:2077–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliman, P., F. Vinals, X. Testar, M. Palacin, and A. Zorzano. 1996. Phosphatidylinositol 3-kinase inhibitors block differentiation of skeletal muscle cells. J. Biol. Chem. 271:19146–19151. [DOI] [PubMed] [Google Scholar]
- Kaspar, B.K., J. Llado, N. Sherkat, J.D. Rothstein, and F.H. Gage. 2003. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 301:839–842. [DOI] [PubMed] [Google Scholar]
- Kiepe, D., S. Ciarmatori, A. Haarmann, and B. Tonshoff. 2006. Differential expression of IGF system components in proliferating vs. differentiating growth plate chondrocytes: the functional role of IGFBP-5. Am. J. Physiol. Endocrinol. Metab. 290:E363–E371. [DOI] [PubMed] [Google Scholar]
- Kohn, A.D., A. Barthel, K.S. Kovacina, A. Boge, B. Wallach, S.A. Summers, M.J. Birnbaum, P.H. Scott, J.C. Lawrence Jr., and R.A. Roth. 1998. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J. Biol. Chem. 273:11937–11943. [DOI] [PubMed] [Google Scholar]
- Lawlor, M.A., and P. Rotwein. 2000. a. Coordinate control of muscle cell survival by distinct insulin-like growth factor activated signaling pathways. J. Cell Biol. 151:1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor, M.A., and P. Rotwein. 2000. b. Insulin-like growth factor-mediated muscle cell survival: central roles for Akt and cyclin-dependent kinase inhibitor p21. Mol. Cell. Biol. 20:8983–8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor, M.A., X. Feng, D.R. Everding, K. Sieger, C.E. Stewart, and P. Rotwein. 2000. Dual control of muscle cell survival by distinct growth factor-regulated signaling pathways. Mol. Cell. Biol. 20:3256–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J.P., J. Baker, A.S. Perkins, E.J. Robertson, and A. Efstratiadis. 1993. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 75:59–72. [PubMed] [Google Scholar]
- Lochrie, J.D., K. Phillips, E. Tonner, D.J. Flint, G.J. Allan, N.C. Price, and J. Beattie. 2006. Insulin-like growth factor binding protein (IGFBP)-5 is upregulated during both differentiation and apoptosis in primary cultures of mouse mammary epithelial cells. J. Cell. Physiol. 207:471–479. [DOI] [PubMed] [Google Scholar]
- Mukherjee, A., E.M. Wilson, and P. Rotwein. 2007. Insulin-like growth factor (IGF) binding protein-5 blocks skeletal muscle differentiation by inhibiting IGF actions. Mol. Endocrinol. 22:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musaro, A., K. McCullagh, A. Paul, L. Houghton, G. Dobrowolny, M. Molinaro, E.R. Barton, H.L. Sweeney, and N. Rosenthal. 2001. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 27:195–200. [DOI] [PubMed] [Google Scholar]
- Musaro, A., C. Giacinti, G. Borsellino, G. Dobrowolny, L. Pelosi, L. Cairns, S. Ottolenghi, G. Cossu, G. Bernardi, L. Battistini, et al. 2004. Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proc. Natl. Acad. Sci. USA. 101:1206–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning, Y., A.G. Schuller, S. Bradshaw, P. Rotwein, T. Ludwig, J. Frystyk, and J.E. Pintar. 2006. Diminished growth and enhanced glucose metabolism in triple knockout mice containing mutations of insulin-like growth factor binding protein-3, -4, and -5. Mol. Endocrinol. 20:2173–2186. [DOI] [PubMed] [Google Scholar]
- Perry, R.L., and M.A. Rudnick. 2000. Molecular mechanisms regulating myogenic determination and differentiation. Front. Biosci. 5:D750–D767. [DOI] [PubMed] [Google Scholar]
- Powell-Braxton, L., P. Hollingshead, C. Warburton, M. Dowd, S. Pitts-Meek, D. Dalton, N. Gillett, and T.A. Stewart. 1993. IGF-I is required for normal embryonic growth in mice. Genes Dev. 7:2609–2617. [DOI] [PubMed] [Google Scholar]
- Rommel, C., B.A. Clarke, S. Zimmermann, L. Nunez, R. Rossman, K. Reid, K. Moelling, G.D. Yancopoulos, and D.J. Glass. 1999. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 286:1738–1741. [DOI] [PubMed] [Google Scholar]
- Rommel, C., S.C. Bodine, B.A. Clarke, R. Rossman, L. Nunez, T.N. Stitt, G.D. Yancopoulos, and D.J. Glass. 2001. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 3:1009–1013. [DOI] [PubMed] [Google Scholar]
- Rosenthal, S.M., and Z.Q. Cheng. 1995. Opposing early and late effects of insulin-like growth factor I on differentiation and the cell cycle regulatory retinoblastoma protein in skeletal myoblasts. Proc. Natl. Acad. Sci. USA. 92:10307–10311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotwein, P., P.L. James, and K. Kou. 1995. Rapid activation of insulin-like growth factor binding protein-5 gene transcription during myoblast differentiation. Mol. Endocrinol. 9:913–923. [DOI] [PubMed] [Google Scholar]
- Sabourin, L.A., and M.A. Rudnicki. 2000. The molecular regulation of myogenesis. Clin. Genet. 57:16–25. [DOI] [PubMed] [Google Scholar]
- Salih, D.A., G. Tripathi, C. Holding, T.A. Szestak, M.I. Gonzalez, E.J. Carter, L.J. Cobb, J.E. Eisemann, and J.M. Pell. 2004. Insulin-like growth factor-binding protein 5 (Igfbp5) compromises survival, growth, muscle development, and fertility in mice. Proc. Natl. Acad. Sci. USA. 101:4314–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov, D.D., R. Stefanova, V.G. Grigoriev, and C.A. Peterson. 1995. Role of insulin-like growth factors and myogenin in the altered program of proliferation and differentiation in the NFB4 mutant muscle cell line. Proc. Natl. Acad. Sci. USA. 92:10874–10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein, L., S.G. Webster, M. Travis, and H.M. Blau. 1986. Developmental progression of myosin gene expression in cultured muscle cells. Cell. 46:1075–1081. [DOI] [PubMed] [Google Scholar]
- Stewart, C.E., P.L. James, M.E. Fant, and P. Rotwein. 1996. Overexpression of insulin-like growth factor-II induces accelerated myoblast differentiation. J. Cell. Physiol. 169:23–32. [DOI] [PubMed] [Google Scholar]
- Tang, H., and D. Goldman. 2006. Activity-dependent gene regulation in skeletal muscle is mediated by a histone deacetylase (HDAC)-Dach2-myogenin signal transduction cascade. Proc. Natl. Acad. Sci. USA. 103:16977–16982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, H., P. Macpherson, L.S. Argetsinger, D. Cieslak, S.T. Suhr, C. Carter-Su, and D. Goldman. 2004. CaM kinase II-dependent phosphorylation of myogenin contributes to activity-dependent suppression of nAChR gene expression in developing rat myotubes. Cell. Signal. 16:551–563. [DOI] [PubMed] [Google Scholar]
- Tanno, B., V. Cesi, R. Vitali, F. Sesti, M.L. Giuffrida, C. Mancini, B. Calabretta, and G. Raschella. 2005. Silencing of endogenous IGFBP-5 by micro RNA interference affects proliferation, apoptosis and differentiation of neuroblastoma cells. Cell Death Differ. 12:213–223. [DOI] [PubMed] [Google Scholar]
- Wilson, E.M., and P. Rotwein. 2007. Selective control of skeletal muscle differentiation by Akt1. J. Biol. Chem. 282:5106–5110. [DOI] [PubMed] [Google Scholar]
- Wilson, E.M., M.M. Hsieh, and P. Rotwein. 2003. Autocrine growth factor signaling by insulin-like growth factor-II mediates MyoD-stimulated myocyte maturation. J. Biol. Chem. 278:41109–41113. [DOI] [PubMed] [Google Scholar]
- Wilson, E.M., J. Tureckova, and P. Rotwein. 2004. Permissive roles of phosphatidyl inositol 3-kinase and Akt in skeletal myocyte maturation. Mol. Biol. Cell. 15:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, A.W., C. Duan, and H.A. Bern. 2005. Insulin-like growth factor signaling in fish. Int. Rev. Cytol. 243:215–285. [DOI] [PubMed] [Google Scholar]
- Xu, Q., and Z. Wu. 2000. The insulin-like growth factor-phosphatidylinositol 3-kinase-Akt signaling pathway regulates myogenin expression in normal myogenic cells but not in rhabdomyosarcoma-derived RD cells. J. Biol. Chem. 275:36750–36757. [DOI] [PubMed] [Google Scholar]
- Xu, Q., S. Li, Y. Zhao, T.J. Maures, P. Yin, and C. Duan. 2004. Evidence that IGF binding protein-5 functions as a ligand-independent transcriptional regulator in vascular smooth muscle cells. Circ. Res. 94:E46–E54. [DOI] [PubMed] [Google Scholar]
- Yin, P., Q. Xu, and C. Duan. 2004. Paradoxical actions of endogenous and exogenous insulin-like growth factor-binding protein-5 revealed by RNA interference analysis. J. Biol. Chem. 279:32660–32666. [DOI] [PubMed] [Google Scholar]
- Zhao, Y., P. Yin, L.A. Bach, and C. Duan. 2006. Several acidic amino acids in the N-domain of insulin-like growth factor-binding protein-5 are important for its transactivation activity. J. Biol. Chem. 281:14184–14191. [DOI] [PubMed] [Google Scholar]