Abstract
Directed cell migration requires the orientation of the Golgi and centrosome toward the leading edge. We show that stimulation of interphase cells with the mitogens epidermal growth factor or lysophosphatidic acid activates the extracellular signal–regulated kinase (ERK), which phosphorylates the Golgi structural protein GRASP65 at serine 277. Expression of a GRASP65 Ser277 to alanine mutant or a GRASP65 1–201 truncation mutant, neither of which can be phosphorylated by ERK, prevents Golgi orientation to the leading edge in a wound assay. We show that phosphorylation of GRASP65 with recombinant ERK leads to the loss of GRASP65 oligomerization and causes Golgi cisternal unstacking. Furthermore, preventing Golgi polarization by expressing mutated GRASP65 inhibits centrosome orientation, which is rescued upon disassembly of the Golgi structure by brefeldin A. We conclude that Golgi remodeling, mediated by phosphorylation of GRASP65 by ERK, is critical for the establishment of cell polarity in migrating cells.
Introduction
Cell migration is essential for diverse physiological processes including embryonic development, wound healing, and immune response (Ridley et al., 2003; Jaffe and Hall, 2005). The generation and maintenance of cellular polarity is essential for directional cell migration and must be established for migration to occur. Rac, Rho, and Cdc42 regulate many aspects of polarization, including actin polymerization and microtubule stabilization at the leading edge (Jaffe and Hall, 2005; Ridley, 2006). The activation of Cdc42 induces the reorganization of the actin and microtubule cytoskeletons, which causes the nucleus to move to the back of the cell and the microtubule organizing center together with the Golgi to orient facing the leading edge (Gomes et al., 2005).
Several lines of evidence suggest important roles for the polarized localization of the Golgi at the leading edge. Cell migration requires the regulated transport of vesicles and the addition of new membranes to the leading edge (Jaffe and Hall, 2005). Polarized secretion at the leading edge is regulated by the exocyst, which is essential for exocytosis, and also organizes actin polarization at the leading edge (Zuo et al., 2006). In addition, the directional delivery of membranes and proteins toward the leading edge is dependent on Golgi-localized protein kinase D, which regulates the formation of transport vesicles from the trans-Golgi network to the plasma membrane (Liljedahl et al., 2001). Inhibition of protein kinase D blocks both membrane delivery to the cell surface and directional cell migration (Prigozhina and Waterman-Storer, 2004). These examples suggest that vesicle formation and fusion are tightly coupled to directed cell motility. Additionally, delivery of adhesion molecules and cytoskeletal components are all important functions of a polarized Golgi (Mellor, 2004).
Results and discussion
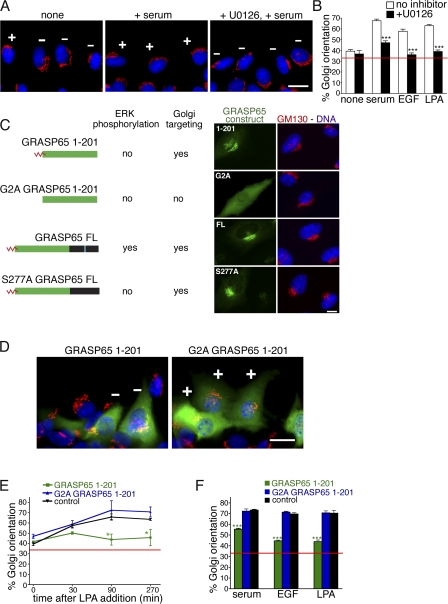
Polarization before cell migration is induced by mitogens that activate extracellular signal–regulated kinase (ERK; Pullikuth and Catling, 2007). Active ERK and H-Ras, as well as scaffolding proteins of MAPK/ERK kinase (MEK)/ERK, have been localized on the Golgi (Torii et al., 2004). Stimulation of cells with EGF activates ERK, which phosphorylates the Golgi protein GRASP65 on Ser277 (Yoshimura et al., 2005). To confirm the role of ERK in mitogen-induced Golgi polarization, we analyzed the orientation of the Golgi toward the leading edge. Confluent monolayers of normal rat kidney (NRK) cells were serum starved for 24 h, wounded with a razor blade, and allowed to recover. Wounding alone had no effect, allowing polarization to be controlled by the addition of mitogens (Gomes and Gundersen, 2006). Lysophosphatidic acid (LPA), serum, or EGF was added for 90 min and the cells were then stained for GM130 to visualize the polarization of the Golgi (Fig. 1 A). Golgi polarization was determined by well-established criteria: the Golgi in cells facing the wound was counted as oriented when the majority of the staining was located within a 120° angle facing the wound (Etienne-Manneville and Hall, 2001; Gomes et al., 2005). With no mitogens added, 39% of the cells showed a polarized Golgi, similar to the predicted random orientation of 33% (Fig. 1 B). We consistently observed Golgi polarization levels above 33%, which is perhaps caused by the stimulation of removing the adjacent cell with the razor blade. Upon stimulation with LPA, serum, or EGF, the Golgi polarized (63, 68, and 58%, respectively). This effect was abolished by preincubation for 30 min with the MEK/ERK inhibitor U0126, suggesting that the polarization of the Golgi toward the wound is dependent on ERK activation.
Figure 1.
ERK-induced Golgi orientation is inhibited by GRASP65 1–201. (A) Mitogen-induced Golgi orientation depends on ERK signaling. NRK monolayers starved for 24 h were wounded and treated with serum for 90 min or pretreated with U0126 for 30 min before stimulation. The Golgi was labeled with an antibody against GM130 (red) and DNA was stained with Hoechst (blue). Golgi were counted as oriented (+) if the majority lay in a 120° angle facing the wound edge at the top of the image, and not oriented if the majority was outside the angle (−). (B) Quantitation. Serum, EGF, or LPA was added for 90 min with or without preincubation with U0126 to inhibit MEK/ERK with an average of 103 cells counted per condition, per experiment. (C) GRASP65 mutants were used in this study. Green, GRASP oligomerization domain; black, phosphorylation and regulatory domain; red, myristoylation site at Gly2; blue, ERK phosphorylation site at Ser277. The S277A point mutant and GRASP65 1–201 lack the ERK phosphorylation site. The constructs were expressed in NRK cells by microinjection of the plasmids into the nuclei. After 2 h, the cells were fixed and stained for Golgi with anti-GM130 (red), DNA (blue), and the expressed GRASP65 construct (green; GRASP65 1–201 and G2A GRASP 1–201 with against-GFP; FL and S277A with anti-GRASP65). Nonmyristoylated G2A GRASP65 is cytosolic, whereas the other constructs are targeted to the Golgi. (D–F) GRASP65 1–201 inhibits Golgi polarization. (D) Serum-starved cells at the wound edge were microinjected with GFP-tagged GRASP65 1–201 or G2A GRASP65 1–201 cDNA, allowed to express proteins for 2 h, and stimulated with LPA for 90 min. Antibodies against GFP (green) and GM130 (red) were used to visualize the expressed proteins and the Golgi, respectively. Nuclei were stained with Hoechst (blue). Golgi were counted as oriented and marked as in A. (E) Quantitation of Golgi orientation of cells expressing GRASP 1–201 or G2A GRASP 1–201 fixed at 0, 30, 90, or 270 min after LPA addition. Nonexpressing cells were counted as an additional control. An average of 64 cells were counted per condition, per experiment. (F) Cells were treated as in D and Golgi orientation was induced with serum, EGF, or LPA and quantified after 90-min stimulation with an average of 100 cells per condition, per experiment. Bars, 10 μm. Red lines mark basal levels expected for random orientation of 33%. Shown are mean ± SEM, from n ≥ 3 independent experiments for each condition or time point. *, P < 0.05; ***, P < 0.001.
To analyze whether ERK affects Golgi polarization by influencing the Golgi structure, we focused on the Golgi protein GRASP65, which is phosphorylated by ERK (Yoshimura et al., 2005). GRASP65 regulates Golgi cisternal stacking in vitro and in intact cells (Barr et al., 1997; Sütterlin et al., 2005) with oligomers of GRASP65 holding adjacent cisternae together (Wang et al., 2003), and its additional roles include lateral linking of Golgi stacks (Puthenveedu et al., 2006). GRASP65 is composed of two distinct domains: the N-terminal GRASP domain, responsible for oligomerization, and the C-terminal regulatory domain, which contains all phosphorylation sites (Preisinger et al., 2005). Mitotic phosphorylation causes a loss of oligomerization, which results in cisternal unstacking followed by an extensive remodeling of the Golgi (Barr et al., 1997).
To test whether ERK modifies the regulatory domain of GRASP65 during Golgi polarization, we expressed the GRASP domain (residues 1–201) fused to GFP (Fig. 1 C). Because GRASP65 1–201 is insensitive to kinase activity, a constitutive oligomer, and prevents cisternae from unstacking or remodeling, the mutant should delay or inhibit Golgi orientation. After expression of the cDNA in wound-edge cells by microinjection, we assessed Golgi orientation in response to mitogens. Fig. 1 D shows a typical immunofluorescence image of cells at the wound edge expressing either GRASP65 1–201 or the control protein G2A GRASP65 1–201, where the glycine at position two is replaced by an alanine (G2A) to abolish the myristoylation, and therefore Golgi targeting (Fig. 1 C; Barr et al., 1998). In control cells and cells expressing G2A GRASP65 1–202, the Golgi achieve maximum orientation after 90-min stimulation with LPA, whereas in cells expressing GRASP65 1–202, inhibition of Golgi orientation is observed after 90 min and maintained after a 270-min addition of LPA (Fig. 1 E). The inhibitory effect of GRASP65 1–201 on Golgi polarization persisted after stimulation with serum, EGF, or LPA for 90 min (Fig. 1 F), suggesting that the regulatory domain of GRASP65 is essential for mitogen-stimulated Golgi orientation.
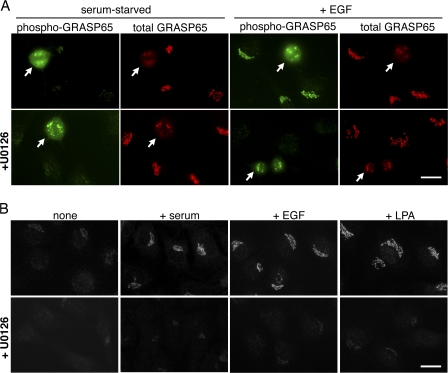
GRASP65 is phosphorylated at Ser277 by ERK (Yoshimura et al., 2005). GRASP65 is also phosphorylated in mitosis at Ser277 and at three other sites by cdk1/cyclinB1 (Preisinger et al., 2005). To test if GRASP65 phosphorylation by ERK is induced by other mitogens known to cause Golgi orientation, we used a previously characterized antibody against phospho-Ser277 of GRASP65, which also recognizes mitotically phosphorylated GRASP65 (Yoshimura et al., 2005). In the absence of mitogens, the phospho-GRASP65 antibody stained mitotic cells (Fig. 2 A), whereas the surrounding interphase cells showed little or no signal (images acquired equally). Stimulation with serum, EGF, or LPA considerably increased phosphorylation of GRASP65 in interphase cells (Fig. 2 B). U0126 abolished GRASP65 phosphorylation in interphase cells, whereas the mitotic signal was unaffected (Fig. 2, A and B), suggesting separate regulation of GRASP65 phosphorylation in mitosis and interphase (Yoshimura et al., 2005).
Figure 2.
ERK phosphorylates GRASP65 in response to mitogens. (A) Antibodies against phosphorylated Ser277 of GRASP65 (phospho-GRASP65) detect mitotic cells (arrows) but not serum-starved interphase cells. Phospho-GRASP65 is detected in interphase cells upon EGF stimulation and is sensitive to U0126. In contrast, mitotic GRASP65 phosphorylation is resistant to U0126. (B) Phospho-GRASP65 antibodies detect Golgi in cells treated for 10 min with serum, EGF, or LPA, but not in serum-starved cells. No signal was detected when cells were preincubated for 30 min with U0126. Exposure time and image processing are equal. Bars, 10 μm.
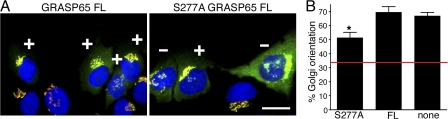
We then tested whether GRASP65 phosphorylation by ERK is required for Golgi orientation. S277A GRASP65 FL, in which the Ser277 is mutated to alanine or the control protein GRASP65 FL, was expressed in wound-edge cells (Fig. 3 A). S277A GRASP65 FL significantly inhibited Golgi polarization compared with GRASP65 FL or nonexpressing cells (Fig. 3 B), supporting the conclusion that phosphorylation of GRASP65 at Ser277 by ERK is critical for Golgi polarization.
Figure 3.
S277A GRASP65 FL inhibits Golgi orientation. (A) GRASP65 FL or S277A GRASP65 FL cDNA were microinjected into serum-starved cells at the wound edge. Proteins were expressed for 2 h before inducing polarization with LPA for 90 min. Cells were then labeled with antibodies against GRASP65 (green) and GM130 (red), and DNA was stained with Hoechst (blue). Injected cells were identified by overexpressed GRASP65. Golgi were counted as oriented and marked as in Fig. 1 A. Bar, 10 μm. (B) Quantitation of A. Shown are mean ± SEM of n = 4 independent experiments with an average of 27 cells per condition, per experiment. *, P < 0.016. Red lines mark basal levels expected for random orientation of 33%.
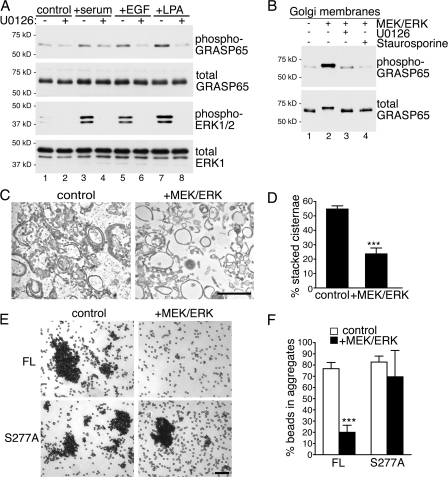
Mitotic GRASP65 phosphorylation causes a vast remodeling of the Golgi, induced by cisternal unstacking (Barr et al., 1997). We therefore investigated whether the phosphorylation of GRASP65 by ERK induces changes in the Golgi structure. Serum-starved NRK cells were stimulated with serum, EGF, or LPA, and lysates were analyzed by immunoblotting for phospho-Ser277 GRASP65. Stimulation led to an increase in active ERK and to phosphorylation of GRASP65 as previously reported (Yoshimura et al., 2005), which was inhibited by preincubation with U0126 (Fig. 4 A). GRASP65 was also phosphorylated when purified rat liver Golgi (RLG) membranes were incubated with recombinant MEK and ERK, which was abolished by preincubation with U0126 or the kinase inhibitor staurosporine, suggesting that these kinases are sufficient for GRASP65 phosphorylation (Fig. 4 B).
Figure 4.
GRASP65 phosphorylation by ERK causes loss of oligomerization and Golgi unstacking. (A) Serum-staved NRK cells were treated with serum, EGF, or LPA for 10 min. Proteins were separated by SDS-PAGE and blotted with antibodies against phospho-GRASP65, GRASP65, phospho-ERK1/2, and ERK1. Serum, EGF, and LPA induced phosphorylation of ERK and GRASP65, which was abolished by U0126 (30-min preincubation). (B) RLG membranes were treated with recombinant MEK/ERK and then subjected to Western blotting for phospho-GRASP65 and GRASP65. Kinase treatment induced GRASP65 phosphorylation, which was inhibited by U0126 and the kinase inhibitor staurosporine. (C) RLG membranes were treated with MEK/ERK as in B and analyzed by EM. Bar, 0.5 μm. (D) The percentage of stacked cisternae in RLG membranes was quantified after EM analysis using the intersection method. MEK/ERK treatment significantly reduced the percentage of stacked cisternae. (E and F) Purified recombinant GRASP65 FL or S277A GRASP65 FL protein was cross-linked to magnetic beads. Incubation with interphase cytosol (control) caused the beads to aggregate. Further incubation with MEK/ERK dispersed the GRASP65 FL beads, but had no effect on S277A GRASP65 FL beads. Bar, 50 μm. Mean ± SEM for n ≥ 4 independent experiments are shown in D and F. ***, P < 0.001.
Mitotic phosphorylation of GRASP65 induces Golgi cisternal separation (Wang et al., 2003). We therefore analyzed the MEK/ERK-treated RLG membranes by EM (Fig. 4 C). Untreated membranes showed 55% of the cisternae in stacks (Fig. 4 D). Addition of MEK/ERK reduced stacking to 24%, suggesting that ERK causes unstacking, which correlates with GRASP65 phosphorylation. GRASP65 regulates stacking through oligomerization of the N-terminal GRASP domain (Wang et al., 2003). In mitosis, phosphorylation of the C-terminal domain causes separation of oligomers and, in turn, cisternae. To determine whether GRASP65 oligomerization is regulated by MEK/ERK in interphase, we performed a bead-aggregation assay (Fig. 4 E). When beads coated with recombinant GRASP65 were treated with interphase cytosol, 77% of GRASP65 FL beads were in aggregates, indicating oligomerization. Upon further incubation with MEK/ERK, aggregation was reduced to 20%, a value similar to treatment with mitotic kinases (Wang et al., 2003). S277A GRASP65 FL beads showed comparable aggregation with interphase cytosol (70%) but were insensitive to MEK/ERK (83%), suggesting that S277A GRASP65 FL is a constitutive oligomer. Therefore, MEK/ERK treatment results in a loss of GRASP65 oligomerization, which correlates with the EM observation that MEK/ERK treatment results in cisternal unstacking of Golgi membranes.
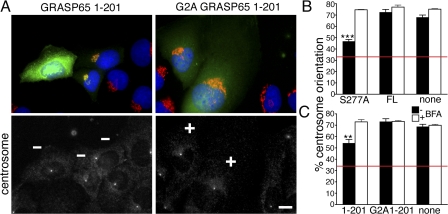
During cell migration, the centrosome orients toward the leading edge, as does the Golgi (Magdalena et al., 2003; Cau and Hall, 2005). Thus, we explored whether centrosome orientation is linked to Golgi orientation using the GRASP65 mutants that inhibit Golgi polarization (Fig. 5 A). In cells expressing S277A GRASP65 FL or GRASP65 1–201, centrosome orientation was significantly inhibited, whereas centrosomes of control protein or nonexpressing cells oriented as expected (Fig. 5, B and C), consistent with our quantitation of Golgi polarization (Fig. 1 E and Fig. 3). The fact that centrosomes always localized with the Golgi suggests that tight coupling between these two organelles is preserved during cell polarization.
Figure 5.
Expression of GRASP65 1–201 inhibits centrosome orientation. (A) Serum-starved cells expressing GFP-tagged GRASP65 1–201 or G2A GRASP65 1–201 (green) were stimulated with LPA for 90 min. The cells were then stained for centrosomes (anti–γ-tubulin; white), Golgi (anti-GM130; red), and DNA (blue). Injected cells were identified by the GFP signal (green). Centrosomes were counted as oriented (+) if they fell into a 120° angle facing the wound, and not oriented if the majority was outside the angle (−). Bar, 10 μm. (B and C) Fragmentation of the Golgi by BFA rescues centrosome orientation. Cells expressing GRASP65 S277A/GRASP65 FL (B) or GFP-tagged GRASP65 1–201/G2A GRASP65 1–201 (C) were treated with BFA for 30 min to fragment the Golgi or treated with vehicle alone. BFA was washed out and the cells were stimulated with LPA for 90 min. Cells were fixed and stained for GRASP65 (B) or GFP (C) to identify injected cells, γ-tubulin to count centrosome orientation, and Hoechst. Mean percentage of centrosome orientation ± SEM was calculated from greater than or equal to three independent experiments, with averages of 64 (A) and 105 (B) cells counted per condition, per experiment. ** P, < 0.01; ***, P < 0.001. Red lines mark basal levels expected for random orientation of 33%.
If Golgi remodeling by GRASP65 phosphorylation is required for centrosome orientation, fragmentation of the Golgi induced by other means should bypass this requirement. We tested this by pharmacologically reorganizing the Golgi with brefeldin A (BFA; Lippincott-Schwartz et al., 1989). Wound-edge cells expressing GRASP65 constructs were treated with BFA for 30 min to break up the Golgi. BFA was removed and LPA was added for 90 min, after which nonexpressing cells and cells expressing GRASP65 FL and G2A GRASP65 1–201 showed centrosome polarization to a similar extent (Fig. 5, B and C). Furthermore, we observed that BFA treatment restored centrosome orientation to control levels in cells expressing S277A GRASP65 FL and GRASP65 1–201 (Fig. 5, B and C), such that the inhibited centrosome polarization was rescued by breaking apart the Golgi structure with BFA. Our results suggest that centrosome polarization cannot be achieved without Golgi reorganization. One possibility is that the bulky Golgi could exert a drag force, which cannot be overcome by the polarization forces acting on the centrosome and cytoskeleton. In any case, we have demonstrated a closely integrated mechanism in which Golgi remodeling by phosphorylation of GRASP65 acts as a negative regulator of Golgi and, surprisingly, centrosome orientation.
We have explored the effects of ERK phosphorylation on GRASP65 during Golgi orientation toward the direction of cell migration. Our data indicate that ERK phosphorylates GRASP65 in interphase cells, resulting in the loss of GRASP65 oligomerization and causing subsequent Golgi cisternal unstacking. Preventing GRASP65 phosphorylation with phosphorylation-resistant mutants inhibits Golgi and centrosome orientation, leading to the conclusion that Golgi remodeling by kinase signaling is critical for the polarization of migrating cells.
Golgi remodeling has been best described in mitosis, where unstacking and vesiculation are driven by the phosphorylation of Golgi proteins including GM130, GRASP65, and GRASP55 (Shorter and Warren, 2002). Phosphorylation of GRASP65 by mitotic kinases leads to the loss of oligomerization followed by unstacking and lateral unlinking of Golgi cisternae (Barr et al., 1997; Wang et al., 2003; Feinstein and Linstedt, 2007). Ser277 of GRASP65 is phosphorylated in mitosis by cdk1/cyclinB1 and by ERK in interphase (Yoshimura et al., 2005), suggesting that GRASP65 phosphorylation may organize similar processes during interphase and mitosis. We report here that inhibiting interphase GRASP65 phosphorylation by ERK prevents Golgi reorganization, which is required for Golgi polarization. We conclude that interphase and mitotic phosphorylation of GRASP65, although differently regulated and required for unique cellular processes, produce similar consequences for Golgi structure, including loss of oligomerization and unstacking of cisternae. Therefore, phosphorylation of GRASP65 functions to regulate Golgi remodeling whether during mitosis or cell polarization.
The fragmented Golgi structure of mitotic cells is, however, not found during interphase by light microscopy (Yoshimura et al., 2005). The amount of Golgi reorganization could be a function of the extent of GRASP65 phosphorylation. GRASP65 phosphorylation in mitosis involves at least four distinct sites (Preisinger et al., 2005), but only Ser277 is known to be phosphorylated during interphase (Yoshimura et al., 2005). Additionally, mitotic Golgi fragmentation depends on the phosphorylation of other Golgi proteins, including GM130 and GRASP55, which are not phosphorylated during interphase (Lowe et al., 1998; Feinstein and Linstedt, 2007). Therefore, decreased GRASP65 phosphorylation and the lack of phosphorylation of other Golgi proteins correlate to the lesser extent of Golgi remodeling during interphase. Maintaining the Golgi structure and organization in polarizing cells would allow for the quick upstart of directed membrane traffic necessary for migration once the Golgi faces the leading edge.
In summary, our work reveals that remodeling of the Golgi structure is required for the polarization of the Golgi toward the direction of cell migration. The Golgi remodeling is achieved by phosphorylation of GRASP65 by ERK, causing loss of GRASP65 oligomerization and cisternal unstacking. Furthermore, we found that centrosome polarization cannot be achieved without Golgi remodeling, showing that Golgi remodeling is critical for cell polarization.
Materials and methods
Reagents and antibodies
The following reagents were used: LPA (Sigma-Aldrich), EGF (PeproTech), U0126 (LC Laboratories), and staurosporin (EMD). The following antibodies were used: polyclonal antibodies against phospho-S277 GRASP65 (Yoshimura et al., 2005), ERK1 (C-16; Santa Cruz Biotechnology, Inc.), phospho-ERK1/2 (Cell Signaling Technology), GRASP65 (Wang et al., 2003), and GFP (Bartz et al., 2008). Monoclonal antibodies against GM130 (BD Biosciences), γ-tubulin (GTU-88; Sigma-Aldrich), and GRASP65 (F. Barr, University of Liverpool, Liverpool, UK) were used. Hoechst 33342 and secondary antibodies conjugated to Alexa Fluor 488, 555, 594, or 643 were purchased from Invitrogen, and HRP-conjugated secondary antibodies were purchased from Thermo Fisher Scientific.
Cell culture and wounding
NRK cells were maintained in medium A (DME 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamate [Invitrogen]) with 10% cosmic calf serum (HyClone) at 37°C and 5% CO2. Cells were grown on glass coverslips until confluency and starved for 24 h in medium A without serum before Western blot analysis or wounding. Wound edges were created with a razor blade and the cells were allowed to recover for 2 h before microinjection. For experiments not involving microinjection, cells were wounded 5 h before addition of serum to be consistent with microinjection experiments. To inhibit ERK activation, 10 μM U0126 was added 30 min before stimulation with 1% serum.
To detect GRASP65 phosphorylation, serum-starved NRK cells were treated for 10 min with 0.1% serum, 2 ng/ml EGF, or 2 μM LPA. To inhibit ERK activation, the cells were preincubated for 30 min with 20 μM U0126. The cells were then analyzed by immunofluorescence or lysed for 15 min on ice in TEGN buffer (10 mM Tris-HCl, pH 7.4, 420 mM NaCl, 10% glycerol, 0.5% NP-40, and 1 mM EDTA), 1 mM DTT, 1 mM PMSF, 10 mM β-glycerophosphate, 10 mM NaF, and protease inhibitor cocktail (Roche). The extracts were cleared by centrifugation for 15 min at 15,000 g and subjected to 10% SDS-PAGE followed by Western blotting analysis.
Microinjection
The following constructs were used: pEGFP 1–201 GRASP65, pEGFP G2A GRASP65 1–201 (Wang et al., 2005), pcDNA3.1 GRASP65, and pcDNA3.1 S277A GRASP65 (Yoshimura et al., 2005). Cells in Hepes medium (DME, 25 mM Hepes, and no calf serum) were microinjected as described previously (Bartz and Seemann, 2008) with 0.1 mg/ml plasmid and incubated at 37°C for 2 h to allow protein expression. 2 μM LPA (bound to fatty-acid free BSA), 2 ng/ml EGF, or 0.1% serum was added to induce cell polarization.
Immunofluorescence and image analysis
Cells were fixed and permeabilized for 10 min in methanol at −20°C and incubated with the indicated antibodies followed by fluorescent secondary antibodies. DNA was stained with Hoechst 33342. Fluorescence analysis was performed with a 40×/1.4 differential interference contrast objective (Plan-Apochromat; Carl Zeiss, Inc.) and a microscope (Axiovert 200 M; Carl Zeiss, Inc.). Images were captured with a camera (Orca-285; Hamamatsu Photonics) and the software package Openlab (4.02; Improvision).
Quantitation of Golgi and centrosome polarization
The Golgi was stained for GM130 and centrosomes for γ-tubulin. Golgi and centrosome orientation was determined for the first row of cells facing the wound as described previously (Etienne-Manneville and Hall, 2001; Gomes et al., 2005) and counted as oriented if the majority was located in a 120° sector emerging from the center of the nucleus and facing the wound edge. Basal levels of expected random orientation of 33% are marked by red lines in the graphs. Percentage of Golgi or centrosome orientation was calculated by dividing the number of oriented cells by the number of total cells for each condition. The mean percentage from three or more experiments were averaged and presented as a mean ± SEM. Statistical analysis was performed using Student's t tests and significance was assigned for P < 0.05. P-values compared with each of the control conditions are represented by asterisks.
Golgi unstacking assay
120 μg of RLG membranes (Wang et al., 2003), with or without pretreatment for 30 min with 20 μM U0126 or 10 μM staurosporine, were incubated with 6 μg of recombinant purified MEK1 and ERK2 in 0.4 ml MEB buffer (50 mM Tris-HCl, pH 7.3, 50 mM KCl, 10 mM MgCl2, 15 mM EGTA, 20 mM β-glycerophosphate, 0.2 M sucrose, 2 mM ATP, 1 mM GTP, and 1 mM glutathione) for 20 min at 37°C. The reactions were then subjected to 10% SDS-PAGE and analyzed by Western blotting. For EM analysis, the reactions were fixed with 2% glutaraldehyde and processed as described previously (Wang et al., 2003). The relative proportion of stacked and unstacked membranes was determined by the intersection method (Wang et al., 2003).
Bead aggregation assay
Phosphorylation-dependent oligomerization of GRASP65 was determined as described previously (Wang et al., 2003). Recombinant GRASP65 FL and S277A mutant were purified as described previously (Wang et al., 2005), cleared by centrifugation at 15,000 g for 30 min, and cross-linked to M500 beads (Invitrogen). The beads were incubated for 60 min at 37°C with interphase HeLa cytosol (Wang et al., 2003) in KHM buffer (25 mM Hepes, pH 7.3, 60 mM KCl, 5 mM Mg(OAc)2, 0.2 M sucrose, and 1 mM glutathione) to allow aggregation. The beads were washed with MEB buffer, incubated for 60 min at 37°C with MEK1 and ERK2 in MEB buffer, and observed by phase-contrast microscopy. The percentage of beads in aggregates was quantified as described previously (Wang et al., 2003).
Acknowledgments
We thank F. Grinnell and W. Snell for helpful comments.
Y. Wang is supported by the Pardee Cancer Research Foundation. J. Seemann is a Virginia Murchison Lithicum Scholar in Medical Research. This work was supported by a grant from the American Cancer Society to J. Seemann (ACS-IRG-02-196).
B. Bisel's present address is European Laboratory for Non-Linear Spectroscopy, University of Florence, Sesto Fiorentino 50019, Italy.
S.-i. Yoshimura's present address is University of Liverpool, Cancer Research Centre, Liverpool L3 9TA, England, UK.
Abbreviations used in this paper: BFA, brefeldin A; ERK, extracellular signal–regulated kinase; LPA, lysophosphatidic acid; MEK, MAPK/ERK kinase; NRK, normal rat kidney; RLG, rat liver Golgi.
References
- Barr, F.A., M. Puype, J. Vandekerckhove, and G. Warren. 1997. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 91:253–262. [DOI] [PubMed] [Google Scholar]
- Barr, F.A., N. Nakamura, and G. Warren. 1998. Mapping the interaction between GRASP65 and GM130, components of a protein complex involved in the stacking of Golgi cisternae. EMBO J. 17:3258–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz, R., and J. Seemann. 2008. Mitotic regulation of SREBP and ATF6 by separation of the Golgi and ER. Cell Cycle. 7:2100–2105. [DOI] [PubMed] [Google Scholar]
- Bartz, R., L.P. Sun, B. Bisel, J.H. Wei, and J. Seemann. 2008. Spatial separation of Golgi and ER during mitosis protects SREBP from unregulated activation. EMBO J. 27:948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau, J., and A. Hall. 2005. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J. Cell Sci. 118:2579–2587. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and A. Hall. 2001. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 106:489–498. [DOI] [PubMed] [Google Scholar]
- Feinstein, T.N., and A.D. Linstedt. 2007. Mitogen-activated protein kinase kinase 1-dependent Golgi unlinking occurs in G2 phase and promotes the G2/M cell cycle transition. Mol. Biol. Cell. 18:594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, E.R., and G.G. Gundersen. 2006. Real-time centrosome reorientation during fibroblast migration. Methods Enzymol. 406:579–592. [DOI] [PubMed] [Google Scholar]
- Gomes, E.R., S. Jani, and G.G. Gundersen. 2005. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 121:451–463. [DOI] [PubMed] [Google Scholar]
- Jaffe, A.B., and A. Hall. 2005. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21:247–269. [DOI] [PubMed] [Google Scholar]
- Liljedahl, M., Y. Maeda, A. Colanzi, I. Ayala, J. Van Lint, and V. Malhotra. 2001. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 104:409–420. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., L.C. Yuan, J.S. Bonifacino, and R.D. Klausner. 1989. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 56:801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, M., C. Rabouille, N. Nakamura, R. Watson, M. Jackman, E. Jamsa, D. Rahman, D.J. Pappin, and G. Warren. 1998. Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell. 94:783–793. [DOI] [PubMed] [Google Scholar]
- Magdalena, J., T.H. Millard, and L.M. Machesky. 2003. Microtubule involvement in NIH 3T3 Golgi and MTOC polarity establishment. J. Cell Sci. 116:743–756. [DOI] [PubMed] [Google Scholar]
- Mellor, H. 2004. Cell motility: Golgi signalling shapes up to ship out. Curr. Biol. 14:R434–R435. [DOI] [PubMed] [Google Scholar]
- Preisinger, C., R. Körner, M. Wind, W.D. Lehmann, R. Kopajtich, and F.A. Barr. 2005. Plk1 docking to GRASP65 phosphorylated by Cdk1 suggests a mechanism for Golgi checkpoint signalling. EMBO J. 24:753–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigozhina, N.L., and C.M. Waterman-Storer. 2004. Protein kinase D-mediated anterograde membrane trafficking is required for fibroblast motility. Curr. Biol. 14:88–98. [DOI] [PubMed] [Google Scholar]
- Pullikuth, A.K., and A.D. Catling. 2007. Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics: a perspective. Cell. Signal. 19:1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveedu, M.A., C. Bachert, S. Puri, F. Lanni, and A.D. Linstedt. 2006. GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat. Cell Biol. 8:238–248. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J. 2006. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 16:522–529. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., M.A. Schwartz, K. Burridge, R.A. Firtel, M.H. Ginsberg, G. Borisy, J.T. Parsons, and A.R. Horwitz. 2003. Cell migration: integrating signals from front to back. Science. 302:1704–1709. [DOI] [PubMed] [Google Scholar]
- Shorter, J., and G. Warren. 2002. Golgi architecture and inheritance. Annu. Rev. Cell Dev. Biol. 18:379–420. [DOI] [PubMed] [Google Scholar]
- Sütterlin, C., R. Polishchuk, M. Pecot, and V. Malhotra. 2005. The Golgi-associated protein GRASP65 regulates spindle dynamics and is essential for cell division. Mol. Biol. Cell. 16:3211–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii, S., M. Kusakabe, T. Yamamoto, M. Maekawa, and E. Nishida. 2004. Sef is a spatial regulator for Ras/MAP kinase signaling. Dev. Cell. 7:33–44. [DOI] [PubMed] [Google Scholar]
- Wang, Y., J. Seemann, M. Pypaert, J. Shorter, and G. Warren. 2003. A direct role for GRASP65 as a mitotically regulated Golgi stacking factor. EMBO J. 22:3279–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., A. Satoh, and G. Warren. 2005. Mapping the functional domains of the Golgi stacking factor GRASP65. J. Biol. Chem. 280:4921–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura, S., K. Yoshioka, F.A. Barr, M. Lowe, K. Nakayama, S. Ohkuma, and N. Nakamura. 2005. Convergence of cell cycle regulation and growth factor signals on GRASP65. J. Biol. Chem. 280:23048–23056. [DOI] [PubMed] [Google Scholar]
- Zuo, X., J. Zhang, Y. Zhang, S.C. Hsu, D. Zhou, and W. Guo. 2006. Exo70 interacts with the Arp2/3 complex and regulates cell migration. Nat. Cell Biol. 8:1383–1388. [DOI] [PubMed] [Google Scholar]