Abstract
We have used coexpression of a salivary basic proline-rich protein (PRP) along with a proline-rich proteoglycan (PRPg) in pituitary AtT-20 cells to examine the regulation of glycosaminoglycan (GAG) biosynthesis and the storage of these secretory products for regulated secretion. The basic PRP caused a dose-dependent increase in sulfation of PRPg and also increased the extent to which PRPg polypeptide backbones are modified by a GAG chain. The sulfation of an endogenous proteoglycan was similarly increased in the presence of basic PRP; however, other sulfated secretory products of AtT-20 cells were unaffected. These results imply that enzymes functioning in elongation and sulfation of proteoglycans are coordinately regulated and that their activities respond to a change in the milieu of the intracellular transport pathway. Analysis of the regulated secretion of both the basic PRP and PRPg has indicated that while the presence of the GAG chain improves the storage of PRPg, the presence of PRPg does not increase the storage of basic PRP. Therefore, sulfation of GAGs does not appear to be a primary factor in regulated secretory sorting.
INTRODUCTION
In the regulated secretory pathway, proteins are stored in secretory granules at very high concentrations prior to exocytosis. The processes of segregation and packaging of these proteins are thought to include aggregation among secretory proteins and interaction with granule membranes (Chanat et al., 1994; Colomer et al., 1994; Kuliawat and Arvan, 1994; Cool et al., 1997). Growing evidence indicates that many of the interactions are selective and progressive during granule maturation and thus constitute part of protein sorting (Huang and Arvan, 1995; Colomer et al., 1996; Natori and Huttner, 1996; Castle et al., 1997). Much less attention has been given to exploring the potential supporting role of charge neutralization in storage of secretory proteins. The process of condensing proteins to high concentration and low osmotic activity is likely to include mechanisms for reducing net fixed charge. Several earlier studies have suggested that the addition of sulfate groups on proteoglycans might serve this role especially where basic polypeptides are packaged (Zanini et al., 1980; Serafin et al., 1986; Blair et al., 1991; Matsumoto et al., 1995).
We have been interested in the potential role of sulfation in the packaging of salivary proline-rich proteins (PRPs) for regulated secretion. PRPs constitute a family of secretory proteins produced by the parotid gland. In rats, chronic administration of the β-adrenergic agonist isoproterenol amplifies the production and storage of basic PRPs (Muenzer et al., 1979). Concomitantly, isoproterenol also increases the production and sulfation of proline-rich proteoglycan (PRPgs; e.g., PRPg1, PRPg2) that have a PRP backbone and are posttranslationally modified by glycosaminoglycan (GAG) side chains (Blair et al., 1991; Castle and Castle, 1993). PRPgs normally are packaged in the parotid secretory granules along with the basic PRPs (Blair et al., 1991). It has been suggested that increased sulfation of PRPgs parallels increased production of basic PRPs (Blair et al., 1991). We have now developed an experimental system to test this possibility using cDNAs encoding the basic PRP and one of the PRPg polypeptides (PRPg2) expressed in pituitary AtT-20 cells. When expressed individually, both products are stored in the dense-core granules in these cells, although less efficiently than the endogenous hormone adrenocorticotropic hormone (ACTH) (Castle et al., 1992; Castle and Castle, 1993). In AtT-20 cells, the PRPg2 polypeptide is posttranslationally modified by either chondroitin sulfate or heparan sulfate (Castle and Castle, 1993). Using coexpression of basic PRP and PRPg2, we now address whether sulfation of PRPg2 is affected by the presence of basic PRP and whether it in turn affects the efficiency of storage of basic PRP in the dense-core granules. We find that, similar to the situation in the parotid, expression of the basic PRP causes an overall enhancement in GAG sulfation of the exogenous PRPg2 and an endogenous granule proteoglycan. Removal of GAG chains from PRPg2 results in reduced storage of the PRPg2 polypeptide, suggesting a possible role of the GAG chains in the packaging of PRPg2 for granule storage. However, the storage of basic PRP was not affected by the expression of PRPg2, suggesting that the role of GAG chains may be protein or system specific.
MATERIALS AND METHODS
Materials
Carrier-free [35S]sulfate and [3H]glucosamine (25 Ci/mmol) were obtained from ICN Biomedicals (Costa Mesa, CA) and [3H]proline (100 Ci/mmol) was obtained from Amersham (Arlington Heights, IL). 8-Bromocyclic AMP (8-Br-cAMP), protein A-Sepharose, chondroitinase AC, and heparinase III were obtained from Sigma (St. Louis, MO). DE52 cellulose and Sephadex G25 were obtained from Pharmacia (Piscataway, NJ). Anti-PRP antibodies recognizing basic PRPs and PRPgs were described previously (Castle and Castle, 1993).
Expression Vectors and Transfections
The cDNAs encoding the basic PRP and acidic PRPg2 have been described previously (Castle et al., 1992; Castle and Castle, 1993). The glycosaminoglycan addition site (Ser-77) in PRPg2 (Castle and Castle, 1993) was mutagenized to alanine by site-directed mutagenesis. The sequence of the mutagenized region was confirmed by sequencing. All cDNAs were inserted into the pRhR1100 expression vector (Stahl et al., 1996) and transfected into the mouse pituitary cell line AtT-20. Culture conditions, transfection procedure, and selection of stable transfectants were as described previously (Castle et al., 1992). Two approaches were used to obtain clones expressing both basic PRP and PRPg2: 1) AtT-20 cells were cotransfected with both constructs and pSV2neo and G418-resistant clones were screened for the presence of basic PRP and PRPg2; and 2) stably transfected G418-resistant clones expressing one of the constructs were cotransfected with the other construct and pREP4 (Invitrogen, Eugene, OR) and selection was carried out using 0.2 mg/ml hygromycin. Isolated clones were screened using Western blotting of conditioned medium.
Radiolabeling of Transfected AtT-20 Cells
Cells were plated in dishes at 4 × 105 cells/cm2 and used 48–60 h later. Prior to labeling with [35S]sulfate, cells were preincubated with sulfate-free Eagle’s minimal essential medium for 1 h and then labeled with 0.2–0.5 mCi/ml carrier-free sodium [35S]sulfate, generally for 3 h. Chases were carried out in DMEM containing 0.5 mM sodium sulfate. For double labeling with [35S]sulfate and [3H]glucosamine, cultures were incubated with 30 μCi/ml [35S]sulfate and 10 μCi/ml [3H]glucosamine for 48 h. All media containing secreted polypeptides were treated with 0.3 mg/ml phenylmethylsulfonyl fluoride and 0.3 mg/ml iodoacetamide. Labeling with [3H]proline, subsequent chases, and stimulation of secretion with secretagogues were described previously (Stahl et al., 1996). Radiolabeled proteins were immunoprecipitated with appropriate antibodies exactly as described before (Castle et al., 1992; Castle and Castle, 1993). For analysis of total protein profile, labeled proteins were precipitated with 10% trichloroacetic acid (TCA) using 0.2 mg/ml sodium deoxycholate as a carrier. Immunoprecipitated or TCA-precipitated proteins were analyzed as follows: [35S]sulfate-labeled proteins by SDS-PAGE (Laemmli, 1970) and fluorography or phosphorimager analysis; [3H]proline-labeled proteins by SDS-PAGE and fluorography or by scintillation counting of sliced tube gels (Castle et al., 1992). For determination of the 35S:3H ratio, immunoprecipitated PRPg2 was run on SDS-polyacrylamide tube gels, the gels were sliced and eluted with 0.1% SDS and 20 mM sodium bicarbonate, and radioactivity in PRPg2 was determined by scintillation counting. 3H cpms were corrected for the spillover of 35S into the 3H channel. cpms from the individual slices were summed and the resulting values were used to calculate the 35S:3H ratio.
Levels of expression of different polypeptides were estimated from the total amount of radiolabeled polypeptide (secreted + cell associated) synthesized during a 15-h labeling with [3H]proline. The values were corrected for the number of prolines in each polypeptide and expressed relative to the level of expression of ACTH-related peptides (ACTH-related peptides = proopiomelanocortin + ACTH biosynthetic intermediate + ACTH + glycosylated ACTH) quantitated from the same experiment.
Chromatography of Radiolabeled Proteoglycans
Medium containing radiolabeled proteoglycans was exchanged into 50 mM sodium acetate (pH 7.0), 50 mM sodium chloride, 0.5% Triton X-100, and 8 M urea (buffer A) by passing over a Sephadex G-25 column equilibrated in buffer A. Fractions containing the excluded material were loaded onto a DE 52 column (2 cm × 0.7 cm) equilibrated in buffer A. The retained material was eluted with a gradient (18-ml total volume) of sodium chloride (0.05–0.6 M) prepared in buffer A. Fractions (0.9 ml) were collected and aliquots were taken for measurements of radioactivity, immunoprecipitations, and analysis by SDS-PAGE.
Enzymatic Digests
Medium containing [35S]sulfate-labeled PRPg2 was treated with chondroitinase AC (1 IU/ml) or heparinase III (1 IU/ml) in the presence of 50 mM HEPES and 10 mM calcium acetate for 1 h at 37°C. Digested material was immunoprecipitated with the antibody against PRPg2 or TCA precipitated and analyzed by SDS-PAGE followed by fluorography.
RESULTS
Coexpression of Basic PRP with PRPg2 Increases the Incorporation of Sulfate into PRPg2
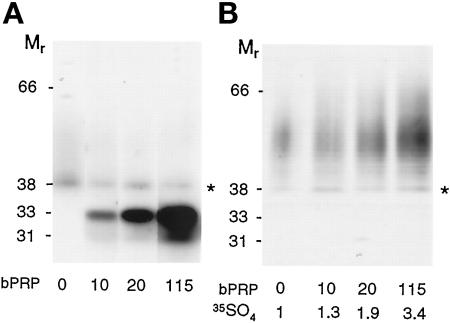
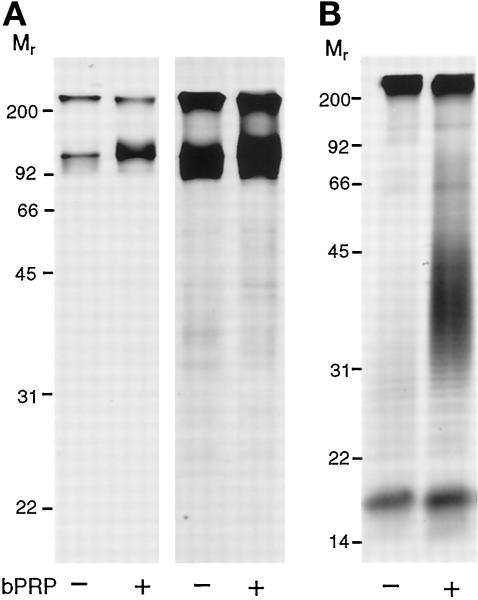
To study the effect of basic PRP expression on the sulfation of PRPg2, it was necessary to obtain cells expressing PRPg2 and varying levels of basic PRP. The cDNAs of basic PRP and PRPg2 were cotransfected into AtT-20 cells and stable cell lines expressing both proteins were selected. Figure 1A shows a Western blot of secreted PRPg2 and basic PRP from selected clones in which PRPg2 is expressed at an approximately constant level, and the level of expression of basic PRP varies by a factor of 10. The level of expression of PRPg2 corresponds to 6–10% of the level of expression of ACTH-related peptides, whereas the level of expression of basic PRP varies between 10% and 115% of the expression of ACTH. As observed previously, PRPg2 appears as a discrete band at Mr = 38,000 representing the proteoglycan core and a faint smear extending to Mr ≥ 60,000, corresponding to the proteoglycan. Analysis of immunoprecipitated PRPg2 from [35S]sulfate-labeled cells shows a progressive increase in the incorporation of [35S]sulfate into PRPg2 in clones expressing progressively higher levels of basic PRP (Figure 1B). Quantitation indicates that incorporation of [35S]sulfate into PRPg2 rose approximately 3.5-fold in the clone expressing the highest level of basic PRP as compared with the clone expressing PRPg2 alone. Interestingly, with increasing levels of expression of basic PRP, the sulfated PRPg2 migrates at increasingly slower electrophoretic mobility. This change could reflect the presence of longer GAG chains, reduced SDS binding due to an increased density of sulfate groups on the GAG chain, or both effects.
Figure 1.
Expression and [35SO4] sulfate labeling of PRPg2 alone and along with basic PRP in AtT-20 cells. (A) Western blot of PRPg2 secreted from cells expressing PRPg2 alone or coexpressing PRPg2 and basic PRP (bPRP). The blot was probed with a mixture of antibodies recognizing PRPg2 and basic PRP followed by biotin-conjugated goat anti-rabbit IgG and 125I-labeled strepavidin. The PRPg2 core (∗) is easily visible, whereas the modified proteoglycan is more diffuse and not easily visible at this exposure. Two forms of basic PRP are detected: the major one (33,000) is glycosylated and the minor one (31,000) is nonglycosylated. The level of expression of PRPg2 is 10% of that of endogenous ACTH except for the clone in lane 2, for which the level of expression of PRPg2 is 6% of that of ACTH. The level of expression of basic PRP is 10, 20, and 115% of that of ACTH. (B) PRPg2 from clones shown in A was labeled with [35S]sulfate for 3 h, and equal amounts of product were immunoprecipitated and analyzed by SDS-PAGE and fluorography. [35S]sulfate incorporation into PRPg2 was quantitated by phosphorimager analysis and the amount relative to that observed when PRPg2 is expressed alone is indicated beneath each lane.
The PRPg2 Polypeptide Is Sulfated and Contains the Linker Oligosaccharide
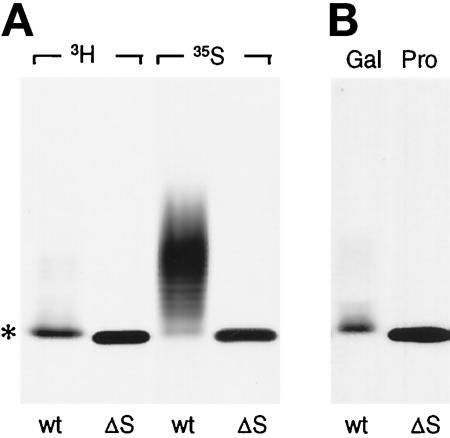
Based on the cDNA sequence, PRPg2 polypeptide contains a single putative site for GAG chain addition (Castle and Castle, 1993) and, consequently, the observed effects on sulfation are expected to involve a single GAG chain. To confirm that GAG chain addition was occurring at a single site, we constructed a PRPg2 mutant in which the relevant Ser was replaced with Ala. The mutant cDNA (PRPg2ΔS) was expressed in AtT-20 cells, and stably transfected cells were labeled with [3H]proline and [35S]sulfate to be able to detect the polypeptide and any residual sulfation. Labeled PRPg2ΔS collected from the secretion migrates as a sharp band with a slightly higher electrophoretic mobility than the PRPg2 core (Figure 2A). The ladder of bands with slower mobility corresponding to the PRPg2 proteoglycan was not detected, confirming that GAG addition was prevented. Surprisingly, the PRPg2ΔS itself was labeled with [35S]sulfate and migrated as a sharp band with an identical mobility to that of [3H]proline-labeled PRPg2ΔS. Most likely the labeling of PRPg2ΔS represents sulfation of the lone tyrosine residue within the PRPg2 polypeptide backbone (Castle and Castle, 1993). This sulfation also occurs in PRPg2 but its extent appears to be variable (Figures 1 and 3).
Figure 2.
Analysis of PRPg2 mutant lacking the GAG addition site. PRPg2 cDNA lacking the Ser used for GAG attachment (PRPg2ΔS) was expressed in AtT-20 cells. (A) Stable cell lines expressing wild-type PRPg2 (wt) and PRPg2ΔS (ΔS) were labeled with 0.4 mCi/ml [3H]proline (3H) for 15 h or with 0.25 mCi/ml [35S]sulfate (35S) for 3 h. PRPg2 and PRPgΔS were immunoprecipitated and analyzed by SDS-PAGE and fluorography. The asterisk indicates the position of the PRPg2 core. (B) Cells expressing PRPg2 were labeled for 4 h with 0.4 mCi/ml [3H]galactose and PRPg2 was immunoprecipitated (Gal). For comparison, [3H]proline-labeled PRPg2ΔS (Pro) obtained as in A is also shown.
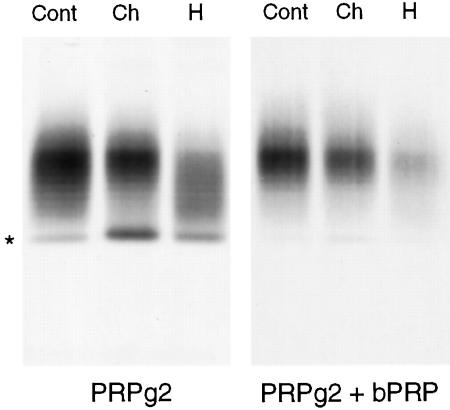
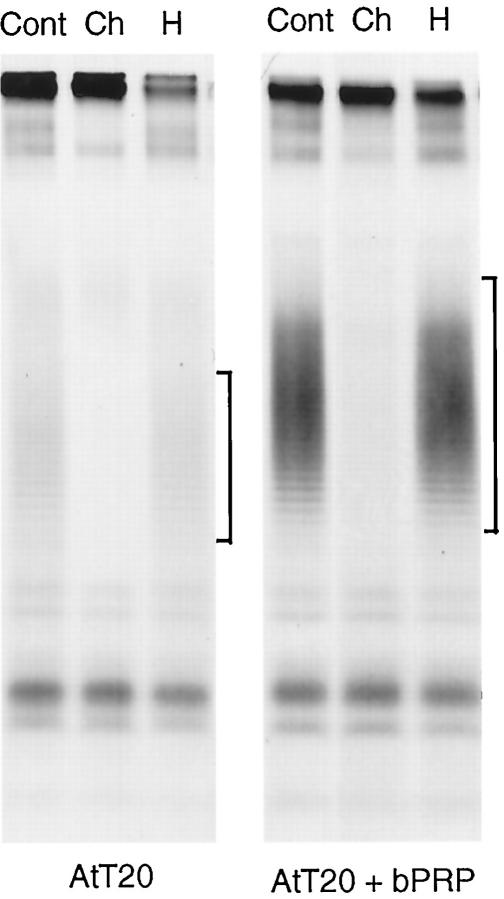
Figure 3.
Enzymatic digests of PRPg2. AtT-20 cells expressing PRPg2 alone or PRPg2 and basic PRP were labeled with 0.25 mCi/ml [35S]sulfate for 3 h with enzymes, as described in MATERIALS AND METHODS, and immunoprecipitated. Cont, control; Ch, chondroitinase AC; H, heparinase III. The asterisk indicates the position of the PRPg2 core. The lanes in the right panel were loaded with one-fourth of the material that was loaded for samples shown in the left panel.
The difference in the apparent electrophoretic mobility of the PRPg2 core and of PRPg2ΔS could not be accounted for by the substitution of Ala for Ser. Therefore, we considered the possibility that the PRPg2 core might contain the tetrasaccharide, xylose-galactose-galactose-glucuronic acid, that links the repeating disaccharides of the GAG chain to the Ser within the polypeptide backbone. If this is the case, then the PRPg2 core should be labeled when the cells are incubated with [3H]galactose. Figure 2B shows that this is true, indicating that the oligosaccharide linker accounts for the difference in the electrophoretic mobilities between the PRPg2 core and PRPg2ΔS.
The Relative Proportions of Chondroitin and Heparan Sulfate Chains Are Not Affected by Expression of Basic PRP
When expressed in AtT-20 cells, PRPg2 contains chondroitin sulfate and heparan sulfate chains. To determine whether or not sulfation of both types of GAG chains is affected by the expression of basic PRP, we performed enzymatic digests of PRPg2 from cells expressing PRPg2 alone and cells expressing PRPg2 and the highest level of basic PRP. The results shown in Figure 3 suggest that there is no shift in the sensitivity to the enzymes upon coexpression with basic PRP. Quantitation of the labeled PRPg2 showed that in both cases ∼35% of the GAG chains are sensitive to chondroitinase AC and ∼65% are sensitive to heparinase III. Consequently, the observed increase in the incorporation [35S]sulfate into the GAG chains of PRPg2 likely involves both chondroitin sulfate and heparan sulfate oligosaccharides.
Expression of Basic PRP Affects Sulfation of PRPg2 GAG Chains
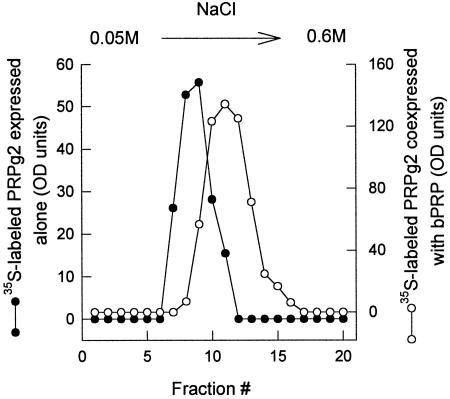
The enhanced incorporation of [35S]sulfate into PRPg2 resulting from coexpression with basic PRP could reflect an increased extent of sulfation of individual GAG chains, an increase in the utilization of the PRPg2 core for GAG chain addition, or both processes. We performed two studies to assess whether the sulfate content per GAG chain is increased. First, we used ion exchange chromatography of [35S]sulfate-labeled PRPg2 to see whether the proteoglycan had a greater charge content. Secreted PRPg2 was passed over a DEAE cellulose column and the retained material was eluted with a gradient of sodium chloride. PRPg2 from cells coexpressing basic PRP was retained on the column and eluted at a higher salt concentration than when it was expressed alone (Figure 4). These elution characteristics indicate that individual PRPg2 molecules on average have a higher content of negatively charged groups when coexpressed with basic PRP, suggesting an increased extent of sulfation per GAG chain.
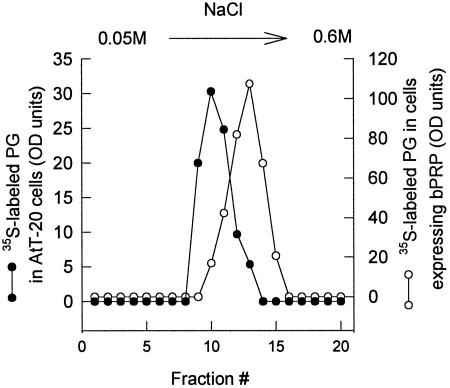
Figure 4.
Ion exchange chromatography of [35S]sulfate-labeled PRPg2. Secreted PRPg2 labeled as described in Figure 3 was loaded onto a DEAE (DE 52) cellulose column and eluted with a 0.05 M to 0.6 M NaCl gradient as described in MATERIALS AND METHODS. Equal amounts of each fraction were analyzed by SDS-PAGE, and [35S]sulfate-labeled PRPg2 was quantitated by phosphorimager analysis. •, PRPg2 expressed alone; ○, PRPg2 coexpressed with basic PRP.
In the second study we performed double labeling with [3H]glucosamine (to label the carbohydrate backbone of the GAG) and [35S]sulfate to determine whether the isotope ratio is altered when PRPg2 is coexpressed with basic PRP. Cells expressing PRPg2 alone or PRPg2 and basic PRP were double labeled with [3H]glucosamine and [35S]sulfate. Secreted PRPg2 was immunoprecipitated and the ratio of [35S]:[3H] was quantitated. The [35S]:[3H] ratio for PRPg2 was found to be 2.5 when it was expressed alone and 4.5 when it was coexpressed with basic PRP. Thus, the GAG chain is more highly sulfated and the enhancement in the degree of sulfation likely accounts for at least part of the overall increase in the incorporation of [35S]sulfate.
Effect of the Ratio of Basic PRP to PRPg2 on the Fractional Modification of the PRPg2 Core
SDS-PAGE of [3H]proline-labeled PRPg2 products shows that a relatively large fraction of the labeled material is present as the proteoglycan core when PRPg2 is expressed alone (Figure 5A). We were interested in evaluating whether this fraction is affected by coexpression of basic PRP. Cells expressing either PRPg2 alone or PRPg2 and basic PRP were metabolically labeled with [3H]proline and secreted PRPg2 products and basic PRP were immunoprecipitated and analyzed by SDS-PAGE, fluorography, and densitometry (Figure 5). The densitometric traces in Figure 5B show quite clearly that a greater proportion of the proteoglycan core is utilized when PRPg2 is expressed with basic PRP (compare top, middle. and bottom panels in Figure 5B). Quantitation indicates that 40% of the labeled material is a proteoglycan when PRPg2 is expressed alone. In clones coexpressing basic PRP and PRPg2, the percentage of labeled PRPg2 products in the form of proteoglycan increases to 55% (middle panel, Figure 5B) and 75% (bottom panel, Figure 5B). Notably, the increase parallels the increasing ratio of basic PRP:PRPg2. Essentially the same results were obtained with clones in which the level of expression of basic PRP was lower. Thus, increased sulfation of PRPg2 upon coexpression with basic PRP reflects a combination of increasing the fraction of the available polypeptide pool that is modified by GAG chains and also increasing the sulfate content of the GAG chains.
Figure 5.
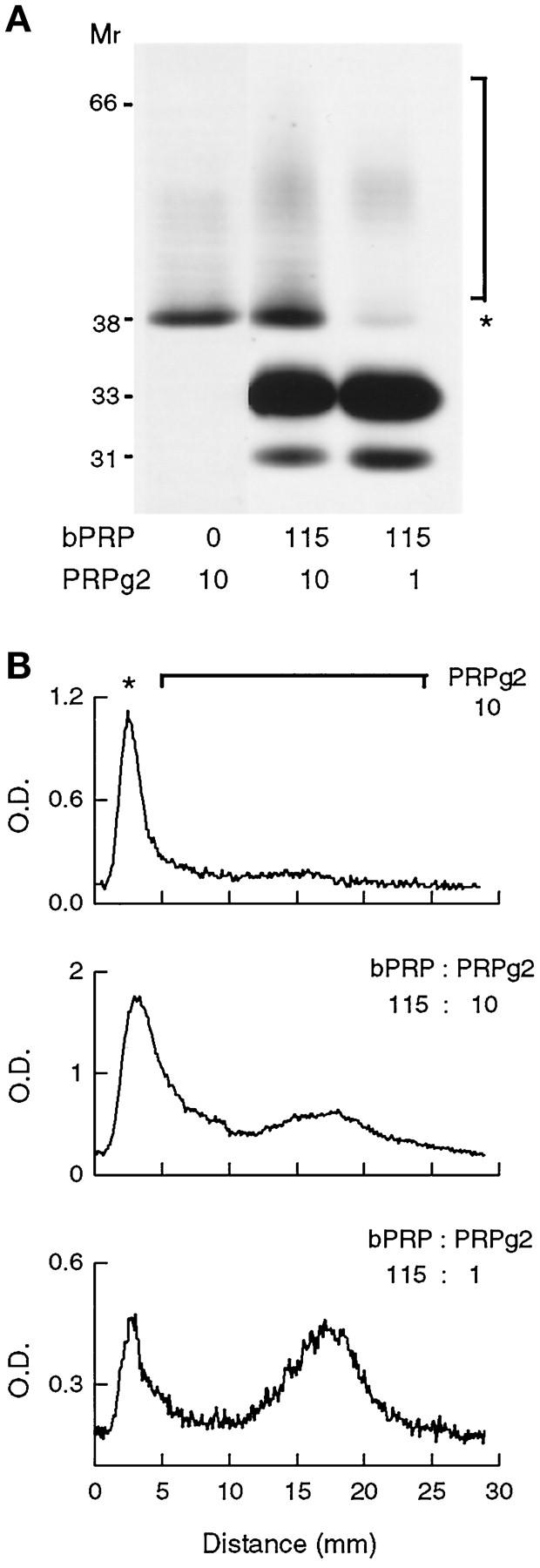
Effect of basic PRP expression on fractional modification of the PRPg2 core. Cells expressing PRPg2 alone or coexpressing PRPg2 and basic PRP (bPRP) were labeled for 15 h with 0.4 mCi/ml [3H]proline and PRPg2 and basic PRP were immunoprecipitated. (A) Immunoprecipitated polypeptides were visualized by fluorography. The asterisk (*) shows the position of the PRPg2 core and the bracket indicates the PRPg2 proteoglycan. The coexpressing clones express the same level of basic PRP (115% of ACTH) and two different levels of PRPg2 (10% and 1% of ACTH). (B) Optical density profile of PRPg2 products shown in the three lanes in A. The line graph used for the analysis includes both the PRPg2 core (asterisk) and PRPg2 proteoglycan (bracket).
Expression of Basic PRP Enhances the Sulfation of the Endogenous Granule Proteoglycan and Its Precursor in AtT-20 Cells
The results presented so far have demonstrated that the expression of basic PRP affects the degree of sulfation and fractional modification of PRPg2. We were curious whether this is a selective effect for PRPg2 or whether it involves other sulfated secretory products in AtT-20 cells. Therefore, we examined the incorporation of [35S]sulfate into sulfated polypeptides discharged constitutively and those secreted upon stimulation with the secretagogue 8-Br-cAMP (Moore et al., 1983) in nontransfected AtT-20 cells and cells expressing basic PRP (Figure 6). Of the sulfated species secreted constitutively, only the major band at 95 kDa showed an enhancement in sulfation when cells are expressing basic PRP (Figure 6A). This band has been shown previously to correspond to the precursor of a chondroitin sulfate proteoglycan (Burgess and Kelly, 1984). Our unpublished observations indicate that protease digestion of this band eluted from a gel gives rise to a ladder of bands characteristic of the proteoglycan as previously demonstrated by Burgess and Kelly (1984). The processed proteoglycan is one of the major sulfated species stored in the secretory granules and is released upon stimulation with 8-Br-cAMP. Examination of the sulfated polypeptides released upon stimulation with 8-Br-cAMP shows that the incorporation of [35S]sulfate into the proteoglycan is increased in cells expressing basic PRP four- to sixfold over the level found in nontransfected AtT-20 cells. However, as in the case of constitutively secreted polypeptides, sulfation of granule polypeptides that are not proteoglycans (Figure 6B) is affected little or not at all, indicating that the effect is specific for proteoglycans.
Figure 6.
Sulfated polypeptides secreted by nontransfected AtT-20 cells and AtT-20 cells expressing basic PRP. Nontransfected AtT-20 cells (−bPRP) and AtT-20 cells expressing basic PRP (+bPRP) were labeled with 0.5 mCi/ml [35S]sulfate for 15 min and chased consecutively first for 20 min, then for 100 min and finally for 60 min. During the last chase, 5 mM 8-Br-cAMP was added to stimulate exocytosis of secretory granules. Labeled polypeptides were precipitated with TCA and analyzed by SDS-PAGE and fluorography. (A) The first chase was used to analyze constitutively secreted polypeptides. The left panel is a short exposure showing a major 95-kDa band and the right panel is a long exposure showing several minor polypeptides. (B) Polypeptides secreted in response to 8-Br-cAMP. The endogenous granule proteoglycan appears as a series of closely spaced bands plus a smear extending from Mr = 22,000 to Mr = 40,000 in the absence of bPRP (−bPRP) and to Mr = 60,000 when basic PRP is expressed.
Enzymatic digests of the endogenous proteoglycan from nontransfected AtT-20 cells and from cells expressing basic PRP reveal that in both cases the GAG chains are sensitive to chondroitinase AC and not to heparinase III (Figure 7). Thus, the enhanced incorporation of [35S]sulfate into endogenous proteoglycan does not involve a change in the type of GAG chain added. When the endogenous proteoglycans from nontransfected cells and from cells expressing basic PRP were analyzed by chromatography on DEAE cellulose, the proteoglycan from cells expressing basic PRP eluted at a higher concentration of sodium chloride (Figure 8), indicating a higher charge content in the proteoglycan. Taken together these results indicate that expression of basic PRP results in increased sulfation of proteoglycans but not of other sulfated polypeptides.
Figure 7.
Enzymatic digests of endogenous granule proteoglycan. Nontransfected AtT-20 cells and AtT-20 cells expressing basic PRP (+bPRP) were labeled and chased as described in Figure 6. Media from 8-Br-cAMP-stimulated cells were digested with chondroitinase AC and heparinase III as described in MATERIALS AND METHODS. Labeled polypeptides were precipitated with TCA and analyzed by SDS-PAGE and fluorography. Cont, control; Ch, chondroitinase AC; H, heparinase III. The bracket indicates the position of the endogenous granule proteoglycan.
Figure 8.
Ion exchange chromatography of endogenous granule proteoglycan. Medium containing the endogenous granule proteoglycan was collected from [35S]sulfate-labeled cells that had been stimulated with 5 mM 8-Br-cAMP as described in Figure 6. The medium was loaded onto a DEAE cellulose column and eluted with a 0.05 M to 0.6 M NaCl gradient as described in MATERIALS AND METHODS. Equal amounts of each fraction were analyzed by SDS-PAGE and [35S]sulfate-labeled endogenous granule proteoglycan (PG) was quantitated by phosphorimager analysis. Nontransfected AtT-20 cells (•) and cells expressing basic PRP (○).
Storage of Basic PRP, PRPg2, and PRPg2ΔS in the Secretory Granules of AtT-20 Cells
Previous studies have shown that when expressed individually, basic PRP is stored in the granules of AtT-20 cells less efficiently than PRPg2 (Castle et al., 1992; Castle and Castle, 1993). To address whether the presence of GAG chains may be responsible for the different degrees of storage, we evaluated the storage of PRPg2ΔS which lacks the GAG acceptor site. Storage in secretory granules was assayed as the secretagogue-dependent discharge of PRPg2ΔS from cells metabolically labeled with [3H]proline. Although treatment of cells with the secretagogue increased the secretory output of PRPg2ΔS, the net percentage of labeled PRPg2ΔS undergoing stimulated secretion was much lower than that for PRPg2 (Table 1). Thus, the presence of the GAG chain improves storage and this posttranslational modification may contribute to the relatively greater storage of PRPg2 than of basic PRP (Castle et al., 1992; Castle and Castle, 1993).
Table 1.
8-Br-cAMP-dependent secretion of PRPg2, PRPg2ΔS, and basic PRPa
| Clone expressing | Stimulated secretion of
|
||
|---|---|---|---|
| PRPg2 | PRPg2ΔS | bPRPb | |
| PRPg2 | 20 ± 2 | NA | NA |
| PRPg2ΔS | NA | 4.4 ± 0.4 | NA |
| bPRP | NA | NA | 10 ± 0 |
| bPRP/PRPg2 | 15 ± 2 | NA | 12 ± 0 |
For each clone, duplicate wells of cells were labeled for 15 h with [3H]proline, chased first for 5 h and then for 2.5 h, and cells were harvested at the end of the experiment. During the second chase, 5 mM 8-Br-cAMP was added to one of the wells. Polypeptides were quantified by densitometry of fluorographs of immunoprecipitates from chases and cell lysates. The amount of each labeled polypeptide in each chase was normalized to the total labeled polypeptide (chases + cell extract). Stimulated secretion represents the difference between percentage of each polypeptide secreted in the presence and that secreted in the absence of 8-Br-cAMP. Data are expressed as mean ± range from two or three experiments each.
bPRP, basic PRP; NA, not applicable.
Since the presence of the GAG chain appears to affect the sorting of PRPg2, we wished to examine whether the presence of PRPg2 could also affect the sorting of basic PRP. Therefore, we compared stimulated secretion of basic PRP expressed alone and coexpressed with PRPg2. Table 1 shows that the percentage of basic PRP undergoing stimulated secretion is essentially the same in the absence and in the presence of PRPg2, suggesting that GAGs do not affect the sorting of basic PRP in AtT-20 cells. Interestingly, the percentage of stimulated secretion of PRPg2 in the presence of basic PRP decreased slightly.
DISCUSSION
In this work, we have shown that expression of a basic PRP correlates with an enhanced incorporation of [35S]sulfate into the GAG chains of exogenous and endogenous proteoglycans in AtT-20 cells. Thus, we reproduced in a cell culture model system the enhanced sulfation of proteoglycans that accompanies the induction of basic PRPs by isoproterenol in the rat parotid (Blair et al., 1991). We now show that the effect is induced by a basic PRP and that it involves increases in both the amount of sulfate per GAG chain as indicated by the ratio of [35S]sulfate:[3H]glucosamine and in the extent of modification of the PRPg2 backbone. We believe that increased sulfation also reflects an increase in the average length of the existing sulfated GAG chain as indicated by the progressively decreased electrophoretic mobility of the ladder-like proteoglycan with increased basic PRP expression (Figure 1B).
Sulfation of proteoglycans is mediated by sulfotransferases which catalyze the transfer of sulfate from adenosine 3′-phosphate, 5′-phosphosulfate (PAPS) to the sugar residues of the GAG chain. Work with purified heparan sulfate and chondroitin sulfate sulfotransferases has shown that their activities in vitro are strongly stimulated by cationic substances such as protamine and histones, presumably by lowering the Km for PAPS (Habuchi and Miyata, 1980; Habuchi et al., 1995). Basic PRP, which contains a high proportion of Lys and has a pI >10, may also be functioning as an cationic stimulator of the sulfotransferases, and our data argue that such stimulation is relevant in vivo. Since the presence of basic PRP affects the sulfation of PRPg2 and an unrelated proteoglycan, the effect appears to be a general one and not due to possible interactions between basic PRP and PRPg2. It is interesting to note that other forms of posttranslational sulfation are unaffected by the expression of basic PRP (Figure 6). This suggests that the cationic proteins do not alter the activities of other sulfotransferases and conversely that specific features of the GAG sulfation process contribute to its unique sensitivity.
To our knowledge, this is the first illustration of a change in the utilization of the polypeptide backbone for GAG chain addition mediated by the expression of a distinct protein, and it is interesting to consider what this may mean mechanistically. GAG chain biosynthesis is a complex multistep process beginning with the formation of the linkage region attached to specific Ser residues within the polypeptide and followed by addition of the repeating disaccharides that become sulfated (reviewed in Salmivirta et al., 1996; Silbert, 1996). Although we have not directly addressed the extent to which the polypeptide backbone of PRPg2 is modified by addition of core xylose and galactose residues, we suspect that this process is fairly complete, regardless of the presence of basic PRP based on two observations. First, the PRPg2 has a slightly slower electrophoretic mobility than does PRPg2ΔS (Figure 2) which cannot be modified. Second, the PRPg2 backbone is labeled by [3H]galactose, indicating the presence of the linkage region. Thus, the change in fractional modification of the PRPg2 core in the presence of basic PRP actually corresponds to a change in the utilization of the linkage region for chain elongation by repeating disaccharide addition. Taken together with the suspected increased elongation of PRPg2 GAG chains in the presence of basic PRP, we deduce that the entire process of repeating disaccharide addition and sulfation seems to be coordinately regulated by the presence of this basic secretory protein. Thus, we infer that the enzymes involved in elongation and modification of the GAG chain are colocalized and may function as a complex. This is consistent with the presence of a GAG biosynthetic complex envisioned previously (Salmivirta et al., 1996; Silbert, 1996). We presume that it is the net positive charge of the basic PRP that regulates the glycosylation and sulfation machinery because expression of PRPg2 (which is structurally related to PRP but has a low pI) alone does not affect the extent of sulfation of endogenous proteoglycan in AtT-20 cells (our unpublished observations).
The enhanced sulfation of proteoglycans is likely to be a response to a change in the intracompartmental environment rather than due to specific interactions between basic PRP and the proteoglycans. The extent of sulfation progressively increases as the expression of basic PRP increases, suggesting that the response is customized and is linked to quantity rather than quality of the basic protein. Apparently, the posttranslational modifications respond to the biophysical conditions during intracellular transport and may be aimed at regulating the effects of basic proteins on post-Golgi compartments. Taken together, these observations suggest that the role of the enhanced sulfation is in homeostasis of intracellular compartments. This is in contrast to other situations where regulation of sulfation of proteoglycans is directed toward extracellular signalling, e.g., cell proliferation (Ruoslahti, 1989; Rapraeger, 1993; Lindahl et al., 1994). In view of these characteristics, we propose that the role of this conditional sulfation is in controlling the osmotic activity within Golgi-derived vesicles. As a mechanism that reduces net fixed positive charge, sulfation would limit intracompartmental electrolyte concentration and water content that accumulate as a result of the Donnan effect.
We began this study with the notion that such a mechanism would be beneficial to the process of condensation of secretory proteins for storage in granules in regulated secretory cells. Absence of the GAG chain on PRPg2 resulted in a substantially reduced storage of PRPg2 backbone, suggesting that the GAG chain may be responsible for ensuring the more efficient storage of PRPg2 than of basic PRP. However, coexpression of PRPg2 with the basic PRP did not significantly improve on the limited storage of the latter in AtT-20 cells (Table 1), suggesting that the proteoglycans are not essential to the sorting process but may be beneficial in some cases. A similar conclusion regarding the nonessential role of proteoglycans for sorting of proteins for regulated secretion in AtT-20 cells was reached previously (Burgess and Kelly, 1984).
We also noted that the presence of basic PRP decreases the storage of PRPg2 (Table 1). Previously, we have shown that basic PRP is removed from the granule pool of AtT-20 cells (Castle et al., 1997). Because PRPs are suspected to interact via their proline-rich domains (Stahl et al., 1996) it is likely that the reduced storage of PRPg2 is a consequence of increased removal of PRPg2 from the granules due to interactions with basic PRP.
In future studies, it will be interesting to examine whether the enhanced glycosylation/sulfation of proteoglycans is a specialization of cells that have a regulated secretory pathway or whether it is a constitutive property of a machinery that is present in all cells. It may be the case, in the latter event, that the proposed osmotic regulation mechanism is important to other post-Golgi carriers. Also, it will be interesting to explore whether a complementary mechanism operates in cases where cells produce and secrete unusually large proportions of acidic proteins.
ACKNOWLEDGMENTS
We are grateful to Charles Hubbard for expert technical assistance in mutagenizing the cDNA of the PRP and to the members of the Castle laboratory for helpful discussion. This work was supported by National Institute of Health grant DE-08941.
Footnotes
Abbreviations used: ACTH, adrenocorticotropic hormone; 8-Br-cAMP, 8-bromocyclic AMP; GAG, glycosaminoglycan; PRP, proline-rich protein; PRPg, proline-rich proteoglycan; TCA, trichloroacetic acid.
REFERENCES
- Blair EA, Castle AM, Castle JD. Proteoglycan sulfation and storage parallels storage of basic secretory proteins in exocrine cells. Am J Physiol. 1991;261:C897–C905. doi: 10.1152/ajpcell.1991.261.5.C897. [DOI] [PubMed] [Google Scholar]
- Burgess TL, Kelly RB. Sorting and secretion of adrenocorticotropin in a pituitary tumor cell line after perturbation of the level of a secretory granule-specific proteoglycan. J Cell Biol. 1984;99:2223–2230. doi: 10.1083/jcb.99.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle AM, Castle JD. Novel secretory proline-rich proteoglycans from rat parotid: cloning and characterization by expression in AtT-20 cells. J Biol Chem. 1993;268:20490–20496. [PubMed] [Google Scholar]
- Castle AM, Huang AY, Castle JD. Passive sorting in maturing granules of AtT-20 cells: the entry and exit of salivary amylase and proline-rich protein. J Cell Biol. 1997;138:45–54. doi: 10.1083/jcb.138.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle AM, Stahl LE, Castle JD. A 13-amino acid N-terminal domain of a basic proline-rich protein is necessary for storage in secretory granules and facilitates exit from the endoplasmic reticulum. J Biol Chem. 1992;267:13093–13100. [PubMed] [Google Scholar]
- Chanat E, Weiss U, Huttner WB. The disulfide bond in chromogranin B, which is essential for its sorting to secretory granules, is not required for its aggregation in the trans-Golgi network. FEBS Lett. 1994;351:225–230. doi: 10.1016/0014-5793(94)00865-5. [DOI] [PubMed] [Google Scholar]
- Colomer V, Kicska GA, Rindler MJ. Secretory granule content proteins and the luminal domains of granule membrane proteins aggregate in vitro at mildly acidic pH. J Biol Chem. 1996;271:48–55. doi: 10.1074/jbc.271.1.48. [DOI] [PubMed] [Google Scholar]
- Colomer V, Lal K, Hoops TC, Rindler MJ. Exocrine granule specific packaging signals are present in the polypeptide moiety of the pancreatic granule membrane protein GP2 and in amylase: implications for protein targeting to secretory granules. EMBO J. 1994;13:3711–3719. doi: 10.1002/j.1460-2075.1994.tb06680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool DR, Normant E, Shen F, Chen H-C, Pannel L, Zhang Y, Loh YP. Carboxypeptidse E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in CPEfat mice. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- Habuchi H, Habuchi O, Kimata K. Purification and characterization of heparan sulfate 6-sulfotransferase from the culture medium of Chinese hamster ovary cells. J Biol Chem. 1995;270:4172–4179. doi: 10.1074/jbc.270.8.4172. [DOI] [PubMed] [Google Scholar]
- Habuchi O, Miyata K. Stimulation of glycosaminoglycan sulfotransferase from chick embryo cartilage by basic proteins and polyamines. Biochim Biophys Acta. 1980;616:208–217. doi: 10.1016/0005-2744(80)90139-4. [DOI] [PubMed] [Google Scholar]
- Huang XF, Arvan P. Intracellular transport of proinsulin in pancreatic beta cells. Structural maturation probed by disulfide accessibility. J Biol Chem. 1995;270:20417–20423. doi: 10.1074/jbc.270.35.20417. [DOI] [PubMed] [Google Scholar]
- Kuliawat R, Arvan P. Distinct molecular mechanisms for protein sorting within immature secretory granules of pancreatic B-cells. J Cell Biol. 1994;126:77–86. doi: 10.1083/jcb.126.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindahl U, Lindholt K, Spilmann D, Kjellen L. More to “heparin” than anticoagulation. Thromb Res. 1994;75:1–32. doi: 10.1016/0049-3848(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Sali A, Ghildyal N, Karplus M, Stevens RL. Packaging of proteases and proteoglycans in the granules of mast cells and other hematopoietic cells. A cluster of histidines on mouse mast cell protease 7 regulates its binding to heparin serglycin proteoglycans. J Biol Chem. 1995;270:19524–19531. doi: 10.1074/jbc.270.33.19524. [DOI] [PubMed] [Google Scholar]
- Moore H-P, Gumbiner B, Kelly RB. A subclass of proteins and sulfated macromolecules secreted by AtT-20 (mouse pituitary tumor) cells is sorted with adrenocorticotropin into dense secretory granules. J Cell Biol. 1983;97:810–817. doi: 10.1083/jcb.97.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenzer J, Bildstein C, Gleason M, Carlson DM. Properties of proline-rich proteins from parotid glands of isoproterenol treated rats. J Biol Chem. 1979;254:5629–5634. [PubMed] [Google Scholar]
- Natori S, Huttner WB. Chromogranin B (secretogranin I) promotes sorting to the regulated secretory pathway of processing intermediates derived from a peptide hormone precursor. Proc Natl Acad Sci USA. 1996;93:4431–4436. doi: 10.1073/pnas.93.9.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger AC. The coordinated regulation of heparan sulfate, syndecans and cell behavior. Curr Opin Cell Biol. 1993;5:844–853. doi: 10.1016/0955-0674(93)90034-n. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Proteoglycans in cell regulation. J Biol Chem, 1989;264:13369–13372. [PubMed] [Google Scholar]
- Salmivirta M, Lindholt K, Lindahl U. Heparan sulfate: a piece of information. FASEB J. 1996;10:1270–1279. doi: 10.1096/fasebj.10.11.8836040. [DOI] [PubMed] [Google Scholar]
- Serafin WE, Katz HR, Austen KF, Stevens RL. Complexes of heparin proteoglycans, chondroitin sulfate E proteoglycans, and [3H]diisopropyl fluorophosphate-binding proteins are exocytosed from activated mouse bone marrow-derived mast cells. J Biol Chem. 1986;261:15017–15021. [PubMed] [Google Scholar]
- Silbert JE. Organization of glycosaminoglycan sulfation in the biosynthesis of proteochondroitin sulfate and proteodermatan sulfate. Glycoconjugate J. 1996;13:907–912. doi: 10.1007/BF01053185. [DOI] [PubMed] [Google Scholar]
- Stahl LE, Wright RL, Castle JD, Castle AM. The unique proline-rich domain of parotid proline-rich proteins functions in secretory sorting. J Cell Sci. 1996;109:1637–1645. doi: 10.1242/jcs.109.6.1637. [DOI] [PubMed] [Google Scholar]
- Zanini A, Giannatasio G, Meldolesi J. Synthesis and Release of Adenohypophyseal Hormones. M. Justuz and K.W. McKerns, New York: Plenum; 1980. Intracellular events in prolactin secretion; pp. 105–123. [Google Scholar]