Abstract
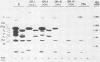
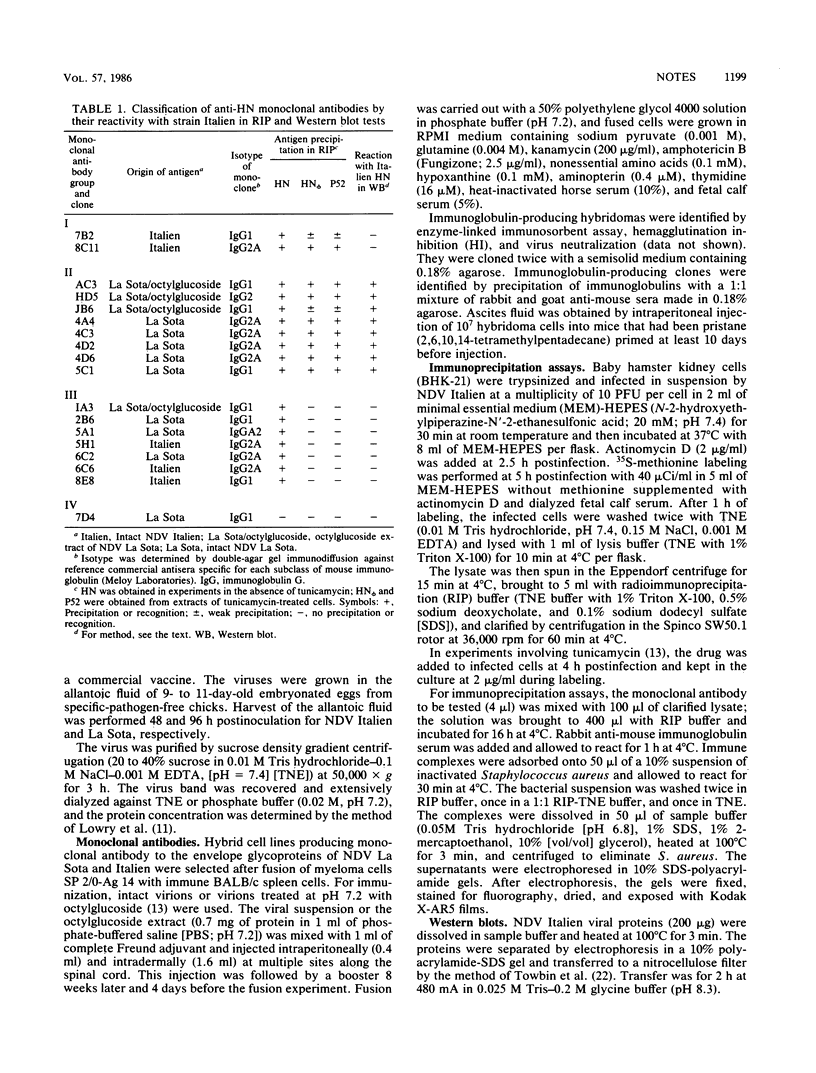
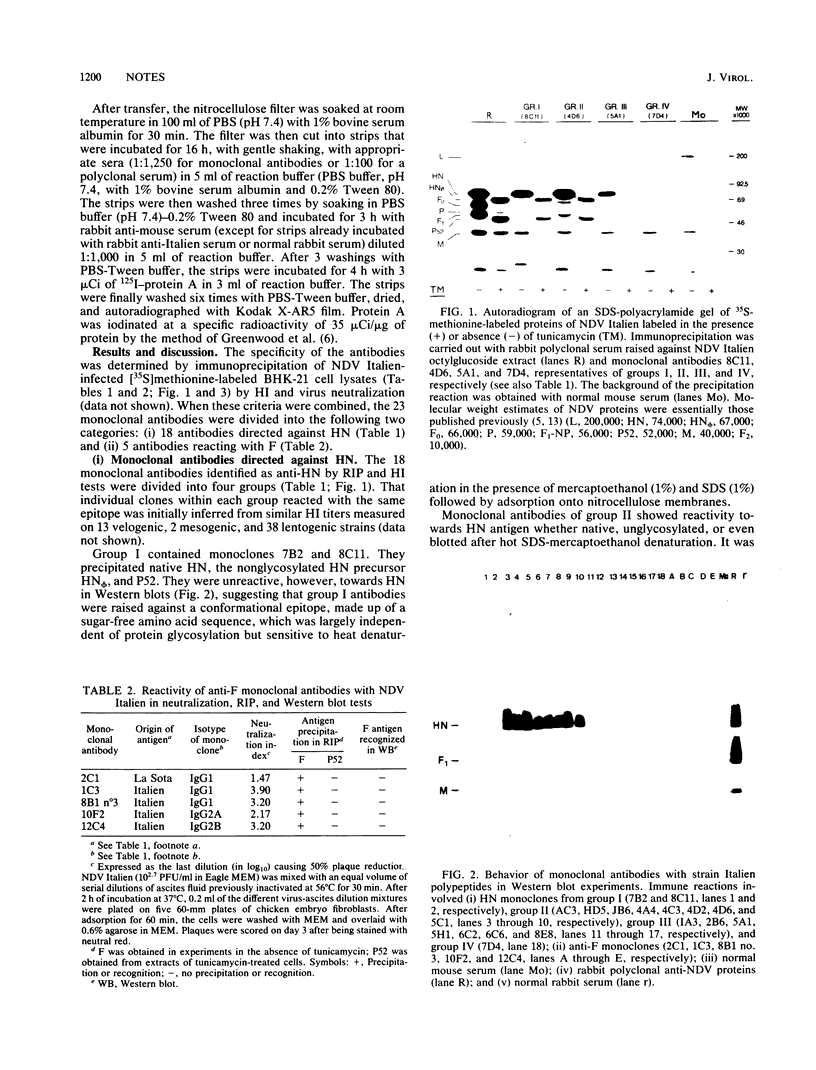
Eighteen hybridoma lines obtained by immunization of mice with Newcastle disease virus (NDV) lentogenic strain La Sota or velogenic strain Italien produced hemagglutinating monoclonal antibodies. The 18 monoclones were divided into four groups according to their reactivity toward native hemagglutinin neuraminidase protein (HN), nonglycosylated HN precursor, and heat-denatured HN blotted on nitrocellulose membranes. Only group II reagents were reactive toward their targets in all conditions tested. They were considered sequence-specific antibodies. Group I antibodies did not require glycosylation but lacked reactivity towards the denatured glycosylated antigen. Monoclonal antibodies from group III recognized only the native HN. Group IV was made up of a single monoclone that lacked reactivity with NDV Italien but recognized the La Sota strain in hemagglutination inhibition and enzyme-linked immunosorbent assays. Five hybridoma lines produced monoclonal antibodies which neutralized viral infectivity but failed to inhibit hemagglutination. One monoclonal antibody obtained after immunization of mice with NDV La Sota showed a low neutralization index versus NDV Italien. Four monoclonal antibodies derived from mice immunized with NDV Italien showed higher neutralization indices towards this strain. Neither the denatured F protein nor its nonglycosylated precursor was reacted against by the five monoclonal antibodies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basak S., Compans R. W. Studies on the role of glycosylation in the functions and antigenic properties of influenza virus glycoproteins. Virology. 1983 Jul 15;128(1):77–91. doi: 10.1016/0042-6822(83)90320-3. [DOI] [PubMed] [Google Scholar]
- Choppin P. W., Richardson C. D., Merz D. C., Hall W. W., Scheid A. The functions and inhibition of the membrane glycoproteins of paramyxoviruses and myxoviruses and the role of the measles virus M protein in subacute sclerosing panencephalitis. J Infect Dis. 1981 Mar;143(3):352–363. doi: 10.1093/infdis/143.3.352. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W., Ball L. A., Hightower L. E. Coding assignments of the five smaller mRNAs of Newcastle disease virus. J Virol. 1982 Sep;43(3):1024–1031. doi: 10.1128/jvi.43.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Fusion of Sendai virus with liposomes: dependence on the viral fusion protein (F) and the lipid composition of liposomes. Virology. 1983 Apr 15;126(1):361–369. doi: 10.1016/0042-6822(83)90485-3. [DOI] [PubMed] [Google Scholar]
- Iorio R. M., Bratt M. A. Monoclonal antibodies to newcastle disease virus: delineation of four epitopes on the HN glycoprotein. J Virol. 1983 Nov;48(2):440–450. doi: 10.1128/jvi.48.2.440-450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohama T., Garten W., Klenk H. D. Changes in conformation and charge paralleling proteolytic activation of Newcastle disease virus glycoproteins. Virology. 1981 Jun;111(2):364–376. doi: 10.1016/0042-6822(81)90340-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Immunological studies of the functions of paramyxovirus glycoproteins. Virology. 1981 Feb;109(1):94–105. doi: 10.1016/0042-6822(81)90474-8. [DOI] [PubMed] [Google Scholar]
- Morrison T. G., Simpson D. Synthesis, stability, and cleavage of Newcastle disease virus glycoproteins in the absence of glycosylation. J Virol. 1980 Oct;36(1):171–180. doi: 10.1128/jvi.36.1.171-180.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D., Rott R. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology. 1976 Jul 15;72(2):494–508. doi: 10.1016/0042-6822(76)90178-1. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Isomura S., Suzuki S., Watanabe E., Hamaguchi M., Yoshida T., Nagai Y. Monoclonal antibodies to the HN glycoprotein of Newcastle disease virus. Biological characterization and use for strain comparisons. Virology. 1983 Oct 30;130(2):318–330. doi: 10.1016/0042-6822(83)90086-7. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Asano A., Okada Y. Biological activities of glycoproteins of HVJ (Sendai virus) studied by reconstitution of hybrid envelope and by concanavalin A-mediated binding: a new function of HANA protein and structural requirement of F protein in hemolysis. Virology. 1979 Nov;99(1):197–202. doi: 10.1016/0042-6822(79)90055-2. [DOI] [PubMed] [Google Scholar]
- Prehm P., Scheid A., Choppin P. W. The carbohydrate structure of the glycoproteins of the paramyxovirus SV5 grown in bovine kidney cells. J Biol Chem. 1979 Oct 10;254(19):9669–9677. [PubMed] [Google Scholar]
- Richardson C. D., Choppin P. W. Oligopeptides that specifically inhibit membrane fusion by paramyxoviruses: studies on the site of action. Virology. 1983 Dec;131(2):518–532. doi: 10.1016/0042-6822(83)90517-2. [DOI] [PubMed] [Google Scholar]
- Russell P. H., Alexander D. J. Antigenic variation of Newcastle disease virus strains detected by monoclonal antibodies. Arch Virol. 1983;75(4):243–253. doi: 10.1007/BF01314890. [DOI] [PubMed] [Google Scholar]
- Russell P. H., Griffiths P. C., Goswami K. K., Alexander D. J., Cannon M. J., Russell W. C. The characterization of monoclonal antibodies to Newcastle disease virus. J Gen Virol. 1983 Sep;64(Pt 9):2069–2072. doi: 10.1099/0022-1317-64-9-2069. [DOI] [PubMed] [Google Scholar]
- Smith G. W., Hightower L. E. Biological consequences of neuraminidase deficiency in Newcastle disease virus. J Virol. 1983 Sep;47(3):385–391. doi: 10.1128/jvi.47.3.385-391.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]