Abstract
Synaptosomal-associated protein of 25 kDa (SNAP-25) is a palmitoylated membrane protein essential for neurotransmitter release from synaptic terminals. We used neuronal cell lines to study the biosynthesis and posttranslational processing of SNAP-25 to investigate how palmitoylation contributes to the subcellular localization of the protein. SNAP-25 was synthesized as a soluble protein that underwent palmitoylation approximately 20 min after synthesis. Palmitoylation of the protein coincided with its stable membrane association. Treatment of cells with brefeldin A or other disrupters of transport inhibited palmitoylation of newly synthesized SNAP-25 and abolished membrane association. These results demonstrate that the processing of SNAP-25 and its targeting to the plasma membrane depend on an intact transport mechanism along the exocytic pathway. The kinetics of SNAP-25 palmitoylation and membrane association and the sensitivity of these parameters to brefeldin A suggest a novel trafficking pathway for targeting proteins to the plasma membrane. In vitro, SNAP-25 stably associated with membranes was not released from the membrane after chemical deacylation. We propose that palmitoylation of SNAP-25 is required for initial membrane targeting of the protein but that other interactions can maintain membrane association in the absence of fatty acylation.
INTRODUCTION
Neurotransmitter release at synaptic terminals is a calcium-regulated process that is critical for nervous system function (Südhof, 1995). A molecular description of this process is emerging with the characterization of proteins that mediate vesicle targeting and fusion. The plasma membrane proteins, syntaxin and synaptosomal-associated protein of 25 kDa (SNAP-25), and the synaptic vesicle protein synaptobrevin/vesicle-associated membrane protein were identified as receptors for cytosolic factors necessary for vesicular transport of proteins (Söllner et al., 1993a,b). The soluble factors are N-ethylmaleimide-sensitive factor (NSF) and soluble NSF-attachment proteins (SNAPs); hence, the receptor proteins are named SNAP receptors (SNAREs).
Although SNAREs associated with synaptic vesicle exocytosis are well characterized, information about the mechanisms of targeting of these proteins to their resident membranes is limited. SNAREs are integral membrane proteins, but do not contain signal sequences for cotranslational insertion into the endoplasmic reticulum (ER). Syntaxin and synaptobrevin contain a hydrophobic domain at their C terminus that is responsible for insertion into membranes (Kutay et al., 1993). Synaptobrevin is posttranslationally inserted into the ER membrane by a novel mechanism (Kutay et al., 1995) and is transported to the Golgi where it is sorted to synaptic vesicles. Syntaxin could potentially follow the same transport route as synaptobrevin, with a specific sorting mechanism from the Golgi to the plasma membrane. SNAP-25 lacks a stretch of hydrophobic amino acids characteristic of transmembrane domains (Oyler et al., 1989). The membrane-binding properties of SNAP-25 are presumed to be due to modification of the protein by palmitate at cysteine residues near the center of the primary amino acid sequence (Hess et al., 1992). Indeed, deletion of 12 amino acids, including the four cysteine residues that are the putative sites of palmitoylation of SNAP-25, abolishes incorporation of radioactive palmitate into the mutant protein and results in a protein that is no longer associated with membranes (Veit et al., 1996). Therefore, it is likely that palmitoylation of SNAP-25 is required for its association with membranes.
Like SNAP-25, a variety of otherwise soluble polypeptides are associated with the inner surface of the plasma membrane through covalent lipid modifications. Membrane targeting of growth-associated protein of 43 kDa (GAP-43) is mediated by palmitoylation of cysteine residues 3 and 4 (Zuber et al., 1989). A dual acylation motif of N-myristoylation in conjunction with palmitoylation of nearby cysteine residues is responsible for plasma membrane targeting of most Src family kinases (Casey, 1995; Timson Gauen et al., 1996), certain G protein α subunits (Wedegaertner et al., 1995), and endothelial nitric oxide synthase (Robinson et al., 1995). H-Ras and N-Ras are also dually modified with lipids; prenylation and palmitoylation near the carboxyl terminus are required for stable membrane association (Hancock et al., 1990). Although these lipid-modified proteins share a common final destination, it is uncertain whether they are targeted there by the same mechanism.
The role that the different lipid moieties play in the intracellular trafficking of newly synthesized proteins is only beginning to be characterized. A recent report suggests that the nonreceptor tyrosine kinase p59fyn is palmitoylated at the plasma membrane very rapidly after synthesis and myristoylation on soluble ribosomes (van’t Hof and Resh, 1997). Switching the membrane targeting sequence in p59fyn from a myristoylation/palmitoylation motif to the dual palmitoylation motif found on GAP-43 resulted in a change in the kinetics of membrane association of the protein (van’t Hof and Resh, 1997). These results raise the possibility of a second pathway for targeting proteins synthesized on soluble ribosomes to the plasma membrane.
How is SNAP-25 targeted to the plasma membrane? Fatty acylation of newly synthesized SNAP-25 could occur at the cell surface, allowing the protein to become stably associated with its resident membrane. Alternatively, palmitoylation could occur on an intracellular organelle, followed by vesicular transport to the plasma membrane. Protein palmitoylation occurs both at the plasma membrane and in the early secretory pathway. However, proteins known to be palmitoylated in the early secretory pathway are integral membrane proteins with transmembrane spans (Schlesinger et al., 1993).
In this study, we sought to define the biosynthetic pathway of SNAP-25, with the goal of understanding how palmitoylation contributes to the subcellular localization of the protein. We present evidence that palmitoylation of SNAP-25 is coincident with initial binding of the protein to membranes, but may not be essential for maintaining stable membrane association. Furthermore, we show that palmitoylation and membrane association of SNAP-25 require an intact secretory pathway, a property shared by GAP-43, but not N-myristoylated proteins that are palmitoylated. This unexpected requirement for transport illustrates that there are at least two distinct pathways that lipid-modified proteins use for plasma membrane targeting.
MATERIALS AND METHODS
Materials
l-[35S]methionine/l-[35S]cysteine (>1000 Ci/mmol) was purchased from Amersham Life Science (Arlington Heights, IL); [3H]palmitate (30–60 Ci/mmol) was obtained from DuPont New England Nuclear (Wilmington, DE). Reagents for affinity purification of the SNAP-25 antibody were purchased from Pharmacia Biotech (Piscataway, NJ). Hydroxylamine and Tris base were obtained from J.T. Baker (Phillipsburg, NJ). Brefeldin A (BFA), monensin, nocodazole, cycloheximide, and ammonium chloride were purchased from Sigma Chemical Company (St. Louis, MO). Brefeldin A and nocodazole were stored at −20°C as 2-mg/ml and 10-mg/ml stock solutions, respectively, in dimethyl sulfoxide (DMSO). Monensin was stored at −20°C as a 2 mM stock in 100% ethanol. Cycloheximide was stored at −20°C as a 2-mg/ml stock in distilled water. Ammonium chloride was prepared fresh as a 2 M stock in distilled water. Immune complexes were precipitated using Staphylococcus aureus (Pansorbin cells, Calbiochem-Novabiochem Corporation, San Diego, CA). Nerve growth factor (NGF) was kindly provided by Dr. Eugene M. Johnson, Jr. (Washington University, St. Louis, MO).
Cell Culture
PC12 cells (ATCC CRL-1721) were cultured in DMEM-high glucose (HG) supplemented with 10% horse serum, 5% fetal bovine serum (FBS), 2 mM glutamine, 150 U/ml penicillin, and 50 μg/ml streptomycin. Horse serum and FBS were heat inactivated at 56°C for 30 min. Cells were seeded in 35-mm dishes coated with poly-l-lysine. Differentiation of PC12 cells into a neuron-like phenotype was induced by incubation of the cells for 2 to 5 d in medium containing 100 ng/ml NGF. N2A cells were obtained from Dr. David Harris (Washington University, St. Louis, MO) and cultured in Eagle’s minimal essential medium (MEM) with nonessential amino acids, 10% heat-inactivated FBS, and antibiotics. NG108 cells were a gift from Dr. Ron Taussig (University of Michigan, Ann Arbor, MI) and were cultured in DMEM-HG supplemented with 10% FBS.
Antibodies
A peptide corresponding to the carboxyl-terminal residues 195–206 of the SNAP-25 protein (Oyler et al., 1989) was synthesized, coupled to rabbit serum albumin, and the resulting conjugate was used to immunize rabbits. The antibody was purified from whole antisera by affinity chromatography on peptide covalently linked to Sepharose (Mumby and Gilman, 1991). Rhodamine-conjugated secondary antibodies were purchased from Cappel (Durham, NC). Goat anti-rabbit IgG labeled with 125I was purchased from ICN (Costa Mesa, CA). Antibody 856 was prepared using the peptide GAGESKSTIVKQMK derived from a common region of G protein α subunits as immunogen (Mumby and Gilman, 1991). The affinity-purified antibody reacts strongly with all forms of Goα and Giα, weakly with Gsα, and does not recognize Gqα or G12α family members. Mouse monoclonal antibody to GAP-43 (clone 91E12) was purchased from Boehringer Mannheim (Indianapolis, IN). Monoclonal antisyntaxin (clone HPC-1) was obtained from Sigma.
Radiolabeling and Inhibition of Transport
[35S]Methionine.
Semiconfluent PC12 cells were incubated in methionine- and serum-depleted DMEM-HG for 1 h. The medium was replaced with methionine-free DMEM-HG containing 2.5% dialyzed FBS and 50 μCi/ml [35S]methionine, and cells were incubated for the times indicated. Cells were then lysed and analyzed directly (pulse) or after incubation for different periods of time in complete culture medium (chase).
[3H]Palmitate.
Cells were incubated with DMEM-HG containing [3H]palmitate (1 mCi/ml), DMSO (1% vol/vol), and horse serum (10% vol/vol) for times indicated in the figure legends (Linder et al., 1995).
Transport Inhibitors.
Transport inhibitors were added to PC12 cells growing in 35-mm dishes along with or before labeling of the cells with [35S]methionine or [3H]palmitate. Brefeldin A (final concentration 10 μg/ml) was added to the cells along with the label, unless indicated. Monensin (10 μM), nocodazole (20 μg/ml), and ammonium chloride (50 mM) were added to the cells 30 min before the addition of the radiolabeled medium. Control cells were incubated with the solvents.
Immunoprecipitations
Cells were washed once with warm phosphate-buffered saline (PBS) and solubilized in RIPA buffer (150 mM NaCl, 10 mM sodium phosphate, pH 7.2, 1% sodium deoxycholate, 1% Nonidet P-40) containing 0.5% SDS and 1 mM dithiothreitol (DTT) (modified RIPA buffer) for 15 min on ice. The lysate was cleared by centrifugation at 100,000 × g for 30 min. Typically, 50–100 μg of [35S]methionine-labeled lysate or 200–300 μg of [3H]palmitate-labeled lysate were incubated with affinity-purified SNAP-25 antibody overnight at 4°C. Inactivated S. aureus were added to precipitate immune complexes by incubation at 4°C for 30 min. The immunoprecipitates were pelleted by centrifugation at 15,850 × g for 5 min, resuspended in 0.1 ml of modified RIPA buffer, and layered on a cushion of 20% sucrose in modified RIPA buffer. The precipitates were then pelleted by centrifugation at 15,850 × g for 7 min and washed once with modified RIPA buffer and once with PBS. The final pellet was dissolved in SDS sample buffer. Proteins were separated by SDS-PAGE on 11% or 13% polyacrylamide gels (16-cm plate size) in a Hoefer apparatus. The long gels were necessary to resolve the SNAP-25 doublet (see text). The gels were stained with Coomassie blue, destained, and soaked in Amplify (Amersham, Arlington Heights, IL) to detect radiolabeled polypeptides by fluorography. [35S]methionine-labeled proteins were detected after overnight exposure of the film. For [3H]palmitate-labeled polypeptides, the exposure times ranged from 2 wk to 1 mo.
Subcellular Fractionation
Cells growing in 35-mm dishes were washed once with warm PBS and scraped off the plates into ice-cold PBS. Cells were then collected by centrifugation at 1000 × g for 10 min and resuspended in a hypotonic buffer (20 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM DTT, 0.1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM benzamidine, and 10 μg/ml pepstatin). After incubation for 15 min, cells were homogenized with 40 strokes of a Dounce homogenizer or alternatively with 20 passes through a ball-bearing homogenizer and pelleted at 600 × g for 5 min. The pellet consisting of unbroken cells and nuclei was designated the low speed pellet. The supernatant was collected and subjected to further centrifugation at 100,000 × g for 30 min. The resulting supernatant contained soluble proteins and was designated the S100 fraction. The 100,000 × g pellet (P100) was considered the membrane fraction. Membranes were solubilized by suspension in modified RIPA buffer, and the cytosol was mixed with an equivalent volume of 2× modified RIPA buffer for immunoprecipitation. Membrane and cytosolic fractions obtained in this manner were also subjected to hydroxylamine treatment.
Hydroxylamine Treatment
Immunoprecipitated SNAP-25 before SDS-PAGE.
After immunoprecipitation of radiolabeled SNAP-25 from PC12 cells, the pellets containing the immune complexes were resuspended in 20 μl of PBS and mixed with 20 μl of 1 M hydroxylamine (pH 7.5) or 1 M Tris-HCl (pH 7.5) or PBS as a control. After incubation at room temperature for 2 h, SDS sample buffer was added and the entire sample was analyzed by SDS-PAGE and fluorography.
Immunoprecipitated SNAP-25 after SDS-PAGE.
Immunoprecipitated SNAP-25 from PC12 cells was subjected to SDS-PAGE. Samples were run on duplicate gels. One gel was immersed in a solution of 1 M hydroxylamine (pH 7.5), the second gel in 1 M Tris-HCl (pH 7.5). After 30 min at room temperature, fresh solutions were added and the gels were incubated for an additional hour. The gels were washed in water for 15 min, changing the water at 5-min intervals before processing for fluorography.
Brain Membranes.
Bovine brain membranes were prepared according to Sternweis and Robishaw (1984). Membranes were resuspended in TEDK buffer (50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM DTT, 50 mM KCl) containing a cocktail of protease inhibitors: 0.1 mM phenylmethylsulfonyl fluoride, 21 μg/ml N-tosyl-l-phenylalanine chloromethyl ketone, 21 μg/ml N-α-p-tosyl-l-lysine chloromethyl ketone, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM benzamidine, and 10 μg/ml pepstatin. The solution was adjusted to 1.4 M sucrose, placed at the bottom of a centrifuge tube, and overlaid with 1.2 M sucrose and finally 0.25 M sucrose solutions. The three-step gradient was subjected to centrifugation at 82,000 × g for 1 h at 4°C in a swinging bucket rotor. Membranes were harvested from the 0.25 M/1.2 M interface. The washed membranes were incubated with an equal volume of 1 M Tris (pH 7.5) or 1 M hydroxylamine (pH 7.5) for 30 min. The samples were diluted in a sucrose solution to a final concentration of 1.4 M sucrose and overlaid with 1.2 M and 0.25 M sucrose solutions as before. The 1.2 M sucrose layer was present to cleanly separate soluble proteins from membrane proteins. The three-step gradient was subjected to centrifugation at 82,000 × g for 1 h at 4°C in a swinging bucket rotor. Nearly all of the membranes accumulated at the 0.25 M/1.2 M interphase, whereas soluble proteins remained in the 1.4 M phase. The amount of SNAP-25 in each of the fractions was analyzed by immunoblotting. SNAP-25 was detected using the C-terminal peptide antibody, followed by incubation with 125I-labeled secondary antibody.
Membranes and Cytosol from Radiolabeled PC12 Cells.
PC12 cells were incubated with [3H]palmitate or [35S]methionine for 90 min and fractionated as described in Subcellular Fractionation. Membranes and cytosol separately or combined were incubated with an equal volume of 1 M Tris or 1 M hydroxylamine for 30 min at room temperature. Membranes were reisolated by a high-speed centrifugation (100,000 × g for 15 min). Soluble proteins were recovered in the supernatant, which was subjected to methanol precipitation for 30 min at −20°C. Membranes (particulate fraction) and soluble fractions were finally solubilized in modified RIPA buffer. SNAP-25 was immunoprecipitated from each of the samples and analyzed by SDS-PAGE followed by fluorography using preflashed film. [3H]palmitate incorporation was quantitated by densitometry using NIH Image 1.6 software.
Immunofluorescence
PC12 cells were plated on poly-l-lysine–coated chamber slides and allowed to differentiate for 2 d in the presence of 100 ng/ml NGF. Following various treatments (see Figure 6 legend), cells were washed once in DMEM-HG and once in warm PBS, fixed, and permeabilized in methanol at −20°C for 5 min. After washing twice with PBS, nonspecific staining was blocked by incubating the cells for 30 min in PBS containing 1% bovine serum albumin. Cells were incubated with affinity-purified SNAP-25 antibody for 1 h. After washing three times in PBS, the cells were incubated for 1 h with rhodamine-conjugated goat anti-rabbit antibody, washed four times with PBS, and mounted. Immunofluorescence microscopy was performed using a Zeiss Axioplan microscope coupled to an MRC-1000 Laser Scanning Confocal Microscope (Bio-Rad Laboratories, Hercules, CA). The images represent single planes obtained from the middle part of the cell with a 63× objective lens. Confocal images were assembled as montages using Adobe Photoshop v. 3.0 and printed using a Kodak ColorEase Printer.
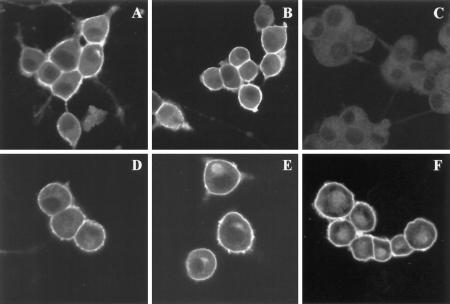
Figure 6.
Immunofluorescence of PC12 cells showing the subcellular distribution of SNAP-25 after treatment with BFA. PC12 cells cultured in four-chamber slides were differentiated with NGF for 2 d and subjected to the following treatments: A, DMSO for 1 h; B, BFA for 1 h; C, DMSO for 1 h; D, cycloheximide for 4 h; E, BFA for 4 h; and F, cycloheximide and BFA for 4 h. The cells were then processed for immunofluorescence and examined by confocal microscopy. SNAP-25 immunostaining was specific, as demonstrated by the lack of labeling in the presence of the SNAP-25 peptide (C) during the incubation with the primary antibody. The staining was unaffected by the presence of an unrelated peptide (A). SNAP-25 was localized to the plasma membrane in cells incubated in the presence (B) or absence (A) of BFA. A 4-h treatment of the cells with BFA (E) resulted in an accumulation of intracellular SNAP-25 when compared with control cells (D). The intracellular accumulation was also apparent when both protein synthesis and transport were inhibited (F).
RESULTS
Palmitoylation of SNAP-25 Begins within 20 Min of Its Biosynthesis
Early in our characterization of SNAP-25 biosynthesis and processing, we observed that palmitoylation of SNAP-25 resulted in an electrophoretic mobility shift of the protein. SNAP-25 immunoprecipitated from cells incubated with [35S]methionine for 1 h migrated on SDS-PAGE as two different forms, suggesting posttranslational modification of the protein (Figure 1, lanes 1 and 3). Confirming previous reports (Hess et al., 1992; Patterson and Skene, 1994), SNAP-25 was labeled with [3H]palmitate in PC12 cells in a hydroxylamine-sensitive manner, consistent with a thioester linkage (Figure 1, lanes 2 and 4). The [3H]palmitate-labeled form comigrated with the upper band labeled with [35S]methionine. To determine whether palmitoylation was responsible for the mobility shift of the protein, [35S]methionine-labeled SNAP-25 was treated with hydroxylamine before SDS-PAGE. This treatment removed palmitate and produced a single, nonacylated form of the protein (Figure 1, lane 6). Although we cannot exclude the possibility of a second modification of the protein that is also sensitive to treatment with neutral hydroxylamine, it seems likely that palmitoylation causes the slower electrophoretic mobility of the protein.
Figure 1.
Palmitoylation of SNAP-25 causes a mobility shift of the protein on SDS-PAGE. PC12 cells were labeled with [35S]methionine or [3H]palmitate for 1 h. SNAP-25 was immunoprecipitated with an affinity-purified polyclonal antibody directed against a C-terminal peptide of SNAP-25. The incorporation of radiolabel was analyzed by SDS-PAGE and fluorography. Incorporation of [3H]palmitate into SNAP-25 was sensitive to treatment of the gels with 1 M hydroxylamine (pH 7.5) after SDS-PAGE, but insensitive to treatment with 1 M Tris (middle and left panels). [35S]methionine incorporation into SNAP-25 was unaffected by soaking the gel in hydroxylamine or Tris (lanes 1 and 3). Treatment of [35S]methionine-labeled SNAP-25 immunoprecipitates with 1 M hydroxylamine before SDS-PAGE removed palmitate, and the protein migrated as a single band (right panel).
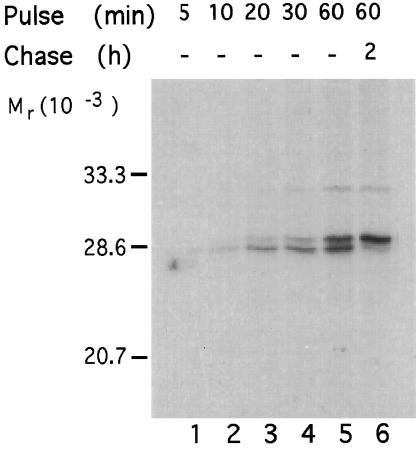
The ability to differentiate fatty acylated and nonacylated forms of SNAP-25 enabled us to establish the time course of palmitoylation of the newly synthesized protein. PC12 cells were metabolically labeled for different periods of time and then lysed immediately or collected after 2 h of chase. The palmitoylation state of SNAP-25 protein was assessed by its mobility on SDS-PAGE. As shown in Figure 2 (lanes 1–4), the protein synthesized during the first 10 min of labeling migrated on SDS-PAGE as the lower, nonpalmitoylated form. Only after 20 min of labeling could the upper, palmitoylated form of SNAP-25 be seen. After 1 h of labeling, 50% of the newly synthesized protein was palmitoylated (Figure 2, lane 5). When the protein labeled during this hour was chased, a complete shift to the palmitoylated form was observed (Figure 2, lane 6). These results demonstrate that newly synthesized nonpalmitoylated SNAP-25 matures into the palmitoylated form within 20 min after its synthesis. At steady state, only a small fraction (<10%) of SNAP-25 is found in the nonpalmitoylated form and this population can be accounted for by the newly synthesized protein that has not yet been processed (our unpublished observations). Thus, there does not appear to be a substantial pool of depalmitoylated SNAP-25 in cells.
Figure 2.
Maturation of SNAP-25 into the palmitoylated form occurs within 20 min of its synthesis. PC12 cells were metabolically labeled with [35S]methionine for various times. Cell lysates were either collected immediately (lanes 1–5) or collected following a chase period of 2 h (lane 6). SNAP-25 was immunoprecipitated and the state of palmitoylation was assessed by the mobility of the protein on SDS-PAGE.
Membrane Association of SNAP-25 Is Coincident with Palmitoylation
To test whether palmitoylated SNAP-25 associates with membranes, we analyzed the subcellular distribution of both the palmitoylated and nonpalmitoylated forms of the protein. Only the palmitoylated form of SNAP-25 was found in the particulate fraction (Figure 3, lanes 2 and 5) whereas the nonpalmitoylated form was in the soluble fraction (lanes 3 and 6). Furthermore, when the subcellular distribution of the protein during maturation was followed, a gradual shift in the localization of the protein from soluble to membrane fractions was observed, and this correlated with the time course of palmitoylation. Our results demonstrate that SNAP-25 is synthesized as a soluble protein that becomes associated with membranes coincident with the posttranslational addition of palmitate.
Figure 3.
Only the palmitoylated form of SNAP-25 associates with membranes. PC12 cells were metabolically labeled with [35S]methionine or [3H]palmitate for 1 h, followed by homogenization and separation into particulate and soluble fractions by high-speed centrifugation. SNAP-25 was immunoprecipitated from the total cell lysate (TCL), particulate fractions (P100), and soluble fractions (S100). The presence of radiolabeled SNAP-25 in each of the fractions was evaluated by SDS-PAGE and fluorography.
Disruption of the Secretory Pathway by BFA Inhibits Palmitoylation and Membrane Association of SNAP-25
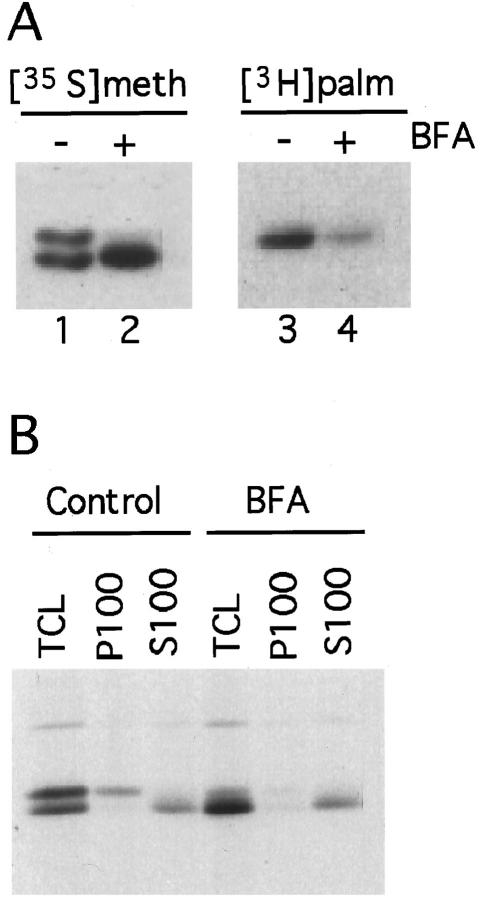
Maturation of newly synthesized SNAP-25 into the palmitoylated form requires the association of the soluble protein with a palmitoyltransferase. Because palmitoylation of proteins can occur during transit through the secretory pathway or at the plasma membrane (Schlesinger et al., 1993), we tested whether disruption of the secretory pathway affected palmitoylation of SNAP-25. PC12 cells were labeled with [35S]methionine or [3H]palmitate in the presence or absence of BFA, a fungal metabolite that causes disassembly of the Golgi and inhibits transport along the secretory pathway (Klausner et al., 1992). BFA significantly inhibited the incorporation of [3H]palmitate into SNAP-25 (Figure 4A, lane 4). As a consequence, the [35S]methionine-labeled protein migrated as a single, nonpalmitoylated form (Figure 4, lane 2). Our results demonstrate that BFA inhibits palmitoylation of newly synthesized SNAP-25, without affecting protein synthesis as assessed by incorporation of [35S]methionine into the protein (Figure 4, lane 2). The minimum concentration of BFA required to see inhibition of palmitoylation was 0.5 μg/ml, a concentration sufficient to collapse the Golgi into the ER (Lippincott-Schwartz et al., 1991). The inhibitory effect of BFA on palmitoylation was time dependent, with a maximal effect after 1 h of incubation in the presence of 10 μg/ml BFA. Inhibition of fatty acylation was reversible; palmitoylation of newly synthesized SNAP-25 was fully restored 2 h after removal of BFA. The effect of BFA on SNAP-25 palmitoylation allowed us to investigate whether fatty acylation was associated with binding of the protein to membranes. Inhibition of palmitoylation by BFA completely abolished the association of newly synthesized SNAP-25 with membranes (Figure 4B), confirming the correlation between palmitoylation and membrane targeting.
Figure 4.
Treatment of PC12 cells with BFA results in inhibition of palmitoylation and membrane association of newly synthesized SNAP-25. PC12 cells were labeled with [35S]methionine or [3H]palmitate for 1 h in the presence or absence of 10 μg/ml BFA. (A) Radiolabeled SNAP-25 was immunoprecipitated and analyzed by SDS-PAGE and fluorography. (B) [35S]methionine-labeled cells were homogenized and fractionated into particulate (P100) and soluble (S100) fractions. The presence of SNAP-25 in each of the fractions as well as in the total cell lysate (TCL) was detected by immunoprecipitation followed by SDS-PAGE and fluorography.
Palmitoylation of Newly Synthesized SNAP-25 Requires an Intact Transport Mechanism along the Secretory Pathway
To determine whether the effect of BFA on palmitoylation of SNAP-25 is a direct consequence of inhibition of transport along the exocytic pathway, other transport inhibitors were tested for their ability to affect the posttranslational processing of the protein. The inhibitors were added to PC12 cells during or before labeling with [35S]methionine or [3H]palmitate (Figure 5A). Monensin is a Na/K/proton ionophore that disrupts ion gradients across biological membranes. A major effect of monensin on cells is to induce a dose-dependent arrest of intracellular transport at the level of the Golgi (Tartakoff, 1983). Treatment with monensin partially inhibited palmitoylation of SNAP-25 (Figure 5A). As in the case of BFA-treated cells, association of SNAP-25 with membranes was inhibited in the presence of monensin, although not completely. Thus, a second agent that disrupts transport through the secretory pathway inhibits palmitoylation of SNAP-25. Nocodazole is a microtubule depolymerizing agent that inhibits retrograde transport of vesicles from the Golgi to the ER (Ho et al., 1989). Pretreatment of cells with nocodazole prevents BFA-induced mixing of ER and Golgi and maintains secretory proteins in the ER compartment (Klausner et al., 1992). SNAP-25 palmitoylation was unaffected by nocodazole alone, but palmitoylation was inhibited by treatment with both BFA and nocodazole (Figure 5A). These data suggest that the block of palmitoylation is associated with disruption of anterograde rather than retrograde transport. Ammonium chloride is a lysosomotropic agent that disrupts the acidic pH of intracellular organelles. Its primary effect is associated with perturbation of transport through the lysosomal, endosomal, and trans-Golgi compartments. Ammonium chloride had no effect on SNAP-25 fatty acylation, suggesting that palmitoylation does not depend on recycling through the endocytic compartment (Figure 5A). We also evaluated whether temperature blocks of 15 and 20°C, which prevent transport from ER to cis-Golgi and from trans-Golgi to plasma membrane (Saraste and Kuismanen, 1992), respectively, affected SNAP-25 palmitoylation. Both partially inhibited the maturation of SNAP-25 into the palmitoylated form (Figure 5B). Because there does not appear to be a selective block in SNAP-25 processing, the inhibitory effects of the temperature blocks may be a result of slowing cellular metabolism. Nonetheless, palmitoylation of SNAP-25 is inhibited by different treatments that perturb the integrity of the Golgi apparatus. Thus, these data support the hypothesis that palmitoylation of SNAP-25 relies on an intact secretory apparatus.
Figure 5.
Effect of transport inhibitors on palmitoylation of SNAP-25. (A) PC12 cells were labeled with [3H]palmitate or [35S]methionine for 1 h in normal media (control) or in media containing 10 μg/ml BFA, 10 μM monensin, 20 μg/ml nocodazole, nocodazole and BFA together, or 50 mM NH4Cl. Radiolabeled SNAP-25 was immunoprecipitated from cell lysates. The effect of these drugs on palmitoylation of SNAP-25 was assessed by incorporation of [3H]palmitate and by analysis of the mobility of the [35S]methionine-labeled protein on SDS-PAGE. (B) PC12 cells were pulse labeled for 20 min with [35S]methionine at 37°C and then chased without label for 0.5, 1, or 2 h at 37°C, 20°C, or 15°C. Radiolabeled SNAP-25 was immunoprecipitated and analyzed by SDS-PAGE and fluorography. Note the inhibition of maturation of SNAP-25 at 15 and 20°C.
BFA Does Not Affect Localization of the Mature Protein
The effects of BFA on palmitoylation of SNAP-25 appear to affect newly synthesized protein, but not the mature form. The subcellular localization of SNAP-25 after a 1-h treatment of PC12 cells with BFA was evaluated by immunofluorescence. As shown in Figure 6, SNAP-25 is localized to the plasma membrane in cells incubated in the presence (B) or absence (A) of BFA. SNAP-25 immunostaining was specific, as demonstrated by the lack of labeling in the presence of competing peptide (C), but not in the presence of an irrelevant peptide (A). In support of the immunofluorescence data, immunoblot analysis of membrane and soluble fractions of PC12 cells revealed that most SNAP-25 was found in the membrane fraction. A 1-h BFA treatment had little effect on the distribution of SNAP-25 between soluble and membrane fractions.
Interestingly, some changes in the distribution of SNAP-25 were observed when cells were treated with BFA for 4 h. Under these conditions, an intense labeling of an intracellular pool of SNAP-25 was seen (Figure 6E). The intracellular staining appeared as a discrete focus adjacent to the nucleus, thus it is likely to represent SNAP-25 association with membranes. To determine whether the intracellular labeling represents accumulation of newly synthesized protein, protein synthesis was inhibited during the 4-h treatment with BFA (Figure 6F). Intracellular accumulation of SNAP-25 was not blocked by cycloheximide (compare Figure 6E and F), indicating that it represents mature, fully processed protein. During the 4-h treatment with BFA, the newly synthesized SNAP-25 remains cytosolic and is detected as a small increase in the amount of soluble SNAP-25 seen by Western blot (our unpublished observations). This modest accumulation of soluble SNAP-25 is not apparent by immunofluorescence (Figure 6E). Thus, longer treatments of PC12 cells with BFA reveal that the drug affects two populations of SNAP-25 protein. The newly synthesized protein is not palmitoylated and remains soluble, while a population of mature SNAP-25 accumulates on intracellular membranes.
BFA Inhibits Palmitoylation of GAP-43 but not G Protein α Subunits
To evaluate whether the effect of BFA on palmitoylation is specific for SNAP-25 or whether the drug inhibits palmitoylation of many cellular proteins, we assayed the incorporation of [3H]palmitate into cellular proteins in PC12 cells. The major substrate for palmitoylation was a polypeptide of 25 kDa (Figure 7A, lane 1) that comigrated with immunoprecipitated SNAP-25. When BFA was added during labeling, there was a great reduction in the incorporation of [3H]palmitate into SNAP-25, but most palmitoylated proteins were unaffected (Figure 7, lane 2). Similar results were observed with monensin (Figure 7, lane 3). Thus, the effect of BFA is not a general inhibition of protein acylation.
Figure 7.
BFA and monensin do not inhibit palmitoylation of all cellular proteins. (A) PC12 cells were labeled with [3H]palmitate for 1 h in the absence (lane 1) or presence of 10 μg/ml BFA (lane 2) or 10 μM monensin (lane 3). Cell lysates were resolved by SDS-PAGE, and the incorporation of [3H]palmitate into proteins was detected by fluorography. (B) N2A cells were labeled with [35S]methionine or [3H]palmitate for 1 h in the absence or presence of BFA. Goα and Giα subunits labeled with [35S]methionine (lanes 1 and 2) or [3H]palmitate (lanes 5 and 6) were immunoprecipitated. Note how palmitoylation of G protein α subunits is not affected by treatment with BFA. SNAP-25 labeled with [35S]methionine (lanes 3 and 4) or [3H]palmitate (lanes 7 and 8) was immunoprecipitated. Inhibition of SNAP-25 palmitoylation by BFA is apparent from reduced labeling with [3H]palmitate (lane 8). (C) NG108 cells were labeled with [3H]palmitate for 1 h in the presence or absence of BFA, and SNAP-25 (lanes 3 and 4) and GAP-43 (lanes 1 and 2) were immunoprecipitated from total cell lysates. Equal amounts of protein in each lane was demonstrated by immunoblotting an aliquot of the immunoprecipitated samples (bottom panel). Palmitoylation of both GAP-43 and SNAP-25 were inhibited by BFA in NG108 cells.
We also tested the effects of BFA on other resident plasma membrane proteins. Goα and Giα share the dual acylation motif of myristoylation and palmitoylation found on p59fyn and other Src family kinases. Palmitoylation of Goα and Giα in a neuroblastoma cell line, N2A, was insensitive to BFA (Figure 7B, lanes 5 and 6). Similar results were observed for Goα and Giα in PC12 cells and NG108 cells. These results are consistent with reports that palmitoylation of p59fyn and endothelial nitric oxide synthase, another dually acylated protein, are not affected by treatment with BFA (van’t Hof and Resh, 1997; Michel, personal communication). Palmitoylation of SNAP-25 was inhibited by BFA in N2A cells (Figure 7B, lanes 7 and 8) and NG108 cells (Figure 7C, lanes 3 and 4), indicating that a BFA-sensitive pathway exists in these cells as well as in PC12 cells. Interestingly, palmitoylation of GAP-43 was also inhibited by BFA (Figure 7C, lane 2). Thus, both a BFA-sensitive and -insensitive pathway for palmitoylation of proteins associated with the inner leaflet of the plasma membrane exists in neuronal cell lines.
Depalmitoylation of SNAP-25 Does Not Release the Protein from Membranes
To characterize further the role of palmitoylation in the membrane binding properties of SNAP-25, we tested the ability of mature SNAP-25 to remain bound to membranes after removal of palmitate. PC12 cells were radiolabeled with [3H]palmitate or [35S]methionine and fractionated into membranes and cytosol. The cytosolic fraction provides a source of newly synthesized, nonpalmitoylated SNAP-25, whereas the membranes provide a source of palmitoylated SNAP-25. Cytosolic and membrane fractions, separately or combined, were subjected to treatment with neutral hydroxylamine. As a control, duplicate samples were treated with Tris at the same pH and concentration. Samples were pelleted to separate soluble proteins from membranes and SNAP-25 was immunoprecipitated from each fraction. Treatment of PC12 membranes with hydroxylamine resulted in approximately 90% removal of [3H]palmitate label from SNAP-25 (Figure 8A, lanes 1 and 3). Analysis of the membrane samples labeled with [35S]methionine confirmed the loss of palmitate by a faster mobility of the protein on SDS-PAGE (Figure 8A, compare lanes 1 and 3). Nevertheless, depalmitoylated SNAP-25 remained associated with the particulate fraction, as did the palmitoylated form that had been treated with Tris. Little or no SNAP-25 was detected in the soluble fraction of [35S]methionine-labeled membranes (Figure 8A, lanes 2 and 4). Cytosolic SNAP-25 subjected to treatment with Tris or hydroxylamine was recovered in the soluble fraction (Figure 8A, lanes 6 and 8). To rule out the possibility that the presence of depalmitoylated SNAP-25 in the particulate fraction was due to aggregation or precipitation of the protein, we tested the ability of cytosolic SNAP-25 to fractionate with the soluble fraction after incubation with membranes. Cytosolic SNAP-25 was recovered in the soluble fraction after treatment with hydroxylamine or Tris (Figure 8A, lanes 10 and 12), whereas depalmitoylated SNAP-25 stayed associated with the particulate fraction (Figure 8A, compare lanes 9 and 11). As a second means of assessing whether depalmitoylated SNAP-25 remains productively associated with membranes, bovine brain membranes were treated with hydroxylamine or Tris and then reisolated by flotation in a sucrose density gradient. An interphase containing membranes was collected as well as the phase containing soluble proteins that remained at the bottom of the gradient. Both fractions were analyzed by immunoblotting (Figure 8B). Again, depalmitoylation of SNAP-25 was evident by the electrophoretic mobility shift of the protein, but SNAP-25 remained associated with membranes (Figure 8B, lane 2). We conclude that palmitoylation is not required to maintain stable association of SNAP-25 with the plasma membrane.
Figure 8.
Depalmitoylation of SNAP-25 does not release the protein from membranes. (A) PC12 cells were labeled with [3H]palmitate or [35S]methionine for 90 min and fractionated into membranes and cytosol. Membranes (Mb) or Cytosol (Cy) or the combined fractions (Mb + Cy) were incubated in 0.5 M hydroxylamine (HA) to remove palmitate or 0.5 M Tris as a control. Each sample was then subjected to a high-speed centrifugation to separate a particulate (P) from a soluble fraction (S). SNAP-25 was immunoprecipitated from each of the samples and analyzed by SDS-PAGE and fluorography. Depalmitoylation of the protein was assessed by loss of [3H]palmitate labeling (upper panel) and by faster mobility of [35S]methionine-labeled protein in SDS-PAGE (lower panel). (B) Brain membranes were treated with hydroxylamine or Tris. A sucrose step gradient was used to separate membranes from soluble proteins. SNAP-25 in membranes (lanes 1 and 2) or soluble fractions (lanes 3 and 4) was detected by immunoblotting. Note the presence of depalmitoylated SNAP-25 in the membrane fraction (lane 2). (C) SNAP-25 was immunoprecipitated from brain membranes treated with hydroxylamine or Tris. Co-immunoprecipitating syntaxin was detected by blotting the SNAP-25 immunoprecipitates with antibodies against syntaxin.
Once the newly synthesized protein is targeted to membranes, other interactions may be sufficient to maintain stable association in the absence of palmitate. One possible mechanism for maintaining SNAP-25 membrane association after depalmitoylation of the protein is through its interactions with syntaxin. We evaluated whether association of SNAP-25 with syntaxin was perturbed by depalmitoylation by coprecipitating both proteins from membranes treated with hydroxylamine or Tris. Coprecipitation of syntaxin was unaffected by the palmitoylation status of SNAP-25 (Figure 8C).
DISCUSSION
In this study, we examined the biosynthesis and membrane targeting of SNAP-25 and how fatty acylation of the protein impinges upon these processes. The primary structure of SNAP-25 predicts a hydrophilic molecule lacking any features that would promote insertion into membranes (Oyler et al., 1989). The discovery that SNAP-25 was palmitoylated provided the simplest explanation for its membrane localization (Hess et al., 1992). However, the hypothesis that palmitoylation mediates membrane association of SNAP-25 has only been tested recently. Veit et al. (1996) provided the first biochemical evidence that palmitoylation of SNAP-25 is required for its membrane association by expressing a nonpalmitoylated mutant in a heterologous cell type. Our finding that translocation of newly synthesized SNAP-25 from cytosol to membranes is temporally correlated with palmitoylation supports this conclusion.
We propose that palmitoylation of SNAP-25 is a mechanism for membrane targeting rather than simply serving as a membrane anchor. Elimination of the palmitoylation sites in the molecule clearly interferes with the ability of SNAP-25 to bind stably to membranes (Veit et al., 1996). However, we have shown in vitro that palmitoylated SNAP-25 stably associated with membranes is not released upon deacylation. A similar result has been reported for Drosophila cysteine string proteins that are extensively fatty acylated (van de Goor and Kelly, 1996). Membrane association of SNAP-25 without palmitate must be maintained by another mechanism, possibly through protein–protein interactions. The demonstration that coimmunoprecipitation of SNAP-25 with syntaxin is not affected by depalmitoylation suggests at least one mechanism by which deacylated SNAP-25 maintains membrane association. We envision palmitoylation as a signal to localize otherwise hydrophilic proteins such as SNAP-25 to a specific membrane compartment. After the initial targeting step, other interactions may come into play to maintain efficient membrane association.
In addition to its role in targeting newly synthesized SNAP-25 to membranes, palmitoylation may also provide a mechanism to modulate the function of the protein. Palmitoylation of many proteins is reversible, and thus has the potential to be regulated. Dynamic fatty acylation of SNAP-25 has been observed in neuronal growth cones (Hess et al., 1992) and synaptosomes (Lansbery and Linder, unpublished results). It has been proposed that reversible fatty acylation may serve as a mechanism to regulate the presence of SNAP-25 at the plasma membrane and therefore to regulate SNARE complex formation during the process of exocytosis (Bark and Wilson, 1994). Our results suggest that deacylation of SNAP-25 is not likely to release it from membranes. It is possible that palmitoylation of SNAP-25 is important for modulating protein–protein interactions within the nerve terminal. Cycles of acylation and deacylation could induce conformational changes in SNAP-25 that affect its affinity with interacting proteins. Alternatively, dynamic fatty acylation of SNAP-25 may result in local remodeling of the lipid bilayer that could have an impact on the process of synaptic vesicle exocytosis.
The requirement for transport through an intact secretory pathway for SNAP-25 palmitoylation and membrane association is surprising. To our knowledge, this is the first demonstration that a protein’s palmitoylation is inhibited by disruption of transport. Indeed, this effect is limited to a small number of palmitoylated proteins (Figure 7). There are at least three potential pathways that could account for our finding that palmitoylation of SNAP-25 requires an intact secretory apparatus. First, SNAP-25 is palmitoylated at some site along the secretory pathway and delivered to the plasma membrane by vesicular transport. In this model, BFA and other inhibitors of transport interfere with palmitoylation of SNAP-25 by disrupting the integrity of a compartment necessary for a productive interaction of SNAP-25 with the palmitoylating machinery. Second, SNAP-25 is palmitoylated at the plasma membrane. Disruption of the secretory pathway prevents a cofactor required for SNAP-25 palmitoylation, such as the palmitoyltransferase, from arriving at the plasma membrane. Third, nonpalmitoylated SNAP-25 binds to an intracellular membrane and is transported to the plasma membrane where it is palmitoylated. Inhibition of transport prevents the nonacylated protein from reaching a palmitoyltransferase at the plasma membrane.
We favor the first hypothesis that SNAP-25 is palmitoylated during transit through the secretory pathway. However, our attempts to directly demonstrate that newly synthesized SNAP-25 transits through the secretory pathway have been frustrated by the inability to adequately separate intracellular membranes from plasma membrane in PC12 cells. Synaptobrevin is posttranslationally inserted into the ER membrane and transported to the Golgi where it is sorted to synaptic vesicles (Kutay et al., 1995). It would not be surprising if other SNARE proteins, including SNAP-25, also trafficked through the secretory pathway.
The second model predicts that the effect of BFA on SNAP-25 palmitoylation is indirect. In this model, BFA interferes with transport of the SNAP-25 palmitoyltransferase or some other cofactor required for palmitoylation of SNAP-25 at the plasma membrane. This model appears unlikely because the cofactor would have to be turning over very rapidly to account for the effects of BFA within 30 min.
The third model proposes that SNAP-25 binds to an intracellular membrane and travels through the secretory pathway to the plasma membrane where it is palmitoylated. If this model is correct, BFA would inhibit palmitoylation of SNAP-25 but not its binding to membranes. We have not detected nonpalmitoylated SNAP-25 associated with membranes by biochemical means. However, it is possible that association of nonpalmitoylated SNAP-25 with membranes is disrupted during subcellular fractionation. Indeed, a population of SNAP-25 on intracellular membranes is detected by immunofluorescence of PC12 cells exposed to BFA for 4 h (Figure 6). However, this pool represents mature, palmitoylated SNAP-25 since it was present also in the absence of protein synthesis. We suggest that this intracellular accumulation of protein is the result of fusion of the endosomal compartment caused by exposure to BFA. Tubulation of endosomes is a well documented effect of BFA (Pelham, 1991).
Because palmitoyltransferase activities are associated with membranes (Schmidt and Burns, 1989; Berthiaume and Resh, 1995; Dunphy et al., 1996), newly synthesized, soluble SNAP-25 most likely associates with the cytoplasmic face of a membrane compartment to be recognized and modified by the palmitoyltransferase. For proteins that undergo sequential modification by myristate or prenyl groups and palmitate, the initial lipid modification provides sufficient hydrophobicity to allow a transient association with membranes, thus allowing interaction with a palmitoyltransferase (Cadwallader et al., 1994; Shahinian and Silvius, 1995; van’t Hof and Resh, 1997). SNAP-25, however, must interact with membranes by some other mechanism, possibly through binding to a chaperone protein or directly to a palmitoyltransferase. Alternatively, syntaxin or synaptobrevin could serve as a membrane protein “receptor” to facilitate the subsequent interaction of SNAP-25 with a palmitoyltransferase. It is possible that newly synthesized SNAREs are cotransported through the secretory pathway for delivery to the plasma membrane.
We and others are beginning to define the trafficking pathways that target newly synthesized lipid-modified proteins to plasma membrane. SNAP-25 becomes palmitoylated and associated with membranes within 20 min of synthesis in a BFA-sensitive pathway. The kinetics of membrane association and sensitivity of this process to BFA contrast with the results reported for p59fyn. On the basis of their recent studies, van’t Hof and Resh (1997) suggest a model where p59fyn is synthesized and myristoylated on soluble ribosomes, then specifically shuttled to the plasma membrane where it encounters a palmitoyltransferase. The dually modified protein is now stably associated with the plasma membrane due to the strong hydrophobicity conferred by the myristoyl and palmitoyl groups. The kinetics of plasma membrane targeting are very fast (within 2 min) and membrane association of p59fyn is insensitive to BFA. The fast kinetics are associated with the myristoylation and palmitoylation motif. A chimeric construct with the first 10 amino acids of Goα replacing those of p59fyn that maintains myristoylation and palmitoylation is targeted to membranes with the same kinetics as wild-type p59fyn. In contrast, a dipalmitoylated chimeric construct (first 10 amino acids of GAP-43) becomes membrane associated within 20 min (van’t Hof and Resh, 1997). The slow kinetics of membrane association of the GAP-43 chimera matches the time course we observe for SNAP-25. Furthermore, we have shown that palmitoylation of both SNAP-25 and GAP-43 is sensitive to BFA. Therefore, palmitoylation and membrane association of GAP-43 and SNAP-25 occur by a pathway distinct from that used by proteins that are dually modified with myristate and palmitate.
A feature common to GAP-43 (Liu et al., 1993) and SNAP-25 (Veit et al., 1996) is fatty acylation at multiple cysteine residues. Biophysical characterization of acylated peptides indicates that the hydrophobicity conferred by a single palmitate residue is sufficient to support stable membrane association (Peitzsch and McLaughlin, 1993). Thus, the presence of multiple palmitate groups on these proteins may serve an additional purpose, perhaps as a sorting signal for packaging into specific transport vesicles. Palmitoylation of SNAP-25 and GAP-43 in neurons precedes fast axonal transport to nerve terminals (Hess et al., 1992) or neuronal growth cones (Skene, 1989). Proteins that undergo fast axonal transport are packaged into membrane vesicles for transport at the trans-Golgi network (Smith and Snyder, 1992). Both GAP-43 and SNAP-25 have been localized at the trans-Golgi network in neuronal cells (Van Hooff et al., 1989; Goslin et al., 1990; Duc and Catsicas, 1995). Multiple palmitoyl groups on SNAP-25 and GAP-43 may facilitate sorting of these proteins at the trans-Golgi network into vesicles for fast axonal transport.
ACKNOWLEDGMENTS
We thank Blaine Olson and Kim Riehle for contributions to this work, Dr. Eugene Johnson for the gift of NGF, and Dr. Thomas Michel for sharing unpublished data. We appreciate suggestions and helpful comments from Drs. Peggy Weidman, David Harris, and Nicholas Ktistakis, and members of our laboratory. This work was supported by United States Public Health Service grant GM50556 and the Alzheimer’s Disease Research Center at Washington University. S.G. was supported by Training Grant 5T32GM07805 to Washington University School of Medicine.
Footnotes
Abbreviations used: BFA, brefeldin A; GAP-43, growth-associated protein 43 kDa; NSF, N-ethylmaleimide-sensitive factor, SNAP-25, synaptosomal-associated protein of 25 kDa; SNAP, soluble NSF attachment protein; SNARE, SNAP receptor.
REFERENCES
- Bark I, Wilson M. Regulated vesicular fusion in neurons: Snapping together the details. Proc Natl Acad Sci USA. 1994;91:4621–4624. doi: 10.1073/pnas.91.11.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume L, Resh M. Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J Biol Chem. 1995;270:22399–22405. doi: 10.1074/jbc.270.38.22399. [DOI] [PubMed] [Google Scholar]
- Cadwallader KA, Paterson H, MacDonald SG, Hancock JF. N-terminally myristoylated Ras proteins require palmitoylation or a polybasic domain for plasma membrane localization. Mol Cell Biol. 1994;14:4722–4730. doi: 10.1128/mcb.14.7.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey PJ. Protein lipidation in cell signaling. Science. 1995;268:221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- Duc C, Catsicas S. Ultrastructural localization of SNAP-25 within the rat spinal cord and peripheral nervous system. J Comp Neurol. 1995;356:152–163. doi: 10.1002/cne.903560111. [DOI] [PubMed] [Google Scholar]
- Dunphy JD, Greentree WK, Manahan CL, Linder ME. G-protein palmitoyltransferase activity is enriched in plasma membranes. J Biol Chem. 1996;271:7154–7159. doi: 10.1074/jbc.271.12.7154. [DOI] [PubMed] [Google Scholar]
- Goslin K, Schreyer D, Skene J, Banker G. Changes in the distribution of GAP-43 during the development of neuronal polarity. J Neurosci. 1990;10:558–602. doi: 10.1523/JNEUROSCI.10-02-00588.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF, Paterson H, Marshall CJ. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- Hess DT, Slater TM, Wilson MC, Skene JHP. The 25 kDa synaptosomal-associated protein SNAP-25 is the major methionine-rich polypeptide in rapid axonal transport and a major substrate for palmitoylation in adult CNS. J Neurosci. 1992;12:4634–4641. doi: 10.1523/JNEUROSCI.12-12-04634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho W, Allan V, van Meer G, Berger E, Kreis T. Reclustering of scattered Golgi elements occurs along microtubules. Eur J Cell Biol. 1989;48:250–263. [PubMed] [Google Scholar]
- Klausner R, Donaldson J, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U, Ahnert-Hilger G, Hartmann E, Wiedenmann B, Rapoport T. Transport route for synaptobrevin via a novel pathway of insertion into the endoplasmic reticulum membrane. EMBO J. 1995;14:217–223. doi: 10.1002/j.1460-2075.1995.tb06994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U, Hartmann E, Rapoport T. A class of membrane proteins with a C-terminal anchor. Trends Cell Biol. 1993;3:72–75. doi: 10.1016/0962-8924(93)90066-a. [DOI] [PubMed] [Google Scholar]
- Linder ME, Kleuss C, Mumby SM. Palmitoylation of G-protein α subunits. Methods Enzymol. 1995;250:314–330. doi: 10.1016/0076-6879(95)50081-2. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Glickman J, Donaldson J, Robbins J, Kreis T, Seamon K, Sheetz M, Klausner R. Forskolin inhibits and reverses the effects of brefeldin A on Golgi morphology by a cAMP-independent mechanism. J Cell Biol. 1991;112:567–577. doi: 10.1083/jcb.112.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fisher DA, Storm DR. Analysis of the palmitoylation and membrane targeting domain of neuromodulin (GAP-43) by site-specific mutagenesis. Biochemistry. 1993;32:10714–10719. doi: 10.1021/bi00091a023. [DOI] [PubMed] [Google Scholar]
- Mumby SM, Gilman AG. Synthetic peptide antisera with determined specificity for G-protein α or β subunits. Methods Enzymol. 1991;195:215–223. doi: 10.1016/0076-6879(91)95168-j. [DOI] [PubMed] [Google Scholar]
- Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989;89:3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SI, Skene JHP. Novel inhibitory action of tunicamycin homologues suggests a role for dynamic protein fatty acylation in growth cone-mediated neurite extension. J Cell Biol. 1994;124:521–536. doi: 10.1083/jcb.124.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitzsch RM, McLaughlin S. Binding of acylated peptides and fatty acids to phospholipid vesicles: pertinence to myristoylated proteins. Biochemistry. 1993;32:10436–10443. doi: 10.1021/bi00090a020. [DOI] [PubMed] [Google Scholar]
- Pelham H. Multiple targets for brefeldin A. Cell. 1991;67:449–451. doi: 10.1016/0092-8674(91)90517-3. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Busconi L, Michel T. Agonist-modulated palmitoylation of endothelial nitric oxide synthase. J Biol Chem. 1995;270:995–998. doi: 10.1074/jbc.270.3.995. [DOI] [PubMed] [Google Scholar]
- Saraste J, Kuismanen E. Pathways of protein sorting and membrane traffic between the rough endoplasmic reticulum and the Golgi complex. Semin Cell Biol. 1992;3:343–355. doi: 10.1016/1043-4682(92)90020-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger MJ, Veit M, Schmidt MF, G. Palmitoylation of cellular and viral proteins. In: Schlesinger MJ, editor. Lipid Modifications of Proteins. Boca Raton: CRC Press; 1993. pp. 2–19. [Google Scholar]
- Schmidt MF G, Burns GR. Hydrophobic modifications of membrane proteins by palmitoylation in vitro. Biochem Soc Trans. 1989;17:625–626. doi: 10.1042/bst0170625. [DOI] [PubMed] [Google Scholar]
- Shahinian S, Silvius JR. Doubly-lipid-modified protein sequence motifs exhibit long-lived anchorage to lipid bilayer membranes. Biochemistry. 1995;34:3813–3822. doi: 10.1021/bi00011a039. [DOI] [PubMed] [Google Scholar]
- Skene JHP. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Smith R, Snyder R. Relationships between the rapid axonal transport of newly synthesized proteins and membranous organelles. Mol Neurobiol. 1992;6:285–300. doi: 10.1007/BF02780558. [DOI] [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993a;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993b;362:318–323. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Sternweis PC, Robishaw JD. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984;259:13806–13813. [PubMed] [Google Scholar]
- Südhof T. The synaptic vesicle cycle: a cascade of protein–protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983;32:1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Timson Gauen LK, Linder ME, Shaw AS. Multiple features of the p59fyn SH4 domain define a motif for ITAM binding and for plasma membrane localization. J Cell Biol. 1996;133:1007–1015. doi: 10.1083/jcb.133.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Goor J, Kelly R. Association of Drosophila cysteine string proteins with membranes. FEBS Lett. 1996;380:251–256. doi: 10.1016/0014-5793(96)00026-9. [DOI] [PubMed] [Google Scholar]
- Van Hooff C, Holthuis J, Oestreicher A, Boonstra J, DeGraan P, Gispen W. Nerve growth factor-induced changes in the intracellular localization of the protein kinase C substrate B-50 in pheochromocytoma PC12 cells. J Cell Biol. 1989;108:1115–1125. doi: 10.1083/jcb.108.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van’t Hof W, Resh MD. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J Cell Biol. 1997;136:1023–1035. doi: 10.1083/jcb.136.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M, Söllner TH, Rothman JE. Multiple palmitoylation of synaptotagmin and the t-SNARE SNAP-25. FEBS Lett. 1996;385:119–123. doi: 10.1016/0014-5793(96)00362-6. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Wilson PT, Bourne HR. Lipid modifications of trimeric G proteins. J Biol Chem. 1995;270:503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]
- Zuber MX, Strittmatter SM, Fishman MC. A membrane-targeting signal in the amino terminus of the neuronal protein GAP-43. Nature. 1989;341:345–348. doi: 10.1038/341345a0. [DOI] [PubMed] [Google Scholar]