Abstract
Myelin sheets originate from distinct areas at the oligodendrocyte (OLG) plasma membrane and, as opposed to the latter, myelin membranes are relatively enriched in glycosphingolipids and cholesterol. The OLG plasma membrane can therefore be considered to consist of different membrane domains, as in polarized cells; the myelin sheet is reminiscent of an apical membrane domain and the OLG plasma membrane resembles the basolateral membrane. To reveal the potentially polarized membrane nature of OLG, the trafficking and sorting of two typical markers for apical and basolateral membranes, the viral proteins influenza virus–hemagglutinin (HA) and vesicular stomatitis virus–G protein (VSVG), respectively, were examined. We demonstrate that in OLG, HA and VSVG are differently sorted, which presumably occurs upon their trafficking through the Golgi. HA can be recovered in a Triton X-100-insoluble fraction, indicating an apical raft type of trafficking, whereas VSVG was only present in a Triton X-100-soluble fraction, consistent with its basolateral sorting. Hence, both an apical and a basolateral sorting mechanism appear to operate in OLG. Surprisingly, however, VSVG was found within the myelin sheets surrounding the cells, whereas HA was excluded from this domain. Therefore, despite its raft-like transport, HA does not reach a membrane that shows features typical of an apical membrane. This finding indicates either the uniqueness of the myelin membrane or the requirement of additional regulatory factors, absent in OLG, for apical delivery. These remarkable results emphasize that polarity and regulation of membrane transport in cultured OLG display features that are quite different from those in polarized cells.
INTRODUCTION
During myelin formation, oligodendrocytes (OLGs), the myelinating cells of the CNS, express large quantities of myelin proteins and lipids that are subsequently transferred from the cell body to the myelin sheath, which is wrapped around the axons (for review, see Kalwy and Smith, 1994). Although primary neonatal rat OLGs in monoculture do not have axons to ensheath, they do differentiate and express all myelin components in a coordinated manner (Baron et al., 1997), and, moreover, they form large myelin-containing networks called myelin sheets. Given their enrichment in glycosphingolipid, these domains are reminiscent of the glycosphingolipid-enriched apical membranes in polarized cells. In epithelial cells the delivery of newly synthesized proteins to the apical surface requires specific sorting mechanisms. Sorting takes place in the trans-Golgi network and involves specific packaging of apical proteins in sphingolipid-enriched rafts (Simons and Wandinger-Ness, 1990). The cellular sorting and transport machineries toward basolateral and apical membranes have been extensively characterized in epithelial cells (Simons and Zerial, 1993; Rothman and Wieland, 1996; Weimbs et al., 1997). These two types of sorting have also been found in neurons, with apical transport occurring to the axons and basolateral transport to the dendrites (De Hoop and Dotti, 1993). Finally, recent evidence indicates that even in nonpolarized cells, both types of sorting occur (Müsch et al., 1996).
At present it is not clear whether parts of the OLG’s plasma membrane, as referred to above, can be considered as apical or basolateral in the classical sense. Nor have tight junctions, separating myelin from the remaining plasma membrane, been described in OLG. About a decade ago, detergent solubility of proteins present in CNS myelin was studied (Pereyra et al., 1988; Gillespie et al., 1989; Wilson and Brophy, 1989). It was found that myelin basic protein and, to a lesser extent, 2′,3′-cyclic nucleotide phosphodiesterase were insoluble in 0.5% and 1% Triton X-100 and were associated with microtubules. Conclusions on the transport of myelin proteins were not drawn then. More recently, the MAL/MVP17 proteolipid, a developmentally regulated protein of oligodendrocytes, kidney cells, and T cells, was found to be detergent insoluble at low temperature, both in OLGs (Kim et al., 1995) and in transfected COS-7 cells (Puertollano et al., 1997). Finally, Krämer et al. (1997) obtained evidence that glycosylphosphatidylinositol (GPI)-anchored proteins are present in Triton X-114-insoluble vesicles, thus indicating that an apical sorting system is operative in OLG. To further investigate whether specific, polarized membrane domains exist in OLGs, we infected primary rat OLGs with two different viruses, influenza virus and vesicular stomatitis virus (VSV), and followed the intracellular fate of their glycoproteins, hemagglutinin (HA) and G protein (VSVG), respectively. In polarized cells such as MDCK cells and neurons, it has been demonstrated that HA is sorted to the apical membrane and that it is transported in detergent-insoluble glycolipid rafts, whereas VSVG localizes to the basolateral membrane and its transport occurs independently of cotransport with glycolipids (Kobayashi et al., 1992).
In this report we demonstrate that in cultured OLGs the destinations of HA and VSVG are different and, consequently, that sorting occurs. Unexpectedly, however, the myelin sheet apparently does not necessarily represent the target membrane of typical raft-like transport. Thus, unlike the trafficking commonly seen in epithelial cells, detergent-soluble VSVG rather than detergent-insoluble HA is effectively transferred to the sphingolipid-containing sheet.
MATERIALS AND METHODS
Materials
Triton X-100 was purchased from Sigma Chemical (St. Louis, MO); Brefeldin A (BFA) and monensin were purchased from Calbiochem (La Jolla, CA).
Antibodies
Monoclonals.
Antibody TuJ1 (IgG2a; Geisert and Frankfurter, 1989) was a kind gift from Dr. A. Frankfurter (Charlottesville, VA); MG-160 (IgG2b; Gonatas et al., 1989) was a gift from Dr. Nicholas Gonatas (Philadelphia, PA); and TGN-38 (IgG1; Luzio et al., 1990) was a gift from Dr. George Banting (Bristol, United Kingdom). Anti-myelin basic protein (MBP; IgG1) and anti-influenza–HA (IgG2b) were purchased from Boehringer Mannheim (Indianapolis, IN), and anti-VSVG (IgG1) was purchased from Sigma Chemical.
Polyclonals.
The antibodies against HA and VSVG were generous gifts from Dr. Ineke Braakman (Amsterdam, the Netherlands) and Dr. Peter Rottier (Utrecht, the Netherlands), respectively.
Appropriate fluroescein isothiocyanate (FITC)-labeled and tetramethylrhodamine isothiocyanate-labeled secondary antibodies were obtained from Southern Biotechnology (Birmingham, AL).
Isolation and Culture of Neonatal Rat OLGs
OLGs were isolated from neonate rat spinal cord and cultured as described by de Vries et al. (1993). In brief, OLG-enriched glia cells were isolated from the spinal cords of Wistar rats (6- to 8-d old). After adhesion for 1 h to poly-l-lysine–coated Petri dishes in DMEM containing 5% fetal calf serum, the medium and nonadhering cells were removed and new medium was added. After 24 h the cells were shifted to a serum-free, chemically defined medium (Van der Pal, 1990) to induce differentiation of progenitor cells into OLGs. At d 2 after plating, 10−5 M cytosine-1-β-d-arabinoside (Ara-C) was added to inhibit overgrowth by astrocytes.
Isolation and Culture of Mixed Brain Cells
Mixed brain cells (MBC) were isolated from 15-d-old rat fetuses and cultured as described by Lubetzki et al. (1993) using as isolation medium NM-MBC [DMEM, pH 7.6, supplemented with 2 mM glutamine, 10 mM HEPES, 22 mM sodium bicarbonate, 25 mM glucose, 0.028% bovine serum albumin, 105 U/l penicillin, and 100 mg/l streptomycin), and as culture medium CDM-MBC (DMEM, pH 7.6, supplemented with 8.85 mg/l insulin, 100 mg/l transferrin, 6.2 μg/l progesterone, 16.1 mg/l putrescine, 0.3 mg/l T3, 0.4 mg/l T4, 38.7 μg/l sodium selenite, 105 U/l penicillin, 100 mg/l streptomycin, and 1% fetal calf serum). Cells were infected after 13 d in culture as described below.
Viral Infection of Cells
Influenza strain X47 and VSV strain San Juan A were kind gifts from Dr. Jan Wilschut (Groningen, the Netherlands) and Dr. Peter Rottier (Utrecht, the Netherlands), respectively. Cells were infected with viruses according to Braakman et al. (1991). In brief, cells were rinsed twice with culture medium (pH 6.8) before adding the virus. Cells were infected for 1 h at 37°C with the virus in culture medium (pH 6.8) without CO2. Viral concentrations were chosen such that most cells in the culture were infected. Then the medium was removed, fresh culture medium (pH 7.6) was added, and the cells were incubated for 4 to 5 h at 37°C under an atmosphere of 5% CO2 before starting the experiment.
Triton X-100 Fractionation and Immunoblotting
Triton X-100 (TX-100) extraction was based on the method described by Skibbens et al. (1989) and is described by Van der Haar et al. (1998). In brief, cells from four spinal cords, i.e., about 2 × 106 cells, were scraped, spun down, and lysed in 100 μl of extraction buffer (25 mM 2-(N-morpholino)ethanesulfonic acid, pH 6.5, 0.15 M NaCl, 1% TX-100, and 1 mM phenylmethylsulfonyl fluoride) by incubation for 45 min at 4°C or at 37°C. The pellet was washed once with 25 μl of extraction buffer and the supernatants were collected. The pellet was solubilized in 25 μl of solubilization buffer (50 mM Tris, pH 8.8, 5 mM EDTA, 1% SDS) by passage through a 21-gauge needle, and diluted by addition of 100 μl of extraction buffer. The TX-100-soluble fraction was adjusted to 0.2% SDS. Soluble and insoluble fractions from infected or uninfected OLGs were separated by 12% SDS-PAGE and transferred to Immobilon-P membrane (Millipore, Bedford, MA). HA and VSVG were detected using the appropriate monoclonal antibody and an anti-mouse IgG antibody coupled to alkaline phosphatase (Boehringer Mannheim).
Immunocytochemistry
Fixation of cells for immunofluorescence was as described by de Vries et al. (1997), using 4% paraformaldehyde fixation for 20 min and 0.1% TX-100 permeabilization for 30 min. Antibodies were diluted to appropriate concentrations. After removal of secondary fluorophore-conjugated antibodies by washing, the cells were washed three times and covered with 2.5% 1,4-diazobicyclooctane (Janssen Chimica, Beerse) in 90% glycerol/10% phosphate-buffered saline. Because the monoclonal antibody against HA always leads to a faint labeling in immunofluorescence and also stains the nucleus, only the results obtained with the polyclonal HA antibody are presented.
RESULTS
Both Influenza HA and VSVG Traffic through the Golgi Apparatus
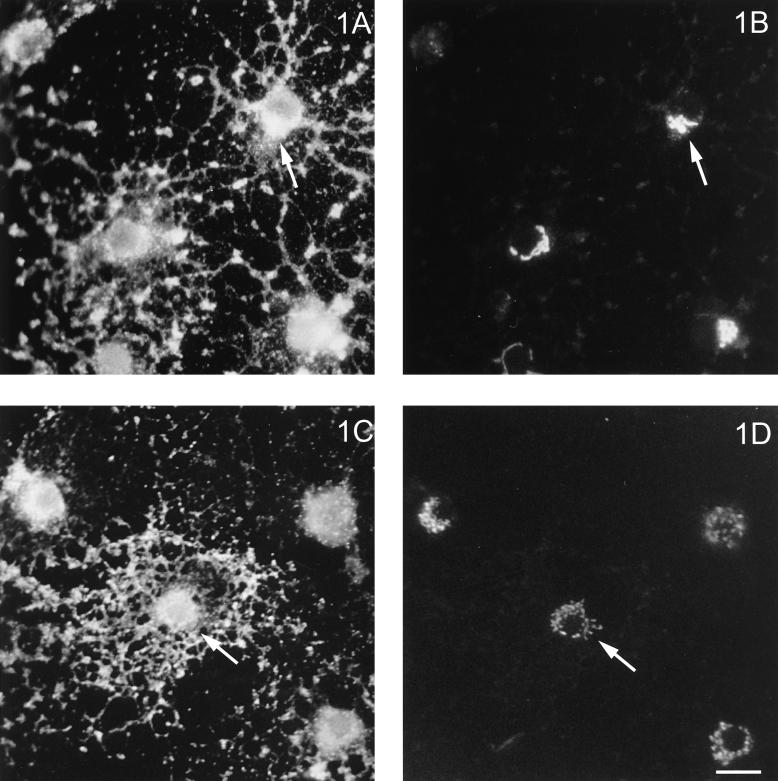
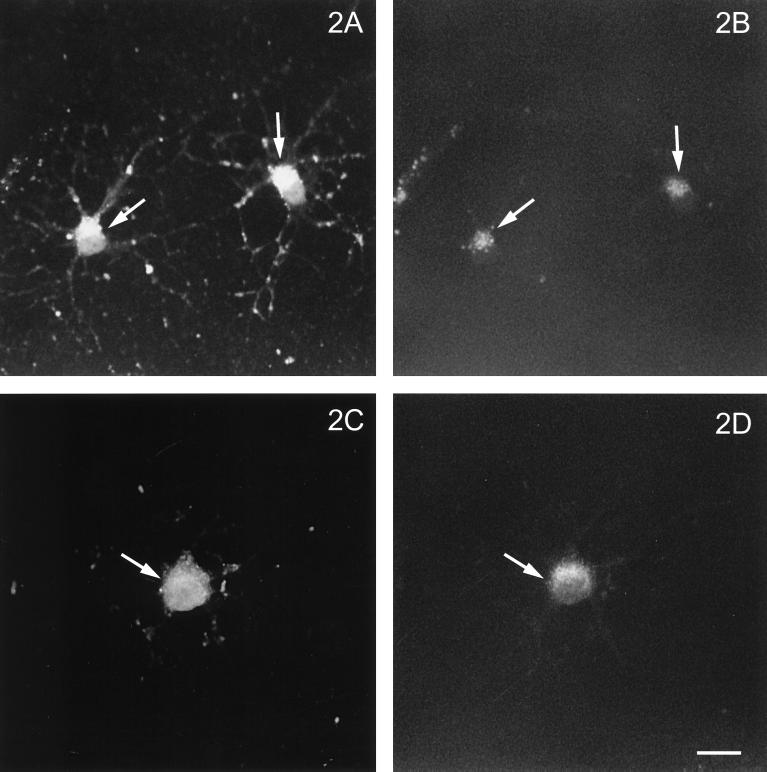
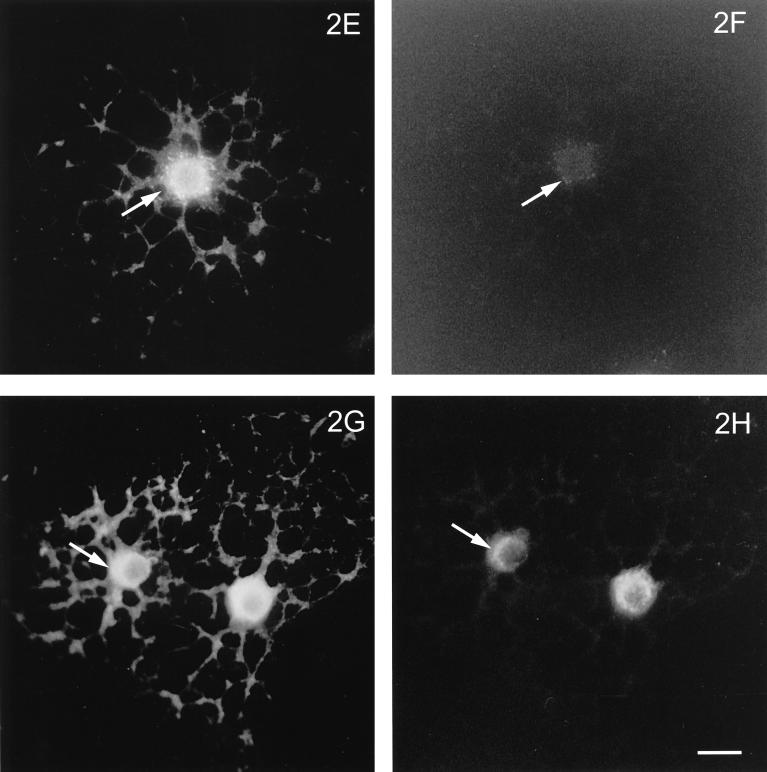
To determine whether OLGs are susceptible to infection by both influenza and VSV and, if so, whether their major membrane glycoproteins (HA and G, respectively) traffic differently, OLGs were infected and costained for these glycoproteins and the medial Golgi-specific marker, MG-160 (Gonatas et al., 1989). Figure 1 shows that both viral glycoproteins were expressed after infection and were present in the cell body and in the processes. The staining intensity increased with time after infection (our unpublished observations), indicating that viral replication occurs in OLGs. Furthermore, HA (Figure 1, A and B) as well as VSVG (Figure 1, C and D) colocalized with MG-160, implying that both proteins pass through the Golgi. Generally, a colocalization of VSVG and the medial Golgi marker could be better discerned than that of HA and MG-160. To obtain further support for a Golgi localization of HA in OLGs, we treated the cells with a concentration of monensin, which is known to disrupt the integrity of the Golgi in HT29 cells (Kok et al., 1991). As shown in Figure 2, 10 μM monensin suffices to fragment the Golgi (cf. Figure 1D), as revealed by the scattered appearance of the Golgi markers MG-160 (Figure 2, B and F) and the trans-Golgi protein TGN-38 (Figure 2, D and H). Note that the scattered appearance of the markers is matched (compare arrows) by a very similar, scattered appearance of HA (Figure 2, A and C) and VSVG (Figure 2, E and G), after monensin treatment. In passing, it is of interest to note that the appearance of VSVG in primary processes and developing sheets is much more pronounced than that of HA (compare Figure 2, E and G with Figure 2, A and B, respectively), the distribution of which appears to be more restricted toward the cell body. Such differences could therefore be a reflection of the existence of different trafficking and sorting (e.g., basolateral versus apical) pathways of these viral proteins in OLGs, as has been noted before in epithelial cells. The fact that both VSVG and HA pass through the Golgi, as shown above, led us to investigate whether raft-mediated transport occurred in OLGs, which, as is generally assumed (Simons and Ikonen, 1997), can be revealed by determining the detergent solubility of the transported entity.
Figure 1.
Intracellular localization of HA and VSVG in virus-infected OLGs. After infection with either influenza (A and B) or VSV (C and D), the intracellular distribution of HA and VSVG was determined by immunostaining as described in MATERIALS AND METHODS. Their distribution was compared with that of medial Golgi-specific MG-160. (A and B) Costaining of HA and MG-160. (C and D) Costaining of VSVG and MG-160. Both HA and VSVG colocalize with MG-160 in the perinuclear region (arrows). Bar, 20 μm.
Figure 2.
(E through H facing page). Effect of monensin on intracellular localization of HA and VSVG in infected OLGs. Two hours before fixation of infected OLGs, monensin was added to a final concentration of 10 μM and the distribution of HA (A and C) was compared with that of MG-160 (B) and the trans-Golgi-network–specific TGN-38 (D). Similarly, the distribution of VSVG (E and G) was compared with that of MG-160 (F) and TGN-38 (H). Monensin treatment induces fragmentation of the Golgi apparatus, as is demonstrated by a marked redistribution of the Golgi markers (B, D, F, and H; compare Figure 1D). This redistribution is matched by a similar redistribution of HA (A and C, arrows) and VSVG (E and G, arrows). Bar, 20 μm.
HA Is Present in a TX-100-Insoluble Fraction, VSVG in a Soluble Fraction
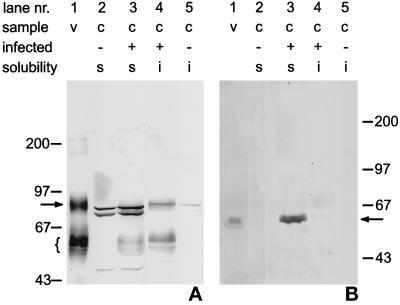
To investigate whether apical and basolateral transport pathways are operating in OLGs, the detergent solubility of HA and VSVG was determined. A hallmark of the apical route is the presence of transported proteins in TX-100-insoluble glycolipid rafts (Simons and Wandinger-Ness, 1990; Brown and Rose, 1992), whereas basolaterally transported proteins do not enter these rafts. Therefore, we extracted virus-infected OLGs with TX-100 and characterized the soluble and insoluble fractions by the immunoblot technique, as shown in Figure 3. As expected, i.e., on the basis of extensive work in epithelial cells, the VSVG immunoblot (Figure 3B) demonstrates that the TX-100-soluble fraction contains VSVG, whereas this viral protein is not present in the insoluble fraction (lane 4). Interpretation of the HA data (Figure 3A) is slightly complicated by the aspecificity of the monoclonal antibody, as is seen in lanes 2 and 5, containing material from uninfected cells. In particular, in the TX-100-soluble fraction (lanes 2 and 3) strong aspecific bands are visible. Nevertheless, it is evident that the HA-derived bands, similar to those present in lane 1 containing extracted influenza virus, are primarily present in the cold-insoluble fraction (lanes 4 and 5). Only a minor HA signal, especially derived from the lower bands, is detected in the soluble fraction. Importantly, at 37°C HA is no longer TX-100 insoluble, indicating that the insolubility is not caused by association with cytoskeletal elements (our unpublished results). Hence, these results strongly indicate that HA, but not VSVG, is present in TX-100 sphingolipid rafts. This distinction would thus be in line with the existence of different sorting pathways for VSVG and HA in OLGs. As established in epithelial cells, the presence of a detergent-insoluble HA fraction would indicate the presence of sphingolipid-enriched rafts thought to mediate apical sorting. The exclusion of VSVG from the raft, as inferred from its solubility in detergent, would be consistent with its entry into a basolateral-directed pathway. The next experiments were undertaken to identify the target membranes for VSVG and HA in OLGs.
Figure 3.
Immunoblots of TX-100-soluble and -insoluble fractions obtained at 4°C from OLGs infected with influenza virus or VSV. Monoclonal antibodies against HA (A) or VSVG (B) were used. Molecular weight markers were run alongside the extracts, as marked by horizontal bars. Samples are from the isolated viruses (v) or from cells (c), either infected (+) or noninfected (−). TX-100-soluble and -insoluble fractions are indicated by “s” and “i,” respectively. (A) HA. Lane 1 (v), solubilized influenza virus. The antibody marks HA at about 80 kDa (arrow) as well as a cluster of smaller proteins (accolade). Lane 2, TX-100-soluble fraction from uninfected OLGs; lane 3, TX-100-soluble fraction from influenza virus-infected OLGs; lane 4, TX-100-insoluble fraction from influenza virus-infected OLGs; and lane 5, TX-100-insoluble fraction from uninfected OLGs. Taking into account some nonspecific staining of the antibody (lanes 2 and 5), it is evident that HA (80-kDa band, arrow) primarily resides in the detergent-insoluble fraction (lane 3 versus lane 4). (B) VSVG. Lane 1 (v), solubilized VSV. The antibody demonstrates the presence of VSVG at about 60 kDa; lane 2, TX-100-soluble fraction from uninfected OLGs; lane 3, TX-100-soluble fraction from VSV-infected OLGs; lane 4, TX-100-insoluble fraction from VSV-infected OLG; and lane 5, TX-100-insoluble fraction from uninfected OLGs. Note that virtually all VSVG is present in the soluble fraction (lane 3).
Myelin Sheets Contain VSVG, But Not HA
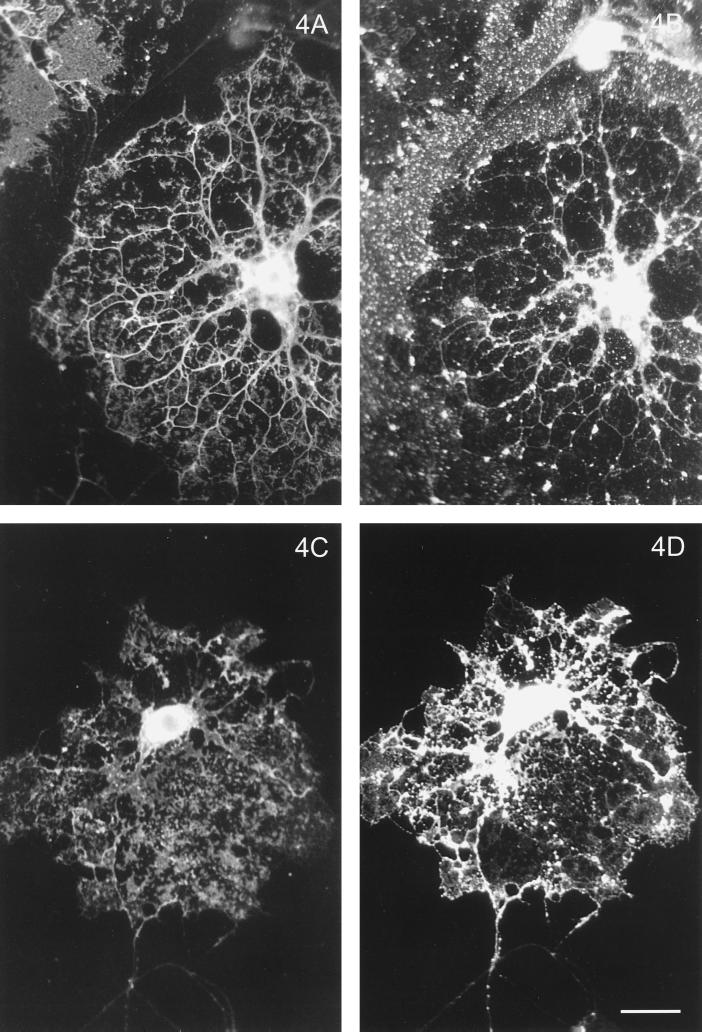
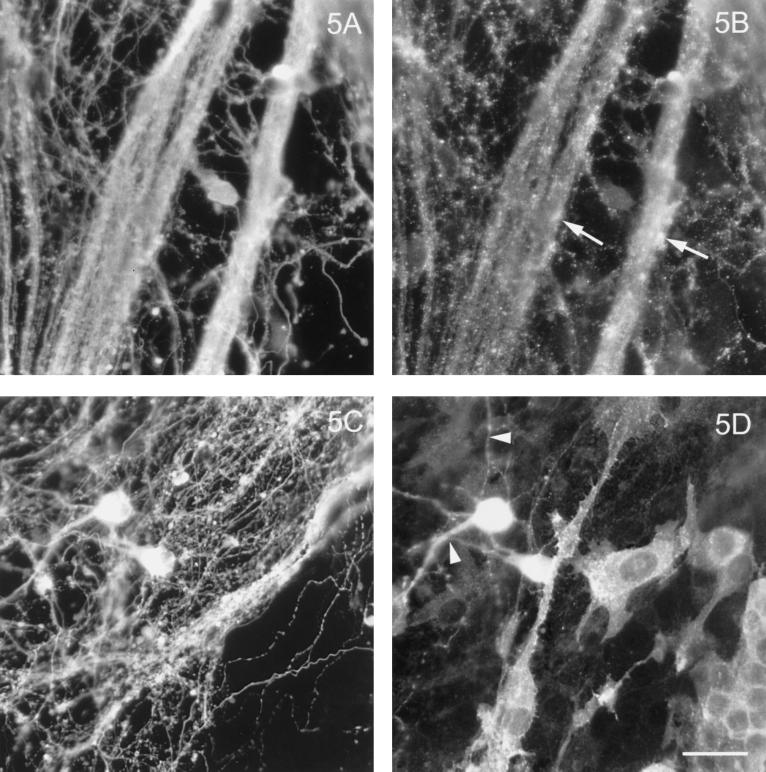
To unequivocally determine their target membranes, we specifically studied HA and G protein transport in those OLGs that possessed elaborate myelin sheets, and their localization was compared with that of MBP, a major constituent of the myelin sheet. In passing, it already became apparent from the data in Figure 1C that a considerable amount of VSVG is present in the myelin membrane. As shown again in Figure 4, a clear difference in the final destination of both viral proteins is evident. Whereas VSVG (Figure 4D), apart from being present in cell body and primary processes, prominently stains the sheet per se as indicated by its colocalization with MBP (Figure 4C), HA, present in cell body and primary processes, is conspicuously absent from this domain (Figure 4, B versus A). Thus, whereas a sharp boundary of the sheet shows up when stained for either MBP (Figure 4, A and C) or VSVG (Figure 4D), no such boundary can be distinguished in the case of HA (Figure 4B). Rather, note that the signal beyond the boundary of the sheet (cf. Figure 4A) seen in Figure 4B originates from an artifact caused by nonspecific sticking of influenza virus to those regions on the culture dish where no cells or cellular material are present, as was also evident from experiments with control, polylysine-coated dishes incubated with influenza virus (our unpublished observations). These results were unexpected, given the TX-100 insolubility of the HA fraction and sphingolipid-enriched nature of the myelin membrane. Therefore, as a control experiment we examined the fate of HA and VSVG in neurons. As shown in Figure 5, in mixed fetal brain cell cultures, which contain neurons as demonstrated by the neuron-specific antibody TuJ1, HA localizes exclusively to the axons whereas VSVG exclusively reaches the dendrites. These data of a polarized transport of both viral proteins in neurons are entirely consistent with previous data reported by Kobayashi et al. (1992). Yet these control experiments provide further support for an aberrant targeting mechanism in OLGs. On the one hand, it is evident that OLGs (like neurons and epithelial cells) are able to sort HA and VSVG to different membrane domains. On the other hand, however, the glycosphingolipid-enriched myelin sheets appear to be the target of the basolateral transport route, rather than the apical pathway, when VSVG and HA, respectively, are considered as representatives of such pathways.
Figure 4.
Differential localization of HA and VSVG in sheet-forming OLGs. OLGs were infected and costained for MBP and the appropriate viral glycoprotein. Note that HA (B) is excluded from the MBP-stained sheets (A), whereas VSVG (D) colocalizes with MBP (C) in the sheets (C and D). Bar, 20 μm.
Figure 5.
Polarized localization of HA and VSVG in neurons. Neurons, present in mixed brain cultures, were infected with influenza virus (A and B) and VSV (C and D), fixed, and costained with the neuron-specific TuJ1 antibody (A and C) and with antibodies against the viral glycoproteins. HA localizes exclusively to axon bundles (B, arrows), whereas G is only present in dendrites (D, arrowheads). Bar, 20 μm.
BFA Does Not Induce a Shift of HA Localization to the Myelin Sheet
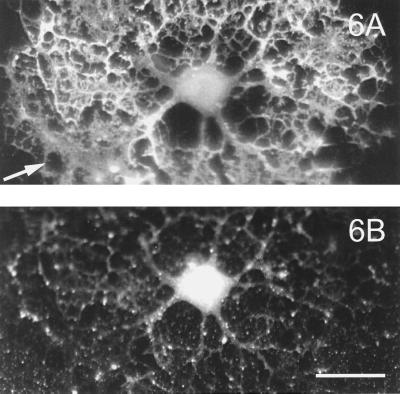
It has been shown that upon treatment of MDCK cells with BFA, an antibiotic that induces fusion of the Golgi apparatus to the endoplasmic reticulum, the sorting of HA is affected whereas its transport is not impaired (Cid-Arregui et al., 1995). Thus, in MDCK cells it was shown that, after this treatment, HA localizes to the basolateral membrane instead of to the apical domain. We therefore investigated whether BFA treatment of infected OLGs leads to a similar mislocalization of HA and to the presence of HA in the sheets as well. Figure 6 shows that even after BFA treatment, the protein is markedly absent from the MBP-stained sheet (Figure 6A). Thus, after addition of BFA the distribution is not distinctly different from that in the absence of the drug (Figure 4); therefore, HA apparently is not able to enter the myelin sheet.
Figure 6.
Effect of BFA on intracellular localization of HA in influenza-infected OLGs. Two hours after infection BFA was added to a final concentration of 18 μM. After another 2 h the OLGs were fixed and costained for MBP (A) and HA (B). No shift in intracellular localization of HA to the myelin sheet was detected. Arrow indicates a densely MBP-labeled part of the sheet. Bar, 20 μm.
DISCUSSION
The present work has provided some remarkable and unexpected features concerning the potential mechanism by which myelin components are sorted and transported in OLGs. In these cells, myelin components (i.e., specific proteins and galactosphingolipids) are transported to the myelin sheet, resulting in a grossly different composition of this membrane as compared with the plasma membrane. The mechanism of transport of glycosphingolipids and myelin proteins, and the possibility of cotransport of these myelin constituents, have been the subject of extensive research. It has been postulated that transport of proteolipid protein (PLP), the major myelin component in the CNS, is coupled to glycosphingolipid synthesis (Pasquini et al., 1989). Moreover, it was concluded that cotransport of PLP with sulfogalactosylceramide (sulfatide; Brown et al., 1993) occurs. However, Bansal and Pfeiffer (1994) have shown that inhibition of sulfatide synthesis does not prevent PLP accumulation in the OLG’s processes. Indeed, more recently PLP was shown not to be associated at all with glycosphingolipids (Van der Haar et al., 1998). In addition, evidence was presented demonstrating that the presence or absence of galactosylceramide or sulfatide was of no consequence to PLP transport to the plasma membrane of Chinese hamster ovary cells transfected with PLP. Yet it has also been reported that the MAL proteolipid (Kim et al., 1995) and GPI-anchored proteins (Krämer et al., 1997) are present in detergent-insoluble, sphingolipid-containing vesicles in myelinating OLGs.
According to the polarity concept, one would intuitively expect the myelin sheet to be the equivalent of the apical membrane, especially since glycosphingolipids are major constituents of myelin and are asymmetrically located, along with cholesterol, in the outer leaflet (Casper and Kirschner, 1971; Braun, 1984). The present report, as well as those by Kim et al. (1995) and Krämer et al. (1997), indicates that an apical-type transport machinery, reflected by detergent-insoluble rafts as described for model polarized cells, is present in the mature OLGs as well. The evidence presented here was derived from experiments in which the fate of the viral glycoproteins HA and VSVG was examined. The results show that both viral glycoproteins are present in the medial Golgi and in the trans-Golgi network and that they are differentially sorted, as evidenced by immunofluorescence microscopy of OLGs. Our results demonstrate the presence of an apical-type sorting pathway, since HA is present in TX-100-insoluble rafts, but at the same time they show that this route is not the one taken for targeting proteins to the sheet. Interestingly, the experiments with BFA did not reveal a shift in sorting of HA to the sheet. Therefore, a BFA-induced shift from apical to basolateral sorting as shown in MDCK cells (Cid-Arregui et al., 1995) does not occur in OLGs, which indicates that for HA a block exists to enter the sheet from the processes. It cannot be concluded, however, that this is caused by an intrinsic resistance of OLGs to the action of BFA, because even at very high BFA concentrations no fragmentation of Golgi could be induced in OLGs (our unpublished observations).
It is clear that a major fraction of HA is present in a TX-100-insoluble fraction, but the presence of a minor fraction in the TX-100 supernatant indicates that HA is presumably located both within and outside rafts. Likely, TX-100-soluble HA represents a fraction that has not yet reached the trans-Golgi, because HA becomes insoluble only upon entry into this compartment. From the abundant presence of VSVG in the myelin sheet, we infer that the sheet bears analogy to the basolateral domain of MDCK cells and neurons and not to the apical domain. However, it is difficult to define an apical membrane in OLGs because no membrane area could be found that is specific for HA. Nevertheless, the MAL proteolipid (Kim et al., 1995) and GPI-anchored proteins (Krämer et al., 1997) are present in detergent-insoluble complexes, at least in mature OLGs, and apparently end up in myelin. Because VSVG also arrives in the myelin sheet, as shown in the present work, it appears that raft-mediated transport does not represent the exclusive transport pathway that leads to the myelin sheet. On the other hand, a localization of a protein in the raft (i.e., HA, as reflected by its TX-100 insolubility) does not necessarily lead to transport into the sheet.
Accordingly, we suggest that additional molecular parameters are necessary for targeting to the myelin sheet (and perhaps to apical membranes in general), the identity of which remains to be determined. Alternatively, it may be equally possible (Krämer et al., 1997; Van der Haar et al., 1998) that myelin is not the equivalent of an (oligodendrocytic) apical membrane. In fact, both proteolipid protein and myelin-associated glycoprotein (MAG) (Krämer et al., 1997), are transported via a different route, i.e., independent of a potential localization in apical GSL rafts. In this respect it may be important that Minuk and Braun (1996) have recently found a difference in sorting between MAG isoforms expressed in MDCK cells. L-MAG, which is expressed early in myelinogenesis in OLGs, invariably sorts to the basolateral membrane, whereas the sorting of S-MAG (expressed in a later developmental stage in the OLG than L-MAG) depends on the type of MDCK transfectant used and on the growth conditions. If extrapolation of these data to OLGs is allowed, they again indicate, in conjunction with our results, that myelin constituents are usually sorted by a basolateral-like route.
Taken together, a picture is emerging for the sorting mechanisms operative in the OLG indicating that polarized expression does exist. This is evidenced by the sorting of VSVG and of MBP and other myelin proteins to the sheets. These proteins, however, do not comply with the apical-type sorting by means of (TX-100-insoluble) rafts. Nevertheless, an apical transport system is present as well, as is reflected by the TX-100 insolubility of HA. This system may localize MAL, some GPI-anchored proteins, and perhaps S-MAG to myelin, but HA, which is present in a TX-100-insoluble complex, is not transported beyond the plasma membrane of the OLG cell body and processes to the myelin sheet. Hence, a most important issue concerns the relevance of an apical-type transport mechanism in the OLGs and the significance of this mechanism for carrying specific proteins to distinct membrane domains. Apparently, the presence of GPI-linked proteins in rafts may lead to their localization to myelin whereas, alternatively, the presence of HA in a raft does not suffice to transfer this protein to the myelin sheet. We propose that additional regulatory mechanisms are involved.
ACKNOWLEDGMENTS
We thank the Stichting Vrienden MS Research (grants 91–87 MS and 93–153 MS) and the Foundation Jan Kornelis de Cock for supporting our research, Dr. Ineke Braakman for her advice during initiation of this project and for providing polyclonal antibodies, and Drs. N.A. Gonatas and G. Banting for the use of their antibodies.
REFERENCES
- Bansal R, Pfeiffer SE. Inhibition of protein and lipid sulfation in oligodendrocytes blocks biological responses to FGF-2 and retards cytoarchitectural maturation, but not developmental lineage progression. Dev Biol. 1994;162:511–524. doi: 10.1006/dbio.1994.1105. [DOI] [PubMed] [Google Scholar]
- Baron W, De Jonge JC, de Vries H, Hoekstra D. Regulation of oligodendrocyte differentiation: protein kinase C activation prevents differentiation of O2A progenitor cells towards oligodendrocytes. Glia. 1998;22:121–129. doi: 10.1002/(sici)1098-1136(199802)22:2<121::aid-glia3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Braakman I, Hoover-Litty H, Wagner KR, Helenius A. Folding of influenza hemagglutinin in the endoplasmic reticulum. J Cell Biol. 1991;114:401–411. doi: 10.1083/jcb.114.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun PE. Molecular organization of myelin. Myelin. 2nd ed. P. Morell, New York: Plenum; 1984. pp. 97–116. [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brown MC, Moreno MB, Bongarzone ER, Cohen PD, Soto EF, Pasquini JM. Vesicular transport of myelin proteolipid and cerebroside sulfates to the myelin membrane. J Neurosci Res. 1993;35:402–408. doi: 10.1002/jnr.490350407. [DOI] [PubMed] [Google Scholar]
- Casper DL, Kirschner DA. Myelin membrane structure at 10 C resolution. Nat New Biol. 1971;231:46–52. doi: 10.1038/newbio231046a0. [DOI] [PubMed] [Google Scholar]
- Cid-Arregui A, Parton RG, Simons K, Dotti CG. Nocodazole-dependent transport, and Brefeldin A-sensitive processing and sorting, of newly synthesized membrane proteins in cultured neurons. J Neurosci. 1995;15:4259–4269. doi: 10.1523/JNEUROSCI.15-06-04259.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hoop MJ, Dotti CG. Membrane traffic in polarized neurons in culture. J Cell Sci. 1993;17:85–92. doi: 10.1242/jcs.1993.supplement_17.13. [DOI] [PubMed] [Google Scholar]
- de Vries H, De Jonge JC, Schrage C, Van der Haar ME, Hoekstra D. Differential and cell-development-dependent localization of myelin mRNAs in oligodendrocytes. J Neurosci Res. 1997;47:479–488. doi: 10.1002/(sici)1097-4547(19970301)47:5<479::aid-jnr3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- de Vries H, Schrage C, Hoekstra K, Kok JW, Van der Haar ME, Kalicharan D, Liem RSB, Copray JCVM, Hoekstra D. Outstations of the Golgi complex are present in the processes of cultured rat oligodendrocytes. J Neurosci Res. 1993;36:336–343. doi: 10.1002/jnr.490360311. [DOI] [PubMed] [Google Scholar]
- Geisert EE, Frankfurter A. The neuronal response to injury as visualized by immunostaining of class III beta-tubulin in the rat. Neurosci Lett. 1989;102:137–141. doi: 10.1016/0304-3940(89)90068-2. [DOI] [PubMed] [Google Scholar]
- Gillespie CS, Wilson R, Davidson A, Brophy PJ. Characterization of a cytoskeletal matrix associated with myelin from rat brain. Biochem J. 1989;260:689–696. doi: 10.1042/bj2600689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas JO, Mezitis SGE, Stieber A, Fleischer B, Gonatas NK. MG-160. A novel sialoglycoprotein of the medial cisternae of the Golgi apparatus. J Biol Chem. 1989;264:646–653. [PubMed] [Google Scholar]
- Kalwy SA, Smith R. Mechanisms of myelin basic protein and proteolipid protein targeting in oligodendrocytes (Review) Mol Membr Biol. 1994;11:67–78. doi: 10.3109/09687689409162223. [DOI] [PubMed] [Google Scholar]
- Kim T, Fiedler K, Madison DL, Krueger WH, Pfeiffer SE. Cloning and characterization of MVP17: a developmentally regulated myelin protein in oligodendrocytes. J Neurosci Res. 1995;42:413–422. doi: 10.1002/jnr.490420316. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Storrie B, Simons K, Dotti CG. A functional barrier to movement of lipids in polarized neurons. Nature. 1992;359:647–650. doi: 10.1038/359647a0. [DOI] [PubMed] [Google Scholar]
- Kok JW, Babia T, Hoekstra D. Sorting of sphingolipids in the endocytic pathway of HT29 cells. J Cell Biol. 1991;114:231–239. doi: 10.1083/jcb.114.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer EM, Koch T, Niehaus A, Trotter J. Oligodendrocytes direct glycosyl phosphatidylinositol-anchored proteins to the myelin sheath in glycosphingolipid-rich complexes. J Biol Chem. 1997;272:8937–8945. doi: 10.1074/jbc.272.14.8937. [DOI] [PubMed] [Google Scholar]
- Lubetzki C, Demerens C, Anglade P, Villarroya H, Frankfurter A, Lee VM, Zalc B. Even in culture, oligodendrocytes myelinate solely axons. Proc Natl Acad Sci USA. 1993;90:6820–6824. doi: 10.1073/pnas.90.14.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Brake B, Banting G, Howell KE, Braghetta P, Stanley KK. Identification, sequencing and expression of an integral membrane protein of the trans-Golgi network (TGN38) Biochem J. 1990;270:97–102. doi: 10.1042/bj2700097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minuk J, Braun PE. Differential intracellular sorting of the myelin-associated glycoprotein isoforms. J Neurosci Res. 1996;44:411–420. doi: 10.1002/(SICI)1097-4547(19960601)44:5<411::AID-JNR1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Müsch A, Xu H, Shields D, Rodriguez-Boulan E. Transport of vesicular stomatitis virus G protein to the cell surface is signal mediated in polarized and nonpolarized cells. J Cell Biol. 1996;133:543–558. doi: 10.1083/jcb.133.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquini JM, Guarna MM, Besio-Moreno MA, Iturregui MT, Oteiza PI, Soto EF. Inhibition of the synthesis of glycosphingolipids affects the translocation of proteolipid protein to the myelin membrane. J Neurosci Res. 1989;22:289–296. doi: 10.1002/jnr.490220309. [DOI] [PubMed] [Google Scholar]
- Pereyra PM, Horvath E, Braun PE. Triton X-100 extractions of central nervous system myelin indicate a possible role for the minor myelin proteins in the stability of the lamellae. Neurochem Res. 1988;6:583–595. doi: 10.1007/BF00973301. [DOI] [PubMed] [Google Scholar]
- Puertollano R, Li S, Lisanti MP, Alonso MA. Recombinant expression of the MAL proteolipid, a component of glycolipid-enriched membrane microdomains, induces the formation of vesicular structures in insect cells. J Biol Chem. 1997;272:18311–18315. doi: 10.1074/jbc.272.29.18311. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990;62:207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Simons K, Zerial M. Rab proteins and the road maps for intracellular transport. Neuron. 1993;11:789–799. doi: 10.1016/0896-6273(93)90109-5. [DOI] [PubMed] [Google Scholar]
- Van der Haar, M.E., Visser, H.W., de Vries, H., and Hoekstra, D. (1998). Transport of proteolipid protein to the plasma membrane does not depend on glycosphingolipid cotransport in oligodendrocyte cultures. J. Neurosci. Res. (in press). [DOI] [PubMed]
- Van der Pal RHM, Vos JP, Van Golde LMG, Lopes-Cardozo M. A rapid procedure for the preparation of oligodendrocyte-enriched cultures from rat spinal cord. Biochim Biophys Acta. 1990;1051:159–165. doi: 10.1016/0167-4889(90)90188-j. [DOI] [PubMed] [Google Scholar]
- Weimbs T, Low S-H, Chapin S, Mostov K. Protein traffic in polarized epithelial cells. Trends Cell Biol. 1997;7:393–399. doi: 10.1016/S0962-8924(97)01130-6. [DOI] [PubMed] [Google Scholar]
- Wilson R, Brophy PJ. Role for the oligodendrocyte cytoskeleton in myelination. J Neurosci Res. 1989;22:439–448. doi: 10.1002/jnr.490220409. [DOI] [PubMed] [Google Scholar]