Abstract
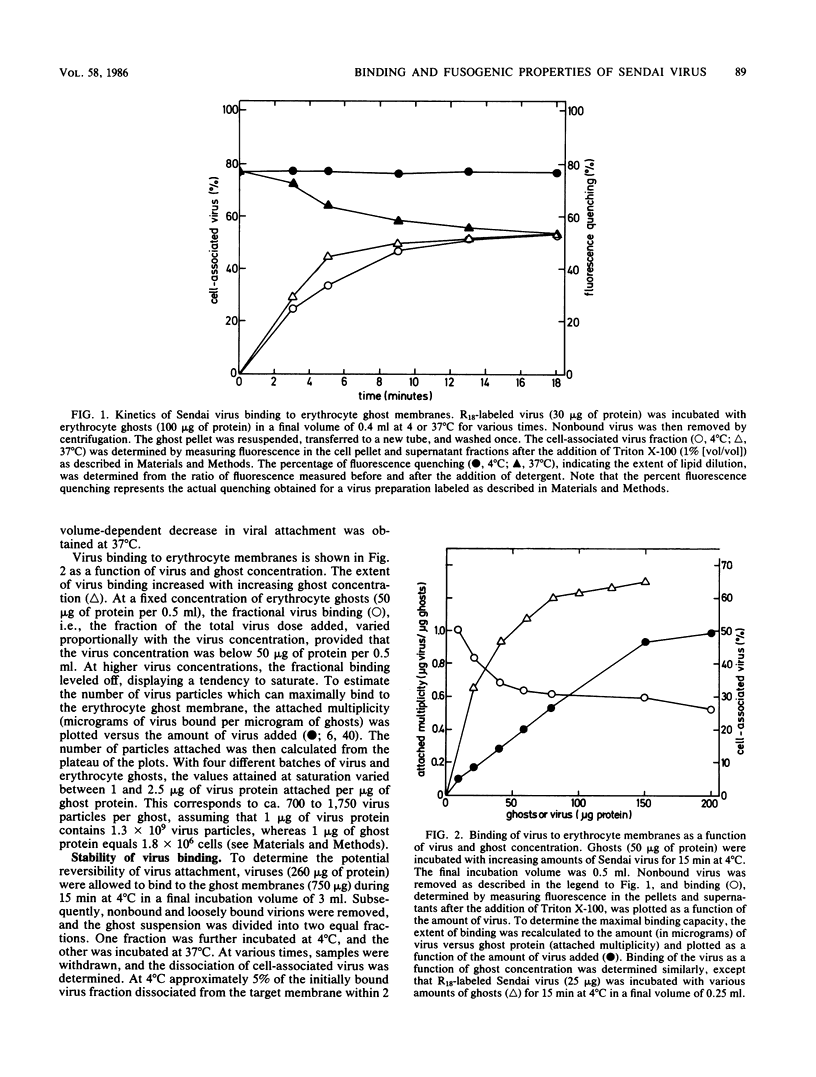
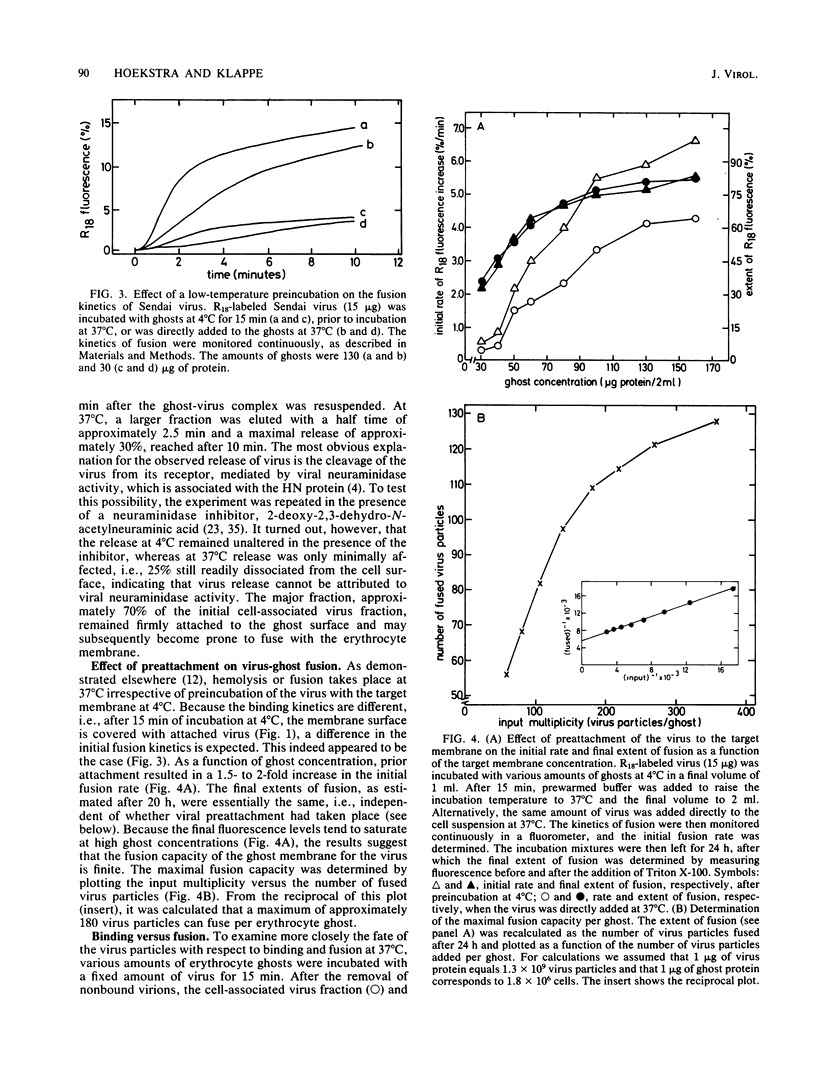
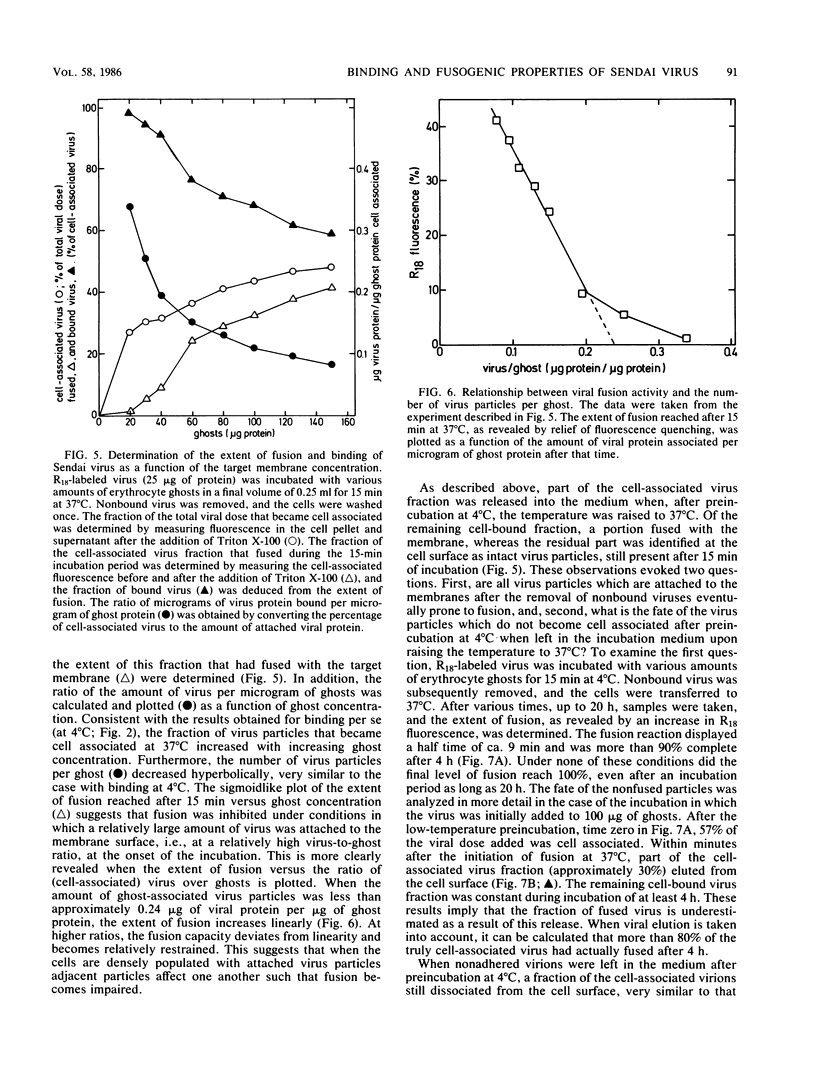
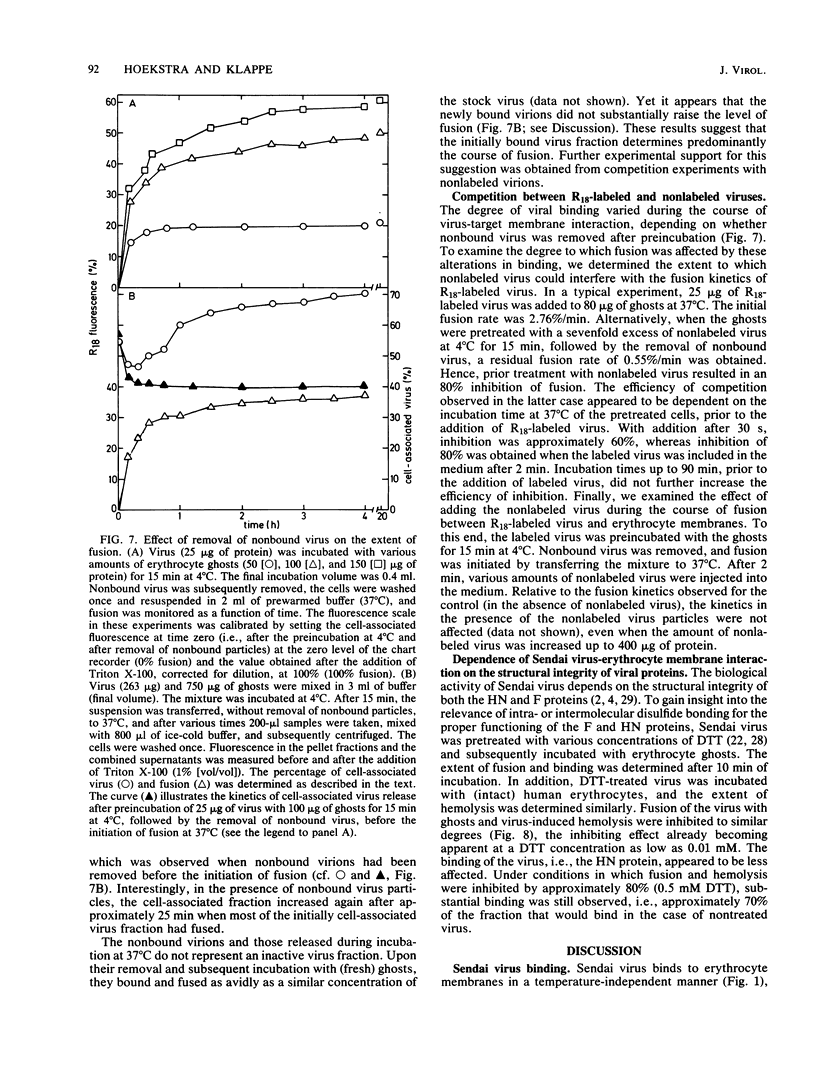
A kinetic and quantitative analysis of the binding and fusion of Sendai virus with erythrocyte membranes was performed by using a membrane fusion assay based on the relief of fluorescence self-quenching. At 37 degrees C, the process of virus association displayed a half time of 2.5 min; at 4 degrees C, the half time was 3.0 min. The fraction of the viral dose which became cell associated was independent of the incubation temperature and increased with increasing target membrane concentration. On the average, one erythrocyte ghost can accommodate ca. 1,200 Sendai virus particles. The stability of viral attachment was sensitive to a shift in temperature: a fraction of the virions (ca. 30%), attached at 4 degrees C, rapidly (half time, ca. 2.5 min) eluted from the cell surface at 37 degrees C, irrespective of the presence of free virus in the medium. The elution can be attributed to a spontaneous, temperature-induced release, rather than to viral neuraminidase activity. Competition experiments with nonlabeled virus revealed that viruses destined to fuse do not exchange with free particles in the medium but rather bind in a rapid and irreversible manner. The fusion rate of Sendai virus was affected by the density of the virus particles on the cell surface and became restrained when more than 170 virus particles were attached per ghost. In principle, all virus particles added displayed fusion activity. However, at high virus-to-ghost ratios, only a fraction actually fused, indicating that a limited number of fusion sites exist on the erythrocyte membrane. We estimate that ca. 180 virus particles maximally can fuse with one erythrocyte ghost.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altstiel L. D., Landsberger F. R. Structural changes in BHK cell plasma membrane caused by the binding of vesicular stomatitis virus. J Virol. 1981 Jul;39(1):82–86. doi: 10.1128/jvi.39.1.82-86.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burness A. T., Pardoe I. U. A sialoglycopeptide from human erythrocytes with receptor-like properties for encephalomyocarditis and influenza viruses. J Gen Virol. 1983 May;64(Pt 5):1137–1148. doi: 10.1099/0022-1317-64-5-1137. [DOI] [PubMed] [Google Scholar]
- Choppin P. W., Richardson C. D., Merz D. C., Hall W. W., Scheid A. The functions and inhibition of the membrane glycoproteins of paramyxoviruses and myxoviruses and the role of the measles virus M protein in subacute sclerosing panencephalitis. J Infect Dis. 1981 Mar;143(3):352–363. doi: 10.1093/infdis/143.3.352. [DOI] [PubMed] [Google Scholar]
- Fries E., Helenius A. Binding of Semliki Forest virus and its spike glycoproteins to cells. Eur J Biochem. 1979 Jun;97(1):213–220. doi: 10.1111/j.1432-1033.1979.tb13105.x. [DOI] [PubMed] [Google Scholar]
- Gething M. J., White J. M., Waterfield M. D. Purification of the fusion protein of Sendai virus: analysis of the NH2-terminal sequence generated during precursor activation. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2737–2740. doi: 10.1073/pnas.75.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood A. M., Boyer B. P. Sendai virus membrane fusion: time course and effect of temperature, pH, calcium, and receptor concentration. Biochemistry. 1982 Nov 23;21(24):6041–6046. doi: 10.1021/bi00267a003. [DOI] [PubMed] [Google Scholar]
- Haywood A. M. Letter to the editor: Fusion of Sendai viruses with model membranes. J Mol Biol. 1974 Aug 15;87(3):625–628. doi: 10.1016/0022-2836(74)90107-7. [DOI] [PubMed] [Google Scholar]
- Henis Y. I., Jenkins T. M. Detection of Sendai virus fusion with human erythrocytes by fluorescence photobleaching recovery. FEBS Lett. 1983 Jan 10;151(1):134–138. doi: 10.1016/0014-5793(83)80358-5. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., Klappe K., de Boer T., Wilschut J. Characterization of the fusogenic properties of Sendai virus: kinetics of fusion with erythrocyte membranes. Biochemistry. 1985 Aug 27;24(18):4739–4745. doi: 10.1021/bi00339a005. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., Wilschut J., Scherphof G. Fusion of erythrocyte ghosts induced by calcium phosphate. Kinetic characteristics and the role of Ca2+, phosphate and calcium-phosphate complexes. Eur J Biochem. 1985 Jan 2;146(1):131–140. doi: 10.1111/j.1432-1033.1985.tb08629.x. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., de Boer T., Klappe K., Wilschut J. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry. 1984 Nov 20;23(24):5675–5681. doi: 10.1021/bi00319a002. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm L., Elwing H., Fredman P., Strannegård O. Sendai virus receptor: proposed recognition structure based on binding to plastic-adsorbed gangliosides. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1947–1950. doi: 10.1073/pnas.77.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M., Scheid A., Choppin P. W. Activation of the Sendai virus fusion protein (f) involves a conformational change with exposure of a new hydrophobic region. J Biol Chem. 1981 Apr 10;256(7):3557–3563. [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Chemical composition of the parainfluenza virus SV5. Virology. 1969 Jan;37(1):155–157. doi: 10.1016/0042-6822(69)90321-3. [DOI] [PubMed] [Google Scholar]
- Lyles D. S., Landsberger F. R. Kinetics of Sendai virus envelope fusion with erythrocyte membranes and virus-induced hemolysis. Biochemistry. 1979 Nov 13;18(23):5088–5095. doi: 10.1021/bi00590a011. [DOI] [PubMed] [Google Scholar]
- Maeda T., Asano A., Oki K., Okada Y., Onishi S. A spin-label study on fusion of red blood cells induced by hemagglutinating virus of Japan. Biochemistry. 1975 Aug 26;14(17):3736–3741. doi: 10.1021/bi00688a003. [DOI] [PubMed] [Google Scholar]
- Maeda T., Eldridge C., Toyama S., Ohnishi S. I., Elson E. L., Webb W. W. Membrane receptor mobility changes by Sendai virus. Exp Cell Res. 1979 Oct 15;123(2):333–343. doi: 10.1016/0014-4827(79)90475-0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Protein-protein interactions within paramyxoviruses identified by native disulfide bonding or reversible chemical cross-linking. J Virol. 1980 Jan;33(1):152–166. doi: 10.1128/jvi.33.1.152-166.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl P., Bodo G., Palese P., Schulman J., Tuppy H. Inhibition of neuraminidase activity by derivatives of 2-deoxy-2,3-dehydro-N-acetylneuraminic acid. Virology. 1974 Apr;58(2):457–463. doi: 10.1016/0042-6822(74)90080-4. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Prehm P., Scheid A., Choppin P. W. Inhibition of the neuraminidase of paramyxoviruses by halide ions: a possible means of modulating the two activities of the HN protein. Virology. 1981 Jul 15;112(1):296–305. doi: 10.1016/0042-6822(81)90635-8. [DOI] [PubMed] [Google Scholar]
- Oku N., Inoue K., Nojima S., Sekiya T., Nozawa Y. Electron microscopic study on the interaction of Sendai virus with liposomes containing glycophorin. Biochim Biophys Acta. 1982 Sep 24;691(1):91–96. doi: 10.1016/0005-2736(82)90217-6. [DOI] [PubMed] [Google Scholar]
- Oku N., Nojima S., Inoue K. Studies on the interaction of Sendai virus with liposomal membranes. Sendai virus-induced agglutination of liposomes containing glycophorin. Biochim Biophys Acta. 1981 Aug 6;646(1):36–42. doi: 10.1016/0005-2736(81)90269-8. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Asano A., Okada Y. The presence and cleavage of interpeptide disulfide bonds in viral glycoproteins. J Biochem. 1979 Nov;86(5):1361–1369. doi: 10.1093/oxfordjournals.jbchem.a132653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. D., Choppin P. W. Oligopeptides that specifically inhibit membrane fusion by paramyxoviruses: studies on the site of action. Virology. 1983 Dec;131(2):518–532. doi: 10.1016/0042-6822(83)90517-2. [DOI] [PubMed] [Google Scholar]
- Roux L., Holland J. J. Role of defective interfering particles of Sendai virus in persistent infections. Virology. 1979 Feb;93(1):91–103. doi: 10.1016/0042-6822(79)90278-2. [DOI] [PubMed] [Google Scholar]
- Solomon A. K. Ca binding to the human red cell membrane: characterization of membrane preparations and binding sites. J Membr Biol. 1976 Nov 29;29(4):345–372. doi: 10.1007/BF01868970. [DOI] [PubMed] [Google Scholar]
- Tozawa H., Watanabe M., Ishida N. Structural components of Sendai virus. Serological and physicochemical characterization of hemagglutinin subunit associated with neuraminidase activity. Virology. 1973 Sep;55(1):242–253. doi: 10.1016/s0042-6822(73)81027-x. [DOI] [PubMed] [Google Scholar]
- Umeda M., Nojima S., Inoue K. Activity of human erythrocyte gangliosides as a receptor to HVJ. Virology. 1984 Feb;133(1):172–182. doi: 10.1016/0042-6822(84)90436-7. [DOI] [PubMed] [Google Scholar]
- Umeda M., Nojima S., Inoue K. HVJ-mediated fusion between erythrocyte membranes and liposomes containing glycophorin. J Biochem. 1983 Dec;94(6):1955–1966. doi: 10.1093/oxfordjournals.jbchem.a134549. [DOI] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- Wolf D., Kahana I., Nir S., Loyter A. The interaction between Sendai virus and cell membranes. A quantitative analysis of 125I-sendai virus particles' association with human red blood cells. Exp Cell Res. 1980 Dec;130(2):361–369. doi: 10.1016/0014-4827(80)90013-0. [DOI] [PubMed] [Google Scholar]
- Wu P. S., Ledeen R. W., Udem S., Isaacson Y. A. Nature of the Sendai virus receptor: glycoprotein versus ganglioside. J Virol. 1980 Jan;33(1):304–310. doi: 10.1128/jvi.33.1.304-310.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunner W. H., Reagan K. J., Koprowski H. Characterization of saturable binding sites for rabies virus. J Virol. 1984 Jun;50(3):691–697. doi: 10.1128/jvi.50.3.691-697.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G., Simons K. An efficient method for introducing defined lipids into the plasma membrane of mammalian cells. J Cell Biol. 1983 Nov;97(5 Pt 1):1365–1374. doi: 10.1083/jcb.97.5.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]



