Abstract
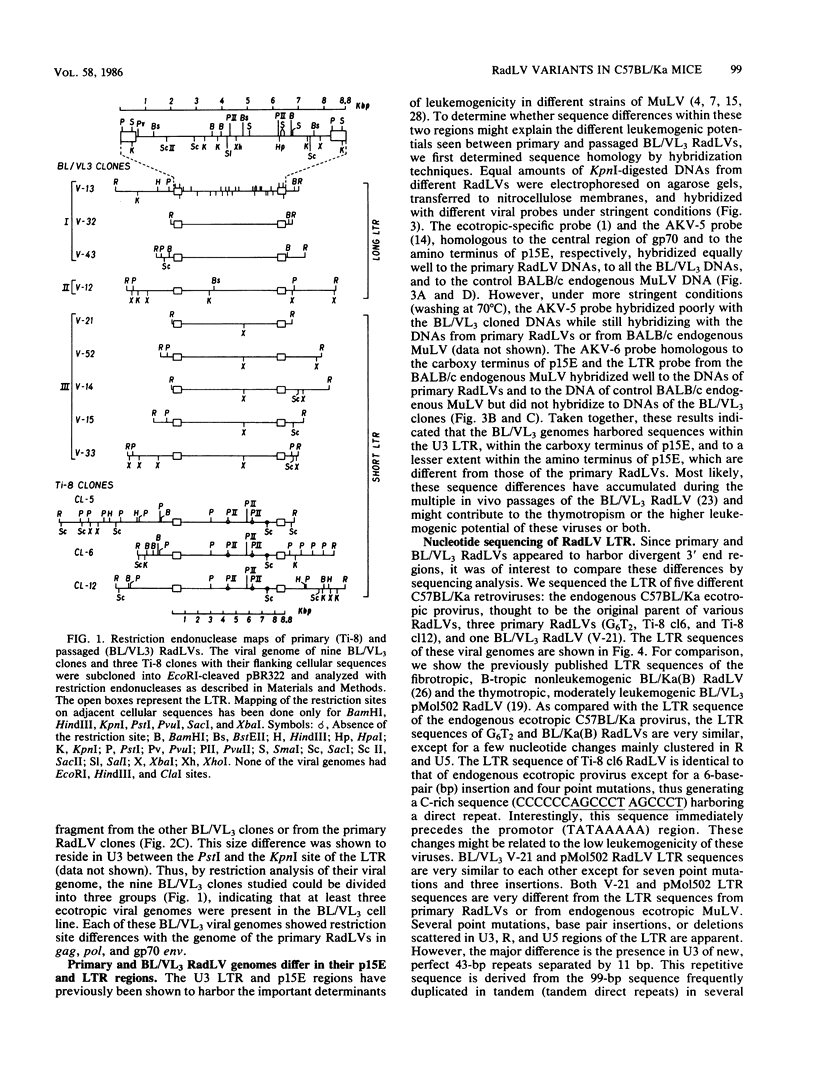
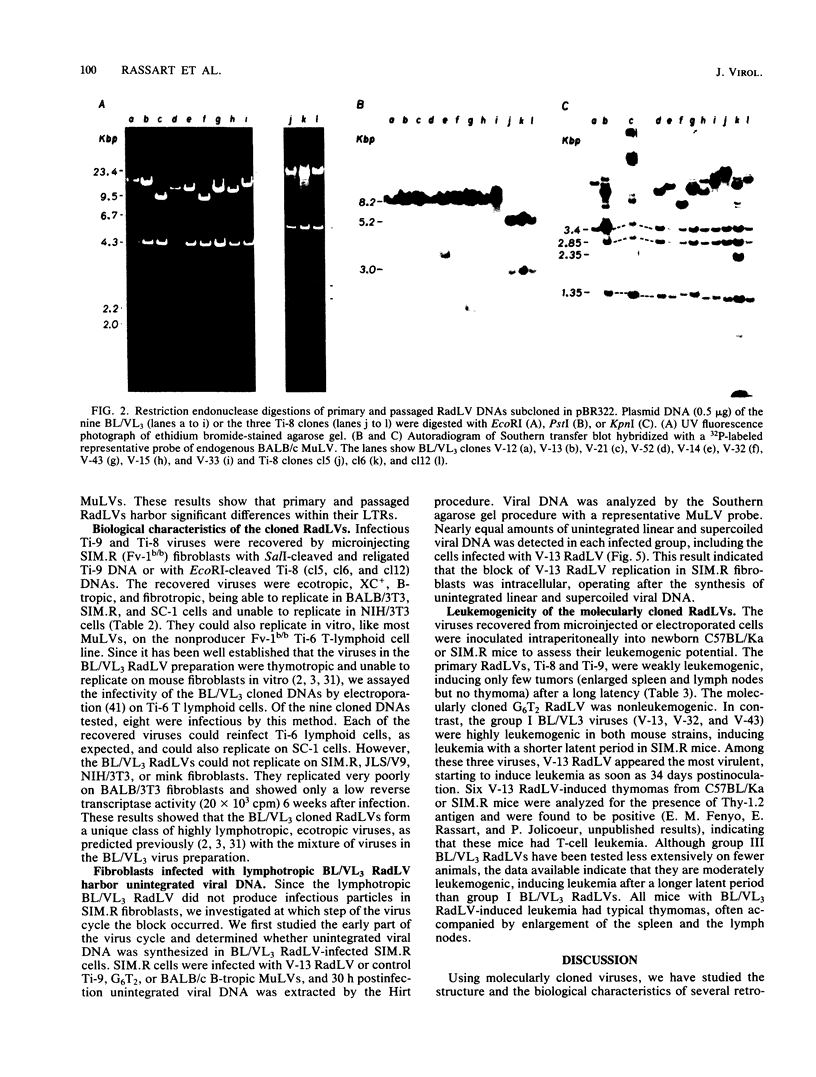
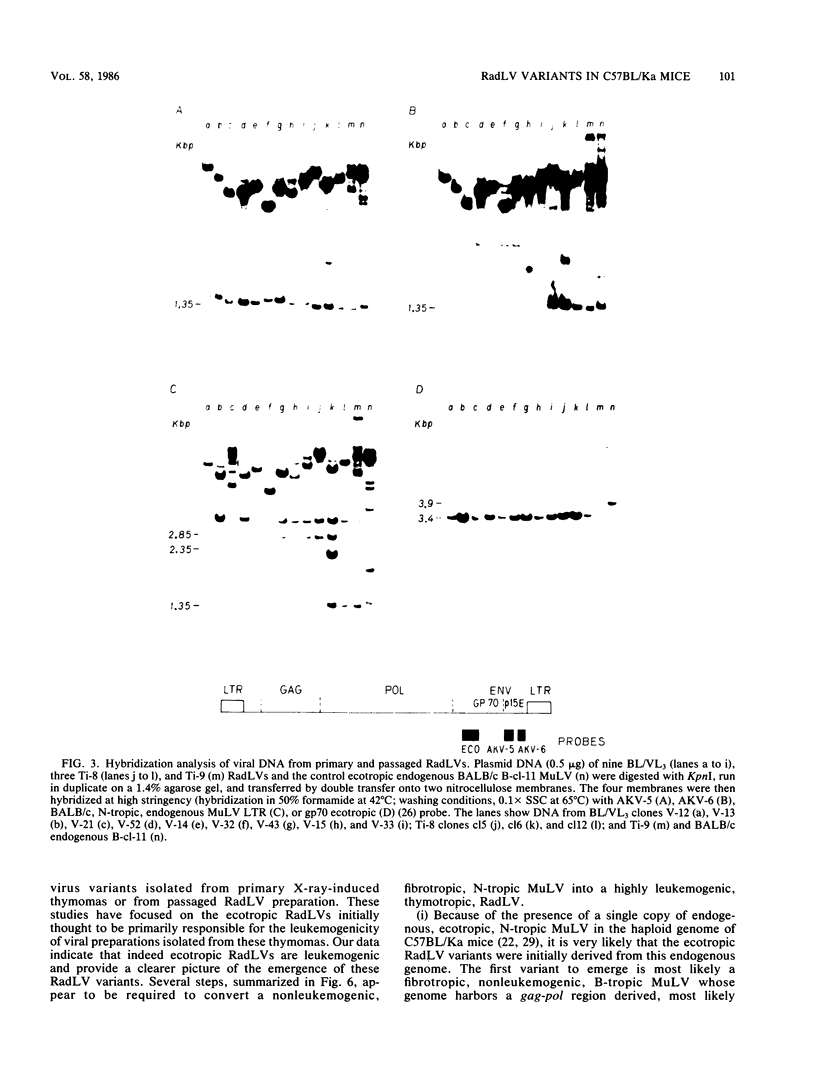
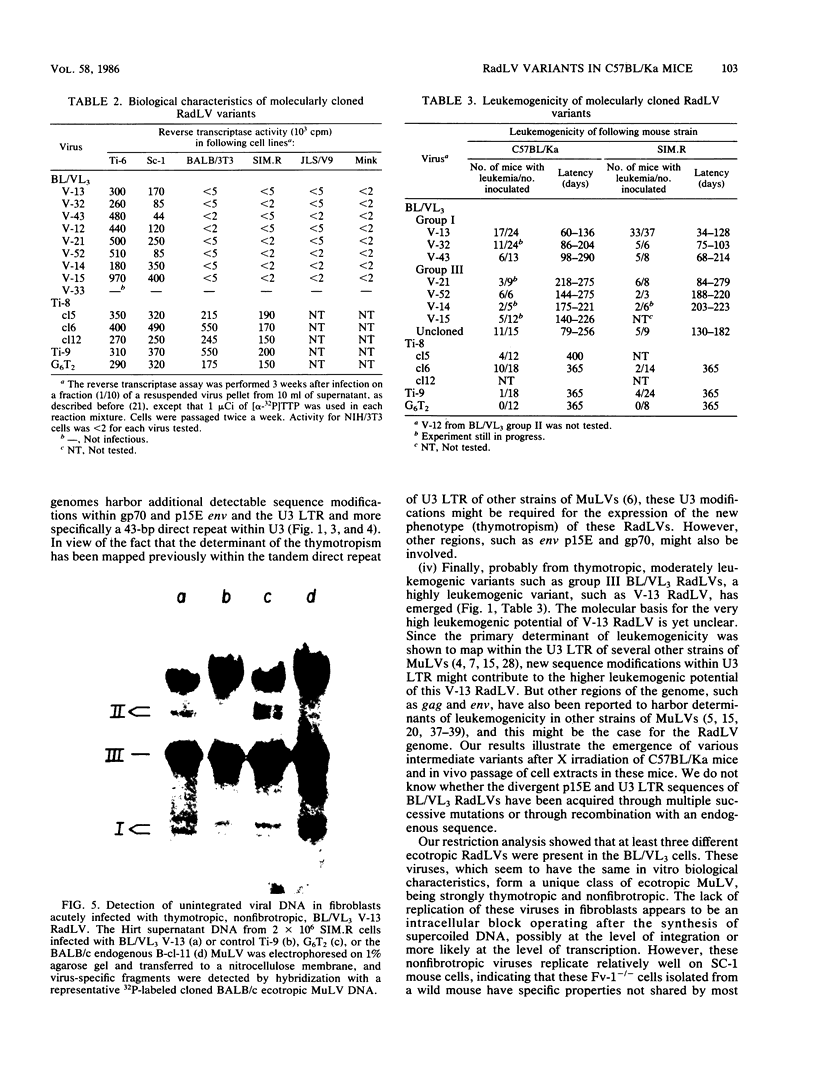
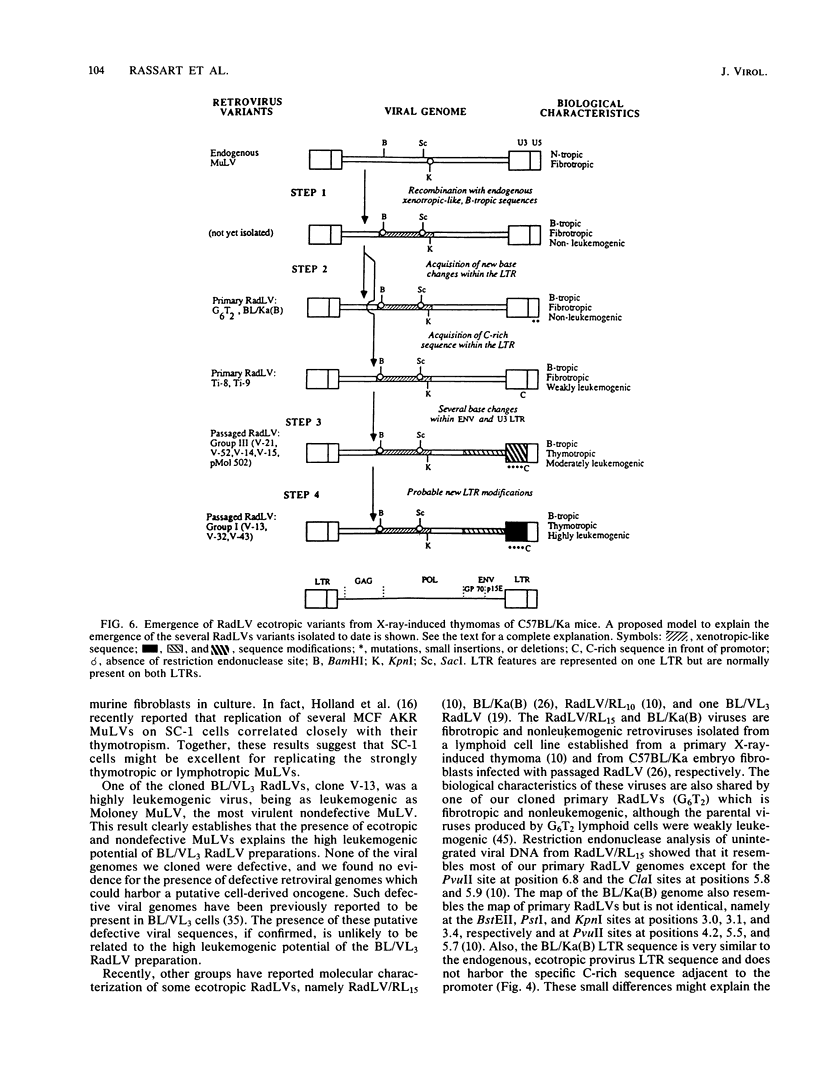
To analyze the emergence of radiation leukemia virus (RadLV) variants in primary X-ray-induced C57BL/Ka thymoma and to identify the virus responsible for the very high leukemogenic potential of passaged Kaplan strain BL/VL3 preparation, we cloned several primary and passaged ecotropic RadLV infectious genomes. By restriction analysis, we found that BL/VL3 cells harbor three related but different ecotropic RadLVs. Their restriction map differs significantly from those of primary RadLVs. Hybridization analysis also indicated that BL/VL3 and primary RadLVs differ in their p15E and long terminal repeat (LTR) regions. As compared with the LTR sequence of the putative parental endogenous ecotropic provirus, the LTR sequence of primary weakly leukemogenic RadLV has only one change, a C-rich sequence, generating a 6-base-pair direct repeat just in front of the promotor. The LTR of the primary nonleukemogenic RadLV only showed few base changes, mainly clustered in R and U5. The LTR from a moderately leukemogenic passaged BL/VL3 RadLV had conserved the C-rich sequence and acquired a 43-base-pair direct repeat in U3 and several other point mutations, small insertions, and deletions scattered in U3, R, and U5. All cloned primary RadLVs were fibrotropic, and some were weakly leukemogenic. All cloned BL/VL3 RadLVs were thymotropic and nonfibrotropic. The block of their replication was found to be after the synthesis of unintegrated linear and supercoiled viral DNA. Most of the BL/VL3 RadLVs were moderately leukemogenic, and one (V-13) was highly leukemogenic, being as virulent as the Moloney strain. We propose a model for the emergence of the RadLV variants and show that the virus responsible for the high leukemogenic potential of BL/VL3 preparation is a nondefective, ecotropic, lymphotropic, nonfibrotropic, unique retrovirus which most likely arose from a parental primary RadLV similar to those studied here.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declève A., Lieberman M., Ihle J. N., Rosenthal P. N., Lung M. L., Kaplan H. S. Physicochemical, biological and serological properties of a leukemogenic virus isolated from cultured RadLV-induced lymphomas of C57BL/Ka mice. Virology. 1978 Oct 1;90(1):23–35. doi: 10.1016/0042-6822(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Declève A., Sato C., Lieberman M., Kaplan H. S. Selective thymic localization of murine leukemia virus-related antigens in C57BL-Ka mice after inoculation with radiation virus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3124–3128. doi: 10.1073/pnas.71.8.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. Mapping the viral sequences conferring leukemogenicity and disease specificity in Moloney and amphotropic murine leukemia viruses. J Virol. 1984 Nov;52(2):448–456. doi: 10.1128/jvi.52.2.448-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. The tandem direct repeats within the long terminal repeat of murine leukemia viruses are the primary determinant of their leukemogenic potential. J Virol. 1984 Dec;52(3):945–952. doi: 10.1128/jvi.52.3.945-952.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Jolicoeur P. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4203–4207. doi: 10.1073/pnas.80.14.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Villemur R., Jolicoeur P. The high leukemogenic potential of Gross passage A murine leukemia virus maps in the region of the genome corresponding to the long terminal repeat and to the 3' end of env. J Virol. 1983 Jul;47(1):24–32. doi: 10.1128/jvi.47.1.24-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T., LaPorte P., Esty A. Nucleotide sequence studies of polyoma DNA. The Hpa II 3/5 junction to the Hpa II 4/Hae III 18 junction, encoding the origin of DNA replication and the 5' end of the early region. J Biol Chem. 1978 Sep 25;253(18):6561–6567. [PubMed] [Google Scholar]
- Grymes R. A., Scott M. L., Kim J. P., Fry K. E., Kaplan H. S. Molecular studies of the radiation leukemia virus (RadLV) and related retroviruses of C57BL/ka mice. Prog Nucleic Acid Res Mol Biol. 1983;29:53–73. doi: 10.1016/s0079-6603(08)60431-6. [DOI] [PubMed] [Google Scholar]
- Haas M. Transient virus expression during murine leukemia induction by X-irradiation. J Natl Cancer Inst. 1977 Feb;58(2):251–257. doi: 10.1093/jnci/58.2.251. [DOI] [PubMed] [Google Scholar]
- Haran-Ghera N. Leukemogenic activity of centrifugates from irradiated mouse thymus and bone marrow. Int J Cancer. 1966 Jan;1(1):81–87. doi: 10.1002/ijc.2910010111. [DOI] [PubMed] [Google Scholar]
- Haran-Ghera N., Peled A. Induction of leukemia in mice by irradiation and radiation leukemia virus variants. Adv Cancer Res. 1979;30:45–87. doi: 10.1016/s0065-230x(08)60894-5. [DOI] [PubMed] [Google Scholar]
- Herr W., Gilbert W. Somatically acquired recombinant murine leukemia proviruses in thymic leukemias of AKR/J mice. J Virol. 1983 Apr;46(1):70–82. doi: 10.1128/jvi.46.1.70-82.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. At least four viral genes contribute to the leukemogenicity of murine retrovirus MCF 247 in AKR mice. J Virol. 1985 Jan;53(1):158–165. doi: 10.1128/jvi.53.1.158-165.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Wozney J., Chatis P. A., Hopkins N., Hartley J. W. Construction of recombinants between molecular clones of murine retrovirus MCF 247 and Akv: determinant of an in vitro host range property that maps in the long terminal repeat. J Virol. 1985 Jan;53(1):152–157. doi: 10.1128/jvi.53.1.152-157.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Joseph D. R., Pazmino N. H. Radiation leukemia in C57BL/6 mice. II. Lack of ecotropic virus expression in the majority of lymphomas. J Exp Med. 1976 Dec 1;144(6):1406–1423. doi: 10.1084/jem.144.6.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., McEwan R., Bengali K. Radiation leukemia in C57BL/6 mice. I. Lack of serological evidence for the role of endogenous ecotropic viruses in pathogenesis. J Exp Med. 1976 Dec 1;144(6):1391–1405. doi: 10.1084/jem.144.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski M., Merregaert J., Boniver J., Maisin J. R. Proviral genome of radiation leukemia virus: molecular cloning of biologically active proviral DNA and nucleotide sequence of its long terminal repeat. J Virol. 1985 Jul;55(1):251–255. doi: 10.1128/jvi.55.1.251-255.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., DesGroseillers L. Neurotropic Cas-BR-E murine leukemia virus harbors several determinants of leukemogenicity mapping in different regions of the genome. J Virol. 1985 Nov;56(2):639–643. doi: 10.1128/jvi.56.2.639-643.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Rassart E. Effect of Fv-1 gene product on synthesis of linear and supercoiled viral DNA in cells infected with murine leukemia virus. J Virol. 1980 Jan;33(1):183–195. doi: 10.1128/jvi.33.1.183-195.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Rassart E., Sankar-Mistry P. Strong selection for cells containing new ecotropic recombinant murine leukemia virus provirus after propagation of C57BL/6 radiation-induced thymoma cells in vitro or in vivo. Mol Cell Biol. 1983 Sep;3(9):1675–1679. doi: 10.1128/mcb.3.9.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN H. S. THE ROLE OF RADIATION ON EXPERIMENTAL LEUKEMOGENESIS. Natl Cancer Inst Monogr. 1964 May;14:207–220. [PubMed] [Google Scholar]
- Kaplan H. S. On the natural history of the murine leukemias: presidential address. Cancer Res. 1967 Aug;27(8):1325–1340. [PubMed] [Google Scholar]
- Khan A. S., Martin M. A. Endogenous murine leukemia proviral long terminal repeats contain a unique 190-base-pair insert. Proc Natl Acad Sci U S A. 1983 May;80(9):2699–2703. doi: 10.1073/pnas.80.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. P., Kaplan H. S., Fry K. E. Characterization of an infective molecular clone of the B-tropic, ecotropic BL/Ka(B) murine retrovirus genome. J Virol. 1982 Oct;44(1):217–225. doi: 10.1128/jvi.44.1.217-225.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATARJET R., DUPLAN J. F. Experiment and discussion on leukaemogenesis by cell-free extracts of radiation-induced leukaemia in mice. Int J Radiat Biol Relat Stud Phys Chem Med. 1962 Aug;5:339–344. doi: 10.1080/09553006214550911. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN M., KAPLAN H. S. Leukemogenic activity of filtrates from radiation-induced lymphoid tumors of mice. Science. 1959 Aug 14;130(3372):387–388. doi: 10.1126/science.130.3372.387. [DOI] [PubMed] [Google Scholar]
- Lenz J., Celander D., Crowther R. L., Patarca R., Perkins D. W., Haseltine W. A. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. 1984 Mar 29-Apr 4Nature. 308(5958):467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J. S-tropic murine type-C viruses: frequency of isolation from continuous cell lines, leukemia virus preparations and normal spleens. Int J Cancer. 1974 May 15;13(5):587–598. doi: 10.1002/ijc.2910130503. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Declève A., Kaplan H. S. Rapid in vitro assay for thymotropic, leukemogenic murine C-type RNA viruses. Virology. 1978 Oct 15;90(2):274–278. doi: 10.1016/0042-6822(78)90311-2. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Declève A., Ricciardi-Castagnoli P., Boniver J., Finn O. J., Kaplan H. S. Establishment, characterization and virus expression of cell lines derived from radiation- and virus-induced lymphomas of C57BL/Ka mice. Int J Cancer. 1979 Aug;24(2):168–177. doi: 10.1002/ijc.2910240208. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Niwa O., Declève A., Kaplan H. S. Continuous propagation of radiation leukemia virus on a C57BL mouse-embryo fibroblast line, with attenuation of leukemogenic activity. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1250–1253. doi: 10.1073/pnas.70.4.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manteuil-Brutlag S., Liu S. L., Kaplan H. S. Radiation leukemia virus contains two distinct viral RNAs. Cell. 1980 Mar;19(3):643–652. doi: 10.1016/s0092-8674(80)80041-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Oliff A., McKinney M. D., Agranovsky O. Contribution of the gag and pol sequences to the leukemogenicity of Friend murine leukemia virus. J Virol. 1985 Jun;54(3):864–868. doi: 10.1128/jvi.54.3.864-868.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Ruscetti S. A 2.4-kilobase-pair fragment of the Friend murine leukemia virus genome contains the sequences responsible for friend murine leukemia virus-induced erythroleukemia. J Virol. 1983 Jun;46(3):718–725. doi: 10.1128/jvi.46.3.718-725.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Signorelli K., Collins L. The envelope gene and long terminal repeat sequences contribute to the pathogenic phenotype of helper-independent Friend viruses. J Virol. 1984 Sep;51(3):788–794. doi: 10.1128/jvi.51.3.788-794.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. L., Sass B., Stephenson J. R., Al-Ghazzouli I. K., Hino S., Donahoe R. M., Kende M., Aaronson S. A., KElloff G. J. Immunoprevention of x-ray-induced leukemias in the C57BL mouse. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1697–1701. doi: 10.1073/pnas.74.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassart E., DesGroseillers L., Jolicoeur P. Molecular cloning of B- and N-tropic endogenous BALB/c murine leukemia virus circular DNA intermediates: isolation and characterization of infectious recombinant clones. J Virol. 1981 Jul;39(1):162–171. doi: 10.1128/jvi.39.1.162-171.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassart E., Sankar-Mistry P., Lemay G., DesGroseillers L., Jolicoeur P. New class of leukemogenic ecotropic recombinant murine leukemia virus isolated from radiation-induced thymomas of C57BL/6 mice. J Virol. 1983 Feb;45(2):565–575. doi: 10.1128/jvi.45.2.565-575.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sankar-Mistry P., Jolicoeur P. Frequent isolation of ecotropic murine leukemia virus after x-ray irradiation of C57BL/6 mice and establishment of producer lymphoid cell lines from radiation-induced lymphomas. J Virol. 1980 Jul;35(1):270–275. doi: 10.1128/jvi.35.1.270-275.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert E., Old L. J. Preleukemic expression of TL antigens in x-irradiated C57BL/6 mice. J Exp Med. 1977 Jul 1;146(1):271–276. doi: 10.1084/jem.146.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]