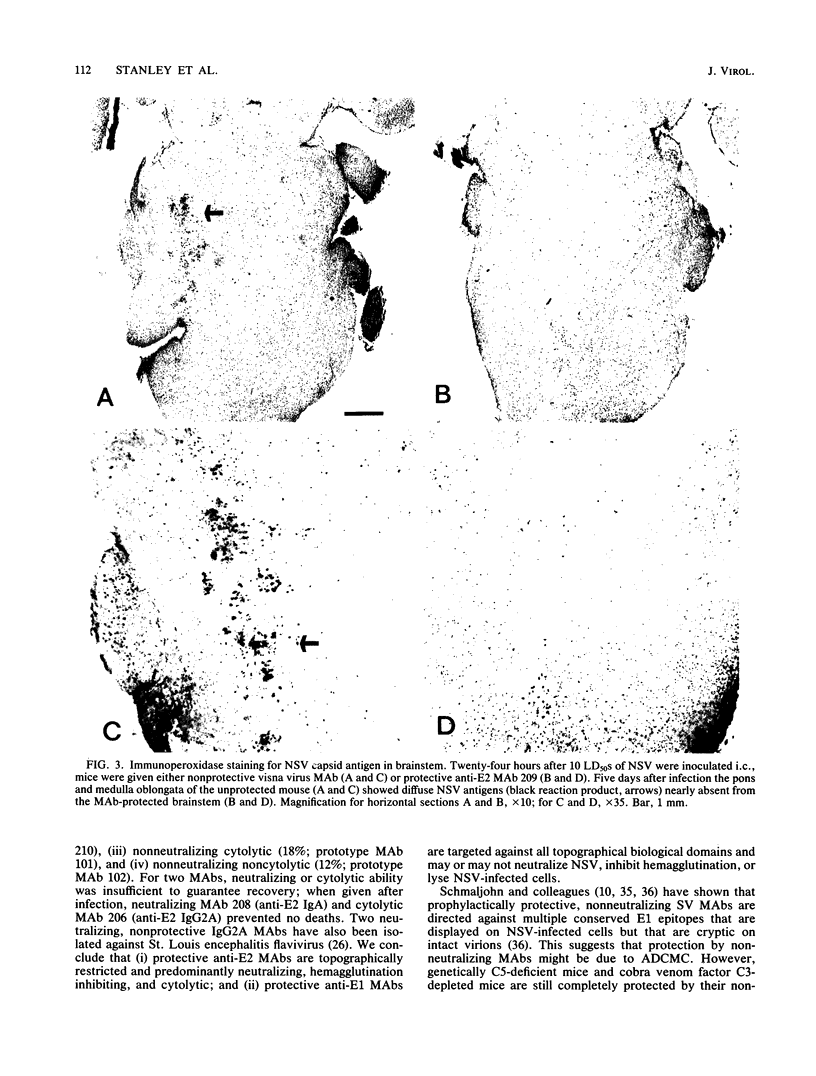
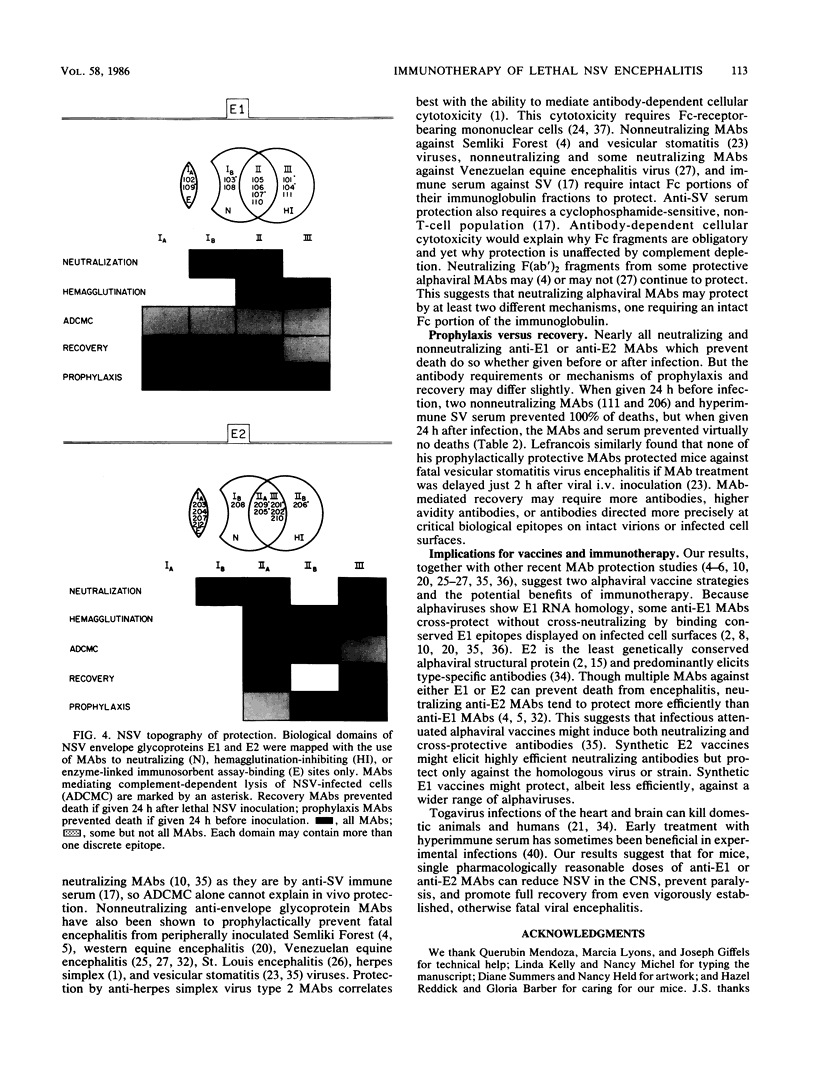
Abstract
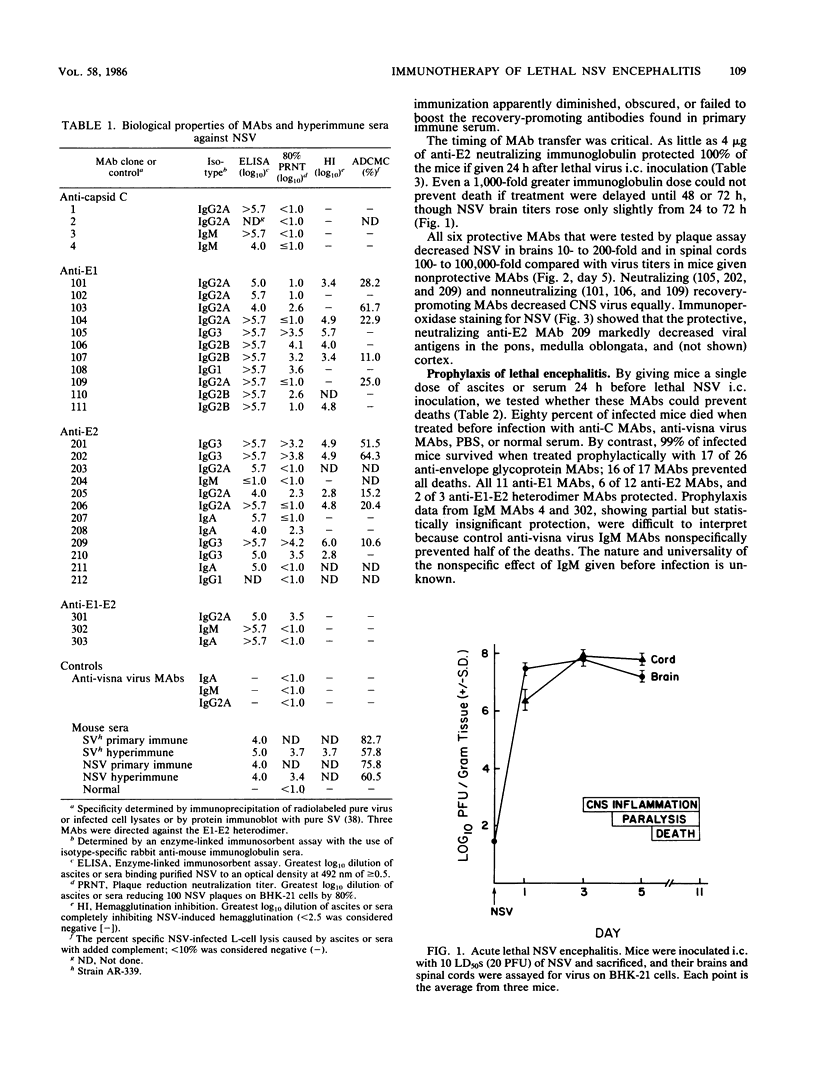
Neuroadapted Sindbis virus (NSV) causes acute encephalitis and paralyzes and kills adult mice unless they are treated with primary immune serum after infection. To study the nature and specificity of curative antibodies, we gave mice 30 different monoclonal antibodies (MAbs) against Sindbis virus (SV) 24 h after lethal intracerebral inoculation of NSV. By the time of MAb treatment, NSV replication in the brain had been well established (7.5 X 10(7) PFU/g). Seventeen MAbs directed against multiple biological domains on the NSV E1 and E2 envelope glycoproteins prevented paralysis and death. Anticapsid MAbs failed to protect. Altogether, 15 of 17 curative MAbs either neutralized NSV infectivity or lysed NSV-infected cells with complement, but neither ability was necessary or sufficient to guarantee recovery. All 5 protective anti-E2 MAbs neutralized NSV infectivity; 6 of 10 protective anti-E1 MAbs neutralized NSV; 4 did not. Plaque assay or immunohistochemical staining showed that neutralizing and nonneutralizing curative MAbs decreased NSV in the brain, brainstem, and spinal cord. Despite high neutralization titers, hyperimmune anti-SV and anti-NSV mouse sera prevented only 6 and 30% of deaths, respectively, while primary immune sera prevented 50 (SV) and 90% (NSV) of deaths. Secondary intravenous immunization with a live virus apparently diminished, obscured, or failed to boost a class of protective antibodies. When separate mouse groups were given these 30 MAbs 24 h before lethal intracerebral inoculation of NSV, a slightly different set of 17 neutralizing or nonneutralizing anti-E1 and anti-E2 antibodies protected. Two nonneutralizing MAbs and hyperimmune anti-SV serum, which had failed to promote recovery, prophylactically protected 100% of the mice. The antibody requirements or mechanisms of prophylaxis and recovery may differ.
Full text
PDF

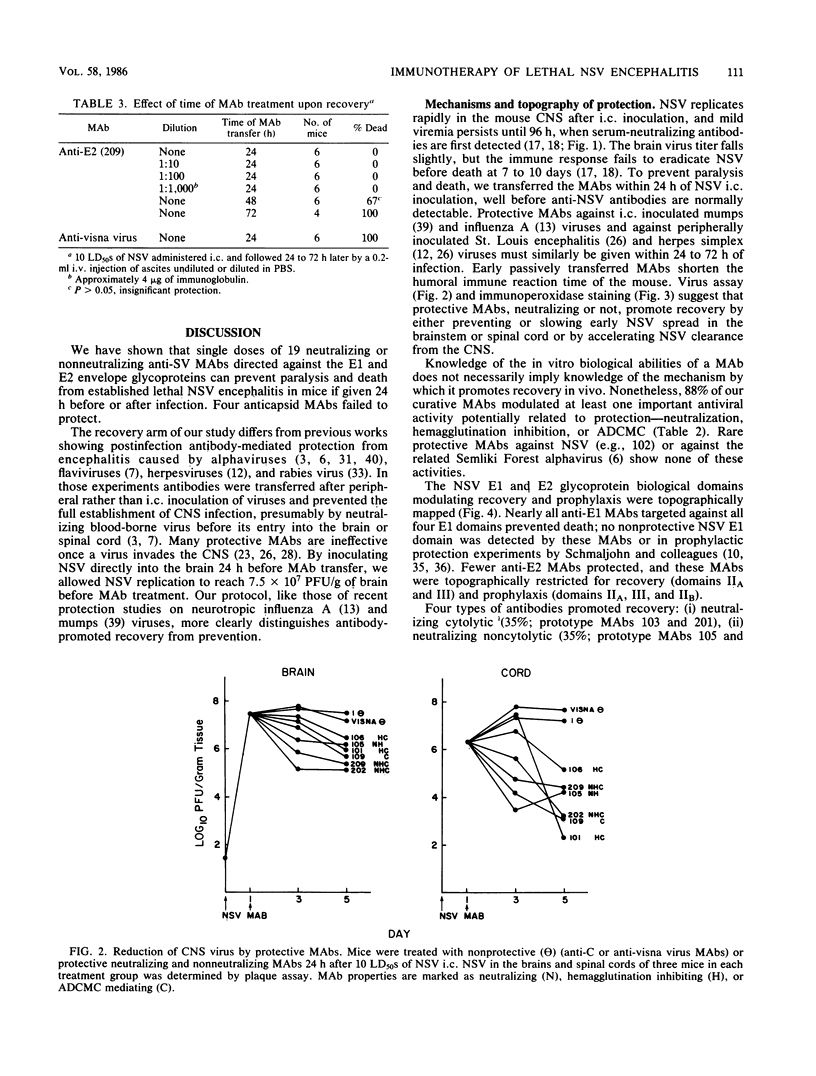


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGE T. O., GLEISER C. A., GOCHENOUR W. S., Jr, MIESSE M. L., TIGERTT W. D. Studies on the virus of Venezuelan equine encephalomyelitis. II. Modification by specific immune serum of response of central nervous system of mice. J Immunol. 1961 Nov;87:509–517. [PubMed] [Google Scholar]
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. R., Kinney R. M., Trent D. W., Strauss E. G., Strauss J. H. An evolutionary tree relating eight alphaviruses, based on amino-terminal sequences of their glycoproteins. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4702–4706. doi: 10.1073/pnas.81.15.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boere W. A., Benaissa-Trouw B. J., Harmsen M., Kraaijeveld C. A., Snippe H. Neutralizing and non-neutralizing monoclonal antibodies to the E2 glycoprotein of Semliki Forest virus can protect mice from lethal encephalitis. J Gen Virol. 1983 Jun;64(Pt 6):1405–1408. doi: 10.1099/0022-1317-64-6-1405. [DOI] [PubMed] [Google Scholar]
- Boere W. A., Benaissa-Trouw B. J., Harmsen T., Erich T., Kraaijeveld C. A., Snippe H. Mechanisms of monoclonal antibody-mediated protection against virulent Semliki Forest virus. J Virol. 1985 May;54(2):546–551. doi: 10.1128/jvi.54.2.546-551.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boere W. A., Harmsen T., Vinjé J., Benaissa-Trouw B. J., Kraaijeveld C. A., Snippe H. Identification of distinct antigenic determinants on Semliki Forest virus by using monoclonal antibodies with different antiviral activities. J Virol. 1984 Nov;52(2):575–582. doi: 10.1128/jvi.52.2.575-582.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASALS J. RELATIONSHIPS AMONG ARTHROPOD-BORNE ANIMAL VIRUSES DETERMINED BY CROSS-CHALLENGE TESTS. Am J Trop Med Hyg. 1963 Jul;12:587–596. doi: 10.4269/ajtmh.1963.12.587. [DOI] [PubMed] [Google Scholar]
- Camenga D. L., Nathanson N., Cole G. A. Cyclophosphamide-potentiated West Nile viral encephalitis: relative influence of cellular and humoral factors. J Infect Dis. 1974 Dec;130(6):634–641. doi: 10.1093/infdis/130.6.634. [DOI] [PubMed] [Google Scholar]
- Chanas A. C., Gould E. A., Clegg J. C., Varma M. G. Monoclonal antibodies to Sindbis virus glycoprotein E1 can neutralize, enhance infectivity, and independently inhibit haemagglutination or haemolysis. J Gen Virol. 1982 Jan;58(Pt 1):37–46. doi: 10.1099/0022-1317-58-1-37. [DOI] [PubMed] [Google Scholar]
- Dalrymple J. M., Schlesinger S., Russell P. K. Antigenic characterization of two sindbis envelope glycoproteins separated by isoelectric focusing. Virology. 1976 Jan;69(1):93–103. doi: 10.1016/0042-6822(76)90197-5. [DOI] [PubMed] [Google Scholar]
- Dix R. D., Pereira L., Baringer J. R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun. 1981 Oct;34(1):192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Gerhard W. Breakdown of the blood--cerebrospinal fluid barrier to immunoglobulin in mice injected intracerebrally with a neurotropic influenza A virus. Post-exposure treatment with monoclonal antibody promotes recovery. J Neuroimmunol. 1981 Sep;1(3):227–237. doi: 10.1016/0165-5728(81)90027-8. [DOI] [PubMed] [Google Scholar]
- Edelman R., Ascher M. S., Oster C. N., Ramsburg H. H., Cole F. E., Eddy G. A. Evaluation in humans of a new, inactivated vaccine for Venezuelan equine encephalitis virus (C-84). J Infect Dis. 1979 Nov;140(5):708–715. doi: 10.1093/infdis/140.5.708. [DOI] [PubMed] [Google Scholar]
- Garoff H., Kondor-Koch C., Riedel H. Structure and assembly of alphaviruses. Curr Top Microbiol Immunol. 1982;99:1–50. doi: 10.1007/978-3-642-68528-6_1. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Moench T. R., Narayan O., Griffin D. E. Selection of a fixative for identifying T cell subsets, B cells, and macrophages in paraffin-embedded mouse spleen. J Immunol Methods. 1983 Dec 16;65(1-2):137–145. doi: 10.1016/0022-1759(83)90310-1. [DOI] [PubMed] [Google Scholar]
- Griffin D. E., Johnson R. T. Role of the immune response in recovery from Sindbis virus encephalitis in mice. J Immunol. 1977 Mar;118(3):1070–1075. [PubMed] [Google Scholar]
- Hirsch R. L., Griffin D. E., Johnson R. T. Interactions between immune cells and antibody in protection from fatal Sindbis virus encephalitis. Infect Immun. 1979 Feb;23(2):320–324. doi: 10.1128/iai.23.2.320-324.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hunt A. R., Roehrig J. T. Biochemical and biological characteristics of epitopes on the E1 glycoprotein of western equine encephalitis virus. Virology. 1985 Apr 30;142(2):334–346. doi: 10.1016/0042-6822(85)90342-3. [DOI] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois L. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J Virol. 1984 Jul;51(1):208–214. doi: 10.1128/jvi.51.1.208-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlan R. I., Burns W. H., White D. O. Two cytotoxic cells in peritoneal cavity of virus-infected mice: antibody-dependent macrophages and nonspecific killer cells. J Immunol. 1977 Nov;119(5):1569–1574. [PubMed] [Google Scholar]
- Mathews J. H., Roehrig J. T. Determination of the protective epitopes on the glycoproteins of Venezuelan equine encephalomyelitis virus by passive transfer of monoclonal antibodies. J Immunol. 1982 Dec;129(6):2763–2767. [PubMed] [Google Scholar]
- Mathews J. H., Roehrig J. T. Elucidation of the topography and determination of the protective epitopes on the E glycoprotein of Saint Louis encephalitis virus by passive transfer with monoclonal antibodies. J Immunol. 1984 Mar;132(3):1533–1537. [PubMed] [Google Scholar]
- Mathews J. H., Roehrig J. T., Trent D. W. Role of complement and the Fc portion of immunoglobulin G in immunity to Venezuelan equine encephalomyelitis virus infection with glycoprotein-specific monoclonal antibodies. J Virol. 1985 Sep;55(3):594–600. doi: 10.1128/jvi.55.3.594-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendall R. R., Klassen T., Baringer J. R. Host defenses in herpes simplex infections of the nervous system: effect of antibody on disease and viral spread. Infect Immun. 1979 Feb;23(2):305–311. doi: 10.1128/iai.23.2.305-311.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench T. R., Griffin D. E. Immunocytochemical identification and quantitation of the mononuclear cells in the cerebrospinal fluid, meninges, and brain during acute viral meningoencephalitis. J Exp Med. 1984 Jan 1;159(1):77–88. doi: 10.1084/jem.159.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted R. A., Baric R. S., Sawyer B. A., Johnston R. E. Sindbis virus mutants selected for rapid growth in cell culture display attenuated virulence in animals. Science. 1984 Jul 27;225(4660):424–427. doi: 10.1126/science.6204381. [DOI] [PubMed] [Google Scholar]
- Rabinowitz S. G., Adler W. H. Host defenses during primary Venezuelan equine encephalomyelitis virus infection in mice. I. Passive transfer of protection with immune serum and immune cells. J Immunol. 1973 May;110(5):1345–1353. [PubMed] [Google Scholar]
- Roehrig J. T., Day J. W., Kinney R. M. Antigenic analysis of the surface glycoproteins of a Venezuelan equine encephalomyelitis virus (TC-83) using monoclonal antibodies. Virology. 1982 Apr 30;118(2):269–278. doi: 10.1016/0042-6822(82)90346-4. [DOI] [PubMed] [Google Scholar]
- SCHINDLER R. Studies on the pathogenesis of rabies. Bull World Health Organ. 1961;25:119–126. [PMC free article] [PubMed] [Google Scholar]
- Schmaljohn A. L., Johnson E. D., Dalrymple J. M., Cole G. A. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982 May 6;297(5861):70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- Schmaljohn A. L., Kokubun K. M., Cole G. A. Protective monoclonal antibodies define maturational and pH-dependent antigenic changes in Sindbis virus E1 glycoprotein. Virology. 1983 Oct 15;130(1):144–154. doi: 10.1016/0042-6822(83)90124-1. [DOI] [PubMed] [Google Scholar]
- Sissons J. G., Oldstone M. B. Antibody-mediated destruction of virus-infected cells. Adv Immunol. 1980;29:209–260. doi: 10.1016/S0065-2776(08)60045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Cooper S. J., Griffin D. E. Alphavirus neurovirulence: monoclonal antibodies discriminating wild-type from neuroadapted Sindbis virus. J Virol. 1985 Oct;56(1):110–119. doi: 10.1128/jvi.56.1.110-119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky J. S., Waxham M. N., Server A. C. Protective effects of glycoprotein-specific monoclonal antibodies on the course of experimental mumps virus meningoencephalitis. J Virol. 1985 Mar;53(3):727–734. doi: 10.1128/jvi.53.3.727-734.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]