Abstract
Background and purpose:
GPR119 is a G protein-coupled receptor that is preferentially expressed in islet cells and mediates insulin secretion. Oleoyl-lysophosphatidylcholine and oleoylethanolamide (OEA) act as endogenous ligands for this receptor, whereas PSN375963 and PSN632408 are two recently reported synthetic agonists. In this study, we explored mechanisms underlying GPR119-induced insulin secretion. In addition, we assessed the potential utility of the synthetic agonists as tools for exploring GPR119 biology.
Experimental approach:
We examined natural and synthetic GPR119 agonist activity at GPR119 in MIN6c4 and RINm5f insulinoma cells. We evaluated insulin secretion, intracellular calcium [Ca2+]i, ion channel involvement and levels of cAMP.
Key results:
We report that increases in insulin secretion induced by OEA were associated with increased cAMP and a potentiation of glucose-stimulated increases in [Ca2+]i. We also demonstrate that ATP-sensitive K+ and voltage-dependent calcium channels were required for GPR119-mediated increases in glucose-stimulated insulin secretion. In contrast to OEA, the synthetic GPR119 agonist PSN375963 and PSN632408 have divergent effects on insulin secretion, cAMP and intracellular calcium in MIN6c4 cells.
Conclusions and implications:
The endogenous ligand OEA signals through GPR119 in a manner similar to glucagon-like peptide-1 (GLP-1) and its receptor with respect to insulin secretion, [Ca2+]i and cAMP. In addition, PSN375963 and PSN632408 substantially differ from OEA and from one another. These studies suggest that the commercially available synthetic agonists, although they do activate GPR119, may also activate GPR119-independent pathways and are thus unsuitable as GPR119-specific pharmacological tools.
Keywords: GPR119, GLP-1, oleoylethanolamide, agonists, insulin secretion, calcium flux, cAMP
Introduction
In the last decade, an increasing number of unliganded G protein-coupled receptors, so-called orphan receptors, with unknown function have been identified, and they present new opportunities for drug discovery. GPR119 is one such orphan receptor, first identified through bioinformatics methods and cloned in 2003 (Fredriksson et al., 2003). Although GPR119 clearly belongs to the family of rhodopsin-like G protein-coupled receptors, it exhibits little overall sequence homology to other receptors. Further distinguishing features of this receptor are the localization of its gene on the X-chromosome and its relatively narrow expression pattern. GPR119 expression is restricted largely to pancreatic islets, although lesser amounts of message are detected in the human gastrointestinal tract and in the rodent brain (Soga et al., 2005; Overton et al., 2006).
Recently, oleoyl-lysophosphatidylcholine (OLPC) and oleoylethanolamide (OEA) were identified as endogenous ligands for GPR119 (Soga et al., 2005; Overton et al., 2006). OLPC was reported to bind to GPR119, causing increased intracellular cAMP accumulation and an increase in glucose-stimulated insulin secretion (GSIS) in NIT-1 insulinoma cells. OEA, a lipid-signalling agent previously shown to be a ligand for peroxisome proliferator-activated receptor (PPAR)-α that suppresses food intake (Fu et al., 2003), is also reported to be an endogenous ligand for GPR119 (Overton et al., 2006). This report also revealed two synthetic GPR119 agonists, PSN375963 and PSN632408, that increased intracellular cAMP in a GPR119-dependent manner. Although the effects of OEA or the synthetic agonists on insulin secretion were not reported, Overton et al. (2006) showed that PSN632408 or OEA could suppress feeding to a similar extent. These findings led the authors to suggest that suppression of feeding by OEA might be mediated through GPR119. More recently, Chu et al. (2007) reported a potent synthetic agonist for GPR119-designated AR231453. They demonstrated that this compound increased intracellular cAMP and insulin secretion in a GPR119-dependent manner, but did not explore other signalling events. Finally, AR231453 was shown to have in vivo effects, improving oral glucose tolerance in wild-type, but not in GPR119 knockout mice. The effects of AR231453 on feeding were not reported.
A growing body of evidence supports an important function for GPR119 in regulating metabolic responses. GPR119 appears similar to the glucagon-like peptide-1 receptor (GLP-1R) in that they are both Gs-coupled receptors and activation of either receptor leads to an increase in GSIS (Soga et al., 2005; Drucker, 2006). In addition, activation of GPR119 in the intestine leads to increased GLP-1 and glucose-dependent insulinotropic polypeptide secretion (Chu et al., 2008). This makes GPR119 of particular interest, as a drug target similar to small molecule agonists of GLP-1R have so far not been identified. Compared with GLP-1R, however, we still know relatively little about the function of GPR119. In particular, the signalling events (beyond cAMP) that lead to GPR119-mediated increases in GSIS have not been explored.
Here, we have examined the endogenous ligand, OEA, and provided further characterization of the GPR119 signalling pathways leading to insulin secretion. In addition, we provide some comparisons between GPR119 signalling and the well-characterized signalling of GLP-1Rs. We show that the endogenous GPR119 ligand, OEA, potentiates glucose-stimulated increases in intracellular calcium [Ca2+]i and cAMP in a manner similar to that of GLP-1, and that the ATP-sensitive K+ (KATP) and the voltage-dependent calcium (VDC) channels involved in GLP-1R-mediated insulin secretion are also required for GPR119-induced insulin release. In contrast, two reported synthetic agonists PSN375963 and PSN632408 appear to have divergent effects on [Ca2+]i, cAMP and insulin secretion that distinguish them from the endogenous GPR119 ligand OEA and would appear to make them unsuitable as GPR119-specific research tools.
Materials and methods
Insulinoma cell culture
Mouse MIN6c4 insulinoma cells (a subclone of Min6 cells selected for high insulin secretion) were licensed from Professor Jun-ichi Miyazaki (Osaka University Medical School, Osaka, Japan). MIN6c4 cells were cultured in Dulbecco's modified Eagle's medium containing 15% (v/v) heat-inactivated foetal bovine serum 4.5 g L−1 glucose, 50 μM 2-mercaptoethanol, 100 U mL−1 penicillin. Cells were incubated in a humidified atmosphere at 37 °C with 5% CO2.
Rat RINm5f insulinoma cells were maintained in RPMI 1640 medium containing 2 mM L-glutamine, 1.5 g L−1 sodium bicarbonate, 4.5 g L−1 glucose, 10 mM HEPES, 1.0 mM sodium pyruvate and 10% FBS. For cells stably expressing human GPR119, RINm5f cells were transfected with 5 mg of human-GPR119 cDNA (pCDNA3.1) using Nucleofector Solution V and an Amaxa Nucleofector II (Amaxa Biosystems, Germany). Transfected cells were then selected and maintained in culture media with 500 mg mL−1 geneticin. Stable clones were obtained by limiting dilution and the expression of human-GPR119 in RINm5f cells was confirmed by quantitative PCR using an ABI 7700 instrument (Perkin-Elmer, Waltham, MA, USA). The clone expressing the highest levels of human GPR119 mRNA was selected from among ten candidates and used for the insulin secretion assays.
Measurement of insulin release
Measurement of insulin release was carried out in HEPES–Krebs–Ringer bicarbonate buffer (KRBH, containing CaCl2, 1.26 mM; KCl, 5.4 mM; KH2PO4, 0.44 mM; MgCl2 6H2O, 0.5 mM; MgSO4 7H2O, 0.4 mM; NaCl, 0.14 M; NaHCO3, 4.1 mM; Na2HPO4, 0.34 mM; HEPES, 20 mM; pH 7.0) without BSA, which can bind to OEA and OLPC and decrease the free concentration of ligand availability. MIN6c4 cells were plated in 96-well plate (3 × 104 cells per well) for 3 days (70–80% confluence). On the day of experiment, the cell culture medium was aspirated and the plate was washed twice with KRBH buffer. Cells were rested at 37 °C for 30 min in KRBH containing 2.8 mM glucose. Compounds were dissolved in either 2.8 or 16 mM glucose and added to cells for 2 h before collecting supernatant for insulin measurements. In some experiments, nitrendipine or diazoxide (in KRBH) were added after the 30 min rest period and incubated for 10 min before the addition of other compounds. Insulin concentration in the supernatant was determined using a mouse insulin assay kit from Meso Scale Diagnostics (Gaithersburg, MD, USA). Data are expressed as percentage of control with control concentrations noted in the figure legends.
Calcium assays
MIN6c4 cells were seeded into poly-D-lysine-coated 384-well plates at 8 × 103 cells per well and cultured at 37 °C for 2 or 3 days (90% confluence). On the day of experiment, cells were loaded for 1 h at 37 °C with 1 μM BD dye (BD Biosciences, Franklin Lakes, NJ, USA) in KRBH buffer solution containing 2.8 mM glucose, 10 mM HEPES, 1 mM CaCl2, 1.25 mM probenecid. The plate was cooled to room temperature and compounds were added. Changes in intracellular calcium content were monitored for up to 20 min using a Fluorometric Imaging Plate Reader (FLIPR, Molecular Devices, Sunnyvale, CA, USA) (Schroeder, 1996). Occasionally, minor changes in calcium were observed after addition of either vehicle or compound. These presumably resulted from mechanical stimulation or displacement of cells following pipetting by the FLIPR. Changes in calcium are only discussed if they were reproducibly observed.
Intracellular cAMP assays
MIN6c4 cells were seeded into poly-D-lysine-coated 96-well plates at 30 × 103 cells per well and cultured at 37 °C for 2 or 3 days (90% confluence). On the day of experiments, cells were incubated in KRBH (without BSA) with 2.8 mM glucose for 30 min. Cells were subsequently brought to 2.8 mM and 16 mM glucose with the addition of the indicated concentrations of forskolin, GLP-1, OEA, PSN632408 and PSN375963. The cAMP levels were measured after 2 h incubation by cAMP EIA kits (Cayman Chemical Co., Ann Arbor, MI, USA), then normalized to the protein concentration in each well. Three replicates were measured for each treatment and data are presented as percentage of the control release in 2.8 mM glucose or 16 mM glucose (mean±s.e.mean).
Gene expression analysis
Total RNA from RINm5f, RINm5f-GPR119 and MIN6c4 were extracted utilizing the Ultraspec RNA isolation kit (Biotecx, Houston, TX, USA). cDNA was generated by reverse transcription using random hexamers (Promega, Madison, WI, USA) and oligo-dT primers (Invitrogen Life Techno;logies, Carlsbad, CA, USA). Quantitative real-time reverse transcriptase PCR analysis (referred to as TaqMan) was performed on an ABI 7700 sequence-detection instrument following manufacturer's instructions. For TaqMan analysis, 25 ng of cDNA was used together with primers at a final concentration of 0.9 μM, and carboxyfluorescein labelled diagnostic probe at a final concentration of 0.25 μM. Ribosomal 18S RNA primers and probe (PE Applied Biosystems, Foster City, CA, USA) were used as an internal control. The sequences of 5′-primer, 3′-primer and the probe were as follows: Gpr119 (NM_181751): cttctactgtgacatgctcaagattg, ccatggctcctgcatgttc, ccatggctcctgcatgttc; data were analysed using Sequence Detection Systems software Version 1.7.
Data analysis
Data are reported as mean±s.e.mean. For the assays of insulin release, statistical analysis was performed using one-way ANOVA, with Bonferroni post-tests. *P<0.05; **P<0.01. Data from cAMP assays were compared using Student's t-test implemented in GraphPad Prism 4.0 (GraphPad Software Inc., La Jolla, CA, USA).
Materials
Glucagon-like peptide-1 (7–37), nitrendipine and diazoxide were obtained from Sigma. OLPC, OEA, PSN375963 and PSN632408 were obtained from Cayman Chemical Company. MSD mouse insulin assay kit was obtained from Meso Scale Diagnostic, LLC (Gaithersburg, MD, USA). The calcium assay kit used in conjunction with FLIPR instrumentation (see below) was obtained from BD Biosciences.
Results
Effect of GPR119 agonists on insulin release in RINm5f and RINm5f-GPR119 cell lines
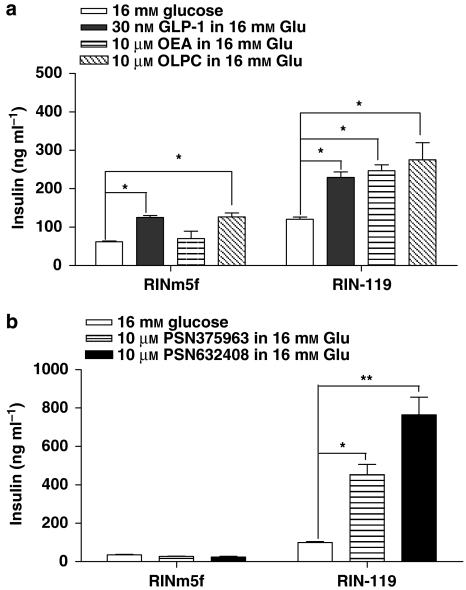
To confirm the specificity of the endogenous and synthetic GPR119 agonists, we utilized the rat RINm5f insulinoma cell line. Although insulin secretion from RINm5f cells is not glucose sensitive (Poitout et al., 1996; McClenaghan and Flatt, 1999), they do not express GPR119 endogenously (Supplementary Figure 1) and provide a good background to assess GPR119-specific signalling. We found that parental RINm5f cells secreted insulin in response to GLP-1 (positive control) and OLPC, but not to OEA (Figure 1a). In contrast, RINm5f cells transfected with human GPR119 (RIN-119 cells) responded to OEA and had an increased response to OLPC (Figure 1a), whereas the response to GLP-1 was unaffected. Similarly, the two commercially available synthetic GPR119 agonists PSN275963 and PSN632408 did not increase insulin secretion in the parental RINm5f but did increase insulin secretion in RIN-119 cells (Figure 1b). These data suggest that all four agonists do indeed signal through GPR119 in a selective manner, although OLPC appears to act through GPR119-independent mechanisms as well. For this reason, OLPC was not used in subsequent studies.
Figure 1.
GPR119 agonists increase insulin secretion in RIN cell lines. (a and b) The RINm5f and RIN-119 cells were stimulated by 16 mM glucose, with or without the presence of 30 nM glucagon-like peptide-1, 10 μM oleoylethanolamide, 10 μM oleoyl-lysophosphatidylcholine, 10 μM PSN375963 and PSN632408 as indicated. The insulin levels from the triplicates were measured for each treatment and data were presented as ng insulin per ml (mean±s.e.mean). *P<0.05; **P<0.01 using ANOVA-Bonferroni, compared with insulin induced by 16 mM glucose alone in the same cell line.
Effect of GPR119 agonists on intracellular cAMP accumulation
GPR119 and GLP-1R are both Gs-coupled receptors and activate intracellular adenylate cyclase. To compare agonist activation of GPR119 with that of GLP-1R, intracellular cAMP levels were explored in non-insulinoma cells (HEK293 and HEK293-GPR119) and insulinoma MIN6c4 cells. MIN6c4 cell line is a subclone of the commonly employed Min6 cell line and was selected for glucose responsiveness and high insulin production. This MIN6 subclone also shows a smaller fold increase in GLP-1-stimulated insulin secretion (Professor J Miyazaki, Osaka University Medical School, Osaka, Japan, personal communication).
Forskolin, used as a positive control, generated a large increase in cAMP in MIN6c4 cells under both high and low glucose conditions. As expected, GLP-1 also induced a significant increase in cAMP in both high and low glucose conditions (Table 1). OEA induced a much lower increase in cAMP in MIN6c4 cells compared with GLP-1 and only in 16 mM glucose. PSN632408 was similar to OEA in this respect. In contrast, PSN375963 suppressed cAMP production in MIN6c4 cells in both high and low glucose (Table 1). In contrast to MIN6c4 cells, both PSN632408 and PSN375963 increased cAMP in a concentration-dependent manner in HEK293 cells overexpressed with GPR119 but not in untransfected HEK293 cells (Supplementary Figure 2). These data suggest that PSN375963, although increasing cAMP through GPR119 in HEK293-GPR119 cells, may suppress cAMP through another mechanism, leading to the net decrease observed in MIN6c4 cells.
Table 1.
cAMP levels in MIN6c4 cells treated with forskolin, GLP-1, OEA, PSN632408 and PSN375963 in presence of 2.8 and 16 mM glucose
| Treatment |
In the presence of 2.8 mM glucose |
In the presence of 16 mM glucose |
||
|---|---|---|---|---|
| Mean±s.e.mean (% of 2.8 mM glucose control) | P-value (vs 2.8 mM glucose) | Mean±s.e.mean (% of 16 mM glucose control) | P-value (vs 16 mM glucose) | |
| Forskolin (40 μM) | 980±116 | 0.002 | 1291±203 | 0.004 |
| GLP-1 (50 nM) | 144±9 | 0.007 | 446±69 | 0.007 |
| OEA (3.3 μM) | 98±10 | 0.87 | 142±13 | 0.027 |
| OEA (10 μM) | 104±5 | 0.5 | 121±6 | 0.026 |
| PSN632408 (3.3 μM) | 85±7 | 0.1 | 161±17 | 0.02 |
| PSN632408 (10 μM) | 112±19 | 0.56 | 141±5 | 0.001 |
| PSN375963 (3.3 μM) | 66±2 | 0.0001 | 87±10 | 0.23 |
| PSN375963 (10 μM) | 62±8 | 0.009 | 64±7 | 0.006 |
Abbreviations: GLP-1, glucagon-like peptide-1;KRBH, HEPES–Krebs–Ringer bicarbonate buffer; OEA, oleoylethanolamide.
MIN6c4 cells in 96-well plates were incubated in KRBH with 2.8 mM glucose for 30 min. Cells were subsequently made up to 2.8 and 16 mM glucose with the addition of the indicated concentrations of forskolin, GLP-1, OEA, PSN632408 and PSN375963. The cAMP levels were measured after 2 h incubation by cAMP EIA kit (Cayman Chemical Co.), then normalized to the protein concentration in each well. Three replicates were measured for every treatment and data are presented as percentage of the level in 2.8 mM glucose or in 16 mM glucose (mean±s.e.mean). The cAMP level induced by 2.8 mM glucose was 739±11.2 pmol mg−1 protein; the cAMP level induced by 16 mM glucose was 963±7.9 pmol mg−1 protein. Data were analysed using Student's t-test implemented in GraphPad Prism 4.0 (GraphPad Software Inc.).
Effect of endogenous GPR119 ligand OEA on insulin secretion and calcium signalling
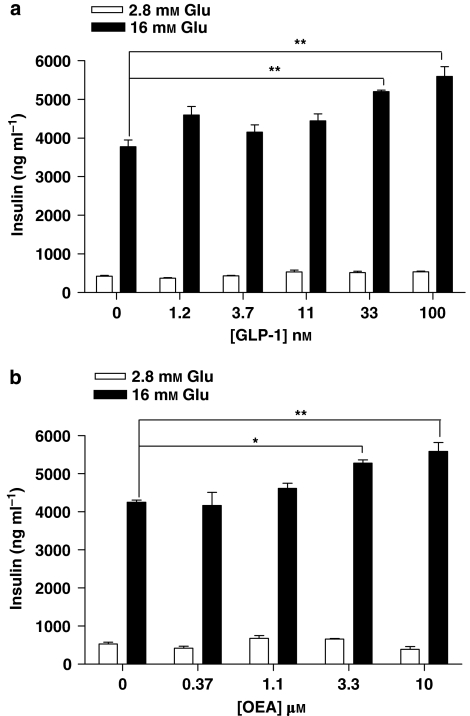
As mentioned above, insulin secretion by the RINm5f cell line is not glucose sensitive. In addition, secreted insulin levels in RINm5f are relatively low. Therefore, to investigate the effects of GPR119 on glucose-dependent signalling, we used MIN6c4 cells, which express GPR119 endogenously and are glucose sensitive (Overton et al., 2006). The signalling of endogenous ligands in 2.8 mM (low) or 16 mM (high) glucose was examined first. Consistent with previous reports (Drucker, 2006), the GLP-1-stimulated insulin secretion from MIN6c4 cells was dependent on high extracellular glucose (Figure 2a). Likewise, OEA also increased insulin secretion in MIN6c4 in a glucose-dependent manner (Figure 2b).
Figure 2.
Glucagon-like peptide-1 (GLP-1) and oleoylethanolamide (OEA) increase glucose-stimulated insulin secretion. MIN6c4 cells were stimulated with the indicated concentrations of (a) GLP-1, (b) OEA, in the presence of 2.8 mM glucose or 16 mM glucose. The insulin levels were measured after 2 h incubation. Three replicates were measured for every treatment and data presented as ng insulin ml−1 (mean±s.e.). *P<0.05; **P<0.01 using ANOVA-Bonferroni, compared with insulin induced by glucose alone.
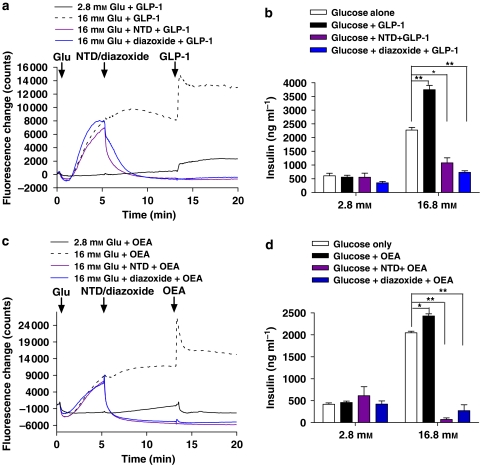
Glucagon-like peptide-1 binding to GLP-1R induces an increase in [Ca2+]i through a mechanism that involves cAMP and the depolarization of the cell through the KATP channel and subsequent opening of VDC channels (Light et al., 2002; Tsuboi et al., 2003). GPR119 signalling also increases cAMP (Soga et al., 2005; Overton et al., 2006) and induces GSIS; however, the function of KATP and VDC channels in GPR119-induced insulin secretion has not been demonstrated. MIN6c4 cells were either maintained in 2.8 mM glucose or stimulated with 16 mM glucose, then treated with the KATP channel activator diazoxide (Ohno-Shosaku and Yamamoto, 1992; Chan et al., 2001) or the VDC channel inhibitor nitrendipine (Ganesan et al., 1992; Zawalich and Zawalich, 1992), followed by the addition of OEA or GLP-1. The resulting changes in [Ca2+]i and insulin secretion were then measured.
As shown in Figure 3a, MIN6c4 exhibit a sustained increase in [Ca2+]i after stimulation with 16 mM glucose. The addition of 10 nM GLP-1 further potentiates this increase in [Ca2+]i. Treatment of the cells with either 5 μM nitrendipine or 200 μM diazoxide resulted in a rapid reduction of the glucose-mediated calcium influx that GLP-1 was unable to rescue (Figure 3a). GLP-1 potentiation of GSIS paralleled the effects on calcium and was almost completely inhibited by either nitrendipine or diazoxide (Figure 3b). OEA was similar to GLP-1 in its effects on [Ca2+]i and insulin release (Figures 3c and d). OEA at 10 μM increased 16 mM glucose-stimulated [Ca2+]i and was unable to overcome the inhibition by nitrendipine or diazoxide (Figure 3c). Similarly, OEA increased GSIS and the addition of nitrendipine or diazoxide dramatically reduced insulin secretion (Figure 3d).
Figure 3.
Glucagon-like peptide-1 (GLP-1)- and oleoylethanolamide (OEA)-stimulated increases in insulin secretion are dependent on ATP-sensitive K+ (KATP) and voltage-dependent calcium channels. (a) MIN6c4 cells rested in HEPES–Krebs–Ringer bicarbonate buffer (KRBH) with 2.8 mM glucose for 30 min. Cells were then alternatively maintained in 2.8 mM glucose and GLP-1 (10 nM) added or brought to 16 mM glucose followed as indicated by GLP-1, nitrendipine (NTD, 5 μM) and then GLP-1 or diazoxide (200 μM) then GLP-1. Additions occurred at the times indicated by the arrows and [Ca2+]i was continuously monitored. (b) MIN6c4 cells were rested in KRBH with 2.8 mM glucose for 30 min. Then in the presence of 2.8 mM or 16 mM glucose (indicated), insulin secretion was stimulated with GLP-1 alone (10 nM), GLP-1 plus nitrendipine (NTD, 5 μM) or GLP-1 plus diazoxide (200 μM) were added. Nitrendipine and diazoxide were added 10 min before GLP-1 and insulin release was measured 2 h later. Each treatment was performed in triplicate and data were presented as ng insulin per ml culture supernatant (mean±s.e.mean). (c) is identical to (a) with the exception that OEA (10 μM) is used in place of GLP-1. (d) is identical to B with the exception that OEA (10 μM) is used in place of GLP-1. *P<0.05; **P<0.01 using ANOVA-Bonferroni, compared with insulin induced by glucose alone.
Effect of PSN375963 and PSN632408 on insulin secretion and calcium signalling in MIN6c4 cells
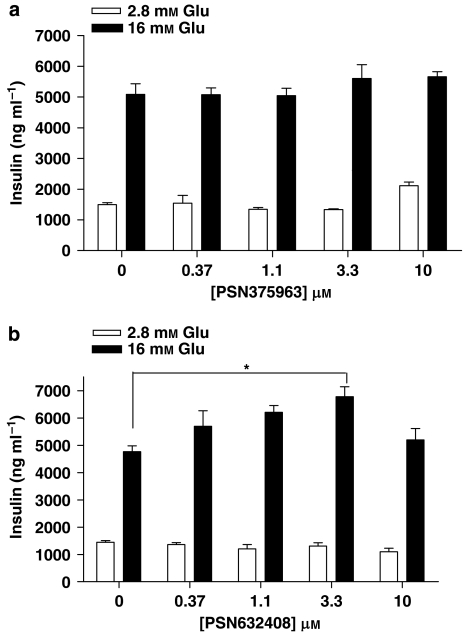
To compare the commercially available synthetic GPR119 agonists with OEA, concentration–response experiments, using PSN375963 and PSN632408 in GSIS, were performed. Surprisingly, PSN375963 had no clear demonstrable effect on insulin secretion in the presence of either 2.8 or 16 mM glucose (Figure 4a). In contrast to PSN375963, PSN632408 increased insulin release in a glucose-dependent manner. PSN632408 does not alter insulin secretion in the presence of 2.8 mM glucose (Figure 4b). In the presence of 16 mM glucose, PSN632408 showed a bell-shaped concentration-dependent effect on GSIS. At 1.1 and 3.3 μM, PSN632408 increased insulin by 25 and 39%, respectively, but at 10 μM, it had no significant effect (Figure 4b).
Figure 4.
PSN375963 and PSN632408 vary in their ability to augment glucose-stimulated insulin secretion in MIN6c4 cells. (a) MIN6c4 cells were stimulated with the indicated concentrations of PSN375963 in the presence of 2.8 mM glucose or 16 mM glucose (b) MIN6c4 cells were stimulated with the indicated concentrations of PSN632408 in the presence of 2.8 mM glucose or 16 mM glucose. For all experiments, the insulin levels were measured after 2 h incubation. Each treatment was performed in triplicate and data were presented as ng insulin per ml of culture supernatant (mean±s.e.mean). *P<0.05; using ANOVA-Bonferroni, compared with insulin induced by glucose alone.
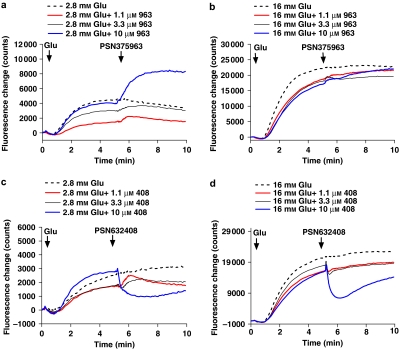
As before, we also examined calcium signalling with the synthetic GPR119 agonists and the effect of diazoxide and nitrendipine on calcium and insulin secretion in MIN6c4 cells. Unlike GLP-1 or OEA, PSN375963 induces an increase in [Ca2+]i in MIN6c4 cells in the presence of 2.8 mM glucose (Figure 5a), but did not potentiate the increase in [Ca2+]i induced by 16 mM glucose (Figure 5b). In contrast, PSN632408 showed the opposite effect and inhibited [Ca2+]i at both 2.8 and 16 mM glucose (Figures 5c and d).
Figure 5.
PSN375963 and PSN632408 have opposite effects on [Ca2+]i in MIN6c4 cells. MIN6c4 cells were incubated in HEPES–Krebs–Ringer bicarbonate buffer with 2.8 mM glucose for 30 min. Cells were subsequently maintained at 2.8 mM glucose or brought to 16 mM glucose followed by the addition of the indicated concentrations of PSN375963 (a and b) or PSN632408 (c and d). [Ca2+]i was continuously monitored by FLIPR.
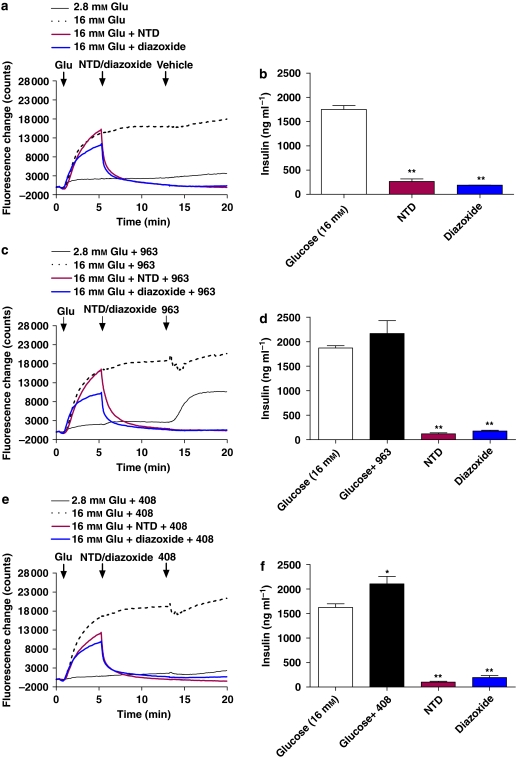
The function of the KATP and VDC channels in mediating the effects of the synthetic agonists on insulin secretion was also investigated. A concentration of 3.3 μM was used for both compounds, as PSN632408 was able to enhance GSIS at this concentration, but did not inhibit [Ca2+]i. Glucose alone (without KATP or VDC channel blockers) was used as a control. As expected, diazoxide and nitrendipine both inhibit the glucose-stimulated increases in [Ca2+]i and insulin secretion (Figures 6a and b). As observed earlier, 3.3 μM PSN375963 increases [Ca2+]i in 2.8 mM glucose but not in 16 mM glucose (Figure 6c). Similarly, no increase in GSIS was observed with PSN375963, and it did not affect either nitrendipine or diazoxide inhibition of GSIS (Figure 6d). PSN632408 at 3.3 μM had no effect on [Ca2+]i at either 2.8 or 16 mM glucose (Figure 6e), whereas PSN632408 did significantly increase GSIS, but it did not alter diazoxide or nitrendipine inhibition of GSIS (Figure 6f).
Figure 6.
PSN375963 and PSN632408 effects are ATP-sensitive K+ and voltage-dependent calcium channel dependent. (a and b) MIN6c4 cells were rested in HEPES–Krebs–Ringer bicarbonate buffer with 2.8 mM glucose for 30 min after which they were stimulated with 16 mM glucose alone, nitrendipine (5 μM) followed by 16 mM glucose, or diazoxide (200 μM) followed by 16 mM glucose as indicated. Channel blockers were pre-incubated with cells for 10 min before stimulation with glucose. Calcium signal was monitored by FLIPR (a) and insulin secretion was measured after 2 h (b). Each point was determined in triplicate. Data are presented as ng insulin per ml of culture supernatant (mean±s.e.mean). **P<0.01; using ANOVA-Bonferroni, compared with insulin induced by glucose alone. (c and e) are identical to (a), with the exception that PSN375963 (3.3 μM; c) or PSN632408 (3.3 μM; e) were added at the indicated time points. (d and f) Min6c4 cells were treated as in (a) and insulin secretion in response to 16 mM glucose alone, glucose plus PSN375963 (3.3 μM; d) or glucose plus PSN632408 (3.3 μM; f) was measured. Insulin secretion in response to glucose plus compound was also assessed in the presence of nitrendipine (5 μM) or diazoxide (200 μM) as indicated. Insulin secretion was measured after 2 h and each point was determined in triplicate. *P<0.05, **P<0.01; using ANOVA-Bonferroni, compared with insulin induced by glucose alone.
Discussion
The ability of GPR119 activation to enhance GSIS has been previously demonstrated (Chu et al., 2007, 2008); however, the subsequent signals leading to insulin secretion have not been reported. In this study, we have examined some of the mechanisms underlying insulin release mediated by the GPR119 ligand OEA and two recently reported synthetic agonists, PSN375963 and PSN632408 (Overton et al., 2006). We show here that GPR119 signalling by the endogenous ligand is similar in many ways to that mediated by GLP-1R, including modulation of [Ca2+]i, production of cAMP and the dependence of insulin release on KATP and VDC channels. The synthetic agonists PSN375963 and PSN632408, however, have divergent effects on calcium signalling, cAMP and insulin secretion in MIN6c4 cells, which suggests that they may not be appropriate tools to study GPR119-specific functions.
Among the GPR119 agonists examined in this study, OEA appears the closest to GLP-1 in terms of its effects on GSIS from MIN6c4 cells as well as in the associated signalling events. In MIN6c4 insulinoma cells, OEA and GLP-1 both increase insulin secretion in a glucose-dependent manner. Consistent with this observation, both OEA and GLP-1 potentiate the increased [Ca2+]i observed in response to 16 mM glucose, whereas [Ca2+]i in 2.8 mM glucose is unaffected. In addition, they both increase cAMP content in Min6c4 cells; however, OEA stimulates intracellular cAMP production only in the presence of 16 mM glucose, whereas GLP-1 produces a more robust increase in cAMP and does so in both low and high glucose. This may simply reflect the relative potency of the ligands or, alternatively, the characteristics of their respective receptors. More potent and selective agonists for GPR119 should aid in determining if this is a receptor- or ligand-based difference.
We also find that the GPR119-mediated increase of insulin secretion from MIN6c4 cells, stimulated by the endogenous ligand OEA requires KATP and VDC channels. Incubation of MIN6c4 cells with diazoxide or nitrendipine not only inhibits the increase in [Ca2+]i induced by glucose but also significantly blocks insulin secretion. The addition of GPR119 ligands (either endogenous or synthetic) is unable to overcome this inhibition. This is similar to the previously reported findings that GLP-1 mediates an increase in [Ca2+]i through VDC channels (Light et al., 2002; Drucker, 2006).
In contrast to OEA, OLPC induces increased [Ca2+]i and increased insulin secretion in MIN6c4 cells in both 2.8 and 16 mM glucose (Supplemental Figure 3). The most likely explanation for this is that OLPC signals in both a GPR119-dependent and GPR119-independent manner. This hypothesis is supported by the fact that OLPC increases insulin secretion in RINm5f cells (which do not express GPR119) but has a greater effect in RINm5f-GPR119 cells (Figure 1).
Although OEA appears to be more selective for GPR119 in our studies, the data we have do not rule out the possibility that OEA may also act independently of GPR119 in other cells or tissues. Certainly, OEA is a known PPAR-α agonist (Fu et al., 2003); however, OEA does not increase insulin secretion from RINm5f unless they are transfected with GPR119. It is also unlikely that a PPAR-α-mediated event would be evident in the short time frame of the insulin secretion assay.
Overall, our study shows that, to the extent that we have examined the question, activation of GPR119 signalling by the endogenous ligands is similar to that of GLP-1-stimulated GLP-1R signalling. The endogenous ligand OEA binding to GPR119 leads to increased intracellular cAMP accumulation (Table 1 and Overton et al., 2006) and, as we have shown, enhances [Ca2+]i and insulin secretion in a glucose-dependent manner. The effect of OEA on calcium and insulin is also dependent on KATP and VDC channels.
Similar to the endogenous ligands, the synthetic GPR119 agonists PSN375963 and PSN632408 both increase insulin secretion from RINm5f-GPR119 cells but not from the parental RINm5f cell line. This suggests that these compounds are indeed selective agonists for GPR119. This conclusion is supported further by the fact that both of these compounds increase intracellular cAMP in GPR119-transfected HEK293 cells but not in untransfected control cells (Supplementary Figure 2; Overton et al., 2006). Despite being a GPR119 agonist, PSN375963 had no significant effect on insulin secretion in MIN6c4 cells except at 10 μM in 2.8 mM glucose (Figure 4a). Consistent with this observation, 10 μM PSN375963 increased [Ca2+]i in the presence of 2.8 mM glucose, but had minimal or no effect at lower concentrations (Figure 5a). In 16 mM glucose, the addition of PSN375963 had no effect at any concentration (Figure 5b). Given our previous observation that PSN375963 increased cAMP in GPR119 transfected cells, we were surprised to see that PSN375963 inhibited cAMP production in MIN6c4 cells. The inhibition of cAMP occurred in both low and high glucose conditions; however, the effect in high glucose was significant only at 10 μM PSN375963 (Table 1). This may be the result of PSN375963 acting at another receptor in MIN6c4 cells that is not present in HEK293 cells to activate a Gi-coupled pathway.
The other commercially available agonist, PSN632408, had no effect on insulin secretion or [Ca2+]i in 2.8 mM glucose, but did increase MIN6c4 cell insulin secretion in the presence of 16 mM glucose. The effect on GSIS observed in MIN6c4 cells showed a bell-shaped response, with a peak at 3.3 μM PSN632408 (Figure 5b). PSN632408 also increased cAMP in MIN6c4 cells, although similar to OEA, this effect was observed only in the presence of 16 mM glucose (Table 1). The loss of enhanced GSIS at 10 uM PSN632408 may be the result of the inhibition of the glucose-stimulated increase in [Ca2+]i that is observed at higher concentrations of PSN632408 (Figures 5c and d). This effect could act to counter the enhancement of GSIS that might otherwise be observed. As with the effects of PSN375963 on cAMP, it is not clear if the effects of PSN632408 on calcium in the MIN6c4 cells is mediated through GPR119 or if it represents an off-target effect of the compound. Given the relatively low potency of these compounds (PSN632408 EC50=1.9 μM; PSN375963 EC50=13.9 μM in HEK293-GPR119 cAMP), off-target effects would not be unexpected. Attempts to address this issue by inhibiting GPR119 expression in MIN6c4 cells with RNAi were unsuccessful, and RINm5f cells are unresponsive to glucose. Thus, at present, we lack a suitable cell-based means for examining this question. The most direct way to assess this might be to compare the response of islets from GPR119−/− mice with those of wild-type littermates. Arguing in favour of an off-target effect, OEA, at 10 μM did not inhibit [Ca2+]i and also increased both cAMP and GSIS, despite having reported EC50 in cAMP assays comparable with those of the synthetic ligands (Overton et al., 2006). These findings are against the use of either PSN375963 or PSN632408 as tools to examine GPR119-specific function. Although AR231453 is not commercially available, the data published by Chu et al. (2007) suggest that it may be a more suitable tool.
Oleoylethanolamide as a ligand for GPR119 is of particular interest, as it has been reported to be a peripherally acting agent that suppresses food intake and reduces body weight gain in rodent-feeding models (Fu et al., 2003; Nielsen et al., 2004; Proulx et al., 2005). OEA has been shown to activate PPAR-α (Fu et al., 2003; Guzman et al., 2004; Astarita et al., 2006), and OEA inhibition of feeding requires the presence of PPAR-α (Desvergne and Wahli, 1999; Fu et al., 2003). The recent paper by Overton et al (2006) suggested that OEA's effects on feeding were mediated by GPR119. The basis for this claim is that PSN632408 inhibited feeding in orally dosed rats. Our data here suggest that caution should be used in interpreting these results, as PSN632408 shows unexpected activities at higher concentrations. Nonetheless, it is still possible that stimulation of GPR119 might alter food intake in rats. Rats have higher relative expression of GPR119 in the brain than mice or humans (JA Hedrick, unpublished data). It cannot be ruled out that signalling through GPR119 in the rat brain might modulate feeding in these animals. GPR119 is also expressed in the intestinal tract, and stimulation of GPR119 in this tissue could in turn stimulate the release of a secondary signal (for example, GLP-1 and glucose-dependent insulinotropic polypeptide) that inhibits feeding (Soga et al., 2005). Indeed, recent data from Chu et al. (2008) suggest this is the case. In this regard, it is of interest to note that we detect mRNA expression of GPR119 in the GLUTag mouse intestinal L cell line as well as in the human line NCI-H716 cells (JA Hedrick, unpublished data). Further exploration into the potential function of GPR119 in controlling food intake will be an interesting avenue of future investigation.
Although this study improves our understanding of the signalling mechanisms that underlie the physiological functions of GPR119, our knowledge of GPR119 biology remains incomplete and additional studies on the mechanism of action of GPR119 are clearly warranted. We have suggested here that GPR119 signalling shares some properties in common with GLP-1R signalling that leads us to further questions. For example, it has been shown that GLP-1R signalling can augment β cell mass in rodents via activation of β cell proliferation and neogenesis (Li et al., 2003). Does GPR119 also have a function in β cell survival and apoptosis? If so, how similar are these effects to those of GLP-1? Moreover, the GLP-1R ligands, GLP-1 and glucose-dependent insulinotropic polypeptide exert actions well beyond the β cell, and the function of these peptides in peripheral organs such as adipose tissue, the brain and the heart is receiving increasing attention. Similarly, how important is GPR119 signalling in other tissues (for example, intestine) and what effects might such signalling have? The availability of high-potency, GPR119-selective synthetic agonists in the future should assist in answering some of these questions. However, it will be important to consider the function of the endogenous ligands as we attempt to comprehend better the function of GPR119 in regulating insulin secretion.
Supplementary Material
Acknowledgments
We acknowledge Drs Timothy Kowalski, Michael Graziano and Marvin Bayne for support and helpful discussion. Dr Yun Ning is a Post-Doctoral Fellow at the Schering-Plough Research Institute.
Abbreviations
- GLP-1
glucagon-like peptide-1
- GSIS
glucose stimulated insulin secretion
- KATP
ATP-sensitive K+
- KRBH
HEPES–Krebs–Ringer bicarbonate buffer
- OEA
oleoylethanolamide
- OLPC
oleoyl-lysophophatidylcholine
- PPAR
peroxisome proliferator-activated receptor
- VDC
voltage-dependent calcium channel
Conflict of interest
All authors are employed by Schering-Plough Corporation.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Astarita G, Di Giacomo B, Gaetani S, Oveisi F, Compton TR, Rivara S, et al. Pharmacological characterization of hydrolysis-resistant analogs of oleoylethanolamide with potent anorexiant properties. J Pharmacol Exp Ther. 2006;318:563–570. doi: 10.1124/jpet.106.105221. [DOI] [PubMed] [Google Scholar]
- Chan SL, Mourtada M, Morgan NG. Characterization of a KATP channel-independent pathway involved in potentiation of insulin secretion by efaroxan. Diabetes. 2001;50:340–347. doi: 10.2337/diabetes.50.2.340. [DOI] [PubMed] [Google Scholar]
- Chu ZL, Carroll C, Alfonso J, Gutierrez V, He H, Lucman A, et al. A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like peptide-1 and glucose-dependent insulinotropic peptide release. Endocrinology. 2008;149:2038–2047. doi: 10.1210/en.2007-0966. [DOI] [PubMed] [Google Scholar]
- Chu ZL, Jones RM, He H, Carroll C, Gutierrez V, Lucman A, et al. A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology. 2007;148:2601–2609. doi: 10.1210/en.2006-1608. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Hoglund PJ, Gloriam DE, Lagerstrom MC, Schioth HB. Seven evolutionarily conserved human rhodopsin G protein-coupled receptors lacking close relatives. FEBS Lett. 2003;554:381–388. doi: 10.1016/s0014-5793(03)01196-7. [DOI] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- Ganesan S, Calle R, Zawalich K, Greenawalt K, Zawalich W, Shulman GI, et al. Immunocytochemical localization of alpha-protein kinase C in rat pancreatic beta-cells during glucose-induced insulin secretion. J Cell Biol. 1992;119:313–324. doi: 10.1083/jcb.119.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman M, Lo Verme J, Fu J, Oveisi F, Blazquez C, Piomelli D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha) J Biol Chem. 2004;279:27849–27854. doi: 10.1074/jbc.M404087200. [DOI] [PubMed] [Google Scholar]
- Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem. 2003;278:471–478. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- Light PE, Manning Fox JE, Riedel MJ, Wheeler MB. Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol Endocrinol. 2002;16:2135–2144. doi: 10.1210/me.2002-0084. [DOI] [PubMed] [Google Scholar]
- McClenaghan NH, Flatt PR. Engineering cultured insulin-secreting pancreatic B-cell lines. J Mol Med. 1999;77:235–243. doi: 10.1007/s001090050344. [DOI] [PubMed] [Google Scholar]
- Nielsen MJ, Petersen G, Astrup A, Hansen HS. Food intake is inhibited by oral oleoylethanolamide. J Lipid Res. 2004;45:1027–1029. doi: 10.1194/jlr.C300008-JLR200. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Yamamoto C. Identification of an ATP-sensitive K+ channel in rat cultured cortical neurons. Pflugers Arch. 1992;422:260–266. doi: 10.1007/BF00376211. [DOI] [PubMed] [Google Scholar]
- Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Poitout V, Olson LK, Robertson RP. Insulin-secreting cell lines: classification, characteristics and potential applications. Diabetes Metab. 1996;22:7–14. [PubMed] [Google Scholar]
- Proulx K, Cota D, Castaneda TR, Tschop MH, D'Alessio DA, Tso P, et al. Mechanisms of oleoylethanolamide-induced changes in feeding behavior and motor activity. Am J Physiol Regul Integr Comp Physiol. 2005;289:R729–R737. doi: 10.1152/ajpregu.00029.2005. [DOI] [PubMed] [Google Scholar]
- Schroeder KS, Neagle BD. FLIPR: a new instrument for accurate, high throughput optical screening. J Biomol Screen. 1996;1:75–80. [Google Scholar]
- Soga T, Ohishi T, Matsui T, Saito T, Matsumoto M, Takasaki J, et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem Biophys Res Commun. 2005;326:744–751. doi: 10.1016/j.bbrc.2004.11.120. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, da Silva Xavier G, Holz GG, Jouaville LS, Thomas AP, Rutter GA. Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 beta-cells. Biochem J. 2003;369:287–299. doi: 10.1042/BJ20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawalich WS, Zawalich KC. Biochemical mechanisms involved in monomethyl succinate-induced insulin secretion. Endocrinology. 1992;131:649–654. doi: 10.1210/endo.131.2.1322278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.