Abstract
Colorectal cancer is the fourth most common non-cutaneous malignancy in the United States and the second most frequent cause of cancer-related death. Over the past 12 years, significant progress has been made in the systemic treatment of this malignant condition. Six new chemotherapeutic agents have been introduced, increasing median overall survival for patients with metastatic colorectal cancer from less than 9 months with no treatment to approximately 24 months. For patients with stage III (lymph node positive) colon cancer, an overall survival benefit for fluorouracil-based chemotherapy has been firmly established, and recent data have shown further efficacy for the inclusion of oxaliplatin in such adjuvant treatment programs. For patients with stage II colon cancer, the use of adjuvant chemotherapy remains controversial, but may be appropriate in a subset of individuals at higher risk for disease recurrence. Ongoing randomized clinical trials are evaluating how best to combine currently available therapies, while smaller studies are evaluating new agents, with the goal of continued progress in prolonging life among patients with metastatic colorectal cancer and increasing cure rates among those with resectable disease.
Keywords: Colon cancer, chemotherapy, targeted therapy
I. Introduction
Colorectal cancer is the fourth most common non-cutaneous malignancy in the United States and the second most frequent cause of cancer-related death. In 2007, an estimated 153,760 cases of colorectal cancer were diagnosed and 52,180 people died from this disease1. Significant progress in the treatment of colorectal cancer has been achieved over the past twelve years, with the approval of six new therapeutic agents in the United States (Table 1). These compounds have greatly improved the outlook for patients diagnosed with resectable and metastatic disease. The current review focuses on advances in the systemic therapy of colorectal cancer.
Table 1.
New Chemotherapeutic Agents in the Systemic Treatment of Colon Cancer
| Drug | Current Indications* | |||
|---|---|---|---|---|
| Metastatic Disease | FDA-approval Date | Adjuvant Therapy | FDA-approval Date | |
| Irinotecan (Camptosar®) | Yes | Jun 1996 | No | - |
| Capecitabine (Xeloda®) | Yes | Apr 2001 | Yes | Jun 2005 |
| Oxaliplatin (Eloxatin®) | Yes | Aug 2002 | Yes | Nov 2004 |
| Cetuximab (Erbitux®)# | Yes | Feb 2004 | No | - |
| Bevacizumab (Avastin®) | Yes | Feb 2004 | No | - |
| Panitumumab (Vectibix®)# | Yes | Sept 2006 | No | - |
U.S. Food and Drug Administration (FDA) data accessed at www.accessdata.fda.gov
Approved for use in patients with tumors that express the epidermal growth factor receptor
II. Staging and Prognosis
Pathologic stage represents the most important prognostic factor for patients with colorectal cancer. The tumor-node-metastasis (TNM) system, as defined by the American Joint Committee on Cancer (AJCC), is the most commonly used staging system and is based on depth of invasion of the bowel wall, extent of regional lymph node involvement, and presence of distant sites of disease (Table 2)2–4. The depth of tumor invasion defines the T stage and increases from T1 (invasion of the submusosa) to T4 (invasion into the serosa or adjacent structures). As the depth of tumor invasion increases, the risk for nodal and distant spread also grows. Pathologic review of surrounding lymph nodes defines the three N categories: N0 (no lymph nodes involved), N1 (1–3 lymph nodes involved), and N2 (greater than 3 lymph nodes involved). Current guidelines recommend the identification of 12 or more lymph nodes in the resected specimen5, 6, as the examination of fewer regional lymph nodes has been linked with poorer outcome in patients with node-negative and node-positive disease7–11. The examination of fewer lymph nodes may reflect a less complete operative procedure or an inadequate inspection of the pathologic specimen, mistakenly leading to “understaging” of the tumor and the subsequent omission of beneficial adjuvant therapy.
Table 2.
| Primary tumor (T) | ||
| Tx | Primary tumor cannot be assessed | |
| Tis | Carcinoma in situ | |
| T1 | Tumor invades submucosa | |
| T2 | Tumor invades muscularis propia | |
| T3 | Tumor invades through the muscularis propria into the subserosa | |
| T4 | Tumor directly invades other organs or structures, or perforates visceral Peritoneum | |
| Regional lymph nodes (N) | ||
| Nx | Regional lymph nodes cannot be assessed | |
| N0 | No regional lymph node metastases | |
| N1 | Metastases in one to three regional lymph nodes | |
| N2 | Metastases in four or more regional lymph nodes | |
| Distant metastases (M) | ||
| Mx | Presence or absence of distant metastases cannot be determined | |
| M0 | No distant metastases detected | |
| M1 | Distant metastases detected | |
| Stage Grouping and Five-year Survival | ||
| Stage | TNM classification | Five-year survival |
| I | T1–2, N0, M0 | > 90 % |
| IIA | T3, N0, M0 | 80–85% |
| IIB | T4, N0, M0 | 70–80% |
| IIIA | T1–2, N1, M0 | 65–80 % |
| IIIB | T3–4, N1, M0 | 50–65 % |
| IIIC | T1–4, N2, M0 | 25–50 % |
| IV | T1–4, N0–2, M1 | 5–8 % |
In patients with resectable colorectal cancer, several other pathologic and clinical features have been identified that are associated with an increased risk for tumor recurrence. These include poorly differentiated histology, lymphovascular invasion, perineural invasion, T4 tumor penetration, bowel perforation, clinical bowel obstruction, and an elevated preoperative plasma level of carcinoembryonic antigen (CEA)12–16. In contrast, hospitals and surgeons with higher patient volume have been associated with improved outcomes for resectable colorectal cancer17–19.
Microsatellite instability and loss of heterozygosity at chromosome 18q are the two best-defined molecular prognostic markers20. Microsatellite instability results from mutations or promoter hypermethylation of DNA mismatch repair genes leading to errors in DNA replication and changes in short, repeated sequences of DNA. It is present in the vast majority of tumors from patients with hereditary nonpolyposis colon cancer (HNPCC), but is also found in 15 to 20 percent of patients with sporadic colon cancer21, 22. Patients with tumors possessing a high degree of microsatellite instability have a more favorable prognosis than those patients whose tumors are microsatellite stable21, 23. Loss of heterozygosity at chromosome 18q has been reported in approximately 50% of colon cancers and has been associated with a worse prognosis24, 25. Although these factors provide prognostic information on the risk of tumor recurrence after primary resection, they have not been prospectively validated as predictive markers for altered outcome with administration of specific chemotherapeutic regimens.
The rectum is located within the pelvis and extends from the transitional mucosa of the anal dentate line to the sigmoid colon, which measures between 10 and 15 centimeters from the anal verge by rigid sigmoidoscopy. The bony constraints of the pelvis limit surgical access to the rectum, leading to a lower likelihood of achieving widely negative margins and a higher risk of local recurrence. Due to the increased risk of local recurrence, the local management of rectal cancer varies somewhat from that of colon cancer. Surgical resection of rectal cancer with sharp dissection of the mesorectum en bloc with the rectum, as part of a total mesorectal excision (TME), has resulted in a lower likelihood of local recurrence26, 27. The mesorectum is the rectal mesentery that contains the rectum’s vascular supply and lymphatic drainage and is the initial site of spread for rectal cancer. Additionally, radiotherapy administered preoperatively or postoperatively has been associated with a lower risk of local recurrence when compared to surgery alone, even when a TME has been performed27, 28.
Spread of tumor beyond the colorectum and regional lymph nodes defines the M stage of the AJCC classification system, with M1 indicating the presence of tumor metastases to distant sites. Approximately 20% of patients present with metastatic disease and 30% to 40% of patients with localized disease ultimately develop metastases. The liver reflects the most common initial site of disease spread, but metastases to other organs during the course of the disease are common, including to the lungs, peritoneum, and intra-abdominal lymph nodes. Patients with a small number of isolated, organ-confined metastases may be cured of their disease by surgical resection29; decisions regarding metastatectomy should be made by a medical oncologist working in close conjunction with an experienced surgeon. Most patients with metastatic disease are candidates for systemic chemotherapy to palliate symptoms and prolong life. As the AJCC stage increases from stage I to stage IV, five-year overall survival declines dramatically: stage I, > 90%; stage II, 70–85%; stage III 25–80%; and stage IV, < 10% (Table 2)2, 30, 31.
III. Fluoropyrimidines
A. Intravenous Fluorouracil
Fluorouracil remains the cornerstone of systemic treatment for colorectal cancer. It is a fluorinated pyrimidine that acts primarily through inhibition of thymidylate synthetase, the rate-limiting enzyme in pyrimidine nucleotide synthesis32 and is commonly administered with leucovorin, a reduced folate that is thought to stabilize fluorouracil’s interaction with this enzyme33–36. A meta-analysis of 3,300 patients from 19 randomized trials found that the likelihood of a greater than 50% tumor shrinkage by bidimensional product measurement doubles when fluorouracil is administered with leucovorin in patients with metastatic colorectal cancer, with a modest but statistically significant improvement in overall survival, when compared to fluorouracil alone37. Among patients with metastatic colorectal cancer receiving fluorouracil and leucovorin, approximately 20% will have a reduction in tumor size by 50% or more, and median survival is increased from approximately 6 months to about 12 months37, 38.
Fluorouracil can be administered by a variety of different schedules, with differing toxicity profiles. Neutropenia and stomatitis are the most frequent side effects when bolus fluorouracil and leucovorin are administered daily for five days every four to five weeks (the “Mayo Clinic regimen”). Higher rates of diarrhea are noted when bolus fluorouracil and leucovorin are administered weekly for six of eight weeks (the “Roswell Park regimen”). Schedules that administer fluorouracil as a continuous infusion are associated with less hematologic and gastrointestinal toxicity, but have a greater incidence of “hand-foot” syndrome, a tender, erythematous rash involving the palms and soles.
Although treatment programs that involve infusional fluorouracil were initially thought to be less convenient and more expensive than bolus regimens, little difference has been noted in quality of life or cost between these two types of regimens39–41. In addition, a meta-analysis of six randomized trials has demonstrated a modest improvement in response rate and median overall survival among patients with metastatic colorectal cancer who received infusional fluorouracil when compared with patients who received a more rapid, bolus approach38.
B. Oral Fluoropyrimidines
Initial attempts to administer fluoropyrimidines orally were unsuccessful. A randomized comparison of oral versus intravenous fluorouracil in patients with metastatic colorectal cancer favored the intravenous route in terms of tumor response rate and mean duration of tumor response42. These differences in response were thought to result from erratic intestinal absorption of fluorouracil, due to differing mucosal concentrations of dihydropyrimidine dehydrogenase (DPD), a major catabolic enzyme of the drug. Two strategies have been employed to circumvent this problem: the administration of an absorbable fluorouracil prodrug that is not catabolized by DPD43 and the co-administration of an inhibitor of DPD with oral fluorouracil44.
Capecitabine (Xeloda®) is an oral prodrug of fluorouracil that is absorbed intact through the gastrointestinal mucosa and undergoes a three-step enzymatic conversion to fluorouracil43. The side effect profile of this drug is similar to that seen with continuous infusion fluorouracil, with the hand-foot syndrome being most prominent. Studies have shown capecitabine to be therapeutically equivalent to bolus fluorouracil and leucovorin (Mayo Clinic schedule) as initial therapy in metastatic colorectal cancer, with no significant differences in median time to tumor progression or median overall survival45, 46.
Tegafur uracil (UFT, [Orzel®]) circumvents the erratic intestinal absorption of fluorouracil by the co-administration of an oral fluoropyrimidine (tegafur) with an inhibitor of DPD (uracil), thereby allowing for a more uniform absorption and bioavailability of tegafur47. In two randomized studies of patients with metastatic colorectal cancer, treatment with UFT and oral leucovorin resulted in similar rates of response and median survival as parental fluorouracil and leucovorin48, 49. Although available in Europe and Asia, UFT is not available in the United States.
While capecitabine, at the recommended dose of 1,250 mg/m2 twice daily, appears therapeutically similar to monthly bolus fluorouracil and leucovorin with a somewhat less severe toxicity profile, it is uncertain whether the differences in toxicity profile would remain if capecitabine were compared with a more tolerable schedule of parenteral fluorouracil (i.e. Roswell Park or infusional schedule). Additionally, results from recent studies of capecitabine administered with other intravenous chemotherapies, such as oxaliplatin and irinotecan, call into question the more favorable convenience and cost effectiveness profile that have been reported with single-agent capecitabine50–52.
C. Adjuvant Therapy with Fluoropyrimidines for Stage III Colon Cancer
Fluorouracil was thought for many years to be ineffective as adjuvant treatment for colon cancer53–56; a meta-analysis of randomized trials published prior to 1987 demonstrated only a small, statistically insignificant benefit for such treatment57. In retrospect, these randomized trials suffered from heterogeneous patient populations, inadequate sample size, and poor compliance with therapy. Two subsequent approaches to adjuvant therapy for colon cancer revived interest in fluorouracil.
In an attempt to reduce the incidence of subsequent liver metastases, several clinical trials evaluated the administration of fluorouracil into the portal circulation during the immediate postoperative period58–63. Although these studies failed to reduce tumor spread to the liver, a meta-analysis of ten such trials did demonstrate a modest overall survival benefit, supporting the value of a short exposure to adjuvant fluorouracil, when compliantly administered64.
Additionally, the merits of adjuvant treatment with fluorouracil were reassessed when levamisole, an antihelminthic, was examined as a putative immunomodulating agent65–67. Since levamisole was eventually shown to be inactive, these studies actually represented a reassessment of the adjuvant administration of fluorouracil. A large trial of 1,296 patients conducted by the Eastern Cooperative Oncology Group (ECOG) demonstrated that adjuvant fluorouracil (and levamisole) reduced the risk of recurrence by 41% and the risk of death by 33% compared with surgery alone in patients with stage III disease68. After a median follow-up of 6.5 years, overall survival was increased from 47% to 60% by the addition of postoperative fluorouracil (and levamisole)69.
Since the antitumor activity of fluorouracil was enhanced in the metastatic setting when administered with leucovorin37, the combination of fluorouracil and leucovorin was evaluated in the adjuvant setting, where it was found to improve disease-free and overall survival70–73. A pooled analysis of seven randomized trials of postoperative fluorouracil-based therapy versus surgery alone demonstrated an increase in five-year disease-free survival from 42% to 58% and five-year overall survival from 51% to 61% in patients with stage III disease74. Subsequent studies showed that adjuvant fluorouracil and leucovorin administered for 6 months was equivalent to fluorouracil and levamisole administered for 12 months, and that the addition of levamisole to fluorouracil and leucovorin did not provide added benefit73, 75, 76. Furthermore, none of the various administration schedules of fluorouracil was found to be superior to any other in the adjuvant setting77–80, although different side effect profiles were noted, similar to those observed in patients treated for metastatic disease.
Oral fluoropyrimidines have also been evaluated in the adjuvant therapy of colon cancer. In the “Xeloda in Adjuvant Colon Cancer Trial” (X-ACT), capecitabine (1,250 mg/m2 administered twice daily on days 1 to 14 every three weeks) was shown to be equally effective when compared to the Mayo Clinic regimen of bolus fluorouracil and leucovorin, in a cohort of patients with stage III colon cancer81. A large, randomized trial comparing UFT and leucovorin with intravenous fluorouracil and leucovorin as adjuvant therapy also demonstrated similar rates of disease-free survival and overall survival between the two treatment arms82.
Although nearly 75% of patients diagnosed with colon cancer are 65 years of age or older83, such patients have been under-represented in clinical trials and are less likely to receive adjuvant therapy84, 85. Pooled data analyses and population-based studies have repeatedly shown a consistent and equivalent survival benefit for adjuvant therapy in all age groups86–90, without an increase in treatment-related toxicity among older patients87, 90–93. When disease outcomes have been analyzed by ethnicity, higher colorectal cancer-specific mortality has been noted in African-American than in Caucasian patients94. Differences in comorbid disease, sociodemographic factors, stage at presentation, tumor biology, and receipt of treatment have been investigated as underlying reasons for the discrepancy in outcomes88, 95–98. Subset analyses of randomized treatment trials have demonstrated similar disease-free survival among African-American and Caucasian patients99, 100, suggesting that African-Americans derive a similar degree of benefit from appropriately administered therapy as do Caucasians.
D. Adjuvant Therapy with Fluoropyrimidines for Stage II Colon Cancer
The benefit of adjuvant fluorouracil-based therapy in patients with stage II colon cancer is less clear. Subset analyses of trials that have included patients with stage II and III disease have repeatedly failed to demonstrate a statistically significant survival benefit for stage II patients receiving adjuvant therapy. A pooled analysis of seven studies demonstrated a five-year overall survival of 81% in patients who received fluorouracil-based adjuvant therapy and 80% in patients who underwent surgery alone (p = 0.11)74.
Two studies have been cited in favor of the use of adjuvant therapy in patients with stage II disease. A retrospective subset analysis of four consecutive National Surgical Adjuvant Breast and Bowel Project (NSABP) trials noted a similar proportional survival benefit for patients with stage II and stage III disease who received fluorouracil-based therapy101, although the statistical approach taken in this analysis has been questioned102. The “Quick and Simple and Reliable” (QUASAR) study, a complex comparison of four different fluorouracil-based regimens with observation alone, demonstrated a statistically significant 3.7% improvement in overall survival (80.8% vs. 77.1%) among patients with predominantly stage II colon and rectal cancer who received adjuvant treatment103. Interpretation of these data are clouded by: the lack of central pathologic review to verify tumor stage; a heterogeneous patient population with inclusion of patients with both colon (71%) and rectal (29%) cancers, patients with stage III disease (8%), and patients who also received radiotherapy or portal vein infusion; multiple different chemotherapy regimens in the treatment arm; and a somewhat lower than expected survival in the two arms, when compared with other recently published adjuvant studies104.
After systematically reviewing the available literature, the Cancer Care Ontario Program in Evidence-Based Care105, an expert panel convened by the American Society of Clinical Oncology (ASCO) 106, and the National Comprehensive Cancer Network107 independently recommended against the routine administration of adjuvant therapy in patients with stage II disease. In addition, the ASCO panel determined that a sample size of 9,680 patients per group would be required to detect a 2% survival difference between treatment and control arms, with 90% power and a significance level of 0.05106.
It has been proposed that adjuvant chemotherapy may provide benefit to those patients with stage II disease and adverse clinical characteristics, such as T4 tumor penetration, bowel perforation, or clinical bowel obstruction106. Although this hypothesis has not yet been validated in a prospective, randomized clinical trial, a retrospective subset analysis of patients with stage II disease enrolled in the previously noted ECOG study which examined the adjuvant value of fluorouracil and levamisole, suggested a survival benefit for postoperative therapy in these high-risk patient subgroups15. Although other high risk features, such as inadequate lymph node sampling, lymphovascular or perineural invasion, poorly differentiated histology, microsatellite stability, and loss of heterozygosity at chromosome 18q are also known to carry a higher risk of recurrence12, the potential benefit of chemotherapy has not been prospectively examined in patients with these risk factors.
E. Adjuvant Therapy with Fluoropyrimidines for Stage II and Stage III Rectal Cancer
Several clinical trials performed in the 1980’s demonstrated that the addition of systemic chemotherapy to postoperative radiation reduced the risk of local recurrence and improved overall survival after the resection of stage II and stage III rectal cancers108–110. In a subsequent study, the administration of infusional fluorouracil with radiotherapy was noted to be more effective than similar radiotherapy with concurrent bolus fluorouracil111. More recently, the German Rectal Cancer Study Group demonstrated that preoperative combined chemoradiation therapy improved local control, decreased toxicity, and reduced the need for colostomy when compared to postoperative chemotherapy and radiation, among patients assessed by preoperative endoscope ultrasound and thought to have stage II and stage III rectal cancer112. No differences in disease-free or overall survival were observed between the preoperative and postoperative treatment arms. Therefore, standard of care for stages II and III rectal cancer is generally considered to be preoperative combined modality therapy with radiation and chemotherapy, followed by surgical resection with TME. Perhaps to parallel the six months of adjuvant therapy utilized among patients with resected colon cancer, an additional four months of postoperative fluorouracil-based chemotherapy are typically administered to patients with stage II or III rectal cancer.
IV. Irinotecan
Irinotecan (Camptosar®) is a semi-synthetic derivative of the natural alkaloid camptothecin that is converted by carboxylesterases to SN-38113. By inhibiting topoisomerase I, an enzyme that catalyzes breakage and rejoining of DNA strands during DNA replication, SN-38 causes DNA fragmentation and programmed cell death114. Metabolism of SN-38 occurs predominantly in the liver, where it is inactivated by glucuronidation and excreted through the biliary system. A polymorphism in the uridine diphosphate glucuronosyltransferase isoform 1A1 (UGTA1A) gene, which is responsible for glururonidation of SN-38, has been identified and leads to decreased inactivation of SN-38 with resultant increases in treatment-related toxicity115. A diagnostic test for this genetic polymorphism is available, although not widely used in the clinic. Elevated serum bilirubin levels have also been associated with excess irinotecan-mediated toxicity and this drug is not typically administered to patients with hyperbilirubinemia116. The most commonly observed toxicities associated with irinotecan are diarrhea, myelosuppression, and alopecia117, 118.
Randomized trials have demonstrated improvements in progression-free and overall survival when irinotecan has been added to either infusional (FOLFIRI)119 or bolus (IFL)120 fluorouracil and leucovorin in the initial treatment of patients with metastatic colorectal cancer. More recently, a randomized trial comparing FOLFIRI, IFL and irinotecan plus capecitabine (CAPIRI) demonstrated that those patients receiving FOLFIRI experienced longer progression-free and overall survival times, supporting the superiority of the infusional approach121. Additionally, CAPIRI was associated with approximately twice the rates of serious vomiting, diarrhea and dehydration, when compared with the two regimens that included intravenous fluorouracil.
Based on the encouraging results with irinotecan in patients with metastatic disease, it was anticipated that irinotecan would be an effective addition to adjuvant treatment programs for colon cancer. Three randomized trials of adjuvant irinotecan with either bolus or infusional fluorouracil and leucovorin have examined this premise122–124. Surprisingly, each of these studies demonstrated increased toxicity without a meaningful improvement in outcome among patients receiving irinotecan. This unanticipated failure of irinotecan to prove beneficial in the adjuvant setting has not been well explained, but underscores the importance of conducting rigorous, randomized clinical trials prior to making changes in clinical practice125.
V. Oxaliplatin
Oxaliplatin (Eloxatin®) is a diaminocyclohexane platinum compound that forms DNA adducts, leading to impaired DNA replication and cellular apoptosis126, 127. In patients with metastatic colon cancer, single-agent oxaliplatin has limited efficacy, but clinical benefit has been observed when it is administered with fluorouracil and leucovorin128–133, possibly due to oxaliplatin-induced “down-regulation” of thymidylate synthetase134. A cumulative sensory neuropathy, characterized by paresthesias of the hands and feet, is the primary toxicity associated with oxaliplatin.
In two randomized clinical trials in patients with metastatic colorectal cancer, the addition of oxaliplatin to infusional fluorouracil and leucovorin (FOLFOX) increased tumor response rates and disease-free survival, with a trend towards an improvement in overall survival130, 131. Further studies have compared the efficacy of oxaliplatin-containing and irinotecan containing combinations. In one such trial of patients with newly diagnosed metastatic disease, FOLFOX was associated with prolongations of progression-free and overall survival when compared with IFL or a combination of irinotecan and oxaliplatin135. Since this outcome may have been influenced by the superiority of infusional fluorouracil (as included in FOLFOX) over bolus fluorouracil (as included in IFL)38, 121, two further studies have compared oxaliplatin and irinotecan in combination with an infusional fluorouracil schedule133, 136. In both of these studies, tumor response rate, progression-free survival and overall survival were statistically indistinguishable among patients receiving FOLFOX or FOLFIRI as first-line therapy. Importantly, patients receiving all three of these drugs – fluorouracil, oxaliplatin and irinotecan –were noted to have a median overall survival of approximately 20 months133, 136, 137. Recent randomized studies have examined whether capecitabine can replace fluorouracil and leucovorin in combination with oxaliplatin as initial therapy among patients with metastatic disease138–140. These trials have shown the two combinations to have similar therapeutic benefit and toxicity, but the capecitabine-containing regimens to be more expensive due to the high cost of capecitabine52.
In contrast to the experience with irinotecan, two randomized trials have demonstrated an improvement in disease-free survival when oxaliplatin has been added to fluorouracil and leucovorin in the adjuvant setting141, 142. Both the “Multicenter International Study of Oxaliplatin/Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer” (MOSAIC) study and the C-07 study of the NSABP have demonstrated a 20% reduction in the rate of colon cancer recurrence with the addition of oxaliplatin. With 6 years of follow-up, the MOSAIC study has also demonstrated a statistically significant 4.4% improvement in overall survival for those patients with stage III disease (73.0% vs. 68.6%)104. No such survival benefit was observed in patients with stage II colon cancer, for whom the likelihood of survival after 6 years was 87% in both treatment arms. However, a non-significant 26% reduction in disease recurrence with the addition of oxaliplatin was observed in patients with high-risk stage II disease, defined as the presence of T4 tumor stage, bowel obstruction, perforation, poorly differentiated histology, venous invasion, or examination of less than 10 lymph nodes in the resected specimen. The addition of oxaliplatin to fluorouracil and leucovorin in these trials did result in increased rates of neutropenia and neurotoxicity. Of note, approximately 10% of patients who received oxaliplatin continued to have symptomatic neuropathy 2 years after completing treatment on these clinical trials104, 143.
VI. Angiogenesis Inhibitors
A more recently recognized strategy to control malignant proliferation and spread involves the inhibition of neoangiogenesis, or new blood vessel formation144. Currently, the most successful anti-angiogenic strategy has focused on inhibiting the vascular endothelial growth factor (VEGF), a soluble protein that stimulates blood vessel proliferation145. Bevacizumab (Avastin®) is a humanized monoclonal antibody directed against VEGF that has been examined in combination with chemotherapy in patients with advanced colorectal cancer (Table 3). In these patients, bevacizumab has been relatively well tolerated, with reversible hypertension and proteinuria representing two of the most common toxicities. Nonetheless, rare, yet serious side effects have been observed with bevacizumab, including a 1 to 2 percent risk of bowel perforation, a 3 percent risk of serious bleeding events, a 2 to 3 percent risk of arterial embolic events, and less than 1 percent risk of reversible posterior leukoencephalopathy syndrome146–148.
Table 3.
Trials of Targeted Therapies in Metastatic Colorectal Cancer
| Study | Study Type | No. of Patients | Response Rate (%) | Median DFS (mo.) | Median OS (mo.) |
|---|---|---|---|---|---|
| Cetuximab | |||||
| Cunningham et al.161 | Phase II* | ||||
| Cetuximab | 111 | 11 | 1.5 | 6.9 | |
| Cetuximab + irinotecan | 218 | 23 | 4.1 | 8.6 | |
| NCIC CO.17160 | Phase III∏ | ||||
| Best supportive care | 285 | 0 | N/A | 4.6 | |
| Cetuximab | 287 | 8 | N/A | 6.1 | |
| CRYSTAL168 | Phase IIIΣ | ||||
| FOLFIRI | 609 | 39 | 8.0 | N/A | |
| FOLFIRI + cetuximab | 608 | 47 | 8.9 | N/A | |
| Panitumumab | |||||
| Van Cutsem et al.169 | Phase III∏ | ||||
| Best supportive care | 232 | 0 | 1.8 | N/A | |
| Panitumumab | 231 | 10 | 2.0 | N/A | |
| Bevacizumab | |||||
| Hurwitz et al.146 | Phase IIIΣ | ||||
| IFL | 402 | 35 | 6.2 | 15.6 | |
| IFL + bevacizumab | 411 | 45 | 10.6 | 20.3 | |
| ECOG E3200151 | Phase III# | ||||
| FOLFOX | 291 | 9 | 4.7 | 10.8 | |
| FOLFOX + bevacizumab | 286 | 23 | 7.3 | 12.9 | |
| Bevacizumab + Anti-EGFR | |||||
| BOND-2124 | Phase II* | ||||
| Cetuximab + bevacizumab | 40 | 20 | 4.9 | 11.4 | |
| Irinotecan + cetuximab + bevacizumab | 43 | 37 | 7.3 | 14.5 | |
| PACCE178 | Phase IIIΣ | ||||
| FOLFOX + bevacizumab | 410 | 46 | 11.0 | 20.6 | |
| FOLFOX + bevacizumab + panitumumab | 413 | 45 | 9.5 | 19.3 | |
N/A denotes that data are not available
EGFR = epidermal growth factor receptor; DFS = disease-free survival; OS = overall survival
Second or third-line therapy after progressive disease on an irinotecan-containing regimen
After progressive disease on a fluoropyrimidine, irinotecan, and oxaliplatin
Second-line therapy after progressive disease on an irinotecan-containing regimen
First-line therapy in previously untreated patients
Initial studies of bevacizumab demonstrated improvements in tumor response rate and progression-free survival among patients with metastatic colorectal cancer, when bevacizumab was added to fluorouracil and leucovorin149, 150. In subsequent randomized trials, bevacizumab was shown to prolong median overall survival (20.3 months versus 15.6 months) in combination with IFL146 as initial treatment, and FOLFOX151 after the failure of a prior irinotecan-containing regimen (12.9 months versus 10.8 months). Further studies have confirmed improved response rates and progression-free survival times with the addition of bevacizumab to FOLFIRI or FOLFOX in patients with untreated, metastatic colorectal cancer121, 152.
Given the efficacy of bevacizumab in patients with metastatic colorectal cancer, the role of bevacizumab in adjuvant therapy is currently being examined in several randomized trials (Table 4). In the United States, the C-08 study of the NSABP is randomizing patients with stage II or III colon cancer to FOLFOX with or without bevacizumab, while a similar study of oxaliplatin-containing regimens with or without bevacizumab is ongoing in Europe. In addition, investigators of the ECOG have incorporated molecular markers into a large, randomized study of FOLFOX versus FOLFOX and bevacizumab in patients with high-risk stage II colon cancer. Until the results of these trials are available, it is premature to recommend the incorporation of bevacizumab into adjuvant treatment programs for colon cancer.
Table 4.
Ongoing Trials of Targeted Therapies in Resected Colon Cancer
| Clinical Trial | AJCC Stage# | Randomization∞ |
|---|---|---|
| NSABP C-08 | II, III | FOLFOX +/− Bevacizumab |
| AVANT | II, III | FOLFOX versus FOLFOX + Bevacizumab versus Capecitabine + Oxaliplatin + Bevacizumab |
| ECOG E5202 | II | Molecular high risk (MSS or MSI-L and 18q LOH): |
| FOLFOX +/− Bevacizumab | ||
| Standard risk: observation | ||
| NCCTG N0147 | III | FOLFOX +/− Cetuximab |
| PETACC-8 | III | FOLFOX +/− Cetuximab |
AJCC = American Joint Committee on Cancer
FOLFOX = fluorouracil, leucovorin, and oxaliplatin
MSS = miscrosatellite stable
MSI-L = microsatellite instability – low
18q LOH = loss of heterozygosity at chromosome 18q
VII. Epidermal Growth Factor Receptor Inhibitors
The epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein that interacts with signaling pathways affecting cellular growth, proliferation and programmed cell death153, and is expressed in malignancies of multiple tissues, including those of the colon, lung, breast, and head and neck154. In colorectal cancer, EGFR expression on the tumor cell surface has been demonstrated in up to 80% of tumors155, 156 and tumors that express EGFR carry a poorer prognosis157. Antibodies directed against the extracellular domain of EGFR and small molecular inhibitors of the intracellular tyrosine kinase domain have been developed to inhibit the function of this transmembrane receptor. Thus far, only the anti-EGFR monoclonal antibodies, cetuximab (Erbitux®) and panitumumab (Vectibix®), have definitively demonstrated efficacy in colorectal cancer (Table 3)158. Although small molecule inhibitors of the intracellular tyrosine kinase domain of EGFR, such as erlotinib (Tarceva®), are effective in other solid tumors, they appear to be inactive in patients with colorectal cancer159.
In a study of patients whose disease had progressed on a fluoropyrimidine, irinotecan, and oxaliplatin, weekly cetuximab demonstrated improvements in progression-free and overall survival (6.1 vs. 4.6 months), when compared with those treated with best supportive care alone160. Other studies of cetuximab in patients with irinotecan-refractory, metastatic colorectal cancer have confirmed tumor response rates of approximately 10% with cetuximab alone and 20% with cetuximab and irinotecan161–163, indicating an ability of cetuximab to overcome irinotecan resistance in tumor cells. The main side effects of treatment with cetuximab are acneiform rash, hypomagnesemia, and infusion reactions, with approximately 3% of patients experiencing serious hypersensitivity reactions to cetuximab infusion. The presence of an acneiform rash has been positively associated with an improved response to cetuximab, among patients with metastatic colorectal cancer164. While initial studies mandated the immunohistochemical detection EGFR on the surface of tumor cells as a prerequisite for enrollment, the degree of surface EGFR expression has been found to correlate poorly with tumor response, and responses to cetuximab have been noted among patients without detectable EGFR by immunohistochemistry165, 166.
Two further studies have evaluated the addition of cetuximab to first-line regimens in patients with previously untreated metastatic colorectal cancer167, 168. Initial results from a CALGB trial have shown an improvement in tumor response rate with the addition of cetuximab to FOLFIRI or FOLFOX (RR, 52% vs. 38%)167. The CRYSTAL trial, a randomized evaluation of FOLFIRI with or without cetuximab, has demonstrated improvements in tumor response rate (RR, 47% vs. 39%) and progression-free survival (median PFS, 8.9 months vs. 8.0 months), among those patients receiving cetuximab168. Although these results do support the efficacy of including cetuximab in first-line treatment programs, how these regimens compare with bevacizumab-containing regimens is currently unknown. Several ongoing studies, described below, have been designed to investigate this question.
The role of cetuximab in the adjuvant therapy of colon cancer has not yet been defined. The North Central Cancer Treatment Group (NCCTG) and the European Organization for Research and Treatment of Cancer (EORTC) are each registering over 2,000 patients with resected stage III colon cancer and randomizing them to receive FOLFOX alone or FOLFOX with cetuximab (Table 4). Until the results of these trials become available, the inclusion of cetuximab in adjuvant treatment programs cannot be recommended outside of a clinical trial.
Panitumumab is a humanized monoclonal antibody to EGFR that has shown similar single-agent activity as cetuximab in metastatic colorectal cancer, but with a biweekly (rather than weekly) administration schedule158, 169. In an initial study, 9% of patients whose cancers had progressed after treatment with fluorouracil and either irinotecan or oxaliplatin experienced a tumor response to panitumumab170. In a randomized trial of 463 patients previously treated with a fluoropyrimidine, irinotecan and oxaliplatin, single-agent panitumumab improved progression-free survival when compared with best supportive care (median PFS, 8.0 vs. 7.3 weeks)169, similar to the previously described experience with cetuximab160. Two ongoing studies are evaluating the addition of panitumumab to FOLFOX and FOLFIRI in patients with metastatic colorectal cancer. Panitumumab has not yet been tested in adjuvant treatment programs among patients with colon cancer, and cannot be recommended in this setting.
As only a subset of patients’ tumors treated with cetuximab or panitumumab will respond to this drug, the identification and characterization of molecular markers to predict tumor response is an area of active investigation. Two such tumor characteristics have emerged from initial studies: EGFR copy number as determined by fluorescence in situ hybridization (FISH), and K-ras gene mutation status. Among patients treated with cetuximab or panitumumab, high EGFR gene copy number by FISH has been associated with higher tumor response rates and prolongation of disease-free and overall survival171, 172. In contrast, patients with tumors having mutations in K-ras appear to be relatively resistant to treatment with cetuximab173–175 or panitumumab176, with lower response rates and poorer survival. These and other molecular features may help define a subset of patients who will derive benefit from treatment with an inhibitor of EGFR.
VIII. Combined Targeted Therapy
Several ongoing studies are assessing the efficacy of combined treatment with monoclonal antibodies to VEGF and EGFR in patients with metastatic colorectal cancer (Table 3). Initial data supporting this treatment approach arose from two studies, in which patients received combinations of irinotecan, cetuximab and bevacizumab161, 177. Those patients receiving both cetuximab and bevacizumab had improvements in tumor response rate and progression-free survival.
In the “Panitumumab Advanced Colorectal Cancer Evaluation” (PACCE) trial, patients with previously untreated, metastatic colorectal cancer received FOLFOX and bevacizumab with or without panitumumab178. Surprisingly, the first planned efficacy interim analysis demonstrated an inferior outcome for those patients receiving panitumumab, with shorter survival times and increased side effects. Since patients receiving panitumumab experienced greater treatment-related toxicity, it remains uncertain whether the combination is therapeutically inferior or whether the toxic effects resulted in less exposure to active drugs. This question should be answered by an ongoing randomized trial coordinated by the National Cancer Institute (CALGB/SWOG 80405), in which patients with previously untreated, metastatic colorectal cancer are receiving FOLFOX or FOLFIRI with the addition of cetuximab; bevacizumab; or cetuximab and bevacizumab.
IX. Summary and Future Directions
Currently available data in 2008 support the use of a fluoropyrimidine, irinotecan, oxaliplatin, bevacizumab, and either cetuximab or panitumumab, in the treatment of patients with metastatic colorectal cancer. The optimal sequence of administration of these drugs remains under investigation, but patients who receive all of these available therapies can now expect a median overall survival of approximately two years (Figure 1). The success of chemotherapy in prolonging survival in the metastatic setting is also being translated to improved cure rates among patients with stage III disease. The goal of ongoing adjuvant trials evaluating bevacizumab and cetuximab is to increase even further the improved rates of survival provided by fluorouracil, leucovorin, and oxaliplatin (Table 5).
Figure 1. Trends in Median Survival Among Patients with Metastatic Colorectal Cancer.
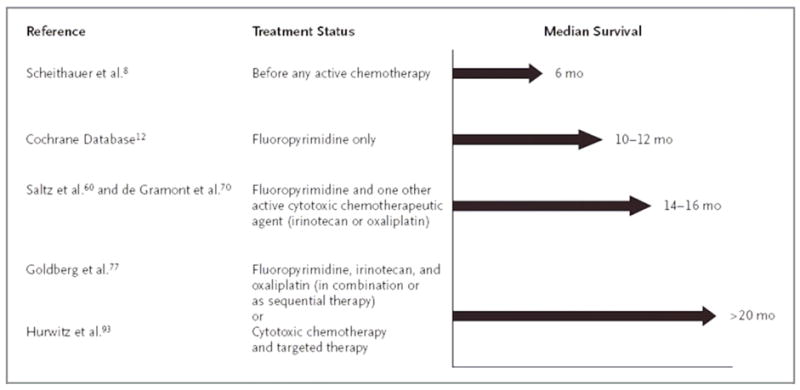
Adapted with permission from Meyerhardt and Mayer31
Scheithauer et al.182
Cochrane Database183
Saltz et al.120 and de Gramont et al.131
Goldberg et al.135 and Fuchs et al.121
Hurwitz et al.146
Table 5.
Postoperative Treatment of Patients with Resected Stage II and Stage III Colon Cancer
Stage III disease:
|
Stage II disease:
|
Over the past fifteen years, deaths due to colorectal cancer in the United States have decreased by approximately nine percent1, 179. This decline in mortality highlights the advances made in screening, prevention, and treatment for colorectal cancer, brought about by the collaboration of gastroenterologists, medical oncologists, pathologists, primary care physicians, and surgeons. Although this progress has occurred relatively rapidly, such cancer care and new chemotherapeutic agents, in particular, have not come without a significant cost to the health care system (Table 6)180, 181. In the near future, physicians and society may be faced with difficult decisions regarding resource allocation and innovative cancer treatment, as we work to maintain our current trajectory of progress181.
Table 6.
Costs of Systemic Treatments for Colorectal Cancer
| Regimen* | Cost per 6 Months# ($) |
|---|---|
| Bolus Fluorouracil/Leucovorin (Mayo Clinic schedule) | 96 |
| Infusional Fluorouracil/Leucovorin | 352 |
| Capecitabine | 11,648 |
| Irinotecan (every 3 weeks) | 30,100 |
| FOLFIRI | 23,572 |
| FOLFOX | 29,989 |
| Bevacizumab | 23,897 |
| Cetuximab | 52,131 |
| Panitumumab | 44,720 |
Adapted with permission from Meropol and Schulman181.
FOLFIRI = infusional fluorouracil, leucovorin, and irinotecan
FOLFOX = infusional fluorouracil, leucovorin, and oxaliplatin
Only drug costs included. Costs based upon average sales price for 70 kg patient with body-surface area of 1.7 m2. Wholesale acquisition costs provided for panitumumab.
Glossary of Relevant Terms
- AJCC TNM system
American Joint Committee on Cancer, Tumor-Node-Metastasis Cancer Staging System
- adjuvant treatment
Treatment delivered after resection of the primary tumor, with the goal of reducing the risk of tumor recurrence by eliminating micrometastatic disease
- IFL
Irinotecan, bolus Fluorouracil (5-FU), and Leucovorin (LV)
- FOLFIRI
Infusional 5-FU, LV, and Irinotecan
- CAPIRI
Capecitabine and Irinotecan
- FOLFOX
Infusional 5-FU, LV, and Oxaliplatin
- XELOX
Capecitabine (Xeloda®) and Oxaliplatin
- targeted therapy
Therapeutic agents designed to perturb specific molecular pathways critical for tumor cell growth and survival
- EGFR
Epidermal growth factor receptor – a transmembrane protein on the surface of tumor cells, targeted by cetuximab and panitumumab
- VEGF
Vascular endothelial growth factor – a serum protein involved in stimulating new blood vessel formation, targeted by bevacizumab
Footnotes
Conflicts of Interest: Brian Wolpin, M.D.: None Robert Mayer, M.D.: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brian M. Wolpin, Instructor in Medicine, Harvard Medical School, Department of Medical Oncology, Dana-Farber Cancer Institute.
Robert J. Mayer, Stephen B. Kay Family Professor of Medicine, Harvard Medical School, Director, Center for Gastrointestinal Oncology, Department of Medical Oncology, Dana-Farber Cancer Institute.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Greene F, Balch CM, Fleming ID, et al., editors. AJCC cancer staging handbook. 6. Springer; 2002. [Google Scholar]
- 3.Greene FL, Stewart AK, Norton HJ. A new TNM staging strategy for node-positive (stage III) colon cancer: an analysis of 50,042 patients. Ann Surg. 2002;236:416–21. doi: 10.1097/00000658-200210000-00003. discussion 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene FL, Stewart AK, Norton HJ. New tumor-node-metastasis staging strategy for node-positive (stage III) rectal cancer: an analysis. J Clin Oncol. 2004;22:1778–84. doi: 10.1200/JCO.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Greene FLBC, Fleming ID, et al., editors. AJCC cancer staging handbook. 6. Springer; 2002. [Google Scholar]
- 6.Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54:295–308. doi: 10.3322/canjclin.54.6.295. [DOI] [PubMed] [Google Scholar]
- 7.Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol. 2003;10:65–71. doi: 10.1245/aso.2003.03.058. [DOI] [PubMed] [Google Scholar]
- 8.Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912–9. doi: 10.1200/JCO.2003.05.062. [DOI] [PubMed] [Google Scholar]
- 9.Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23:8706–12. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]
- 10.Stocchi L, Nelson H, Sargent DJ, O’Connell MJ, Tepper JE, Krook JE, Beart R., Jr Impact of surgical and pathologic variables in rectal cancer: a United States community and cooperative group report. J Clin Oncol. 2001;19:3895–902. doi: 10.1200/JCO.2001.19.18.3895. [DOI] [PubMed] [Google Scholar]
- 11.Tepper JE, O’Connell MJ, Niedzwiecki D, Hollis D, Compton C, Benson AB, 3rd, Cummings B, Gunderson L, Macdonald JS, Mayer RJ. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol. 2001;19:157–63. doi: 10.1200/JCO.2001.19.1.157. [DOI] [PubMed] [Google Scholar]
- 12.Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88:1739–57. doi: 10.1002/(sici)1097-0142(20000401)88:7<1739::aid-cncr30>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 13.Wanebo HJ, Rao B, Pinsky CM, Hoffman RG, Stearns M, Schwartz MK, Oettgen HF. Preoperative carcinoembryonic antigen level as a prognostic indicator in colorectal cancer. N Engl J Med. 1978;299:448–51. doi: 10.1056/NEJM197808312990904. [DOI] [PubMed] [Google Scholar]
- 14.Wolmark N, Fisher B, Wieand HS, Henry RS, Lerner H, Legault-Poisson S, Deckers PJ, Dimitrov N, Gordon PH, Jochimsen P, et al. The prognostic significance of preoperative carcinoembryonic antigen levels in colorectal cancer. Results from NSABP (National Surgical Adjuvant Breast and Bowel Project) clinical trials. Ann Surg. 1984;199:375–82. doi: 10.1097/00000658-198404000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, Ungerleider JS, Emerson WA, Tormey DC, Glick JH, et al. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/Dukes’ B2 colon cancer. J Clin Oncol. 1995;13:2936–43. doi: 10.1200/JCO.1995.13.12.2936. [DOI] [PubMed] [Google Scholar]
- 16.Krasna MJ, Flancbaum L, Cody RP, Shneibaum S, Ben Ari G. Vascular and neural invasion in colorectal carcinoma. Incidence and prognostic significance. Cancer. 1988;61:1018–23. doi: 10.1002/1097-0142(19880301)61:5<1018::aid-cncr2820610527>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 17.Hodgson DC, Zhang W, Zaslavsky AM, Fuchs CS, Wright WE, Ayanian JZ. Relation of hospital volume to colostomy rates and survival for patients with rectal cancer. J Natl Cancer Inst. 2003;95:708–16. doi: 10.1093/jnci/95.10.708. [DOI] [PubMed] [Google Scholar]
- 18.Schrag D, Cramer LD, Bach PB, Cohen AM, Warren JL, Begg CB. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA. 2000;284:3028–35. doi: 10.1001/jama.284.23.3028. [DOI] [PubMed] [Google Scholar]
- 19.Meyerhardt JA, Tepper JE, Niedzwiecki D, Hollis DR, Schrag D, Ayanian JZ, O’Connell MJ, Weeks JC, Mayer RJ, Willett CG, MacDonald JS, Benson AB, 3rd, Fuchs CS. Impact of hospital procedure volume on surgical operation and long-term outcomes in high-risk curatively resected rectal cancer: findings from the Intergroup 0114 Study. J Clin Oncol. 2004;22:166–74. doi: 10.1200/JCO.2004.04.172. [DOI] [PubMed] [Google Scholar]
- 20.Lurje G, Zhang W, Lenz HJ. Molecular prognostic markers in locally advanced colon cancer. Clin Colorectal Cancer. 2007;6:683–90. doi: 10.3816/CCC.2007.n.037. [DOI] [PubMed] [Google Scholar]
- 21.Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, Redston M, Gallinger S. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 22.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J, Panescu J, Fix D, Lockman J, Comeras I, de la Chapelle A. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–60. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe T, Wu TT, Catalano PJ, Ueki T, Satriano R, Haller DG, Benson AB, 3rd, Hamilton SR. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196–206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popat S, Houlston RS. A systematic review and meta-analysis of the relationship between chromosome 18q genotype, DCC status and colorectal cancer prognosis. Eur J Cancer. 2005;41:2060–70. doi: 10.1016/j.ejca.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 25.Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC., Jr ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–27. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 26.Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg. 1998;133:894–9. doi: 10.1001/archsurg.133.8.894. [DOI] [PubMed] [Google Scholar]
- 27.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–46. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 28.Adjuvant radiotherapy for rectal cancer: a systematic overview of 8, 507 patients from 22 randomised trials. Lancet. 2001;358:1291–304. doi: 10.1016/S0140-6736(01)06409-1. [DOI] [PubMed] [Google Scholar]
- 29.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH, D’Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–80. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 30.O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–5. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 31.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–87. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 32.Sobrero A, Guglielmi A, Grossi F, Puglisi F, Aschele C. Mechanism of action of fluoropyrimidines: relevance to the new developments in colorectal cancer chemotherapy. Semin Oncol. 2000;27:72–7. [PubMed] [Google Scholar]
- 33.Zhang ZG, Harstrick A, Rustum YM. Modulation of fluoropyrimidines: role of dose and schedule of leucovorin administration. Semin Oncol. 1992;19:10–5. [PubMed] [Google Scholar]
- 34.de Gramont A, Bosset JF, Milan C, Rougier P, Bouche O, Etienne PL, Morvan F, Louvet C, Guillot T, Francois E, Bedenne L. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol. 1997;15:808–15. doi: 10.1200/JCO.1997.15.2.808. [DOI] [PubMed] [Google Scholar]
- 35.Petrelli N, Douglass HO, Jr, Herrera L, Russell D, Stablein DM, Bruckner HW, Mayer RJ, Schinella R, Green MD, Muggia FM, et al. The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: a prospective randomized phase III trial. Gastrointestinal Tumor Study Group. J Clin Oncol. 1989;7:1419–26. doi: 10.1200/JCO.1989.7.10.1419. [DOI] [PubMed] [Google Scholar]
- 36.Poon MA, O’Connell MJ, Moertel CG, Wieand HS, Cullinan SA, Everson LK, Krook JE, Mailliard JA, Laurie JA, Tschetter LK, et al. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989;7:1407–18. doi: 10.1200/JCO.1989.7.10.1407. [DOI] [PubMed] [Google Scholar]
- 37.Thirion P, Michiels S, Pignon JP, Buyse M, Braud AC, Carlson RW, O’Connell M, Sargent P, Piedbois P. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol. 2004;22:3766–75. doi: 10.1200/JCO.2004.03.104. [DOI] [PubMed] [Google Scholar]
- 38.Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. Meta-analysis Group In Cancer. J Clin Oncol. 1998;16:301–8. doi: 10.1200/JCO.1998.16.1.301. [DOI] [PubMed] [Google Scholar]
- 39.Lokich JJ, Moore CL, Anderson NR. Comparison of costs for infusion versus bolus chemotherapy administration: analysis of five standard chemotherapy regimens in three common tumors--Part one. Model projections for cost based on charges. Cancer. 1996;78:294–9. doi: 10.1002/(SICI)1097-0142(19960715)78:2<294::AID-CNCR16>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 40.Lokich JJ, Moore CL, Anderson NR. Comparison of costs for infusion versus bolus chemotherapy administration--Part two. Use of charges versus reimbursement for cost basis. Cancer. 1996;78:300–3. doi: 10.1002/(SICI)1097-0142(19960715)78:2<300::AID-CNCR17>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Kohne CH, Wils J, Lorenz M, Schoffski P, Voigtmann R, Bokemeyer C, Lutz M, Kleeberg C, Ridwelski K, Souchon R, El-Serafi M, Weiss U, Burkhard O, Ruckle H, Lichnitser M, Langenbuch T, Scheithauer W, Baron B, Couvreur ML, Schmoll HJ. Randomized phase III study of high-dose fluorouracil given as a weekly 24-hour infusion with or without leucovorin versus bolus fluorouracil plus leucovorin in advanced colorectal cancer: European organization of Research and Treatment of Cancer Gastrointestinal Group Study 40952. J Clin Oncol. 2003;21:3721–8. doi: 10.1200/JCO.2003.11.122. [DOI] [PubMed] [Google Scholar]
- 42.Hahn RG, Moertel CG, Schutt AJ, Bruckner HW. A double-blind comparison of intensive course 5-flourouracil by oral vs. intravenous route in the treatment of colorectal carcinoma. Cancer. 1975;35:1031–5. doi: 10.1002/1097-0142(197504)35:4<1031::aid-cncr2820350403>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 43.Pentheroudakis G, Twelves C. The rational development of capecitabine from the laboratory to the clinic. Anticancer Res. 2002;22:3589–96. [PubMed] [Google Scholar]
- 44.Meropol NJ. Oral fluoropyrimidines in the treatment of colorectal cancer. Eur J Cancer. 1998;34:1509–13. doi: 10.1016/s0959-8049(98)00226-3. [DOI] [PubMed] [Google Scholar]
- 45.Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, Maroun J, Walde D, Weaver C, Harrison E, Burger HU, Osterwalder B, Wong AO, Wong R. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19:2282–92. doi: 10.1200/JCO.2001.19.8.2282. [DOI] [PubMed] [Google Scholar]
- 46.Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, Bugat R, Findlay M, Frings S, Jahn M, McKendrick J, Osterwalder B, Perez-Manga G, Rosso R, Rougier P, Schmiegel WH, Seitz JF, Thompson P, Vieitez JM, Weitzel C, Harper P. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19:4097–106. doi: 10.1200/JCO.2001.19.21.4097. [DOI] [PubMed] [Google Scholar]
- 47.Sulkes A, Benner SE, Canetta RM. Uracil-ftorafur: an oral fluoropyrimidine active in colorectal cancer. J Clin Oncol. 1998;16:3461–75. doi: 10.1200/JCO.1998.16.10.3461. [DOI] [PubMed] [Google Scholar]
- 48.Carmichael J, Popiela T, Radstone D, Falk S, Borner M, Oza A, Skovsgaard T, Munier S, Martin C. Randomized comparative study of tegafur/uracil and oral leucovorin versus parenteral fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2002;20:3617–27. doi: 10.1200/JCO.2002.10.129. [DOI] [PubMed] [Google Scholar]
- 49.Douillard JY, Hoff PM, Skillings JR, Eisenberg P, Davidson N, Harper P, Vincent MD, Lembersky BC, Thompson S, Maniero A, Benner SE. Multicenter phase III study of uracil/tegafur and oral leucovorin versus fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2002;20:3605–16. doi: 10.1200/JCO.2002.04.123. [DOI] [PubMed] [Google Scholar]
- 50.Ward S, Kaltenthaler E, Cowan J, Brewer N. Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation. Health Technol Assess. 2003;7:1–93. doi: 10.3310/hta7320. [DOI] [PubMed] [Google Scholar]
- 51.Twelves C, Gollins S, Grieve R, Samuel L. A randomised cross-over trial comparing patient preference for oral capecitabine and 5-fluorouracil/leucovorin regimens in patients with advanced colorectal cancer. Ann Oncol. 2006;17:239–45. doi: 10.1093/annonc/mdj023. [DOI] [PubMed] [Google Scholar]
- 52.Mayer RJ. Should capecitabine replace infusional fluorouracil and leucovorin when combined with oxaliplatin in metastatic colorectal cancer? J Clin Oncol. 2007;25:4165–7. doi: 10.1200/JCO.2007.11.6582. [DOI] [PubMed] [Google Scholar]
- 53.Higgins GA, Humphrey E, Juler G, LeVeen HH, McCaughan J, Keehn RJ. Adjuvant chemotherapy in the surgical treatment of large bowel cancer. Cancer. 1976;38:1461–7. doi: 10.1002/1097-0142(197610)38:4<1461::aid-cncr2820380402>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 54.Higgins GA, Donaldson RC, Humphrey EW, Rogers LS, Shields TW. Adjuvant therapy for large bowel cancer: update of Veterans Administration Surgical Oncology Group Trials. Surg Clin North Am. 1981;61:1311–20. doi: 10.1016/s0039-6109(16)42586-7. [DOI] [PubMed] [Google Scholar]
- 55.Grossi CE, Wolff WI, Nealon TF, Jr, Pasternack B, Ginzburg L, Rousselot LM. Intraluminal fluorouracil chemotherapy adjunct to surgical procedures for respectable carcinoma of the colon and rectum. Surg Gynecol Obstet. 1977;145:549–54. [PubMed] [Google Scholar]
- 56.Grage TB, Moss SE. Adjuvant chemotherapy in cancer of the colon and rectum: demonstration of effectiveness of prolonged 5-FU chemotherapy in a prospectively controlled, randomized trial. Surg Clin North Am. 1981;61:1321–9. doi: 10.1016/s0039-6109(16)42587-9. [DOI] [PubMed] [Google Scholar]
- 57.Buyse M, Zeleniuch-Jacquotte A, Chalmers TC. Adjuvant therapy of colorectal cancer. Why we still don’t know. JAMA. 1988;259:3571–8. [PubMed] [Google Scholar]
- 58.Taylor I, Machin D, Mullee M, Trotter G, Cooke T, West C. A randomized controlled trial of adjuvant portal vein cytotoxic perfusion in colorectal cancer. Br J Surg. 1985;72:359–63. doi: 10.1002/bjs.1800720509. [DOI] [PubMed] [Google Scholar]
- 59.Wereldsma JC, Bruggink ED, Meijer WS, Roukema JA, van Putten WL. Adjuvant portal liver infusion in colorectal cancer with 5-fluorouracil/heparin versus urokinase versus control. Results of a prospective randomized clinical trial (colorectal adenocarcinoma trial I) Cancer. 1990;65:425–32. doi: 10.1002/1097-0142(19900201)65:3<425::aid-cncr2820650309>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 60.Beart RW, Jr, Moertel CG, Wieand HS, Leigh JE, Windschitl HE, van Heerden JA, Fitzgibbons RJ, Jr, Wolff BG. Adjuvant therapy for resectable colorectal carcinoma with fluorouracil administered by portal vein infusion. A study of the Mayo Clinic and the North Central Cancer Treatment Group. Arch Surg. 1990;125:897–901. doi: 10.1001/archsurg.1990.01410190095015. [DOI] [PubMed] [Google Scholar]
- 61.Wolmark N, Rockette H, Wickerham DL, Fisher B, Redmond C, Fisher ER, Potvin M, Davies RJ, Jones J, Robidoux A, et al. Adjuvant therapy of Dukes’ A, B, and C adenocarcinoma of the colon with portal-vein fluorouracil hepatic infusion: preliminary results of National Surgical Adjuvant Breast and Bowel Project Protocol C-02. J Clin Oncol. 1990;8:1466–75. doi: 10.1200/JCO.1990.8.9.1466. [DOI] [PubMed] [Google Scholar]
- 62.Fielding LP, Hittinger R, Grace RH, Fry JS. Randomised controlled trial of adjuvant chemotherapy by portal-vein perfusion after curative resection for colorectal adenocarcinoma. Lancet. 1992;340:502–6. doi: 10.1016/0140-6736(92)91708-g. [DOI] [PubMed] [Google Scholar]
- 63.Long-term results of single course of adjuvant intraportal chemotherapy for colorectal cancer. Swiss Group for Clinical Cancer Research (SAKK) Lancet. 1995;345:349–53. [PubMed] [Google Scholar]
- 64.Portal vein chemotherapy for colorectal cancer: a meta-analysis of 4000 patients in 10 studies. Liver Infusion Meta-analysis Group. J Natl Cancer Inst. 1997;89:497–505. [PubMed] [Google Scholar]
- 65.Bedikian AY, Valdivieso M, Mavligit GM, Burgess MA, Rodriguez V, Bodey GP. Sequential chemoimmunotherapy of colorectal cancer: evaluation of methotrexate, Baker’s Antifol and levamisole. Cancer. 1978;42:2169–76. doi: 10.1002/1097-0142(197811)42:5<2169::aid-cncr2820420513>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 66.Buroker TR, Moertel CG, Fleming TR, Everson LK, Cullinan SA, Krook JE, Mailliard JA, Marschke RF, Klaassen DJ, Laurie JA, et al. A controlled evaluation of recent approaches to biochemical modulation or enhancement of 5-fluorouracil therapy in colorectal carcinoma. J Clin Oncol. 1985;3:1624–31. doi: 10.1200/JCO.1985.3.12.1624. [DOI] [PubMed] [Google Scholar]
- 67.Windle R, Bell PR, Shaw D. Five year results of a randomized trial of adjuvant 5-fluorouracil and levamisole in colorectal cancer. Br J Surg. 1987;74:569–72. doi: 10.1002/bjs.1800740707. [DOI] [PubMed] [Google Scholar]
- 68.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, Ungerleider JS, Emerson WA, Tormey DC, Glick JH, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352–8. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 69.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, Ungerleider JS, Emerson WA, Tormey DC, Glick JH, Veeder MH, Mailliard JA. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995;122:321–6. doi: 10.7326/0003-4819-122-5-199503010-00001. [DOI] [PubMed] [Google Scholar]
- 70.Wolmark N, Rockette H, Fisher B, Wickerham DL, Redmond C, Fisher ER, Jones J, Mamounas EP, Ore L, Petrelli NJ, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol. 1993;11:1879–87. doi: 10.1200/JCO.1993.11.10.1879. [DOI] [PubMed] [Google Scholar]
- 71.Francini G, Petrioli R, Lorenzini L, Mancini S, Armenio S, Tanzini G, Marsili S, Aquino A, Marzocca G, Civitelli S, et al. Folinic acid and 5-fluorouracil as adjuvant chemotherapy in colon cancer. Gastroenterology. 1994;106:899–906. doi: 10.1016/0016-5085(94)90748-x. [DOI] [PubMed] [Google Scholar]
- 72.O’Connell MJ, Mailliard JA, Kahn MJ, Macdonald JS, Haller DG, Mayer RJ, Wieand HS. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol. 1997;15:246–50. doi: 10.1200/JCO.1997.15.1.246. [DOI] [PubMed] [Google Scholar]
- 73.Wolmark N, Rockette H, Mamounas E, Jones J, Wieand S, Wickerham DL, Bear HD, Atkins JN, Dimitrov NV, Glass AG, Fisher ER, Fisher B. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol. 1999;17:3553–9. doi: 10.1200/JCO.1999.17.11.3553. [DOI] [PubMed] [Google Scholar]
- 74.Gill S, Loprinzi CL, Sargent DJ, Thome SD, Alberts SR, Haller DG, Benedetti J, Francini G, Shepherd LE, Francois Seitz J, Labianca R, Chen W, Cha SS, Heldebrant MP, Goldberg RM. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 75.O’Connell MJ, Laurie JA, Kahn M, Fitzgibbons RJ, Jr, Erlichman C, Shepherd L, Moertel CG, Kocha WI, Pazdur R, Wieand HS, Rubin J, Vukov AM, Donohue JH, Krook JE, Figueredo A. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol. 1998;16:295–300. doi: 10.1200/JCO.1998.16.1.295. [DOI] [PubMed] [Google Scholar]
- 76.Haller DGCP, MacDonald JS, et al. Fluorouracil (FU), leucovorin (LV), and levimasole (LEV) adjuvant therapy for colon cancer: Five year report of INT-0089. Proc Am Soc Clin Oncol. 1998;17:256a. (abstract 982) [Google Scholar]
- 77.Haller DG, Catalano PJ, Macdonald JS, O’Rourke MA, Frontiera MS, Jackson DV, Mayer RJ. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol. 2005;23:8671–8. doi: 10.1200/JCO.2004.00.5686. [DOI] [PubMed] [Google Scholar]
- 78.Andre T, Colin P, Louvet C, Gamelin E, Bouche O, Achille E, Colbert N, Boaziz C, Piedbois P, Tubiana-Mathieu N, Boutan-Laroze A, Flesch M, Buyse M, de Gramont A. Semimonthly versus monthly regimen of fluorouracil and leucovorin administered for 24 or 36 weeks as adjuvant therapy in stage II and III colon cancer: results of a randomized trial. J Clin Oncol. 2003;21:2896–903. doi: 10.1200/JCO.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 79.Chau I, Norman AR, Cunningham D, Tait D, Ross PJ, Iveson T, Hill M, Hickish T, Lofts F, Jodrell D, Webb A, Oates JR. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol. 2005;16:549–57. doi: 10.1093/annonc/mdi116. [DOI] [PubMed] [Google Scholar]
- 80.Poplin EA, Benedetti JK, Estes NC, Haller DG, Mayer RJ, Goldberg RM, Weiss GR, Rivkin SE, Macdonald JS. Phase III Southwest Oncology Group 9415/Intergroup 0153 randomized trial of fluorouracil, leucovorin, and levamisole versus fluorouracil continuous infusion and levamisole for adjuvant treatment of stage III and high-risk stage II colon cancer. J Clin Oncol. 2005;23:1819–25. doi: 10.1200/JCO.2005.04.169. [DOI] [PubMed] [Google Scholar]
- 81.Twelves C, Wong A, Nowacki MP, Abt M, Burris H, 3rd, Carrato A, Cassidy J, Cervantes A, Fagerberg J, Georgoulias V, Husseini F, Jodrell D, Koralewski P, Kroning H, Maroun J, Marschner N, McKendrick J, Pawlicki M, Rosso R, Schuller J, Seitz JF, Stabuc B, Tujakowski J, Van Hazel G, Zaluski J, Scheithauer W. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696–704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- 82.Lembersky BC, Wieand HS, Petrelli NJ, O’Connell MJ, Colangelo LH, Smith RE, Seay TE, Giguere JK, Marshall ME, Jacobs AD, Colman LK, Soran A, Yothers G, Wolmark N. Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project Protocol C-06. J Clin Oncol. 2006;24:2059–64. doi: 10.1200/JCO.2005.04.7498. [DOI] [PubMed] [Google Scholar]
- 83.Yancik R, Ries LA. Aging and cancer in America. Demographic and epidemiologic perspectives. Hematol Oncol Clin North Am. 2000;14:17–23. doi: 10.1016/s0889-8588(05)70275-6. [DOI] [PubMed] [Google Scholar]
- 84.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 85.Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93:850–7. doi: 10.1093/jnci/93.11.850. [DOI] [PubMed] [Google Scholar]
- 86.Sundararajan V, Mitra N, Jacobson JS, Grann VR, Heitjan DF, Neugut AI. Survival associated with 5-fluorouracil-based adjuvant chemotherapy among elderly patients with node-positive colon cancer. Ann Intern Med. 2002;136:349–57. doi: 10.7326/0003-4819-136-5-200203050-00007. [DOI] [PubMed] [Google Scholar]
- 87.Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, Shepherd LE, Seitz JF, Francini G. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–7. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 88.Jessup JM, Stewart A, Greene FL, Minsky BD. Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA. 2005;294:2703–11. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]
- 89.Iwashyna TJ, Lamont EB. Effectiveness of adjuvant fluorouracil in clinical practice: a population-based cohort study of elderly patients with stage III colon cancer. J Clin Oncol. 2002;20:3992–8. doi: 10.1200/JCO.2002.03.083. [DOI] [PubMed] [Google Scholar]
- 90.Popescu RA, Norman A, Ross PJ, Parikh B, Cunningham D. Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older. J Clin Oncol. 1999;17:2412–8. doi: 10.1200/JCO.1999.17.8.2412. [DOI] [PubMed] [Google Scholar]
- 91.Ayanian JZ, Zaslavsky AM, Fuchs CS, Guadagnoli E, Creech CM, Cress RD, O’Connor LC, West DW, Allen ME, Wolf RE, Wright WE. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol. 2003;21:1293–300. doi: 10.1200/JCO.2003.06.178. [DOI] [PubMed] [Google Scholar]
- 92.Aschele C, Guglielmi A, Tixi LM, Bolli E, Mori AM, Lionetto R, Rosso R, Sobrero A. Adjuvant Treatment of Colorectal Cancer in the Elderly. Cancer Control. 1995;2:36–38. [PubMed] [Google Scholar]
- 93.Sargent D, Goldberg RM, Bleiberg H, De Gramont A, Tournigand C, Andre T, Rothenberg ML, Tabah-Fisch IM. A pooled safety and efficacy analysis of the FOLFOX4 regimen (bi-monthly oxaliplatin plus fluorouracil/leucovorin) in elderly compared to younger patients with colorectal cancer. J Clin Oncol. 2006;24:3517. Abstract. [Google Scholar]
- 94.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer and race: understanding the differences in outcomes between African Americans and whites. Med Clin North Am. 2005;89:771–93. doi: 10.1016/j.mcna.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 95.Marcella S, Miller JE. Racial differences in colorectal cancer mortality. The importance of stage and socioeconomic status. J Clin Epidemiol. 2001;54:359–66. doi: 10.1016/s0895-4356(00)00316-4. [DOI] [PubMed] [Google Scholar]
- 96.Mayberry RM, Coates RJ, Hill HA, Click LA, Chen VW, Austin DF, Redmond CK, Fenoglio-Preiser CM, Hunter CP, Haynes MA, et al. Determinants of black/white differences in colon cancer survival. J Natl Cancer Inst. 1995;87:1686–93. doi: 10.1093/jnci/87.22.1686. [DOI] [PubMed] [Google Scholar]
- 97.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287:2106–13. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 98.Baldwin LM, Dobie SA, Billingsley K, Cai Y, Wright GE, Dominitz JA, Barlow W, Warren JL, Taplin SH. Explaining black-white differences in receipt of recommended colon cancer treatment. J Natl Cancer Inst. 2005;97:1211–20. doi: 10.1093/jnci/dji241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCollum AD, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB, 3rd, Fuchs CS. Outcomes and toxicity in african-american and caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. J Natl Cancer Inst. 2002;94:1160–7. doi: 10.1093/jnci/94.15.1160. [DOI] [PubMed] [Google Scholar]
- 100.Dignam JJ, Ye Y, Colangelo L, Smith R, Mamounas EP, Wieand HS, Wolmark N. Prognosis after rectal cancer in blacks and whites participating in adjuvant therapy randomized trials. J Clin Oncol. 2003;21:413–20. doi: 10.1200/JCO.2003.02.004. [DOI] [PubMed] [Google Scholar]
- 101.Mamounas E, Wieand S, Wolmark N, Bear HD, Atkins JN, Song K, Jones J, Rockette H. Comparative efficacy of adjuvant chemotherapy in patients with Dukes’ B versus Dukes’ C colon cancer: results from four National Surgical Adjuvant Breast and Bowel Project adjuvant studies (C-01, C-02, C-03, and C-04) J Clin Oncol. 1999;17:1349–55. doi: 10.1200/JCO.1999.17.5.1349. [DOI] [PubMed] [Google Scholar]
- 102.Harrington DP. The tea leaves of small trials. J Clin Oncol. 1999;17:1336–8. doi: 10.1200/JCO.1999.17.5.1336. [DOI] [PubMed] [Google Scholar]
- 103.Quasar Collaborative G, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–9. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 104.de Gramont A, Boni C, Navarro M, et al. Oxaliplatin/5-FU/LV in adjuvant colon cancer: updated efficacy results of the MOSAIC trial, including survival, with a median follow-up of six years. J Clin Oncol. 2007;25:165s. (abstr 4007) [Google Scholar]
- 105.Figueredo A, Charette ML, Maroun J, Brouwers MC, Zuraw L. Adjuvant therapy for stage II colon cancer: a systematic review from the Cancer Care Ontario Program in evidence-based care’s gastrointestinal cancer disease site group. J Clin Oncol. 2004;22:3395–407. doi: 10.1200/JCO.2004.03.087. [DOI] [PubMed] [Google Scholar]
- 106.Benson AB, 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J, McAllister P, Van Cutsem E, Brouwers M, Charette M, Haller DG. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–19. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 107.Winn R, McClure J. The NCCN clinical practice guidelines in oncology. J of the National Comprehensive Cancer Network. 2003;1:9. doi: 10.6004/jnccn.2003.0003. [DOI] [PubMed] [Google Scholar]
- 108.Prolongation of the disease-free interval in surgically treated rectal carcinoma. Gastrointestinal Tumor Study Group. N Engl J Med. 1985;312:1465–72. doi: 10.1056/NEJM198506063122301. [DOI] [PubMed] [Google Scholar]
- 109.Douglass HO, Jr, Moertel CG, Mayer RJ, Thomas PR, Lindblad AS, Mittleman A, Stablein DM, Bruckner HW. Survival after postoperative combination treatment of rectal cancer. N Engl J Med. 1986;315:1294–5. doi: 10.1056/NEJM198611133152014. [DOI] [PubMed] [Google Scholar]
- 110.Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, Kubista TP, Poon MA, Meyers WC, Mailliard JA, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–15. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 111.O’Connell MJ, Martenson JA, Wieand HS, Krook JE, Macdonald JS, Haller DG, Mayer RJ, Gunderson LL, Rich TA. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502–7. doi: 10.1056/NEJM199408253310803. [DOI] [PubMed] [Google Scholar]
- 112.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 113.Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11) Clin Cancer Res. 2001;7:2182–94. [PubMed] [Google Scholar]
- 114.Garcia-Carbonero R, Supko JG. Current perspectives on the clinical experience, pharmacology, and continued development of the camptothecins. Clin Cancer Res. 2002;8:641–61. [PubMed] [Google Scholar]
- 115.Ando M, Hasegawa Y, Ando Y. Pharmacogenetics of irinotecan: a promoter polymorphism of UGT1A1 gene and severe adverse reactions to irinotecan. Invest New Drugs. 2005;23:539–45. doi: 10.1007/s10637-005-4022-6. [DOI] [PubMed] [Google Scholar]
- 116.Wasserman E, Myara A, Lokiec F, Goldwasser F, Trivin F, Mahjoubi M, Misset JL, Cvitkovic E. Severe CPT-11 toxicity in patients with Gilbert’s syndrome: two case reports. Ann Oncol. 1997;8:1049–51. doi: 10.1023/a:1008261821434. [DOI] [PubMed] [Google Scholar]
- 117.Rougier P, Van Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R, Navarro M, Morant R, Bleiberg H, Wils J, Awad L, Herait P, Jacques C. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet. 1998;352:1407–12. doi: 10.1016/S0140-6736(98)03085-2. [DOI] [PubMed] [Google Scholar]
- 118.Cunningham D, Pyrhonen S, James RD, Punt CJ, Hickish TF, Heikkila R, Johannesen TB, Starkhammar H, Topham CA, Awad L, Jacques C, Herait P. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998;352:1413–8. doi: 10.1016/S0140-6736(98)02309-5. [DOI] [PubMed] [Google Scholar]
- 119.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–7. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 120.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–14. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 121.Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol. 2007;25:4779–86. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 122.Van Cutsem ELR, Hossfel D, Bodoky G, et al. Randomized phase III trial comparing infused irinotecan/5-fluorouracil (5-FU)/folinic acid (IF) versus 5-FU/FA (F) in stage III colon cancer patients (PETACC 3) J Clin Oncol. 2005;23(Suppl):8. abstract. [Google Scholar]
- 123.Ychou MRJ, Douillard J, Bugat R, et al. A phase III randomized trial of LV5FU2+CPT-11 vs. LV5FU2 alone in adjuvant high risk colon cancer (FNCLCC Accord02/FFCD9802) J Clin Oncol. 2005;23(Suppl):3502. abstract. [Google Scholar]
- 124.Saltz LB, Niedzwiecki D, Hollis D, Goldberg RM, Hantel A, Thomas JP, Fields AL, Mayer RJ. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol. 2007;25:3456–61. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 125.Meropol NJ. A renewed call for equipoise. J Clin Oncol. 2007;25:3392–4. doi: 10.1200/JCO.2007.11.9503. [DOI] [PubMed] [Google Scholar]
- 126.Raymond E, Chaney SG, Taamma A, Cvitkovic E. Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol. 1998;9:1053–71. doi: 10.1023/a:1008213732429. [DOI] [PubMed] [Google Scholar]
- 127.Raymond E, Faivre S, Woynarowski JM, Chaney SG. Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol. 1998;25:4–12. [PubMed] [Google Scholar]
- 128.Becouarn Y, Ychou M, Ducreux M, Borel C, Bertheault-Cvitkovic F, Seitz JF, Nasca S, Nguyen TD, Paillot B, Raoul JL, Duffour J, Fandi A, Dupont-Andre G, Rougier P. Phase II trial of oxaliplatin as first-line chemotherapy in metastatic colorectal cancer patients. Digestive Group of French Federation of Cancer Centers. J Clin Oncol. 1998;16:2739–44. doi: 10.1200/JCO.1998.16.8.2739. [DOI] [PubMed] [Google Scholar]
- 129.Diaz-Rubio E, Sastre J, Zaniboni A, Labianca R, Cortes-Funes H, de Braud F, Boni C, Benavides M, Dallavalle G, Homerin M. Oxaliplatin as single agent in previously untreated colorectal carcinoma patients: a phase II multicentric study. Ann Oncol. 1998;9:105–8. doi: 10.1023/a:1008200825886. [DOI] [PubMed] [Google Scholar]
- 130.Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B, Bertheaut-Cvitkovic F, Larregain-Fournier D, Le Rol A, Walter S, Adam R, Misset JL, Levi F. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–47. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 131.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 132.Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK, Hart LL, Gupta S, Garay CA, Burger BG, Le Bail N, Haller DG. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003;21:2059–69. doi: 10.1200/JCO.2003.11.126. [DOI] [PubMed] [Google Scholar]
- 133.Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 134.Raymond E, Faivre S, Chaney S, Woynarowski J, Cvitkovic E. Cellular and molecular pharmacology of oxaliplatin. Mol Cancer Ther. 2002;1:227–35. [PubMed] [Google Scholar]
- 135.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 136.Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, Carteni G, Agostara B, Pezzella G, Manzione L, Borsellino N, Misino A, Romito S, Durini E, Cordio S, Di Seri M, Lopez M, Maiello E, Montemurro S, Cramarossa A, Lorusso V, Di Bisceglie M, Chiarenza M, Valerio MR, Guida T, Leonardi V, Pisconti S, Rosati G, Carrozza F, Nettis G, Valdesi M, Filippelli G, Fortunato S, Mancarella S, Brunetti C. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol. 2005;23:4866–75. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 137.Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–14. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 138.Porschen R, Arkenau HT, Kubicka S, Greil R, Seufferlein T, Freier W, Kretzschmar A, Graeven U, Grothey A, Hinke A, Schmiegel W, Schmoll HJ. Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO Colorectal Study Group. J Clin Oncol. 2007;25:4217–23. doi: 10.1200/JCO.2006.09.2684. [DOI] [PubMed] [Google Scholar]
- 139.Diaz-Rubio E, Tabernero J, Gomez-Espana A, Massuti B, Sastre J, Chaves M, Abad A, Carrato A, Queralt B, Reina JJ, Maurel J, Gonzalez-Flores E, Aparicio J, Rivera F, Losa F, Aranda E. Phase III study of capecitabine plus oxaliplatin compared with continuous-infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. J Clin Oncol. 2007;25:4224–30. doi: 10.1200/JCO.2006.09.8467. [DOI] [PubMed] [Google Scholar]
- 140.Cassidy J, Clarke S, Diaz-Rubio E, et al. XELOX vs. FOLFOX 4: Efficay results from XELOX-1/NO16966, a randomized phase III trial in first-line metastatic colorectal cancer (MCRC) Gastrointestinal Cancer Symposium. 2007:219. (abstr 270) [Google Scholar]
- 141.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–51. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 142.Kuebler JP, Wieand HS, O’Connell MJ, Smith RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE, Atkins JN, Zapas JL, Goodwin JW, Fehrenbacher L, Ramanathan RK, Conley BA, Flynn PJ, Soori G, Colman LK, Levine EA, Lanier KS, Wolmark N. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25:2198–204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 143.Land SR, Kopec JA, Cecchini RS, Ganz PA, Wieand HS, Colangelo LH, Murphy K, Kuebler JP, Seay TE, Needles BM, Bearden JD, 3rd, Colman LK, Lanier KS, Pajon ER, Jr, Cella D, Smith RE, O’Connell MJ, Costantino JP, Wolmark N. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol. 2007;25:2205–11. doi: 10.1200/JCO.2006.08.6652. [DOI] [PubMed] [Google Scholar]
- 144.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 145.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 146.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 147.Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, Hambleton J, Novotny WF, Kabbinavar F. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23:3502–8. doi: 10.1200/JCO.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 148.Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354:980–2. doi: 10.1056/NEJMc052954. discussion 980–2. [DOI] [PubMed] [Google Scholar]
- 149.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–5. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 150.Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:3706–12. doi: 10.1200/JCO.2005.00.232. [DOI] [PubMed] [Google Scholar]
- 151.Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB., 3rd Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–44. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 152.Saltz L, Clarke S, Diaz-Rubio E, et al. Bevacizumab (Bev) in combination with XELOX or FOLFOX4: Updated efficacy results from XELOX-1/NO16966, a randomized phase III trial in first-line metastatic colorectal cancer. J Clin Oncol. 2007;25(18S) Abstr 4028. [Google Scholar]
- 153.Baselga J. Why the epidermal growth factor receptor? The rationale for cancer therapy. Oncologist. 2002;7 (Suppl 4):2–8. doi: 10.1634/theoncologist.7-suppl_4-2. [DOI] [PubMed] [Google Scholar]
- 154.Spaulding DC, Spaulding BO. Epidermal growth factor receptor expression and measurement in solid tumors. Semin Oncol. 2002;29:45–54. doi: 10.1053/sonc.2002.35647. [DOI] [PubMed] [Google Scholar]
- 155.Messa C, Russo F, Caruso MG, Di Leo A. EGF, TGF-alpha, and EGF-R in human colorectal adenocarcinoma. Acta Oncol. 1998;37:285–9. doi: 10.1080/028418698429595. [DOI] [PubMed] [Google Scholar]
- 156.Porebska I, Harlozinska A, Bojarowski T. Expression of the tyrosine kinase activity growth factor receptors (EGFR, ERB B2, ERB B3) in colorectal adenocarcinomas and adenomas. Tumour Biol. 2000;21:105–15. doi: 10.1159/000030116. [DOI] [PubMed] [Google Scholar]
- 157.Mayer A, Takimoto M, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993;71:2454–60. doi: 10.1002/1097-0142(19930415)71:8<2454::aid-cncr2820710805>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 158.Messersmith WA, Hidalgo M. Panitumumab, a monoclonal anti epidermal growth factor receptor antibody in colorectal cancer: another one or the one? Clin Cancer Res. 2007;13:4664–6. doi: 10.1158/1078-0432.CCR-07-0065. [DOI] [PubMed] [Google Scholar]
- 159.Venook AP. Epidermal growth factor receptor-targeted treatment for advanced colorectal carcinoma. Cancer. 2005;103:2435–46. doi: 10.1002/cncr.21123. [DOI] [PubMed] [Google Scholar]
- 160.Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, Tebbutt NC, van Hazel G, Wierzbicki R, Langer C, Moore MJ. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–8. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 161.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 162.Saltz L, Rubin M, Hochster H, Tchekmeydian NS, Waksal H, Needle M, LoBuglio A. Cetuximab (IMC-C225) Plus Irinotecan (CPT-11) is Active in CPT-11-Refractory Colorectal Cancer (CRC) that Expresses Epidermal Growth Factor Receptor (EGFR) Proc Am Soc Clin Oncol. 2001;20:3a. [Google Scholar]
- 163.Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–8. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 164.Perez-Soler R, Saltz L. Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol. 2005;23:5235–46. doi: 10.1200/JCO.2005.00.6916. [DOI] [PubMed] [Google Scholar]
- 165.Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L, Klimstra DS, Fridman D, Kelsen DP, Saltz LB. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–10. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 166.Hebbar M, Wacrenier A, Desauw C, Romano O, Cattan S, Triboulet JP, Pruvot FR. Lack of usefulness of epidermal growth factor receptor expression determination for cetuximab therapy in patients with colorectal cancer. Anticancer Drugs. 2006;17:855–7. doi: 10.1097/01.cad.0000217425.44584.9f. [DOI] [PubMed] [Google Scholar]
- 167.Venook A, Niedzwiecki D, Hollis D, et al. Phase III trial of irinotecan/5FU/LV (FOLFIRI) or oxaliplatin/5FU/LV (FOLFOX) =/− cetuximab for patients (pts) with untreated metastatic adenocarcinoma of the colon or rectum (MCRC): CALGB 80203 preliminary results. J Clin Oncol. 2006;24:148s. (abstr 3509) [Google Scholar]
- 168.Van Cutsem E, Nowacki M, Lang I, et al. Randomized phase III study of irinotecan and 5-FU/LV with or without cetuximab in the first line treatment of patients with metastatic colorectal cancer. J Clin Oncol. 2007;25(164s) abstr 4000. [Google Scholar]
- 169.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 170.Malik I, Hecht JR, Patnaik A, Venook A, Berlin J, Croghan G, Navale L, MacDonald M, Jerian S, Meropol NJ. Safety and efficacy of panitumumab monotherapy in patients with metastatic colorectal cancer. In Proc Am Soc Clin Oncol. 2005 [Google Scholar]
- 171.Sartore-Bianchi A, Moroni M, Veronese S, Carnaghi C, Bajetta E, Luppi G, Sobrero A, Barone C, Cascinu S, Colucci G, Cortesi E, Nichelatti M, Gambacorta M, Siena S. Epidermal growth factor receptor gene copy number and clinical outcome of metastatic colorectal cancer treated with panitumumab. J Clin Oncol. 2007;25:3238–45. doi: 10.1200/JCO.2007.11.5956. [DOI] [PubMed] [Google Scholar]
- 172.Cappuzzo F, Finocchiaro G, Rossi E, Janne PA, Carnaghi C, Calandri C, Bencardino K, Ligorio C, Ciardiello F, Pressiani T, Destro A, Roncalli M, Crino L, Franklin WA, Santoro A, Varella-Garcia M. EGFR FISH assay predicts for response to cetuximab in chemotherapy refractory colorectal cancer patients. Ann Oncol. 2007 doi: 10.1093/annonc/mdm492. [DOI] [PubMed] [Google Scholar]
- 173.Finocchiaro G, Cappuzzo F, Janne PA, et al. EGFR, HER2, Kras as predictive factors for cetuximab sensitivity in colorectal cancer. J Clin Oncol. 2007;25(18S) Abstr 4021. [Google Scholar]
- 174.Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, Wong TW, Huang X, Takimoto CH, Godwin AK, Tan BR, Krishnamurthi SS, Burris HA, 3rd, Poplin EA, Hidalgo M, Baselga J, Clark EA, Mauro DJ. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–7. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 175.Lievre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, Ychou M, Bouche O, Landi B, Louvet C, Andre T, Bibeau F, Diebold MD, Rougier P, Ducreux M, Tomasic G, Emile JF, Penault-Llorca F, Laurent-Puig P. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–9. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 176.Amado R, Wolf M, Freeman D, et al. Panitumumab (pmab) efficacy and patient-raeported outcomes (PRO) in metastatic colorectal cancer (mCRC) patients (pts) with wild-type (WT) KRAS tumor status. Presented at: the 2008 American Society of Clincal Oncology Gastrointestinal Cancers Symposium; January 25–27, 2008; Orlando, FL. abstract 278. [Google Scholar]
- 177.Saltz LB, Lenz HJ, Kindler HL, Hochster HS, Wadler S, Hoff PM, Kemeny NE, Hollywood EM, Gonen M, Quinones M, Morse M, Chen HX. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J Clin Oncol. 2007;25:4557–61. doi: 10.1200/JCO.2007.12.0949. [DOI] [PubMed] [Google Scholar]
- 178.Hecht J, Mitchell EP, Chidiac T, et al. An updated analysis of safety and efficacy of oxaliplatin (Ox)/bevacizumab (bev) +/− panitumumab (pmab) for first-line treatment (tx) of metastatic colorectal cancer (mCRC) from a randomized controlled trial (PACCE). Presented at: the 2008 American Society of Clincal Oncology Gastrointestinal Cancers Symposium; January 25–27, 2008; Orlando, FL. abstract 273. [Google Scholar]
- 179.Boring CC, Squires TS, Tong T. Cancer statistics, 1992. CA Cancer J Clin. 1992;42:19–38. doi: 10.3322/canjclin.42.1.19. [DOI] [PubMed] [Google Scholar]
- 180.Schrag D. The price tag on progress--chemotherapy for colorectal cancer. N Engl J Med. 2004;351:317–9. doi: 10.1056/NEJMp048143. [DOI] [PubMed] [Google Scholar]
- 181.Meropol NJ, Schulman KA. Cost of cancer care: issues and implications. J Clin Oncol. 2007;25:180–6. doi: 10.1200/JCO.2006.09.6081. [DOI] [PubMed] [Google Scholar]
- 182.Scheithauer W, Rosen H, Kornek GV, Sebesta C, Depisch D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. Bmj. 1993;306:752–5. doi: 10.1136/bmj.306.6880.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Palliative chemotherapy for advanced or metastatic colorectal cancer: Colorectal Meta-analysis Collaboration. Cochrane Datavase Syst Rev. 2000;2:CD001545. doi: 10.1002/14651858.CD001545. [DOI] [PMC free article] [PubMed] [Google Scholar]


