Abstract
Pannexins are mammalian orthologs of innexins and have a predicted topological folding pattern similar to that of connexins, except they are glycosylated. We previously showed that rat pannexin1 is glycosylated at N254 and this residue is important for plasma membrane targeting. Here we demonstrate that cell surface expression levels of the rat pannexin1 N254Q mutant are rescued by co-expression with the wild-type protein. In paired Xenopus oocytes, the functional effect of this rescue is inconsequential, however cell surface de-glycosylation by PNGase F significantly enhanced functional gap junction formation. In mammalian cells, wild-type oligomers traffic at a slower rate than Myc or tetracysteine domain tagged versions, a behavior opposite to that of tagged connexins. The temporal differences of Panx1 trafficking correlate with spatial differences of intracellular localizations induced by Golgi-blockage by Brefeldin-A or glycosylation prevention by tunicamycin. Therefore, Panx1 has kinetics and dynamics that make it unique to serve distinct functions separate from connexin-based channels.
Keywords: pannexins, innexins, connexins, gap junctions, hemichannel
Introduction
Pannexins are the mammalian orthologs of innexins, the proteins that constitute invertebrate gap junctions (Panchin, 2005) as well as functional hemichannels (Bao et al., 2007). These two protein families show a predicted membrane topology similar to that of connexins, the constitutive proteins in vertebrate gap junctions. However, there is evidence that pannexin channels may fulfill a different function than connexins (Dahl and Locovei, 2006; Locovei et al., 2006). We recently showed that rat pannexin1 (rPanx1) is N-glycosylated at a unique residue on the second extracellular loop N254 and forms hexamers localized at the plasma membrane (Boassa et al., 2007). Inhibition of Panx1 glycosylation either pharmacologically or by site directed mutagenesis perturbs its expression at the cell surface. Taken together with electron microscopic data these studies indicated that glycosylated Panx1 does not form canonical gap junction structures. Furthermore, the glycosylation-deficient N254Q mutant of rPanx1 is retained in the endoplasmic reticulum strongly suggesting that this residue is important for plasma membrane targeting and that the carbohydrate moieties may serve to interfere with intercellular channel formation. Electrophysiological studies conducted in Xenopus oocytes pairs indicated that while Panx1 can form junctional channels (Boassa et al., 2007; Bruzzone et al., 2003), it does so at much reduced levels than connexins in either oocytes (Boassa et al., 2007) or in N2A cells (Penuela et al., 2007) implying that the functional form of Panx1 is a hemichannel (pannexon) in a single plasma membrane.
It has recently been reported that unlike connexins, murine Panx1 is unaffected by Brefeldin A treatment (Penuela et al., 2007) and turns over more slowly than connexins (Boassa et al., 2007; Penuela et al., 2007). In addition, varying amounts of cell surface expression of the N254Q mutant were also reported (Boassa et al., 2007; Penuela et al., 2007). Specifically, in our studies we used Myc-tagged N254Q mutant rPanx1 while Penuela and collaborators analyzed untagged mPanx1 suggesting the differences observed could be due to the effect of the tag on the protein.
Following up on our observations, we investigated the trafficking dynamics of native, mutant and tagged versions of Panx1. We show that cell surface expression levels of the N254Q mutant are rescued by co-expression with rPanx1 wild-type (WT) proteins. This rescue, however, was limited to the amount of unglycosylated Panx1 in the plasma membrane and did not result in an increase in gap junction formation. On the other hand, we show that de-glycosylation with the glycosidase PNGase F resulted in significantly higher junctional conductances in pairs of oocytes expressing Panx1.
Furthermore, using different pharmacological agents we analyzed the trafficking kinetics of tagged rPanx1 versus WT following inhibition of protein glycosylation or membrane traffic and secretion. It is well known that connexins have a short half-life estimated at 1.5-5 hours depending on the cell type (Laird, 2006) and that tagged connexins traffic at a slightly slower rate than WT (Jordan et al., 1999). While native Panx1 turns over significantly slower than connexins, here we demonstrate that in contrast to tagged connexins, Myc or tetracysteine (referred to as 4C) tagged Panx1 move more quickly through the cells.
Methods
Plasmids and mutagenesis
The cDNAs encoding rat Panx1 wild-type, -myc tagged were kindly provided by Dr. Roberto Bruzzone. Site-directed mutagenesis was done with the Quikchange kit (Stratagene, La Jolla, CA) as previously described (Boassa et al., 2007).
Cell culture and transfections
Human embryonic kidney (HEK) -293T and Madin-Darby canine kidney (MDCK) cells were maintained at 37°C, and 10 % CO2 in Dulbecco’s Modified Eagle’s medium containing 10% fetal bovine serum (GIBCO-BRL, Invitrogen, Carlsbad, CA). Transfections were performed by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Transductions were carried out using a retroviral system according to the protocols from the Nolan laboratory (www.stanford.edu/group/nolan). Experiments were conducted either 1-2 days after transient transfection or on stably expressing cell lines generated by transduction followed by selection with the antibiotic hygromycin (Gibco-BRL, Invitrogen).
Antibodies
We used the following primary antibodies: chicken anti-Panx1 (4515, characterized in (Locovei et al., 2006); mouse monoclonal anti-myc (Sigma, St. Louis, MO); rabbit polyclonal anti-giantin (Covance Research Products, Denver, PA).
Immunocytochemistry and confocal microscopy
Cells were grown on poly-D-lysine-coated glass coverslips, fixed in 4% paraformaldehyde/phosphate buffered saline for 20 minutes, washed and labeled for immunofluorescence. The primary antibodies (as described in figure legends) were mixed and diluted with blocking buffer diluted 5-fold in PBS. The secondary antibodies (fluorescein isothiocyanate-conjugated anti-mouse or anti-chicken, Cy5-conjugated anti-mouse and rhodamine red-X anti-rabbit) were diluted 1:100 in the same buffer. Data acquisition was done with an Olympus FluoView1000 laser-scanning confocal microscope (Olympus, Center Valley, PA).
Electrophysiology
The oocyte cell-cell channel assay was performed as described earlier (Dahl, 1992). The follicle layer was removed from oocytes by collagenase treatment. The oocytes were injected with in vitro transcribed Panx1 cRNA and incubated for 2-3 days. The vitelline membrane was removed with forceps and the oocytes paired. Junctional conductance was determined with the dual voltage clamp technique (Spray et al., 1981) 6 hours after pairing. Pharmacological treatment of oocytes consisted of 30 minute incubations treated with either soybean glycine max (10 μg/ml, Sigma, St. Louis, MO), tunicamycin (1 μg/ml, Sigma, St. Louis, MO), or PNGase F (10 units/ml, New England Biolabs, Ipswich, MA) prior to pairing.
Gel electrophoresis and immunoblotting
Western blot were performed as described earlier (Towbin et al., 1979). Proteins were extracted from cells in SDS buffer containing 4% β-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride, and a protease inhibitor cocktail (Sigma, St. Louis, MO), separated on a 4-20% Tris-glycine gel (Invitrogen, Carlsbad, CA), and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA). Membranes were blocked overnight in 5% nonfat dry milk and 0.5% Tween 20 in PBS, and incubated for 1 hr with the primary antibody chicken anti-Panx1 (1:1000). Blots were developed with an ECL kit (Amersham Biosciences, Arlington Heights, IL). The intensity of signals was quantified using the ImageJ software (Rasband, W.S., National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997-2004).
Tunicamycin and Brefeldin-A treatment
MDCK cells stably expressing rPanx1-WT, rPanx1-Myc, or rPanx1-4C were incubated with tunicamycin (2 μg/ml, Sigma, St. Louis, MO) or BFA (5 μg/ml, Sigma, St. Louis, MO) for specified incubation times. After treatment, cells were lysed in SDS buffer for Western blotting as described above. For immunocytochemistry, cells were fixed, immunolabeled and imaged using a confocal microscope.
In vivo labeling with FlAsH-EDT2 and ReAsH-EDT2
MDCK cells stably expressing a tetracysteine domain (FLNCCPGCCME) tagged rPanx1 (rPanx1-4C) (Martin et al., 2005) were labeled for 1 hour at 37°C with 0.9 μM FlAsH-EDT2 /12.5 μM EDT in HBSS. Free and nonspecifically bound FlAsH was removed by washing with EDT (600 μM, 20 minutes at 37°C in HBSS). Cells were then incubated for 6 hours (chase time) in the presence of complete medium or with BFA. The newly synthesized recombinant proteins were stained at the end of the chase time with the second label (0.5 μM ReAsH-EDT2). After washing, cells were fixed with 4% paraformaldehyde for light microscopic imaging as previously described (Gaietta et al., 2002).
Results
In our recent studies, we identified a glycosylation site on the second extracellular loop of Panx1 and investigated how it affects Panx1 trafficking to the plasma membrane (Boassa et al., 2007). Another recent report by (Penuela et al., 2007) investigated the glycosylation of Panx1 and Panx3 and came to similar conclusions. In this follow up study, we investigate the cell surface expression rescue of the N254Q mutant by WT Panx1 into heteromeric pannexons and also the kinetics of how different tags can affect trafficking and cell surface expression of Panx1. In our initial work, we found that significant mis-trafficking, such as inclusion bodies with large protein aggregates, occurs with the addition of large fluorescent proteins, specifically EGFP or mCherry to the C-terminus or the N-terminus of the protein (data not shown); on the other hand minimal mis-trafficking artifacts occur with small tags such as Myc or the latest generation of 4C tags. Therefore, while large fluorescent proteins are useful to identify protein expression and trafficking using fluorescence microscopy, in our experiments we aimed to use only short tags or WT proteins in order to approach as near-to-native conditions as possible.
Cell surface expression of the glycosylation-deficient Panx1 N254Q mutant is rescued by co-expression with Panx1-wild type proteins
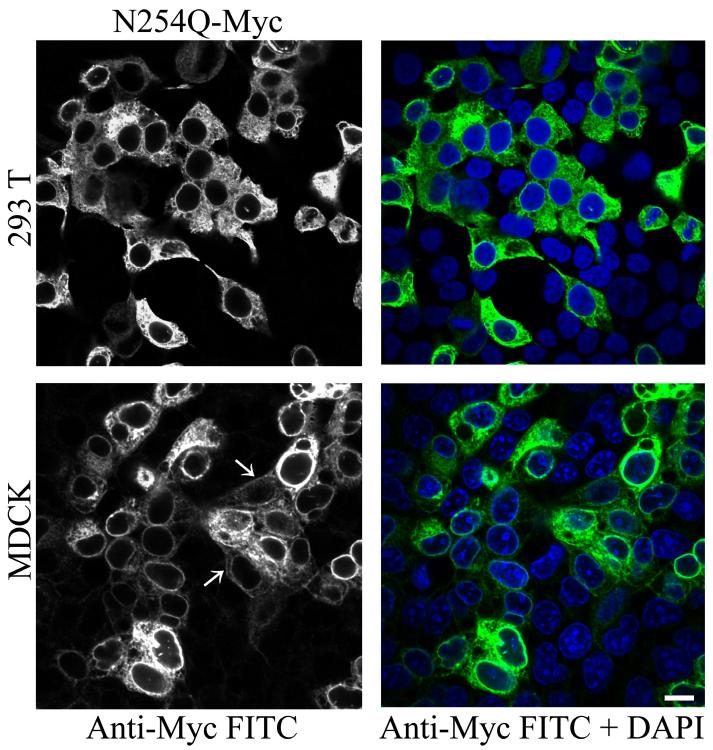
We previously reported that preventing glycosylation of rPanx1 by site-directed mutagenesis at the N254 residue results in high endoplasmic reticulum (ER) retention of the protein. Different cell types, such as HEK-293T and MDCK, transiently transfected with the Myc-tagged N254Q mutant showed a predominant intracellular localization (Figure 1). However, in MDCK cells, a small sub-population of the mutant proteins appeared to be expressed at the cell surface, as indicated by white arrows in Figure 1.
Figure 1. Preventing N-glycosylation at the N254 residue induces high ER retention and inhibits cell surface expression.
Immunolabeling of HEK-293T and MDCK cells transiently expressing Myc-tagged N254Q mutant rPanx1 proteins. Anti-Myc staining (left column in black and white) and the merged view with a nuclear stain DAPI (right column) are shown, and indicate high efficiency of transfection. White arrows show staining of the mutants localized at the cell surface in MDCK cells. The scale bar equals 10 μm.
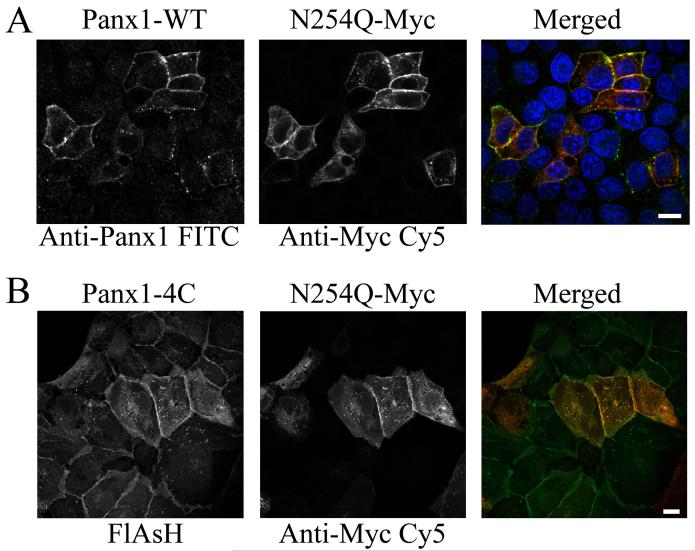
Because MDCK cells endogenously express Panx1, we tested whether the co-expression with WT protein could affect the localization pattern of the N254Q mutant. We did not observe endogenous protein expression of Panx1 in HEK293T cells although others have reported detection of Panx1 mRNA in HEK293 cells (Pelegrin and Surprenant, 2006). Co-transfection of MDCK cells with rPanx1-WT and the N254Q mutant (Figure 2A) clearly showed the rescuing effect on the cell surface expression of the mutant. Furthermore, MDCK cells stably expressing rPanx1-4C and transiently transfected with Myc-tagged N254Q mutant Panx1 (Figure 2B) also reconfirmed the ability of the mutant protein to be targeted to the cell surface when co-expressed with the 4C tagged WT.
Figure 2. Cell surface expression of the rPanx1 N254Q mutant is rescued by co-expression with rPanx1-wild type proteins.
(A) MDCK cells transiently co-expressing rPanx1-WT and Myc-tagged N254Q mutant proteins were stained with anti-Panx1 (FITC, left side in black and white), and anti-Myc (CY5, middle in black and white). Cell surface expression of the mutant proteins was rescued as shown by the yellow color in the merged view (right side, green for Anti-Panx1, red for anti-Myc and blue for nuclear stain DAPI). (B) MDCK cells stably expressing Panx1-4C and transiently transfected with Myc-tagged N254Q mutant Panx1 were stained with green profluorescent FlAsH-EDT2 (left side in black and white) and anti-Myc (CY5, middle in black and white). The cell surface expression of the mutant protein was also rescued by co-expression with the 4C-tagged Panx1 protein as displayed by the yellow in the merged view (FlAsH-EDT2 in green and anti-Myc in red). The scale bars equal 10 μm.
When expressed in Xenopus oocytes Panx1 is responsible for a large voltage-dependent non-junctional membrane conductance and fails to form a significant number of gap junction channels despite high expression levels (Boassa et al., 2007). Furthermore, N254Q -expressing oocytes exhibited a membrane conductance like uninjected oocytes validating a mechanism of impaired trafficking due to the lack of glycosylation. To test whether the co-expression of Panx1-WT and the N254Q mutant could have an impact on the ability of these proteins to predominantly form functional unapposed membrane channels versus intercellular channels, we co-injected oocytes with Panx1-WT and N254Q cRNA. After 2-3 days, the oocytes were paired and 6 hours later we determined junctional and non-junctional conductances by dual voltage clamp. No differences in junctional conductance were detected when compared to Panx1-WT alone. However, the membrane conductance (non-junctional conductance) was rescued by the co-injection validating the ability of the mutant to be targeted to the cell surface when co-expressed with the WT (Table 1).
Table 1.
Membrane (gm) and junctional (gj) conductance in μS of non-injected Xenopus oocytes (n=7) and oocytes expressing rPanx1-WT (n=14), N254Q mutant (n=10) and a combination of WT and N254Q mutant (n=11). The co-injected oocytes received the same total amount of cRNA as the ones injected with WT or mutant only
| Non-injected | rPanx1 WT | N254Q mutant | WT + mutant | |
|---|---|---|---|---|
| gm | 1.7 ± 0.6 | 3.7 ± 0.6 | 2.6 ± 0.7 | 4.0 ± 0.8 |
| gj | 0.01±0.001 | 0.26 ± 0.14 | 0.06 ± 0.03 | 0.21 ± 0.10 |
| gj/gm | 0.001 | 0.07 | 0.02 | 0.05 |
Carbohydrate cleavage facilitates Panx1 intercellular channel formation
In our previous publication, we found that Panx1 can form gap junction channels but at a highly reduced rate compared to Cx46. The presence of bulky carbohydrate groups on the extracellular domain of Panx1 would prevent the close apposition of two hemichannels impeding the formation of a gap junction, except for Panx1 in its de-glycosylated form. Even in heteromeric pannexons consisting of N254Q and WT, carbohydrate moieties should still sterically hinder docking at the extracellular surface.
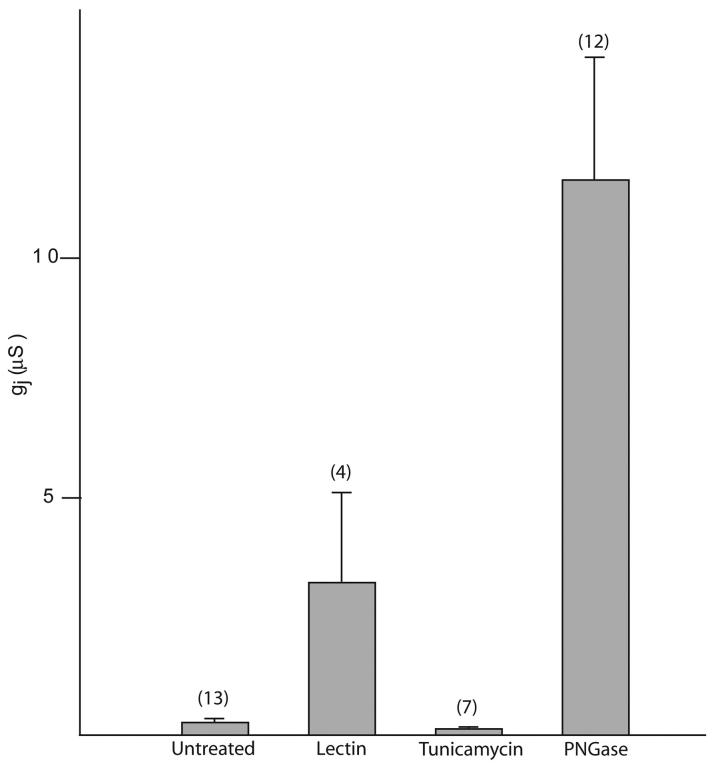
To further test this hypothesis, we analyzed the effect of deglycosylation treatments using different agents on the junctional conductance of oocyte pairs expressing mPanx1. The oocytes were treated with lectin (soybean glycine max, 10 μg/ml), tunicamycin (1 μg/ml), or PNGase F (10 units/ml) for 30 minutes before pairing. The junctional conductance was determined 6 hours after pairing.
As shown in Figure 3, after tunicamycin treatment a minimal decrease, instead of the expected increase, in junctional conductance (cell-to-cell channels) was observed compared to untreated oocytes, most likely due to a lower expression level at the cell surface linked to impaired trafficking due to lack of glycosylation. Consistently, the membrane conductance (single hexameric channels) after tunicamycin treatment was slightly decreased but not significantly different from the one recorded for untreated oocytes. Wang and colleagues previously showed that cell-cell coupling in Cx43 overexpressing cells can be induced by treatment with tunicamycin presumably because bulky glycoproteins fail to traffic to the cell membrane increasing the amount of close appositional areas suitable for connexin hemichannel docking (Wang et al., 1995).
Figure 3. Effect of deglycosylation treatments on the junctional conductance of oocyte pairs expressing mPanx1.
Panx1-WT cRNA was injected 2-3 days before pairing. The oocytes were treated with lectin (glycine max, 10 μg/ml), tunicamycin (1 μg/ml), or PNGase F (10 units/ml) for 30 minutes before pairing. Junctional conductance was determined 6 hours after pairing. Treatments that promoted the removal of carbohydrate groups from the extracellular surface significantly increased junctional conductance.
Both lectin and PNGase treatment induced a dramatic increase in junctional conductance, with the latter being 3 times higher. Lectins are carbohydrate-binding proteins, Levine and colleagues previously showed that a major effect of lectin treatment is the internalization of bulky glycoproteins facilitating intercellular channel formation (Levine et al., 1991). In our case, intercellular channel formation could occur by either non-glycosylated Panx1 or the endogenous Cx38 or a combination of both due to the removal of the bulky glycoproteins from the plasma membrane including the fully glycosylated Panx1. Because of our results on trafficking it is unlikely that non-glycosylated Panx1 proteins contribute to intercellular channel formation since glycosylation appears to be an important step in promoting expression of Panx1 proteins at the cell surface. On the other hand, PNGase is an enzyme that hydrolyzes nearly all types of N-glycan chains from glycoproteins. By removing all steric hindrance due to the carbohydrate moieties only, it might facilitate the formation of a tight seal between two hemichannels in order to assemble into a gap junction, including the contribution of the abundant de-glycosylated Panx1 as well as other endogenously expressed connexins.
Trafficking kinetics of rPanx1-WT, rPanx1-Myc and -4C tagged proteins following inhibition of protein glycosylation
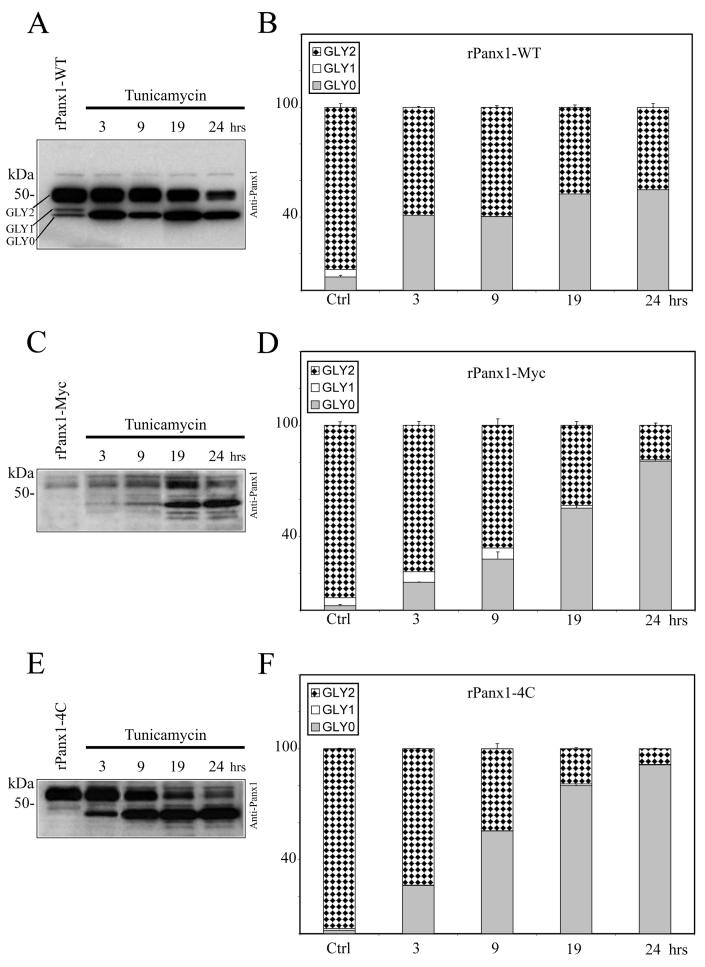
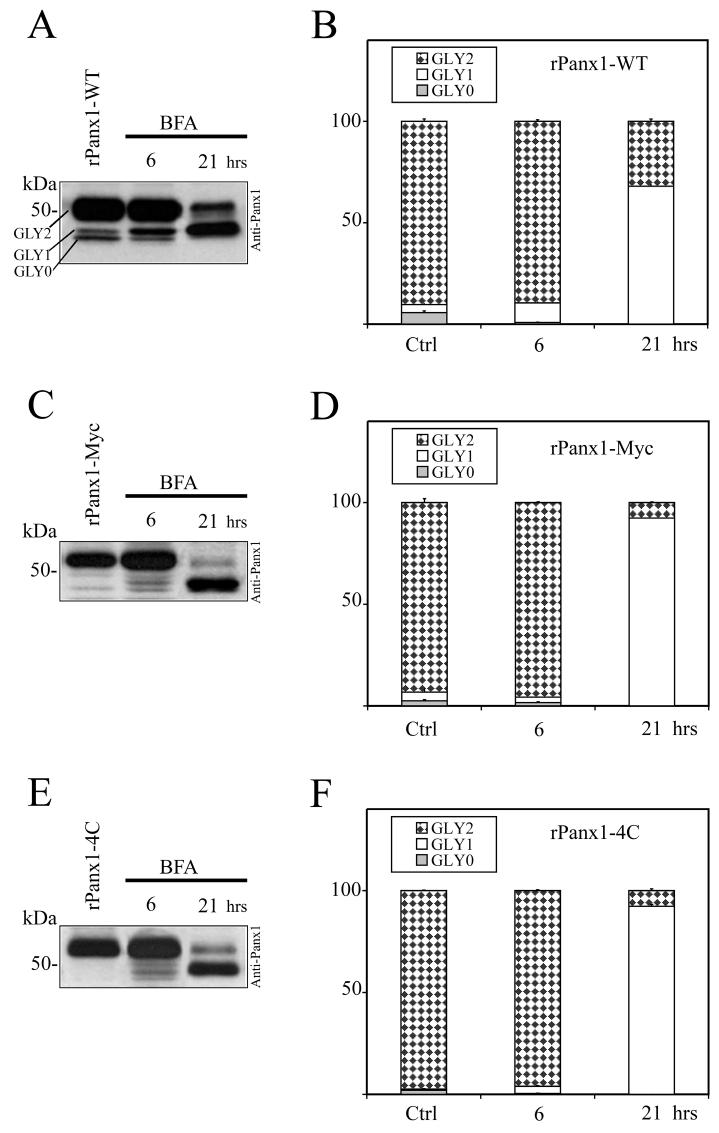
We compared the trafficking dynamics of rPanx1-WT versus tagged proteins following deglycosylation by pharmacological treatment. MDCK cells stably expressing rPanx1-WT, rPanx1-Myc, or rPanx1-4C were treated with tunicamycin (2 μg/ml) for different incubation times (Figure 4A-F). Over the periods of time analyzed, both the rPanx1-WT and the tagged proteins showed a significant increase in the non-glycosylated core protein (GLY0), and a decrease in the fully processed glycoprotein (GLY2, which is expressed at the cell surface). However, comparison of the trafficking kinetics of tagged rPanx1 versus WT revealed that WT GLY2 hexamers appear to be degraded at a slower rate than Myc or tetracysteine domain tagged versions. After 24 hours incubation with tunicamycin the WT GLY2 species composed 45% of the total proteins (Figure 4B) while the tagged GLY2 proteins were less than 20% (Figure 4D, F). Interestingly, Western blot analysis reveals the presence of additional bands of lower molecular weight for rPanx1-Myc and rPanx1-4C at 19 and 24 hours incubation times (Figure 4C, E) most likely linked to protein degradation. These bands were not observed for the rPanx1-WT (Figure 4A) consistent with a slower rate of degradation of the pre-existing glycosylated (GLY2) Panx1-WT at the cell surface.
Figure 4. Comparison of the effect of glycosylation inhibition on rPanx1-WT, rPanx1-Myc and rPanx1-4C tagged proteins.
Western blot analysis of cellular lysates from MDCK cells stably expressing rPanx1-WT (A), rPanx1-Myc (C), or rPanx1-4C (E) before and after tunicamycin treatment (2 μg/ml) for the indicated time periods (hrs). The blots were hybridized with anti-Panx1 antibody. The relative densities of the different forms of the protein (GLY0, non-glycosylated core protein, GLY1, high mannose type glycoprotein, and GLY2, fully processed glycoprotein) were quantified (y axis, normalized value) and represent the mean ± S.E. of three independent Western blots respectively for rPanx1-WT (B), rPanx1-Myc (D), or rPanx1-4C (F). In these samples under control conditions we observed some variations in GLY0 and GLY1 bands in tagged Panx1 while the majority of the proteins (more than 90%) were found in the GLY2 form. Glycosylation inhibition over periods of time induced a significant increase in the GLY0, and a decrease in the GLY2 species for both rPanx1-WT and tagged proteins. However, comparison of the trafficking kinetics of tagged rPanx1 versus WT revealed that WT GLY2 oligomers appear to be degraded at a slower rate than Myc or tetracysteine domain tagged versions.
Trafficking kinetics of Panx1-WT, Panx1-Myc and -4C tagged proteins following inhibition of protein secretion
BFA is a fungal antibacterial reagent typically used to block protein trafficking at the Golgi by promoting the breakdown of Golgi compartments (Lippincott-Schwartz et al., 1991; Lippincott-Schwartz et al., 1989). It has been previously used to uncouple gap junction assembly and study where oligomerization or phosphorylation events take place (Laird et al., 1995; Musil and Goodenough, 1993; Sosinsky et al., 2007). Because BFA has been reported to have no effect on the trafficking of pannexins as opposed to that of connexins (Penuela et al., 2007), we investigated the differences in trafficking dynamics of rPanx1-WT versus tagged proteins following treatment with BFA for 6 and 21 hours (Figure 5A-F). After 6 hours of incubation no major differences were reported for the different versions of rPanx1 protein analyzed, despite a partial increase in the high-mannose type glycoprotein (GLY1) that is consistent with its distribution in the ER. However, at 21 hours a significant decrease in GLY2 and increase in GLY1 was observed, and to a greater extent for tagged rPanx1 versus rPanx1-WT. These results are indicative of an accumulation of partially glycosylated GLY1 Panx1 proteins in the ER, and a slower rate of degradation of pre-existing WT GLY2 oligomers with respect to the tagged versions. In fact, the WT GLY2 composed 32% of the total proteins (Figure 5B) while tagged GLY2 were less than 8% (Figure 5D, F).
Figure 5. Comparison of the effect of BFA treatment on rPanx1-WT, rPanx1-Myc and rPanx1-4C tagged proteins.
Western blot analysis of cellular lysates from MDCK cells stably expressing rPanx1-WT (A), rPanx1-Myc (C), or rPanx1-4C (E) before and after BFA treatment (5 μg/ml) for the indicated time periods (hrs). The blots were hybridized with anti-Panx1 antibody and the relative densities (in the y axis as normalized values) of the different forms of protein analyzed as in Figure 3. Summary from three independent Western blots (mean ± S.E.) respectively for rPanx1-WT (B), rPanx1-Myc (D), or rPanx1-4C (F) is reported. A significant decrease in GLY2 and increase in GLY1 was observed with longer incubation time only, and to a greater extent for the tagged rPanx1 versus the rPanx1-WT.
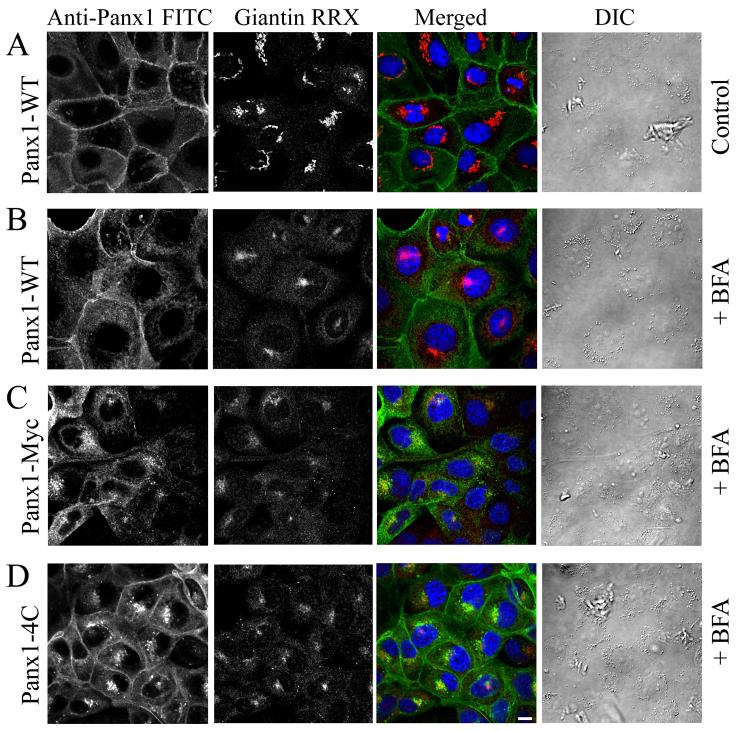
To further demonstrate that cell surface expression of tagged rPanx1 is affected more than rPanx1-WT by inhibition of protein secretion, immunocytochemistry was performed on MDCK cells stably expressing rPanx1-WT, rPanx1-Myc or rPanx1-4C tagged proteins treated with BFA for 21 hours (Figure 6). Immunostaining of the Golgi protein giantin was performed and served as positive control for the effectiveness of the drug in causing and maintaining Golgi fragmentation. Cell surface expression is more prominent for rPanx1-WT. On the contrary, the intracellular localization of newly synthesized proteins is predominant for the rPanx1-Myc and rPanx1-4C confirming that these tagged versions turn over at a more rapid pace.
Figure 6. Cell surface expression of tagged rPanx1 is affected more than rPanx1-WT by long incubation time with BFA.
(A) MDCK cells stably expressing rPanx1-WT in control conditions (not treated with BFA). To block protein secretion MDCK cells stably expressing rPanx1-WT (B), rPanx1-Myc (C) or rPanx1-4C (D) tagged proteins were treated with 5 μg/ml BFA for 21 hours. Confocal images of immunolabeled cells stained for anti-Panx1 (FITC) and with antibodies for the Golgi protein giantin (RRX) are displayed. The Golgi marker serves as positive control for the effectiveness of the drug in causing and maintaining Golgi fragmentation. Differential interference contrast (DIC) images show that cells morphology and viability was unaffected by the treatment. Cell surface expression is more prominent for rPanx1-WT, however the intracellular localization of newly synthesized proteins is predominant for the rPanx1-Myc and rPanx1-4C. The scale bar equals 10 μm.
Temporal and spatial segregation of different pools of rPanx1-4C following inhibition of protein secretion
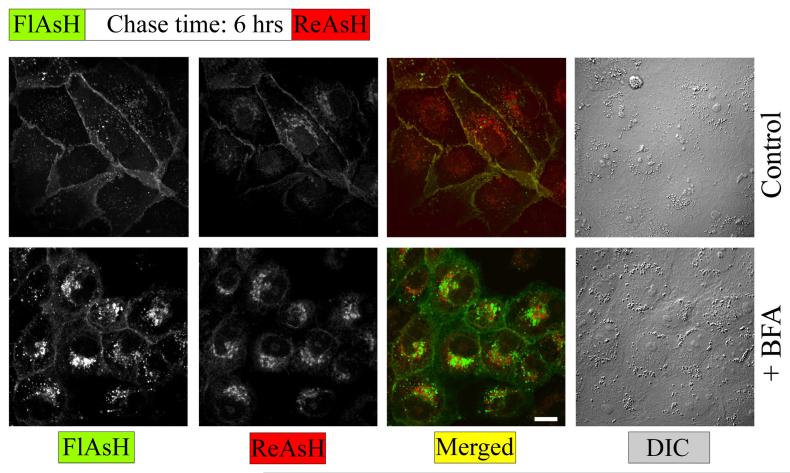
Using a pulse-chase labeling protocol with green profluorescent FlAsH-EDT2, and red profluorescent ReAsH-EDT2, we were able to segregate spatially and temporally distinct pools of rPanx1-4C (Figure 7). Under control conditions (regular medium) the newer proteins were distributed throughout the plasma membrane mixing with the older ones. However, after BFA treatment the nascent rPanx1-4C proteins (labeled with ReAsH-EDT2) were accumulated within intracellular compartments. The older proteins (labeled with FlasH-EDT2) were in part internalized from the cell surface although the majority was still localized at the cell surface.
Figure 7. Pulse chase experiments using FlAsH and ReAsH reveals temporal and spatial segregation of different pools of rPanx1-4C following BFA treatment.
MDCK cells stably expressing rPanx1-4C were labeled with green profluorescent FlAsH-EDT2, incubated for 6 hrs in regular medium (control) or in the presence of 5 μg/ml BFA, stained with red profluorescent ReAsH-EDT2, fixed, and imaged. After BFA treatment the nascent rPanx1-4C proteins (labeled with ReAsH) are accumulated within intracellular compartments. The older proteins (labeled with FlasH) are partially internalized although the majority is still localized at the cell surface. The scale bar equals 10 μm.
Discussion
The trafficking and function of membrane channels are highly regulated and finely tuned processes. For connexins, there are many examples of mutations causing intracellular aggregation of specific isoforms (Gerido and White, 2004; Laird, 2006). Moreover, while the overall trafficking patterns of Cx43 remains the same with the addition of GFP tags, C-terminal additions result in channels with altered gating properties while N-terminal additions result in non-functional gap junction channels or hemichannels (Contreras et al., 2003). The addition of GFP to Cx43 interferes with ZO-1 binding, causing the formation of abnormally large gap junction plaques (Hunter et al., 2005). In the case of pannexins, the post-translational modification of glycosylation adds another level of regulation to the expression and localization of these proteins.
Glycosylation can affect membrane proteins in several ways: changing their folding and stability, influencing their trafficking dynamics, and also interfering with their functional properties (Khanna et al., 2001; Petrecca et al., 1999; Watanabe et al., 2004). Our study shows that cell surface expression of Panx1 changes as a result of glycosylation. Glycosylation-deficient mutant proteins are retained in intracellular compartments and cell surface localization is rescued when the mutant is co-expressed with Panx1-WT proteins, indicating that lack of glycosylation does not influence the folding of the protein subunits therefore their ability to be assembled into oligomers. However, no functional rescuing was observed in the form of intercellular channels in Xenopus oocytes pairs co-expressing the N254Q mutant and WT proteins. This suggests that the carbohydrate groups attached at the extracellular domain of WT may suffice in heteromeric pannexons as a steric barrier to intercellular channel formation. This was confirmed by the observation that carbohydrate cleavage of Panx1 expressed at the plasma membrane using pharmacological agents (which we refer to as de-glycosylated species) induced Panx1 intercellular channel formation. Western blot analysis of different tissue lysates revealed multiple banding pattern due to the presence of glycosylated and non-glycosylated forms of Panx1 (Boassa et al., 2007; Huang et al., 2007; Penuela et al., 2007). This could be an in vivo strategy that specialized cell types have evolved to accommodate cell-to-cell communication needs. For instance, the glycosylated species, which we demonstrated is targeted to the plasma membrane, would be predominantly expressed to form unapposed membrane channels that have been implicated in intracellular calcium flux (Vanden Abeele et al., 2006), ATP release (Bao et al., 2004; Locovei et al., 2006), and large pore formation by P2X7 receptors (Locovei et al., 2007; Pelegrin and Surprenant, 2006, 2007). Alternatively, it could be speculated that the non-glycosylated species could potentially form intercellular channels if localized at the cell surface. This may apply to certain cell types whose functions involve extensive cell communication and/or coupling, such as astrocytes and endothelial cells. However, although we showed that de-glycosylated Panx1 indeed can form intercellular channels in oocytes pairs, it is unclear whether the non-glycosylated species can be targeted to the mammalian cell surface in vivo. In any event, these situations would be expected to impact cell-cell communication mechanisms.
It has been reported that Panx1 turns over more slowly than connexins (Boassa et al., 2007; Penuela et al., 2007). In this study we provide evidence that Panx1 is initially glycosylated in the ER and modified later in the Golgi apparatus where it resides en route to the plasma membrane. Both WT and Myc or 4C-tagged rPanx1 are N-glycosylated and properly trafficked to the plasma membrane. Using tunicamycin treatment to inhibit N-glycosylation, we found a significant increase in the non-glycosylated GLY0 core protein. Furthermore, we used BFA to impair protein secretion at the ER to Golgi transfer step, and identify intermediate processing of Panx1. Our results show a dramatic increase in the high-mannose type glycoprotein (GLY1) which is consistent with an accumulation of partially glycosylated GLY1 Panx1 proteins in the ER. This approach allowed us to follow the fate of pre-existing glycosylated Panx1 at the cell surface. Based on our observations the WT oligomers expressed at the cell surface appear to be degraded at a slower rate than Myc or 4C-tagged versions suggesting that the tags might interfere with some molecular chaperones important for stabilizing the protein at the cell surface. A more in depth analysis is necessary to fully characterize this trafficking pathway.
In summary, these newly discovered proteins represent a new paradigm for regulation of unapposed membrane channels and cell-cell channels by their biosynthetic pathway. For connexins, it has been proposed that their rapid turnover combined with the low number of active channels acts as a regulation mechanism for intercellular communication (Bukauskas et al., 2000; Musil et al., 2000). In this case, while the original expectation was that pannexins would be similar to connexins, in essence, new biosynthetic regulatory mechanisms have evolved as a way of modulating the expression of pannexin channels at the cell surface.
Acknowledgments
We are grateful to Dr. Roberto Bruzzone for the cDNA for the rPanx1-Myc and rPanx1-WT. This work is supported by NIH grants GM065937, GM072881 and NSF grant MCB-0131425 (GES) and NIH grant GM48610 (GD). The work presented here, except the electrophysiological experiments, was conducted at the National Center for Microscopy and Imaging Research at San Diego, which is supported by National Institutes of Health Grant RR04050 awarded to Dr. Mark Ellisman.
References
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Bao L, Samuels S, Locovei S, Macagno ER, Muller KJ, Dahl G. Innexins form two types of channels. FEBS Lett. 2007;581:5703–5708. doi: 10.1016/j.febslet.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem. 2007;282:31733–31743. doi: 10.1074/jbc.M702422200. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Jordan K, Bukauskiene A, Bennett MV, Lampe PD, Laird DW, Verselis VK. Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proc Natl Acad Sci U S A. 2000;97:2556–2561. doi: 10.1073/pnas.050588497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JE, Saez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci U S A. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G. The Xenopus oocyte cell-cell channel assay for functional analysis of gap junction proteins. In: Stevenson B, Paul D, editors. Cell-cell interactions A pratical approach. IRL; Oxfor: 1992. pp. 143–165. GW. [Google Scholar]
- Dahl G, Locovei S. Pannexin: to gap or not to gap, is that a question? IUBMB Life. 2006;58:409–419. doi: 10.1080/15216540600794526. [DOI] [PubMed] [Google Scholar]
- Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–507. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- Gerido DA, White TW. Connexin disorders of the ear, skin, and lens. Biochim Biophys Acta. 2004;1662:159–170. doi: 10.1016/j.bbamem.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Huang Y, Grinspan JB, Abrams CK, Scherer SS. Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia. 2007;55:46–56. doi: 10.1002/glia.20435. [DOI] [PubMed] [Google Scholar]
- Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16:5686–5698. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan K, Solan JL, Dominguez M, Sia M, Hand A, Lampe PD, Laird DW. Trafficking, assembly, and function of a connexin43-green fluorescent protein chimera in live mammalian cells. Mol Biol Cell. 1999;10:2033–2050. doi: 10.1091/mbc.10.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Myers MP, Laine M, Papazian DM. Glycosylation increases potassium channel stability and surface expression in mammalian cells. J Biol Chem. 2001;276:34028–34034. doi: 10.1074/jbc.M105248200. [DOI] [PubMed] [Google Scholar]
- Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DW, Castillo M, Kasprzak L. Gap junction turnover, intracellular trafficking, and phosphorylation of connexin43 in brefeldin A-treated rat mammary tumor cells. J Cell Biol. 1995;131:1193–1203. doi: 10.1083/jcb.131.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine E, Werner R, Dahl G. Cell-cell channel formation and lectins. Am J Physiol. 1991;261:C1025–1032. doi: 10.1152/ajpcell.1991.261.6.C1025. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner RD. Brefeldin A’s effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvin O, Tiunova A, Connell-Alberts Y, Panchin Y, Baranova A. What is hidden in the pannexin treasure trove: the sneak peek and the guesswork. J Cell Mol Med. 2006;10:613–634. doi: 10.1111/j.1582-4934.2006.tb00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Giepmans BN, Adams SR, Tsien RY. Mammalian cell-based optimization of the biarsenical-binding tetracysteine motif for improved fluorescence and affinity. Nat Biotechnol. 2005;23:1308–1314. doi: 10.1038/nbt1136. [DOI] [PubMed] [Google Scholar]
- Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74:1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- Musil LS, Le AC, VanSlyke JK, Roberts LM. Regulation of connexin degradation as a mechanism to increase gap junction assembly and function. J Biol Chem. 2000;275:25207–25215. doi: 10.1074/jbc.275.33.25207. [DOI] [PubMed] [Google Scholar]
- Panchin YV. Evolution of gap junction proteins--the pannexin alternative. J Exp Biol. 2005;208:1415–1419. doi: 10.1242/jeb.01547. [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. Embo J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 2007;282:2386–2394. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci. 2007;120:3772–3783. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- Petrecca K, Atanasiu R, Akhavan A, Shrier A. N-linked glycosylation sites determine HERG channel surface membrane expression. J Physiol. 1999;515(Pt 1):41–48. doi: 10.1111/j.1469-7793.1999.041ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosinsky GE, Solan JL, Gaietta GM, Ngan L, Lee GJ, Mackey MR, Lampe PD. The C-terminus of Connexin43 adopts different conformations in the golgi and gap junction as detected with structure specific antibodies. Biochem J. 2007 doi: 10.1042/BJ20070550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Harris AL, Bennett MV. Equilibrium properties of a voltage-dependent junctional conductance. Journal of General Physiology. 1981;77:77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Abeele F, Bidaux G, Gordienko D, Beck B, Panchin YV, Baranova AV, Ivanov DV, Skryma R, Prevarskaya N. Functional implications of calcium permeability of the channel formed by pannexin 1. J Cell Biol. 2006;174:535–546. doi: 10.1083/jcb.200601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Mehta PP, Rose B. Inhibition of glycosylation induces formation of open connexin-43 cell-to-cell channels and phosphorylation and triton X-100 insolubility of connexin-43. J Biol Chem. 1995;270:26581–26585. doi: 10.1074/jbc.270.44.26581. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Zhu J, Recio-Pinto E, Thornhill WB. Glycosylation affects the protein stability and cell surface expression of Kv1.4 but Not Kv1.1 potassium channels. A pore region determinant dictates the effect of glycosylation on trafficking. J Biol Chem. 2004;279:8879–8885. doi: 10.1074/jbc.M309802200. [DOI] [PubMed] [Google Scholar]