Abstract
One function of spatial attention is to enable goal-directed interactions with the environment through the allocation of neural resources to motivationally relevant parts of space. Studies have shown that responses are enhanced when spatial attention is predictively biased towards locations where significant events are expected to occur. Previous studies suggest that the ability to bias attention predictively is related to posterior cingulate cortex (PCC) activation (Small et al., 2003). Sleep deprivation (SD) impairs selective attention and reduces PCC activity (Thomas et al., 2000). Based on these findings, we hypothesized that SD would affect PCC function and alter the ability to predictively allocate spatial attention. Seven healthy, young adults underwent functional magnetic resonance imaging (fMRI) following normal rest and 34–36 hours of SD while performing a task in which attention was shifted in response to peripheral targets preceded by spatially informative (valid), misleading (invalid), or uninformative (neutral) cues. When rested, but not when sleep-deprived, subjects responded more quickly to targets that followed valid cues than those after neutral or invalid cues. Brain activity during validly cued trials with a reaction time benefit was compared to activity in trials with no benefit. PCC activation was greater during trials with a reaction time benefit following normal rest. In contrast, following SD, reaction time benefits were associated with activation in the left intraparietal sulcus, a region associated with receptivity to stimuli at unexpected locations. These changes may render sleep-deprived individuals less able to anticipate the locations of upcoming events, and more susceptible to distraction by stimuli at irrelevant locations.
Keywords: sleep deprivation, attention, functional imaging
1. Introduction
Sleep loss is common among the adult population. Only 26% of adults report getting the recommended 8 or more hours of sleep per night, and fifty percent of adults report feeling so sleepy that it interferes with their daily activities at least 1–2 times per week (National Sleep Foundation, 2005). Behavioral studies have demonstrated that sleep loss adversely affects a number of neurobehavioral domains, and in some cases this impairment is as great as that observed in individuals who are intoxicated (Dawson and Reid, 1997; Dinges and Kribbs, 1991).
Deficits in attention appear to underlie many of the performance impairments associated with sleep deprivation (Dinges and Kribbs, 1991). Sleep-deprived individuals are impaired in both shifting attention towards relevant stimuli (Gunter et al., 1987; Norton, 1970) and ignoring irrelevant or potentially misleading information (McCarthy and Waters, 1997; Norton, 1970).
One physiological correlate of attentional responses is the electrodermal orienting response to auditory stimuli. Following sleep deprivation, it is delayed, shows reduced amplitude, and habituates faster (McCarthy and Waters, 1997). These findings have been taken to indicate that sleep deprivation results in slower shifts to novel stimuli, decreased attentional allocation to stimuli, and a more rapid loss of attention to repeated stimuli, respectively. In addition, event related potentials during a cueing task in sleep-deprived subjects showed delayed latency at P255 and N350 at Cz and P3b at Pz, suggesting delayed covert orienting (Gunter et al., 1987). These studies support the notion that sleep loss impairs the effective allocation of attention to relevant target stimuli.
In a previous functional magnetic resonance imaging (fMRI) study, we demonstrated that activity in the posterior cingulate cortex (PCC) was related to the speed of response to spatially cued targets (Mesulam et al., 2001). Our group subsequently demonstrated that PCC activity was more specifically related to the degree that attention can be allocated, predictively (Small et al., 2003). This study calculated cue benefits as a metric of anticipatory attentional biasing. Cue benefits were defined as the reduction in response speed (i.e., faster responses) to targets preceded by directionally informative versus directionally uninformative cues. Greater cue benefits were associated with both faster reaction times to the spatially informative cues, consistent with the anticipatory biasing of spatial attention, and increased PCC activity (Mesulam et al., 2001; Small et al., 2003). In contrast, as cue benefits disappeared, intraparietal sulcus (IPS) activation was increased. This suggests that predictive attentional biasing was reduced on these trials, and that subjects were instead using a more global spatial strategy, potentially increasing their susceptibility to distracting stimuli.
Previous functional imaging studies have demonstrated reduced resting cerebral metabolism in posterior cingulate cortex both during sleep (Vogt and Laureys, 2005) and following sleep deprivation (Thomas et al., 2000). Furthermore, left parietal but not PCC activation was seen when subjects performed tasks following sleep deprivation versus a normal night of sleep (Strangman et al., 2005). These studies indicate that PCC activity is reduced by sleep deprivation. Based on our previous studies showing an association of PCC activity with predictive attentional orienting, we hypothesized that sleep loss would affect this relationship, leading to reduced PCC activity and an impaired ability to expectantly bias attention.
2. Results
2.1 Behavioral Data
In order to examine the effects of sleep deprivation on the relationship between PCC activity and attentional orienting, seven subjects underwent fMRI scanning while performing a Posner-type task of attentional orienting (see methods) in rested (R) and sleep-deprived (SD) conditions. While performing the task, subjects fixated centrally and were presented a series of peripheral target and foil symbols preceded by informative (valid), misleading (invalid), or uninformative (neutral) cues. To avoid the generation of temporal expectancy, cue-target stimulus onset asynchronies (SOA) were 200, 400, or 800 ms. Behavioral measures of errors of omission and commission, and mean reaction times for each trial type (valid, invalid, neutral) with errors removed were calculated (see methods). Following, the analysis of Small et al., (2003) valid trials were separated into those conferring a cue benefit on reaction time and valid trials that did not confer a cue benefit (V+ and V− respectively, see methods). These behavioral metrics were used to examine the effects of sleep deprivation on sustained attention and attentional orienting.
Performance was impaired in subjects following 34–36 hours of sleep deprivation as opposed to when they had a full night of sleep. Following Sleep deprivation, subjects made more errors of omission (R: 1.48% ± 0.41% vs SD: 24.35% ± 4.93%, p = 0.004) but showed no difference in commission errors (R: 3.88% ± 0.43% vs SD: 4.64% ± 0.51%, p = 0.28) compared with following a normal night of sleep. There were no differences in omission or commission errors by trial type (invalid, neutral, valid), side of target or SOA.
A two way ANOVA revealed significant differences in reaction times across cue types (F2,12 = 14.27, p < 0.001), but not between sleep states (F1,6 = 0.068, p > 0.8), see Table 1. There was a significant Sleep State by Cue Type interaction (F2,12 = 6.15, p = 0.014). All other main effects (side of target, SOA) and interactions were not significant. Bonferroni post hoc testing revealed no significant difference between sleep states for any cue type (valid cues p = 0.15; neutral cues p = 0.07; invalid cues p = 0.466). Within sleep state post hoc testing using the Games-Howell correction for repeated measures revealed that valid trials were faster than both neutral and invalid trials (p < 0.001 for valid versus neutral, and p = 0.024 for valid versus invalid) when subjects were rested, but not after 34–36 hours of sleep deprivation (p = 0.109 for valid versus neutral; p = 0.207 for valid versus invalid). Invalids were also faster than neutral trials for rested but not sleep deprived subjects (p = 0.031 for rested; p = 0.982 for sleep-deprived). Sleep-deprived subjects had significantly fewer valid trials showing a cue benefit (R: 66.0% ± 5.99% vs. SD: 46.4% ± 8.24%, p = 0.003). Of note, there was no significant difference between sleep states in the means or variances of the distribution of reaction times (see table 1). Within subject variance for neutral trials was not different across sleep states (Rested 8.0±1.4 ms; Sleep-deprived 10.7±3.4 ms, tpaired = −1.91, p > 0.1). This was important, since neutral trials were used to categorize valid trials.
Table 1.
Behavioral Results
| Mean RT (SE) by Sleep State (msecs) | ||
|---|---|---|
| Rested | Sleep Deprived | |
| Valid | 272 (2.7) | 281 (3.0) |
| Neutral | 301 (3.3) | 292 (3.6) |
| Invalid | 285 (5.3) | 293 (6.1) |
2.2 fMRI Data
As in our previous studies, blood oxygen level dependent (BOLD) responses during valid trials (i.e., trials with valid cues) were analyzed (Gitelman et al., 1999; Mesulam et al., 2001; Small et al., 2003). Specifically, BOLD responses were examined for contrasts between V+ and V− trials in SD and R states Small et al. (2003). Contrasting these events allows for the examination of the effects of sleep deprivation on neural mechanisms relevant to attentional orienting. During data collection, functional data was lost in one subject leaving six subjects for all fMRI analyses.
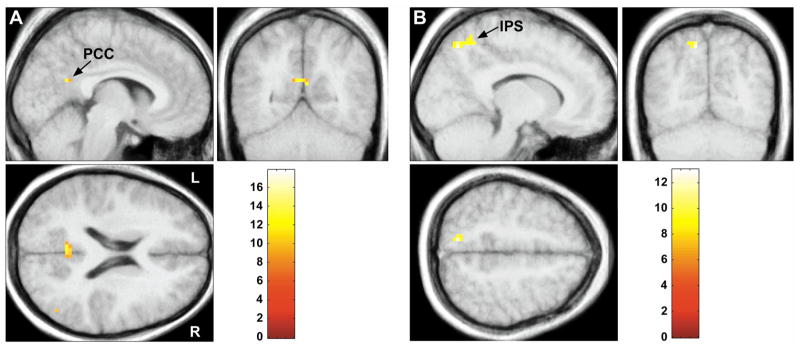
Simple main effects relating to the presence of a cue benefit (V+ − V−) in the rested state showed greater activation within the PCC, Table 2. The “positive” interaction of sleep state (R - SD) with cue benefit (V+ − V−) showed significantly greater BOLD responses within the posterior cingulate and the bilateral middle-temporal gyri (mTG), Table 2 and Figure 2A. In contrast, the “negative” interaction of sleep state (SD - R) with cue benefit (V+ − V−) demonstrated greater activation within the medial intraparietal sulcus, Table 2 and Figure 2B.
Table 2.
Activations by sleep state and contrast
| Sleep State and Brain Region | MNI coordinates | Z score | # of voxels | Mean (SPM ) and cluster FWHM mm | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Rested (V+ > V− ) | (9.10) | |||||
| Posterior cingulate cortex | 0 | −39 | 45 | 3.83 | 12 | 7.30 |
| Rested (V−> V+) | (9.37) | |||||
| Right inferior parietal lobule | 30 | −63 | 33 | 4.09 | 35 | 7.12 |
| Right inferior parietal lobule | 36 | −75 | 27 | 3.78 | 18 | 5.66 |
| SD (V−> V+) | (9.37) | |||||
| Right middle temporal gyrus | 51 | −57 | 0 | 4.10 | 29 | 7.49 |
| Left inferior frontal gyrus | −57 | 12 | 24 | 3.88 | 13 | 6.10 |
| Rested - SD (V+ > V−) | (9.37) | |||||
| Left posterior cingulate cortex | −6 | −57 | 21 | 3.72 | 14 | 7.96 |
| Left middle temporal gyrus | −54 | −9 | −24 | 4.41 | 61 | 7.25 |
| Right middle temporal gyrus | 63 | −6 | −15 | 4.03 | 17 | 7.37 |
| SD – Rested (V > V−) | (9.37) | |||||
| Left intraparietal sulcus | −12 | −69 | 51 | 4.06 | 26 | 6.15 |
Figure 2.
Activation related to the presence of cue benefit (V+ − V−). A) PCC activation in the rested state greater than the sleep-deprived state. B) IPS activation in the sleep-deprived state greater than the rested state. All peaks are significant at p < 0.05 corrected at the cluster level. The color bar identifies the t-values.
In order to examine the interaction effects more closely, BOLD signal was extracted for both sleep states from the maxima in the PCC (xyz: −6 −57 21) and IPS (xyz: −12 −69 51) clusters, and plotted as peri-stimulus time histograms, Figure 3. In the rested state, activity in the PCC was increased for V+ trials and reduced for V− trials. Following SD, activity in the PCC did not change significantly for either V+ or V− trials. In contrast, in the SD state IPS activity was increased during V+ trials and reduced during V− trials.
Figure 3.
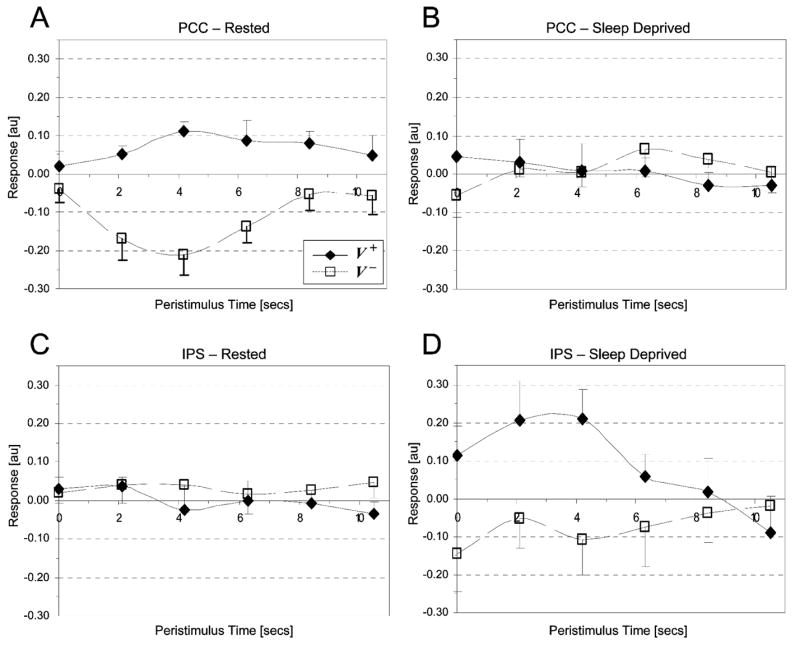
Peri-stimulus time histograms for V+ (diamonds) and V− (squares) trials in Rested and Sleep Deprived states for the maxima from the PCC (xyz: −6 −57 21) and IPS (xyz: −12 −69 51) clusters. (A) Mean group BOLD responses in the rested state within the PCC for V+ (solid line) and V− (dashed line) trials. (B) Mean group BOLD response in the sleep-deprived state within the PCC for V+ and V− trials. (C) Mean group BOLD response in the rested state within the IPS for V+ and V− trials. (D) Mean group BOLD response in the sleep-deprived state within the IPS for V+ and V− trials. Values are group means ± standard error of the mean. The graphs demonstrate the greatest cue benefits (difference between V+ and V−) in the PCC when subjects are rested (3A) and the IPS when they are sleep deprived (3D).
The V− − V+ contrast was also examined to determine areas that were more active in the absence of a cue benefit. In the rested state, activations were seen in right inferior parietal cortex, while in the SD state, activations were seen in the right middle temporal gyrus and left inferior frontal gyrus.
In SPM the calculation for cluster statistics is dependent on the “smoothness” of the region, i.e. the average number of voxels containing BOLD signals that significantly correlate with each other (Hayasaka et al., 2004). Under lower degrees of freedom, this value is more likely to vary across the image. Regions that are “smoother” have a greater chance of containing false positive clusters. In order to reduce the chance of a Type I error, a measure of smoothness, full width at half maximum (FWHM), was examined. Clusters with a mean FWHM larger than the one used by SPM, were designated as non-significant because of the greater chance of a false positive cluster in these regions. The mean FWHM for each cluster is listed in Table 2. The FWHM values used by SPM for each of the contrasts are in parentheses. As shown in Table 2, the actual FWHM of the clusters was smaller than the FWHM used by SPM, suggesting it was unlikely that the Type I error rate was increased for these clusters.
3. Discussion
This preliminary report examined the influence of sleep state on the neural mechanisms underlying the anticipatory biasing of spatial attention. Previous reports have shown that sleep deprivation impairs performance and alters brain activity when subjects perform tasks targeting attention, verbal learning, and working memory (Chee and Choo, 2004; Drummond et al., 2000; Drummond et al., 2001; Thomas et al., 2000; Wu et al., 1991). The current results suggest that sleep deprivation may also impair the anticipatory allocation of attention in response to spatially predictive cues and alter the underlying neural correlates.
Following sleep deprivation, subjects performed less accurately and made significantly more errors of omission than when rested, which has been seen in other studies of sleep deprivation (Williams et al., 1959). Although there was no main effect of sleep state on reaction time, an interaction with the type of cue did affect subjects’ responses such that valid trials were significantly faster than both neutral and invalid trials when subjects were rested but not when they were sleep-deprived. Sleep-deprived subjects also had significantly fewer trials conferring a cue benefit, despite the lack of differences in means and variances of reaction times when cue types were compared individually between sleep states. These results suggest that sleep deprivation may lead to an impairment in the anticipatory allocation of spatial attention through interacting effects on both spatial and non-spatial attentional components (de Gonzaga Gawryszewski et al., 1987; Jongen et al., 2006; Mesulam et al., 2001; Small et al., 2003).
In the rested state, the presence of a cue benefit was associated with activity within the PCC, whereas following sleep deprivation cue benefits were associated with activity within the IPS. Previous reports have also shown increased PCC activity when attention is shifted in response to spatially predictive cues (Hopfinger et al., 2001; Mesulam et al., 2001; Small et al., 2003). This relationship between performance and brain activity was demonstrated to be independent of reaction time, per se (Small et al., 2003). Instead, it was suggested that PCC activity was associated with the generation of a motivational bias for attending to a focal location in space (Small et al., 2003).
In contrast, studies have shown that IPS activity may display the opposite relationship to spatial cues, by demonstrating decreased activity when attention is allocated predictively to a location in space (Constantinidis and Steinmetz, 2001; Robinson et al., 1995; Small et al., 2003). These data have been taken to suggest IPS suppression may be necessary in order limit attentional receptivity to stimuli at unexpected locations. Consistent with these reports, the current study showed no change in IPS activity to spatial cues when subjects were rested, Figure 3C. However, in the SD state, IPS activity was increased for trials showing a cue benefit. Thus, when sleep-deprived, subjects appear to preferentially recruit the IPS when spatially orienting attention.
It is unclear why the relationship between cue benefit and brain activation is altered by sleep deprivation. One possibility is that PCC recruitment is impaired by sleep deprivation. This notion is supported by studies demonstrating reduced activity within the PCC following SD as compared to the rested state when subjects performed serial addition/subtraction and complex navigation tasks (Strangman et al., 2005; Thomas et al., 2000). Furthermore, positron emission tomography (PET) imaging of wake and non-rapid eye movement sleep (NREM) states have shown that activity in posterior cingulate is significantly reduced in NREM sleep, while medial parietal regions remain as active as when awake (Nofzinger et al., 2002). It is often argued that in a sleep-deprived state, errors of omission predominantly represent a brief transition to NREM sleep (Dinges and Kribbs, 1991; Williams et al., 1959). Taken together, these data suggest that it is possible that PCC recruitment is impaired in the sleep-deprived state due to the intermittent suppression of PCC activity when subjects generate errors of omission.
Because the recruitment of the PCC in the sleep-deprived state is impaired, cue benefits may depend on a strategy other than generating a motivational bias for attending to a focal location in space. We have shown that this strategy is associated with IPS activity. Greater IPS activity in the sleep-deprived state may reflect a strategy that relies on increasing receptivity to stimuli at unexpected locations. Thus, sleep-deprived individuals may shift from a focal endogenous orienting strategy to a more global exogenous orienting strategy.
Another possibility is that sleep-deprived subjects rely more on eye-movements to perform the task than when rested. Eye movements were not monitored in the present experiment, thus this possibility cannot be definitively addressed. However, we suggest that if this was the case we would have expected to see greater activity in the frontal eye fields and lateral IPS, which was not found (Corbetta et al., 1998; Nobre et al., 2000). In order to examine further the linkage between the generation of cue benefits and subject performance a cue benefit score calculated as previously described (Small et al., 2003) and regressed against BOLD responses. Results were similar to those seen in the categorical V+ and V− analysis further supporting the possibility that PCC and IPS activations were associated with cue benefits (see Table S1).
One limitation of the current study is the small number of subjects, which may potentially affect both the generalizability of the findings and the assumptions underlying the parametric statistics used to analyze the fMRI data. Random effects statistics were used to address the issue of population inference, and activations were found in the PCC and IPS. Similar sites of activation were also seen previously in other studies examining the anticipatory allocation of spatial attention (Mesulam, 2003; Small et al., 2003).
In order to address our use of cluster level parametric statistics in the setting of low degrees of freedom, we also calculated the mean FWHM values for each of the clusters, and compared these values with the average value used by SPM. In all cases, the FWHM in the cluster was smaller than the value used by SPM, suggesting that clusters identified as significant were unlikely to be false positives.
In the setting of low degrees of freedom, non-parametric statistics could have been used to analyze the fMRI data (Hayasaka et al., 2004). However, the power of non-parametric statistics may also be reduced by the small number of subjects (n=6), which would have only allowed a limited number of resamplings (26 = 64). The constrained number of resamplings limits the lowest possible p-value to 1/64 = 0.0156, thereby reducing the power of the technique (S. Hayasaka, personal communication). In the face of limitations to both parametric and non-parametric techniques, we chose to utilize standard parametric statistics, while attempting to minimize the chance of a Type I error. Nevertheless, replication and extension of these findings in a larger study will be important.
The standard reaction time pattern (Valid RT < Neutral RT < Invalid RT) was not replicated in the rested condition. Instead, neutral RT was slower than both valid and invalid trials in the rested state. We do not have a definitive explanation for this phenomenon. This pattern of reaction times with neutral trials being slower than invalid trials has been previously reported, albeit infrequently (Amir et al., 2003; Perchet et al., 2001; Posner et al., 1987). In some of these reports “neutral” trials were uncued, which was not the case in the present study. Posner (1987) attributed this reaction time pattern to diminished transient arousal due to the lack of an alerting signal (Posner et al., 1987). Nevertheless, we still believe that the neutral trials served as a valid comparison in the current study. The neutral trials were used to calculate V+ and V− trials as a way of normalizing subjects’ response times, i.e., to provide a baseline across subjects for dividing valid trials into those with (V+) and without (V−) cueing effects. Since subjects showed similar patterns of responses across runs for each sleep condition, we were reassured by the consistency of subjects’ responses. We therefore suggest that the neutral trials represent a reasonable choice for a baseline both within and across sleep conditions. We also note that standard errors were similar in both sleep states for neutral trials, making it unlikely that changes in variance affected the determination of V+ and V−.
To address further concerns about the neutral trials the analysis was also performed by using invalid trial reaction times as a baseline to categorize the valid trials Results were similar to those reported with neutral trials (see Table S2). This result provides further support that the current analysis demonstrates an effect of sleep deprivation on the anticipatory spatial biasing of attention.
In conclusion, sleep deprivation impairs the ability to utilize a predictive cue to shift attention towards relevant locations in space. This impairment is reflected in a lack of PCC activation, which has been implicated in the generation of an anticipatory bias for target location. Instead, it appears that SD subjects recruit the IPS when allocating spatial attention. This alternate strategy may depend on enhancing receptivity to stimuli in unexpected locations, thus shifting to more global, exogenous, attentional mechanisms that would rely more on IPS recruitment. Nevertheless, this strategy appears to be less effective overall, as there was no benefit of informative cues on reaction time (comparison of valid vs. neutral cues) in the SD state. These data suggest that sleep loss may affect performance by interfering with the ability to predictively allocate attention and to suppress distractibility to irrelevant spatial events. The consequence of this is that sleep-deprived individuals may miss predictive environmental cues and react impulsively to behaviorally irrelevant stimuli. Both responses are likely to increase errors and result in accidents even while individuals appear to be awake and responding.
4. Experimental Procedures
4.1 Subjects
Seven young, healthy adult subjects (19.5±2.3 years) participated in the study. Subjects were screened with questionnaires and in person interviews and no subject had a history of significant medical, neurological, or psychiatric illness. All subjects reported being right-handed. Their mean abridged Edinburgh handedness score was 57.1 ± 1.5 on a scale of −60 to +60 (Oldfield, 1971). All subjects gave written informed consent, and this study was approved by the Institutional Review Board at Northwestern University.
4.2 Procedure
Subjects completed two visits to the General Clinical Research Center (GCRC) at Northwestern Memorial Hospital. Before each visit, subjects were instructed to maintain a diary of their sleep-wake habits. Sleep habits were also monitored for 1–2 weeks prior to each GCRC visit using wrist actigraphy (Cambridge Neurotechnology, Cambridge, England). Subjects were instructed to obtain an average of 7–8 hours of sleep per night. The order of the GCRC visits was counter-balanced in a cross-over design with subjects acting as their own controls. In the rested condition (R), subjects entered the GCRC on the evening before scanning, and spent 8 hours time in bed (23:00-07:00). Subjects were scanned in the afternoon, 10–12 hours after awakening, between 17:00-19:00 h.
In the sleep-deprived condition (SD), subjects entered the GCRC on the evening before sleep deprivation began, and spent 8 hours time in bed (23:00-07:00). Subjects were allowed to leave during the first day between 08:00 - 17:00 h, but were instructed to remain awake. Wrist actigraphy recordings and sleep diaries were used to monitor subject compliance. Subjects were instructed to log the occurrence of any sleep episodes or naps during the study period. Subjects were also specifically questioned about naps, and none reported napping within the 3 days prior to scanning. Following 34–36 hours of wakefulness, subjects underwent functional MRI scanning. Subjects were required to sleep for at least eight hours in the GCRC, to recover from sleep deprivation, before they were sent home after scanning.
Prior to their first scanning session, subjects were familiarized with the task during a training session in the psychophysics laboratory. Subjects completed 3 training runs. There were 152 trials per run, which lasted just over 7 minutes. These data were only reviewed to make sure the subjects could perform the task. None of the subjects failed to perform adequately during the training session. The data are not discussed further here. Details of the training environment have been previously reported (Mesulam et al., 2001; Small et al., 2003).
4.3 Behavioral Task
The task used in this study was a variant of one developed by Posner for examining endogenously triggered shifts of spatial attention.(Posner, 1980; Small et al., 2003) Figure 1 illustrates trial organization and timing. Subjects were instructed to keep their eyes fixed on the central diamond throughout the experiment, and to respond to the appearance of targets “X” but not foils “+” in their peripheral vision. One-hundred milliseconds into a trial subjects saw a directional or non-directional cue. Directional cues involved the bolding of one-half of the central diamond, while non-directional cues, termed “neutral”, involved the bolding of the entire central diamond. Directional cues that correctly indicated the side of target or foil appearance, were termed “valid” or informative. Cues that pointed opposite to the side of target or foil appearance were termed “invalid” or misleading. To avoid the generation of temporal expectancy, the time between the appearance of the cue and the appearance of the target (stimulus onset asynchrony or SOA) could be 200, 400 or 800 ms. The target (or foil) then appeared for 100 msecs, and was followed by a variable end-trial interval of 1700, 1500 or 1100 msec respectively, so that the total trial duration was always 2100 msecs. Trials with reaction times less than 100 ms or greater than 1000 ms were discarded. Reaction times less than 100 ms or responses to foils were considered “errors of commission” or false positives. Trials with no response or reaction times greater than 1000 ms were considered “lapses” or false negatives.
Figure 1.
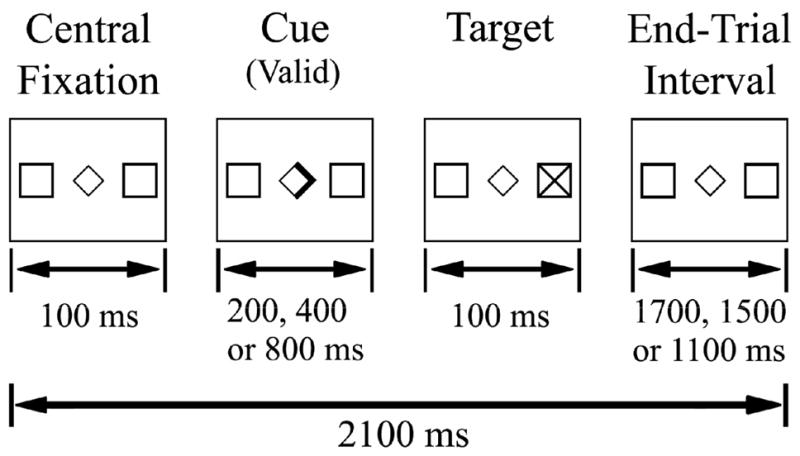
Schematic representation of the Posner task, and the timing parameters of a single trial. Subjects performed three runs in the scanner in each session. An event-related design was used. Each trial consisted of fixation followed by a cue presented for 200, 400, or 800 msecs. Following the cue, the target was presented for 100 msecs. The intertrial interval varied as a function of the SOA length to maintain an overall trial length of 2100 msecs, matching TR length.
An event-related design was used. Subjects completed three experimental runs in each scanning session. Each experimental run contained 152 trials (138 targets and 14 foils), of which 66% were directional (valid or invalid) and 34% were non-directional (neutral). Valid cues made up 80% of all directional cues. Trials lasted for 2.1 seconds, and each run lasted seven minutes, Figure 1. Fifty null events were distributed throughout the run to allow deconvolution of the hemodynamic response function (HRF) (Burock et al., 1998). Null events consisted of a fixation display for 2.1–6.3 seconds.
4.4 MRI Scanning
Subjects were imaged using a Siemens Vision 1.5-T scanner. Both anatomical (T1) and functional scans were acquired. T1-weighted anatomical images were obtained using a 3D FLASH (fast low angle shot) sequence with an inferior saturation band to reduce flow artifacts. The T1 imaging parameters were [repetition time/echo time (TR/TE) 22 ms/5.6 ms, flip angle 25°, field of view (FOV) 240 mm, matrix 256 ×256, 160 slices with a thickness of 1.0 mm]. Anatomical scans were obtained in transaxial planes parallel to the anterior commissure-posterior commissure (AC—PC) line. Twenty-four contiguous 5-mm slices aligned to the AC-PC line (3×3×5 mm resolution) were acquired using a susceptibility-weighted single-shot EPI method in order to image the regional distribution of the BOLD signal (TR/TE 2100/40ms, flip angle 90°, FOV 240, 64×64 matrix). In all functional runs, the MR signal was allowed to reach equilibrium over the six initial scans, which were excluded from analysis.
In the scanner subjects viewed images that were projected onto a nonmagnetic screen located approximately 170 cm from their eyes. Head movement was reduced by using a vacuum pillow (VacFix, Toledo, OH). Subjects responded using a fiber-optically linked button.
4.5 Data analysis
4.5.1 Behavioral analysis
Only behavioral data collected within the scanner are reported. Total errors of commission and omission were calculated for each subject in each sleep state, and adjusted by the number of responses. Paired t-tests were used to compare these errors between sleep states. Reaction times were examined using an ANOVA model that included fixed factors of sleep state (R and SD), trial type (valid, neutral, invalid), side (left, right) and SOA (200, 400, and 800) and a random factor of subject. Bonferroni corrected post hoc t-tests were used to determine specific significant effects.
In order to examine the effects of sleep state on the anticipatory biasing of attention we used the method previously described by Small et al. (2003): valid trials were categorized into those that conferred a cue benefit (V+) from those that did not (V−). It was assumed that the anticipatory biasing of spatial attention was present if reaction time to a valid cue was significantly faster (at least one standard error) than the mean reaction time to neutral cues in the corresponding SOA for that run. A cue considered to confer a cue benefit was termed (V+), and all trials that did not meet the (V+) criteria were considered to show no cue benefit (V−). Categorization of the valid-cue trial-type in this manner was done so that the fMRI signal could be compared among validly-cued trials that were identical except for the presence of an anticipatory bias. We have previously used this calculation as a measure of whether or not the predictive cue caused an anticipatory bias in spatial attention (Small et al., 2003). The percentage of valid trials categorized as V+ for each sleep state was compared using a paired t-test. The number of lapses and false positive errors as a function of the number of responses was also compared across sleep states. All statistical analyses of behavioral data were performed using SPSS version 15.0 (SPSS, Inc., Chicago, IL).
4.5.2 fMRI analysis
During data acquisition, the functional image data for one subject was lost. This subject was excluded leaving six subjects for the fMRI analysis. Functional data were analyzed using SPM2 (Wellcome Department of Imaging Neuroscience http://www.fil.ion.ucl.ac.uk/spm) running in the Matlab environment (Mathworks, Inc., Sherborn, MA). Functional images were preprocessed as previously described (Small et al., 2003). Functional images were slice time corrected, realigned and coregistered to the anatomic T1 volume. The T1 volume and functional images were normalized to the MNI-305 template supplied with SPM2. The template approximates the space described in the atlas of Talairach and Tournoux (Talairach and Tournoux, 1988). Functional images were smoothed with a 7 mm Gaussian kernel.
In order to minimize the effect of head movements on the analyzed BOLD signal, trials occurring during the 16 seconds preceding any head movements over 1mm were “excluded” by modeling them as an effect of no interest. Only trials that preceded a movement were modeled this way because movements instantaneously disrupt the measurement of BOLD signal by shifting voxel positions. The effects of movements are immediate as motion effects are not filtered by the hemodynamic response as is the case for neural activity (Barch et al., 1999; Small et al., 2004). Residual movement-related variance was further modeled by including affine movement parameters in the design matrix.
As in our previous studies, only valid trials (i.e., trials with valid cues) were analyzed (Gitelman et al., 1999; Mesulam et al., 2001; Small et al., 2003). Specifically, BOLD responses were examined for contrasts between V+ and V− trials in SD and R states. Although there are fewer trials in the SD state due to the increase in lapses, there were still at least 130 valid responses per subject.
The fMRI design matrix did not include a global covariate, as it can bias the parameter estimates (Aguirre et al., 1998). Instead, a voxel-level linear model of the global signal (LMGS), which has been shown not to introduce bias, was used to remove the global effects (Macey et al., 2004).
Group activations were assessed by a random effects analysis. However, one concern with having only 6 subjects in this study is that the low degrees of freedom might violate assumptions underlying parametric statistics used to analyze the fMRI data. By smoothing at more than double the normalized voxel size (7 mm3 for 3 mm3 voxels) the assumptions underlying voxel-level statistics should be preserved (Friston et al., 1996). However, under low degrees of freedom the random field distributional assumptions underlying cluster level statistics may still be violated. Cluster-level p-values depend on the statistical smoothness of the image, measured in RESELS (resolution elements) at each voxel, and the consistency of this smoothness or stationariness across the image (Worsley et al., 1996).
SPM uses the average RESELS per voxel (RPV) value for calculating cluster statistics. Under lower degrees of freedom, however, this value is more likely to vary across the image. Regions that have fewer RESELS per voxel than the average used by SPM are considered smoother, and have a greater probability of containing larger clusters. False positive clusters are more likely to occur in these regions. Conversely, areas of an image that have more RESELS per voxel than average are rougher, and less likely to contain false positive clusters (Hayasaka et al., 2004).
In order to reduce the chance of a Type I error, mean RPV values were calculated for each cluster passing the cluster-height threshold of p < 0.05 corrected for multiple comparisons across the brain. RPV was then transformed to the more intuitive measure of smoothness, full width at half maximum (FWHM), using the relationship FWHM = RPV (−1/3). This value was expressed in mm by multiplying by the normalized, isotropic voxel size of 3 mm. Clusters with a mean FWHM larger than the one used by SPM (larger FWHM is equivalent to smaller RPV), were designated as non-significant because of the greater chance of a false positive cluster in these regions.
In order to better visualize the BOLD responses within the PCC and the IPS, BOLD signal time courses from the most significant voxels in the PCC and the IPS were extracted for both sleep states and transformed to peri-stimulus time histograms (PSTH).
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Lisa Wolfe who conducted the history and physicals for the study participants, Satoru Hayasaka for helpful discussions on random field and non-parametric statistics, the GCRC staff for their assistance in running the protocol, and Jasmine Koita for her help with the data analysis. We would also like to thank the anonymous reviewer whose comments helped to considerably improve the manuscript. This work was supported by research grants M01 RR-00048, P01 AG11412, and F31 MH074291 from the National Institutes of Health and the Northwestern University Cross-School Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre GK, et al. The inferential impact of global signal covariates in functional neuroimaging analyses. Neuroimage. 1998;8:302–6. doi: 10.1006/nimg.1998.0367. [DOI] [PubMed] [Google Scholar]
- Amir N, et al. Attentional bias to threat in social phobia: facilitated processing of threat or difficulty disengaging attention from threat? Behav Res Ther. 2003;41:1325–35. doi: 10.1016/s0005-7967(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Barch DM, et al. Overt verbal responding during fMRI scanning: empirical investigations of problems and potential solutions. Neuroimage. 1999;10:642–57. doi: 10.1006/nimg.1999.0500. [DOI] [PubMed] [Google Scholar]
- Burock MA, et al. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. NeuroReport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–7. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA. Neuronal responses in area 7a to multiple stimulus displays: II. responses are suppressed at the cued location. Cereb Cortex. 2001;11:592–7. doi: 10.1093/cercor/11.7.592. [DOI] [PubMed] [Google Scholar]
- Corbetta M, et al. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–73. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Dawson D, Reid K. Fatigue, alcohol and performance impairment. Nature. 1997;388:235. doi: 10.1038/40775. [DOI] [PubMed] [Google Scholar]
- de Gonzaga Gawryszewski L, et al. Movements of attention in the three spatial dimensions and the meaning of “neutral” cues. Neuropsychologia. 1987;25:19–29. doi: 10.1016/0028-3932(87)90040-6. [DOI] [PubMed] [Google Scholar]
- Dinges D, Kribbs N. Performing while sleepy: effects of experimentally-induced sleepiness. In: Monk TH, editor. Sleep, Sleepiness and Performance. John Wiley & Sons; New York: 1991. pp. 97–128. [Google Scholar]
- Drummond SP, et al. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–7. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- Drummond SP, et al. Increased cerebral response during a divided attention task following sleep deprivation. J Sleep Res. 2001;10:85–92. doi: 10.1046/j.1365-2869.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, et al. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–35. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, et al. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122(Pt 6):1093–106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Gunter TC, et al. Visual selective attention during meaningful noise and after sleep deprivation. Electroencephalogr Clin Neurophysiol Suppl. 1987;40:99–107. [PubMed] [Google Scholar]
- Hayasaka S, et al. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–87. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, et al. Dissociating top-down attentional control from selective perception and action. Neuropsychologia. 2001;39:1277–91. doi: 10.1016/s0028-3932(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Jongen EM, et al. Varieties of attention in neutral trials: linking RT to ERPs and EEG frequencies. Psychophysiology. 2006;43:113–25. doi: 10.1111/j.1469-8986.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- Macey PM, et al. A method for removal of global effects from fMRI time series. Neuroimage. 2004;22:360–6. doi: 10.1016/j.neuroimage.2003.12.042. [DOI] [PubMed] [Google Scholar]
- McCarthy ME, Waters WF. Decreased attentional responsivity during sleep deprivation: orienting response latency, amplitude, and habituation. Sleep. 1997;20:115–23. doi: 10.1093/sleep/20.2.115. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia--a language-based dementia. N Engl J Med. 2003;349:1535–42. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, et al. Heterogeneity of cingulate contributions to spatial attention. Neuroimage. 2001;13:1065–72. doi: 10.1006/nimg.2001.0768. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. Sleep in America Poll. National Sleep Foundation; Washington, D.C: 2005. pp. 1–55. [Google Scholar]
- Nobre AC, et al. Covert Visual Spatial Orienting and Saccades: Overlapping Neural Systems. Neuroimage. 2000;11:210–216. doi: 10.1006/nimg.2000.0539. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, et al. Human regional cerebral glucose metabolism during non-rapid eye movement sleep in relation to waking. Brain. 2002;125:1105–15. doi: 10.1093/brain/awf103. [DOI] [PubMed] [Google Scholar]
- Norton R. The effects of acute sleep deprivation on selective attention. Br J Psychol. 1970;61:157–61. doi: 10.1111/j.2044-8295.1970.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Perchet C, et al. Attention shifts and anticipatory mechanisms in hyperactive children: an ERP study using the Posner paradigm. Biol Psychiatry. 2001;50:44–57. doi: 10.1016/s0006-3223(00)01119-7. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, et al. Isolating attentional systems: A cognitive-anatomical analysis. Psychobiology. 1987;15:107–121. [Google Scholar]
- Robinson DL, et al. Covert orienting of attention in macaques. II. Contributions of parietal cortex. J Neurophysiol. 1995;74:698–712. doi: 10.1152/jn.1995.74.2.698. [DOI] [PubMed] [Google Scholar]
- Small DM, et al. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuroimage. 2003;18:633–41. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Small DM, et al. Experience-dependent neural integration of taste and smell in the human brain. J Neurophysiol. 2004;92:1892–903. doi: 10.1152/jn.00050.2004. [DOI] [PubMed] [Google Scholar]
- Strangman G, et al. Functional brain imaging of a complex navigation task following one night of total sleep deprivation: a preliminary study. J Sleep Res. 2005;14:369–75. doi: 10.1111/j.1365-2869.2005.00488.x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical; New York: 1988. [Google Scholar]
- Thomas M, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205–17. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HL, et al. Impaired performance with acute sleep loss. Psychological Monographs: General and Applied. 1959;73:1–26. [Google Scholar]
- Worsley KJ, et al. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wu JC, et al. The effect of sleep deprivation on cerebral glucose metabolic rate in normal humans assessed with positron emission tomography. Sleep. 1991;14:155–62. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.