Abstract
Human pluripotent stem cells have the unique properties of being able to proliferate indefinitely in their undifferentiated state and to differentiate into any somatic cell type. These cells are thus posited to be extremely useful for furthering our understanding of both normal and abnormal human development, providing a human cell preparation that can be used to screen for new reagents or therapeutic agents, and generating large numbers of differentiated cells that can be used for transplantation purposes. Critical among the applications for the latter are diseases and injuries of the nervous system, medical approaches to which have been, to date, primarily palliative in nature. Differentiation of human pluripotent stem cells into cells of the neural lineage, therefore, has become a central focus of a number of laboratories. This has resulted in the description in the literature of several dozen methods for neural cell differentiation from human pluripotent stem cells. Among these are methods for the generation of such divergent neural cells as dopaminergic neurons, retinal neurons, ventral motoneurons, and oligodendroglial progenitors. In this review, we attempt to fully describe most of these methods, breaking them down into five basic subdivisions: 1) starting material, 2) induction of loss of pluripotency, 3) neural induction, 4) neural maintenance and expansion, and 5) neuronal/glial differentiation. We also show data supporting the concept that undifferentiated human pluripotent stem cells appear to have an innate neural differentiation potential. In addition, we evaluate data comparing and contrasting neural stem cells differentiated from human pluripotent stem cells with those derived directly from the human brain.
Keywords: Human pluripotent stem cells, human embryonic stem cells, human neural stem cells, differentiation
Introduction
It is widely appreciated that the central nervous system (CNS) has little capacity for self-repair after loss of cellular elements due to disease or injury. Indeed, it has long been held that any restoration of lost neurological function is primarily due to the subjugation of that function by intact redundant, parallel, and/or contralateral nervous pathways[10;19;22;35;52;73;91;97]. CNS diseases and injuries, therefore, represent a huge class of human maladies that have largely not been fully treatable using conventional medical approaches. Although progress has been made in the pharmacological treatment of some CNS diseases, such as depression, Parkinson's disease, and phenylketonuria, most of the approaches taken are less than robust in their overall outcome or do little to address the underlying cause of neurological dysfunction. Despite these advances, many CNS diseases and injuries have little to no therapy available. These include as traumatic injury, neurodegenerative diseases, and stroke. Among the obstacles to devising effective therapies for CNS disease and injuries are: a lack of knowledge about the basic pathophysiologic mechanisms involved; methods for the timely evaluation of new therapeutic drugs and devices; a suitable transplantable cell population; and the very simple fact that the CNS is orders of magnitude more complicated than any other organ system. Much of the transplantation research directed toward devising therapies for CNS diseases and injuries has been based on neural tissue derived from the fetal brain or neural cells derived from neuroectodermal tumors[20;29;34;44;120]. Neither of these sources is entirely satisfactory. Therefore, alternative sources of transplantable material are needed.
The dawn of an entirely new approach to CNS diseases and injuries was heralded by two seminal discoveries: 1) there exists within the human brain a population of harvestable undifferentiated neurogenic cells (neural stem cells, NSCs, or brain-derived NSCs, bNSCs[36;96]) and 2) a population of pluripotent stem cells (PSCs) with virtually unlimited proliferation potential and proven neurogenic potential can be harvested from the very early human embryo (embryo-derived pluripotent[16;94;115] stem cells, ePSCs). The former discovery strongly suggests that there actually exists within the brain an innate repair mechanism and that medical interventions based on such a mechanism might be possible. The latter suggests that there exist readily replenishable sources of cells for such medical interventions. Therefore, a new and much invigorated effort has ensued to devise therapies for CNS diseases and injuries. Differentiation of ePSCs down the neural lineage, in fact, was one of the earliest described potentials of the PSC. Given the two basic properties of the PSC, therefore, an entirely new line of research has emerged: the use of the PSC to generate a replenishable population of neural cells not only for transplantation but also as a tool for drug and small-molecule reagent discovery and for furthering our understanding of the development of the nervous system in health and disease[114].
Three preclinical examples (among many) serve to illustrate the breadth of the transplantation effort and the breadth of possibility of the clinical efficacy of transplanted stem cells or their derivatives:
1) Use of neural cells differentiated from human ePSCs to treat an animal model of Parkinson's disease[98]
In this study, dopamine-producing neural cells differentiated from ePSCs were injected into the lesioned parenchyma of rats whose striatal tissue had been lesioned with 6-hydroxydopamine to produce a rat model of Parkinson's disease. The implants gave rise to a long-lasting restitution of motor function and transplanted cells engrafted as tyrosine hydroxylase-positive neurons. Thus, transplanted cells gave rise to bone fide, functional, albeit ectopic, neuroregeneration.
2) Use of neural cells differentiated from ePSCs or harvested from human brain to treat an animal model of a lethal lysosomal storage disease[62]
In this study, human neural cells were injected into the lateral cerebral ventricles of newborn mice that carried a lysosomal enzyme mutation producing the mouse-equivalent of the lethal human lysosomal storage disorder, Sandhoff's disease. The primary goal of the study was to use the cells not to regenerate tissue or replace dead cells in the brain, but to efficiently deliver the missing enzyme to as large an extent of the brain as possible[102]. The study showed that a single intracerebroventricular cell injection, on the day of birth, significantly prolonged the lives of these mice and, at the same time, delayed the onset of the loss of motor skills. The mechanisms by which this neuroprotection occurred appeared to be manifold and included decreasing neuroinflammation as well as increasing the local activities of the defective enzyme. That mouse NSCs, human bNSCs and human ePSC-derived neural stem cells (eNSCs) had virtually identical efficacies and that the observed numbers of donor-derived differentiated neurons and glia were quite low supports the premise that the primary mechanism may be neuroprotection rather than neuroregeneration[102].
3) Use of neural cells differentiated from ePSCs to treat and animal model of spinal cord injury[78]
In this study, oligodendroglial progenitors, differentiated from ePSCs, were implanted into the area of injury of adult rats whose spinal cords had been contused – a rat model of human spinal cord injury. The implanted cells caused an improvement of motor function in these animals and enhanced remyelination in the general area of the injury/implant. The implanted progenitors differentiated in situ into oligodendrocytes and induced an increase of oligodendrocyte remyelination, much of which was directly due to the implanted cells themselves. Thus, this example could be seen as a combination of neuroprotection and neuroregeneration as the progenitors replaced those lost in the host during the injury process and both remyelinated host axons and induced remyelination by the host, protecting those axons from secondary degeneration, one of the known sequelae to the injury[51].
The human PSC, therefore, because of its capacity for neural differentiation and because of its potential wide application to CNS therapeutics and basic research has received considerable attention from neuroscientists. As a result, there are a number of reports of methodologies for neural differentiation of this unique cell. Because of the plethora of neural cell types, most particularly neuronal cell types, the methodologies reported vary widely in their composition[8;9;11;31;37;43;45;49;59;61;63;67;68;76;81;84;93;100;101;109;117;123]. The purpose of this review is to comprehensively present the extant methodologies in a neurodevelopmental, step-wise manner, starting with the PSC and ending with the differentiated neural cell. The methodological steps, five in all, will be presented one at a time, comparing and contrasting the various methodological approaches at each step. The ultimate goal, therefore, is to give the interested reader a comprehensive, but organized, overview of the extant neural differentiation methodologies (summarized in Figure 1 and Table 1) so that appropriate methodologies pertinent to their particular interests can be more easily appreciated and applied. A detailed methodology can be found in our recently published stem cell manual[39;64;82;110].
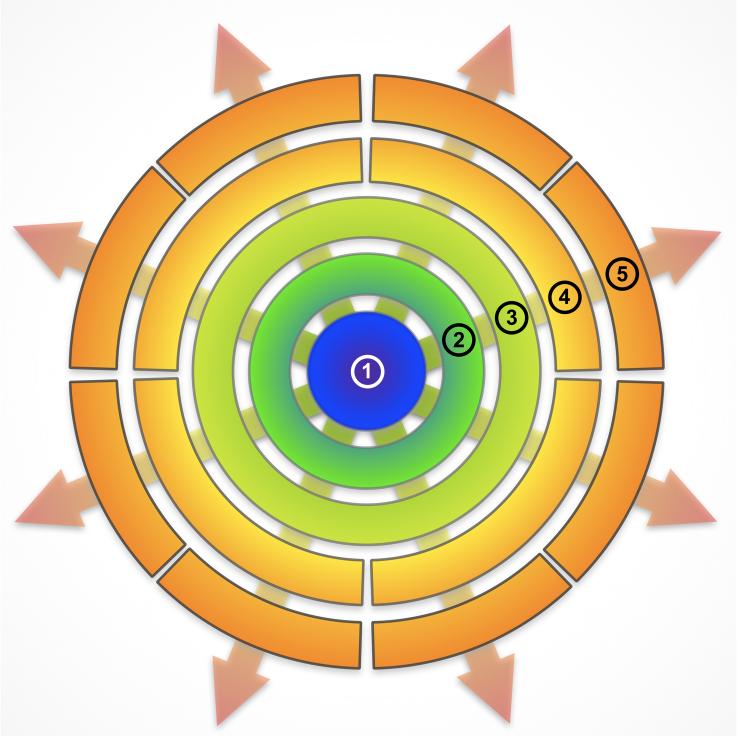
Figure 1.
Schematic of the stages in the differentiation of PSCs. The starting material, undifferentiated PSCs (1), progresses through four transitions, each time becoming more lineage restricted. The first transition is loss of pluripotency (stage 2), which is usually accomplished by the formation of embryoid bodies. At stage (3), neural rosettes have formed. These are the anlage of the nervous system and can be thought of as consisting of neural stem cells with the greatest degree of multipotentiality. At stage (4), a certain degree of lineage restriction, within the neural lineage, has taken place. This is indicated by a broken ring. These cells tend to further differentiate toward a particular mature neural lineage, although under some circumstances a residual plasticity can still be demonstrated. At stage (5), neural cells of a particular lineage have been produced and there is little interchangeability among them, again illustrated by the breaks in the circle. These include oligodendroglia, astroglia and neurons of anterior, posterior, or retinal derivation.
Table 1.
Methods for neural differentiation of PSCs. The methods have been broken down into 5 stages (illustrated in Figure 1). Each stage is discussed in the text.
| 1) Starting Material | ||||
| Reference | Park, et al.[84] | Iacovitti, et al.[43] | Gerrard et al.[37] | Schultz, et al.[100] |
| Lines | HSF-6, SNU-hes-3, Miz | H9, BG01, HUES7, HUES8 | H1, H7, H9 | SSEA4-enriched BG01 |
| Medium | STD | STD, HUES | MEF-CM | STD |
| Feeders/Substrate | iMEF | iMEF | Matrigel | mmcMEF |
| Passaging | Mechanical | Mechanical, trypsin | Collagenase | Collagenase/Trypsin |
| 2) Induced loss of pluripotency | ||||
| Passage | Collagenase | Collagenase | EDTA | Collagenase+Mechanical |
| Substrate | PA6-SHH Stromal Cells | LA polystyrene | Matrigel or PLL/laminin | LA Polystyrene |
| Media | FGF-free | STD, HUES | DMEM/F12 w/N2B27 | DMEM/F12, 1X N2 |
| Factors | ITS+AA | bFGF | 100ng/mL Noggin | MedII conditioned, BFGF |
| Duration | 3 days | |||
| Selection | Bulk passage | |||
| 3) NSC Induction | ||||
| Passage | Pipetted | |||
| Substrate | PA6-SHH Stromal Cells | 100ng/cm2 collagen IV | Matrigel or PLL/laminin | LA Polystyrene |
| Media | FGF-free | DMEM/F12, Glucose, 1% ITS | DMEM/F12 w/N2B27 | DMEM/F12, 1X N2 |
| Factors | ITS+AA | Noggin, fibronectin | 100ng/mL Noggin | MedII conditioned, BFGF |
| Duration | 2 weeks | 4−14 days | 3 passages | |
| Selection | passive | Rosettes | Bulk enzymatic passage | |
| 4) NSC Maintenance&Expansion | ||||
| Passage | Collagenase | Mechanical. Dispase after 1 week | TrypLE Express | |
| Substrate | FN | TC-treated polystyrene | Matrigel or PLL/laminin | LA Polystyrene |
| Media | DMEM/F12 | DMEM/F12, Glucose, 1% ITS | DMEM/F12 w/N2B27 | DMEM/F12, 1X N2 |
| Factors | N2+AA+bFGF. ITS after 3−4 days | 20ng/mL bFGF | 100ng/mL Noggin+20ng/mL FGF | MedII conditioned, BFGF |
| Duration | 1.5 weeks | 7−10 days | 2+ passages | 2−6 weeks |
| Selection | bulk passage | rosettes | Passive | passive |
| 5) Neural differentiation | ||||
| Passage | Collagenase | Dispase | TrypLE Express | Pipetted |
| Substrate | PLO+FN | PLO | PLL/laminin | PLO/ Laminin |
| Media | DMEM/F12 | DMEM/F12 | N2B27 | MedII/BFGF or Neurobasel.B27 |
| Added Factors | ITS+AA | dbcAMP | I:SHH+FGF8+AA; ii:BDNF+GDNF+AA+Laminin) | 5% FBS+GDNF+BDNF |
| Duration | 2−3 days | 1 week | i:1−2 weeks plus ii: 2 weeks | Undefined |
| Selection | Midbrain DA TuJ1+ neurons | TH+DA B-tubulin III+ neurons | TH positive neurons | TH+MAP2+ and VMAT2+MAP2+ |
| 1) Starting Material | ||||
| Reference | Pankrantz, et al.[81] | Lim et al.[66] | Baharvand, et al.[8] | Park et al.[86] |
| Lines | H1, H9, monkey R366.4 | hES 3.1, 3.2, 3.3 | Royan H5 | MB03 |
| Medium | STD | HES/mod | MOD | MBO3/MBO3 mod |
| Feeders/Substrate | iMEF | iHFF | mmcMEF | mmcSTO |
| Passaging | Bulk enzymatic passage | Bulk enzymatic passage | Mechanical | Trypsin |
| 2)Induced loss of pluripotency | ||||
| Passage | Collagenase | Collagenase | Mechanical | Trypsin |
| Substrate | LA Polystyrene | LA Polystyrene | Matrigel | LA Polystyrene |
| Media | DMEM/F12 20%KSR | DMEM/HG 20%KSR | DMEM/F12+5%KSR | MB03 |
| Factors | bFGF | 4ng/mL bFGF | bFGF, Noggin | - |
| Duration | 4 days | 6 days | 1 passage | 4 days |
| Selection | bulk | bulk | ePSC colonies | bulk |
| 3) NSC Induction | ||||
| Passage | Pipetted | Pipetted | Mechanical | Pipetted |
| Substrate | suspension for 2 days, laminin for 15 | tissue culture-treated polystyrene | Matrigel | Gelatin |
| Media | (DMEM/F12+N2) | DMEM/HG 20%KSR | DMEM/F12+5%KSR | Serum-free |
| Factors | 20ng/mL bFGF | bFGF, RA, Shh, Wnt3a, BMP-2 | RA first 6 days | ITS+Fibronectin |
| Duration | 15 days | 12 days | 8 days | |
| Selection | Neural tubes | Rosettes | bulk | |
| 4) NSC Maintenance&Expansion | ||||
| Passage | Enzymatic | Mechanical | Trypsin | |
| Substrate | PO+laminin or suspension | tissue culture-treated polystyrene | Matrigel | Laminin |
| Media | (DMEM/F12+N2) | DMEM/HG 20%KSR | DMDM/F12+5%KSR | N2 |
| Factors | 20ng/mL bFGF or RA | bFGF, RA, Shh, Wnt3a, BMP-2 | bFGF | Laminin, bFGF |
| Duration | 14−16 days | 2 weeks | 6 days | 6 days |
| Selection | Spheres or rosettes | bulk | Neural Tubes | bulk |
| 5) Neural differentiation | ||||
| Passage | Enzymatic | Undefined | Mechanical isol. of Neural Tubes | Trypsin |
| Substrate | Suspension then PLO/Laminin | tissue culture-treated polystyrene | PLO/Laminin | Glass Coverslips |
| Media | Neurobasel, N2, NEAA | Gibco Neurobasal medium+B27 | Neurobasel Medium. 1% N2, 2% B27 | N2 |
| Added Factors | BDNF, GCNF, cAMP, AA and Laminin | 4ng/mL bFGF | bFGF and RA | RA, bFGF, BDNF, TGF-alpha |
| Duration | 8 days | 3 weeks | 14 days | 7, 14, 21 days |
| Selection | b-tubulin+, otx-2, Pax6 | b-tubulin III +, NSE and HB9 | b-tubulin III, Map-2, Synaptophysin, NF | NF200, TH, GAD |
| 1) Starting Material | ||||
| Reference | Joannides, et al.[47] | Nistor, et al.[78] | Sonntag, et al.[109] | Kang, et al.[50] |
| Lines | H9, HUES9 | H7 | H7, H9 | SNUhES1 |
| Medium | STD, HUES | STD-CM | Std/MOD | MOD |
| Feeders/Substrate | iMEF | Matrigel | mmcHFF | mmcSTO |
| Passaging | Mech; collagenase | Collagenase | Collagenase | Mechanical |
| 2) Induced loss of pluripotency | ||||
| Passage | Collagenase+chopping | Collagenase | Collagenase MS5 Stromal Cells | Collagenase LA Polystyrene |
| Substrate | LA Polystyrene | LA Polystyrene | SRM | ePSC media w/o bFGF |
| Media | 50%STD, 50%GRM | - | ||
| Factors | 2 days | 4 days | ||
| Duration | EGF, bFGF, B27, ITS, many hormones | Passive | ||
| Selection | bulk | |||
| 3) NSC Induction | ||||
| Passage | Pipetted | Pipetted | ||
| Substrate | Suspension+Shaking | Suspension | MS5 Stromal Cells | Matrigel |
| Media | DMEM+Human Serum | 1 day 50%STD, 50%GRM. 100% GRM 7 days | SRM 14 days, N2 7 days | DMEM/F12 |
| Factors | ITS+EGF+bFGF | EGF, bFGF, B27, hormones + RA | Noggin 300ng/mL 7−21 days | ITS+Fibronectin |
| Duration | 8 days | 8 days | 21 days | 5 days |
| Selection | bulk | Yellow spheres | rosettes | |
| 4) NSC Maintenance&Expansion | ||||
| Passage | Pipetted+chopping | |||
| Substrate | Suspension+Shaking | Matrigel | ||
| Media | DMEM+Human Serum | DMEM/F12 | ||
| Factors | ITS+EGF+bFGF | 1X N2, bFGF | ||
| Duration | >8 days | 5 days | ||
| Selection | bulk | rosettes+neural tubes | ||
| 5) Neural differentiation | ||||
| Passage | Pipetted+chopping | Pipetted | Mechanical | Mechanical |
| Substrate | poly(D-lysine)/Laminin | poly (L-Lysine)/Laminin | PLO/`Laminin | Matrigel |
| Media | DMEM/2% B27 | GRM | N2 | DMEM/F12 |
| Added Factors | none | None | Shh, FGF8, BDNF, bFGF, TGF-B3, GDNF, cAMP, AA | N2 and T3 |
| Duration | 14 days | 1 week | 16 days | 20 days |
| Selection | b-tubulinIII, Map2ab, Synapsin 1, GABA | GAL C, RIP, O4 | NCAM, TuJ1, TH | O4, O1, MBP |
| 1) Starting Material | ||||
| Reference | Banin, et al.[9] | Kim, et al.[54] | Lee, et al[60] | |
| Lines | HES-1, EF1α-GFP | HSF6, Miz-hES4, Miz-hES6 | H9 | |
| Medium | HES | STD | STD | |
| Feeders/Substrate | iMEF | iMEF | mmcMEF | |
| Passaging | Mechanical | Collagenase | Collagenase | |
| 2)Induced loss of pluripotency | ||||
| Passage | Collagenase | Collagenase | Collagenase | |
| Substrate | iMEF | LA Polystyrene | MS-5 Stromal cells | |
| Media | DMEM-glucose w/ 10% FBS | ePSC media w/o bFGF | SRM | |
| Factors | 50ng/mL Noggin | - | - | |
| Duration | 8 days | 16 days | ||
| Selection | Passive | |||
| 3) NSC Induction | ||||
| Passage | Pipetted | |||
| Substrate | iMEFs | TC-treated polystyrene | MS-5 Stromal cells | |
| Media | DMEM-glucose w/ 10% FBS | ITS medium | DMEM, 1X N2 | |
| Factors | 500ng/mL Noggin | 5ug/mL fibronectin | Shh, FGF8, BDNF, AA | |
| Duration | 8 days w/noggin, 5 days w/o | 8 days | 12 days | |
| Selection | “Gray, opaque morphology” | bulk | Rosettes | |
| 4) NSC Maintenance&Expansion | ||||
| Passage | Mechanical | Mechanical | ||
| Substrate | LA Polystyrene | PO/Laminin | ||
| Media | DMEM/F12 | DMEM, 1X N2 | ||
| Factors | B27, 20ng/mL EGF, bFGF | Initial passage BFGF, AA, BDNF. Subsequent passages BFGF, EGF. | ||
| Duration | >1 week | 6−7 day passages | ||
| Selection | spheres | p75+, HNK1+ cells for first passage. Bulk for subsequent passages. | ||
| 5) Neural differentiation | ||||
| Passage | HBSS | |||
| Substrate | Poly D-Lysine/Laminin | PO/Laminin | ||
| Media | DMEM/F12. B27 | DMEM, 1X N2 | ||
| Added Factors | NT3, NT4, BDNF | BDNF, GDNF, NGF, dbcAMP | ||
| Duration | 2−3 weeks | >2weeks | ||
| Selection | B-tubulin III, NF 160, MAP2ab, GABA,TH | TuJ1, islet1, TH, GABA, PLP | TH, Peripherin, Brn3a |
Description of Methods
A. Human pluripotent stem cells
Most current lines of human PSCs have been obtained from the inner cell mass (ICM) of supernumerary embryos produced by in vitro fertilization (IVF[114]). In this procedure, eggs are harvested from a woman after she has been treated with follicular hormones to stimulate the ovaries. The eggs are fertilized either by combining them with sperm in a dish, or by mechanically injecting the sperm into the egg (intra-cytoplasmic sperm injection or ICSI). The fertilized eggs are incubated to allow them to progress to the blastocyst stage of embryonic development. At the blastocyst stage, the trophectoderm of the embryo is removed and the ICM is plated on to a “feeder layer” of mouse or human embryonic fibroblasts [115], which are essential for the survival of the ICM [28]. The ICM then flattens into a compact colony which is mechanically dissociated and replated several times to eventually give rise to a stable cell line of ePSCs.
ePSCs in culture have a specific morphology, and they express characteristic surface antigens and nuclear transcription factors. The colonies are very compact with many tightly spaced cells of large nucleus-to-cytoplasm ratios. The surface antigens include the stage-specific pluripotent antigens SSEA-4 and SSEA-3 and the teratocarcinoma recognition antigens TRA-1−60 and TRA-1−81 [25]. Specific transcription factors expressed that are associated with the expression of particular elements of the pluripotent genome [115] include the POU (pit-oct-unc)-domain transcription factor Octamer-4 (Oct-4), Nanog, and Sox2.
When undifferentiated ePSC colonies are detached from the feeder layer and transferred into serum containing medium, they form multicellular aggregates called embryoid bodies (EBs) which can contain cell types representing all three germ layers of the body - endoderm, mesoderm, and ectoderm. Many EBs tend to show cell types of only one or two germ layers, but in an unpredictable manner. Thus, with appropriate subculture conditions and physical removal of colonies showing specific morphologies, behaviors, or proteins, it is possible to establish cultures that are enriched for particular cell types or mixtures of cell types [25]. However, this cell behavior is unpredictable and the sorting is not completely effective. Many labs have therefore attempted to develop protocols for directly controlling the differentiation of ePSCs.
Exogenous differentiating factors have been useful in favoring differentiation into specific derivatives: retinoic acid and nerve growth factor for neuronal differentiation [99]; basic fibroblast growth factor and platelet-derived growth factor for glial precursors [21]; 5-aza-2'-deoxycytidine for cardiomyocytes [121]; bone morphogenetic protein-4 and transforming growth factor-beta for trophoblast cells [24]; sodium butyrate for hepatocytes [90]; and various cytokines for hematopoietic cells [124]. Differentiation into particular tissue types can also be elicited by over expressing genes encoding transcription factors that function in cell commitment during normal development: MyoD1 for skeletal muscle [30]; and Nurr1 for dopamine producing neurons [54]. However, these methods still usually only give enrichment rather than total induction, so additional sorting is often necessary. This has been performed based on lineage-specific gene expression: PS-NCAM and A2B5 as cell-surface markers for neural precursors [26]; or hygromycin resistance driven by a myosin heavy chain promoter for cardiomyocytes [58].
Several groups [21;93;112;118] have produced neuronal precursors from either mouse or human ePSCs and tested them following injection into the developing brain of newborn mice or rats. The transplanted cells were incorporated into the host brain, migrated along appropriate tracks, differentiated into neurons in a region-specific manner and made synaptic contacts with host neurons. In some cases the transplanted cells also gave rise to glia and astrocytes. This procedure has been shown to promote recovery in animal models of Parkinson's Disease and spinal cord injury [108].
Four other sources of PSCs that meet all of the above criteria have recently become available. The first is the blastomere. At the cleavage stage (8−16 cells) of embryonic development, a single cell (blastomere) is removed and placed in co-culture with ePSCs. The blastomere then divides and develops into a blastomere-derived PSC colony (bPSCs)[57]. In addition, parthenogenically-derived PSCs (pPSCs) have been described. In this case, single human eggs are stimulated, by incubation in low oxygen and application of calcium ionophores, to replicate their DNA and then divide to form a PSC colony[95]. Next, there are PSCs derived from spermatogonial tissue (sPSCs). While these have been described in the literature for mice[40], the methods for the derivation of human cells have not yet been. Finally, there are the induced PSCs (iPSCs). In this case, somatic cells are genetically transduced to express once-silent genes that reprogram these cells into PSCs directly, without going through a blastocyst stage. Two recent publications have demonstrated this with human cells. In one case, the cells were transduced to express Nanog, Oct4, Sox2, and Lin28[123]. In the other, c-Myc, Oct4, Sox2, and Klf4 were required for the reprogramming[113]. The iPSCs are of particular importance as they provide a potential source of patient-specific cells for transplantation as well as the opportunity to create PSCs for study from cells of patients with specific diseases. In addition, these cells do not have ethical concerns associated with the production of PSCs from human embryos[48;105].
B. Inherent Neural Potential of human ePSCs (Figure 2)
Figure 2.
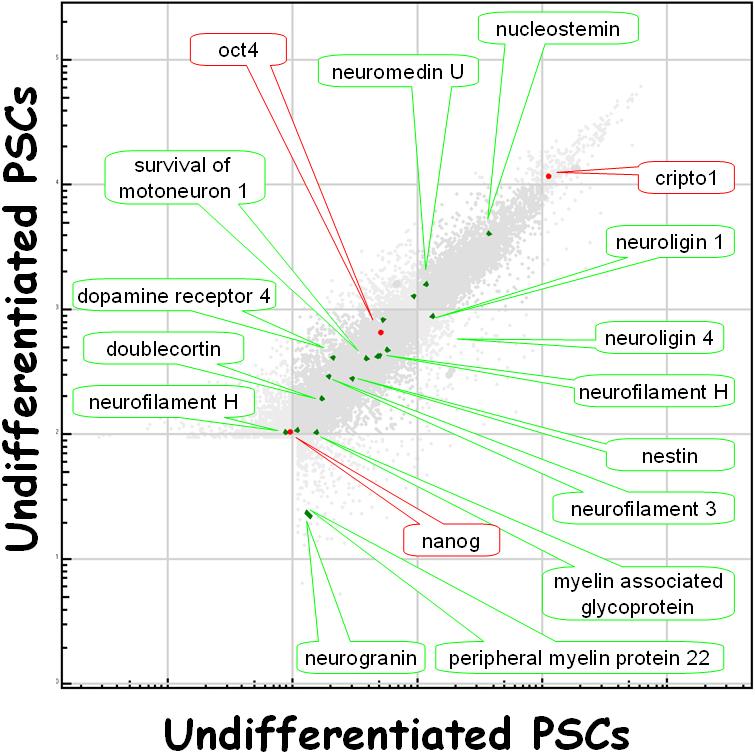
Gene expression microarray data from two PSC cultures grown under STD conditions, plotted against each other. Note the rather loose grouping of the data. The data points in gray (shaded area at the bottom-left of the graph) represent those transcripts whose expression levels fell below the limit of detection. Although classic markers of undifferentiated PSCs can easily be detected, such as OCT4, Nanog, and teratocarcinoma derived growth factor 1 (cripto 1), markers of more differentiated stem cells and their progeny are also seen, as described in the text. These data represent two pairs of four different cultures of the same PSC line (H9).
PSCs appear to be an artifact of tissue culture, albeit a useful one. That is, no bone fide in vivo representation of the PSC has yet been convincingly described. Among their many unique properties, PSCs appear to be cells on the very verge of differentiating. Indeed, reliably maintaining PSCs in their undifferentiated state has been one of the central challenges of PSC culture. Even in their “undifferentiated” state, however, PSCs express a number of mRNAs that are specifically found in particular, more differentiated cells (Figure 2). Thus, PSCs, as a population, express a number of different multipotent stem cell markers: nucleostemin and nestin, found in neural stem cells, for example. It is not yet clear, however, whether particular subpopulations of undifferentiated PSCs singly express a lineage specific set of stem cell markers or whether all PSCs express all stem cell markers (or some iteration between these two extremes). Moreover, undifferentiated PSCs also express mRNAs specific to more fully differentiated cell types. Thus, PSCs, as a population, express the mRNAs for neurofilament H, dopamine receptor 4, and doublecortin, which are neuronal markers; and for peripheral myelin protein and myelin-associated glycoprotein, which are glial markers. Again, it is not clear if subpopulations or the population as a whole are responsible. What is clearly suggested by these data, however, is that there is a relatively short distance between the gene expression state of PSCs and the gene expression state of their more differentiated progeny – PSCs appear to be on the verge of differentiation and only the operation of a particular pluripotent expression program keeps them from differentiating.
Whether or not there is protein expression that corresponds to the mRNA expression described above has not been explored to any great extent. A simple experiment done in our laboratory, however, seems to show that, at least for some neuronal markers, not only is the protein expressed, but some cells show morphological and functional evidence of neuronal maturation in conditions that favor the undifferentiated state of PSCs. Thus, small, round, doublecortin-positive cells were found at the juncture of two colliding PSCs colonies (Figure 3) and doublecortin-positive cells with morphologies exactly the same as those described for cells cultured from the human brain were found outside the PSC colony in the surrounding MEFs (Figure 3). This strongly suggests that, even under conditions that favor the pluripotent state, functional, maturing neural cells can develop, as doublecortin is known to be associated with migratory neuroblasts[38]. Although other cells types have also been shown to spontaneously differentiate from PSC colonies, none have been associated with a convincingly functional state. Thus, neural differentiation appears to be a lineage pathway easily accessible to PSCs under conditions commonly used to maintain these cells. This is certainly not surprising, given that the anlage of the nervous system, the neural tube, is one of the first tissues to form in the developing embryo.
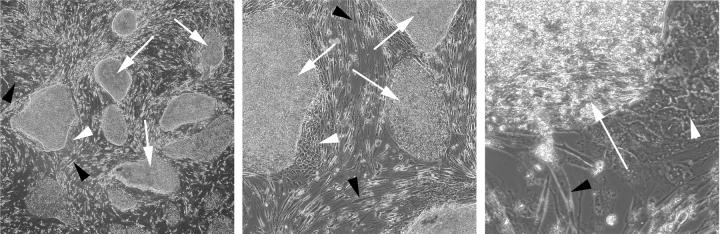
Figure 3.
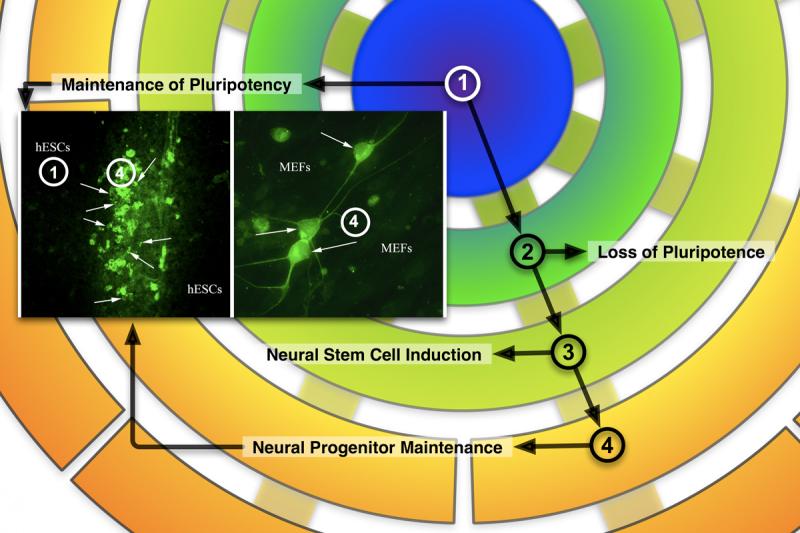
Immunocytochemical staining of doublecortin(DCX)-positive cells (inset) overlaid on the differentiation schema of Figure 1. The inset-left panel (10x) shows DXC+ cells (green, arrows) at the juncture of two colliding PSC colonies (unstained, hESCs), growing under STD conditions. The inset-right panel (40x) shows DCX+ cells (green, arrows) with typical morphologies found in the MEFs (unstained, MEFs) surrounding the colonies. These photomicrographs illustrate that, even under conditions favoring pluripotency, some cells progress through stages 2 and 3 to stage 4 and show, therefore, the propensity of PSCs to differentiate down the neural lineage.
ePSCs, therefore, can be considered the most immature human cell type that can be cultured for an extended period of time. The gene expression patterns in ePSCs are unique when compared to any other cell type that has been isolated and cultured so far. The last two years have seen a surge of landmark papers which have advanced our phenomenological knowledge of ePSCs by taking advantage of the latest developments in microarray and sequencing technologies. At least one common theme appears to emerge from these studies: the ePSC's singular position within the “cell kingdom” depends upon seemingly opposite transcriptional characteristics: expression of most lineage fate genes that are known from differentiating cells is strongly repressed[6;17;18;71] while, at the same time, most other genes from differentiated and, surprisingly, non-coding elements and regions in the eukaryotic genome are actively transcribed[4;41;70]. At the moment, this paradox can be only explained with teleological, high level concepts, since our ability to mechanistically dissect global system functions has yet to catch up with the pace at which we can phenotype cell types.
The current models propose that ePSCs are defined by a “bivalent” state on many levels of transcriptional control. These cells are “about to” readily differentiate, so every differentiation fate (which means the transcription of differentiation-inducing or -defining transcripts) is readily available, but also under tight, repressive control, since when “unleashed”, these genes and transcriptional elements will invariably tip the equilibrium towards differentiation and thus terminate the pluripotent state. When differentiation occurs, expression levels of specific, developmental fate-related genes can be rapidly up-regulated. The ability to access and transcribe other regions necessary for other developmental paths is, at the very same time, severely restricted. Stochastic resonance theory provides another interesting view of this low level transcription of most genetic elements in ePSCs: adding background noise to signals in unstable systems can better define their context and lead to more robust fate decisions[14;15]. It is important, therefore, not to rely on the expression of single, or even a few, genes or proteins as differentiation markers, but to attempt to measure the readout quantitatively and include proper controls for comparison. Most importantly, any system theory analysis of this process must follow function, not just markers.
As mentioned above, there appears to be a “default” mechanism for neuronal differentiation. We believe this observation needs further scrutiny, however; “defaultness” may be also a function of current PSC culture technologies, the small “developmental distance” between PSC and neuroepithelial cells, and a higher level of in vitro robustness of neural progenitors. The continuity of in vitro phenotypes when ePSC differentiate into neural stem cells appears more relevant to us. The recent work on inducing pluripotency in somatic cells suggests that a, yet undefined, “pluripotency program” is booted up into the “read-and-write memory” of the cells. This picture can help understand that, assuming the whole hypothetical regulatory network is not shut down all at once, there may be intermediate states, which could be restored to the starting point if the right signals are present in the culture medium. To what extent fates are reversible or cells can shift phenotypes is yet unknown. Recent work on rosette cells (see below) points towards much more plasticity of differentiated cells than previously assumed[33], at least in vitro.
C. Overview of Techniques (Figure 1, Table 1)
Figure 1 schematically illustrates the overall organization of the methodologies used to differentiate PSCs down the neural lineage. In the first stage is the undifferentiated PSC. Since not all PSCs are cultured under similar conditions, the nature of this starting material will be described in some detail (Tables 1, 2, and 3). In addition, newer culture media, with growth factors and co-factors already included, are radically changing the standard culture conditions for PSCs but there are as yet few manuscripts describing them so they will not be included here. During the second stage, the PSCs are induced to lose their pluripotentiality (We use this descriptor, rather than “induced to differentiate” as the method used is rather passive in nature and the latter term suggests a more intentional design.). Until recently, this step has consisted simply of depriving the PSCs of the feeder layer, culture substrate, and mitogens, thereby transforming them into the so-called embryoid bodies described above. While this method has its merits, some newer methodologies more fully direct the cells down the neural lineage rather than haphazardly down all three lineages simultaneously (see Table 1), although most of these methods result in initial lineages of more restricted potential. During stage 3, neural cells themselves are isolated and their fate becomes sealed by a specific change in culture conditions, similar to those used for brain-derived neural stem cells. During this stage, generally speaking, the cells still have the potential to generate any of the three basic lineages of neural cells – neurons, astrocytes, or oligodendrocytes. By stage 4, the cells’ fates have become more restricted: they are now fated to become either neurons or glia and by stage 5, a cell of restricted lineage is produced. In this stage, however, the cell may still be at the progenitor stage. If so, it is a progenitor for a single cell type.
Table 2.
PSC lines used for neural differentiation and the cultures methods used for maintaining pluripotence.
| Line ID | NIH Registry Cell Line? | Culture Medium (Table 3) | Substrate (Table 1) | Passage Method | Derivation Reference |
|---|---|---|---|---|---|
| BG01 | Y | STD | iMEF | Mechanical | [72] |
| BG01 | Y | STD | mmcMEF | Mechanical | [72] |
| BG01 | Y | STD | mmcMEF | Coll/Tryp | [72] |
| BG01 | Y | STD | mmcMEF | Collagenase | [72] |
| H1 | Y | STD- | iMEF | Mechanical | [115] |
| H1 | Y | STD/CM | Matrigel | Collagenase | [115] |
| H7 | Y | STD/Mod | mmcHFF | Collagenase | [115] |
| H7 | Y | STD/CM | Matrigel | Collagenase | [115] |
| H9 | Y | STD- | iMEF | Mechanical | [115] |
| H9 | Y | STD/Mod | mmcHFF | Collagenase | [115] |
| H9 | Y | STD/CM | Matrigel | Collagenase | [115] |
| H9 | Y | STD | iMEF | Mechanical | [115] |
| HES-1 | Y | HES | mmcMEF | Mechanical | [88] |
| HES3.1 | Y | HES/Mod | iHFF | Collagenase | [88] |
| HES3.2 | Y | HES/Mod | iHFF | Collagenase | [88] |
| HES3.3 | Y | HES/Mod | iHFF | Collagenase | [88] |
| HUES7 | N | HUES | iMEF | Trypsin | [28] |
| HUES8 | N | HUES | iMEF | Trypsin | [28] |
| HUES9 | N | HUES | iMEF | Trypsin | [28] |
| HSF6 | Y | STD | iMEF | Collagenase | [1] |
| HSF-6 | Y | STD | iMEF | Mechanical | [1] |
| MB03 | Y | MB03 | mmcSTO | Trypsin | [87] |
| MB03 | Y | MB03/Mod | mmcSTO | Trypsin | [87] |
| Miz-hES-1 | N | STD | iMEF | Mechanical | [85] |
| Miz-hES4 | N | STD | iMEF | Collagenase | [85] |
| Miz-hES6 | N | STD | iMEF | Collagenase | [85] |
| Royan H5 | N | STD/Mod | mmcMEF | Mechanical | [7] |
| SNU-hes-1 | N | STD/Mod | mmcSTO | Mechanical | [53] |
| SNU-hes-3 | N | STD | iMEF | Mechanical | [53] |
Table 3.
Various media formulations used in the culture of undifferentiated ePSCs (from Table 1).
| Medium Component | STD | STD- | STD/Mod | HES | HES/Mod | HUES | MB03 | MB03/Mod |
|---|---|---|---|---|---|---|---|---|
| DMEM/F12 | + | − | + | − | − | − | − | − |
| KO-DMEM | − | + | − | − | − | + | + | + |
| DMEM-HG | − | − | − | + | + | − | − | − |
| KOSR, % | 20 | 20 | 20 | − | 20 | 10 | − | 20 |
| FBS, % | − | − | − | 20 | − | − | 20 | − |
| Plasmanate, % | − | − | − | − | − | 10 | − | − |
| ITS | − | − | − | − | 2x | − | − | − |
| 2-ME, uM | 100 | 100 | 100 | 100 | 55 | 55 | 100 | 100 |
| NEAAs, % | 1 | − | 1 | 1 | 1 | − | 1 | 1 |
| GLN, mM | 1−2 | − | 1−2 | − | 2 | 1 | 1 | 1 |
| Ribonucleosides, % | − | − | − | − | − | − | 1 | 1 |
| Heparin, ug/mL | − | + | − | − | − | − | − | − |
| LIF, ng/mL | − | − | − | 2000U/mL | − | 12 | − | − |
| bFGF, ng/mL | 4−20 | 4 | 40 | − | 4 | 10 | 4 | 4 |
| Noggin, ng/mL | − | − | 100 | − | − | − | − | − |
1) Starting material (Table 1)
As stated above, all PSCs, to date, for which neural differentiation methodologies have been published, are ePSCs. The neural differentiation of at least 16 different ePSC lines has so far been described (Table 2). These lines include many on the NIH Stem Cell Registry as well as other more recently created lines. All described lines were either grown on irradiation- or mitomycin C-inactivated feeder cells or on Matrigel with medium conditioned by these feeder cells. Most ePSCs are mechanically passaged but some are passaged (as clumps of cells) after partial enzymatic digestion with trypsin, dispase, or collagenase. The culture media used for the various lines vary somewhat and the ingredients for those media are shown in Table 3. It should be noted that a recently published laboratory manual details the most common methods for ePSC culture and passaging[39;110;111;119].
Despite the variation in culture conditions for the starting material, all the PSCs described show same basic set of criteria: 1) morphology: compact colonies of small phase bright cells with high nucleus-to-cytoplasm ratios (Figure 4); 2) gene expression: cells uniformly expressing Oct4, Nanog, Sox2, SSEA4, Tra-1−81; 3) differentiation potential: cells generate tumors containing cells of endodermal, ectodermal, and mesodermal lineages. In addition, PSCs are routinely karyotyped to assure chromosomal stability as these cells can easily progress to aneuploidy. Finally, it should be noted that most cell lines, but not all, can be conditioned to thrive in any of the culture media described.
Figure 4.
ePSCs in culture. Photomicrographs are taken through the phase-contrast microscope at 40x (left panel), 100x (center panel), and 400x (right panel) magnification. These colonies of ePSCs, as is commonly the case, are grown in the presence of a feeder layer of cells (black arrowheads), in this case mouse embryonic fibroblasts. Even when grown under conditions that do not favor differentiation, spontaneous differentiation occurs and is seen as groups of less tightly packed cells emanating from the sides of the ePSC colonies (white arrowheads).
2) Induced loss of pluripotency (Table 1)
The most widely used method for inducing loss of pluripotence/inducing differentiation in PSCs is to enzymatically or mechanically lift the PSC colonies and place them, as clumps of cells, into low-adherence culture dishes or flasks without substrate, feeder cells, or mitogens. The exact media formulations vary (Table 1) but the formation of floating, spherical clusters or balls of cells of varying degrees and lineages of differentiation and known as embryoid bodies (EBs) is the result. EBs, as a general rule, have been used as the source of partially differentiated cells for the production of a variety of tissue/cell types including neural cell types. EBs, even though the earliest differentiated progeny of PSCs, show a great degree of gene expression divergence from that of PSCs (Figure 5) and immunocytochemical staining of EBs shows cells of all three germ lineages[12;32].
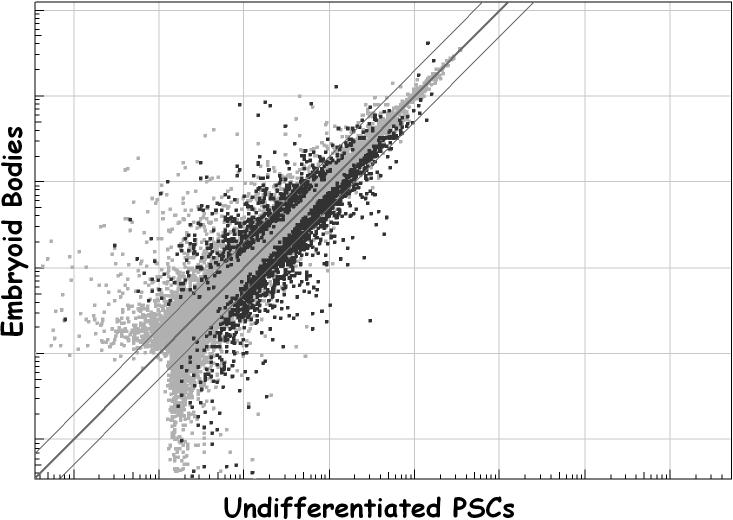
Figure 5.
Formation of embryoid bodies induces a dramatic shift in gene expression in PSCs. The line of identity (thick line) and the bounds of 2-fold expression differences or less (thin lines) are shown. The expression of many gene products falls well outside the boundaries. Note the increased spread of the data compared to that in Figure 2. In this particular case, EBs express some 617 genes at significantly higher levels (p<0.0001) compared to PSCs, while PSCs express some 1305 genes at higher levels than EBs. The data represent pooled mRNA from the H1, H7, H9, BG01, BG02, BG03, and BG01v cell lines.
Non-EB approaches have also been used. The most common of these is the use of specific growth factor and/or antagonists to accelerate differentiation towards one cell type or lineage of interest (see Table 1). Other avenues of directed differentiation that have been employed involve the coculture of PSCs with cells of a particular origin that have been found to produce factors that enhance a specific lineage type phenotype. Future options may lie in the direction of specific lineage differentiation through the selective expression of specific transcription factors and/or combinations of transcription factors or through the use of small molecules that activate growth factor and or developmental pathways independent of the growth factors’ presence.
3) Neural Induction (Table 1)
The EBs are differentiated to neuroepithelial cells in simple, serum-free culture media. EBs first are plated onto laminin-coated dishes to generate an adherent culture. The earliest reports of neural induction of EBs showed the formation of unique clusters of small, elongated (or columnar) cells surrounding a central, small, but cell-free zone. These neural rosettes[81;82], which resemble the morphology of the very early neural tube in cross-section, express early neural marker antigens such as nestin and Musashi-1, but not markers of more mature neural cells, which are found adjacent to the rosettes. Rosettes form the basis of most published methods of neural induction of PSCs. This method allows control of developmental stages and generation of primitive neuroepithelial cells which can be further induced to neuronal and glial progenitors with forebrain, mid/hindbrain, and spinal cord identities. Thus, this neuroepithelial differentiation method can be used to broadly generate neural progenitors and mature neural subtypes, and is well-adapted to the needs of individual investigators who intend to differentiate ePSC to specific types of neurons and glial cells. The protocol comprises three major steps: EB formation, differentiation of primitive neuroepithelial cells (i.e. neural rosette cells[33]), and generation of more definitive neuroepithelial cells. Each step is morphologically distinct and is readily identifiable under a regular phase contrast microscope. The typical yield of neuroepithelial cells, defined by immunostaining for the neuroepithelial transcription factors PAX6, SOX1, and SOX2, is about 90% of the total differentiated progenies[64;82].
4) Neural Maintenance and Expansion (Table 1)
The primitive neuroepithelial cells, or NSCs, are largely maintained and expanded in the presence of standard media containing growth factors. Basic fibroblast growth factor (bFGF) has been the predominant mitogen of choice[8;13;43;61;100]. Epidermal growth factor (EGF)[9;93], laminin[54] and ascorbic acid (AA)[61] have also been used in combination with FGF and other proprietary supplements. NSCs are commonly expanded as either rosettes or neural tubes on adherent substrates such as laminin, poly ornithine (PLO), or FN, or as neurospheres (clonal clusters of cells) in suspension on low attachment polystyrene. Rosettes are typically expanded over a 7−10 day period prior to their isolation and plating for induction of terminal differentiation (Table 1). Neurospheres typically expand over the same time period; however, they are more amenable to passaging over several weeks. The neurospheres are dissociated into a cell suspension and replated under identical conditions. Here they may undergo another round of expansion prior to the induction of terminal differentiation (Table 1).
5) Neuronal/Glial Differentiation (Table 1)
Although the formation of neural tissue types from spontaneously differentiating PSCs has long been considered a non-specialized default pathway, the promotion of differentiation towards one neural lineage subtype or the other may be manipulated through varied means. While a multiple of terminal neuronal differentiation methods have been documented (see Table 1 and references therein), a few common denominators among them exist. The NSCs, either as rosettes and/or neural tubes or in suspension as neurospheres, are plated on an adhesive substrate, typically laminin in combination with PLO or poly-lysine. The NSCs are typically grown in low-serum or serum free/ serum replacement media that is tailored with defined and specific combinations of growth factors, such as bFGF, and/or signaling molecules. Neural differentiation typically occurs over a period of 2−3 weeks after which cells may be evaluated for differentiation using a variety of methods. These typically involve immunocytochemistry, staining for the emergence of neural lineage specific markers accompanied by the loss of pluriopotency markers (see Table 4) and, ultimately, the demonstration of functional activity such as action potentials for neurons.
Table 4.
Markers for neural differentiation.
| Cell Type | Markers |
|---|---|
| Pluripotent Stem Cell [PSCs] | POU5F1 (Oct-3/4) |
| NANOG | |
| SOX-1 | |
| SOX-2 | |
| TDGF | |
| GDF-3 | |
| SSEA-3/−4 | |
| Tra-1−81 | |
| Tra-1−60 | |
| CD9 | |
| Thy-1 [CD90] | |
| Neural Stem Cell [Rosettes] | PAX-6 |
| SOX-1 | |
| Nestin | |
| FORSE-1 | |
| N-CAD | |
| CD133 | |
| FOXG1 [BF1] | |
| 3CB2 | |
| Neural Precursor Cell [Other] | CD133 |
| SSEA-1 [CD15] | |
| A2B5 | |
| FORSE-1 | |
| Integrin B-1 [CD29] | |
| CD146 | |
| p75[CD271] | |
| Neural Crest Cell | AP2 |
| HNK1 | |
| p75 | |
| Mature Neural Cell | TuJ1 |
| NSE | |
| Map2a | |
| Gap43 | |
| NF | |
| CD24 | |
| NCAM [CD56] | |
| Dopaminergic Neurons | TH |
| AaDC | |
| Dat | |
| Otx-2 | |
| VMAT2 | |
| Cholinergic Neurons | NGF |
| ChAT | |
| Motor Neurons | HB9 |
| SMN | |
| ChAT | |
| NKX6 | |
| Peripheral Neurons | TH, Peripherin |
| Sensory Neurons | POU4F1 [Brn3A] |
| Peripherin | |
| Glial Cells | S100beta |
| Astrocytes | GFAP |
| Tapa1[CD81] | |
| Oligodendrocytes | O1 |
| O4 |
Retinoic acid (RA) plays important roles in the development, regeneration and maintenance of the nervous system[69]. In particular, RA promotes the induction of caudally fated neuroepithelial cells[125]. Adherent cultures of PSCs grown in serum-free media supplemented with RA, in combination with bFGF, have been shown to give rise to an enriched neural population (>90%) following mechanical isolation and replating of neural tube like structures[8]. The effectiveness of RA in promoting differentiation is enhanced by the presence of additional signaling molecules, such as sonic hedgehog (SHH). SHH is a critical factor for patterning of the early embryo, particularly the ventral neural tube. Treatment of human PSC-derived neuroectodermal cells with retinoic acid (RA) and sonic hedgehog (SHH) has resulted in the generation of spinal motor neurons[61;64;66], Remarkably, purmorphamine, a small molecule activator of the SHH pathway, can substitute for SHH and, in association with RA, promote the formation of motor neurons[65].
The SHH signaling pathway is also involved in the specification of dopamine (DA) neural subtypes. Treatment of PSC-derived neuroepithelial cells with SHH in combination with fibroblast growth factor-8 (FGF-8) has been shown to promote differentiation of DA neurons with a forebrain phenotype[122]. However, early exposure to FGF during neuroepithelial specification promotes the formation of midbrain dopaminergic neurons[122]. Thus, the sequence and timing of FGF-8 and SHH exposure is critical in determining the choice of neuronal subtype produced.
Tyrosine hydroxylase positive (TH+) midbrain dopamine (DA) neurons have also been successfully differentiated from PSCs following co-culture with PA6 stromal cells and directed differentiation with bFGF and AA. Although these PSC-derived DA neurons demonstrated functionality in vitro, they failed to have any beneficial effect following transplantation into the striatum of hemi-parkinsonian rats[86]. It has become evident that there are many combinations of neural differentiation agents that can produce a similar, marker-defined, output. For example, TH+ DA neurons have also been induced by culture in the presence of noggin and dibutyryl cAMP (dbcAMP)[43]. Transplantation of these neuronal progenitors into the striata of 6-hydroxydopamine treated rats produced functionally differentiated DA cells in vivo 2−3 weeks post-transplantation.
A variety of neural differentiation protocols have utilized the developmental signaling molecule Noggin[9;37;109]. Noggin is critical for neural tube fusion during embryogenesis. It is a secreted polypeptide that binds and inactivates members of the transforming growth factor-beta (TGF-beta) superfamily of signaling proteins. In most culture methods, Noggin is thought to act to suppress non-neural differentiation pathways. Noggin enhances the directed differentiation of PSCs into neuroectoderm through its antagonistic action on bone morphogenetic protein-4 (BMP4) signaling pathways[45].
Recently, neural crest stem (NCS) cells have also been derived from PSCs at the neural rosette stage. Neural crest precursors (expressing p75 and HNK1) were isolated from neural rosettes by fluorescence activated cell sorting (FACS) and expanded in culture using BFGF and EGF. Neural differentiation was induced by the withdrawal of BFGF/EGF and exposure to BDNF, GDNF, NGF, dbcAMP. This treatment regimen yielded both peripheral sympathetic neurons (TH+/Peripherin+) and sensory neurons (Brn3a+/Peripherin+). Schwann cell differentiation was also induced from these expanded p75+/HNK1+ cultures by treatment with CNTF, neuregulin 1 beta, and dbcAMP. Critically, transplantation of the human NCS cells into the developing chick embryo and the adult mouse demonstrated survival, migration and differentiation compatible with neural crest identity[60].
The directed differentiation of PSCs towards oligodendrocytes has also been achieved. In one method oligodendrocyte progenitor cells (OPCs) have been produced using from neurospheres cultured with a combination of bFGF, EGF and RA[78]. These OPCs are reported to be capable of functional integration and compact myelin formation following transplantation into the shiverer mouse model of dysmyelination[78]. OPCs have also been differentiated from mechanically isolated rosettes and neural tubes that were cultured with a regiment of bFGF and EGF for four days followed by N2, bFGF and PDGF for an additional 8 days, prior to growth factor withdrawal and treatment with the thyroid hormone 3,3’,5-triido L-thyronine[50]. Using this method, over 80% of the total cell population were oligodendrocytes. Importantly, these OPCs, when co-cultured with rat hippocamal neurons, were capable of myelinating axons.
Astrocytes are the most abundant cell type in the brain and are critical for the normal physiology of the CNS. Although methods for the directed differentiation of PSCs towards the astrocyte lineage remain scant, neural progenitors derived from PSCs, however, do generate astrocytes during gliogenic stages in the absence of any defined treatment[116]. The generation of astrocytes is still poorly understood. The identification of specific markers for the identification of astrocyte precursors is a necessary first step. Indeed, the molecular pathways involved in the differentiation of many specialized neural subtypes remain to be fully unraveled.
D. Comparison of heNSCs and hbNSCs (Figure 6)
Figure 6.
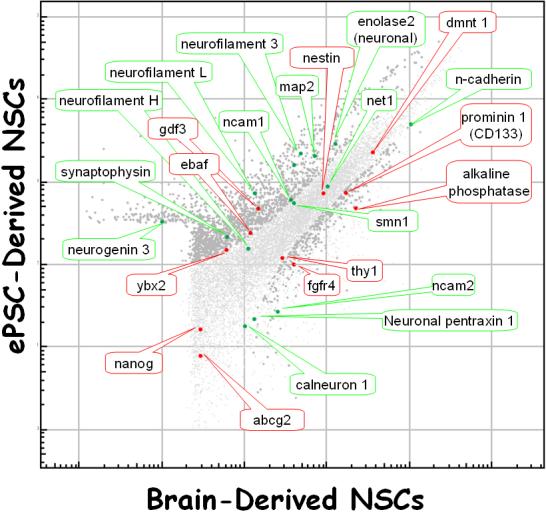
bNSC gene expression versus that of eNSCs. The former were derived from human brain[103] and the latter were derived from ePSCs[23]. Both cell types were cultured in bFGF/EGF-containing media. In the graph, a select few stem cell-specific (red) and neural cell-specific (green) genes are highlighted. The darker gray dots are those gene products that are significantly different between the two cell populations (p<0.0001). What is clear is that there is a wide digression of gene expression between the two cell types: there are both a large scatter of the data as well as a lack of identity of stem cell-specific and neural cell-specific genes.
Neural stem cells which were derived from PSCs or from somatic tissues most likely represent two (or even a large number of) phenotypes with different biological properties (Figure 6)[2;27;33;107]. The only commonalities among them are the expression of certain markers (Table 4) and neuronal-like functional properties when differentiated in vitro or in vivo[62]. Not only do these two cell types display very different expression patterns, even when looking at “marker” genes, they may vary considerably in the neuronal phenotypes which evolve after differentiation in vitro or after transplantation in vivo[2;27;33;107]. Currently, there is considerable discordance between what we assume about the identity and fate of neural stem cells (from any source) and experimental, especially quantitative, insight into what each possible stem cell type actually does mature into when subjected to differentiating conditions.
What we now know is that neural progenitors and stem cells derived from somatic sources or PSCs can form neuronal connections and participate in functional neuronal circuits[62;75;126]. We do not know for sure how physiological or “normal” these circuits are. Data from models of abnormal function of endogeneous neural progenitors in epilepsy indicate that even minute alterations in morphology, connectivity, and signal-transmission properties of a few cells can result in a severe chronic disorder[46;83]. And, as mentioned, these cells represent an endogeneous population of neural cells, which weren't extracted from tissue, derived from ePSCs, or cultured and manipulated in vitro.
Under certain conditions in preclinical models, neural stem cells can participate in normal brain development and correct neurological pathology[62;74]. Neural stem cells do offer many more possibly disease ameliorating properties than just cell replacement, however, as has been shown in many studies: they can act as anti inflammatory cells, correct metabolic defects by supplying lacking enzymatic activities and can support sick neuronal populations with trophic support[62;80;89;92;127]. These beneficial effects make our current experimental data difficult to interpret because all of these effects can actually contribute to neurological improvement in preclinical models. Thus, we cannot predict from these results what a given cell preparation will or will not do in a slightly different preclinical or clinical setting.
The assumption that neural stem cells derived from ePSCs and neural stem cells from somatic sources may have a shared phenotype, does beg the question what the actual molecular neural progenitor and/or stem cell is. This question has never been resolved satisfactorily in experiments since there is currently no accepted method to prospectively identify neural stem cells (of any source). The lack of such an assay is mostly due to the fact that there is no experimental method, such as the repopulation experiments in the hematopoietic stem cell field, which can unequivocally demonstrate that a single cell or cell preparation does indeed have the ability to differentiate and assume the function of every cell type within a specific organ system. One major reason for this is that the neurological equivalent of the hematopoietic experiments raises some profound ethical issues[48;104].
At present, only the neural rosette cell can currently be regarded as the “true” neural stem cell[33]; neural stem cells from somatic sources are considerably different from neural stem cells from ePSCs (Figure 6)[2;27;33;107]. Every neural progenitor and stem cell type that has been derived, even just from slightly different sources (age of subject, marker based sorting, ePSC-line of origin, brain region, culture method), may possibly represent its own neural stem cell phenotype with unique properties within a wide spectrum of possible functions. Only an unbiased, scientific phenotyping examination of every reported neural stem cell preparation using systematic, high-throughput, functional genomics, combined with in vitro and in vivo assays, will be able to tell us if any generalizations can be made about what we now call “neural stem cells”.
E. Concluding Remarks
1. Differentiation ability of different ePSC lines
One issue that is particularly important is whether all PSC lines have equal capacity to differentiate into a particular cell type. There have been many anecdotal reports of cell lines that are better than others at producing neurons, or glia, or pancreatic islet cells, but there have been only a few publications on the multiple cell line comparisons that would be necessary to demonstrate differences in potency. In one study[56], HSF6, Miz-hES4, and Miz-hES6 were compared for their ability to give rise to cells of ectodermal, mesodermal, and endodermal lineages following EB formation and subsequent plating. The data convincingly show that there were marked differences in differentiation potential among the three lines. In the second study[79], HUES lines 1−17 were examined with a finding similar to the previous study: some lines showed a marked propensity to differentiate into specific lineages. Importantly, even within a given line, the variability of specific lineage differentiation appears to be quite large.
Although no specific causes for the variability in differentiation potential have yet been found, the differences are most likely due to genetic variations, epigenetic modifications[3;13;55], or both. There is abundant evidence that PSC lines accumulate genetic alterations during culture[3]. These may be gross aneuploidies or subtle differences that can be detected only by high-resolution genetic mapping techniques such as SNP genotyping. Genetic changes could affect the levels of expression of genes, either directly through mutations in protein-coding sequences, or indirectly through affecting regulatory elements such as transcription factors and miRNAs. Epigenetic factors, such as DNA methylation and chromatin modifications are known to change during cellular differentiation, but it is unclear which changes are causal and which are the result of the differentiation. Both genetic and epigenetic changes could occur very early in the derivation of PSCs, and influence their future direction. Such changes would not be detectable by currently available analysis methods. Since most culture conditions are developed with the goal of preventing PSCs from differentiating, early events that predestine their later differentiation would not be noticed.
2. Homogeneity of final product
Production of a “pure” population of differentiated cells may, in fact, be possible in vitro. What remains, however, is to determine whether or not a homogeneous final product is actually desirable. This will depend entirely on its application. It is probable that a homogeneous final product will not be best for most applications. In the transplantation arena, for example, although a pure population of oligodendrocyte progenitors may be desirable for the demyelination subsequent to spinal cord injury, this form of therapy alone does not address the many other consequences of spinal cord injury on neuronal populations. Indeed, in most forms of nervous system injury more than one type of cell is affected, not only glia and neurons, but different types of neurons, both intrinsic and extrinsic. A related consideration can be made for in vitro studies, whether they be aimed at understanding neurobiology in health or disease or whether they are designed to screen for small, therapeutic, or reagent molecules. That is, no part of the nervous system is comprised of a single cell type; thus, multiple cell types are not only desired, but required, to recapitulate normal nervous function in vitro. At present, only relatively passive methods for directing an appropriate multi-lineage differentiation sequence exist and these depend entirely on the abilities of the resulting cells to self-assemble into semi-functional neural nets.
3. Expandability of final product
Generation of sufficient quantities of appropriately differentiated cells will drive the methodology. That is, whether or not any of the progenitors to the cells desired, besides the PSCs themselves, can be expanded in vitro will determine which step in the differentiation process will also be the expansion step. Other considerations include ease and cost of expansion and untoward effects of expansion such as genetic instability. For example, while it seems to be relatively straightforward to expand neural progenitors of limited differentiation potential (i.e. stage 4 cells) in vitro, expansion of neural rosette cells with more differentiation potential (i.e. stage 3 cells) appears to be more difficult. This may be due to a requirement of these cells for a particular two- (or three-)dimensional arrangement with particularly close cell-to-cell contacts. Generation of large quantities of these cells, therefore, may require a large quantity of their immediate precursors, EBs and, by extension, PSCs. Expansion of PSCs, however, can give rise to aneuploidies; thus, additional care must be taken in characterizing the starting material before a large scale differentiation protocol is instituted.
4. Tumorigenicity of final product
One of the basic properties of undifferentiated PSCs is their ability to form a certain type of tumor called a teratoma, which is a tumor comprised of disorganized foci of differentiated tissues of different lineages. The differentiation potential of PSC lines is typically determined by injecting a bolus of undifferentiated PSCs into the muscle of a SCID mouse and then evaluating the resulting tumor some weeks later. So far, all ePSC lines have demonstrated the ability to form teratomas. The implantation of undifferentiated PSCs, therefore, carries substantial risk [5] . Undifferentiated PSCs are thus unlikely to be used directly in therapeutic applications. Differentiation of a PSC, however, is thought to eliminate its capacity to form a tumor. Therefore, PSCs will be used to generate populations of cells that are at least partially differentiated, and these differentiated cells will be used therapeutically [42]. Two possible confounds to this general principle, however, have recently been described in the literature: 1) the continued in vivo proliferation of neural cells differentiated from PSCs[33;98] and 2) the production of a much more malignant tumor when PSCs were implanted into human, rather then mouse, tissue[106]. Thus it is not only unclear which differentiated progeny of PSCs are appropriate for transplantation but also how, exactly, to even determine human tumorigenicity of these differentiated populations.
Acknowledgements
We gratefully acknowledge Boback Ziaeian for performing the immunocytochemistry and generating the fluorescence photomicrographs and Hubert Nethercott, Michele Sheridan, and Heather Maxwell for performing ePSC culture. We also thank Robin Wesselschmidt for critically reading the manuscript. The work in the communicating author's laboratory is supported by the CHOC Foundation for Children and the National Institutes for Health.
Abbreviations
A
- A2B5
neuronal cell surface antigen A2B5 clone 105
- AA
Ascorbic acid
- AaDC
aromatic amino acid decarboxylase
- ABCG2
ATP-binding cassette
B
- B27
B27 Neural growth supplement
- BDNF
brain-derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- BMP
bone morphogenic protein
- bNSC
brain-derived neural stem cell
- bPSCs
blastomere-derived pluripotent stem cells
C
- cAMP
cyclic adenosine monophosphate
- CD
cluster of differentiation antigen
- ChAT
choline acetyltransferase
- CM
Conditioned medium
- CNS
Central Nervous System
D
- DA
Dopamine
- Dat
dopamine transporter
- DCX
doublecortin
- DMEM
Dulbecco Modified Eagle Medium
- DMEM/F12
Dulbecco Modified Eagle Medium w/ F12 Supplementi
E
- EB
Embryoid body
- EDTA
ethylenediaminetetraacetic acid
- EGF
epidermal growth factor
- ePSCs
embryo-derived pluripotent stem cells
- ESC
embryonic stem cell
F
- FBS
fetal bovine serum
- FGF
fibroblast growth factor
- FN
fibronectin
- FORSE
forebrain surface embryonic antigen
G
- GABA
Gamma aminobutyric acid (receptor)
- Gap43
growth associated protein-43
- GDNF
glial-derived neurotrophic factor
- GFAP
glial fibrillary acidic protein;
- GRM
Glial restriction media
H
- HB9
homeobox 9 protein
- hbNSCs
human brain-derived neural stem cells
- heNSCs
human ePSC-derived neural stem cells
I
- iHFF
irradiated human fibroblast feeders
- iMEF
irradiated mouse embryo feeders (CF-1 strain)
- iPSCs
induced pluripotent stem cells
- ITS
insulin, transferin, & selenium
- IVF
in vitro fertilization
J
K
- Klf4
Kruppel-like factor 4
L
- LA polystyrene
low attachment polystyrene
M
- MAG
myelin associated glycoprotein
- MAP2a
microtubule associated protein 2a
- mmcMEF
mitomycin c-treated mouse embryo feeders
- mmcSTO
mitomycin c-treated mouse embryo feeders (STO strain)
- mRNA
messenger ribonucleic acid
- MyoD1
myogenic differentiation 1
N
- N2
N2 neural growth supplement
- NCAM
neural cell adhesion molecule
- NF
neurofilament
- NGF
nerve growth factor
- NKX6
NK6 homeobox protein
- NPM
Neural Progenitor Medium
- NSC
Neural Stem Cell
- NSE
neuron specific enolase
O
- O1, O4
oligodendrocyte antigens 1 and 4
- Oct-4
octamer binding transcription factor 4
- Otx-2
orthodenticle homolog-2
P
- PDGF
Platelet-derived growth factor
- PGD
preimplantation genetic diagnosis
- PLL
Poly-L-Lysine
- PMP22
Peripheral myelin protein
- PO
Polyornithine
- POU
Pit-oct-unc
- pPSCs
Parthenogenic-derived pluripotent stem cells
- PSC
Pluripotent Stem Cells
- PS-NCAM
Polysialated neural cell adhesion molecule
R
- RA
Retinoic Acid
S
- SHH
Sonic Hedgehog
- SMN
survival motor neuron protein
- SOX
SRY box containing gene
- sPSCs
spermatogonial tissue-derived plurpotent stem cells
- SRM
serum replacement medium
- SSEA
stage specific embryonic antigen
- STD
standard (media or condition)
T
- Tapa1
target of the antiproliferative antibody 1
- TC
tissue culture
- TGF-beta
transforming growth factor beta
- TH
tyrosine hydroxylase
- Thy1
Thymocyte differentiation antigen 1
- Tra-1−60
teratocarcinoma related antigen 1−60
- Tra-1−81
teratocarcinoma related antigen 1−81
- TuJ1
Beta tubulin III antibody clone TuJ1
U
V
- VMAT-2
vesicle monamine transporter 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Abeyta MJ, Clark AT, Rodriguez RT, Bodnar MS, Pera RA, Firpo MT. Unique gene expression signatures of independently-derived human embryonic stem cell lines. Hum Mol Genet. 2004 Mar 15;13(6):601–8. doi: 10.1093/hmg/ddh068. [DOI] [PubMed] [Google Scholar]
- 2.Aiba K, Sharov AA, Carter MG, Foroni C, Vescovi AL, Ko MS. Defining a developmental path to neural fate by global expression profiling of mouse embryonic stem cells and adult neural stem/progenitor cells. Stem Cells. 2006;24(4) doi: 10.1634/stemcells.2005-0332. [DOI] [PubMed] [Google Scholar]
- 3.Allegrucci C, Wu YZ, Thurston A, Denning CN, Priddle H, Mummery CL, et al. Restriction landmark genome scanning identifies culture-induced DNA methylation instability in the human embryonic stem cell epigenome. Hum Mol Genet. 2007 May 15;16(10):1253–68. doi: 10.1093/hmg/ddm074. [DOI] [PubMed] [Google Scholar]
- 4.Araki R, Fukumura R, Sasaki N, Kasama Y, Suzuki N, Takahashi H, et al. More than 40,000 transcripts, including novel and noncoding transcripts, in mouse embryonic stem cells. Stem Cells. 2006;24(11) doi: 10.1634/stemcells.2006-0005. [DOI] [PubMed] [Google Scholar]
- 5.Arnhold S, Klein H, Semkova I, Addicks K, Schraermeyer U. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004 Dec;45(12):4251–5. doi: 10.1167/iovs.03-1108. [DOI] [PubMed] [Google Scholar]
- 6.Azuara V, Perry P, Sauer S, Spivakov M, Jorgensen HF, John RM, et al. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8(5) doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- 7.Baharvand H, Ashtiani SK, Valojerdi MR, Shahverdi A, Taee A, Sabour D. Establishment and in vitro differentiation of a new embryonic stem cell line from human blastocyst. Differentiation. 2004 Jun;72:224–9. doi: 10.1111/j.1432-0436.2004.07205005.x. [DOI] [PubMed] [Google Scholar]
- 8.Baharvand H, Mehrjardi NZ, Hatami M, Kiani S, Rao M, Haghighi MM. Neural differentiation from human embryonic stem cells in a defined adherent culture condition. Int J Dev Biol. 2007;51(5):371–8. doi: 10.1387/ijdb.72280hb. [DOI] [PubMed] [Google Scholar]
- 9.Banin E, Obolensky A, Idelson M, Hemo I, Reinhardtz E, Pikarsky E, et al. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells. Stem Cells. 2006 Feb;24(2):246–57. doi: 10.1634/stemcells.2005-0009. [DOI] [PubMed] [Google Scholar]
- 10.Barrett RD, Bennet L, Davidson J, Dean JM, George S, Emerald BS, et al. Destruction and reconstruction: hypoxia and the developing brain. Birth Defects Res C Embryo Today. 2007 Sep;81(3):163–76. doi: 10.1002/bdrc.20095. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Hur T, Idelson M, Khaner H, Pera M, Reinhartz E, Itzik A, et al. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells. 2004;22(7):1246–55. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharya B, Cai J, Luo Y, Miura T, Mejido J, Brimble SN, et al. Comparison of the gene expression profile of undifferentiated human embryonic stem cell lines and differentiating embryoid bodies. BMC Dev Biol. 2005 Oct 5;5:22. doi: 10.1186/1471-213X-5-22. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bibikova M, Chudin E, Wu B, Zhou L, Garcia EW, Liu Y, et al. Human embryonic stem cells have a unique epigenetic signature. Genome Res. 2006 Sep;16(9):1075–83. doi: 10.1101/gr.5319906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blake WJ, Balazsi G, Kohanski MA, Isaacs FJ, Murphy KF, Kuang Y, et al. Phenotypic consequences of promoter-mediated transcriptional noise. Mol Cell. 2006;24(6) doi: 10.1016/j.molcel.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Blake WJ, Collins JJ. And the noise played on: stochastic gene expression and HIV-1 infection. Cell. 2005;122(2) doi: 10.1016/j.cell.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Bongso A, Fong CY, Ng SC, Ratnam S. Isolation and culture of inner cell mass cells from human blastocysts. Hum Reprod. 1994 Nov;9(11):2110–7. doi: 10.1093/oxfordjournals.humrep.a138401. [DOI] [PubMed] [Google Scholar]
- 17.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122(6) doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441(7091) doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 19.Bracci R, Perrone S, Buonocore G. The timing of neonatal brain damage. Biol Neonate. 2006;90(3):145–55. doi: 10.1159/000092517. [DOI] [PubMed] [Google Scholar]
- 20.Branch DW, Ducat L, Fantel A, Low WC, Zhou FC, Dayton DH, et al. Suitability of fetal tissues from spontaneous abortions and from ectopic pregnancies for transplantation. Human Fetal Tissue Working Group [see comments]. JAMA. 1995 Jan 4;273:66–8. [PubMed] [Google Scholar]
- 21.Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, et al. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999 Jul 30;285(5428):754–6. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 22.Buccino G, Solodkin A, Small SL. Functions of the mirror neuron system: implications for neurorehabilitation. Cogn Behav Neurol. 2006 Mar;19(1):55–63. doi: 10.1097/00146965-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Cai J, Chen J, Liu Y, Miura T, Luo Y, Loring JF, et al. Assessing self-renewal and differentiation in human embryonic stem cell lines. Stem Cells. 2006 Mar;24(3):516–30. doi: 10.1634/stemcells.2005-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpenter MK, Rosler E, Rao MS. Characterization and differentiation of human embryonic stem cells. Cloning Stem Cells. 2003;5:79–88. doi: 10.1089/153623003321512193. [DOI] [PubMed] [Google Scholar]
- 25.Carpenter MK, Rosler ES, Fisk GJ, Brandenberger R, Ares X, Miura T, et al. Properties of four human embryonic stem cell lines maintained in a feeder-free culture system. Dev Dyn. 2004 Feb;229:243–58. doi: 10.1002/dvdy.10431. [DOI] [PubMed] [Google Scholar]
- 26.Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, Rao MS. Enrichment of Neurons and Neural Precursors from Human Embryonic Stem Cells. Experimental Neurology. 2001 Dec;172(2):383–97. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- 27.Colombo E, Giannelli SG, Galli R, Tagliafico E, Foroni C, Tenedini E, et al. Embryonic stem-derived versus somatic neural stem cells: a comparative analysis of their developmental potential and molecular phenotype. Stem Cells. 2006;24(4) doi: 10.1634/stemcells.2005-0313. [DOI] [PubMed] [Google Scholar]
- 28.Cowan CA, Klimanskaya I, McMahon J, Atienza J, Witmyer J, Zucker JP, et al. Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med. 2004 Mar 25;350:1353–6. doi: 10.1056/NEJMsr040330. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham M, McKay R. Transplantation strategies for the analysis of brain development and repair. J Neurol. 1994 Dec;242:S40–S42. doi: 10.1007/BF00939241. [DOI] [PubMed] [Google Scholar]
- 30.Dekel I, Magal Y, Pearson-White S, Emerson CP, Shani M. Conditional conversion of ES cells to skeletal muscle by an exogenous MyoD1 gene. New Biol. 1992 Mar;4(3):217–24. [PubMed] [Google Scholar]
- 31.Du ZW, Zhang SC. Neural differentiation from embryonic stem cells: which way? Stem Cells Dev. 2004 Aug;13(4):372–81. doi: 10.1089/scd.2004.13.372. [DOI] [PubMed] [Google Scholar]
- 32.Dvash T, Mayshar Y, Darr H, McElhaney M, Barker D, Yanuka O, et al. Temporal gene expression during differentiation of human embryonic stem cells and embryoid bodies. Hum Reprod. 2004 Dec;19:2875–83. doi: 10.1093/humrep/deh529. [DOI] [PubMed] [Google Scholar]
- 33.Elkabetz Y, Panagiotakos G, Al SG, Socci ND, Tabar V, Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008 Jan 15;22(2):152–65. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freed CR, Leehey MA, Zawada M, Bjugstad K, Thompson L, Breeze RE. Do patients with Parkinson's disease benefit from embryonic dopamine cell transplantation? J Neurol. 2003 Oct;250(Suppl 3):11144–6. doi: 10.1007/s00415-003-1308-5. [DOI] [PubMed] [Google Scholar]
- 35.Fregni F, Pascual-Leone A. Hand motor recovery after stroke: tuning the orchestra to improve hand motor function. Cogn Behav Neurol. 2006 Mar;19(1):21–33. doi: 10.1097/00146965-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci. 1995;18:159–92. doi: 10.1146/annurev.ne.18.030195.001111. 159−92. [DOI] [PubMed] [Google Scholar]
- 37.Gerrard L, Rodgers L, Cui W. Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells. 2005 Oct;23(9):1234–41. doi: 10.1634/stemcells.2005-0110. [DOI] [PubMed] [Google Scholar]
- 38.Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999 Jun;23(2):257–71. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez R, Wesselschmidt RL, Schwartz PH, Loring JF. Human Embryonic Stem Cell Culture. In: Loring JF, Wesselschmidt RL, Schwartz PH, editors. Human Stem Cell Manual. 1 ed. Elsevier: 2007. pp. 3–17. [Google Scholar]
- 40.Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006 Apr 27;440(7088):1199–203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 41.Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130(1) doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heng BC, Cao T, Haider HK, Wang DZ, Sim EK, Ng SC. An overview and synopsis of techniques for directing stem cell differentiation in vitro. Cell Tissue Res. 2004 Mar;315(3):291–303. doi: 10.1007/s00441-003-0847-5. [DOI] [PubMed] [Google Scholar]
- 43.Iacovitti L, Donaldson AE, Marshall CE, Suon S, Yang M. A protocol for the differentiation of human embryonic stem cells into dopaminergic neurons using only chemically defined human additives: Studies in vitro and in vivo. Brain Res. 2007 Jan 5;1127(1):19–25. doi: 10.1016/j.brainres.2006.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Itakura T, Nakai E, Nakao N, Nakai K. Transplantation of neural tissue into the brain--a new therapeutic modality for the 21st century. Neurol Med Chir (Tokyo ) 1998 Nov;38:756–62. doi: 10.2176/nmc.38.756. [DOI] [PubMed] [Google Scholar]
- 45.Itsykson P, Ilouz N, Turetsky T, Goldstein RS, Pera MF, Fishbein I, et al. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Mol Cell Neurosci. 2005 Sep;30(1):24–36. doi: 10.1016/j.mcn.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Jessberger S, Zhao C, Toni N, Clemenson GD, Jr., Li Y, Gage FH. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci. 2007;27(35) doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joannides AJ, Fiore Heriche C, Battersby AA, thauda-Arachchi P, Bouhon IA, Williams L, et al. A scaleable and defined system for generating neural stem cells from human embryonic stem cells. Stem Cells. 2007 Mar;25(3):731–7. doi: 10.1634/stemcells.2006-0562. [DOI] [PubMed] [Google Scholar]
- 48.Kalichman MW, Schwartz PH. Guidelines for Embryonic Stem Cell Research Oversight (ESCRO) Committees. In: Loring JF, Wesselschmidt RL, Schwartz PH, editors. Human Stem Cell Manual. 1 ed. Elsevier: 2007. pp. 437–48. [Google Scholar]
- 49.Kameda T, Smuga-Otto K, Thomson JA. A severe de novo methylation of episomal vectors by human ES cells. Biochem Biophys Res Commun. 2006 Nov 3;349(4):1269–77. doi: 10.1016/j.bbrc.2006.08.175. [DOI] [PubMed] [Google Scholar]
- 50.Kang SM, Cho MS, Seo H, Yoon CJ, Oh SK, Choi YM, et al. Efficient induction of oligodendrocytes from human embryonic stem cells. Stem Cells. 2007 Feb;25(2):419–24. doi: 10.1634/stemcells.2005-0482. [DOI] [PubMed] [Google Scholar]
- 51.Keirstead HS, Blakemore WF. The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination. Adv Exp Med Biol. 1999;468:183–97. doi: 10.1007/978-1-4615-4685-6_15. 183−97. [DOI] [PubMed] [Google Scholar]
- 52.Khot S, Tirschwell DL. Long-term neurological complications after hypoxic-ischemic encephalopathy. Semin Neurol. 2006 Sep;26(4):422–31. doi: 10.1055/s-2006-948323. [DOI] [PubMed] [Google Scholar]
- 53.Kim HS, Oh SK, Park YB, Ahn HJ, Sung KC, Kang MJ, et al. Methods for derivation of human embryonic stem cells. Stem Cells. 2005 Oct;23:1228–33. doi: 10.1634/stemcells.2004-0296. [DOI] [PubMed] [Google Scholar]
- 54.Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002 Jul 4;418(6893):50–6. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 55.Kim KP, Thurston A, Mummery C, Ward-van OD, Priddle H, Allegrucci C, et al. Gene-specific vulnerability to imprinting variability in human embryonic stem cell lines. Genome Res. 2007 Dec;17(12):1731–42. doi: 10.1101/gr.6609207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim SE, Kim BK, Gil JE, Kim SK, Kim JH. Comparative analysis of the developmental competence of three human embryonic stem cell lines in vitro. Mol Cells. 2007 Feb 28;23(1):49–56. [PubMed] [Google Scholar]
- 57.Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R. Derivation of human embryonic stem cells from single blastomeres. Nat Protoc. 2007;2(8):1963–72. doi: 10.1038/nprot.2007.274. [DOI] [PubMed] [Google Scholar]
- 58.Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest. 1996 Jul 1;98(1):216–24. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ko JY, Park CH, Koh HC, Cho YH, Kyhm JH, Kim YS, et al. Human embryonic stem cell-derived neural precursors as a continuous, stable, and on-demand source for human dopamine neurons. J Neurochem. 2007 Sep 13; doi: 10.1111/j.1471-4159.2007.04898.x. [DOI] [PubMed] [Google Scholar]
- 60.Lee G, Kim H, Elkabetz Y, Al SG, Panagiotakos G, Barberi T, et al. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007 Dec;25(12):1468–75. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- 61.Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, et al. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007 Aug;25(8):1931–9. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- 62.Lee JP, Jeyakumar M, Gonzalez R, Takahashi H, Lee PJ, Baek RC, et al. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat Med. 2007 Apr;13:439–47. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- 63.Levenstein ME, Ludwig TE, Xu RH, Llanas RA, VanDenHeuvel-Kramer K, Manning D, et al. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006 Mar;24(3):568–74. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li XJ, Yang D, Zhang SC. Motor Neuron and Dopamine Neuron Differentiation. In: Loring JF, Wesselschmidt RL, Schwartz PH, editors. Human Stem Cell Manual. 1 ed. Elsevier: 2007. pp. 199–209. [Google Scholar]
- 65.Li XJ, Hu BY, Jones SA, Zhang YS, Lavaute T, Du ZW, et al. Directed Differentiation of Ventral Spinal Progenitors and Motor Neurons from Human Embryonic Stem Cells by Small Molecules. Stem Cells. 2008 Jan 31; doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lim UM, Sidhu KS, Tuch BE. Derivation of Motor Neurons from three Clonal Human Embryonic Stem Cell Lines. Curr Neurovasc Res. 2006 Nov;3(4):281–8. doi: 10.2174/156720206778792902. [DOI] [PubMed] [Google Scholar]
- 67.Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006 Aug;3(8):637–46. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 68.Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006 Feb;24(2):185–7. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 69.Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007 Oct;8(10):755–65. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- 70.Meshorer E, Misteli T. Chromatin in pluripotent embryonic stem cells and differentiation. Nat Rev Mol Cell Biol. 2006;7(7) doi: 10.1038/nrm1938. [DOI] [PubMed] [Google Scholar]
- 71.Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448(7153) doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mitalipova M, Calhoun J, Shin S, Wininger D, Schulz T, Noggle S, et al. Human embryonic stem cell lines derived from discarded embryos. Stem Cells. 2003;21:521–6. doi: 10.1634/stemcells.21-5-521. [DOI] [PubMed] [Google Scholar]
- 73.Morita I, Keith MW, Kanno T. Dorsal column stimulation for persistent vegetative state. Acta Neurochir Suppl. 2007;97(Pt 1):455–9. doi: 10.1007/978-3-211-33079-1_59. [DOI] [PubMed] [Google Scholar]
- 74.Muller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nat Rev Neurosci. 2006;7(1) doi: 10.1038/nrn1829. [DOI] [PubMed] [Google Scholar]
- 75.Muotri AR, Nakashima K, Toni N, Sandler VM, Gage FH. Development of functional human embryonic stem cell-derived neurons in mouse brain. Proc Natl Acad Sci U S A. 2005;102(51) doi: 10.1073/pnas.0509315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Narayan AD, Chase JL, Lewis RL, Tian X, Kaufman DS, Thomson JA, et al. Human embryonic stem cell-derived hematopoietic cells are capable of engrafting primary as well as secondary fetal sheep recipients. Blood. 2006 Mar 1;107(5):2180–3. doi: 10.1182/blood-2005-05-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nethercott H, Maxwell H, Schwartz PH. Neural Progenitor Cell Culture. In: Loring JF, Wesselschmidt RL, Schwartz PH, editors. Human Stem Cell Manual. 1 ed. Elsevier: 2007. pp. 309–31. [Google Scholar]
- 78.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. GLIA. 2005 Feb;49:385–96. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 79.Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008 Feb 17; doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- 80.Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002;20(11) doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- 81.Pankratz MT, Li XJ, Lavaute TM, Lyons EA, Chen X, Zhang SC. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007 Jun;25(6):1511–20. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pankratz MT, Zhang SC. Embryoid Bodies and Neuroepithelial Development. In: Loring JF, Wesselschmidt RL, Schwartz PH, editors. Human Stem Cell Manual. 1 ed. Elsevier: 2007. pp. 185–98. [Google Scholar]
- 83.Parent JM. Adult neurogenesis in the intact and epileptic dentate gyrus. Prog Brain Res. 2007;163 doi: 10.1016/S0079-6123(07)63028-3. [DOI] [PubMed] [Google Scholar]
- 84.Park CH, Minn YK, Lee JY, Choi DH, Chang MY, Shim JW, et al. In vitro and in vivo analyses of human embryonic stem cell-derived dopamine neurons. J Neurochem. 2005 Mar;92(5):1265–76. doi: 10.1111/j.1471-4159.2004.03006.x. [DOI] [PubMed] [Google Scholar]
- 85.Park JH, Kim SJ, Oh EJ, Moon SY, Roh SI, Kim CG, et al. Establishment and maintenance of human embryonic stem cells on STO, a permanently growing cell line. Biol Reprod. 2003 Dec;69(6):2007–14. doi: 10.1095/biolreprod.103.017467. [DOI] [PubMed] [Google Scholar]
- 86.Park S, Lee KS, Lee YJ, Shin HA, Cho HY, Wang KC, et al. Generation of dopaminergic neurons in vitro from human embryonic stem cells treated with neurotrophic factors. Neurosci Lett. 2004 Apr 8;359(1−2):99–103. doi: 10.1016/j.neulet.2004.01.073. [DOI] [PubMed] [Google Scholar]
- 87.Park SP, Lee YJ, Lee KS, Ah SH, Cho HY, Chung KS, et al. Establishment of human embryonic stem cell lines from frozen-thawed blastocysts using STO cell feeder layers. Hum Reprod. 2004 Mar;19(3):676–84. doi: 10.1093/humrep/deh102. [DOI] [PubMed] [Google Scholar]
- 88.Pera MF, Filipczyk AA, Hawes SM, Laslett AL. Isolation, characterization, and differentiation of human embryonic stem cells. Methods Enzymol. 2003;365:429–46. doi: 10.1016/s0076-6879(03)65030-5. 429−46. [DOI] [PubMed] [Google Scholar]
- 89.Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436(7048) doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- 90.Rambhatla L, Chiu CP, Kundu P, Peng Y, Carpenter MK. Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplant. 2003;12:1–11. doi: 10.3727/000000003783985179. [DOI] [PubMed] [Google Scholar]
- 91.Raymont V, Grafman J. Cognitive neural plasticity during learning and recovery from brain damage. Prog Brain Res. 2006;157:199–206. doi: 10.1016/S0079-6123(06)57013-X. [DOI] [PubMed] [Google Scholar]
- 92.Redmond DE, Jr., Bjugstad KB, Teng YD, Ourednik V, Ourednik J, Wakeman DR, et al. Behavioral improvement in a primate Parkinson's model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci U S A. 2007;104(29) doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, et al. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001 Dec;19(12):1134–40. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 94.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000 Apr;18(4):399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 95.Revazova ES, Turovets NA, Kochetkova OD, Kindarova LB, Kuzmichev LN, Janus JD, et al. Patient-specific stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 2007;9(3):432–49. doi: 10.1089/clo.2007.0033. [DOI] [PubMed] [Google Scholar]
- 96.Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996 Apr 10;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 97.Romero JR, Babikian VL, Katz DI, Finklestein SP. Neuroprotection and stroke rehabilitation: modulation and enhancement of recovery. Behav Neurol. 2006;17(1):17–24. doi: 10.1155/2006/137532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006 Nov;12:1259–68. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 99.Schuldiner M, Eiges R, Eden A, Yanuka O, Itskovitz-Eldor J, Goldstein RS, et al. Induced neuronal differentiation of human embryonic stem cells. Brain Research. 2001 Sep 21;913(2):201–5. doi: 10.1016/s0006-8993(01)02776-7. [DOI] [PubMed] [Google Scholar]
- 100.Schulz TC, Noggle SA, Palmarini GM, Weiler DA, Lyons IG, Pensa KA, et al. Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture. Stem Cells. 2004;22(7):1218–38. doi: 10.1634/stemcells.2004-0114. [DOI] [PubMed] [Google Scholar]
- 101.Schulz TC, Palmarini GM, Noggle SA, Weiler DA, Mitalipova MM, Condie BG. Directed neuronal differentiation of human embryonic stem cells. BMC Neurosci. 2003 Oct 22;4:27. doi: 10.1186/1471-2202-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schwartz PH. The potential of stem cell therapies for neurological diseases. Expert Rev Neurother. 2006 Feb;6:153–61. doi: 10.1586/14737175.6.2.153. [DOI] [PubMed] [Google Scholar]
- 103.Schwartz PH, Bryant PJ, Fuja TJ, Su H, O'Dowd DK, Klassen H. Isolation and characterization of neural progenitor cells from post-mortem human cortex. J Neurosci Res. 2003 Dec 15;74:838–51. doi: 10.1002/jnr.10854. [DOI] [PubMed] [Google Scholar]
- 104.Schwartz PH, Kalichman MW. Ethical Concerns for Stem Cell Research. In: Loring JF, Wesselschmidt RL, Schwartz PH, editors. Human Stem Cell Manual. 1 ed. Elsevier: 2007. pp. 426–36. [Google Scholar]
- 105.Schwartz PH, Kalichman MW. Ethical Concerns for Stem Cell Research. In: Loring JF, Wesselschmidt RL, Schwartz PH, editors. Human Stem Cell Manual. 1 ed. Elsevier: 2007. pp. 426–36. [Google Scholar]
- 106.Shih CC, Forman SJ, Chu P, Slovak M. Human embryonic stem cells are prone to generate primitive, undifferentiated tumors in engrafted human fetal tissues in severe combined immunodeficient mice. Stem Cells Dev. 2007 Dec;16(6):893–902. doi: 10.1089/scd.2007.0070. [DOI] [PubMed] [Google Scholar]
- 107.Shin S, Sun Y, Liu Y, Khaner H, Svant S, Cai J, et al. Whole genome analysis of human neural stem cells derived from embryonic stem cells and stem and progenitor cells isolated from fetal tissue. Stem Cells. 2007;25(5) doi: 10.1634/stemcells.2006-0660. [DOI] [PubMed] [Google Scholar]
- 108.Shufaro Y, Reubinoff BE. Therapeutic applications of embryonic stem cells. Best Pract Res Clin Obstet Gynaecol. 2004 Dec;18(6):909–27. doi: 10.1016/j.bpobgyn.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 109.Sonntag KC, Pruszak J, Yoshizaki T, van AJ, Sanchez-Pernaute R, Isacson O. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells. 2007 Feb;25(2):411–8. doi: 10.1634/stemcells.2006-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stubban C, Wesselschmidt RL. Mouse Embryonic Fibroblast Feeder Cells. In: Loring JF, Wesselschmidt RL, Schwartz PH, editors. Human Stem Cell Manual. 1 ed. Elsevier: 2007. pp. 34–46. [Google Scholar]
- 111.Stubban C, Wesselschmidt RL, Katkov I, Loring JF. Cryopreservation of Human Embryonic Stem Cells. In: Loring JF, Wesselschmidt RL, Schwartz PH, editors. Human Stem Cell Manual. 1 ed. Elsevier: 2007. pp. 47–56. [Google Scholar]
- 112.Tabar V, Panagiotakos G, Greenberg ED, Chan BK, Sadelain M, Gutin PH, et al. Migration and differentiation of neural precursors derived from human embryonic stem cells in the rat brain. Nat Biotech. 2005 May;23(5):601–6. doi: 10.1038/nbt1088. [DOI] [PubMed] [Google Scholar]
- 113.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov 30;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 114.Terskikh AV, Bryant PJ, Schwartz PH. Mammalian stem cells. Pediatr Res. 2006 Apr;59(4 Pt 2):13R–20R. doi: 10.1203/01.pdr.0000205154.86517.2a. [DOI] [PubMed] [Google Scholar]
- 115.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov 6;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 116.Trounson A. The production and directed differentiation of human embryonic stem cells. Endocr Rev. 2006 Apr;27(2):208–19. doi: 10.1210/er.2005-0016. [DOI] [PubMed] [Google Scholar]
- 117.Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006 Sep 15;108(6):2095–105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wernig M, Benninger F, Schmandt T, Rade M, Tucker KL, Bussow H, et al. Functional integration of embryonic stem cell-derived neurons in vivo. J Neurosci. 2004 Jun 2;24:5258–68. doi: 10.1523/JNEUROSCI.0428-04.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wesselschmidt RL, Loring JF. Human Feeder Cells, Feeder-free, and Defined Culture Systems. In: Loring JF, Wesselschmidt RL, Schwartz PH, editors. Human Stem Cell Manual. 1 ed. Elsevier: 2007. pp. 18–33. [Google Scholar]
- 120.Westgren M, Ek S, Bui TH, Hagenfeldt L, Markling L, Pschera H, et al. Establishment of a tissue bank for fetal stem cell transplantation. Acta Obstet Gynecol Scand. 1994 May;73:385–8. doi: 10.3109/00016349409006248. [DOI] [PubMed] [Google Scholar]
- 121.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002 Sep;91:501–8. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 122.Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005 Jun;23(6):781–90. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu J, Vodyanik MA, Smuga-Otto K, ntosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007 Dec 21;318(5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 124.Zhan X, Dravid G, Ye Z, Hammond H, Shamblott M, Gearhart J, et al. Functional antigen-presenting leucocytes derived from human embryonic stem cells in vitro. Lancet. 2004 Jul 10;364:163–71. doi: 10.1016/S0140-6736(04)16629-4. [DOI] [PubMed] [Google Scholar]
- 125.Zhang SC. Neural subtype specification from embryonic stem cells. Brain Pathol. 2006 Apr;16(2):132–42. doi: 10.1111/j.1750-3639.2006.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19(12) doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 127.Ziv Y, Avidan H, Pluchino S, Martino G, Schwartz M. Synergy between immune cells and adult neural stem/progenitor cells promotes functional recovery from spinal cord injury. Proc Natl Acad Sci U S A. 2006;103(35) doi: 10.1073/pnas.0603747103. [DOI] [PMC free article] [PubMed] [Google Scholar]