Abstract
The human sodium-dependent vitamin C transporters (hSVCT1 and hSVCT2) mediate cellular uptake of ascorbic acid. Both these transporters contain potential sites for N-glycosylation in their extracellular domains (Asn-138, Asn-144 [hSVCT1]; Asn-188, Asn-196 [hSVCT2]), however the role of N-glycosylation in transporter function is unexplored. On the basis of the result that tunicamycin decreased 14C-ascorbic acid uptake in HepG2 cells, we systematically ablated all consensus N-glycosylation sites in hSVCT1 and hSVCT2 to resolve any effects on ascorbic acid uptake, transporter expression and targeting. We show that removal of individual N-glycosylation sites significantly impairs protein expression and consequently ascorbic acid uptake for hSVCT1 mutants (which N138Q is retained intracellularly) and for hSVCT2 mutants (all of which reach the cell surface). N-glycosylation is therefore essential for vitamin C transporter functionality.
Keywords: hSVCT1, hSVCT2, transport, ascorbic acid
Introduction
Vitamin C (ascorbic acid) is an essential water-soluble micronutrient required for normal cellular function and growth. It acts as a cofactor for several important enzymes and as an antioxidant [1]. Vitamin C synthesis occurs in the liver of most mammals, however, humans lost the capability of endogenous synthesis during the course of evolution and obtain it via intestinal absorption/circulation. Vitamin C deficiency leads to a variety of clinical abnormalities including scurvy, fatigue, delayed wound healing, bone and connective tissue damage as well as vasomotor instability [1, 2]. Recent studies have shown that optimal vitamin C body homeostasis protects against gallbladder diseases, nonalcoholic steatohepatities, cardiovascular diseases, cancer, and cataract formation [2–4].
The mechanism of ascorbic acid uptake by mammalian cells appears to involve Na+ and temperature-dependent specialized carrier-mediated system(s) [5–8]. The molecular identity of the system(s) involved has been delineated following the cloning of two transporters: the human sodium-dependent vitamin C transporters-1 and -2 (hSVCT1 and hSVCT2; the products of the SLC23A1 and SLC23A2 genes, respectively). Both transporters mediate ascorbic acid uptake when expressed in a variety of systems [9–12]. Hydropathy plot analyses predict that these two proteins have a 12 transmembrane spanning topology with cytoplasmic C and N-terminal domains as well as multiple consensus sites for glycosylation and phosphorylation [13].
N-glycosylation has been shown to be required for transport activity, targeting, folding, and stability of several transporters [14–17]. To date, however, nothing is known about the role of glycosylation in ascorbic acid transport. Given the importance of the liver in regulation and maintenance of vitamin C homeostasis, we examined the effect of tunicamycin treatment and mutation of potential N-glycosylation sites in hSVCT1 and -2 on functionality and cellular expression in human hepatic liver cells (HepG2), in parallel with a well characterized polarized renal cell line.
Materials and methods
Materials
YFP-N3 fluorescent protein and YFP polyclonal antibodies were from BD Biosciences (Palo Alto, CA). Cell lines were from ATCC (Manassas, VA). DNA oligonucleotides were from Sigma Genosys (Woodlands, TX). [14C] Ascorbic acid (specific activity ~13mCi/mmol) was from Amersham (Arlington, IL).
Generation of hSVCT1 and -2-YFP mutants
The Quik change™ site-directed mutagenesis kit (Stratagene, CA) was used to introduce insertions or deletions of nucleotides into the open reading frame (ORF) of SLC23A1 and SLC23A2. Paired sense-and anti-sense primer oligonucleotides encompassing the specified mutation sites (Table 1, Supplementary Table), as well as a plasmid containing hSVCT1 and -2 fused to YFP-N1 were used as a template for PCR mutagenesis. Nucleotide changes were confirmed by sequencing (Laragen, CA).
Cell culture, transient and stable transfections
MDCK cells were maintained in minimal essential medium and HepG2 cells were maintained in Dulbecco’s modified Eagle’s medium. For transient transfection, cells were grown on sterile glass-bottomed Petri dishes (MatTek, MA) and transfected at 90% confluency (2µg plasmid DNA) using Lipofectamine2000 (Invitrogen, CA). After 24–48 hrs, cells were analyzed by confocal microscopy. For stable transfection, cells were selected using G418 (0.5mg/ml) for 6–8 weeks.
Tunicamycin and PNGaseF treatment
Stable hSVCT1 and -2-YFP expressing HepG2 cells were treated with 2µg/ml tunicamycin for 48 hrs and then cells were used for uptake, FACS analysis, imaging and membrane isolation. Total membrane protein (150µg) was incubated with denaturing buffer at 100°C for 10 mins and then incubated with PNGaseF (10 µg protein/µl of PNGaseF, NEB Inc., MA) for 1 hr at 37 °C.
Confocal Imaging of hSVCT1/2 mutants
Cell monolayers grown on cover-slip dishes were imaged using a Nikon C-1 confocal scanner head attached to a Nikon Inverted phase contrast microscope. Fluorophores were excited using the 488-nm line from an argon ion-laser, and emitted fluorescence was monitored with a 530±20nm band pass.
Uptake Assays
[14C]-ascorbic acid uptake assay was performed on confluent, stable hSVCT1-YFP, hSVCT2-YFP and mutant expressing HepG2 cell lines following established procedures [6, 18]. Protein contents were estimated on parallel wells using a Bio-Rad protein assay kit.
Western blot analysis
Crude membrane fractions were isolated from stable hSVCT1 and -2-YFP expressing cell lines. Protein was resolved in (150µg) 10% SDS-PAGE and blotted on PVDF membrane (Bio-Rad, CA) [18]. Membrane was hybridized with anti-YFP polyclonal antibodies (Clontech, CA) and immunodetection performed utilizing anti-mouse IgG secondary antibodies conjugated to horseradish peroxidase (Santa Cruz Biotechnology, CA) and an enhanced chemiluminescence kit (Amersham, IL).
Flow cytometry
Flow cytometry was performed using a FACScalibur benchtop cytometer (BD Biosciences, San Jose, CA). hSVCT1 and -2 wild-type and mutants stably expressing HepG2 cells were grown within T25 tissue culture flasks. Monolayers were trypsinized and cells pelleted, resuspended in 2ml aliquots of Ca2+-free media at a density of 1×106 cells/ml as described previously [6].
Results
1. Predicted location of N-glycosylation sites in hSVCT1 and -2
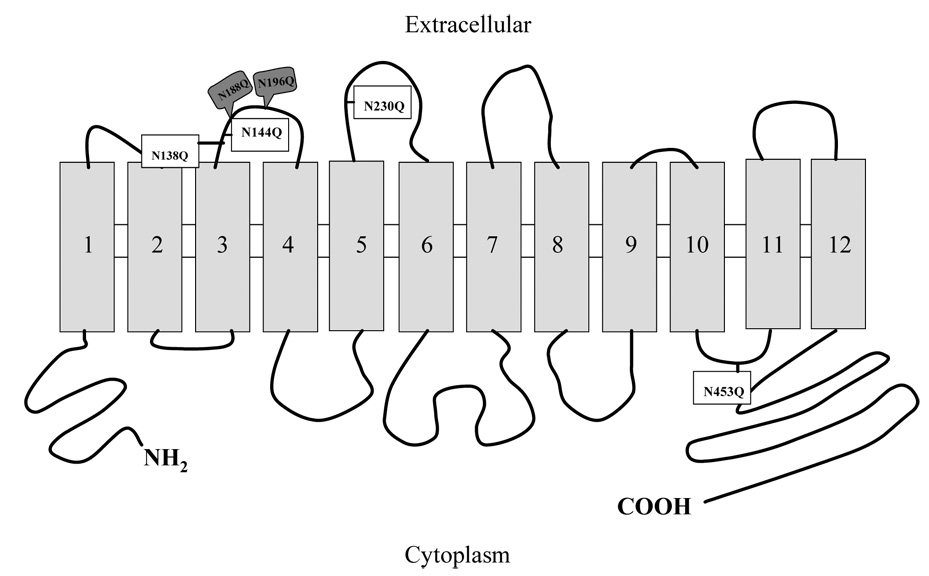
Hydropathy analyses of the sequences of hSVCT1 and -2 predict that each polypeptide has two potential N-glycosylation sites [Asn138 (N-W-S), Asn144 (N-T-S) for hSVCT1 and Asn188 (N-T-T), Asn196 (N-G-T) for hSVCT2] within the extracellular loops between the predicted transmembrane (TM) domains 3 and 4. Additionally, a third site [Asn230 (N-L-T)] is predicted only in hSVCT1 within the extracellular loops between TM5 and 6 [13] (Fig. 1). These predicted glycosylation sites conform to the established human, rat and mouse species consensus (N-X-S/T) [13].
Fig. 1. Schematic of putative hSVCT1 and -2 secondary structure.
Topological plot showing predicted N-glycosylation sites in hSVCT1 (open) and hSVCT2 (shaded).
2. Tunicamycin treatment inhibits hSVCT1 and -2 transport activity, expression and targeting in HepG2 cells
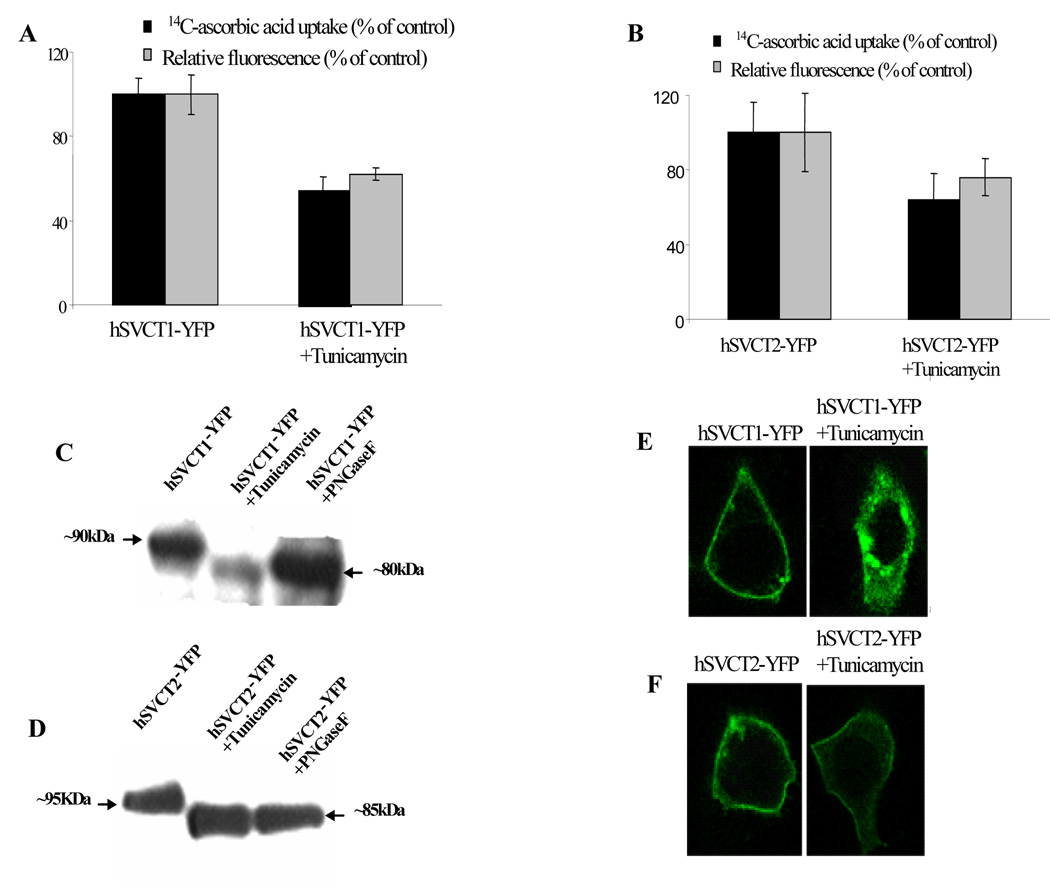
First, we examined the effect of tunicamycin on [14C]-ascorbic acid uptake in HepG2 cells. Results showed that tunicamycin (2µg/ml) inhibited endogenous ascorbic acid uptake (~45%) compared to controls. Similarly in hSVCT1 and -2-YFP stably expressing HepG2 cells, tunicamycin significantly decreased (p<0.05) ascorbic acid uptake (Fig. 2A & B). Notably, mean fluorescence intensity decreased in parallel (Fig. 2A & B) suggesting a decreased level of transporter expression was responsible for the impaired uptake. Western blot analysis showed that hSVCT1 and -2-YFP (~90 and ~95 kDa, respectively) were shifted to a lower size (representative of their predicted molecular weight ~ 80 and ~85 kDa) when treated either with tunicamycin or PNGaseF (Fig. 2C & D). Finally, HepG2 cells transiently transfected with hSVCT1-YFP and simultaneously treated with tunicamycin revealed that hSVCT1-YFP expression at the cell surface was impaired such that hSVCT1-YFP was retained intracellularly compared to control (untreated) cells (Fig. 2E). In contrast, the same treatment in hSVCT2-YFP expressing cells did not prevent hSVCT2 targeting to the cell surface, although fluorescence intensity was decreased (Fig. 2F). In parallel, experiments revealed that tunicamycin did not prevent membrane targeting of the folate transporter (hRFC) in HepG2 cells (data not shown) as reported previously [19]. These results suggest that glycosylation is important for hSVCT1 and -2 expression. To gain molecular insight into the role of specific glycosylation sites in hSVCT properties we subsequently utilized a mutagenesis approach.
Fig. 2. Effect of tunicamycin treatment on hSVCT1 and -2 properties in HepG2 cells.
[14C]-ascorbic acid uptake and flow cytometry measurements of hSVCT1-YFP (A) or hSVCT2-YFP (B) expressing stable HepG2 cells with or without tunicamycin treatment (2µg/ml, 48 hrs). C& D, Western blot analysis after tunicamycin and PNGaseF treatment of stable HepG2 cells as described in “Materials and Methods”. E&F, lateral (xy) images of hSVCT1 and hSVCT2-YFP transiently transfected cells treated with tunicamycin (2µg/ml, 48 hrs)
3. Function, expression and membrane targeting of hSVCT1 glycosylation site mutants
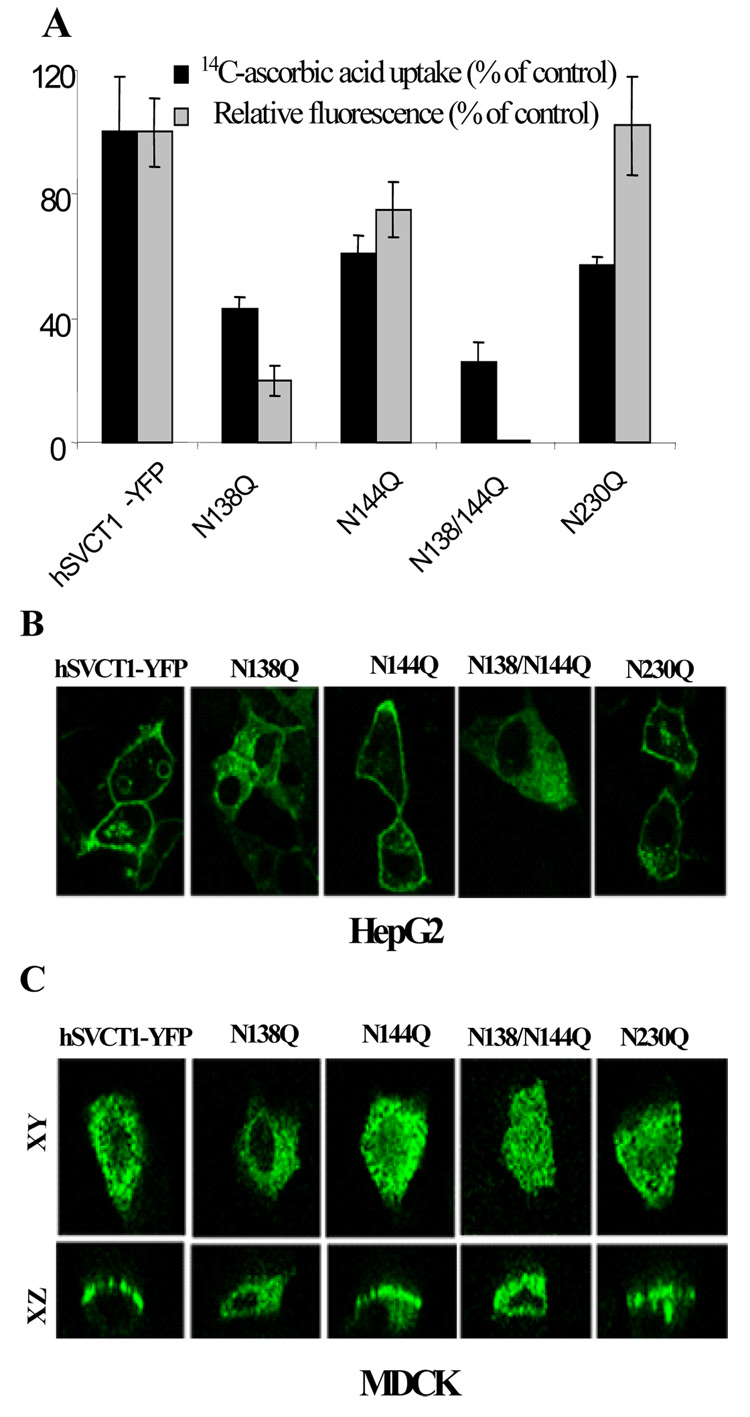
We mutated each of the three consensus sites for glycosylation predicted in the extracellular loop of hSVCT1 (N138Q, N144Q and N230Q, Fig. 1). N138Q and N138/N144Q stably expressing HepG2 cells displayed lower (p<0.01) [14C]-ascorbic acid uptake and mean fluorescence intensity relative to controls (Fig. 3A). On the other hand, N144Q and N230Q did not affect steady state expression (as judged by mean fluorescence intensity), but significantly (p<0.01) decreased [14C]-ascorbic acid uptake (Fig. 3A). In parallel to the uptake and FACS analysis, the N138Q mutation showed significant retention in intracellular membranes and reached the cell surface in only a few cells reminiscent of the tunicamycin result (Fig. 2E). In contrast, mutations at N144Q and N230Q did not affect cell surface targeting (Fig. 3B). As a control, we also mutated N453Q, located in the TM10-TM11 cytoplasmic loop. This mutation proved to have no effect on rate of [14C]-ascorbic acid accumulation, expression or the cell membrane localization (data not shown).
Fig. 3. Properties of hSVCT1 glycosylation mutants.
A, measurements of [14C]-ascorbic acid uptake and flow cytometric analysis of the mean fluorescence intensity of stable HepG2 cells expressing indicated constructs. B, lateral confocal images of the hSVCT1 glycosylation mutants in stable HepG2 cells. C, lateral (xy; top) and axial (xz; bottom) images captured 24–48 hrs after transient transfection of constructs into MDCK cells.
In polarized MDCK cells, we previously reported that hSVCT1 was localized apically [6]. Results showed that N138Q prevented apical targeting with the majority of cells retaining hSVCT1 intracellularly (Fig. 3C). Similarly the double N138Q/N144Q mutant displayed an intracellular expression profile in MDCK cells (Fig. 3C). In contrast, N144Q and N230Q, did not impair the apical targeting of hSVCT1 (Fig. 3C).
4. Functionality, expression and membrane targeting of hSVCT2 glycosylation site mutants
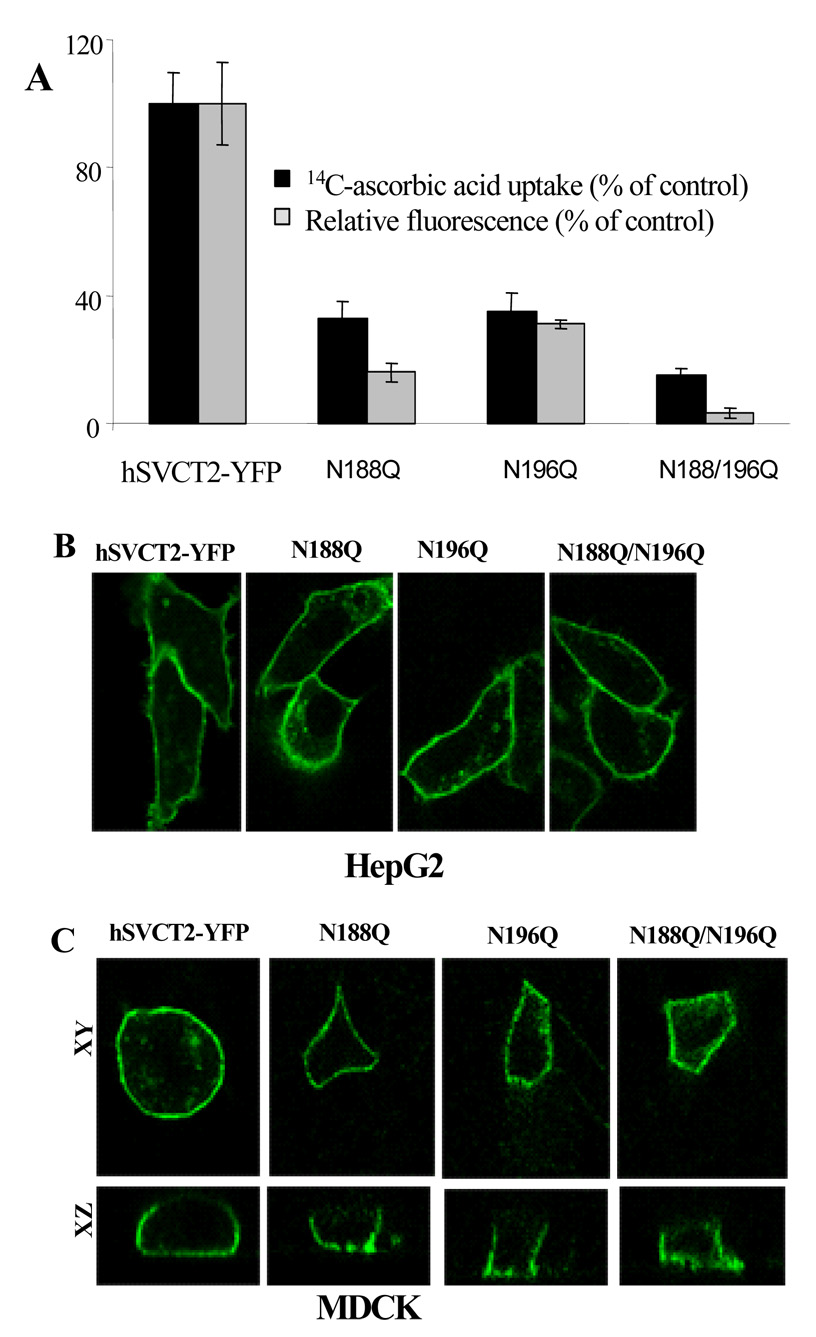
We next examined the effects of glycosylation mutants N188Q and N196Q on expression and function of hSVCT2. N188Q, N196Q and N188/N196Q expressed in HepG2 cells showed significantly (p<0.01) decreased [14C]-ascorbic acid uptake and steady-state expression levels (Fig. 4A) compared to wild-type hSVCT2. Confocal imaging revealed that there was no effect of these mutations either individually (N188Q and N196Q) or in tandem (N188Q/N196Q) on membrane targeting compared to the wild-type (Fig. 4B). Previous studies have established that hSVCT2 is expressed in the basolateral membrane of MDCK cells [8]. In MDCK cells transfected with mutant constructs the results showed that individual mutations (N188Q, N196Q) or mutations in tandem (N188Q/N196Q) did not affect the basolateral membrane targeting of hSVCT2 (Fig. 4C).
Fig. 4. Properties of hSVCT2 glycosylation mutants.
A, measurements of [14C]-ascorbic acid uptake and flow cytometric analysis of the mean fluorescence intensity of stable HepG2 cells expressing indicated constructs. B, lateral confocal images of hSVCT2 glycosylation mutants in stable HepG2 cells. C, lateral (xy; top) and axial (xz; bottom) images captured 24–48 hrs after transient transfection of constructs into MDCK cells.
Conclusions
Glycosylation is important for protein function, folding and membrane targeting [17, 20–22]. Previous studies have shown that both hSVCT1 and -2 are glycosylated [7] and our data confirms that hSVCT1 and -2 are glycosylated in human liver and renal cells. However, nothing is known about the role of glycosylation in hSVCT functionality. Here we show that tunicamycin treatment of hSVCT1 and -2 expressing HepG2 cells inhibited ascorbic acid transport activity, likely via decreased expression efficiency for both hSVCT1 and -2. To gain a better understanding of the roles of specific glycosylation sites we mutated all predicted glycosylation sites in both hSVCT1 and -2. We found that mutations in hSVCT1 caused a decrease in ascorbic acid uptake and notably N138Q prevented cell surface targeting. The tunicamycin treatment and N138Q both resulted in intracellular retention suggested in vivo relevance of this site for glycosylation. When we examined individual (N188Q, N196Q) or in tandem (N188/196Q) mutations in hSVCT2 glycosylation sites we found significant inhibition in the transport function and expression, but no affect on the membrane targeting of hSVCT2 in HepG2 cells or in polarized MDCK cells. Therefore our results suggest that glycosylation is important for functional expression of this family of ascorbic acid transporters.
Supplementary Material
Acknowledgements
This study is supported by the Dept of VA, NIH grants DK 056061 (HMS), DK-73032 (JCR) and DK-71538 (VSS). Work in the Marchant lab is supported by NIH (#NS046783) and NSF (CAREER #0237946).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Packer L, Fuchs J. Vitamin C in health and diseases. New York: Marcel Dekker Inc; 1997. [Google Scholar]
- 2.Li Y, Schellhorn HE. New developments and noval therapeutic perspectives for vitamin C. J. Nutr. 2007;137:2171–2184. doi: 10.1093/jn/137.10.2171. [DOI] [PubMed] [Google Scholar]
- 3.Simon JA, Hudes ES. Serum ascorbic acid and gallbladder disease prevalence among US adults. Arch. Intern. Med. 2000;160:931–936. doi: 10.1001/archinte.160.7.931. [DOI] [PubMed] [Google Scholar]
- 4.Harrision SA, Torgerson S, Hayashi P, ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2003;98:2485–2490. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 5.Upston JM, Karjalainen A, Bygrave FL, Stocker R. Efflux of hepatic ascorbate: a potential contributor to the maintenance of plasma vitamin C. Biochem. J. 1999;342:49–56. [PMC free article] [PubMed] [Google Scholar]
- 6.Subramanian VS, Marchant JS, Boulware MJ, Said HM. A C-terminal region dictates the apical plasma membrane targreting of the human sodium-dependent vitamin C transporter-1 in polarized epithelia. J. Biol. Chem. 2004;279:27719–27728. doi: 10.1074/jbc.M400876200. [DOI] [PubMed] [Google Scholar]
- 7.Liang WJ, Johnson D, Ma LS, Jarvis SM. Regulation of the human vitamin C transporters expressed in COS-1 cells by protein kinase C. Am. J. Physiol. 2002;283:C1696–C1704. doi: 10.1152/ajpcell.00461.2001. [DOI] [PubMed] [Google Scholar]
- 8.Boyer JC, Campbell CE, Sigurdson WJ, Kuo SM. polarized localization of vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochem. Biophys Res. Commun. 2005;334:150–156. doi: 10.1016/j.bbrc.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 9.Daruwala R, Song J, Koh WS, Rumsey SC, Levine M. Cloning and functional characterization of the human sodium-dependent vitamin C transporters hSVCT1 and hSVCT2. FEBS Lett. 1999;460:480–484. doi: 10.1016/s0014-5793(99)01393-9. [DOI] [PubMed] [Google Scholar]
- 10.Rajan PD, Huang W, Dutta B, Leibach LD, Ganapathy V, Prasad PD. Human placental sodium-dependent vitamin C transporter (SVCT2): molecular cloning and transport function. Biochem. Biophys. Res. Commun. 1999;262:762–768. doi: 10.1006/bbrc.1999.1272. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Mackenzie B, Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Human vitamin C (L-ascorbic acid) transporter SVCT1. Biochem. Biophys. Res. Commun. 2000;267:488–494. doi: 10.1006/bbrc.1999.1929. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Dutta B, Huang W, Dovoe LD, Leibach FH, Ganapathy V, Prasad PD. Human Na+-dependent vitamin C transporter 1 (hSVCT1): primary structure, functional characteristics and evidence for a non-functional splice variant. Biochem. Biophys. Acta. 1999;1461:1–9. doi: 10.1016/s0005-2736(99)00182-0. [DOI] [PubMed] [Google Scholar]
- 13.Liang WJ, Johnson DD, Jarvis SM. Vitamin C transport systems of mammalian cells. Mol. Membr. Biol. 2001;18:87–95. doi: 10.1080/09687680110033774. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Xu W, Zhou F, You G. Role of glycosylation in the organic anion transporter OAT1. J. Biol. Chem. 2004;279:14961–14966. doi: 10.1074/jbc.M400197200. [DOI] [PubMed] [Google Scholar]
- 15.Zhou F, Xu W, Hong M, Pan Z, Sinko PJ, Ma J, You G. The role of N-linked glycosylation in protein folding, membrane targeting, and substrate binding of human organic anion transporter hOAT4. Mol. Pharmacol. 2005;67:868–876. doi: 10.1124/mol.104.007583. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Maza R, Poyatos I, Lopez-Coreuera B, Nunez E, Gimenez C, Zafra F, Aragon C. The role of N-glycosylation in transport to the plasma membrane and sorting of the neuronal glycine transporter GLYT2. J. Biol. Chem. 2001;259:2168–2173. doi: 10.1074/jbc.M006774200. [DOI] [PubMed] [Google Scholar]
- 17.Kuze K, Graves P, Leahy A, Wilson P, Stuhlmann H, You G. Heterologous expression and functional characterization of a mouse renal organic anion transporter in mammalian cells. J. Biol. Chem. 1999;274:1519–1524. doi: 10.1074/jbc.274.3.1519. [DOI] [PubMed] [Google Scholar]
- 18.Subramanian VS, Chatterjee N, Said HM. Folate uptake in the human intestine: promoter activity and effect of Folate deficiency. J. Cell. Physiol. 2003;196:403–408. doi: 10.1002/jcp.10324. [DOI] [PubMed] [Google Scholar]
- 19.Wong SC, Zhang L, Proefke SA, Matherly LH. Effect of the loss of capacity for N-glycosylation on the transport activity and cellular localization of the human reduced folate carrier. Biochim. Biophys. Acta. 1998;1375:6–12. doi: 10.1016/s0005-2736(98)00118-7. [DOI] [PubMed] [Google Scholar]
- 20.Gahmberg CG, Tolvanen M. Why mammalian cell surface protein are glycoproteins. Trends Biochem. Sci. 1996;21:308–311. [PubMed] [Google Scholar]
- 21.Vagin O, Turdikulova S, Sachs G. The H, K-ATPase B subunit as a model to study the role of N-glycosylation in membrane trafficking and apical sorting. J. Biol. Chem. 2004;279:39026–39034. doi: 10.1074/jbc.M405453200. [DOI] [PubMed] [Google Scholar]
- 22.Scheiffele P, Peranen J, Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.