Abstract
This review summarizes the recent advancements that have improved our understanding of the functions of prostatic stem/progenitor cells in maintaining homeostasis of the prostate gland. We also describe the oncogenic events that may contribute to their malignant transformation into prostatic cancer stem/progenitor cells during cancer initiation and progression to metastatic disease stages. The molecular mechanisms that may contribute to the intrinsic or the acquisition of a resistant phenotype by the prostatic cancer stem/progenitor cells and their differentiated progenies with a luminal phenotype to the current therapies and disease relapse are also reviewed. The emphasis is on the critical functions of distinct tumorigenic signaling cascades induced through the epidermal growth factor system, hedgehog, Wnt/β-catenin, and/or stromal cell-derived factor-1/CXC chemokine receptor-4 pathways as well as the deregulated apoptotic signaling elements and ATP-binding cassette multidrug transporter. Of particular therapeutic interest, we also discuss the potential beneficial effects associated with the targeting of these signaling elements to overcome the resistance to current treatments and prostate cancer recurrence. The combined targeted strategies toward distinct oncogenic signaling cascades in prostatic cancer stem/progenitor cells and their progenies as well as their local microenvironment, which could improve the efficacy of current clinical chemotherapeutic treatments against incurable, androgen-independent, and metastatic prostate cancers, are also described.
I. Introduction
II. Functions of Normal and Malignant Adult Prostatic Stem/Progenitor Cells
- III. Functions of Normal Adult Prostatic Stem/Progenitor Cells in the Homeostatic Maintenance of the Prostate Gland
- A. In vivo and ex vitro characterization of adult prostatic stem/progenitor cell properties
- B. Paracrine and autocrine regulation of prostatic stem/progenitor cell proliferation and differentiation by the interplay of androgens, growth factors, and integrins
- IV. Functions of Prostatic Cancer Stem/Progenitor Cells in Prostate Cancer Initiation and Progression
- A. Heterogeneity of prostate cancers derived from distinct prostatic cancer stem/progenitor cells
- B. In vitro and in vivo prostatic cancer stem/progenitor cell models
V. Molecular Mechanisms Involved in the Therapeutic Resistance of Prostatic Cancer Stem/Progenitor Cells and Their Further Differentiated Progenies
- VI. Novel Therapeutic Strategies against the Locally Advanced and Metastatic Hormone-Refractory Prostate Cancers
- A. Targeting of PC stem/progenitor cells and their further differentiated progenies
- B. Targeting of the local tumor microenvironment of PC stem/progenitor cells and their further differentiated progenies
VII. Conclusions and Perspectives
I. Introduction
THE DEVELOPMENT OF earlier diagnostic tests in the past few years has led to a more effective therapeutic intervention for patients diagnosed with prostate cancer (PC) (1,2,3,4,5,6). Among the current clinical treatments, radical prostatectomy, hormonal therapies, radiotherapy, and/or adjuvant chemotherapy generally show beneficial effects and a significant curative rate for the patients diagnosed with localized PCs in the early stages (3,5,6,7). Unfortunately, the accumulation of genetic and/or epigenic alterations leading to an enhanced expression of numerous growth factor and cytokine signaling cascades during PC progression may confer an androgen-independent (AI) phenotype to PC cells and thereby contribute to the resistance to androgen deprivation therapies and disease relapse (3,8,9,10,11,12,13). For patients at high risk of progression to more advanced PCs or those diagnosed in the late stages with metastatic and hormone-refractory PCs (HRPCs), the nonhormonal systemic chemotherapeutic regimens may thus represent another clinical therapeutic option (3,7,9,11,12,13,14,15). The current chemotherapeutic treatments against the metastatic HRPCs include docetaxel plus prednisone and/or estramustine phosphate, or mitoxantrone plus prednisone (3,11,12,13,14,15,16,17,18,19,20,21). The choice of using the docetaxel- or mitoxantrone-based therapeutic regimens in first- or second-line treatments against metastatic HRPCs is based on the observations indicating that these chemotherapeutic treatments may improve the quality of life of the patients. However, these chemotherapies, which principally offer palliative care of metastatic bone pain, generally show no significant, or in case of docetaxel-based regimens, modest beneficial effects for improving the overall survival rate and outcome of patients in the clinics, and they ultimately result in the death of patients (16,17,18,21,22). At present, few therapeutic options exist to treat patients with metastatic HRPCs after a lack of response to docetaxel- or mitoxantrone-based therapies. Therefore, the identification of novel drug targets and cytotoxic agents for improving the efficacy of current chemotherapeutic treatments against the locally advanced and metastatic HRPCs is essential to counteract PC progression and thereby to prevent the formation of metastases at distant sites, including bone marrow, and disease relapse.
Numerous recent lines of evidence indicated that the persistence of androgen receptor negative (AR−) PC stem/progenitor cells, also designated as PC-initiating cells, in primary and secondary neoplasms, which might be resistant to current antihormonal therapy, radiotherapy, and/or chemotherapeutic treatments, may contribute to PC recurrence (10,23,24,25,26,27,28,29,30,31). More specifically, a very small subpopulation of CD133+/CD44+/α2β1high-integrin/AR− PC stem/progenitor cells, comprising about 0.1–3.0% of total PC cells, has been identified and isolated from primary and metastatic PCs (23,31,32). These isolated PC stem/progenitor cells gave rise to differentiated tumor cells in vitro and in vivo possessing a secretory luminal phenotype like the original tumor cells, such as the expression of AR (23,31). Moreover, an intermediate PC cell subpopulation expressing the basal and luminal cell-specific markers, such as cytokeratins, CK5 and CK18, respectively, and which represent only a minor fraction in the total PC cell population, has been identified in primary and metastatic PCs (33,34,35,36). Importantly, the sustained activation of numerous developmental signaling cascades in PC cells, including PC stem/progenitor cells, may cooperate in inducing a more complete epithelial-mesenchymal transition (EMT) program and thereby provide to them a more malignant behavior (Figs. 1 and 2). Among them, there are epidermal growth factor (EGF)/EGF receptor (EGFR), sonic hedgehog (SHH/PTCH/GLI), Wnt/β-catenin, stromal cell-derived factor-1 (SDF-1) also designated as CXCL12/CXC chemokine receptor-4 (CXCR4) and/or TGF-β cascades (3,10,37,38,39,40,41,42,43,44,45,46,47,48). These oncogenic pathways may contribute to the sustained growth, migration, invasion, and metastasis of PC cells as well as the resistance to current clinical therapies and disease relapse. Additionally, the changes in the local microenvironment of PC cells, including the release of diverse soluble factors by activated myofibroblasts and immune cells in tumor-reactive stroma, may also influence their malignant transformation and thereby promote the PC transition to a more aggressive disease state (3,28,40,44,45,49,50,51,52). In this review, we provide a particular view on the potential implications of normal and malignant prostatic stem/progenitor cells in tissue homeostasis and disease development with respect to new stem cell concepts and the accumulating body of experimental evidence in this research field. Specifically, we describe recent advancements related to the establishment of physiological functions of prostatic stem/progenitor cells in maintaining homeostasis of the prostate gland during the adult lifespan. Moreover, we discuss the possible pathophysiological implication of their malignant counterpart, the PC stem/progenitor cells, in PC initiation and progression to aggressive and recurrent disease states. We also review the recent works that have led to the identification of novel drug targets for the development of more effective combination therapies against metastatic HRPCs. The emphasis is on new combined targeted therapies aimed at eliminating the total mass of PC cells, which consist of PC stem/progenitor cells with a basal or intermediate phenotype and their further differentiated progenies with a luminal phenotype.
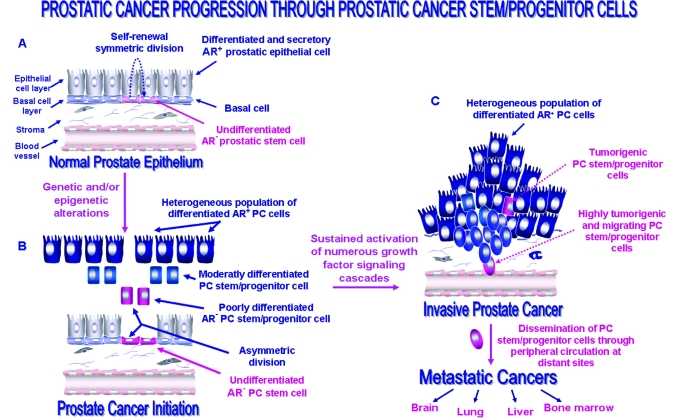
Figure 1.
Proposed model of the molecular events associated with the PC initiation and progression through the malignant transformation of prostatic stem/progenitor cells. A, The anatomic localization of undifferentiated prostatic adult stem/progenitor cells expressing different stem cell-like markers including CD133/CD44/α2β1high-integrin in basal cell compartment in normal prostate epithelium and their possible symmetric division in homeostatic conditions. B, The possible malignant transformation of prostate stem/progenitor cells induced through genetic and/or epigenic alterations resulting via their asymmetric division to the generation of a heterogenous population of poorly, moderately, and well-differentiated PC stem/progenitor cells and PC initiation. C, The possible accumulation of genetic and/or epigenetic alterations in tumorigenic PC stem progenitor cells leading to the sustained activation of numerous growth factor signaling cascades and their acquisition of a migratory phenotype during EMT program and PC progression to the invasive and metastatic disease stages.
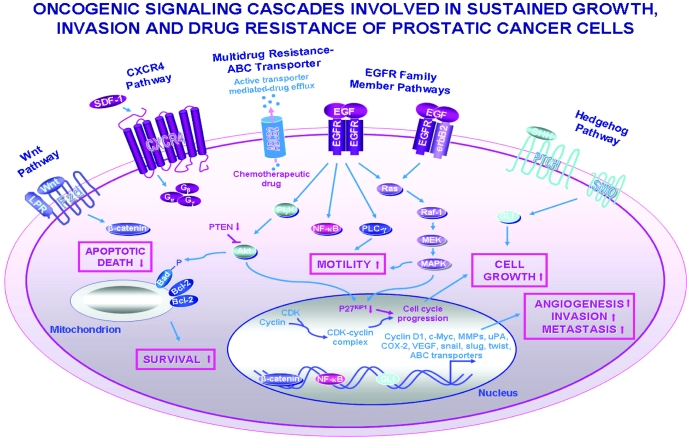
Figure 2.
Scheme showing the possible oncogenic signaling pathways involved in the stimulation of sustained growth, survival, migration, invasion, and drug resistance of PC cells. The intracellular signal transducing elements and expression of target gene products induced through the activation of EGFR family members, sonic hedgehog (SHH/PTCH/GLI), Wnt/β-catenin, and SCF-1/CXCR4 pathways and possible cross-talks between these cascades are shown. Moreover, the chemotherapeutic drug efflux mediated through the ABC-multidrug transporters such as ABCG2 pump is also illustrated. BCRP-1/ABCG2, Breast cancer resistance protein-1; CDK, cyclin-dependent kinase; LRP, low-density lipoprotein receptor-related protein; MEK, extracellular signal-related kinase kinase; MMPs, matrix metalloproteinases; PLC-γ, phospholipase C-γ; PTCH, hedgehog-patched receptor; SHH, sonic hedgehog ligand; uPA, urokinase plasminogen activator; Wnt, wingless ligand.
II. Functions of Normal and Malignant Adult Prostatic Stem/Progenitor Cells
The prostatic epithelium is derived during embryogenesis from the differentiation of a stem/progenitor cell population localized in the embryonic endodermal urogenital sinus (UGS) epithelium from which prostatic epithelial buds develop under the influence of UGS mesenchyme (51,53,54,55,56,57). The normal prostate gland is constituted essentially of three cell types, including basal and secretory luminal epithelial cells and neuroendocrine cells within the prostatic stratified epithelium. Accumulating lines of evidence suggest that the prostatic stem/progenitor cells may also persist in the mature prostatic epithelium in adults within the specialized microenvironments, or “niches” localized in the basal compartment (Fig. 1) (53,55,56,58,59). The genetic and/or epigenic alterations occurring in prostatic stem/progenitor cells combined with the changes in their local microenvironment consisting of stromal cells and extracellular matrix components, and more particularly during aging, may however lead to their acquisition of aberrant functions and malignant transformation (60). These prostatic stem/progenitor cells with a deregulated behavior can contribute to certain hyperproliferative disorders affecting prostate gland epithelium, such as basal cell hyperplasia, benign prostatic hypertrophy or hyperplasia (BPH also designated as BHP) and PCs, that commonly afflict the middle-aged and elderly men (3,23,24,53,61,62,63,64,65,66,67,68). Based on the experimental observations indicating the critical importance of prostatic stem/progenitor cells in the homeostatic maintenance of prostate gland as well as their implication in PC development, several recent works have been carried out to enrich, isolate, and characterize these cells and their malignant counterpart, PC stem/progenitor cells with stem cell-like properties. Among the methodologies frequently used, there are the immunohistochemical analyses and diverse enrichment and isolation strategies, including magnetic-activated cell sorting and fluorescence-activated cell sorting (FACS) with antibodies directed against certain stem cell-like surface markers such as CD133, CD44, α2β1-integrin, and/or CXCR4 (23,29,49,64,65,69,70,71,72,73,74,75). Moreover, the DNA label-retaining cell analyses have also been performed to detect slow-cycling prostatic stem cells and the Hoechst side population (SP) method to characterize the prostatic epithelial cells with the stem cell-like properties including their high dye efflux ability associated with the high expression levels of ATP-binding cassette (ABC) multidrug efflux pumps (27,29,32,73,74,76,77). Hence, it has been observed that a very small subpopulation of nonmalignant or malignant prostatic stem/progenitor cells may be detected and isolated from normal and malignant prostatic tissue specimens from healthy individuals and patients with PCs and well-established nonmalignant or malignant human prostatic cell lines. In regard to this, we are reporting the recent works that have led to the identification and in vivo and in vitro characterization of specific functional properties of human and rodent normal prostatic stem/progenitor cells and the molecular events that may contribute to their malignant transformation during PC initiation and progression.
III. Functions of Normal Adult Prostatic Stem/Progenitor Cells in the Homeostatic Maintenance of the Prostate Gland
A. In vivo and ex vitro characterization of adult prostatic stem/progenitor cell properties
Several recent investigations revealed that a very small subpopulation of multipotent and undifferentiated prostate stem/progenitor cells, comprising about 0.1–3.0% of the total prostatic epithelial cell population, principally reside within specialized areas or “niches” localized in the basal cell layer of acinar and ductal regions of the human prostate gland (Fig. 1) (24,30,32,49,55,56,59,64,70,74,78,79,80,81,82,83). This small fraction of human adult prostatic stem/progenitor cells, which is enriched near the basement membrane, expresses several specific markers, including CD133 (also designated as prominin-1 or AC133), CD44, α2β1high-integrin, breast cancer resistance protein-1/ABCG2, telomerase reverse transcriptase (TERT), and CK5/14 (Fig. 1) (24,32,49,55,56,58,59,64,70,74,78,80,81,82,83). Adult prostatic stem/progenitor cells, however, did not express detectable levels of luminal markers such as AR and prostate-specific antigen (PSA). Therefore, these cells did not directly depend on androgens for their survival and proliferation. Moreover, human CD133/α2β1high-integrin prostatic stem/progenitor cells possess a high in vitro proliferative potential and are able to reconstitute fully differentiated prostatic structure-like acini in male nude mice in vivo (70). In rodents, the adult prostatic stem/progenitor cells, which express CD133, stem cell antigen-1 (Sca-1), TERT, α6-integrin, Notch 1, ABCG2, and antiapoptotic factor Bcl-2 but not AR, are enriched in the proximal region in prostatic ducts and the urethra (3,30,69,84,85,86,87,88,89). The mature prostatic tissue normally undergoes limited regeneration under physiological conditions, and thus the prostatic stem/progenitor cells may be maintained in a quiescent state due to their local microenvironment through a complex network of soluble growth factors, such as TGF-β secreted by the nearboring stromal mesenchymal cells, that tightly regulate their functions in a paracrine manner (3,51,90). The prostatic stem/progenitor cells may display a high self-renewal capacity and give rise, by asymmetric division, to distinct highly proliferative transit-amplifying (TA)/intermediate cell subpopulations in pathological conditions, such as intense injuries (3,30,59,64,78,80,85). The TA/intermediate cells, which are organized as a hierarchical population, may express different levels of CD44 and/or α2β1-integrin, which are stem cell-like markers, and an intermediate phenotype [p63, CK5/14, CK8/18, prostate stem cell antigen (PSCA) and AR−/low] between the basal and secretory cells of prostatic epithelium (3,36,59,64,78,80,83,85,91,92,93). In fact, a decline in the expression of stem/progenitor cell-like markers on TA/intermediate cells upon acquisition of a more differentiated phenotype during prostatic epithelium regeneration appears to occur gradually. The TA/intermediate cell subpopulations may ultimately regenerate by subsequent divisions all of the mature cell lineages in stratified epithelium consisting of basal, neuroendocrine, and luminal cells in vitro and in vivo, and thereby they can participate in restoring the prostate gland after injuries (59,78,80). More specifically, the majority of secretory luminal terminally differentiated epithelial cells localized at the apical region of the prostate epithelium only express luminal markers, such as high levels of AR, PSA, and CK8/18, and are dependent on androgens for their survival and growth (Fig. 1) (30,58,59,80,83,92). In contrast, the basal and neuroendocrine cells, which are devoid of nuclear AR, are AI for their survival and proliferation (30,83,92,94). The mature basal epithelial cell subpopulation found within the basal compartment adjacent to basement membrane also expresses several specific markers, including p63, CK5/14, CK19, and glutathione S-transferase π (GST π), whereas neuroendocrine cells express chromogranin A and synaptophysin (30,55,56,58,83,92). Additionally, the intermediate prostatic stem/progenitor cells expressing basal-luminal markers CK5/18 and/or p63 have also been detected in normal and malignant prostate epithelium, suggesting that these cell types may play an important role in prostate regeneration and pathogenesis (33,34,35,36,67,95,96,97,98). Along these lines of thought, it is noteworthy that a positive staining of a very small subpopulation (clusters) of PC cells has been observed in certain primary and metastatic prostate adenocarcinoma specimens using basal cell-specific 34βE12 antibody directed against CK1/5/10/14 (35,99,100,101). In fact, 34βE12 antibody is generally used as a clinical diagnostic tool to distinguish between the PC lesions in which the basal cells are generally destroyed and benign prostatic lesions that retain the cytokeratin-expressing basal cells. Therefore, this observation underlines the importance of considering the possible presence of a small population of PC cells expressing the basal cell-like markers such as cytokeratins during the clinical diagnosis of patients, and more particularly, when the basal cell-reactive 34βE12 antibody is used.
B. Paracrine and autocrine regulation of prostatic stem/progenitor cell proliferation and differentiation by the interplay of androgens, growth factors, and integrins
Numerous investigations indicated that an interplay of signaling cascades initiated by the androgens, integrins, and growth factors, such as keratinocyte growth factor (KGF), also designated as fibroblast growth factor (FGF)-7, EGF, TGF-α/EGFR, IGF-I, sonic hedgehog SHH/PTCH, and Notch, may influence the prostatic epithelial cell proliferation and/or differentiation in paracrine and/or autocrine manners (3,8,39,49,53,59,65,70,80,81,85,102,103,104,105,106,107,108,109,110,111). These factors can participate in prostate organogenesis during embryogenesis or prostate gland development and regeneration in fetal, postnatal, and adult life (8,49,53,65,70,80,81,85,105,107,110). More specifically, androgens may induce a differentiation of prostate epithelial cells by acting on the adjacent stromal cells, causing them to secrete the soluble growth factors that stimulate the differentiation of prostatic stem/progenitor cells. In support of this, the tissue recombination experiments with AR-negative epithelium and normal UGS mesenchyme expressing wild-type AR showed that androgens may mediate their effects on AR+ mesenchymal cells by stimulating the release of diffusible growth factors such as KGF, which in turn may induce the growth and differentiation of prostatic epithelium (51,59,112). Further experimental evidence supporting the paracrine role of androgens on prostatic epithelium differentiation is based on the observation that the adult rodent prostate undergoes involution after the surgical castration, and the exogenous androgen treatment may reverse this degenerative effect (113,114,115,116,117). In this regard, the castration triggers apoptotic death of the luminal prostatic epithelial cells that are dependent on androgens for their survival. In contrast, the cells within the basal compartment near basement membrane, including AR− prostatic stem/progenitor cells may survive and subsequently be restimulated by exogenous androgen application to regenerate the functional prostatic epithelium (113,116,117). The results from a recent study have also revealed that the castration may be accompanied by the up-regulated expression of EGFR in the prostatic epithelial cells, suggesting that the EGF and TGF-α/EGFR system may contribute to the regeneration of prostatic epithelium (107).
In addition, the androgens can also mediate their differentiating effect through a direct action on the prostatic epithelial cells. For instance, the results from recent studies have shown a direct effect of androgens on prostatic TA/intermediate cells isolated from human prostatic tissue obtained after transurethral resection of the prostates of BPH patients or cystoprostatectomy for bladder cancer (64,65). More specifically, the treatment of CD133−/α2β1high-integrin/AR−/low prostatic TA/intermediate cell subpopulations, which have been enriched from the total basal cells of the prostate gland with an androgen analog, R1881, down-regulated the α2β1-integrin expression level and induced their differentiation into epithelial cells with a terminally differentiated epithelial cell-like phenotype including the expression of AR, PSA, prostatic acid phosphatase, and CK18 (65). The treatment of CD133−/α2β1high-integrin/AR−/low cells, which express the FGF receptor tyrosine kinase (FGFR2) possessing a high affinity for KGF, with an anti-β1-integrin antibody or KGF also suppressed α2β1high-integrin expression and induced their differentiation into cells expressing the luminal markers including AR, prostatic acid phosphatase, and CK18 (64). In rodents, it has also been observed that α6-integrin positive-murine prostate stem cells, when grown in Matrigel media containing its ligand, extracellular protein laminin, can form the clonogenic spheroid structures termed as prostate spheres that spontaneously differentiate into basal cells or PSCA-expressing TA cells (88). The addition of 5α-dihydrotestosterone in cell culture media, which stabilized AR and induced its nuclear translocation, also triggered the differentiation of cells into progenies possessing a luminal phenotype (88). A fraction of sphere cells endowed with the self-renewal capacity and multilineage differentiating potential can form prostate gland-like structures containing the basal and secretory luminal cell layers in vivo (88).
In light of these experimental observations, it appears that a very small subpopulation of undifferentiated and multipotent AR− prostatic stem/progenitor cells can participate along the lifespan to generate moderately differentiated TA/intermediate cell subpopulations. The TA cells, in turn, can replenish the bulk mass of further differentiated AR+ epithelial cells with the secretory functions and thereby maintain the prostate homeostasis or regenerate the prostatic epithelium after tissue injuries. Unfortunately, the changes in prostatic epithelium-mesenchyme interactions may be accompanied by the malignant transformation of prostatic stem/progenitor cells into PC-initiating cells (3,30,36,40,50,67,83,118).
IV. Functions of Prostatic Cancer Stem/Progenitor Cells in Prostate Cancer Initiation and Progression
The accumulation of genetic and/or epigenic alterations occurring in prostatic stem/progenitor cells during the lifespan combined with the changes in their local microenvironment, and more particularly after intense prostatic epithelium injuries such as chronic proliferative inflammatory atrophies of the prostate gland, may result in their malignant transformation into PC stem/progenitor cells showing aberrant growth and differentiation potential (Fig. 1) (3,30,31,36,50,67,83,118). In general, PC development is accompanied by a continued transition from low- to high-grade prostatic intraepithelial neoplasias that may culminate in well-established localized PCs. The primary prostatic neoplasms may subsequently progress into locally invasive forms whose event may be accompanied by the spread of PC cells at the surrounding tissues, including the lymph nodes, and/or metastasize to distant tissues/organs such as the bone marrow, brain, lungs, and/or liver (Fig. 1) (30,40). This notion is well supported by the identification of a rare subpopulation of human PC stem/progenitor cells comprising about 0.1–3.0% of total PC cells by immunohistochemical staining in malignant prostatic adenocarcinomas and metastatic neoplasms (23,24,29,32,82). The PC stem/progenitor cells isolated from primary neoplasms express several prostatic stem cell-like markers such as CD133, CD44, α2β1+/high-integrin, CK5/14, CK18, and/or CXCR4 but lack AR and PSA luminal marker expression (23,24,29,31,32,82). In regard to this, it has been reported that the CD133/α2β1+/high-integrin PC stem cells isolated from primary PC (P4E6), when grafted orthotopically in a matrigel plug containing human prostatic stroma, were able to form multiple intraprostatic tumors in nude mice showing a histology like the original Gleason 4 grade PC (31). Importantly, certain PC stem/progenitor cells, unlike their normal counterpart, prostatic stem/progenitor cells with a basal phenotype (CK5/14), appear to possess an intermediate phenotype based on the coexpression of basal (CK5/14) and luminal (CK8/18) cytokeratin markers (33,34,35,36,95,96,98). These findings suggest that these PC cells may represent early progenies with an intermediate phenotype, which have been preserved even after the destruction of the basal epithelial cell layer during PC progression. This line of thought is well supported by the observation that the hedgehog signaling elements, receptor Patched 1 (PTCH1) and GLI transcription factor, were frequently found to colocalize with p63 in CD44/CK8/14-expressing prostatic hyperplasia basal cells and in the PC cells but were rarely detected in normal basal cells (67). On the basis of these observations, it has been proposed that the hedgehog signaling activation may induce a transitory differentiation of prostatic stem/progenitor cells into CD44+/p63−/+ hyperplasia basal cells with an intermediate phenotype (CK8/14), and this early transforming event may culminate in tumorigenesis by giving rise to CD44-, PTCH1-, and GLI-expressing PC cells (67).
Hence, it seems that certain CD133+/AR− PC stem/progenitor cells may successfully complete the multistep selective process and, more particularly, the acquisition of a migratory phenotype during the EMT program at primary neoplasms that may allow them to invade and migrate at distant metastatic sites such as bone marrow where they can form the established metastases (Fig. 1). Further studies are necessary to establish more precisely the specific gene expression pattern that characterizes CD133+/AR− PC stem/progenitor cells and to distinguish them from their further differentiated progenies detected in different poorly to moderately differentiated PC subtypes during the disease progression.
A. Heterogeneity of prostate cancers derived from distinct prostate cancer stem/progenitor cells
Although the PC stem/progenitor cells may arise from the malignant transformation of normal prostatic stem cells that acquire an aberrant growth and differentiation potential, it is likely that the mutations in a wide variety of oncogenes and tumor suppressor genes may lead to different deregulated molecular pathways during PC initiation and progression (3,28,30,40,44,109,119,120,121,122,123,124,125). Therefore, the occurrence of distinct genetic and/or epigenic alterations in PC stem/progenitor cells concomitant with the changes in their local microenvironment may activate different oncogenic cascades during prostate carcinogenesis and thereby contribute to the intratumoral heterogeneity and/or development of diverse PC subtypes. For instance, the results from a study consisting of the deletion of the tumor suppressor gene, phosphatase and tensin homolog deleted on chromosome 10 (PTEN) in murine prostatic epithelium, showed that the loss of the PTEN gene product, lipid phosphatase, is accompanied by an expansion of Sca-1/Bcl-2/CK5/p63+-expressing prostatic stem/progenitor cells and their differentiation into a Sca-1/Bcl-2/CK5/p63−/low cell subpopulation (124). These early progenies, which are localized near the basement membrane of the prostatic epithelial compartment, can give rise to a further differentiated cell subpopulation that dissociates from the basement membrane (124). The PTEN inactivation in a murine model in vivo, which activates the phosphatidylinositol 3′-kinase (PI3K)/Akt signaling pathway, may result in the prostatic intraepithelial neoplasia lesions that progress into invasive and metastatic PCs recapitulating the molecular events seen during human PC progression (120,123,124). Furthermore, the combined loss of PTEN and other tumor suppressor genes, such as p53, or a lack of cyclin-dependent kinase inhibitor, p27KiP1 expression may promote tumorigenesis and rapidly lead to the aggressive, invasive, and lethal PCs in murine models in vivo (120,122). Similarly, the deletion of both p53 and retinoblastoma (Rb) tumor suppressor genes in mouse prostatic epithelium may also induce a malignant transformation of AI Sca-1+ prostatic stem/progenitor cells in the proximal region of the ducts (121). These malignant Sca-1+ prostatic stem/progenitor cells, in turn, can give rise to a heterogeneous population of PC cells that express the neuroendocrine (chromogranin A and synaptophysin) and luminal epithelial (AR and CK8) markers but lack expression of basal cell marker CK5 in a microenvironment-dependent manner (121). In addition, the recombination of human prostatic carcinoma-associated fibroblasts (CAFs), which secrete the high levels of SDF-1 and TGF-β1, with the nonmalignant human prostatic epithelial cell line BHP-1 also induced their malignant transformation in vivo (45,50). This oncogenic event was mediated at least in part through the activation of TGF-β1 that stimulated the Akt pathway via the up-regulation of CXCR4 expression on PC cells (45,50). The p63/CK14/CK8/18-expressing BHP-1 cell sublines established from the tumors generated by transformed BHP-1 cells, gave the colonies in soft agar assays in vitro whereas parental BHP-1 cells did not. Moreover, p63/CK14/CK8/18 BHP-1 cells formed the poorly to moderately differentiated tumors resembling the original primary tumors when grafted beneath the renal capsule of athymic mice (50). Several lines of evidence have indicated that the PC stem/progenitor cells, like their normal counterpart, prostatic stem/progenitor cells, may reexpress the markers specific to mature and differentiated basal (CK5 and p63), luminal (AR), and/or neuroendocrine cells (nestin) depending upon the particular microenvironment prevalent at the primary and secondary neoplasms. For instance, the human TERT-overexpressing PC epithelial cell lines termed HPET or clonally derived HPET cells with stem cell-like characteristics (CD44+, CD133+, nestin+, Oct-4+, Nanog+, Sox2+ and AR/PSA−) have recently been established from prostatic adenocarcinoma specimens. These TERT+ HPET cells gave rise to the differentiated PC cells that expressed the markers specific to mature basal, neuroendocrine, and AR+ luminal cells in vivo (101). The parental and clonal HPET cells endowed with a self-renewal potential were also able to reconstitute the human prostatic tumors with a histopathological architecture and Gleason grade 8 to 9 similar to the original patients’ adenocarcinomas from which they were derived after serial transplantation in vivo (101). Similarly, human TERT-immortalized primary malignant RC-92a/hTERT showing a CD133+/CD44+/CK1/5/10/14+/CK18+/CXCR4+ and AR/PSA− phenotype and endowed with a self-renewal potential were also able to differentiate into AR-expressing cells (29).
In addition, an enhanced expression and activation of TERT and/or diverse growth factor and chemokine signaling pathways such as EGF-EGFR, hedgehog, Wnt/β-catenin, estrogen/estrogen receptor, TGF-β, and/or SDF-1/CXCR4 in PC cells may also contribute to PC initiation and/or its progression to a metastatic state (Figs. 1 and 2) (3,28,29,37,44,67,87,108,109,126). The stimulation of these oncogenic cascades may enhance the expression of diverse signaling elements such as cyclin D1, MYC, Bcl-2, survivin, matrix metalloproteinases, urokinase-plasminogen activator, cyclooxygenase-2 (COX-2), ABC transporters, and transcriptional repressors of E-cadherin, snail, slug, and twist in PC stem/progenitor cells and/or their differentiated progenies. Hence, the activation of these tumorigenic cascades may contribute to the sustained growth, survival, migration, invasion, and/or metastasis of PC cells to distant sites as well as their resistance to current therapeutic treatments and disease relapse. Furthermore, the results of numerous recent studies have also revealed that the chromosomal rearrangements implicating the oncogenic ETS family transcription factors, and which fuse the 3′-end ERG, ETV1, ETV4, or ETV5 gene to the 5′-region of TMPRSS2 as well as ETV1 and untranslated region from SLC45A3, HERV-K 22q11.3, C15ORF21, or HNRPA2B1 gene, may be detected in certain PC subtypes (127,128,129,130,131,132,133,134,135,136,137,138,139,140). More particularly, a gene fusion consisting of androgen-regulated TMPRSS2 (21q22.2) and ERG (21q22.3) appears frequently to occur during PC development (127,128,129,130,131,132,133,134,136,137,138,139). Importantly, although the expression of TMPRSS2-ERG gene fusion may be up-regulated in response to the androgen treatment in AR+ PC cells, it has also been observed that an AR− NCI-H660 prostatic cell line derived from a lymph node metastasis taken from a patient before therapy harbored TMPRSS2-ERG gene fusion and expressed ERG oncoprotein in an AI manner (132,141). Together these observations suggest that these fusion oncoptoteins like the wild-type ETS oncoproteins could mediate the transactivation of ETS target genes that may be involved in the malignant transformation of PC cells. Further studies are however essential to establish whether certain of these chromosomal rearrangements including TMPRSS2-ERG gene fusion may occur in PC stem/progenitor cells and/or their early progenies, and thereby contribute to their acquisition of more aggressive behavior during PC progression.
On the other hand, the malignant transformation of PC cells, and more particularly during the EMT process at the primary neoplasm, as well as their abilities to form the metastases at distant sites may also be influenced by their local microenvironment (3,28,30,40,44,45,49,50,51,52). The reciprocal PC cell-microenvironment interactions implicate the interplay of a complex network of diverse diffusible soluble growth factors and cytokines released by the tumor and host cells in reactive stroma and changes in the extracellular matrix components. Among host stromal cells, there are tumor microvasculature-associated endothelial cells, activated myofibroblasts, and immune cells such macrophages that may be recruited at the primary and secondary neoplastic sites (Fig. 1) (40). All of these molecular events may contribute to the progression of localized PCs to metastatic disease stages in promoting the tumor growth, angiogenesis and PC cell migration, invasion, and adhesion at distant tissues/organs where they can form the well-established metastases.
Hence, the specific oncogenic alterations occurring in PC stem/progenitor cells concomitant with the changes in their local microenvironment during PC progression may influence the phenotype of their progenies at the primary neoplasm and metastatic sites and thereby alter the differentiation state of the tumor subtype and the aggressiveness of the disease.
B. In vitro and in vivo prostate cancer stem/progenitor cell models
Recent experimental lines of evidence have revealed that several well-established nonmalignant and malignant prostatic cell lines may contain a prostatic stem/progenitor cell subpopulation that may be enriched or isolated by SP Hoechst technique and FACS with the specific antibodies directed against prostatic stem cell-like surface markers (27,58,71,72,73,75,83,86,142). These well-characterized nonmalignant and malignant prostatic stem/progenitor cells may then constitute the in vitro and in vivo cell models to define the molecular events involved in maintaining the prostatic epithelium differentiation and inducing the malignant transformation of prostatic stem/progenitor cells during PC progression. In this respect, a recent FACS study has revealed the presence of a small prostatic stem/progenitor cell subpopulation that expressed CD133/ABCG2/α2β1+/high-integrin but lacked AR and PSA protein expression in nonmalignant and nonimmortalized human PrEC and immortalized parental RWPE-1 and PZ-HPV-7 epithelial cells corresponding to about 1.3, 0.24, and 1.75% of the total cell population, respectively (143). It has also been observed that the prostatic stem/progenitor cells from these well-established normal prostatic epithelial cell lines differentiated in vitro in low Ca2+ medium but with a limited potential into the TA/intermediate cells expressing different markers such as PSCA protein, a late intermediate marker (83). Importantly, the poorly differentiated and tumorigenic AR− PC3 cell line with intermediate phenotype (CK5/18), which contains a small subpopulation of PC progenitor cells expressing stem cell-like markers (CD133+ and CD44high), can also form poorly differentiated tumors in vivo that resemble the original patient’s tumor (72,144,145). Similarly, it has been reported that the PC cells constituting xenograft tumors established from LAPC9 or DU145 cells may be organized as a functional hierarchy where CD44+/α2β1+/high or α2β1−/low-integrin-expressing cells display a higher tumorigenic potential than the CD44− cell fraction in nude mice (71,72,73). Additionally, the results from a recent study have revealed that the very small subpopulation of CD133+/CD44+/α2β1+-integrin DU145 isolated from the parental AR− DU145 cell mass, which expresses a high level of β-catenin and MYC but a low level of apoptotic factor Bax, showed a self-renewal, extensive differentiating, and proliferative abilities and a higher tumorigenic potential relative to the CD133−/CD44+/α2β1−/low-integrin DU145 cell fraction in vivo (75).
Hence, the use of PC stem/progenitor cells with stem cell-like properties from well-established cell lines should constitute useful preclinical in vivo cell models to establish the molecular mechanisms involved in the treatment resistance as well as to estimate the efficacy of new antitumoral drugs to eliminate these PC-initiating cells that can contribute to tumor formation, metastasis, and disease relapse. In this matter, we describe the molecular mechanisms that may be responsible, at least in part, for the drug resistance of PC stem/progenitor cells and their further differentiated progenies and disease relapse as well as the novel targeting therapies for overcoming treatment resistance and improving the current clinical therapies for patients diagnosed with locally advanced and metastatic HRPCs.
V. Molecular Mechanisms Involved in the Therapeutic Resistance of Prostatic Cancer Stem/Progenitor Cells and Their Further Differentiated Progenies
An understanding of the molecular mechanisms involved in the resistance of highly tumorigenic and metastatic PC stem/progenitor cells and their further differentiated progenies to current therapeutic regimens is of immense clinical interest. This information will enable us to establish novel strategies for a more effective treatment of locally advanced and/or metastatic HRPCs. Indeed, the intrinsic or acquired therapeutic resistance of PC stem/progenitor cells to current clinical antiandrogen, radiation, and chemotherapeutic treatments may be causally linked and contribute to treatment failure and disease relapse (Fig. 3). The cancer stem/progenitor cell concept suggests that the bulk mass of differentiated PC cells with a luminal phenotype does not possess the ability to form new tumors. Based on this, it is likely that AR− PC stem/progenitor cells can contribute to PC recurrence (27,28,30,31,44). This concept is consistent with the observations that the patients with PCs initially respond to androgen deprivation therapy, which induces a massive rate of apoptotic death of bulk mass of androgen-dependent AR+ PC cells in the luminal compartment, and thereby clinically leads to tumor regression and a partial remission (Fig. 3). However, the emergence of HRPCs, which may be due to the persistence of AI and chemoresistant PC cells, including AR− PC stem/progenitor cells that are able to initiate and drive tumor growth in primary and secondary neoplasms, may cause the disease relapse even after several years (Fig. 3) (8,27,28,30,44,86).
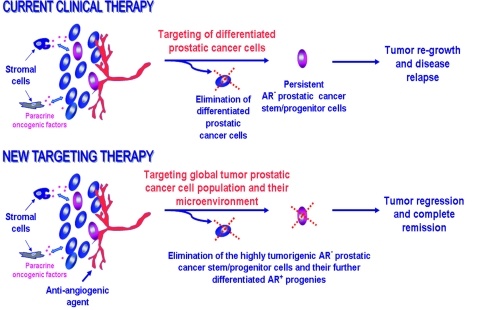
Figure 3.
Scheme showing the possible molecular events associated with the resistance of PC cells to current clinical treatments and new targeting therapies against locally advanced and/or metastatic HRPCs. Based on the PC stem/progenitor concept, it is likely that the androgen-independent and highly tumorigenic and migrating PC cells including AR− PC stem/progenitor cells may persist after the current therapeutic treatments including androgen-deprivation therapies. Hence, they can contribute to the tumor regrowth and disease relapse. In contrast, novel combination therapies targeting the total mass of PC cells including AR− PC stem/progenitor cells and their differentiated AR+ progenies as well as their local microenvironment including the host stromal cells such as activated myofibroblasts and endothelial cells could lead to an eradication of the global PC cell population and inhibition of the neoangiogenesis process. These new targeting therapies could induce a total tumor regression and thereby result in a complete remission of patients diagnosed with aggressive and incurable PCs.
In addition, the acquisition of drug resistance by PC cells may also result from the genetic and/or epigenic changes occurring in tumor cells during disease progression after continued exposure to therapeutic agents for a long time (3,9,27,28,30,44,77,146,147,148,149,150,151,152,153,154,155,156). The development of simultaneous resistance to several drugs and/or radiation therapy may also occur during the transition from localized PC into invasive and metastatic stages. Among the molecular events associated with the occurrence of multidrug resistance (MDR) phenomenon, there are the enhanced expression and/or activity of ABC superfamily transporter genes that encode the transmembrane proteins acting as the drug efflux pumps. The MDR phenomenon is frequently involved in the resistance to diverse unstructurally related chemical compounds, such as distinct cytotoxic chemotherapeutic agents including docetaxel and mitoxantrone (Fig. 2) (27,77,86,146,147,148,149,150,151,155,156,157,158,159). In this regard, the immunohistochemical analyses have revealed that the expression of a MDR transporter, P-glycoprotein encoded by the MDR1 gene, was elevated in 96 patients with PC, and this enhanced expression correlated with tumor grade, stage, and PSA levels (160). Moreover, several studies revealed that the metastatic PC cell lines, DU145 and PC3 cells, express high levels of multidrug efflux pumps including ABCG2 and MDR-associated protein-1 that may contribute to the SP fraction detected by the Hoechst technique (147,148,149,150,151,156,158,159,161). This suggests then the possible implication of these ABC multidrug transporters in the drug resistance of PC cells, including PC stem/progenitor cells, and in disease recurrence in certain patients. Additionally, the drug resistance may also be caused by the changes in the expression and/or activity of drug targets including the β-tubulin and/or topoisomerase II-α and -β, modification in GST π, DNA repair enzymes, and/or antiapoptotic vs. apoptotic signaling cascade elements in PC cells during PC progression (9,77,146,147,149,162,163). As a matter of fact, it has been observed that the expression levels of MDR-associated protein-1, topoisomerase IIα, GST π, p53, and Bcl-2 detected in PC tissue specimens at the time of surgical intervention enhanced with the Gleason grade (146). The up-regulation of glucosylceramide synthase, cholesterol, caveolin-1 and antiapoptotic factors such as Bcl-2, survivin, nuclear factor-κB (NF-κB), and PI3K/Akt concomitant with the down-regulation of cellular ceramide levels may also occur during PC progression (3,8,9,77,146,156,162,163,164,165,166,167). These molecular events may increase the resistance of PC cells including PC stem/progenitor cells to current therapeutic treatments. In addition, the enhanced expression and/or sustained activation of numerous growth factor, chemokine, and cytokine signaling pathways, including EGF-EGFR, hedgehog SHH-PTCH, Wnt/β-catenin, estrogen/estrogen receptor, TGF-β, and SDF-1/CXCR4 axes in PC cells may also contribute to their sustained growth, survival, migration, invasion, and/or metastasis during the initiation and progression from localized PCs into advanced and metastatic disease states (Fig. 2) (3,8,37,38,39,42,43,45,47,48,68,168,169,170,171,172,173,174,175,176,177,178). In particular, the enhanced expression of EGFR and its ligands, EGF, TGF-α, and amphiregulin, hedgehog, and β-catenin signaling elements occurring in PC cells during the PC progression has been associated with the development of AI disease, a high rate of disease relapse, and decreased survival of patients after therapy (38,109,169,179,180,181,182,183,184,185,186). Importantly, several recent lines of evidence have revealed that the activation of these oncogenic signaling pathways in AI PC stem/progenitor cells may contribute to their malignant transformation into migrating, invasive, and metastatic PC cells and disease recurrence (29,67,102,156,187). In support of this, it has been reported that an accumulation of EGFR-positive tumor cells expressing intermediate markers CK5/18 occurred in a subset of patients with HRPCs (33,96). This suggests that the number of CK5/18- and EGFR-positive PC progenitor cells with an intermediate phenotype may rise during androgen deprivation and disease relapse, supporting the benefit of using the EGFR inhibitor to target this neoplastic cell subpopulation. Similarly, the CD44+ marker and hedgehog signaling element, PTCH1, were expressed and colocalized in PC cells with an intermediate phenotype CK14/8 (156). Moreover, the expression levels of β-catenin and hedgehog signaling element, smoothened (SMO), were higher in highly tumorigenic CD44+ DU145 cell subpopulation relative to the weakly tumorigenic CD44− DU145 cell fraction (72). These observations suggest that the EGFR, hedgehog, and Wnt/β-catenin signaling cascades may be involved in the malignant transformation of PC stem/progenitor with an intermediate phenotype and disease relapse. Therefore, the development of a novel therapy by the combined targeting of these tumorigenic signaling elements, which are activated in AI and metastatic PC stem/progenitor cells as well as their supporting local microenvironment, could represent a more promising strategy for improving the current therapeutic treatments and the overall survival of patients diagnosed with metastatic HRPCs.
VI. Novel Therapeutic Strategies against the Locally Advanced and Metastatic Hormone-Refractory Prostate Cancers
Although surgical tumor resection, hormonal therapies, radiotherapy, and adjuvant chemotherapy, alone or in combination, show beneficial effects and a significant curative rate in treating patients with localized PCs in the early stages, the development of locally advanced and/or metastatic HRPCs eventually results in disease recurrence. Because most patients who undergo potentially curative resection for advanced and/or metastatic HRPCs subsequently relapse due to the persistence of foci and micrometastases, systemic chemotherapy may represent another option to eradicate the PC cells, including the highly tumorigenic PC stem/ progenitor cells that can drive tumor growth at primary neoplasms and distant metastatic sites. In this regard, we are reporting the recent advances that have led to the identification of novel therapeutic strategies targeting PC stem/progenitor cells and their further differentiated progenies as well as their local microenvironment, which would allow us to improve the efficacy of current antiandrogen and chemotherapeutic treatments against locally advanced and/or metastatic HRPCs.
A. Targeting of PC stem/progenitor cells and their further differentiated progenies
Several investigations revealed that EGFR, hedgehog, Wnt/β-catenin, and/or SDF-1/CXCR4 signaling cascades as well as ABC multidrug efflux pumps could represent important targets for overcoming the intrinsic or acquired resistance of PC stem/progenitor cells and/or their further differentiated progenies to current clinical therapies (Fig. 4) (3,9,37,45,46,47,108,109,126,155,172,184,185,186,188,189,190,191,192,193). Particularly, recent studies have revealed that the blockade of these tumorigenic signaling cascades could be beneficial as adjuvant therapy in the early phases of PC for decreasing the risk of relapse as well as at the late stages for improving the efficacy of current androgen deprivation therapy, radiotherapy, and/or systemic chemotherapy and patient survival rates. In this regard, it has been observed that the blockade of the EGFR pathway by anti-EGFR antibody (cetuximab, also designated erbitux, mAb-C225, and IMC-C225) or EGFR tyrosine kinase inhibitor (gefinib, erlotinib, and EKB-569) caused an arrest in the G1 phase of the cell cycle, inhibited invasion, and/or induced apoptosis in metastatic PC cells in vitro and in vivo (3,169,171,190,191,192,193,194,195,196,197,198,199,200,201,202). More specifically, the preclinical and clinical trials with a low dose of specific inhibitor of EGFR tyrosine kinase activity, gefitinib, have indicated a potential benefit of using this type of agent, which generally shows a good bioavailability and few side effects in combination with other chemotherapeutic drugs used for numerous cancer types overexpressing EGFR, including PCs (203). Although the use of gefitinib as a monotherapy is generally not very effective, it has been observed that this agent may increase the cytotoxic effects induced by diverse chemotherapeutic agents, such as docetaxel, paclitaxel, and platinum compounds, cisplatin and carboplatinum, on metastatic and AI PC3 cells in vitro and in vivo (170,204). Moreover, gefitinib might also inhibit the AR activity and act in cooperation with the antiandrogenic agents such as bicalutamide (205,206). Similarly, the inhibition of the hedgehog cascade, either by using SMO signaling element inhibitor, cyclopamine, or the anti-SHH antibody, has been observed to result in an inhibition both of the growth via a blockade in the G1 phase of the cell cycle and the invasion of metastatic PC cells in vitro and in vivo, whereas the normal prostate epithelial cells were insensitive to the cytotoxic effects of these agents (3,38,39,42,43,169,188,207). Of particular therapeutic interest, the continuous cyclopamine treatment of poorly differentiated and highly tumorigenic PC3 cell xenografts established in nude mice in vivo also resulted in apoptotic cell death and tumor regression without a sign of the adverse effects on normal cells and tumor recurrence after 72 h of treatment cessation, suggesting that PC-initiating cells have been eradicated (39). The expression levels of ABCG2 and MDR1 (ABCB1) multidrug efflux pumps in PC3 cells were also decreased after the cyclopamine treatment, supporting the potential benefit of using the cyclopamine to counteract the MDR mediated by these transporters in PC cells (156). In this regard, the results from our recent studies have also indicated that the combined use of lower doses of gefinitib and cyclopamine with docetaxel or mitoxantrone induced a growth arrest and a higher rate of apoptotic death on diverse metastatic and androgen-sensitive AR+ LNCaP-C33 cells and AI AR+ LNCaP-C81 and AR− DU145 and PC3 cells than the individual drugs or two-drug combination treatment (170,208). Additionally, the combined use of the conventional treatments including antiandrogenic therapies with the differentiating agents or epigenic modulators of gene transcription that are able to promote the differentiation of PC cells including PC stem/progenitor cells and/or their early TA progenies into their further differentiated progenies and induce a growth arrest and/or apoptotic death of PC cells also may represent a potential therapeutic strategy against locally advanced and aggressive PC forms (209,210,211,212,213,214). In support of this, the result from numerous studies indicated that the use of differentiating agents such as retinoic acids, including natural active metabolite of vitamin A, all-trans-retinoic acid, and other retinoids, retinoic acid metabolism-blocking agents, and/or histone deacetylase inhibitors, alone in combination therapies, induced the differentiation, growth inhibition, and/or apoptotic death of PC cells in vitro and in vivo (210,215,216,217,218,219,220,221,222,223,224,225,226,227,228). Additional investigations are however necessary to establish more precisely the molecular mechanism(s) of anticarcinogenic effects mediated by these agent types as well as to ascertain whether they are able to eradicate the total mass of PC cells including immature PC stem/progenitor cells in vitro and in vivo without major systemic toxicity.
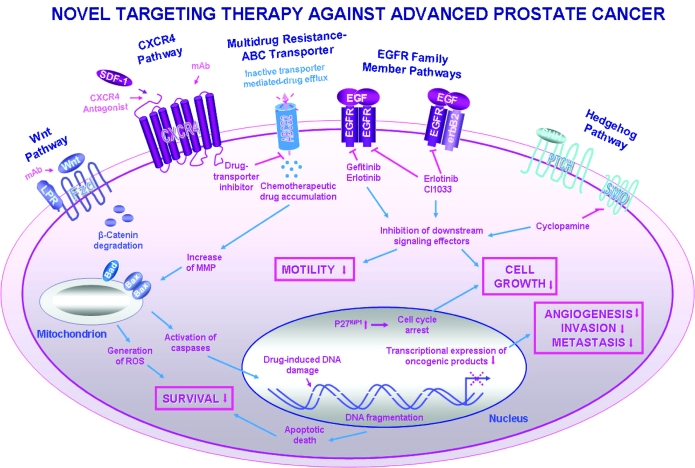
Figure 4.
Scheme showing the novel therapeutic strategies against locally advanced and/or metastatic HRPCs by targeting different oncogenic signaling cascade elements in PC cells. The pharmacological agents acting as the potent inhibitors of the tumorigenic signaling cascades including the selective inhibitors of the EGF-EGFR system (gefitinib, erlotinib, and CI1033), SMO hedgehog signaling element (cyclopamine), CXCR4 antagonist, and monoclonal antibody (mAb) directed against CXCR4 or Wnt ligand are indicated. Moreover, the potential anticarcinogenic effects induced in PC cells through the blockade of these tumorigenic cascades including the inhibition of cell proliferation, survival, migration, invasion, and metastasis are also indicated. Particularly, the possible stimulation of the increase of mitochondrial membrane permeability (MMP), activation of caspase cascades, production of reactive oxygen species (ROS), and/or DNA damage induced by the chemotherapeutic drugs are illustrated. These molecular events may contribute to triggering the activation of apoptotic signaling cascades leading to PC cell death. In addition, the potent inhibitory effect mediated by a specific inhibitor on ABCG2-multidrug efflux pump and whose event may lead to the intracellular chemotherapeutic drug accumulation is shown.
In addition, the altered expression and/or activity of TERT and apoptosis-regulatory signaling elements including the up-regulation of antiapoptotic factors (Bcl-2 family proteins, MYC, survivin, clusterin, PI3K/Akt, and NF-kB), and/or the down-regulation of tumor suppressor gene products (p53 and PTEN), as well as the enzymes involved in the metabolism of proapoptotic ceramide may also occur in PC cells during the progression to AI disease and thus contribute to treatment resistance (3,9,46,77,123,124,146,164,165,168,229,230,231,232,233). Therefore, the targeting of one or several of these deregulated signaling elements may constitute promising approaches for improving the current androgen deprivation, radiation, and docetaxel- or mitoxantrone-based therapies. Particularly, the inhibition of TERT activity, which is up-regulated in proliferating PC cells including the immature PC progenitor cells during the disease progression, has great therapeutic promise (214,234,235). For instance, it has been observed that a combination of the active form of vitamin D3, 1α,25-dihydroxyvitamin D3, and 9-cis-retinoic acid inhibited TERT activity and growth of PC3 cell xenografts established in nude mice in vivo (235). Moreover, the inhibition of the ceramide degradation by using a specific inhibitor of sphingosine kinase-1 significantly improved the growth inhibitory and antimetastatic effects induced by docetaxel on the orthotopically implanted PC3 cells in nude mice in vivo (232). The combination of docetaxel plus bcl-2 and c-myc antisense oligodeoxynucleotides also produced a significant tumor growth inhibition of PC3 cell xenografts that resulted in a significant increase in mice survival (233). Similarly, the combined use of mitoxantrone plus adenoviral-mediated p53 gene transfer, Ad5CMV-p53 and antisense oligodeoxynucleotide targeting clusterin gene, also completely inhibited sc implanted AI PC3 cell-derived tumor growth as well as lymph node metastases from orthotopic PC3 cell-derived tumors in 60 and 100% of mice, respectively (230). Several recent works have revealed the substantial benefit to targeting the supporting tumor microenvironment of PC cells for improving the efficacy of treatments against aggressive PCs.
B. Targeting of the local tumor microenvironment of PC stem/progenitor cells and their further differentiated progenies
The targeting of the local microenvironment “niche” and stromal components that may influence the malignant transformation of PC stem/progenitor cells and their further differentiated progenies during PC progression and the angiogenic process also represent other promising therapeutic strategies (Figs. 3 and 4) (40,45,46,52,119,236). The combined use of antiangiogenic agents such as a specific antibody or an inhibitor of vascular epidermal growth factor (VEGF), VEGF receptor VEGFR), and/or COX-2 with other cytotoxic drugs acting on PC cells may notably counteract the tumor growth and invasion, and thereby prevent disease relapse (3,46,194,237,238). For instance, it has been observed that the combination of docetaxel, or anti-EGFR antibody, cetuximab, with sunitinib malate (SU11248), an oral multitarget tyrosine kinase inhibitor targeting VEGFR-1, -2, and -3, platelet-derived growth factor receptors α and β, stem cell factor receptor (KIT), and FMS-like tyrosine kinase-3 receptor (FLT3), induced a supra-additive inhibitory effect on tumor growth of PC3 xenografts established in mice in vivo (239). New therapeutic strategies targeting the prostatic CAFs and/or bone marrow-derived or vascular wall-resident endothelial progenitor cells or mesenchymal stem cells, which can contribute to the tumorigenesis by giving rise to new tumor endothelial cells or activated myofibroblasts, respectively, also merit further investigation (240,241,242). In this regard, the results from a recent study have revealed that the combined use of low doses of a specific inhibitor of fibroblast-myofribroblast transition, halofuginone and docetaxel, synergistically inhibited the PC cell xenograft-derived tumor development in nude mice (243). Additionally, because it has been reported that the secretion of the high levels of SDF-1 and TGF-β1 by human CAFs in tumor stroma may cooperate for inducing the malignant transformation of CXCR4-expressing BPH-1 cells, the blockade of both the SDF-1-CXCR4 and TGF-β1 pathways by using the specific antagonists or antibodies could also represent a potential strategy to counteract PC development (Fig. 4) (45,50).
Hence, it appears that the simultaneous targeting of PC stem/progenitor cells and their further differentiated progenies as well as their local microenvironment could be more effective to counteract the PC transition to invasive and metastatic stages. Future works with the isolated and well-characterized PC stem/progenitor cells from patients’ malignant tissues or established cell lines should allow us to estimate the efficacy of novel therapeutic agents to eliminate the highly tumorigenic PC stem/progenitor cells and their differentiated progenies, and thereby to improve the current therapeutic treatments against metastatic HRPCs.
VII. Conclusions and Perspectives
Altogether, these recent investigations have revealed that normal and malignant CD133+/CD44+/α2β1high-integrin/AR− prostatic stem/progenitor cells can give rise to different TA/intermediate cell types with distinct proliferative and differentiating capabilities between the prostatic stem/progenitor cells and terminally differentiated secretory epithelial cells during the multiple stages associated with the prostatic tissue regeneration process and PC development, respectively. Further studies are necessary to establish and define more precisely the specific markers and functional properties of normal CD133+/CD44+/α2β1high-integrin/AR− prostatic stem/progenitor cells vs. their more committed progenies and malignant counterpart, PC stem/progenitor cells. The identification of the intrinsic and extrinsic factors that may regulate the self-renewal ability and/or differentiating potential of normal and malignant prostatic stem/progenitor in vitro and in vivo, and in particular, the combined effects of the activation of diverse signaling cascades on their behavior, should provide insight into the molecular events that may be involved in prostate tissue homeostasis and pathologies. It will be of interest to establish the differences between the gene expression patterns observed for normal and malignant CD133+ prostatic stem/progenitor cells compared with the CD133− subpopulation to elucidate their unique functions in prostate physiology and pathophysiology.
In addition, the determination of the implications of AR− PC stem/progenitor cells in tumor growth, metastases, dormancy phenomenon, and PC recurrence after the clinical therapies should also provide important information for overcoming treatment resistance and disease relapse. Further in vitro and in vivo characterization of functional properties of human PC stem/progenitor cells isolated from primary or secondary tumors from patients and well-established PC cell lines should lead to the establishment of new in vitro and in vivo PC cell models in which the genetic and phenotypic changes observed could be comparable to those seen during human PC initiation, progression, and metastasis in the clinics. These novel PC stem/progenitor cell models should help in identifying the deregulated gene products associated with the molecular etiology and progression of benign vs. malignant prostatic diseases as well as the novel drug targets for the development of new therapeutic options to prevent or treat the BPH and the aggressive and recurrent PCs. The in vitro and in vivo evaluation of anticarcinogenic effects induced by the current androgen deprivation, radiation, and/or chemotherapeutic treatments, alone or in combination with novel therapeutic agents targeting the AR− PC stem/progenitor cells and their local microenvironment, also merits future investigation. Particularly, it will be of interest to estimate the specific implication of the tumorigenic signaling cascades induced through EGFR, hedgehog, and/or SDF-1/CXCR4 pathways, which provide a critical role in PC progression and disease recurrence, in the malignant transformation of PC stem/progenitor cells and their progenies. The establishment of the PC stem/progenitor and their progenies made resistant to specific or multiple chemotherapeutic drugs should also provide important information regarding the diverse molecular mechanisms that may be responsible for the acquisition of drug resistance after therapeutic intervention consisting of a long exposition of PC cells to drugs currently used in the clinics. These additional investigations should allow us to identify new signaling elements in PC stem/progenitor cells that are involved in treatment resistance and which could be targeted for improving the current clinical antiandrogen deprivation therapies as well as docetaxel- or mitoxantrone-based chemotherapies against metastatic HRPCs.
Hence, these further investigations should lead to the development of more effective and safe clinical treatments for patients diagnosed with locally advanced and/or metastatic HRPCs by blocking the tumor angiogenic process and eradicating the total mass of PC cells consisting of PC stem/progenitor cells and their further differentiated progenies at primary and secondary neoplasms. These novel treatments could prevent disease relapse, and thereby improve the overall survival of patients with metastatic HRPCs that remain incurable with the current therapeutic regimen options.
Footnotes
The authors are supported by grants from the U.S. Department of Defense (PC04502, OC04110) and the National Institutes of Health (CA78590, CA111294, CA113903).
Disclosure Statement: The authors have nothing to disclose.
First Published Online February 21, 2008
Abbreviations: ABC, ATP-binding cassette; AI, androgen-independent; AR, androgen receptor; BPH or BHP, benign prostatic hypertrophy (or hyperplasia); CAFs, carcinoma-associated fibroblasts; CK, cytokeratin; COX-2, cyclooxygenase-2; CXCR4, CXC chemokine receptor-4; EGF, epidermal growth factor; EGFR, EGF receptor; EMT, epithelial-mesenchymal transition; FACS, fluorescence-activated cell sorting; FGF, fibroblast growth factor; GST π, glutathione S-transferase π; HRPCs, hormone-refractory prostate cancers; KGF, keratinocyte growth factor; MDR, multidrug resistance; NF-κB, nuclear factor-κB; PC, prostate cancer; PI3K, phosphatidylinositol-3′ kinase; PSA, prostate-specific antigen; PSCA, prostate stem cell antigen; PTCH1, Patched 1; PTEN, phosphatase and tensin homolog deleted on chromosome 10; Sca-1, stem cell antigen-1; SDF-1, stromal cell-derived factor-1; SHH, sonic hedgehog; SMO, smoothened; SP, side population; TA, transit-amplifying; TERT, telomerase reverse transcriptase; UGS, urogenital sinus; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
References
- Lobel B 2005 Screening for prostate cancer–how to manage in 2006? Acta Chir Iugosl 52:19–21 [DOI] [PubMed] [Google Scholar]
- Bradford TJ, Wang X, Chinnaiyan AM 2006 Cancer immunomics: using autoantibody signatures in the early detection of prostate cancer. Urol Oncol 24:237–242 [DOI] [PubMed] [Google Scholar]
- Mimeault M, Batra SK 2006 Recent advances on multiple tumorigenic cascades involved in prostatic cancer progression and targeting therapies. Carcinogenesis 27:1–22 [DOI] [PubMed] [Google Scholar]
- Thompson IM, Ankerst DP 2007 Prostate-specific antigen in the early detection of prostate cancer. CMAJ 176:1853–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin MS, Gore JL, Kwan L, Brandeis JM, Lee SP, Withers HR, Reiter RE 2007 Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer 109:2239–2247 [DOI] [PubMed] [Google Scholar]
- Gondi V, Deutsch I, Mansukhani M, O’Toole KM, Shah JN, Schiff PB, Katz AE, Benson MC, Goluboff ET, Ennis RD 2007 Intermediate-risk localized prostate cancer in the PSA era: radiotherapeutic alternatives. Urology 69:541–546 [DOI] [PubMed] [Google Scholar]
- Ye XC, Choueiri M, Tu SM, Lin SH 2007 Biology and clinical management of prostate cancer bone metastasis. Front Biosci 12:3273–3286 [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C 2004 Androgen receptor in prostate cancer. Endocr Rev 25:276–308 [DOI] [PubMed] [Google Scholar]
- Rau KM, Kang HY, Cha TL, Miller SA, Hung MC 2005 The mechanisms and managements of hormone-therapy resistance in breast and prostate cancers. Endocr Relat Cancer 12:511–532 [DOI] [PubMed] [Google Scholar]
- Kasper S, Cookson MS 2006 Mechanisms leading to the development of hormone-resistant prostate cancer. Urol Clin North Am 33:201–210 [DOI] [PubMed] [Google Scholar]
- Arlen PM, Gulley JL 2005 Docetaxel-based regimens, the standard of care for metastatic androgen-insensitive prostate cancer. Future Oncol 1:19–22 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo G, De Placido S 2006 Hormone refractory prostate cancer (HRPC): present and future approaches of therapy. Int J Immunopathol Pharmacol 19:11–34 [PubMed] [Google Scholar]
- Winquist E, Waldron T, Berry S, Ernst DS, Hotte S, Lukka H 2006 Non-hormonal systemic therapy in men with hormone-refractory prostate cancer and metastases: a systematic review from the Cancer Care Ontario Program in Evidence-based Care’s Genitourinary Cancer Disease Site Group. BMC Cancer 6:112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry W, Eisenberger M 2005 Achieving treatment goals for hormone-refractory prostate cancer with chemotherapy. Oncologist 10:30–39 [DOI] [PubMed] [Google Scholar]
- Clarke NW 2006 Management of the spectrum of hormone refractory prostate cancer. Eur Urol 50:428–438 [DOI] [PubMed] [Google Scholar]
- Petrylak DP, Tangen CM, Hussain MH, Lara Jr PN, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED 2004 Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351:1513–1520 [DOI] [PubMed] [Google Scholar]
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA 2004 Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–1512 [DOI] [PubMed] [Google Scholar]
- Petrylak DP 2005 Future directions in the treatment of androgen-independent prostate cancer. Urology 65:8–12 [DOI] [PubMed] [Google Scholar]
- Berry DL, Moinpour CM, Jiang CS, Ankerst DP, Petrylak DP, Vinson LV, Lara PN, Jones S, Taplin ME, Burch PA, Hussain MH, Crawford ED 2006 Quality of life and pain in advanced stage prostate cancer: results of a Southwest Oncology Group randomized trial comparing docetaxel and estramustine to mitoxantrone and prednisone. J Clin Oncol 24:2828–2835 [DOI] [PubMed] [Google Scholar]
- Collins R, Trowman R, Norman G, Light K, Birtle A, Fenwick E, Palmer S, Riemsma R 2006 A systematic review of the effectiveness of docetaxel and mitoxantrone for the treatment of metastatic hormone-refractory prostate cancer. Br J Cancer 95:457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro F, Sternberg CN 2007 Current indications for chemotherapy in prostate cancer patients. Eur Urol 51:17–26 [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ 2007 Cancer statistics, 2007. CA Cancer J Clin 57:43–66 [DOI] [PubMed] [Google Scholar]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ 2005 Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 65:10946–10951 [DOI] [PubMed] [Google Scholar]
- Collins AT, Maitland NJ 2006 Prostate cancer stem cells. Eur J Cancer 42:1213–1218 [DOI] [PubMed] [Google Scholar]
- Sharifi N, Kawasaki BT, Hurt EM, Farrar WL 2006 Stem cells in prostate cancer: resolving the castrate-resistant conundrum and implications for hormonal therapy. Cancer Biol Ther 5:901–906 [DOI] [PubMed] [Google Scholar]
- Lam JS, Reiter RE 2006 Stem cells in prostate and prostate cancer development. Urol Oncol 24:131–140 [DOI] [PubMed] [Google Scholar]
- Mimeault M, Hauke R, Mehta PP, Batra SK 2007 Recent advances on cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancers. J Mol Cell Med 11:981–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Batra SK 2007 Functions of tumorigenic and migrating cancer progenitor cells in cancer progression and metastasis and their therapeutic implications. Cancer Metastasis Rev 26:203–214 [DOI] [PubMed] [Google Scholar]
- Miki J, Furusato B, Li H, Gu Y, Takahashi H, Egawa S, Sesterhenn IA, McLeod DG, Srivastava S, Rhim JS 2007 Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res 67:3153–3161 [DOI] [PubMed] [Google Scholar]
- Nikitin AY, Matoso A, Roy-Burman P 2007 Prostate stem cells and cancer. Histol Histopathol 22:1043–1049 [DOI] [PubMed] [Google Scholar]
- Maitland NJ, Bryce SD, Stower MJ, Collins AT 2006 Prostate cancer stem cells: a target for new therapies. Ernst Schering Found Symp Proc 5:155–179 [DOI] [PubMed] [Google Scholar]
- Brown MD, Gilmore PE, Hart CA, Samuel JD, Ramani VA, George NJ, Clarke NW 2007 Characterization of benign and malignant prostate epithelial Hoechst 33342 side populations. Prostate 67:1384–1396 [DOI] [PubMed] [Google Scholar]
- van Leenders GJ, Aalders TW, Hulsbergen-van de Kaa CA, Ruiter DJ, Schalken JA 2001 Expression of basal cell keratins in human prostate cancer metastases and cell lines. J Pathol 195:563–570 [DOI] [PubMed] [Google Scholar]
- van Leenders GJ, Gage WR, Hicks JL, van Balken B, Aalders TW, Schalken JA, De Marzo AM 2003 Intermediate cells in human prostate epithelium are enriched in proliferative inflammatory atrophy. Am J Pathol 162:1529–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ, Lecksell K, Gaudin P, Epstein JI 1999 Rare expression of high-molecular-weight cytokeratin in adenocarcinoma of the prostate gland: a study of 100 cases of metastatic and locally advanced prostate cancer. Am J Surg Pathol 23:147–152 [DOI] [PubMed] [Google Scholar]
- Schalken JA, van Leenders G 2003 Cellular and molecular biology of the prostate: stem cell biology. Urology 62:11–20 [DOI] [PubMed] [Google Scholar]
- Kambhampati S, Ray G, Sengupta K, Reddy VP, Banerjee SK, Van Veldhuizen PJ 2005 Growth factors involved in prostate carcinogenesis. Front Biosci 10:1355–1367 [DOI] [PubMed] [Google Scholar]
- Sheng T, Li C, Zhang X, Chi S, He N, Chen K, McCormick F, Gatalica Z, Xie J 2004 Activation of the hedgehog pathway in advanced prostate cancer. Mol Cancer 3:29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA 2004 Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature 431:707–712 [DOI] [PubMed] [Google Scholar]
- Chung LW, Baseman A, Assikis V, Zhau HE 2005 Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol 173:10–20 [DOI] [PubMed] [Google Scholar]
- Stecca B, Mas C, Altaba AR 2005 Interference with HH-GLI signaling inhibits prostate cancer. Trends Mol Med 11:199–203 [DOI] [PubMed] [Google Scholar]
- Sanchez P, Hernandez AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, Beyna M, Datta MW, Datta S, Altaba A 2004 Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci USA 101:12561–12566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Pepicelli CV, Dibble CC, Catbagan W, Zarycki JL, Laciak R, Gipp J, Shaw A, Lamm ML, Munoz A, Lipinski R, Thrasher JB, Bushman W 2004 Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology 145:3961–3970 [DOI] [PubMed] [Google Scholar]
- Mimeault M, Batra SK 2007 Interplay of distinct growth factors during epithelial-mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann Oncol 18:1605–1619 [DOI] [PubMed] [Google Scholar]
- Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW 2007 Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res 67:4244–4253 [DOI] [PubMed] [Google Scholar]
- Singh RP, Agarwal R 2006 Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocr Relat Cancer 13:751–778 [DOI] [PubMed] [Google Scholar]
- Sun YX, Fang M, Wang J, Cooper CR, Pienta KJ, Taichman RS 2007 Expression and activation of α(v) β(3) integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate 67:61–73 [DOI] [PubMed] [Google Scholar]
- Chinni SR, Sivalogan S, Dong Z, Filho JC, Deng X, Bonfil RD, Cher ML 2006 CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate 66:32–48 [DOI] [PubMed] [Google Scholar]
- Liu AY, True LD, LaTray L, Nelson PS, Ellis WJ, Vessella RL, Lange PH, Hood L, van den EG 1997 Cell-cell interaction in prostate gene regulation and cytodifferentiation. Proc Natl Acad Sci USA 94:10705–10710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, Sudilovsky D, Cunha GR 2001 Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res 61:8135–8142 [PubMed] [Google Scholar]
- Cunha GR, Ricke W, Thomson A, Marker PC, Risbridger G, Hayward SW, Wang YZ, Donjacour AA, Kurita T 2004 Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J Steroid Biochem Mol Biol 92:221–236 [DOI] [PubMed] [Google Scholar]
- Tomas D, Kruslin B 2004 The potential value of (Myo)fibroblastic stromal reaction in the diagnosis of prostatic adenocarcinoma. Prostate 61:324–331 [DOI] [PubMed] [Google Scholar]
- Bonkhoff H, Remberger K 1996 Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model. Prostate 28:98–106 [DOI] [PubMed] [Google Scholar]
- Hayward SW, Haughney PC, Rosen MA, Greulich KM, Weier HU, Dahiya R, Cunha GR 1998 Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation 63:131–140 [DOI] [PubMed] [Google Scholar]
- Wang Y, Hayward S, Cao M, Thayer K, Cunha G 2001 Cell differentiation lineage in the prostate. Differentiation 68:270–279 [DOI] [PubMed] [Google Scholar]
- Tokar EJ, Ancrile BB, Cunha GR, Webber MM 2005 Stem/progenitor and intermediate cell types and the origin of human prostate cancer. Differentiation 73:463–473 [DOI] [PubMed] [Google Scholar]
- Zhu G, Zhau HE, He H, Zhang L, Shehata B, Wang X, Cerwinka WH, Elmore J, He D 2007 Sonic and desert hedgehog signaling in human fetal prostate development. Prostate 67:674–684 [DOI] [PubMed] [Google Scholar]
- Peehl DM 2005 Primary cell cultures as models of prostate cancer development. Endocr Relat Cancer 12:19–47 [DOI] [PubMed] [Google Scholar]
- Singh P, Uzgare A, Litvinov I, Denmeade SR, Isaacs JT 2006 Combinatorial androgen receptor targeted therapy for prostate cancer. Endocr Relat Cancer 13:653–666 [DOI] [PubMed] [Google Scholar]
- Zhao H, Patra A, Yeh CC, Tanaka Y, Oh BR, Dahiya R 2002 Effects of aging on growth factors gene and protein expression in the dorsal and ventral lobes of rat prostate. Biochem Biophys Res Commun 292:482–491 [DOI] [PubMed] [Google Scholar]
- Isaacs JT, Coffey DS 1989 Etiology and disease process of benign prostatic hyperplasia. Prostate 2:33–50 [DOI] [PubMed] [Google Scholar]
- Feldman BJ, Feldman D 2001 The development of androgen-independent prostate cancer. Nat Rev Cancer 1:34–45 [DOI] [PubMed] [Google Scholar]
- Hudson DL 2004 Epithelial stem cells in human prostate growth and disease. Prostate Cancer Prostatic Dis 7:188–194 [DOI] [PubMed] [Google Scholar]
- Heer R, Collins AT, Robson CN, Shenton BK, Leung HY 2006 KGF suppresses α2β1 integrin function and promotes differentiation of the transient amplifying population in human prostatic epithelium. J Cell Sci 119:1416–1424 [DOI] [PubMed] [Google Scholar]
- Heer R, Robson CN, Shenton BK, Leung HY 2007 The role of androgen in determining differentiation and regulation of androgen receptor expression in the human prostatic epithelium transient amplifying population. J Cell Physiol 212:572–578 [DOI] [PubMed] [Google Scholar]
- Montironi R, Mazzucchelli R, Stramazzotti D, Scarpelli M, Lopez BA, Bostwick DG 2005 Basal cell hyperplasia and basal cell carcinoma of the prostate: a comprehensive review and discussion of a case with c-erbB-2 expression. J Clin Pathol 58:290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BY, Liu JY, Chang HH, Chang CP, Lo WY, Kuo WH, Yang CR, Lin DP 2007 Hedgehog is involved in prostate basal cell hyperplasia formation and its progressing towards tumorigenesis. Biochem Biophys Res Commun 357:1084–1089 [DOI] [PubMed] [Google Scholar]
- Begley L, Monteleon C, Shah RB, Macdonald JW, Macoska JA 2005 CXCL12 overexpression and secretion by aging fibroblasts enhance human prostate epithelial proliferation in vitro. Aging Cell 4:291–298 [DOI] [PubMed] [Google Scholar]
- Burger PE, Xiong X, Coetzee S, Salm SN, Moscatelli D, Goto K, Wilson EL 2005 Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc Natl Acad Sci USA 102:7180–7185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT 2004 CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci 117:3539–3545 [DOI] [PubMed] [Google Scholar]
- Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG 2007 Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+α2β1+ cell population is enriched in tumor-initiating cells. Cancer Res 67:6796–6805 [DOI] [PubMed] [Google Scholar]
- Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L, Tang DG 2006 Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 25:1696–1708 [DOI] [PubMed] [Google Scholar]
- Tang DG, Patrawala L, Calhoun T, Bhatia B, Choy G, Schneider-Broussard R, Jeter C 2007 Prostate cancer stem/progenitor cells: identification, characterization, and implications. Mol Carcinog 46:1–14 [DOI] [PubMed] [Google Scholar]
- Pascal LE, Oudes AJ, Petersen TW, Goo YA, Walashek LS, True LD, Liu AY 2007 Molecular and cellular characterization of ABCG2 in the prostate. BMC Urol 7:6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Guomin W, Yujun L, Ruizhe Q 2007 Cancer stem-like cells in human prostate carcinoma cells DU145: the seeds of the cell line? Cancer Biol Ther 6:763–768 [DOI] [PubMed] [Google Scholar]
- Bhatt RI, Brown MD, Hart CA, Gilmore P, Ramani VA, George NJ, Clarke NW 2003 Novel method for the isolation and characterisation of the putative prostatic stem cell. Cytometry A 54:89–99 [DOI] [PubMed] [Google Scholar]
- Mimeault M, Hauke R, Batra SK 29 August 2007 Recent advances on the molecular mechanisms involved in drug-resistance of cancer cells and novel targeting therapies. Clin Pharmacol Ther 10.1038/sj.clpt.6100296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson DL, O’Hare M, Watt FM, Masters JR 2000 Proliferative heterogeneity in the human prostate: evidence for epithelial stem cells. Lab Invest 80:1243–1250 [DOI] [PubMed] [Google Scholar]
- Hudson DL, Masters JR 2003 Prostate epithelial stem cell isolation and culture. Methods Mol Med 81:59–67 [DOI] [PubMed] [Google Scholar]
- Robinson EJ, Neal DE, Collins AT 1998 Basal cells are progenitors of luminal cells in primary cultures of differentiating human prostatic epithelium. Prostate 37:149–160 [DOI] [PubMed] [Google Scholar]
- Collins AT, Habib FK, Maitland NJ, Neal DE 2001 Identification and isolation of human prostate epithelial stem cells based on α(2)β(1)-integrin expression. J Cell Sci 114:3865–3872 [DOI] [PubMed] [Google Scholar]
- Rizzo S, Attard G, Hudson DL 2005 Prostate epithelial stem cells. Cell Prolif 38:363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov IV, Vander Griend DJ, Xu Y, Antony L, Dalrymple SL, Isaacs JT 2006 Low-calcium serum-free defined medium selects for growth of normal prostatic epithelial stem cells. Cancer Res 66:8598–8607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Shou J, Wong P, French DM, Gao WQ 2004 Notch1-expressing cells are indispensable for prostatic branching morphogenesis during development and re-growth following castration and androgen replacement. J Biol Chem 279:24733–24744 [DOI] [PubMed] [Google Scholar]
- Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, Moscatelli D, Shapiro E, Lepor H, Sun TT, Wilson EL 2002 Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J Cell Biol 157:1257–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss WJ, Gray DR, Greenberg NM, Mohler JL, Smith GJ 2005 Breast cancer resistance protein-mediated efflux of androgen in putative benign and malignant prostate stem cells. Cancer Res 65:6640–6650 [DOI] [PubMed] [Google Scholar]
- Tsujimura A, Fujita K, Komori K, Takao T, Miyagawa Y, Takada S, Matsumiya K, Nonomur N, Okuyama A 2007 Prostatic stem cell marker identified by cDNA microarray in mouse. J Urol 178:686–691 [DOI] [PubMed] [Google Scholar]
- Xin L, Lukacs RU, Lawson DA, Cheng D, Witte ON 2007 Self renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells 25:2760–2769 [DOI] [PubMed] [Google Scholar]
- Barclay WW, Axanova LS, Chen W, Romero L, Maund SL, Soker S, Lees CJ, Cramer SD 29 November 2007 Characterization of adult prostatic progenitor/stem cells exhibiting self-renewal and multilineage differentiation. Stem Cells 10.1634/stemcells.2007-0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salm SN, Burger PE, Coetzee S, Goto K, Moscatelli D, Wilson EL 2005 TGF-β maintains dormancy of prostatic stem cells in the proximal region of ducts. J Cell Biol 170:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran CP, Lin C, Yamashiro J, Reiter RE 2002 Prostate stem cell antigen is a marker of late intermediate prostate epithelial cells. Mol Cancer Res 1:113–121 [PubMed] [Google Scholar]
- Garraway LA, Lin D, Signoretti S, Waltregny D, Dilks J, Bhattacharya N, Loda M 2003 Intermediate basal cells of the prostate: in vitro and in vivo characterization. Prostate 55:206–218 [DOI] [PubMed] [Google Scholar]
- Bhatia B, Tang S, Yang P, Doll A, Aumueller G, Newman RA, Tang DG 2005 Cell-autonomous induction of functional tumor suppressor 15-lipoxygenase 2 (15-LOX2) contributes to replicative senescence of human prostate progenitor cells. Oncogene 24:3583–3595 [DOI] [PubMed] [Google Scholar]
- Bonkhoff H 2001 Neuroendocrine differentiation in human prostate cancer. Morphogenesis, proliferation and androgen receptor status. Ann Oncol 12:S141–S144 [DOI] [PubMed] [Google Scholar]
- Verhagen AP, Ramaekers FC, Aalders TW, Schaafsma HE, Debruyne FM, Schalken JA 1992 Colocalization of basal and luminal cell-type cytokeratins in human prostate cancer. Cancer Res 52:6182–6187 [PubMed] [Google Scholar]
- Gil-Diez de Medina S, Salomon L, Colombel M, Abbou CC, Bellot J, Thiery JP, Radvanyi F, Van der Kwast TH, Chopin DK 1998 Modulation of cytokeratin subtype, EGF receptor, and androgen receptor expression during progression of prostate cancer. Hum Pathol 29:1005–1012 [DOI] [PubMed] [Google Scholar]
- Parsons JK, Gage WR, Nelson WG, De Marzo AM 2001 p63 protein expression is rare in prostate adenocarcinoma: implications for cancer diagnosis and carcinogenesis. Urology 58:619–624 [DOI] [PubMed] [Google Scholar]
- van Leenders G, van BB, Aalders T, Hulsbergen-van de Kaa C, Ruiter D, Schalken J 2002 Intermediate cells in normal and malignant prostate epithelium express c-MET: implications for prostate cancer invasion. Prostate 51:98–107 [DOI] [PubMed] [Google Scholar]
- Googe PB, McGinley KM, Fitzgibbon JF 1997 Anticytokeratin antibody 34 β E12 staining in prostate carcinoma. Am J Clin Pathol 107:219–223 [DOI] [PubMed] [Google Scholar]
- Oliai BR, Kahane H, Epstein JI 2002 Can basal cells be seen in adenocarcinoma of the prostate? An immunohistochemical study using high molecular weight cytokeratin (clone 34βE12) antibody. Am J Surg Pathol 26:1151–1160 [DOI] [PubMed] [Google Scholar]
- Gu G, Yuan J, Wills M, Kasper S 2007 Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res 67:4807–4815 [DOI] [PubMed] [Google Scholar]
- Bello-DeOcampo D, Kleinman HK, Webber MM 2001 The role of α 6 β 1 integrin and EGF in normal and malignant acinar morphogenesis of human prostatic epithelial cells. Mutat Res 480–481:209–217 [DOI] [PubMed] [Google Scholar]
- Abbott BD, Lin TM, Rasmussen NT, Albrecht RM, Schmid JE, Peterson RE 2003 Lack of expression of EGF and TGF-α in the fetal mouse alters formation of prostatic epithelial buds and influences the response to TCDD. Toxicol Sci 76:427–436 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Jones FE 2004 The ErbB signaling network is coordinately expressed and activated in the mouse prostate. Prostate 60:68–75 [DOI] [PubMed] [Google Scholar]
- Huss WJ, Gray DR, Werdin ES, Funkhouser Jr WK, Smith GJ 2004 Evidence of pluripotent human prostate stem cells in a human prostate primary xenograft model. Prostate 60:77–90 [DOI] [PubMed] [Google Scholar]
- Wang XD, Leow CC, Zha J, Tang Z, Modrusan Z, Radtke F, Aguet M, de Sauvage FJ, Gao WQ 2006 Notch signaling is required for normal prostatic epithelial cell proliferation and differentiation. Dev Biol 290:66–80 [DOI] [PubMed] [Google Scholar]
- Hammarsten P, Rudolfsson SH, Henriksson R, Wikstrom P, Bergh A 2007 Inhibition of the epidermal growth factor receptor enhances castration-induced prostate involution and reduces testosterone-stimulated prostate growth in adult rats. Prostate 67:573–581 [DOI] [PubMed] [Google Scholar]
- Shaw A, Bushman W 2007 Hedgehog signaling in the prostate. J Urol 177:832–838 [DOI] [PubMed] [Google Scholar]
- Bruxvoort KJ, Charbonneau HM, Giambernardi TA, Goolsby JC, Qian CN, Zylstra CR, Robinson DR, Roy-Burman P, Shaw AK, Buckner-Berghuis BD, Sigler RE, Resau JH, Sullivan R, Bushman W, Williams BO 2007 Inactivation of Apc in the mouse prostate causes prostate carcinoma. Cancer Res 67:2490–2496 [DOI] [PubMed] [Google Scholar]
- Heemers HV, Tindall DJ 2007 Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev 28:778–808 [DOI] [PubMed] [Google Scholar]
- Li W, Wu CL, Febbo PG, Olumi AF 2007 Stromally expressed c-Jun regulates proliferation of prostate epithelial cells. Am J Pathol 171:1189–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura Y, Foster BA, Hom YK, Lipschutz JH, Rubin JS, Finch PW, Aaronson SA, Hayashi N, Kawamura J, Cunha GR 1996 Keratinocyte growth factor (KGF) can replace testosterone in the ductal branching morphogenesis of the rat ventral prostate. Int J Dev Biol 40:941–951 [PubMed] [Google Scholar]
- Isaacs JT 1984 Antagonistic effect of androgen on prostatic cell death. Prostate 5:545–557 [DOI] [PubMed] [Google Scholar]
- Evans GS, Chandler JA 1987 Cell proliferation studies in the rat prostate. II. The effects of castration and androgen-induced regeneration upon basal and secretory cell proliferation. Prostate 11:339–351 [DOI] [PubMed] [Google Scholar]
- English HF, Kyprianou N, Isaacs JT 1989 Relationship between DNA fragmentation and apoptosis in the programmed cell death in the rat prostate following castration. Prostate 15:233–250 [DOI] [PubMed] [Google Scholar]
- Kwong J, Choi HL, Huang Y, Chan FL 1999 Ultrastructural and biochemical observations on the early changes in apoptotic epithelial cells of the rat prostate induced by castration. Cell Tissue Res 298:123–136 [DOI] [PubMed] [Google Scholar]
- Kyprianou N, English HF, Isaacs JT 1988 Activation of a Ca2+-Mg2+-dependent endonuclease as an early event in castration-induced prostatic cell death. Prostate 13:103–117 [DOI] [PubMed] [Google Scholar]
- De Marzo AM, DeWeese TL, Platz EA, Meeker AK, Nakayama M, Epstein JI, Isaacs WB, Nelson WG 2004 Pathological and molecular mechanisms of prostate carcinogenesis: implications for diagnosis, detection, prevention, and treatment. J Cell Biochem 91:459–477 [DOI] [PubMed] [Google Scholar]
- Langley RR, Fidler IJ 2007 Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev 28:297–321 [DOI] [PubMed] [Google Scholar]
- Carver BS, Pandolfi PP 2006 Mouse modeling in oncologic preclinical and translational research. Clin Cancer Res 12:5305–5311 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Flesken-Nikitin A, Nikitin AY 2007 Prostate cancer associated with p53 and Rb deficiency arises from the stem/progenitor cell-enriched proximal region of prostatic ducts. Cancer Res 67:5683–5690 [DOI] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP 2005 Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436:725–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H 2003 Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 4:209–221 [DOI] [PubMed] [Google Scholar]
- Wang S, Garcia AJ, Wu M, Lawson DA, Witte ON, Wu H 2006 Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc Natl Acad Sci USA 103:1480–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner T, de Las PA, Parrondo R, Perez-Stable C 2007 Progression of prostate cancer from a subset of p63-positive basal epithelial cells in FG/Tag transgenic mice. Mol Cancer Res 5:1171–1179 [DOI] [PubMed] [Google Scholar]
- van Oort I, Tomita K, van Bokhoven, Bussemakers MJ, Kiemeney LA, Karthaus HF, Witjes JA, Schalken JA 2007 The prognostic value of E-cadherin and the cadherin-associated molecules α-, β-, γ-catenin and p120(ctn) in prostate cancer specific survival: a long-term follow-up study. Prostate 67:1432–1438 [DOI] [PubMed] [Google Scholar]
- Cerveira N, Ribeiro FR, Peixoto A, Costa V, Henrique R, Jeronimo C, Teixeira MR 2006 TMPRSS2-ERG gene fusion causing ERG overexpression precedes chromosome copy number changes in prostate carcinomas and paired HGPIN lesions. Neoplasia 8:826–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgeson BE, Tomlins SA, Shah N, Laxman B, Cao Q, Prensner JR, Cao X, Singla N, Montie JE, Varambally S, Mehra R, Chinnaiyan AM 2008 Characterization of TMPRSS2:ETV5 and SLC45A3:ETV5 gene fusions in prostate cancer. Cancer Res 68:73–80 [DOI] [PubMed] [Google Scholar]
- Wang J, Cai Y, Ren C, Ittmann M 2006 Expression of variant TMPRSS2/ERG fusion messenger RNAs is associated with aggressive prostate cancer. Cancer Res 66:8347–8351 [DOI] [PubMed] [Google Scholar]
- Attard G, Clark J, Ambroisine L, Fisher G, Kovacs G, Flohr P, Berney D, Foster CS, Fletcher A, Gerald WL, Moller H, Reuter V, de Bono JS, Scardino P, Cuzick J, Cooper CS 2008 Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene 27:253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosquera JM, Perner S, Demichelis F, Kim R, Hofer MD, Mertz KD, Paris PL, Simko J, Collins C, Bismar TA, Chinnaiyan AM, Rubin MA 2007 Morphological features of TMPRSS2-ERG gene fusion prostate cancer. J Pathol 212:91–101 [DOI] [PubMed] [Google Scholar]
- Mertz KD, Setlur SR, Dhanasekaran SM, Demichelis F, Perner S, Tomlins S, Tchinda J, Laxman B, Vessella RL, Beroukhim R, Lee C, Chinnaiyan AM, Rubin MA 2007 Molecular characterization of TMPRSS2-ERG gene fusion in the NCI-H660 prostate cancer cell line: a new perspective for an old model. Neoplasia 9:200–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perner S, Demichelis F, Beroukhim R, Schmidt FH, Mosquera JM, Setlur S, Tchinda J, Tomlins SA, Hofer MD, Pienta KG, Kuefer R, Vessella R, Sun XW, Meyerson M, Lee C, Sellers WR, Chinnaiyan AM, Rubin MA 2006 TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res 66:8337–8341 [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM 2005 Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310:644–648 [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Mehra R, Rhodes DR, Smith LR, Roulston D, Helgeson BE, Cao X, Wei JT, Rubin MA, Shah RB, Chinnaiyan AM 2006 TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res 66:3396–3400 [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Laxman B, Dhanasekaran SM, Helgeson BE, Cao X, Morris DS, Menon A, Jing X, Cao Q, Han B, Yu J, Wang L, Montie JE, Rubin MA, Pienta KJ, Roulston D, Shah RB, Varambally S, Mehra R, Chinnaiyan AM 2007 Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature 448:595–599 [DOI] [PubMed] [Google Scholar]
- Demichelis F, Fall K, Perner S, Andren O, Schmidt F, Setlur SR, Hoshida Y, Mosquera JM, Pawitan Y, Lee C, Adami HO, Mucci LA, Kantoff PW, Andersson SO, Chinnaiyan AM, Johansson JE, Rubin MA 2007 TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene 26:4596–4599 [DOI] [PubMed] [Google Scholar]
- Nam RK, Sugar L, Yang W, Srivastava S, Klotz LH, Yang LY, Stanimirovic A, Encioiu E, Neill M, Loblaw DA, Trachtenberg J, Narod SA, Seth A 2007 Expression of the TMPRSS2:ERG fusion gene predicts cancer recurrence after surgery for localised prostate cancer. Br J Cancer 97:1690–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soller MJ, Isaksson M, Elfving P, Soller W, Lundgren R, Panagopoulos I 2006 Confirmation of the high frequency of the TMPRSS2/ERG fusion gene in prostate cancer. Genes Chromosomes Cancer 45:717–719 [DOI] [PubMed] [Google Scholar]
- Clark J, Attard G, Jhavar S, Flohr P, Reid A, De-Bono J, Eeles R, Scardino P, Cuzick J, Fisher G, Parker MD, Foster CS, Berney D, Kovacs G, Cooper CS 8 October 2007 Complex patterns of ETS gene alteration arise during cancer development in the human prostate. Oncogene 10.1038/sj.onc.1210843 [DOI] [PubMed] [Google Scholar]
- Clark J, Merson S, Jhavar S, Flohr P, Edwards S, Foster CS, Eeles R, Martin FL, Phillips DH, Crundwell M, Christmas T, Thompson A, Fisher C, Kovacs G, Cooper CS 2007 Diversity of TMPRSS2-ERG fusion transcripts in the human prostate. Oncogene 26:2667–2673 [DOI] [PubMed] [Google Scholar]
- Li H, Zhou J, Miki J, Furusato B, Gu Y, Srivastava S, McLeod DG, Vogel JC, Rhim JS 2008 Telomerase-immortalized non-malignant human prostate epithelial cells retain the properties of multipotent stem cells. Exp Cell Res 314:92–102 [DOI] [PubMed] [Google Scholar]
- Lithgow D, Covington C 2005 Chronic inflammation and breast pathology: a theoretical model. Biol Res Nurs 7:118–129 [DOI] [PubMed] [Google Scholar]
- Yang M, Jiang P, Sun FX, Hasegawa S, Baranov E, Chishima T, Shimada H, Moossa AR, Hoffman RM 1999 A fluorescent orthotopic bone metastasis model of human prostate cancer. Cancer Res 59:781–786 [PubMed] [Google Scholar]
- van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS 2003 Molecular characterization of human prostate carcinoma cell lines. Prostate 57:205–225 [DOI] [PubMed] [Google Scholar]
- Sullivan GF, Amenta PS, Villanueva JD, Alvarez CJ, Yang JM, Hait WN 1998 The expression of drug resistance gene products during the progression of human prostate cancer. Clin Cancer Res 4:1393–1403 [PubMed] [Google Scholar]
- Makarovskiy AN, Siryaporn E, Hixson DC, Akerley W 2002 Survival of docetaxel-resistant prostate cancer cells in vitro depends on phenotype alterations and continuity of drug exposure. Cell Mol Life Sci 59:1198–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rago RP, Einstein Jr A, Lush R, Beer TM, Ko YJ, Henner WD, Bubley G, Merica EA, Garg V, Ette E, Harding MW, Dalton WS 2003 Safety and efficacy of the MDR inhibitor Incel (biricodar, VX-710) in combination with mitoxantrone and prednisone in hormone-refractory prostate cancer. Cancer Chemother Pharmacol 51:297–305 [DOI] [PubMed] [Google Scholar]
- van Brussel JP, Oomen MA, Vossebeld PJ, Wiemer EA, Sonneveld P, Mickisch GH 2004 Identification of multidrug resistance-associated protein 1 and glutathione as multidrug resistance mechanisms in human prostate cancer cells: chemosensitization with leukotriene D4 antagonists and buthionine sulfoximine. BJU Int 93:1333–1338 [DOI] [PubMed] [Google Scholar]
- van Brussel JP, Mickisch GH 2003 Multidrug resistance in prostate cancer. Onkologie 26:175–181 [DOI] [PubMed] [Google Scholar]
- Lee Jr JT, Steelman LS, McCubrey JA 2004 Phosphatidylinositol 3′-kinase activation leads to multidrug resistance protein-1 expression and subsequent chemoresistance in advanced prostate cancer cells. Cancer Res 64:8397–8404 [DOI] [PubMed] [Google Scholar]
- Nomura T, Yamasaki M, Nomura Y, Mimata H 2005 Expression of the inhibitors of apoptosis proteins in cisplatin-resistant prostate cancer cells. Oncol Rep 14:993–997 [PubMed] [Google Scholar]
- Jones HE, Goddard L, Gee JM, Hiscox S, Rubini M, Barrow D, Knowlden JM, Williams S, Wakeling AE, Nicholson RI 2004 Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer 11:793–814 [DOI] [PubMed] [Google Scholar]
- Patterson SG, Wei S, Chen X, Sallman DA, Gilvary DL, Zhong B, Pow-Sang J, Yeatman T, Djeu JY 2006 Novel role of Stat1 in the development of docetaxel resistance in prostate tumor cells. Oncogene 25:6113–6122 [DOI] [PubMed] [Google Scholar]
- Mathew P, Dipaola R 2007 Taxane refractory prostate cancer. J Urol 178:S36–S41 [DOI] [PubMed] [Google Scholar]
- Sims-Mourtada J, Izzo JG, Ajani J, Chao KS 2007 Sonic hedgehog promotes multiple drug resistance by regulation of drug transport. Oncogene 26:5674–5679 [DOI] [PubMed] [Google Scholar]
- van Brussel JP, Jan Van Steenbrugge G, Van Krimpen C, Bogdanowicz JF, Van der Kwast TH, Schroder FH, Mickisch GH 2001 Expression of multidrug resistance related proteins and proliferative activity is increased in advanced clinical prostate cancer. J Urol 165:130–135 [DOI] [PubMed] [Google Scholar]
- Zalcberg J, Hu XF, Slater A, Parisot J, El-Osta S, Kantharidis P, Chou ST, Parkin JD 2000 MRP1 not MDR1 gene expression is the predominant mechanism of acquired multidrug resistance in two prostate carcinoma cell lines. Prostate Cancer Prostatic Dis 3:66–75 [DOI] [PubMed] [Google Scholar]
- Ting HJ, Hsu J, Bao BY, Lee YF 2007 Docetaxel-induced growth inhibition and apoptosis in androgen independent prostate cancer cells are enhanced by 1α,25-dihydroxyvitamin D3. Cancer Lett 247:122–129 [DOI] [PubMed] [Google Scholar]
- Bhangal G, Halford S, Wang J, Roylance R, Shah R, Waxman J 2000 Expression of the multidrug resistance gene in human prostate cancer. Urol Oncol 5:118–121 [DOI] [PubMed] [Google Scholar]
- David-Beabes GL, Overman MJ, Petrofski JA, Campbell PA, De Marzo AM, Nelson WG 2000 Doxorubicin-resistant variants of human prostate cancer cell lines DU 145, PC-3, PPC-1, and TSU-PR1: characterization of biochemical determinants of antineoplastic drug sensitivity. Int J Oncol 17:1077–1086 [DOI] [PubMed] [Google Scholar]
- DiPaola RS, Chenven ES, Shih WJ, Lin Y, Amenta P, Goodin S, Shumate A, Capanna T, Cardiella M, Cummings KB, Aisner J, Todd MB 2001 Mitoxantrone in patients with prostate specific antigen progression after local therapy for prostate carcinoma. Cancer 92:2065–2071 [DOI] [PubMed] [Google Scholar]
- Lavie Y, Fiucci G, Liscovitch M 2001 Upregulation of caveolin in multidrug resistant cancer cells: functional implications. Adv Drug Deliv Rev 49:317–323 [DOI] [PubMed] [Google Scholar]
- Mimeault M 2002 New advances on structural and biological functions of ceramide in apoptotic/necrotic cell death and cancer. FEBS Lett 530:9–16 [DOI] [PubMed] [Google Scholar]
- Wang H, Charles AG, Frankel AJ, Cabot MC 2003 Increasing intracellular ceramide: an approach that enhances the cytotoxic response in prostate cancer cells. Urology 61:1047–1052 [DOI] [PubMed] [Google Scholar]
- Karam JA, Lotan Y, Roehrborn CG, Ashfaq R, Karakiewicz PI, Shariat SF 2007 Caveolin-1 overexpression is associated with aggressive prostate cancer recurrence. Prostate 67:614–622 [DOI] [PubMed] [Google Scholar]
- Oh HY, Lee EJ, Yoon S, Chung BH, Cho KS, Hong SJ 2007 Cholesterol level of lipid raft microdomains regulates apoptotic cell death in prostate cancer cells through EGFR-mediated Akt and ERK signal transduction. Prostate 67:1061–1069 [DOI] [PubMed] [Google Scholar]
- Mimeault M, Pommery N, Henichart JP 2003 New advances on prostate carcinogenesis and therapies: involvement of EGF-EGFR transduction system. Growth Factors 21:1–14 [DOI] [PubMed] [Google Scholar]
- Mimeault M, Moore E, Moniaux N, Henichart JP, Depreux P, Lin MF, Batra SK 2006 Cytotoxic effects induced by a combination of cyclopamine and gefitinib, the selective hedgehog and epidermal growth factor receptor signaling inhibitors, in prostate cancer cells. Int J Cancer 118:1022–1031 [DOI] [PubMed] [Google Scholar]
- Mimeault M, Johansson SL, Venkatraman G, Moore E, Henichart JP, Depreux P, Lin MF, Batra SK 2007 Combined targeting of epidermal growth factor receptor and hedgehog signaling by gefitinib and cyclopamine cooperatively improves the cytotoxic effects of docetaxel on metastatic prostate cancer cells. Mol Cancer Ther 6:967–978 [DOI] [PubMed] [Google Scholar]
- Mimeault M, Venkatraman G, Johansson SL, Moore E, Henichart JP, Depreux P, Lin MF, Batra SK 2007 Novel combination therapy against metastatic and androgen-independent prostate cancer by using gefitinib, tamoxifen and etoposide. Int J Cancer 120:160–169 [DOI] [PubMed] [Google Scholar]
- Nasu K, Tanji N, Nishioka R, Wang J, Yanagihara Y, Ozawa A, Yokoyama M, Sakayama K 2006 Expression of ErbB proteins in human prostate. Arch Androl 52:185–190 [DOI] [PubMed] [Google Scholar]
- Nanni S, Narducci M, Della PL, Moretti F, Grasselli A, De Carli P, Sacchi A, Pontecorvi A, Farsetti A 2002 Signaling through estrogen receptors modulates telomerase activity in human prostate cancer. J Clin Invest 110:219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SM 2004 Estrogens and anti-estrogens: key mediators of prostate carcinogenesis and new therapeutic candidates. J Cell Biochem 91:491–503 [DOI] [PubMed] [Google Scholar]
- Fixemer T, Remberger K, Bonkhoff H 2003 Differential expression of the estrogen receptor β (ERβ) in human prostate tissue, premalignant changes, and in primary, metastatic, and recurrent prostatic adenocarcinoma. Prostate 54:79–87 [DOI] [PubMed] [Google Scholar]
- Torlakovic E, Lilleby W, Berner A, Torlakovic G, Chibbar R, Furre T, Fossa SD 2005 Differential expression of steroid receptors in prostate tissues before and after radiation therapy for prostatic adenocarcinoma. Int J Cancer 117:381–386 [DOI] [PubMed] [Google Scholar]
- Lai JS, Brown LG, True LD, Hawley SJ, Etzioni RB, Higano CS, Ho SM, Vessella RL, Corey E 2004 Metastases of prostate cancer express estrogen receptor-β. Urology 64:814–820 [DOI] [PubMed] [Google Scholar]
- Takase Y, Levesque MH, Luu-The V, El-Alfy M, Labrie F, Pelletier G 2006 Expression of enzymes involved in estrogen metabolism in human prostate. J Histochem Cytochem 54:911–921 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo G, Tortora G, D’Armiento FP, De Rosa G, Staibano S, Autorino R, D’Armiento M, De Laurentiis M, De Placido S, Catalano G, Bianco AR, Ciardiello F 2002 Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin Cancer Res 8:3438–3444 [PubMed] [Google Scholar]
- Bostwick DG, Qian J, Maihle NJ 2004 Amphiregulin expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 93 cases. Prostate 58:164–168 [DOI] [PubMed] [Google Scholar]
- Hernes E, Fossa SD, Berner A, Otnes B, Nesland JM 2004 Expression of the epidermal growth factor receptor family in prostate carcinoma before and during androgen-independence. Br J Cancer 90:449–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger T, Ninck C, Bloch M, Mirlacher M, Koivisto PA, Helin HJ, Mihatsch MJ, Gasser TC, Bubendorf L 2005 Expression patterns of potential therapeutic targets in prostate cancer. Int J Cancer 113:619–628 [DOI] [PubMed] [Google Scholar]
- Bartlett JM, Brawley D, Grigor K, Munro AF, Dunne B, Edwards J 2005 Type I receptor tyrosine kinases are associated with hormone escape in prostate cancer. J Pathol 205:522–529 [DOI] [PubMed] [Google Scholar]
- Shah RB, Ghosh D, Elder JT 2006 Epidermal growth factor receptor (ErbB1) expression in prostate cancer progression: correlation with androgen independence. Prostate 66:1437–1444 [DOI] [PubMed] [Google Scholar]
- Schafer W, Funke PJ, Kunde D, Rausch U, Wennemuth G, Stutzer H 2006 Intensity of androgen and epidermal growth factor receptor immunoreactivity in samples of radical prostatectomy as prognostic indicator: correlation with clinical data of long-term observations. J Urol 176:532–537 [DOI] [PubMed] [Google Scholar]
- Creighton CJ 2007 A gene transcription signature associated with hormone independence in a subset of both breast and prostate cancers. BMC Genomics 8:199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BY, Lin DP, Liu JY, Chang H, Huang PH, Chen YL, Chang HH 2006 A mouse prostate cancer model induced by Hedgehog overexpression. J Biomed Sci 13:373–384 [DOI] [PubMed] [Google Scholar]
- Levitt RJ, Zhao Y, Blouin MJ, Pollak M 2007 The hedgehog pathway inhibitor cyclopamine increases levels of p27, and decreases both expression of IGF-II and activation of Akt in PC-3 prostate cancer cells. Cancer Lett 255:300–306 [DOI] [PubMed] [Google Scholar]
- Attard G, Sarker D, Reid A, Molife R, Parker C, de Bono JS 2006 Improving the outcome of patients with castration-resistant prostate cancer through rational drug development. Br J Cancer 95:767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Grandis JR, Wells A 2006 STAT3 is required but not sufficient for EGF receptor-mediated migration and invasion of human prostate carcinoma cell lines. Br J Cancer 95:164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci A, Gravina GL, Rucci N, Millimaggi D, Festuccia C, Muzi P, Teti A, Vicentini C, Bologna M 2006 Suppression of EGF-R signaling reduces the incidence of prostate cancer metastasis in nude mice. Endocr Relat Cancer 13:197–210 [DOI] [PubMed] [Google Scholar]
- Kharait S, Dhir R, Lauffenburger D, Wells A 2006 Protein kinase Cδ signaling downstream of the EGF receptor mediates migration and invasiveness of prostate cancer cells. Biochem Biophys Res Commun 343:848–856 [DOI] [PubMed] [Google Scholar]
- Normanno N, Gullick WJ 2006 Epidermal growth factor receptor tyrosine kinase inhibitors and bone metastases: different mechanisms of action for a novel therapeutic application? Endocr Relat Cancer 13:3–6 [DOI] [PubMed] [Google Scholar]
- Ross JS, Gray KE, Webb IJ, Gray GS, Rolfe M, Schenkein DP, Nanus DM, Millowsky MI, Bander NH 2005 Antibody-based therapeutics: focus on prostate cancer. Cancer Metastasis Rev 24:521–537 [DOI] [PubMed] [Google Scholar]
- Lau KM, LaSpina M, Long J, Ho SM 2000 Expression of estrogen receptor (ER)-α and ER-β in normal and malignant prostatic epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res 60:3175–3182 [PubMed] [Google Scholar]
- Sgambato A, Camerini A, Faraglia B, Ardito R, Bianchino G, Spada D, Boninsegna A, Valentini V, Cittadini A 2004 Targeted inhibition of the epidermal growth factor receptor-tyrosine kinase by ZD1839 (‘Iressa’) induces cell-cycle arrest and inhibits proliferation in prostate cancer cells. J Cell Physiol 201:97–105 [DOI] [PubMed] [Google Scholar]
- Shenouda NS, Zhou C, Browning JD, Ansell PJ, Sakla MS, Lubahn DB, Macdonald RS 2004 Phytoestrogens in common herbs regulate prostate cancer cell growth in vitro. Nutr Cancer 49:200–208 [DOI] [PubMed] [Google Scholar]
- Sirotnak FM, She Y, Lee F, Chen J, Scher HI 2002 Studies with CWR22 xenografts in nude mice suggest that ZD1839 may have a role in the treatment of both androgen-dependent and androgen-independent human prostate cancer. Clin Cancer Res 8:3870–3876 [PubMed] [Google Scholar]
- Vicentini C, Festuccia C, Gravina GL, Angelucci A, Marronaro A, Bologna M 2003 Prostate cancer cell proliferation is strongly reduced by the epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 in vitro on human cell lines and primary cultures. J Cancer Res Clin Oncol 129:165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Uehara H, Karashima T, Shepherd DL, Killion JJ, Fidler IJ 2003 Blockade of epidermal growth factor receptor signaling in tumor cells and tumor-associated endothelial cells for therapy of androgen-independent human prostate cancer growing in the bone of nude mice. Clin Cancer Res 9:1200–1210 [PubMed] [Google Scholar]
- Mimeault M, Pommery N, Henichart JP 2003 Synergistic antiproliferative and apoptotic effects induced by epidermal growth factor receptor and protein kinase A inhibitors in human prostatic cancer cell lines. Int J Cancer 106:116–124 [DOI] [PubMed] [Google Scholar]
- Mimeault M, Jouy N, Depreux P, Henichart JP 2005 Synergistic antiproliferative and apoptotic effects induced by mixed epidermal growth factor receptor inhibitor ZD1839 and nitric oxide donor in human prostatic cancer cell lines. Prostate 62:187–199 [DOI] [PubMed] [Google Scholar]
- Penne K, Bohlin C, Schneider S, Allen D 2005 Gefitinib (Iressa, ZD1839) and tyrosine kinase inhibitors: the wave of the future in cancer therapy. Cancer Nurs 28:481–486 [DOI] [PubMed] [Google Scholar]
- Sirotnak FM, Zakowski MF, Miller VA, Scher HI, Kris MG 2000 Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res 6:4885–4892 [PubMed] [Google Scholar]
- Sugita S, Kawashima H, Tanaka T, Kurisu T, Sugimura K, Nakatani T 2004 Effect of type I growth factor receptor tyrosine kinase inhibitors on phosphorylation and transactivation activity of the androgen receptor in prostate cancer cells: ligand-independent activation of the N-terminal domain of the androgen receptor. Oncol Rep 11:1273–1279 [PubMed] [Google Scholar]
- Festuccia C, Gravina GL, Angelucci A, Millimaggi D, Muzi P, Vicentini C, Bologna M 2005 Additive antitumor effects of the epidermal growth factor receptor tyrosine kinase inhibitor, gefitinib (Iressa), and the nonsteroidal antiandrogen, bicalutamide (Casodex), in prostate cancer cells in vitro. Int J Cancer 115:630–640 [DOI] [PubMed] [Google Scholar]
- Xie J 2005 Hedgehog signaling in prostate cancer. Future Oncol 1:331–338 [DOI] [PubMed] [Google Scholar]
- Mimeault MM, Parmender PP, Hauke R, Moore E, Henichart JP, Depreux P, Batra SK 21 February 2008 Improvement of cytotoxic effects of mitoxantrone on hormone-refractory metastatic prostate cancer cells by co-targeting epidermal growth factor receptor and hedgehog signaling cascades. Growth Factors 10.1080/08977190801930935 [DOI] [PubMed] [Google Scholar]
- Li LC, Carroll PR, Dahiya R 2005 Epigenetic changes in prostate cancer: implication for diagnosis and treatment. J Natl Cancer Inst 97:103–115 [DOI] [PubMed] [Google Scholar]
- Pasquali D, Rossi V, Bellastella G, Bellastella A, Sinisi AA 2006 Natural and synthetic retinoids in prostate cancer. Curr Pharm Des 12:1923–1929 [DOI] [PubMed] [Google Scholar]
- Perry AS, Foley R, Woodson K, Lawler M 2006 The emerging roles of DNA methylation in the clinical management of prostate cancer. Endocr Relat Cancer 13:357–377 [DOI] [PubMed] [Google Scholar]
- Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK 2001 Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 1:194–202 [DOI] [PubMed] [Google Scholar]
- Marks PA, Richon VM, Miller T, Kelly WK 2004 Histone deacetylase inhibitors. Adv Cancer Res 91:137–168 [DOI] [PubMed] [Google Scholar]
- Koeneman KS 2006 Prostate cancer stem cells, telomerase biology, epigenetic modifiers, and molecular systemic therapy for the androgen-independent lethal phenotype. Urol Oncol 24:119–121 [DOI] [PubMed] [Google Scholar]
- Kuefer R, Hofer MD, Altug V, Zorn C, Genze F, Kunzi-Rapp K, Hautmann RE, Gschwend JE 2004 Sodium butyrate and tributyrin induce in vivo growth inhibition and apoptosis in human prostate cancer. Br J Cancer 90:535–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Wieder R 2004 All-trans retinoic acid potentiates Taxotere-induced cell death mediated by Jun N-terminal kinase in breast cancer cells. Oncogene 23:426–433 [DOI] [PubMed] [Google Scholar]
- Huss WJ, Lai L, Barrios RJ, Hirschi KK, Greenberg NM 2004 Retinoic acid slows progression and promotes apoptosis of spontaneous prostate cancer. Prostate 61:142–152 [DOI] [PubMed] [Google Scholar]
- Appleyard VC, O’Neill MA, Murray KE, Bray SE, Thomson G, Kernohan NM, Varani J, Zhang J, Thompson AM 2004 Activity of MDI-301, a novel synthetic retinoid, in xenografts. Anticancer Drugs 15:991–996 [DOI] [PubMed] [Google Scholar]
- Rephaeli A, Blank-Porat D, Tarasenko N, Entin-Meer M, Levovich I, Cutts SM, Phillips DR, Malik Z, Nudelman A 2005 In vivo and in vitro antitumor activity of butyroyloxymethyl-diethyl phosphate (AN-7), a histone deacetylase inhibitor, in human prostate cancer. Int J Cancer 116:226–235 [DOI] [PubMed] [Google Scholar]
- Camphausen K, Cerna D, Scott T, Sproull M, Burgan WE, Cerra MA, Fine H, Tofilon PJ 2005 Enhancement of in vitro and in vivo tumor cell radiosensitivity by valproic acid. Int J Cancer 114:380–386 [DOI] [PubMed] [Google Scholar]
- Kulp SK, Chen CS, Wang DS, Chen CY, Chen CS 2006 Antitumor effects of a novel phenylbutyrate-based histone deacetylase inhibitor, (S)-HDAC-42, in prostate cancer. Clin Cancer Res 12:5199–5206 [DOI] [PubMed] [Google Scholar]
- Floryk D, Huberman E 2005 Differentiation of androgen-independent prostate cancer PC-3 cells is associated with increased nuclear factor-κB activity. Cancer Res 65:11588–11596 [DOI] [PubMed] [Google Scholar]
- Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E 2006 Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis 27:811–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaiyan P, Varambally S, Tomlins SA, Ray S, Huang S, Chinnaiyan AM, Harari PM 2006 Enhancing the antitumor activity of ErbB blockade with histone deacetylase (HDAC) inhibition. Int J Cancer 118:1041–1050 [DOI] [PubMed] [Google Scholar]
- Jiang AL, Zhang PJ, Chen WW, Liu WW, Yu CX, Hu XY, Zhang XQ, Zhang JY 2006 Effects of 9-cis retinoic acid on human homeobox gene NKX3.1 expression in prostate cancer cell line LNCaP. Asian J Androl 8:435–441 [DOI] [PubMed] [Google Scholar]
- Qian DZ, Ren M, Wei Y, Wang X, van de GF, Rasmussen C, Nakanishi O, Sacchi N, Pili R 2005 In vivo imaging of retinoic acid receptor β2 transcriptional activation by the histone deacetylase inhibitor MS-275 in retinoid-resistant prostate cancer cells. Prostate 64:20–28 [DOI] [PubMed] [Google Scholar]
- Qian DZ, Wei YF, Wang X, Kato Y, Cheng L, Pili R 2007 Antitumor activity of the histone deacetylase inhibitor MS-275 in prostate cancer models. Prostate 67:1182–1193 [DOI] [PubMed] [Google Scholar]
- Gediya LK, Belosay A, Khandelwal A, Purushottamachar P, Njar VC 8 December 2007 Improved synthesis of histone deacetylase inhibitors (HDIs) (MS-275 and CI-994) and inhibitory effects of HDIs alone or in combination with RAMBAs or retinoids on growth of human LNCaP prostate cancer cells and tumor xenografts. Bioorg Med Chem 10.1016/j.bmc.2007.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Koeneman KS, Corey DR 2003 Consequences of telomerase inhibition and combination treatments for the proliferation of cancer cells. Cancer Res 63:5917–5925 [PubMed] [Google Scholar]
- Yamanaka K, Gleave ME, Hara I, Muramaki M, Miyake H 2005 Synergistic antitumor effect of combined use of adenoviral-mediated p53 gene transfer and antisense oligodeoxynucleotide targeting clusterin gene in an androgen-independent human prostate cancer model. Mol Cancer Ther 4:187–195 [PubMed] [Google Scholar]
- Yamanaka K, Rocchi P, Miyake H, Fazli L, So A, Zangemeister-Wittke U, Gleave ME 2006 Induction of apoptosis and enhancement of chemosensitivity in human prostate cancer LNCaP cells using bispecific antisense oligonucleotide targeting Bcl-2 and Bcl-xL genes. BJU Int 97:1300–1308 [DOI] [PubMed] [Google Scholar]
- Pchejetski D, Golzio M, Bonhoure E, Calvet C, Doumerc N, Garcia V, Mazerolles C, Rischmann P, Teissie J, Malavaud B, Cuvillier O 2005 Sphingosine kinase-1 as a chemotherapy sensor in prostate adenocarcinoma cell and mouse models. Cancer Res 65:11667–11675 [DOI] [PubMed] [Google Scholar]
- Leonetti C, Biroccio A, D’Angelo C, Semple SC, Scarsella M, Zupi G 2007 Therapeutic integration of c-myc and bcl-2 antisense molecules with docetaxel in a preclinical model of hormone-refractory prostate cancer. Prostate 67:1475–1485 [DOI] [PubMed] [Google Scholar]
- Suenaga M, Soda H, Oka M, Yamaguchi A, Nakatomi K, Shiozawa K, Kawabata S, Kasai T, Yamada Y, Kamihira S, Tei C, Kohno S 2002 Histone deacetylase inhibitors suppress telomerase reverse transcriptase mRNA expression in prostate cancer cells. Int J Cancer 97:621–625 [DOI] [PubMed] [Google Scholar]
- Ikeda N, Uemura H, Ishiguro H, Hori M, Hosaka M, Kyo S, Miyamoto K, Takeda E, Kubota Y 2003 Combination treatment with 1α,25-dihydroxyvitamin D3 and 9-cis-retinoic acid directly inhibits human telomerase reverse transcriptase transcription in prostate cancer cells. Mol Cancer Ther 2:739–746 [PubMed] [Google Scholar]
- Tang C, Ang BT, Pervaiz S 2007 Cancer stem cell: target for anti-cancer therapy. FASEB J 21:3777–3785 [DOI] [PubMed] [Google Scholar]
- Wang W, Bergh A, Damber JE 2005 Cyclooxygenase-2 expression correlates with local chronic inflammation and tumor neovascularization in human prostate cancer. Clin Cancer Res 11:3250–3256 [DOI] [PubMed] [Google Scholar]
- Gignac GA, Morris MJ, Hussain M 2007 Castration resistant, taxane naive metastatic prostate cancer: current clinical approaches and future directions. J Urol 178:S30–S35 [DOI] [PubMed] [Google Scholar]
- Guerin O, Formento P, Lo NC, Hofman P, Fischel JL, Etienne-Grimaldi MC, Merlano M, Ferrero JM, Milano G 2007 Supra-additive antitumor effect of sunitinib malate (SU11248, Sutent(R)) combined with docetaxel. A new therapeutic perspective in hormone refractory prostate cancer. J Cancer Res Clin Oncol 134:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Hauke R, Batra SK 2007 Stem cells—a revolution in therapeutics. Recent advances on the stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther 82:252–264 [DOI] [PubMed] [Google Scholar]
- Lin VK, Wang SY, Vazquez DV, Xu C, Zhang S, Tang L 2007 Prostatic stromal cells derived from benign prostatic hyperplasia specimens possess stem cell like property. Prostate 67:1265–1276 [DOI] [PubMed] [Google Scholar]
- Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, Sherlock G, Lewicki J, Shedden K, Clarke MF 2007 The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med 356:217–226 [DOI] [PubMed] [Google Scholar]
- Sheffer Y, Leon O, Pinthus JH, Nagler A, Mor Y, Genin O, Iluz M, Kawada N, Yoshizato K, Pines M 2007 Inhibition of fibroblast to myofibroblast transition by halofuginone contributes to the chemotherapy-mediated antitumoral effect. Mol Cancer Ther 6:570–577 [DOI] [PubMed] [Google Scholar]