Abstract
Breast cancer evolution and tumor progression are governed by the complex interactions between steroid receptor [estrogen receptor (ER) and progesterone receptor] and growth factor receptor signaling. In recent years, the field of cancer therapy has witnessed the emergence of multiple strategies targeting these specific cancer pathways and key molecules (ER and growth factor receptors) to arrest tumor growth and achieve tumor eradication; treatment success, however, has varied and both de novo (up front) and acquired resistance have proven a challenge. Recent studies of ER biology have revealed new insights into ER action in breast cancer and have highlighted the role of an intimate crosstalk between the ER and HER family signaling pathways as a fundamental contributor to the development of resistance to endocrine therapies against the ER pathway. The aim of this review article is to summarize the current knowledge on mechanisms of resistance of breast cancer cells to endocrine therapies due to the crosstalk between the ER and the HER growth factor receptor signaling pathways and to explore new available therapeutic strategies that could prolong duration of response and circumvent endocrine resistant tumor growth.
I. Introduction
- II. Biology of the Estrogen Receptor (ER)
- A. ERα and ERβ subtypes
- B. Genomic action of ER
- C. Nongenomic rapid ER activity
- III. The HER Tyrosine Kinase Receptor Family and Its Role in the Development of Endocrine Resistance: Studies in Preclinical Models
- A. HER/ER crosstalk as a mechanism for endocrine therapy resistance: molecular determinants
- B. HER2/ER crosstalk in de novo endocrine resistance models
- C. HER2/ER crosstalk in acquired endocrine resistance models
- IV. The HER Tyrosine Kinase Receptor Family and Its Role in the Development of Endocrine Resistance: Clinical Evidence
- A. De novo endocrine resistance
- B. Progesterone receptor (PgR) negativity associated with endocrine resistance and HER signaling
- C. HER2/ER crosstalk in acquired endocrine resistance
- V. Novel Therapeutic Strategies to Overcome HER/ER Pathway Crosstalk and Endocrine Resistance
- A. Preclinical studies
- B. Clinical studies
VI. Future Challenges
I. Introduction
BREAST CANCER EVOLUTION and progression are deeply influenced by both estrogen receptor (ER) and growth factor receptor signaling. In recent years, the field of cancer therapy has witnessed the emergence of multiple targeted strategies that inhibit specific key molecules and pathways important for tumor growth and progression. Among them, endocrine therapy to block ER activity and signaling, the first targeted therapy in oncology, is still the most successful systemic therapy in the management of ER-positive breast cancer.
Tamoxifen, which binds to and antagonizes ER, has been the mainstay of endocrine (hormonal) therapy in both early and advanced breast cancer patients for almost three decades (1,2). Recently, its role has also expanded to preventive therapy in patients at high risk of developing the disease (3). Unfortunately, however, approximately 50% of patients with advanced disease do not respond to first line treatment with the selective estrogen receptor modulator (SERM) tamoxifen (de novo or up front, intrinsic resistance). Furthermore, almost all patients with metastatic disease and many that receive tamoxifen as adjuvant therapy eventually experience tumor relapse and die from their disease (acquired resistance). Thus, de novo and acquired resistance to tamoxifen occur frequently in breast cancer patients and seriously limit the efficacy of this treatment.
Aromatase inhibitors (AIs) are a class of drugs that inhibit the enzyme aromatase, which is responsible for converting androgens (produced by women in the adrenal glands) to estrogen, thereby lowering the circulating estrogen, and perhaps the tumor levels of estrogen. By depriving ER of its estrogen ligand (4,5,6), AIs inhibit tumor growth and are proving superior to tamoxifen at least in certain patient subsets (2,7,8,9). However, the response rate to these compounds is only slightly higher than the response rate to tamoxifen in patients with advanced breast cancer, and both de novo (i.e., immediate) and acquired resistance after an initial response commonly occur.
The membrane tyrosine kinase HER2 (c-ErbB2, HER2/neu) is gene-amplified in 20–25% of ER-positive breast cancer (10). There is clinical evidence that tamoxifen is less effective in HER2-positive tumors (11,12,13). Furthermore, preclinical models show that HER2 overexpression can cause tamoxifen-stimulated growth as a mechanism of de novo resistance (14,15,16,17) and that the HER family receptors are also implicated in acquired resistance to this drug (18). Advanced studies of ER biology have revealed new insights into ER action in breast cancer and have highlighted the role of an intimate crosstalk between the ER and the epidermal growth factor receptor (EGFR/HER1)/HER2 signaling pathways as a fundamental contributor to the development of resistance to endocrine therapies (19,20,21).
Accumulating knowledge of the mechanisms by which breast cancer cells become resistant to endocrine therapy, coupled with the availability of new compounds that can interfere with the growth factor-driven signaling pathways involved in resistance to endocrine therapy, might lead to the development of new strategies for treatment of ER-positive breast cancer patients (5). The aim of this review article is to summarize the current knowledge on mechanisms of breast cancer resistance to endocrine therapies, particularly those that are due to the crosstalk between the ER and growth factor receptor signaling pathways (focusing on the EGFR/HER2 pathway), and to explore emerging therapeutic strategies that prolong duration of response and circumvent endocrine resistant tumor growth.
II. Biology of the Estrogen Receptor (ER)
A. ERα and ERβ subtypes
The ER signaling pathway and its estrogen ligands are believed to be key players in the etiology and progression of breast cancer. There are two different ER proteins, ERα and ERβ, that are produced by distinct genes. Whereas clinical and experimental studies have confirmed the crucial role of ERα (22) in breast malignancies, the role of ERβ in breast cancer is still controversial (23). Nonetheless, studies indicate that ERβ can antagonize ERα activity (24) and suggest that reduced levels of ERβ protein are associated with resistance to tamoxifen therapy (25). If not otherwise specified, “ER” will refer to “ERα” in the remainder of the manuscript.
B. Genomic action of ER
ER is mainly a nuclear protein that shares a common structural and functional organization with many other nuclear receptors (22). Through its genomic nuclear activity, also known as nuclear-initiated steroid signaling (NISS) (26), ER functions as a ligand-dependent transcription factor and promotes expression of a variety of genes (19). Many of these gene products directly promote breast cancer cell proliferation and survival and tumor progression. Examples are the IGF-I receptor (IGFR), the cell cycle regulator cyclin D1, the antiapoptotic factor Bcl-2 (21,27,28), and the proangiogenic vascular endothelial growth factor (20,29). Nuclear ER also induces the expression of different HER and other growth factor receptor ligands including TGFα and amphiregulin (30), which are able to bind and activate EGFR (31). Recently, microarray analysis of gene expression in the human breast cancer ER-positive MCF-7 cell lines suggests that in response to estrogen, ER is also able to inhibit expression of a subclass of genes (32), many of these being transcriptional repressors, or genes with antiproliferative or proapoptotic function. The ER protein is a composite of multiple domains including a DNA-binding domain and two major transcriptional activation function (AF) domains, AF-1 and AF-2, which usually act synergistically, although some gene promoters have been shown to be activated independently by AF-1 or AF-2 (22,33). The antineoplastic effects of endocrine therapeutic agents are largely mediated by countering or eliminating the transcriptional effects on gene expression of estrogen when bound to ER.
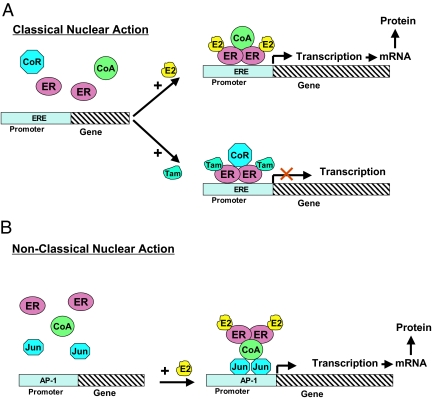
Ligand binding to ER induces a specific conformational change in the receptor, releases it from an inhibitory complex consisting of several chaperone proteins (34), and triggers receptor dimerization (34). This change further facilitates the binding of coregulatory proteins (35) that alter ER transcriptional activity on specific consensus DNA elements [also known as estrogen response elements (EREs)], which are present in the promoter regions of target genes (classic action, Fig. 1A). In particular, the transcriptional activity of ER is enhanced by the binding of coactivators such as members of the p160 family of nuclear receptor coactivators [e.g., nuclear receptor coactivator 1 (NCoA1 or SRC-1), NCoA2 (SRC-2), and NCoA3 (AIB1, SRC-3, TRAM1, RAC3, p/CIP or ACTR) (36,37) (Fig. 1A)]. These proteins lead to the formation of large complexes that enhance ER-driven transcription by different mechanisms, including recruitment of histone-acetyltransferases that modulate the chromatin structure at the promoter site (36).
Figure 1.
Nuclear genomic ER activity. ER, in its classical action (A), directly binds to DNA sequences called estrogen response elements (EREs) residing in the promoter region of target genes, and by recruiting coregulatory proteins regulates gene transcription. Estrogen (E2)-bound ER generally recruits coactivator (CoA) complexes to induce gene transcription, whereas estrogen antagonists such as tamoxifen (Tam) mostly lead to ER association with corepressor (CoR) complexes, thereby turning off gene transcription. In its nonclassical action (B), ER regulates gene transcription via protein-protein interaction (e.g., with Fos/Jun family members) that tether ER to DNA sites responsive to other transcription factors such as AP-1. Together, all of these nuclear ER genomic activities also are called nuclear-initiated steroid signaling (NISS).
In contrast to estrogens, estrogen antagonists induce a distinct receptor conformation leading to ER association with corepressor complexes, such as nuclear-receptor corepressor 1 (NCoR1) and NCoR2 (SMRT), rather than with coactivators, thereby shutting off gene transcription (38,39) (Fig. 1A). Interestingly, SERMs, including tamoxifen and raloxifene, have mixed agonist/antagonist activity and may either stimulate or antagonize ER function depending on the tissue, cell, and gene context (40).
Many coregulatory proteins may be present at rate-limiting levels in the nucleus, so that changes in their level of expression and/or activity can lead to alterations of ER signaling. In particular, overexpression of coactivators and down-regulation of corepressors can negate the inhibitory effects of endocrine therapy, especially in the case of SERMs (41,42,43,44,45). In this regard, recent studies have observed that high AIB1 expression in patients who received tamoxifen adjuvant therapy was associated with an inferior clinical outcome, which is indicative of tamoxifen resistance (12,46).
In addition to the “classical” mode of action of ER regulating the expression of genes that harbor EREs in their promoter region, ER can also regulate gene transcription at DNA sites responsive to other transcription factors (40). Via this so-called “nonclassical” mode, nuclear ER protein interacts with other transcription factors such as specificity protein 1 (SP-1) and members of the Fos/Jun activating protein 1 (AP-1) transcription complex, leading to regulation of gene expression at non-ERE regulatory DNA sequences (Fig. 1B) (40,47,48).
Importantly, signaling from different growth factor receptor-dependent kinases phosphorylates various factors in the ER pathway, including ER itself; this potentiates ER genomic signaling activity on gene transcription. As an example, kinase-induced phosphorylation of nuclear ER on serine 305 (49,50,51) enhances cyclin D1 transcription in breast cancer. Similarly, activation of the growth factor-dependent signaling of p42/44 MAPK (ERK 1/2) and phosphatidyl inositol 3-kinase (PI3K)/AKT leads to an increase in ER serine 118 and serine 167 phosphorylation and ER AF-1 activity (52,53,54). This phosphorylation of ER and its coregulatory proteins by growth factor receptor-dependent kinases is an essential component of the ordinary regulation and function of genomic ER activity. However, in the presence of hyperactive growth factor receptor signaling, as often occurs in breast cancer (e.g., HER2 overexpression), an excessive phosphorylation of ER and its coregulators may severely weaken the inhibitory effects of various endocrine therapies and lead to endocrine resistance, as will be detailed in Sections II and III of this review.
C. Nongenomic rapid ER activity
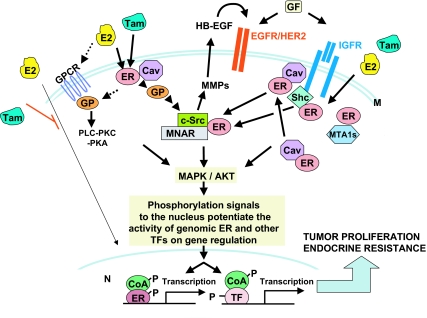
Estrogen, as well as some SERMs like tamoxifen, has also been shown to exert rapid stimulatory effects on a variety of signal transduction pathways and molecules. This rapid nongenomic activity, also called MISS (for membrane-initiated steroid signaling) (26), begins outside the nucleus and is initially independent of gene transcription. The identity and mode of function of the receptors responsible for this steroid-induced rapid signaling are not completely clear at this point. In the case of estrogen, however, it has been shown that this activity is mediated, at least in part, by a small fraction of the traditional ER protein or perhaps by its closely related short-form splicing/translational variants (55,56,57) that are localized near or at the plasma membrane. Cytoplasmic signaling molecules related to growth factor receptor signaling such as the short form variant of metastasis associated gene 1 (17) and the modulator of nongenomic action of estrogen receptor (MNAR) (58) can increase this non-nuclear fraction of ER. Molecular evidence suggests several mechanisms by which non-nuclear/membrane or cytoplasmic ER couples with components of signaling complexes and triggers their responses (59) (Fig. 2). Membrane ER may exist as a cytoplasmic pool tethered to the inner face of the plasma membrane bilayer through binding to membrane proteins of lipid rafts such as caveolin-1 (60,61), flotillin-2 (62), or the caveolin-binding protein striatin (63), or possibly through association with other membrane receptors [e.g., IGFR (64), EGFR (65,66), or HER2 (62,67)], or with signaling adaptor molecules such as Shc (64). Recent laboratory data further suggest that the ER protein within isolated caveolar vesicles typically spreads throughout the cell membrane in a similar fashion to growth factor receptors (60,68,69,70) and assembles as part of a large signalsome complex that includes receptor tyrosine kinases (EGFR, IGFR, HER2 receptor), nonreceptor tyrosine kinases such as Src (71), and G proteins (68,72,73). Additional evidence from endothelial (56,74) and breast cancer (75) cell culture models suggests that estrogen-bound ER coimmunoprecipitates with and/or activates distinct G protein subunits. These interactions trigger secondary downstream signaling pathways including effectors of p21Ras, leading to activation of Raf/Mek/MAPK and of the AKT/PI3K modules.
Figure 2.
Integration of genomic and nongenomic/rapid ER signaling and its crosstalk with growth factor receptor and cell kinase pathways in endocrine resistance: a working model. The ligand estrogen (E2) induces genomic ER activity in the nucleus (N), which results in increased gene transcription, including important genes in the growth factor receptor pathways. The SERM tamoxifen (Tam) antagonizes this activity. However, both estrogen and tamoxifen can turn on nongenomic signaling acting on ER that resides at the membrane (M) and/or cytoplasm (a signaling mode also known as membrane-initiated steroid signaling, or MISS). This induction, in turn, through multiple interactions with signaling intermediate molecules such as Shc and MNAR, can activate growth factor (GF) tyrosine kinase receptors (TKRs), such as EGFR and HER2, and the IGFR and cellular kinases such as c-Src. Cytoplasmic signaling molecules such as metastasis-associated gene 1 (MTA1) can increase this non-nuclear fraction of ER. The interaction between membrane/cytoplasmic ER and TKRs turns on the TKR pathways and their downstream kinases, e.g., p42/44 MAPK and AKT. Other potential MISS activity involves the activation, either directly or indirectly, of G protein (GP)-coupled receptors (GPCRs), which can then trigger various signaling processes including the activation of c-Src and MMPs and subsequent cleavage and release of HB-EGF. The HB-EGF can then stimulate and activate the EGFR/2 signaling pathway. TKR-induced kinases phosphorylate (P) nuclear ER and its coactivators (CoA) as well as other transcription factors (TF), thus potentiating genomic ER activity, which results in enhanced gene expression including genes in the TKR pathways. These gene products in turn further augment GF-TKR signaling, thus completing the cooperative cycle between the two activities of ER and their crosstalk with the growth factor receptor and cellular kinase pathways. In the presence of excessive TKR signaling, such as in HER2-overexpressing tumors, the nongenomic rapid ER action may become more prominent. The resulting activation of downstream kinases can lead to endocrine resistance by modifying the activity of various transcription factors and/or negating the inhibitory effects of tamoxifen on nuclear ER. PKA, Protein kinase A.
More detailed recent work has identified and defined motifs in a specific domain of ER that are critical to membrane localization and function of the receptor (61,76,77). This domain, also known as the E domain, resides in the carboxyl (C) part of ER and harbors the ligand-binding domain and AF-2 function. Mutations in these motifs prevent ER dimerization, association with caveolin-1, and nongenomic ER signaling to activate p42/44 MAPK, PI3K, and cAMP (76,78). Similarly, specific deletions at the caveolin-1 scaffolding domain largely prevent the localization of ER at the plasma membrane (60,76). Interestingly, however, ER interaction with the signaling molecule MNAR involves both the amino (N) terminus and portions of the E domain of ER (79). In contrast, interaction of ER with Shc and striatin has been reported to involve the N terminus of the receptor (64). ER with deleted portions of its N terminus still localizes to the membrane and signals to p42/44 MAPK equivalently to wild-type ER (76), consistent with the idea that it is the E domain at the C terminus of the receptor protein that contains most of the information for both localization and function of ER at the membrane.
Studies in breast cancer culture models have shown that the endogenous membrane ER can directly or indirectly activate EGFR, HER2, and IGFR1 (80). This process involves the sequential activation of the cellular tyrosine kinase Src (81), matrix metalloproteinases (MMPs) 2 and 9, and the release of the EGFR ligand heparin-binding epidermal growth factor-like growth factor (HB-EGF), which, in turn, activates the EGFR downstream kinase cascades (i.e., Ras/Mek/MAPK and PI3K/AKT) (75,76). These downstream activated kinases, consequently, phosphorylate and thereby activate ER and its coregulators, thus augmenting genomic activities of ER on gene transcription (20,52,82) (Fig. 2). The genomic and nongenomic mechanisms of action of ER, therefore, do not appear to be mutually exclusive, but rather are complementary to one another, and many interactions between these two signaling forms exist. These two ER activities also intimately interact at multiple levels with many cellular (growth factor-dependent and other) kinase networks to sustain bidirectional crosstalk that augments signaling of both ER and kinase-related pathways.
In breast cancer, it is becoming evident that this crosstalk of ER with the EGFR/HER2 pathway, and presumably with additional growth factor receptor pathways, plays a very important role not only in the physiological action of ER as well as growth factor receptor signaling, but also in endocrine therapy resistance (42), as will be discussed in the next section.
III. The HER Tyrosine Kinase Receptor Family and Its Role in the Development of Endocrine Resistance: Studies in Preclinical Models
As briefly discussed above, whereas the ER, via its membrane and nuclear activities, up-regulates growth factor signaling, the molecular crosstalk between these two pathways is continuous and bidirectional. Signaling from multiple signal transduction pathways can modulate both the genomic and nongenomic activities of the ER pathway and their ligand dependency. In the section that follows, we will discuss in detail how this crosstalk, especially between the ER and HER family, contributes to endocrine resistance.
A. HER/ER crosstalk as a mechanism for endocrine therapy resistance: molecular determinants
Growth factor signaling plays an important role in the development of both de novo and acquired resistance of breast cancer cells to endocrine manipulation.
It has been demonstrated that ER can be phosphorylated and activated by several intracellular kinases (42,83). In particular, ER is phosphorylated at key residues (including serine 106/107, 118, 167, 305, and threonine 311) residing mainly in the AF-1 domain, after p42/44 MAPK, PI3K/AKT, p90rsk, p21-activated kinase 1 Pak1, protein kinase A, or p38 MAPK pathway activation by various cytokines and growth factors including ligands of EGFR or IGFR (42,49,50,51,52,54,84). ER phosphorylation has been shown to change ER pharmacology and can result in ligand-independent or tamoxifen-mediated activation of the receptor (53,54,85). Phosphorylation of ER coregulators is probably no less important than phosphorylation of ER itself in communicating these signal transduction effects on the ER pathway. Phosphorylation of coactivators, similarly to that of the receptor, enhances the activity of the coactivators themselves on the genomic ER even in the absence of its ligand or in the presence of antiestrogens (42,86). This phosphorylation potentiates the ability of estrogen and SERMs to interact with ER and to recruit other transcriptional coregulators to its transcriptional complex (87); furthermore, it can directly activate their intrinsic enzymatic activities (88). Phosphorylation of corepressors, on the other hand, can result in their export from the nucleus, thereby preventing their access to and inhibition of ER transcriptional complexes in the nucleus (89).
The ER coactivator AIB1 has been shown to be phosphorylated and activated by multiple kinases including MAPKs and other cellular kinases (86,87,90). Two independent recent retrospective studies demonstrate that tumors with high levels of both AIB1 and HER receptors (HER2 or HER3) are less responsive to tamoxifen therapy, probably because of increased estrogen agonistic activity of tamoxifen-bound ER (12,46). Such findings support the hypothesis that increased signaling from the HER family activates downstream kinases, which in turn activates ER and AIB1, increasing transcriptional activity, including in the presence of tamoxifen.
Finally, membrane functions of ER appear to depend not only on ER, but also on the levels of growth factor receptors and their ligands (14,17). This mode of ER signaling might, therefore, be predominant in breast cancer cells that express high levels of tyrosine kinase receptors such as EGFR and HER2 (Fig. 2). Importantly, it has also been suggested that SERMs such as tamoxifen may behave as estrogen agonists for these membrane effects of ER (14,20).
B. HER2/ER crosstalk in de novo endocrine resistance models
The involvement of HER2 in de novo resistance of breast cancer cells to tamoxifen has long been hypothesized (67,91). In the BT474 HER2-overexpressing breast cancer model, HER2 signaling has been shown to induce resistance to tamoxifen by inhibiting the apoptotic effects of the drug (15). Most recently, using the MCF7/HER2–18 model, which is a derivative line of MCF7 cells that stably overexpresses endogenous AIB1 and exogenous HER2, Shou et al. (14) demonstrated that in a low estrogen environment, tamoxifen acts as a potent agonist on tumor growth. In both the above HER2-positive breast cancer cell lines, both estrogen and tamoxifen induce rapid (nongenomic) activation of EGFR/HER2 signaling, which leads to activation of both p42/44 MAPK and AKT signal transduction pathways. These intracellular kinases, as shown in the MCF7/HER2–18 cells, then phosphorylate and functionally activate both ER and the coactivator AIB1. Furthermore, culture of these MCF-7/HER2–18 cells under short-term tamoxifen treatment increases the expression of estrogen-regulated genes nearly as well as estradiol itself. This phenomenon is due to the ability of tamoxifen-ER complexes to recruit coactivators such as AIB1 rather than corepressors to ER-targeted promoters in these HER2-overexpressing cells. Interestingly, all of these phenomena could be blocked by treatment with the selective EGFR-tyrosine kinase inhibitor (TKI) gefitinib (Iressa; AstraZeneca, Macclesfield, Cheshire, UK), which can block signaling from EGFR/HER2 heterodimers, suggesting that EGFR/HER2 signaling is directly involved in the growth-promoting activity of tamoxifen in HER2-overexpressing cells. In this respect, gefitinib was highly effective in inhibiting the tamoxifen-induced growth of MCF-7/HER2–18 cells, whereas it had only a modest effect on estrogen-induced growth (14). The above-mentioned findings are in agreement with the clinical observations noted earlier indicating that tumors that coexpress HER2 and AIB1 have poor outcome when treated with tamoxifen (12,46).
C. HER2/ER crosstalk in acquired endocrine resistance models
Recent laboratory and clinical studies have shown that acquired resistance to tamoxifen in tumors that originally express low levels of EGFR and HER2 is also associated with increased EGFR/HER2 signaling, including HER2 gene amplification (92,93). Experimental evidence by Nicholson and Gee’s group (94) has demonstrated that EGFR/HER2 signaling is involved in acquired tamoxifen resistance of breast cancer cells in long-term culture. These cells show increased levels of expression of EGFR and HER2, increased activation of EGFR/HER2 heterodimers, and increased phosphorylation of p42/44 MAPK, AKT, and nuclear ER (on serine residues 118 and 167) (95,96). As in the de novo experimental models of tamoxifen resistance, the growth of these cells after acquiring resistance is significantly inhibited by treatment with the EGFR/HER2 inhibitor gefitinib or the monoclonal anti-HER2 antibody trastuzumab (Herceptin; Genentech, Inc., South San Francisco, CA) (96,97). Another tyrosine kinase receptor, the IGFR, has also been associated with tamoxifen resistance. In fact, it has recently been reported that IGF-II treatment activates both IGFR and EGFR/HER2 in tamoxifen-resistant cells (98). Taken together, these findings suggest that enhanced growth factor signaling, which up-regulates both the genomic and nongenomic activities of ER, is a key contributor to the mechanism of acquired resistance to tamoxifen.
Enhanced expression of EGFR and HER2, together with subsequent downstream activation of signaling pathways regulated by p42/44 MAPK, has also been identified in breast cancer cell models that have become resistant over time to estrogen depletion or to AI therapy (99,100,101). Interestingly, MCF-7 cells adapted to grow in the presence of the potent antiestrogen fulvestrant also show increased EGFR signaling, suggesting that growth factor receptors play central roles in resistance to various endocrine therapies such as AIs and pure ER antagonists in addition to tamoxifen (94,102,103).
IV. The HER Tyrosine Kinase Receptor Family and Its Role in the Development of Endocrine Resistance: Clinical Evidence
A. De novo endocrine resistance
Cumulative clinical data suggest that patients with HER2- and EGFR-overexpressing tumors have a poorer outcome when treated with tamoxifen (13,104,105). In a subset of patients with metastatic breast cancer, results of published studies have been somewhat inconsistent due to different study designs, different techniques for measuring HER2, and the relatively small numbers of patients included in each study. Despite this heterogeneity, a recently published meta-analysis examining the interaction between HER2 expression and response to endocrine treatment in metastatic disease clearly shows that HER2-positive breast cancer is less responsive to endocrine treatment (13). EGFR generates similar, although not identical, downstream signals as HER2. In metastatic breast cancer patients, EGFR overexpression is also predictive of a decreased benefit from tamoxifen (18,106). In a recent study (104), tumors with higher EGFR were less likely to respond to tamoxifen, and these patients had a significantly shorter time to treatment failure. Even when ER and progesterone receptor (PgR) levels were taken into consideration, EGFR remained predictive of a less sustained response, supporting the hypothesis that signaling from other HER family members besides HER2 can also contribute to the development of tamoxifen resistance.
In patients with early breast cancer, several studies suggest that patients with tumors overexpressing HER2 may derive less benefit from adjuvant tamoxifen than those with HER2-negative tumors (107) and that HER2 expression is a risk factor for tamoxifen failure (108). However, this is not a universal finding, probably because in many adjuvant studies chemotherapy use may have obscured the interaction (109,110). The difficulty in making a conclusive judgment is illustrated by data from the collection of samples from the NATO and Cancer Research Campaign adjuvant breast cancer trials with 2 yr of tamoxifen vs. no treatment (111). In this study, the relative risk of recurrence for tamoxifen was 0.54 for patients negative for both HER2 and EGFR and 1.17 for HER2-positive and/or EGFR-positive patients. However, despite the big difference in the relative risk of recurrence between the different patient groups and the more than 800 patients in the study overall, the small size of the EGFR-/HER2-positive tumor group resulted in se estimates that overlapped with those of the HER-negative population. A positive association between overexpression of HER family receptors (EGFR and/or HER2 and/or HER3) and tamoxifen outcome has recently been shown in two additional independent datasets of patients treated with adjuvant tamoxifen (112,113).
At present there are no clear outcome data related to HER2 status from adjuvant trials of AIs. Data on the influence of HER2 status on the relative benefits of tamoxifen and the AI letrozole as adjuvant therapy in the Breast International Group (BIG) 1-98 trial (114,115) are, therefore, of considerable interest. Both preliminary and updated reports indicated that HER2-positive status was associated with significantly higher relapse rate in the BIG 1-98 trial, regardless of whether letrozole or tamoxifen was used. Additional studies are currently underway to determine the significance of HER2 status in the large randomized adjuvant ATAC (Arimidex, Tamoxifen, alone or in combination) trial comparing the AI anastrozole, the SERM tamoxifen, and the combination (116).
Neoadjuvant trials conducted in women with locally advanced breast cancer before surgery provide a unique opportunity to integrate the molecular determinants of response and resistance with the clinical response of primary breast cancer to medical therapy. Results in the neoadjuvant setting are less controversial and provide solid evidence for the role of HER2 and to a lesser extent of EGFR in tamoxifen resistance (Table 1). Ellis et al. (117), in a neoadjuvant study that randomized for treatment with the AI letrozole vs. tamoxifen, provided a context to study in further detail the relationship between EGFR and HER2 expression and response to AIs vs. tamoxifen. In this report, biopsy-confirmed ER-positive and/or PgR-positive cases that received letrozole had a statistically significant better response rate compared with those receiving tamoxifen (60 vs. 41%, respectively). In retrospective analysis, differences in response rates between the two drugs were most marked for the ER-positive tumors that were also positive for EGFR and/or HER2 (88 vs. 21%), suggesting that EGFR and HER2 signaling through ER is ligand-dependent (estrogen or tamoxifen) and that the growth-promoting effects of these receptor tyrosine kinases on ER-positive breast cancer can be inhibited by potent estrogen deprivation therapy.
Table 1.
Response to estrogen deprivation/SERMs in the neoadjuvant setting
In a different neoadjuvant study in locally advanced breast cancer patients, Zhu et al. (118) analyzed levels of HER2 expression before and after AI treatment and correlated them to the clinical outcome of patients. Using both immunohistochemistry and fluorescence in situ hybridization (FISH), data from this study showed that response to the treatment was significantly influenced by HER2 status, with a response rate of 75% for HER2-positive and 35% for HER2-negative tumors (P = 0.017). In addition, the response rate was also significantly affected by the decrease in HER2 status after the treatment, with a response rate of 73% for tumors showing decreased HER2 overexpression and 38% for tumors showing no change in HER2 expression (P = 0.037).
Most recently, Smith and associates (11,107) carried out a series of biomarker studies in the IMPACT neoadjuvant trial, a double-blind study that randomized 330 patients to 3-month treatment with the AI anastrozole or tamoxifen or a combination of the two agents. The primary marker of biological efficacy in this study was the nuclear proliferation antigen Ki67 (119). Of particular importance, it was shown that suppression of Ki67 was greater at both 2- and 12-wk timepoints when using anastrozole compared with either tamoxifen or the combination, confirming the results obtained in the earlier ATAC trial (7) in which the superiority of anastrazole over both tamoxifen and the combination was also demonstrated. This study indicates that analysis of Ki67 may be considered a useful marker of early response or resistance to endocrine agents. Further subanalysis of the IMPACT study investigated the effect of ER, PgR, and HER2 on the antiproliferative effects of, and the clinical response to, the agents. Only four patients were EGFR-positive and ER-positive, so the analysis was confined to the 34 patients who were both HER2- and ER-positive. Seven of 12 (58%) HER2-positive patients responded to anastrazole compared with two of nine (22%, P = 0.09) to tamoxifen and four of 13 (31%) to the combination (11). In the HER2-negative population, responses were 28 of 68 (41%) for anastrazole, 31 of 73 (43%) for tamoxifen, and 33 of 64 (52%) for the combination. Thus, although the numbers of patients are small in this subgroup analysis, in the HER2-positive group, the data tend to support the findings of the letrozole study (117). Regarding Ki67, in the overall population, there was marginally greater Ki67 suppression in the HER2-negative group than in the HER2-positive group after 2 wk, and this difference seemed to be confined to the group treated with tamoxifen (120). At 12 wk, there was a statistically significant greater suppression by anastrazole of Ki67 in the HER2-negative group compared with the HER2-positive group: mean Ki67 suppression in the HER2-positive group was 45% and in the HER2-negative group was 85%. This contrasts with the good clinical response witnessed in this small HER2-positive group of patients and may suggest that resistance dependent on HER2 signaling might rapidly emerge as was reported in a recent preclinical model (102). Furthermore, a recent follow-up report by Ellis et al. (121) also supports the idea that in ER-positive, HER2-positive tumors, estrogen-independent signaling that leads to increased proliferation is present even during therapy with AIs. Therefore, in such tumors, although estrogen deprivation may be highly effective initially, the addition of HER signaling inhibitors may be needed for a sustained response.
B. Progesterone receptor (PgR) negativity associated with endocrine resistance and HER signaling
Progesterone regulates cell growth in normal breast tissue and in the uterus. The PgR, which is transcriptionally up-regulated as a downstream effect of activated ER, plays an important role in mammary growth and development, especially during pregnancy. Its role in breast cancer is somewhat less established than that of ER, but epidemiological, experimental, clinical, and molecular data suggest that PgR signaling plays a critical role in breast cancer development and progression (122,123,124,125,126). Numerous studies have identified different mechanisms of PgR activation resembling ER signaling, including ligand-dependent and ligand-independent activation (124,125,126,127). Ligand-dependent action is based on PgR functioning as a classical ligand-activated transcription factor in the nucleus (genomic action), and PgR can up-regulate the expression of various genes involved in cell proliferation, survival, and tumor progression, including cyclin D1, Bcl2, and vascular endothelial growth factor. Progesterone action is also based on PgR activating p42/44 MAPK pathways by direct interaction with c-Src-family tyrosine kinases, an event that requires progesterone binding to PgR and occurs in the cytoplasm and/or in association with cell membranes (nongenomic or MISS action) (124,127).
Intriguing preclinical and clinical studies have recently suggested that PgR negativity in ER-positive breast cancer may be a marker of hyperactive growth factor signaling (128) rather than a result of a nonfunctional ER signaling pathway, as previously suggested (129). Recent laboratory studies suggest that growth factors in the IGF and EGF families that activate the PI3K-AKT-mTOR (mammalian target of Rapamycin) pathway can reduce expression of PgR at its transcriptional (mRNA) level (129). Alternatively, studies from Lange et al. (130,131) have demonstrated a coupling between transcriptional hyperactivity of PgR and increased PgR protein turnover. Accordingly, HER downstream kinases, such as p42/44 MAPK and cyclin-dependent kinase 2, which phosphorylate PgR and its accessory proteins and dramatically increase PgR transcriptional activity including in the presence of low levels of progesterone, also greatly increase PgR turnover, resulting in PgR-low or PgR-negative tumors (128,129,132,133,134,135). Finally, increased ligand-independent activation and transcriptional hypersensitivity of PgR associated with breast cancer cell growth have also been recognized as deriving from the phosphorylation of PgR under EGFR/EGF signaling (134).
These molecular data, in concert with clinical findings that follow, suggest that loss of PgR expression in some tumors, at the mRNA and/or the protein level, may be due to increased growth factor receptor activity potentially leading to endocrine therapeutic resistance. Although more studies are needed on this topic, these results suggest that suppressed/reduced PgR levels may derive from and indicate hyperactivity in the signaling cascade generated by EGFR, HER2, and other kinase activation.
Studies in the clinical setting in patients with metastatic breast cancer indicate that PgR is a predictive factor for the outcome of endocrine therapy (136). However, there is still no definitive evidence on the predictive role of PgR in women with early breast cancer receiving adjuvant endocrine therapy. The overview data from 2005 (137) shows a 41% reduction in recurrence in ER-positive, PgR-poor patients receiving about 5 yr of tamoxifen compared with a 40% reduction in the ER-positive, PgR-positive patients. This suggests that there is no underperformance of tamoxifen in ER-positive, PgR-negative patients, although this data set originally suggested benefit from tamoxifen in patients with ER-negative tumors, raising questions about receptor assay quality. The overview data conflict with those from a large, albeit nonrandomized series, where biochemical ER and PgR assays were identically performed in two different central laboratories and in which benefit from tamoxifen was substantially less in PgR-negative tumors (138).
Many clinical studies have confirmed in both metastatic and early breast cancer that the benefit of tamoxifen is less in ER-positive/PgR-negative vs. ER-positive/PgR-positive tumors (138,139,140,141,142). A retrospective analysis from the ATAC trial showed that patients with ER-positive/PgR-positive tumors had a lower recurrence rate than those with ER-positive/PgR-negative tumors (7.6 vs. 14.8%) (143,144). This difference, however, was largely due to the reduced efficacy of tamoxifen in the subgroup of patients with ER-positive/PgR-negative tumors. In contrast, there was little difference in the recurrence rate with anastrozole in the PgR-positive vs. PgR-negative subsets. The observation that patients with ER-positive/PgR-negative tumors respond nearly as well to the AI as those with PgR-positive tumors suggests that the ER signaling pathway is functional in many ER-positive/PgR-negative tumors and that these tumors are still dependent on estrogen for growth despite somewhat lower ER levels. Importantly however, a recently updated conference report of this trial, analyzing the differential response of PgR-negative tumors to AIs vs. tamoxifen only in specimens with centrally reviewed steroid receptor expression, failed to confirm the selective benefit of AIs vs. tamoxifen in this group of tumors. A worse outcome was, nonetheless, demonstrated in patients with PgR-negative tumors, regardless of the endocrine therapy used (145). A similar interaction between PgR-negative status and endocrine resistance in the adjuvant tamoxifen setting was also found in a recent study in an independent group of patients (112). Finally, a report from the BIG 1-98 trial that compared letrozole and tamoxifen with sequences of each agent, while confirming the finding that patients with PgR-negative tumors have a worse clinical outcome, failed to demonstrate an effect of PgR expression on the relative efficacy of letrozole over tamoxifen (114). Whether PgR negativity is a predictive factor for poor clinical outcome on endocrine therapy or a prognostic factor, as recently suggested by RT-PCR-based assays (146), remains controversial and needs to be further investigated.
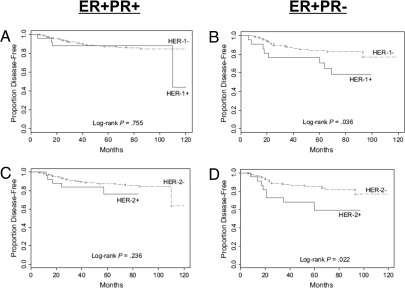
A recent retrospective analysis from Arpino et al. (147) considered a large number of patients with tumor receptor assays all performed centrally by standardized techniques; this work provides clues to the origin of the distinct ER-positive/PgR-negative phenotype. PgR-negative tumors have more aggressive features: they are larger, have more nodal metastases, are more likely to be aneuploid, and are more rapidly proliferating. Interestingly, PgR-negative tumors are also associated with a significantly higher frequency of HER2 overexpression and high expression of EGFR. About 30% of ER-positive, PgR-negative tumors are HER2- and/or EGFR-positive compared with only 10% of ER-positive, PgR-positive tumors (147). As indicated in the preclinical setting, several recent clinical reports also suggest that high growth factor receptor activity may be associated with reduced PgR levels in breast cancer (148,149,150,151). For example, tamoxifen-treated women with ER-positive/PgR-negative and either EGFR or HER2-positive tumors are found to have a significantly worse outcome than patients in the same subgroup whose tumors display low EGFR or HER2. In contrast, deleterious effects of high EGFR or HER2 are less apparent in patients with ER-positive/PgR-positive tumors (147) (Fig. 3). One possible explanation is that less active EGFR and HER2 signaling despite receptor overexpression occurs in ER-positive/PgR-positive tumors and results in a smaller impact on response to tamoxifen. In this context, PgR loss may be a marker of active EGFR/HER2 signaling networks in these HER2-positive tumors.
Figure 3.
Kaplan-Meier curves for disease-free survival (DFS) in tamoxifen-treated patients according to the HER family receptor and PgR status. Among tamoxifen-treated patients with ER+/PR+ tumors, neither EGFR (HER1) nor HER2 had a significant effect on DFS, although there was a suggestion of a trend in HER2-positive tumors. In contrast, among patients whose tumors were ER+/PR−, both EGFR and HER2 expression were associated with significantly poorer DFS (for HER-1, HR = 2.4, 95% CI = 1.0 to 5.4, P = 0.04; and for HER2, HR = 2.6, 95% CI = 1.1 to 6.0, P = 0.03). [Adapted with permission of Oxford University Press from Arpino et al. (147).]
C. HER2/ER crosstalk in acquired endocrine resistance
Recent provocative clinical studies document that acquired resistance to tamoxifen may also be associated with an increase in HER2 expression and/or gene amplification as shown in preclinical models. One study analyzed the changes in growth factor receptors and their associated downstream kinases in tumors after the development of tamoxifen resistance (92). A tissue microarray was constructed of pairs of samples from the same patient, first at presentation, and second at the development of tamoxifen resistance during adjuvant therapy. A total of 39 patients had sufficient tissue in both samples on the arrays to provide near-complete sets of data. Pretreatment, strong positive correlations between ER, PgR, and Bcl-2, and an inverse correlation between ER and HER2, were found as expected. These correlations were lost in the tamoxifen-resistant tumors and replaced by strong correlations between ER and phosphorylated (p) p38 MAPK (p-p38) and phosphorylated p42/44 MAPK (p-p42/44 MAPK). ER expression was lost in 17% of resistant tumors. Three (11%) of the 26 tumors originally negative for HER2 became amplified and/or overexpressed at resistance. All ER-positive tumors that overexpressed HER2 originally or at resistance expressed high levels of p-p38. In the pretreatment and tamoxifen-resistant specimens, there were strong correlations between p-p38 and p-p42/44 MAPK. Therefore, the molecular pathways driving tumor growth can change as the tumor progresses, and the HER family may play an important role in the development of acquired tamoxifen resistance.
Acquired HER2 gene amplification during cancer progression after tamoxifen adjuvant therapy has also been recently reported in patients’ circulating tumor cells (93). Similarly, serum HER2 conversion from negative to positive has also been shown in patients with advanced disease at the time of disease progression on endocrine therapy (152). These data suggest that acquired HER2 overexpression can occur during endocrine treatment, perhaps as an adaptive mechanism for tumor cell survival on these therapies or as a consequence of reversing ER-induced down-regulation of HER expression by endocrine therapy (152).
At present, there are no biological data on samples derived at relapse from patients on AIs. This is an area of substantial clinical importance over the next few years, because these agents are more frequently used in the adjuvant setting. Sampling tumors for molecular studies before and after treatment will enhance our understanding of the mechanisms of response and resistance to these therapies (11,116).
V. Novel Therapeutic Strategies to Overcome HER/ER Pathway Crosstalk and Endocrine Resistance
A. Preclinical studies
Treatment with various growth factor receptor inhibitors and other signal transduction inhibitors (STIs) has been used in preclinical tumor models as an attempt to block growth factor signaling pathways (96,153,154,155,156). However, several reports have shown that in hormone-sensitive, ER-positive breast cancer, these inhibitors as monotherapy (used one at a time) may have only a minimal effect on tumor growth (14,102,157,158). As discussed above, during prolonged endocrine therapy, adaptative changes occur such as up-regulation of growth factor signaling (96,153,154,155,156), which may lead to endocrine resistance. Thus, strategies to combine endocrine therapies with STIs against growth factor receptor downstream signaling molecules such as farnesyltransferase inhibitors (directed against the Ras signaling pathway) and mTOR inhibitors (159,160), have been used in hopes of preventing resistance and improving therapeutic efficacy of endocrine agents (83).
In vitro, the combination of tamoxifen and the selective EGFR TKI gefitinib provided nearly complete inhibition of p42/44 MAPK and AKT phosphorylation, greater suppression of the cell-survival protein Bcl-2, and more complete G0/G1 cell-cycle arrest than that observed with tamoxifen alone (97). In particular, this combined therapy prevented acquired EGFR/p42/44 MAPK signaling and significantly delayed the subsequent resistance that started to develop after 5 wk in tamoxifen-alone-treated cells. For de novo tamoxifen-resistant breast cancer models made by stable transfection of HER2, the strategy of combining endocrine therapy with different HER inhibitors or other STIs is also more effective than using either therapy alone (14,154,157). A synergistic effect has also been reported for the anti-HER2 antibody trastuzumab combined with tamoxifen in ER-positive, endogenously HER2-positive BT474 breast cancer cells, with enhanced accumulation of cells in G0/G1 and a reduction in S-phase compared with either therapy alone (161). Recently, the dual EGFR/HER2 TKI lapatinib (Tykerb, GW57016; GlaxoSmithKline, Brentford, UK) has been shown to cooperate with tamoxifen and other endocrine agents to provide more rapid and profound cell-cycle arrest than either therapy alone in endocrine-resistant cells (162,163).
In vivo, it has been shown that gefitinib added to tamoxifen can completely overcome the agonist activity of tamoxifen and significantly delay the growth of stably transfected HER2-positive MCF-7 xenografts (14). Similar beneficial effects were seen with gefitinib combined with estrogen deprivation. The combination more effectively inhibited growth and delayed the acquired resistance that develops rapidly with estrogen deprivation alone (102).
Finally, data from a more recent study (157) demonstrated in both MCF7/HER2–18 and BT474 ER-positive, HER2-positive xenograft tumors that a combination of several HER inhibitors designed to completely inhibit signaling from all HER dimer pairs, together with, where applicable (MCF7-HER2–18), either tamoxifen or estrogen deprivation, is much more effective in inducing apoptosis and slowing proliferation than each individual drug. Multidrug anti-HER combinations such as those used in the above study can completely eradicate tumors in a substantial number of mice. These provocative data suggest that one type of resistance to the HER inhibitors used currently in the clinic is incomplete blockade of downstream signals generated by the various homo- and heterodimers of the HER family, and also suggest that tumors, to survive, can switch or use an alternative HER dimer pathway when the pathway being used by the cells is blocked. Importantly, however, in one of the above two ER-positive, HER2-positive xenograft models, this potent combination anti-HER therapy was only modestly effective when implemented without endocrine therapy, supporting the concept that initial complete blockade of all HER dimer pairs, together with ER blockade, may be necessary for optimal treatment in some breast tumors.
B. Clinical studies
Taken together, the experimental data indicate that various growth factor receptor and other intracellular signaling pathways may be activated or overexpressed in breast cancer, especially in endocrine-resistant cells, and suggest that targeting such pathways simultaneously with the ER pathway may be an effective therapy.
Proof of principle for therapies that specifically target growth factor pathways in breast cancer has already been provided with the HER2 monoclonal antibody trastuzumab (Herceptin). Successful clinical use of trastuzumab has been demonstrated in HER2-positive breast cancer, and significant activity has been seen as first-line monotherapy, with up to a 48% clinical benefit rate in HER2-positive tumors and a 34% objective response rate in tumors reported positive for HER2 amplification by fluorescence in situ hybridization (164). Thus, it is hoped that various small molecule TKIs and STIs might also prove effective anticancer strategies in the appropriate setting. Early phase II clinical studies have been initiated with EGFR/HER2 TKIs, farnesyltransferase inhibitors, and more recently mTOR antagonists, as monotherapy in patients with advanced (often heavily pretreated) disease; such studies, however, have been somewhat disappointing, with low clinical response rates and rapid disease progression (83).
The EGFR inhibitor gefitinib, an orally active, low-molecular-weight, selective EGFR-TKI, has been studied in patients with breast cancer. There have been several phase II monotherapy studies of gefitinib in patients with advanced breast cancer (165,166,167). Overall, the data are relatively disappointing. The only trial in the metastatic setting to report a significant number of responses includes patients with ER-positive tumors progressing on tamoxifen (167), a setting in which preclinical models have shown the best evidence of activity for gefitinib. Pharmacodynamic studies performed in one of these trials confirmed that EGFR tyrosine kinase signaling is inhibited in both skin and tumor biopsies by the doses of gefitinib delivered orally (166). However, discordance in the effect of gefitinib on downstream intracellular signaling in treated tumor biopsies has been noted, with lack of inhibition of Ki67 in tumors but not in matched skin biopsies. This suggests that activation of other intracellular pathways downstream of EGFR (especially in breast cancer as opposed to normal skin cells) may determine the clinical response to gefitinib. More research is required to establish tumor phenotypes and specific predictive biomarkers in responding vs. nonresponding patients (168). Indeed, a recent neoadjuvant trial that randomized ER-positive, EGFR-positive patients to gefitinib plus the AI anastrazole or to gefitinib alone (169) demonstrated greater reduction in Ki67 with the combination. Fewer clinical data exist regarding other EGFR-TKIs in breast cancer, although a phase II monotherapy trial of the selective EGFR TKI erlotinib (OSI-774) in breast cancer has proven relatively disappointing (170).
As mentioned above, cooperative activation of different growth factor receptors, and especially between HER receptors (EGFR/HER1-HER4), may limit the efficacy of targeting just one single receptor (157). Lapatinib is a potent dual inhibitor of both EGFR and HER2 and blocks autophosphorylation of both receptors (171,172,173). As noted above, it was effective with tamoxifen in cell culture studies of hormone-resistant cells (162,163,171). In phase I/II clinical studies, clinical activity was reported in heavily pretreated patients with EGFR-positive and/or HER2-posistive tumors and in trastuzumab-resistant breast cancer patients (172,173,174,175), and diarrhea and skin rash were the main toxicities. Several biological and pharmacological reasons may account for the efficacy of a small molecule inhibitor of HER2 in patients resistant to therapy with trastuzumab. A phase II trial of lapatinib has been completed in heavily pretreated patients with advanced breast cancer that progressed on prior trastuzumab-containing regimens. A recent analysis of the first 41 patients confirmed clinical activity for lapatinib in breast cancer, with partial response in 7% of patients and/or stable disease in 24% of patients after 16 wk of therapy (174).
Based on the preclinical and clinical evidence suggesting benefit, several phase II/III trials have been initiated with TKIs or monoclonal antibodies in combination with tamoxifen, fulvestrant, or AIs (Table 2). Some of these trials are in the second-line setting, including patients whose tumors were progressing on tamoxifen, and then lapatinib was added to tamoxifen to see whether clinical responses could be observed and resistance reversed. The majority, however, are randomized phase II studies with only 100–200 patients, and in some studies the primary efficacy endpoint is objective response rate rather than time to progression. Such studies are asking whether the combination may provide greater initial antineoplastic activity than endocrine therapy alone, expecting to enhance or restore the response in tumors with de novo endocrine resistance. However, given the preclinical data, prolongation of time to progression (i.e., delaying resistance onset) rather than response rate might be a better endpoint for these trials.
Table 2.
Selected recent ongoing and completed phase II/III clinical trials of inhibitors of HER family receptors in combination with endocrine therapy
| Trial design | Disease setting | Trial phase | Primary endpoints | Outcomes |
|---|---|---|---|---|
| Gefitinib | ||||
| Tamoxifen ± gefitinib | Metastatic | II RCT | TTP | Completed |
| Anastrazole ± gefitinib (178) | Neoadjuvant | II RCT | ORR | NS ORR favoring the AI alone instead of the combination |
| Gefitinib ± anastrazole (169)a | Neoadjuvant | II RCT | ↓ Ki67 labeling index | ↓ Ki67 labeling index 98% vs. 92.4% |
| ↓ in tumor size | ↓ in tumor size no difference | |||
| Gefitinib ± anastrazole | Metastatic | II RCT | TTP | Ongoing |
| Gefitinib + anastrazole vs. gefitinib + fulvestrant | Metastatic | II RCT | ORR | Ongoing |
| Lapatinib | ||||
| Lapatinib + tamoxifen | Metastatic 2nd line | II | ORR/CBR | Ongoing |
| Lapatinib ± letrozole | Metastatic | III RCT | TTP | Ongoing |
| Lapatinib ± fulvestrant | Metastatic | II RCT | ORR/PK | Ongoing |
| Trastuzumab | ||||
| Trastuzumab + letrozole (176) | Metastatic | II | ORR/TTP | ORR 26% |
| TTP 5.5 months | ||||
| Anastrazole vs. anastrazole + trastuzumab (177) | Metastatic | III | ORR/TTP | TTP 2.4 months vs. 4.8 months |
| ORR 6.8% vs. 20.3% | ||||
| Trastuzumab + exemestane | Metastatic | II | TTP | Completed |
RCT, Randomized controlled trial; ORR, objective response rate; CBR, clinical benefit rate; NS, not significant; Tam, tamoxifen; PK, pharmacokinetics. Also see information in Johnston et al. (83).
Only in EGFR-positive patients.
The first safety and efficacy data for the combination of an AI (letrozole) with trastuzumab for the treatment of ER and/or PgR-positive and HER2-positive advanced breast cancer have recently been published (176). In this study, although the response rate to the combination of trastuzumab plus letrozole was not enhanced over trastuzumab monotherapy from previous data, the responses were more durable. Further phase III studies that compare letrozole alone vs. trastuzumab alone vs. the combination will be necessary to fully outline the efficacy of the combination regimen. In addition, results from an open label phase III trial evaluating the safety and efficacy of trastuzumab plus anastrazole vs. anastrazole alone, demonstrated that trastuzumab prolonged progression-free survival in hormone-dependent/HER2-positive metastatic breast cancer (177).
Recently, a randomized phase II neoadjuvant trial of anastrazole ± gefitinib explored whether the AI + gefitinib combination might improve clinical outcome (178). The results of this trial were disappointing in that there was no significant difference in biological or clinical outcomes between the two treatment arms. In fact, there was a nonsignificant trend in response rate favoring the AI alone instead of the combination (response rate, anastrazole 61%, vs. anastrazole + gefitinib 48%; P = 0.067) (169,178). Whether this result might be different in patients selected for EGFR or HER2 overexpression remains to be seen in other studies. The results of the aforementioned trial (169), which demonstrated greater suppression of Ki67 by the addition of AI therapy to the EGFR inhibitor gefitinib in patients with ER-positive/EGFR-positive breast cancer, strongly supports this concept. A large randomized phase III trial comparing the dual EGFR and HER2 inhibitor lapatinib with or without letrozole in ER-positive advanced metastatic breast cancer is currently ongoing. Again, the recently published preclinical data showing the ability of lapatinib to reverse hormone resistance and to cooperate with tamoxifen in providing maximal growth arrest provide the rationale for this study (162).
It will be important in all of the trials mentioned in Table 2 and similar other studies to stratify for prior endocrine therapy (usually tamoxifen) given in the adjuvant setting and, in particular, the interval since completion of such therapy. This is important because it may have implications for the presence or absence of activated growth factor signaling in the relapsed tumor, which could determine the efficacy of added gefitinib or lapatinib. In addition, biological studies are required to help predict those patients who are more likely to benefit from combined endocrine/anti-HER therapy. For example, the tamoxifen/gefitinib trial (Table 2) will undertake studies to look at downstream intracellular signaling components of the HER family, in addition to assessment of ER and ER coactivators such as AIB1.
VI. Future Challenges
As the complexity of ER biology is being revealed, so is the complexity of the mechanisms responsible for endocrine sensitivity and resistance in breast cancer. The genomic and nongenomic activities of ER, as well as their complex interplay with many other growth factors and cellular kinase pathways, influence tumor sensitivity to various types of endocrine therapies. Although ER was first identified more than 30 yr ago, we are still trying to clarify and understand its multiple roles in normal physiology and in disease. In breast cancer, there is convincing evidence that ER does not act alone to stimulate tumor growth; rather, a complex interacting network operates to ensure the viability of cancer cells. Understanding this network will offer therapeutic advantages. The crosstalk between ER and growth factor receptor pathways is the cause of endocrine therapy resistance in many patients. The combination of ER-targeted therapies with growth factor receptor inhibitors or inhibitors of more downstream kinases is a novel strategy currently undergoing more intensive clinical investigation. Future and ongoing clinical trials will determine the true potential and applicability of this combined therapeutic approach. Further clinical trials are needed to evaluate various signaling elements from the multiple networks that crosstalk with and modulate ER activity as predictive markers for initial endocrine therapy. In the future, a molecular profile of these different components in a given patient’s tumor immediately before treatment or at the time of disease progression after therapy might permit the individualization of both the initial type of endocrine therapy and the appropriate signaling inhibitor needed to block de novo or acquired resistance, a strategy that should improve the treatment of breast cancer in the future.
Footnotes
This work was supported in part by National Cancer Institute Grant P50 CA058183 (Breast Cancer SPORE Grant) and by a grant from AstraZeneca Pharmaceuticals.
Disclosure Statement: C.K.O. and R.S. have received research grants from AstraZeneca and Glaxo Smith Kline. G.A. and L.W. have nothing to disclose.
First Published Online January 23, 2008
Abbreviations: AF, Activation function; AI, aromatase inhibitor; EGFR, epidermal growth factor receptor; ER, estrogen receptor; ERE, estrogen response element; HB-EGF, heparin-binding epidermal growth factor-like growth factor; IGFR, IGF-I receptor; MISS, membrane-initiated steroid signaling; MMP, matrix metalloproteinase; MNAR, modulator of nongenomic action of estrogen receptor; mTOR, mammalian target of Rapamycin; NCoA, nuclear receptor coactivator; PgR, progesterone receptor; PI3K, phosphatidyl inositol 3-kinase; SERM, selective ER modulator; STI, signal transduction inhibitor; TKI, tyrosine kinase inhibitor.
References
- Group EBCTC 1998 Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 351:1451–1467 [PubMed] [Google Scholar]
- Gradishar WJ 2004 Tamoxifen—what next? Oncologist 9:378–384 [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N 1998 Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90:1371–1388 [DOI] [PubMed] [Google Scholar]
- Osborne CK, Schiff R 2005 Aromatase inhibitors: future directions. J Steroid Biochem Mol Biol 95:183–187 [DOI] [PubMed] [Google Scholar]
- Johnston SR, Head J, Pancholi S, Detre S, Martin LA, Smith IE, Dowsett M 2003 Integration of signal transduction inhibitors with endocrine therapy: an approach to overcoming hormone resistance in breast cancer. Clin Cancer Res 9:524S–532S [PubMed] [Google Scholar]
- Lonning PE 2004 Aromatase inhibitors in breast cancer. Endocr Relat Cancer 11:179–189 [DOI] [PubMed] [Google Scholar]
- Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, Sahmoud T 2002 Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359:2131–2139 [DOI] [PubMed] [Google Scholar]
- Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Cameron DA, Palmer MJ, Pater JL 2005 Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA. 17. J Natl Cancer Inst 97:1262–1271 [DOI] [PubMed] [Google Scholar]
- Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL 2003 A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349:1793–1802 [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, Press MF 1989 Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244:707–712 [DOI] [PubMed] [Google Scholar]
- Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer JU, Ashley SE, Francis S, Boeddinghaus I, Walsh G 2005 Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 23:5108–5116 [DOI] [PubMed] [Google Scholar]
- Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R 2003 Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 95:353–361 [DOI] [PubMed] [Google Scholar]
- De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, Tortora G, D’Agostino D, Caputo F, Cancello G, Montagna E, Malorni L, Zinno L, Lauria R, Bianco AR, De Placido S 2005 A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res 11:4741–4748 [DOI] [PubMed] [Google Scholar]
- Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R 2004 Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 96:926–935 [DOI] [PubMed] [Google Scholar]
- Chung YL, Sheu ML, Yang SC, Lin CH, Yen SH 2002 Resistance to tamoxifen-induced apoptosis is associated with direct interaction between Her2/neu and cell membrane estrogen receptor in breast cancer. Int J Cancer 97:306–312 [DOI] [PubMed] [Google Scholar]
- Stoica GE, Franke TF, Wellstein A, Czubayko F, List HJ, Reiter R, Morgan E, Martin MB, Stoica A 2003 Estradiol rapidly activates Akt via the ErbB2 signaling pathway. Mol Endocrinol 17:818–830 [DOI] [PubMed] [Google Scholar]
- Kumar R, Wang RA, Mazumdar A, Talukder AH, Mandal M, Yang Z, Bagheri-Yarmand R, Sahin A, Hortobagyi G, Adam L, Barnes CJ, Vadlamudi RK 2002 A naturally occurring MTA1 variant sequesters oestrogen receptor-α in the cytoplasm. Nature 418:654–657 [DOI] [PubMed] [Google Scholar]
- Nicholson RI, McClelland RA, Finlay P, Eaton CL, Gullick WJ, Dixon AR, Robertson JF, Ellis IO, Blamey RW 1993 Relationship between EGF-R, c-erbB-2 protein expression and Ki67 immunostaining in breast cancer and hormone sensitivity. Eur J Cancer 7:1018–1023 [DOI] [PubMed] [Google Scholar]
- Osborne CK, Schiff R 2005 Estrogen-receptor biology: continuing progress and therapeutic implications. J Clin Oncol 23:1616–1622 [DOI] [PubMed] [Google Scholar]
- Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK 2004 Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res 10:331S–336S [DOI] [PubMed] [Google Scholar]
- Lee AV, Cui X, Oesterreich S 2001 Cross-talk among estrogen receptor, epidermal growth factor, and insulin-like growth factor signaling in breast cancer. Clin Cancer Res 7:4429s–4435s; discussion 4411s–4412s [PubMed] [Google Scholar]
- Osborne CK, Schiff R, Fuqua SA, Shou J 2001 Estrogen receptor: current understanding of its activation and modulation. Clin Cancer Res 7:4338s–4342s; discussion 4411s–4412s [PubMed] [Google Scholar]
- Speirs V, Carder PJ, Lansdown MR 2002 Oestrogen receptor β: how should we measure this? Br J Cancer 87:687; author reply 688–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP 1999 The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140:5566–5578 [DOI] [PubMed] [Google Scholar]
- Hopp TA, Weiss HL, Parra IS, Cui Y, Osborne CK, Fuqua SA 2004 Low levels of estrogen receptor β protein predict resistance to tamoxifen therapy in breast cancer. Clin Cancer Res 10:7490–7499 [DOI] [PubMed] [Google Scholar]
- Nemere I, Pietras RJ, Blackmore PF 2003 Membrane receptors for steroid hormones: signal transduction and physiological significance. J Cell Biochem 88:438–445 [DOI] [PubMed] [Google Scholar]
- Klinge CM 2001 Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res 29:2905–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez R, Nguyen D, Rocha W, White JH, Mader S 2002 Diversity in the mechanisms of gene regulation by estrogen receptors. Bioessays 24:244–254 [DOI] [PubMed] [Google Scholar]
- Klinge CM, Jernigan SC, Smith SL, Tyulmenkov VV, Kulakosky PC 2001 Estrogen response element sequence impacts the conformation and transcriptional activity of estrogen receptor α(1). Mol Cell Endocrinol 174:151–166 [DOI] [PubMed] [Google Scholar]
- Saeki T, Cristiano A, Lynch MJ, Brattain M, Kim N, Normanno N, Kenney N, Ciardiello F, Salomon DS 1991 Regulation by estrogen through the 5′-flanking region of the transforming growth factor α gene. Mol Endocrinol 5:1955–1963 [DOI] [PubMed] [Google Scholar]
- Salomon DS, Brandt R, Ciardiello F, Normanno N 1995 Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 19:183–232 [DOI] [PubMed] [Google Scholar]
- Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS 2003 Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144:4562–4574 [DOI] [PubMed] [Google Scholar]
- Gronemeyer H 1991 Transcription activation by estrogen and progesterone receptors. Annu Rev Genet 25:89–123 [DOI] [PubMed] [Google Scholar]
- Jensen EV 1991 Overview of the nuclear receptor family. In: Parker M, ed. Nuclear hormone receptors. London: Academic Press; 1–13 [Google Scholar]
- Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL 1998 The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95:927–937 [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O’Malley BW 1999 Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20:321–344 [DOI] [PubMed] [Google Scholar]
- Leo C, Chen JD 2000 The SRC family of nuclear receptor coactivators. Gene 245:1–11 [DOI] [PubMed] [Google Scholar]
- Chen JD, Evans RM 1995 A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377:371–374 [DOI] [PubMed] [Google Scholar]
- Horlein AJ, Naar AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamel Y, Soderstrom M, Glass CK, Rosenfeld MG 1995 Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377:397–404 [DOI] [PubMed] [Google Scholar]
- Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P 2000 Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol 74:311–317 [DOI] [PubMed] [Google Scholar]
- Lavinsky RM, Jepsen K, Heinzel T, Torchia J, Mullen TM, Schiff R, Del-Rio AL, Ricote M, Ngo S, Gemsch J, Hilsenbeck SG, Osborne CK, Glass CK, Rosenfeld MG, Rose DW 1998 Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc Natl Acad Sci USA 95:2920–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff R, Massarweh S, Shou J, Osborne CK 2003 Breast cancer endocrine resistance: how growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res 9:447S–454S [PubMed] [Google Scholar]
- Graham JD, Bain DL, Richer JK, Jackson TA, Tung L, Horwitz KB 2000 Nuclear receptor conformation, coregulators, and tamoxifen-resistant breast cancer. Steroids 65:579–584 [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Montano MM, Ediger TR, Sun J, Ekena K, Lazennec G, Martini PG, McInerney EM, Delage-Mourroux R, Weis K, Katzenellenbogen JA 2000 Estrogen receptors: selective ligands, partners, and distinctive pharmacology. Recent Prog Horm Res 55:163–193; discussion 194–195 [PubMed] [Google Scholar]
- Smith CL, Nawaz Z, O’Malley BW 1997 Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol 11:657–666 [DOI] [PubMed] [Google Scholar]
- Kirkegaard T, McGlynn LM, Campbell FM, Muller S, Tovey SM, Dunne B, Nielsen KV, Cooke TG, Bartlett JM 2007 Amplified in breast cancer 1 in human epidermal growth factor receptor-positive tumors of tamoxifen-treated breast cancer patients. Clin Cancer Res 13:1405–1411 [DOI] [PubMed] [Google Scholar]
- Ray P, Ghosh SK, Zhang DH, Ray A 1997 Repression of interleukin-6 gene expression by 17 β-estradiol: inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-κB by the estrogen receptor. FEBS Lett 409:79–85 [DOI] [PubMed] [Google Scholar]
- Safe S 2001 Transcriptional activation of genes by 17β-estradiol through estrogen receptor-Sp1 interactions. Vitam Horm 62:231–252 [DOI] [PubMed] [Google Scholar]
- Balasenthil S, Barnes CJ, Rayala SK, Kumar R 2004 Estrogen receptor activation at serine 305 is sufficient to upregulate cyclin D1 in breast cancer cells. FEBS Lett 567:243–247 [DOI] [PubMed] [Google Scholar]
- Rayala SK, Talukder AH, Balasenthil S, Tharakan R, Barnes CJ, Wang RA, Aldaz M, Khan S, Kumar R 2006 P21-activated kinase 1 regulation of estrogen receptor-α activation involves serine 305 activation linked with serine 118 phosphorylation. Cancer Res 66:1694–1701 [DOI] [PubMed] [Google Scholar]
- Zwart W, Griekspoor A, Berno V, Lakeman K, Jalink K, Mancini M, Neefjes J, Michalides R 2007 PKA-induced resistance to tamoxifen is associated with an altered orientation of ERα towards co-activator SRC-1. EMBO J 26:3534–3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P 1995 Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270:1491–1494 [DOI] [PubMed] [Google Scholar]
- Ali S, Coombes RC 2002 Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer 2:101–112 [DOI] [PubMed] [Google Scholar]
- Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H 2001 Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor α: a new model for anti-estrogen resistance. J Biol Chem 276:9817–9824 [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF 2006 A variant of estrogen receptor-α, hER-α36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci USA 103:9063–9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figtree GA, McDonald D, Watkins H, Channon KM 2003 Truncated estrogen receptor α 46-kDa isoform in human endothelial cells: relationship to acute activation of nitric oxide synthase. Circulation 107:120–126 [DOI] [PubMed] [Google Scholar]
- Li L, Haynes MP, Bender JR 2003 Plasma membrane localization and function of the estrogen receptor α variant (ER46) in human endothelial cells. Proc Natl Acad Sci USA 100:4807–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ 2002 Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci USA 99:14783–14788 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Levin ER, Pietras RJ, Estrogen receptors outside the nucleus in breast cancer. Breast Cancer Res Treat, in press [DOI] [PubMed] [Google Scholar]
- Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER 2002 ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol 16:100–115 [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER 2007 A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem 282:22278–22288 [DOI] [PubMed] [Google Scholar]
- Marquez DC, Chen HW, Curran EM, Welshons WV, Pietras RJ 2006 Estrogen receptors in membrane lipid rafts and signal transduction in breast cancer. Mol Cell Endocrinol 246:91–100 [DOI] [PubMed] [Google Scholar]
- Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH 2004 Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor α. Proc Natl Acad Sci USA 101:17126–17131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ 2004 The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor α to the plasma membrane. Proc Natl Acad Sci USA 101:2076–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez DC, Lee J, Lin T, Pietras RJ 2001 Epidermal growth factor receptor and tyrosine phosphorylation of estrogen receptor. Endocrine 16:73–81 [DOI] [PubMed] [Google Scholar]
- Marquez DC, Pietras RJ 2001 Membrane-associated binding sites for estrogen contribute to growth regulation of human breast cancer cells. Oncogene 20:5420–5430 [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, Gorman CM, Parker MG, Sliwkowski MX, Slamon DJ 1995 HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene 10:2435–2446 [PubMed] [Google Scholar]
- Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER 2004 Plasma membrane estrogen receptors exist and function as dimers. Mol Endocrinol 18:2854–2865 [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Rosen EM, Levin ER 2004 BRCA1 inhibits membrane estrogen and growth factor receptor signaling to cell proliferation in breast cancer. Mol Cell Biol 24:5900–5913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Aitkenhead M, Hughes CC, Levin ER 2002 Integration of the non-genomic and genomic actions of estrogen. Membrane-initiated signaling by steroid to transcription and cell biology. J Biol Chem 277:50768–50775 [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Levin ER 2000 Plasma membrane estrogen receptors signal to antiapoptosis in breast cancer. Mol Endocrinol 14:1434–1447 [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER 1999 Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol 13:307–319 [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER 2006 Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol 20:1996–2009 [DOI] [PubMed] [Google Scholar]
- Wyckoff MH, Chambliss KL, Mineo C, Yuhanna IS, Mendelsohn ME, Mumby SM, Shaul PW 2001 Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through Gα (i). J Biol Chem 276:27071–27076 [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Park ST, Levin ER 2003 Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem 278:2701–2712 [DOI] [PubMed] [Google Scholar]
- Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER 2003 Identification of a structural determinant necessary for the localization and function of estrogen receptor α at the plasma membrane. Mol Cell Biol 23:1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambliss KL, Simon L, Yuhanna IS, Mineo C, Shaul PW 2005 Dissecting the basis of nongenomic activation of endothelial nitric oxide synthase by estradiol: role of ERα domains with known nuclear functions. Mol Endocrinol 19:277–289 [DOI] [PubMed] [Google Scholar]
- Levin ER 2003 Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol 17:309–317 [DOI] [PubMed] [Google Scholar]
- Barletta F, Wong CW, McNally C, Komm BS, Katzenellenbogen B, Cheskis BJ 2004 Characterization of the interactions of estrogen receptor and MNAR in the activation of cSrc. Mol Endocrinol 18:1096–1108 [DOI] [PubMed] [Google Scholar]
- Lee AV, Guler BL, Sun X, Oesterreich S, Zhang QP, Curran EM, Welshons WV 2000 Oestrogen receptor is a critical component required for insulin-like growth factor (IGF)-mediated signalling and growth in MCF-7 cells. Eur J Cancer 36(Suppl 4):109–110 [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Kehrl J, Levin ER 2000 Natriuretic peptides inhibit G protein activation. Mediation through cross-talk between cyclic GMP-dependent protein kinase and regulators of G protein-signaling proteins. J Biol Chem 275:7365–7372 [DOI] [PubMed] [Google Scholar]
- Martin MB, Franke TF, Stoica GE, Chambon P, Katzenellenbogen BS, Stoica BA, McLemore MS, Olivo SE, Stoica A 2000 A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology 141:4503–4511 [DOI] [PubMed] [Google Scholar]
- Johnston SR 2005 Combinations of endocrine and biological agents: present status of therapeutic and presurgical investigations. Clin Cancer Res 11:889s–899s [PubMed] [Google Scholar]
- Bunone G, Briand PA, Miksicek RJ, Picard D 1996 Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J 15:2174–2183 [PMC free article] [PubMed] [Google Scholar]
- Weigel NL, Zhang Y 1998 Ligand-independent activation of steroid hormone receptors. J Mol Med 76:469–479 [DOI] [PubMed] [Google Scholar]
- Wu RC, Qin J, Hashimoto Y, Wong J, Xu J, Tsai SY, Tsai MJ, O’Malley BW 2002 Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) coactivator activity by IκB kinase. Mol Cell Biol 22:3549–3561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Font de Mora J, Brown M 2000 AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol 20:5041–5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez GN, Turck CW, Schaufele F, Stallcup MR, Kushner PJ 2001 Growth factors signal to steroid receptors through mitogen-activated protein kinase regulation of p160 coactivator activity. J Biol Chem 276:22177–22182 [DOI] [PubMed] [Google Scholar]
- Hong SH, Privalsky ML 2000 The SMRT corepressor is regulated by a MEK-1 kinase pathway: inhibition of corepressor function is associated with SMRT phosphorylation and nuclear export. Mol Cell Biol 20:6612–6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RC, Qin J, Yi P, Wong J, Tsai SY, Tsai MJ, O’Malley BW 2004 Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic reponses to multiple cellular signaling pathways. Mol Cell 15:937–949 [DOI] [PubMed] [Google Scholar]
- Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osborne CK 1993 Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat 24:85–95 [DOI] [PubMed] [Google Scholar]
- Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou J, Allred DC, Schiff R, Osborne CK, Dowsett M 2005 Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol 23:2469–2476 [DOI] [PubMed] [Google Scholar]
- Meng S, Tripathy D, Shete S, Ashfaq R, Haley B, Perkins S, Beitsch P, Khan A, Euhus D, Osborne C, Frenkel E, Hoover S, Leitch M, Clifford E, Vitetta E, Morrison L, Herlyn D, Terstappen LW, Fleming T, Fehm T, Tucker T, Lane N, Wang J, Uhr J 2004 HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci USA 101:9393–9398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson RI, Staka C, Boyns F, Hutcheson IR, Gee JM 2004 Growth factor-driven mechanisms associated with resistance to estrogen deprivation in breast cancer: new opportunities for therapy. Endocr Relat Cancer 11:623–641 [DOI] [PubMed] [Google Scholar]
- Jordan NJ, Gee JM, Barrow D, Wakeling AE, Nicholson RI 2004 Increased constitutive activity of PKB/Akt in tamoxifen resistant breast cancer MCF-7 cells. Breast Cancer Res Treat 87:167–180 [DOI] [PubMed] [Google Scholar]
- Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, Barrow D, Wakeling AE, Nicholson RI 2003 Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology 144:1032–1044 [DOI] [PubMed] [Google Scholar]
- Gee JM, Harper ME, Hutcheson IR, Madden TA, Barrow D, Knowlden JM, McClelland RA, Jordan N, Wakeling AE, Nicholson RI 2003 The antiepidermal growth factor receptor agent gefitinib (ZD1839/Iressa) improves antihormone response and prevents development of resistance in breast cancer in vitro. Endocrinology 144:5105–5117 [DOI] [PubMed] [Google Scholar]
- Knowlden JM, Hutcheson IR, Barrow D, Gee JM, Nicholson RI 2005 Insulin-like growth factor-I receptor signaling in tamoxifen-resistant breast cancer: a supporting role to the epidermal growth factor receptor. Endocrinology 146:4609–4618 [DOI] [PubMed] [Google Scholar]
- Brodie A, Sabnis G, Macedo L 2007 Xenograft models for aromatase inhibitor studies. J Steroid Biochem Mol Biol 106:119–124 [DOI] [PubMed] [Google Scholar]
- Jeng MH, Yue W, Eischeid A, Wang JP, Santen RJ 2000 Role of MAP kinase in the enhanced cell proliferation of long term estrogen deprived human breast cancer cells. Breast Cancer Res Treat 62:167–175 [DOI] [PubMed] [Google Scholar]
- Martin MB, Reiter R, Pham T, Avellanet YR, Camara J, Lahm M, Pentecost E, Pratap K, Gilmore BA, Divekar S, Dagata RS, Bull JL, Stoica A 2003 Estrogen-like activity of metals in MCF-7 breast cancer cells. Endocrinology 144:2425–2436 [DOI] [PubMed] [Google Scholar]
- Massarweh S, Osborne CK, Jiang S, Wakeling AE, Rimawi M, Mohsin SK, Hilsenbeck S, Schiff R 2006 Mechanisms of tumor regression and resistance to estrogen deprivation and fulvestrant in a model of estrogen receptor-positive, HER-2/neu-positive breast cancer. Cancer Res 66:8266–8273 [DOI] [PubMed] [Google Scholar]
- McClelland RA, Barrow D, Madden TA, Dutkowski CM, Pamment J, Knowlden JM, Gee JM, Nicholson RI 2001 Enhanced epidermal growth factor receptor signaling in MCF7 breast cancer cells after long-term culture in the presence of the pure antiestrogen ICI 182,780 (Faslodex). Endocrinology 142:2776–2788 [DOI] [PubMed] [Google Scholar]
- Arpino G, Green SJ, Allred DC, Lew D, Martino S, Osborne CK, Elledge RM 2004 HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: a southwest oncology group study. Clin Cancer Res 10:5670–5676 [DOI] [PubMed] [Google Scholar]
- Ellis M 2004 Overcoming endocrine therapy resistance by signal transduction inhibition. Oncologist 9(Suppl 3):20–26 [DOI] [PubMed] [Google Scholar]
- Archer SG, Eliopoulos A, Spandidos D, Barnes D, Ellis IO, Blamey RW, Nicholson RI, Robertson JF 1995 Expression of ras p21, p53 and c-erbB-2 in advanced breast cancer and response to first line hormonal therapy. Br J Cancer 72:1259–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett M, Johnston S, Martin LA, Salter J, Hills M, Detre S, Gutierrez MC, Mohsin SK, Shou J, Allred DC, Schiff R, Osborne CK, Smith I 2005 Growth factor signalling and response to endocrine therapy: the Royal Marsden Experience. Endocr Relat Cancer 12(Suppl 1):S113–S117 [DOI] [PubMed] [Google Scholar]
- Hu JC, Mokbel K 2001 Does c-erbB2/HER2 overexpression predict adjuvant tamoxifen failure in patients with early breast cancer? Eur J Surg Oncol 27:335–337 [DOI] [PubMed] [Google Scholar]
- Love RR, Duc NB, Havighurst TC, Mohsin SK, Zhang Q, DeMets DL, Allred DC 2003 Her-2/neu overexpression and response to oophorectomy plus tamoxifen adjuvant therapy in estrogen receptor-positive premenopausal women with operable breast cancer. J Clin Oncol 21:453–457 [DOI] [PubMed] [Google Scholar]
- Berry DA, Muss HB, Thor AD, Dressler L, Liu ET, Broadwater G, Budman DR, Henderson IC, Barcos M, Hayes D, Norton L 2000 HER-2/neu and p53 expression versus tamoxifen resistance in estrogen receptor-positive, node-positive breast cancer. J Clin Oncol 18:3471–3479 [DOI] [PubMed] [Google Scholar]
- Dowsett M, Houghton J, Baum M, Salter J, A’Hern R, Iden C, Farndon J 1999 ER, PgR, c-ERB2 and EGFR in patients randomized to adjuvant tamoxifen: combinations of biomarkers as discriminants of treatment benefit. Breast Cancer Res Treat 57:61 [Google Scholar]
- Tovey S, Dunne B, Witton CJ, Forsyth A, Cooke TG, Bartlett JM 2005 Can molecular markers predict when to implement treatment with aromatase inhibitors in invasive breast cancer? Clin Cancer Res 11:4835–4842 [DOI] [PubMed] [Google Scholar]
- Giltnane JM, Ryden L, Cregger M, Bendahl PO, Jirstrom K, Rimm DL 2007 Quantitative measurement of epidermal growth factor receptor is a negative predictive factor for tamoxifen response in hormone receptor positive premenopausal breast cancer. J Clin Oncol 25:3007–3014 [DOI] [PubMed] [Google Scholar]
- Viale G, Regan M, Dell’Orto P, Del Curto B, Braye S, Orosz Z, Brown R, Olszewski WP, Knox F, Oehlschlegel C, Thürlimann B 2005 Central review of ER, PgR and HER-2 in BIG 1-98 evaluating letrozole vs. tamoxifen as adjuvant therapy for postmenopausal women with receptor positive breast cancer. 28th San Antonio Breast Cancer Symposium, San Antonio, Texas (Abstract 44). Breast Cancer Res Treat 94 (Suppl 1):S13–S14 [Google Scholar]
- Mauriac L, Keshaviah A, Debled M, Mouridsen H, Forbes JF, Thurlimann B, Paridaens R, Monnier A, Lang I, Wardley A, Nogaret JM, Gelber RD, Castiglione-Gertsch M, Price KN, Coates AS, Smith I, Viale G, Rabaglio M, Zabaznyi N, Goldhirsch A 2007 Predictors of early relapse in postmenopausal women with hormone receptor-positive breast cancer in the BIG 1-98 trial. Ann Oncol 18:859–867 [DOI] [PubMed] [Google Scholar]
- Dowsett M 2004 Biomarker investigations from the ATAC trial: the role of TA01. 27th San Antonio Breast Cancer Symposium. Breast Cancer Res Treat 88(Suppl 1):S11 [DOI] [PubMed] [Google Scholar]
- Ellis MJ, Coop A, Singh B, Mauriac L, Llombert-Cussac A, Janicke F, Miller WR, Evans DB, Dugan M, Brady C, Quebe-Fehling E, Borgs M 2001 Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol 19:3808–3816 [DOI] [PubMed] [Google Scholar]
- Zhu L, Chow LW, Loo WT, Guan XY, Toi M 2004 Her2/neu expression predicts the response to antiaromatase neoadjuvant therapy in primary breast cancer: subgroup analysis from celecoxib antiaromatase neoadjuvant trial. Clin Cancer Res 10:4639–4644 [DOI] [PubMed] [Google Scholar]
- Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A’Hern R, Salter J, Detre S, Hills M, Walsh G 2007 Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 99:167–170 [DOI] [PubMed] [Google Scholar]
- Dowsett M, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I, Salter J, Detre S, Hills M, Ashley S, Francis S, Walsh G, Smith IE 2005 Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer—a study from the IMPACT trialists. J Clin Oncol 23:2477–2492 [DOI] [PubMed] [Google Scholar]
- Ellis MJ, Tao Y, Young O, White S, Proia AD, Murray J, Renshaw L, Faratian D, Thomas J, Dowsett M, Krause A, Evans DB, Miller WR, Dixon JM 2006 Estrogen-independent proliferation is present in estrogen-receptor HER2-positive primary breast cancer after neoadjuvant letrozole. J Clin Oncol 24:3019–3025 [DOI] [PubMed] [Google Scholar]
- Lakhani SR, Van De Vijver MJ, Jacquemier J, Anderson TJ, Osin PP, McGuffog L, Easton DF 2002 The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol 20:2310–2318 [DOI] [PubMed] [Google Scholar]
- Poole AJ, Li Y, Kim Y, Lin SC, Lee WH, Lee EY 2006 Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science 314:1467–1470 [DOI] [PubMed] [Google Scholar]
- Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, Feuer EJ, Edwards BK, Berry DA 2007 The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 356:1670–1674 [DOI] [PubMed] [Google Scholar]
- Cordera F, Jordan VC 2006 Steroid receptors and their role in the biology and control of breast cancer growth. Semin Oncol 33:631–641 [DOI] [PubMed] [Google Scholar]
- Aupperlee M, Kariagina A, Osuch J, Haslam SZ 2005 Progestins and breast cancer. Breast Dis 24:37–57 [DOI] [PubMed] [Google Scholar]
- Lange CA 2008 Integration of progesterone receptor action with rapid signaling events in breast cancer models. J Steroid Biochem Mol Biol 108:203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Schiff R, Arpino G, Osborne CK, Lee AV 2005 Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol 23:7721–7735 [DOI] [PubMed] [Google Scholar]
- Cui X, Zhang P, Deng W, Oesterreich S, Lu Y, Mills GB, Lee AV 2003 Insulin-like growth factor-I inhibits progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway: progesterone receptor as a potential indicator of growth factor activity in breast cancer. Mol Endocrinol 17:575–588 [DOI] [PubMed] [Google Scholar]
- Lange CA, Richer JK, Horwitz KB 1999 Hypothesis: progesterone primes breast cancer cells for cross-talk with proliferative or antiproliferative signals. Mol Endocrinol 13:829–836 [DOI] [PubMed] [Google Scholar]
- Lange CA, Richer JK, Shen T, Horwitz KB 1998 Convergence of progesterone and epidermal growth factor signaling in breast cancer. Potentiation of mitogen-activated protein kinase pathways. J Biol Chem 273:31308–31316 [DOI] [PubMed] [Google Scholar]
- Lange CA, Shen T, Horwitz KB 2000 Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci USA 97:1032–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T, Horwitz KB, Lange CA 2001 Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol Cell Biol 21:6122–6131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel AR, Qiu M, Faivre EJ, Ostrander JH, Skildum A, Lange CA 2007 Linkage of progestin and epidermal growth factor signaling: phosphorylation of progesterone receptors mediates transcriptional hypersensitivity and increased ligand-independent breast cancer cell growth. Steroids 72:188–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson-Mullany LK, Lange CA 2004 Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activation of cyclin-dependent protein kinase 2. Mol Cell Biol 24:10542–10557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin PM, Green S, Dorr TM, McGuire WL, Fabian C, Pugh RP, Carter RD, Rivkin SE, Borst JR, Belt RJ, Metch B, Osborne CK 1992 Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: Results of a prospective Southwest Oncology Group study. J Clin Oncol 10:1284–1291 [DOI] [PubMed] [Google Scholar]
- Group EBCTC 2005 Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717 [DOI] [PubMed] [Google Scholar]
- Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM 2003 Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol 21:1973–1979 [DOI] [PubMed] [Google Scholar]
- McGuire WL 1978 Steroid receptors in human breast cancer. Cancer Res 38:4289–4291 [PubMed] [Google Scholar]
- Elledge RM, Green S, Pugh R, Allred DC, Clark GM, Hill J, Ravdin P, Martino S, Osborne CK 2000 Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group Study. Int J Cancer 89:111–117 [PubMed] [Google Scholar]
- Lamy P, Pujol P, Thezenas S, Kramar A, Rouanet P, Guilleux F, Grenier J2002 Progesterone receptor quantification as a strong prognostic determinant in postmenopausal breast cancer women under tamoxifen therapy. Breast Cancer Res Treatment 76:65–71 [DOI] [PubMed] [Google Scholar]
- Osborne CK, Yochmowitz MG, Knight 3rd WA, McGuire WL 1980 The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer 46:2884–2888 [DOI] [PubMed] [Google Scholar]
- Dowsett M, Cuzick J, Wale C, Howell T, Houghton J, Baum M 2005 Retrospective analysis of time to recurrence in the ATAC trial according to hormone receptor status: an hypothesis-generating study. J Clin Oncol 23:7512–7517 [DOI] [PubMed] [Google Scholar]
- Dowsett M 2003 Analysis of time to recurrence in the ATAC (arimidex, tamoxifen, alone or in combination) trial according to estrogen receptor and progesterone receptor status. Breast Cancer Res Treatment 82(Suppl 1):S7 [Google Scholar]
- Dowsett M, Allred DC 2006 Relationship between quantitative ER and PgR expression and HER2 status with recurrence in the ATAC trial. 29th San Antonio Breast Cancer Symposium, San Antonio, Texas (Abstract 48). Breast Cancer Res Treat 100(Suppl 1):S21 [Google Scholar]
- Baehner FL 2006 Quantitative RT-PCR analysis of ER and PR by oncotype DX indicates distinct and different associations with prognosis and prediction of tamoxifen benefits. 29th San Antonio Breast Cancer Symposium, San Antonio, Texas (Abstract 45). Breast Cancer Res Treat 100:S20 [Google Scholar]
- Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, Elledge RM 2005 Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst 97:1254–1261 [DOI] [PubMed] [Google Scholar]
- Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, Wilson C, Rong HM, Bauerfeind I, Felber M, Wang HJ, Beryt M, Seshadri R, Hepp H, Slamon DJ 2003 Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst 95:142–153 [DOI] [PubMed] [Google Scholar]
- Dowsett M, Bundred NJ, Decensi A, Sainsbury RC, Lu Y, Hills MJ, Cohen FJ, Veronesi P, O’Brien ME, Scott T, Muchmore DB 2001 Effect of raloxifene on breast cancer cell Ki67 and apoptosis: a double-blind, placebo-controlled, randomized clinical trial in postmenopausal patients. Cancer Epidemiol Biomarkers Prev 10:961–966 [PubMed] [Google Scholar]
- Balleine RL, Earl MJ, Greenberg ML, Clarke CL 1999 Absence of progesterone receptor associated with secondary breast cancer in postmenopausal women. Br J Cancer 79:1564–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger AM, Milde-Langosch K, Schulte HM, Loning T 2000 Progesterone receptor isoforms, PR-B and PR-A, in breast cancer: correlations with clinicopathologic tumor parameters and expression of AP-1 factors. Horm Res 54:32–37 [DOI] [PubMed] [Google Scholar]
- Lipton A, Leitzel K, Ali SM, Demers L, Harvey HA, Chaudri-Ross HA, Evans D, Lang R, Hackl W, Hamer P, Carney W 2005 Serum HER-2/neu conversion to positive at the time of disease progression in patients with breast carcinoma on hormone therapy. Cancer 104:257–263 [DOI] [PubMed] [Google Scholar]
- Nicholson RI, Hutcheson IR, Knowlden JM, Jones HE, Harper ME, Jordan N, Hiscox SE, Barrow D, Gee JM 2004 Nonendocrine pathways and endocrine resistance: observations with antiestrogens and signal transduction inhibitors in combination. Clin Cancer Res 10:346S–354S [DOI] [PubMed] [Google Scholar]
- Kurokawa H, Lenferink AE, Simpson JF, Pisacane PI, Sliwkowski MX, Forbes JT, Arteaga CL 2000 Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res 60:5887–5894 [PubMed] [Google Scholar]
- Witters L, Engle L, Lipton A 2002 Restoration of estrogen responsiveness by blocking the HER-2/neu pathway. Oncol Rep 9:1163–1166 [PubMed] [Google Scholar]
- Martin MB, Saceda M, Garciamorales P, Gottardis MM 1994 Regulation of estrogen receptor expression. Breast Cancer Res Treat 31:183–189 [DOI] [PubMed] [Google Scholar]
- Arpino G, Gutierrez C, Weiss H, Rimawi M, Massarweh S, Bharwani L, De Placido S, Osborne CK, Schiff R 2007 Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst 99:694–705 [DOI] [PubMed] [Google Scholar]
- Massarweh S, Shou J, DiPietro M, Mohsin SK, Hilsenbeck SG, Wakeling AE, Osborne CK, Schiff R 2002 Targeting the epidermal growth factor receptor pathway improves the anti-tumor effect of tamoxifen and delays acquired resistance in a xenograft model of breast cancer. 25th Annual San Antonio Breast Cancer Symposium, San Antonio, Texas (Abstract 18). Breast Cancer Res Treat 76(Suppl 1):S33 [Google Scholar]
- Martin LA, Head JE, Pancholi S, Salter J, Quinn E, Detre S, Kaye S, Howes A, Dowsett M, Johnston SR 2007 The farnesyltransferase inhibitor R115777 (tipifarnib) in combination with tamoxifen acts synergistically to inhibit MCF-7 breast cancer cell proliferation and cell cycle progression in vitro and in vivo. Mol Cancer Ther 6:2458–2467 [DOI] [PubMed] [Google Scholar]
- de Graffenried LA, Friedrichs WE, Russell DH, Donzis EJ, Middleton AK, Silva JM, Roth RA, Hidalgo M 2004 Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt activity. Clin Cancer Res 10:8059–8067 [DOI] [PubMed] [Google Scholar]
- Argiris A, Wang CX, Whalen SG, DiGiovanna MP 2004 Synergistic interactions between tamoxifen and trastuzumab (Herceptin). Clin Cancer Res 10:1409–1420 [DOI] [PubMed] [Google Scholar]
- Chu I, Blackwell K, Chen S, Slingerland J 2005 The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res 65:18–25 [PubMed] [Google Scholar]
- Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, Paulazzo G, Lyass L, Trusk P, Hill J, Harris J, Spector NL 2006 A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci USA 103:7795–7800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ 2001 First-line, single-agent Herceptin (trastuzumab) in metastatic breast cancer: a preliminary report. Eur J Cancer 37(Suppl 1):S25–S29 [PubMed] [Google Scholar]
- Albain K, Elledge R, Gradishar WJ, Hayes A, Rowinsky EK, Hudis C, Pusztai L, Tripathy D, Modi S, Rubi S 2002 Open-label phase II multicenter trial of ZD1839 (Iressa) in patients with advanced breast cancer. Breast Cancer Res Treat 76:A20 [Google Scholar]
- Baselga J, Albanelli J, Ruiz A, Lluch A, Gascon P, Gonzales S, Guillan V, Sauleda S, Averbuch SD, Rojo F 2003 Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. Proc Am Soc Clin Oncol 22:A24 [DOI] [PubMed] [Google Scholar]
- Robertson JFR, Gutteridge E, Cheung KL, Owers R, Koehler M, Hamilton L, Gee JMW, Nicholson RI 2003 Gefitinib (ZD1839) is active in acquired tamoxifen-resistant oestrogen receptor positive and ER-negative breast cancer: results from a phase II study. Proc Am Soc Clin Oncol 22:A23 [Google Scholar]
- Dancey JE, Freidlin B 2003 Targeting epidermal growth factor receptor—are we missing the mark? Lancet 362:62–64 [DOI] [PubMed] [Google Scholar]
- Polychronis A, Sinnett HD, Hadjiminas D, Singhal H, Mansi JL, Shivapatham D, Shousha S, Jiang J, Peston D, Barrett N, Vigushin D, Morrison K, Beresford E, Ali S, Slade MJ, Coombes RC 2005 Preoperative gefitinib versus gefitinib and anastrozole in postmenopausal patients with oestrogen-receptor positive and epidermal-growth-factor-receptor-positive primary breast cancer: a double-blind placebo-controlled phase II randomised trial. Lancet Oncol 6:383–391 [DOI] [PubMed] [Google Scholar]
- Winer E, Cobleigh MA, Dickler M, Miller K, Fehrenbacher L, Jones C, Justice R 2002 Phase II multicenter study to evaluate the efficacy and safety of Tarceva (erlotinib, OSI-774) in women with previously treated locally advanced or metastatic breast cancer. Breast Cancer Res Treat 76:A445 [Google Scholar]
- Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, Keith BR, Murray DM, Knight WB, Mullin RJ, Gilmer TM 2001 The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther 1:85–94 [PubMed] [Google Scholar]
- Spector NL, Xia W, Burris 3rd H, Hurwitz H, Dees EC, Dowlati A, O’Neil B, Overmoyer B, Marcom PK, Blackwell KL, Smith DA, Koch KM, Stead A, Mangum S, Ellis MJ, Liu L, Man AK, Bremer TM, Harris J, Bacus S 2005 Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol 23:2502–2512 [DOI] [PubMed] [Google Scholar]
- Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, Owens G, Alligood KJ, Spector NL 2002 Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 21:6255–6263 [DOI] [PubMed] [Google Scholar]
- Blackwell K, Kaplan EH, Franco SX, Marcom PK, Maleski JE, Sorensen MJ, Berger MS 2004 A phase II, open label, multicenter study of GW572016 in patients with trastuzumab-refractory metastatic breast cancer. Proc Am Soc Clin Oncol 22:3006 [Google Scholar]
- Burris 3rd HA, Hurwitz HI, Dees EC, Dowlati A, Blackwell KL, O’Neil B, Marcom PK, Ellis MJ, Overmoyer B, Jones SF, Harris JL, Smith DA, Koch KM, Stead A, Mangum S, Spector NL 2005 Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol 23:5305–5313 [DOI] [PubMed] [Google Scholar]
- Marcom PK, Isaacs C, Harris L, Wong ZW, Kommarreddy A, Novielli N, Mann G, Tao Y, Ellis MJ 2006 The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancers. Breast Cancer Res Treat 102:43–49 [DOI] [PubMed] [Google Scholar]
- Mackey JR, Kaufman B, Clemens M, Bapsy PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Jones A 2006 Trastuzumab prolongs progression-free survival in hormone-dependent and HER2-positive metastatic breast cancer. San Antonio Breast Cancer Symposium, San Antonio, Texas (Abstract 3). Breast Cancer Res Treat 100(Suppl 1):S5 [Google Scholar]
- Smith IE, Walsh G, Skene A, Llombart A, Ignacio Mayordomo J, Detre S, Salter J, Clark E, Magill P, Dowsett M 2006 Neoadjuvant anastrozole alone or with gefitinib in early breast cancer: a phase II placebo-controlled trial (study 223) with biological and clinical outcomes. Proc Annual Meeting of American Society of Clinical Oncology, Part I (24). J Clin Oncol Suppl 18S:515 [DOI] [PubMed] [Google Scholar]