Abstract
With the aging of the population, there is a growing recognition that osteoporosis and fractures in men are a significant public health problem, and both hip and vertebral fractures are associated with increased morbidity and mortality in men. Osteoporosis in men is a heterogeneous clinical entity: whereas most men experience bone loss with aging, some men develop osteoporosis at a relatively young age, often for unexplained reasons (idiopathic osteoporosis). Declining sex steroid levels and other hormonal changes likely contribute to age-related bone loss, as do impairments in osteoblast number and/or activity. Secondary causes of osteoporosis also play a significant role in pathogenesis. Although there is ongoing controversy regarding whether osteoporosis in men should be diagnosed based on female- or male-specific reference ranges (because some evidence indicates that the risk of fracture is similar in women and men for a given level of bone mineral density), a diagnosis of osteoporosis in men is generally made based on male-specific reference ranges. Treatment consists both of nonpharmacological (lifestyle factors, calcium and vitamin D supplementation) and pharmacological (most commonly bisphosphonates or PTH) approaches, with efficacy similar to that seen in women. Increasing awareness of osteoporosis in men among physicians and the lay public is critical for the prevention of fractures in our aging male population.
I. Introduction
- II. Epidemiology of Fractures in Men
- A. Overview
- B. Fracture incidence in men and comparisons with women
- C. Hip fractures
- D. Vertebral fractures
- E. Other fractures
- F. Prevalence of osteoporosis in men
- III. Pathogenesis of Bone Loss in Men
- A. Overview
- B. Age-related bone loss in men
- C. Idiopathic osteoporosis in men
- D. Secondary osteoporosis in men
- IV. Diagnostic Criteria
- A. Overview
- B. Which men should be selected for evaluation?
- C. Measures of bone mineral density
- D. Laboratory evaluation
- V. Treatment
- A. Overview
- B. Bisphosphonates
- C. Parathyroid hormone (teriparatide)
- D. Calcitonin
- E. Thiazide diuretics
- F. Strontium ranelate
- G. Sex steroid therapy
- H. Calcium and vitamin D
- I. Exercise
VI. Unresolved Issues Concerning Osteoporosis in Men
VII. Summary and Conclusions
I. Introduction
OSTEOPOROSIS IN MEN is not a rare problem and is an important clinical issue for men, just as it is for women. Men are estimated to lose bone mineral density (BMD) at a rate of up to 1% per year with advancing age (1,2), and one in eight men over age 50 yr will experience an osteoporosis-related fracture in their lifetime (3). Of all osteoporotic fractures, hip fractures contribute to the greatest morbidity as well as mortality, both of which are much greater in men than in women (4,5,6,7). Almost 30% of all hip fractures occur in men (8,9). With the increasing size of our aging population and the improving longevity of men, osteoporosis in men will soon become an even greater burden to society and health care systems worldwide. Thus, a better understanding of the epidemiology, pathogenesis, diagnosis, and treatment of osteoporosis in men will become increasingly important both to the practicing endocrinologist and the primary care provider.
II. Epidemiology of Fractures in Men
A. Overview
Fractures represent the primary clinical consequence of osteoporosis. In both men and women, osteoporosis is defined as an asymptomatic systemic bone disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture (10). However, it is important to recall that both bone strength and the amount of force applied to the bone contribute to fracture occurrence in an individual. The difference in fracture incidence observed between men and women is due not only to a difference in their bone strength but also to the type and frequency of trauma experienced by men compared with women over life. Nonetheless, certain fractures, particularly of the hip, vertebrae, forearm, and humerus, are more likely to occur after minimal trauma in aging men. Of these fractures, those involving the hip and vertebrae are associated with some of the greatest morbidity and mortality for men, as well as health care expenditure.
B. Fracture incidence in men and comparisons with women
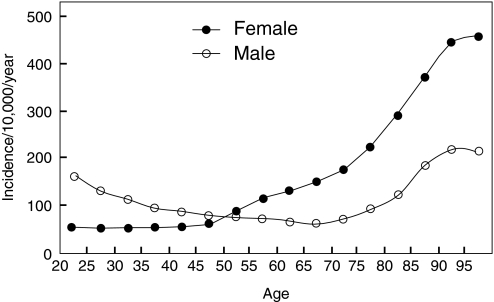
The observed fracture incidence in men follows a bimodal distribution, tending to peak in adolescence and again with advanced age. Although women have a greater incidence of fractures with aging, men are actually more likely than women to sustain a fracture at younger ages. In a prospective study from Scotland of fracture incidence among 15,000 adults (11), there was a higher incidence of overall fractures in men than women in all age groups from 15 to 49 yr, with males in this age range 2.9 times more likely to sustain a fracture than females [95% confidence interval (CI), 2.7 to 3.1]. Several studies have, in fact, demonstrated that before the age of 50 yr, males are more likely to sustain a fracture than females (11,12,13,14,15,16) (Fig. 1), which is felt to be related, in part, to the greater frequency of severe trauma associated with their fractures. One study found that injuries related to sports activities were a major source of limb fractures in males, football injuries being the single most important cause (12), and fights accounted for a number of fractures, in marked contrast to women (12). A study from the United States noted that the incidence rate for a work-related fracture for male employees was more than twice that for female employees (76.3 vs. 30.7 per 10,000 workers), with this higher rate for fracture in men noted across all industrial sectors (17). Fractures in the workplace for men most commonly involved the hands and feet (17). Overall, whereas fracture incidence in men is higher than women below the age of 50 yr, these fractures, in general, appear to be related to high-energy trauma events.
Figure 1.
Age- and gender-specific incidence of fractures at any site among 5 million adults registered in the General Practice Research Database of the UK, 1988–1998. [Reproduced from T. P. van Staa et al.: Bone 29:517–522, 2001 (15), with permission from Elsevier.]
After the age of 50 yr, the trend reverses, with women tending to have a higher incidence of overall fractures than men, although there are geographic differences (9,11,12,13,14,15,16,18,19,20). In both men and women, there is an exponential rise in fracture incidence after age 75 yr, particularly for hip fractures; however, the absolute incidence tends to be lower in men (Fig. 1) (11,12,13,14,15,16). Certain fractures appear to increase in aging men more than others and therefore appear more likely to be related to osteoporosis. In 5 million men and women registered in the General Practice Research Database in the UK, age- and gender-specific incidence of fracture at selected sites was determined from 1988–1998 (15). Among all fractures, those that increased with aging in men included fractures of the femur, vertebrae, radius/ulna, humerus, clavicle, scapula, ribs, and pelvis (15). Fractures of the skull, hands, feet, and distal lower extremity did not appear to increase with aging and therefore were considered less likely to be associated with osteoporosis in men (15). In a study of fracture incidence from the Royal Infirmary of Edinburgh, fractures of the proximal femur, humerus, and clavicle were among those typically seen in elderly men (20). Fractures of the proximal femur, forearm, and humerus were among the most frequent sites of incident nonvertebral fractures in both men and women (aged 55 yr or older) in the Rotterdam Study (20). In general, similar findings were also noted in older men from a population-based study of fracture incidence from the United States (21). In a study from Iceland in which more than 4000 men were followed for almost 20 yr, fractures of the hip (proximal femur), forearm, and humerus resulted from low-energy trauma in 75, 77, and 72% of cases, respectively (22). Fractures of the pelvis and vertebrae were caused by low-energy trauma in 36 and 35% of cases, respectively (22). Collectively, these studies suggest that osteoporotic fractures in men appear to involve fractures of the hip, vertebrae, forearm, and humerus, although fragility fractures at other sites, including the pelvis, ribs, and clavicle, also occur in aging men. Using data from published sources around the world, Johnell and Kanis estimated that 9.0 million new osteoporotic fractures occurred worldwide in the year 2000, of which 39% were in men (9). Of all the hip, forearm, clinical vertebral, and humerus fractures, approximately 30, 20, 42, and 25% were in men, respectively (9).
The observed lower absolute incidence in osteoporotic fractures in older men compared with women may also be due to the fact that with aging, women appear to have an increased frequency of falls relative to men (23,24). Indeed, among U.S. adults aged 65 yr and older, nonfatal, unintentional fall-related injuries disproportionately affected women (25), and fractures after a fall were 2.2 times higher in women than men (25). The reason for this difference between men and women in frequency of falls remains unclear, but speculation includes better muscle strength or balance in men.
C. Hip fractures
Among all osteoporotic fractures, hip fractures account for the greatest morbidity and mortality for men. Overall, the incidence of hip fractures in men is uncommon until after the age of 75 yr, when the risk increases exponentially. Although the absolute incidence varies geographically (26), this marked increase in age-specific hip fracture incidence in elderly men is documented worldwide in population-based studies from North America (8,27,28,29,30), South America (31,32), Europe (11,15,16,18,20,22,31,33,34,35,36,37,38,39,40,41,42), Australia (19,43,44), Asia (31,45,46,47,48,49,50), and Africa (51,52). The highest incidence for hip fracture in men was found in Scandinavian and other northern European countries, as well as in whites from North America (53,54). On the other hand, some of the lowest incidence rates have been reported among blacks and Asians (53,54). In more recent reports, hip fracture incidence was also observed to be low in South America and equatorial areas (26). Johnell et al. (55) reported a 0.3% increase (P < 0.001) in 10-yr probability of hip fracture risk per 10° latitude increment in men (0.8% in women). Results remained similar after adjustment for economic prosperity (55), which was also found to be associated with increased hip fracture risk (countries with greater economic prosperity having a higher risk of hip fracture) (55). The age-adjusted female to male ratio for hip fractures has been observed to be highest for whites, with a ratio up to 3–4:1 (53), but in parts of Asia and among blacks from South Africa, the female to male ratio may be closer to 1:1, with men even having a higher incidence of hip fractures than women in some reports (45,53,56). More recent findings from some parts of Asia, however, report a female to male ratio of 2.5, which is only slightly lower than the 2.9 ratio observed in the United States (49). Differences in trauma exposure, not just bone strength, between men and women and among men from different parts of the world may also explain some of the geographic differences observed in hip fracture rates. For example, in a study from China, where the incidence of fracture in men and women was almost equal, hip fractures in older Chinese men were more likely to be due to accidents compared with women, especially falls from bicycles (28 vs. 10%) (47). Genetic, environmental and lifestyle factors may all contribute to the variability in hip fracture incidence observed worldwide.
Over the past several decades, there has also been a secular increase in the age-adjusted incidence of hip fractures documented worldwide (28,33,34,35,46,57,58,59), particularly in men (33,57,58,59). More recently, this increase has started to level off in some areas (36,37,38,41,60,61), although not everywhere (42,50,62). With the improving longevity of men and the increasing size of the population, the number of men with hip fracture worldwide is estimated to reach 1.8 million in 2050 (8), whereas others suggest that it could even reach 6.8 million, if modest assumptions regarding future secular trends are considered (63).
The mortality and morbidity associated with hip fractures are greater for men than women (4,5,6,7,64,65,66,67,68,69,70,71). Men are twice as likely to die in hospital after a hip fracture than women (6,7). Estimates for the 1-yr mortality rate after hip fracture ranges from 31–35% in men compared with 17–22% in women (4,64,65,70). Greater number of comorbid conditions at the time of fracture contribute to mortality risk (6,72,73,74), which may account for the difference observed between men and women. It has also been reported that up to 50% of men may need institutionalized care after hip fracture (7,72), whereas among those who do return home, many are unable to regain their level of function before fracture (72). Men are less likely to return to autonomous living circumstances than women at 1 yr after hip fracture (64). In men ages 60–69 yr, the decrease in life expectancy after a hip fracture is 11.5 yr, compared with men ages 70–79 yr and age 80 yr or older, where the decrease in life expectancy is 5 and 1.5 yr, respectively (5). Despite these facts, men are less likely to be investigated or treated for osteoporosis after hip fracture (65). In one study, only 7% of men compared with 31% of women were given osteoporosis therapy of any kind at the time of hospital discharge (65). At 1 to 5 yr of follow-up, 27% of men were receiving osteoporosis treatment in contrast to 71% of women (65), whereas 67% of these men receiving treatment were only on calcium and vitamin D (65).
D. Vertebral fractures
The epidemiology of vertebral fractures is more challenging to determine because vertebral fractures are not always associated with pain that would bring them to clinical attention. Many are noted incidentally on radiographs, so the true incidence of these fractures is often underestimated. Nevertheless, even “silent” vertebral fractures, noted as vertebral deformities on radiographs, are clinically relevant because they are associated with low bone density and an increased risk for subsequent osteoporotic fracture.
The European Vertebral Osteoporosis Study (EVOS) determined the prevalence of radiographically defined vertebral deformity in 15,570 subjects (of whom 46% were men) ages 50–79 yr, from 19 countries in Europe. The age-standardized prevalence of vertebral deformity was estimated to be the same for both men and women, either 12 or 20%, depending on the criteria used to define vertebral deformity (75). However, below age 65 yr, men had a higher prevalence of vertebral deformity than women, whereas after this age, the trend was reversed (75). In both men and women, the prevalence of vertebral deformity increased with age, although the increase was greater in women after age 65 yr (75). Similar trends were noted in a U.S. study of 899 women and 529 men over age 50 yr (76), as well as in a study of thoracic spine fractures in 27,000 women and 30,000 men in Finland (77). In a study of men in the MINOS cohort, there was an age-related increase in prevalence of vertebral deformity, regardless of the definition for vertebral deformities (78). The prevalence of vertebral deformity also was noted to increase with decreasing bone density at the total hip (78).
The European Prospective Osteoporosis Study (EPOS) determined the incidence of vertebral fractures in men and women from Europe (79). A total of 14,011 men and women age 50 yr and older were recruited from population-based registers in 29 European centers, and after a mean follow-up of 3.8 yr (1.4–7.9 yr), 6788 (3174 men) had baseline and follow-up spinal radiographs (79). Vertebral fractures were defined by both morphometric criteria and qualitative assessment (79). The age-standardized incidence of morphometric vertebral fractures was 5.7 per 1000 person years at risk (pyr) in men vs. 10.7/1000 pyr in women (79). Using the qualitative assessment, the incidence of vertebral fracture was 6.8/1000 pyr and 12.1/1000 pyr for men and women, respectively (79). Others have also shown that the age-adjusted incidence for radiographically defined vertebral fractures in men is half the rate of women (80,81). Incident fractures at T12 and L1 were the most frequent in both men and women (81). Similar to hip fractures, the incidence of vertebral fractures increases markedly with aging (15,19,29,43,79). There is a geographic variation in vertebral fracture distribution, with the highest rates reported in Sweden compared with the rest of Europe (79).
Vertebral fractures are predictive of subsequent fracture incidence in both men and women (82,83,84,85). In addition, in a 10-yr study from the Swedish cohort of EVOS, prevalent vertebral deformity was a predictor of mortality in men during the forthcoming decade (age-adjusted hazard ratio, 2.4; 95% CI, 1.6 to 3.9) (85). The presence of vertebral deformity in men is also associated with lower energy, poorer sleep, pain, immobility, and social isolation (86). Severe vertebral deformity is related to functional impairment, particularly in men (87), and the association between vertebral deformity and negative health outcomes appears to be even stronger in men than in women (88).
E. Other fractures
Data on the incidence and prevalence of osteoporotic fractures at sites other than the hip and spine are more limited, but there is growing awareness that non-hip, non-spine osteoporotic fractures combined account for significant morbidity and health care costs for both aging men and women (89). In men, the incidence of distal radius fractures after puberty remains relatively stable with age, with perhaps a slight increase in the very elderly (11,13,90,91), in contrast to women, where the increase occurs around the time of the menopause (11,13,90,91). Nevertheless, men with a distal forearm fracture are at an increased risk for a subsequent hip fracture (hazard ratio, 2.27; 95% CI, 1.15 to 4.5 in men age ≥ 40 yr) (92). Others have also demonstrated that men have an increased risk for both vertebral and hip fractures after a distal forearm fracture (93). Fractures of the proximal humerus are also frequently noted in older men (11,29,39,43,94), and the incidence appears to be increasing over time (94). Fractures of the proximal humerus have also been associated with increased mortality within the first 5 yr after fracture, and this risk was higher in men than women (95). Low trauma fractures of the upper limb, in general, increased the risk for subsequent fracture in men (96).
Clavicular fractures occur more frequently in young men than women (15,97). With aging, the location of clavicular fractures differs. Fractures of the proximal clavicle are more likely in the elderly, whereas fractures of the midclavicle occur predominantly in children (97). Fractures of the pelvis in men increase with aging and are also associated with osteoporosis (98,99).
There is a bimodal peak for the incidence rates of femoral and tibial shaft fractures in men, being greatest in both young (ages 15–34 yr) and elderly (over 70 yr) men (11). Although ankle fractures have not traditionally been considered osteoporotic, they do appear to predict subsequent fracture risk in men (relative risk, 4.59; 95% CI, 2.45–8.61), although not in women (96). Low trauma fractures at the ankle may also be increasing in incidence (94).
F. Prevalence of osteoporosis in men
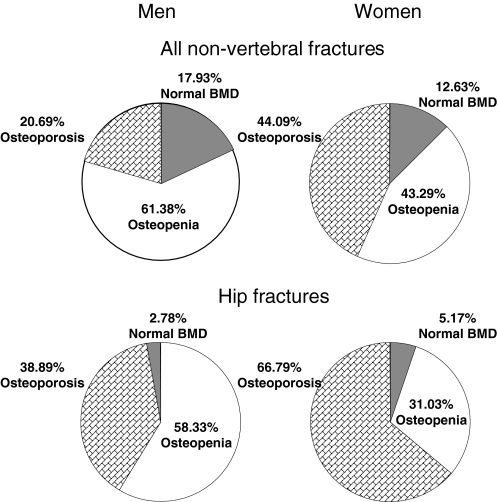
Estimating the prevalence of osteoporosis in the population provides a perspective on the magnitude of the population at risk for an osteoporotic fracture. However, the prevalence of osteoporosis in men has been a challenge to determine, because there had previously been no consensus on an established definition, an issue that is discussed in detail in Section IV. In 1997, investigators estimated the prevalence of osteopenia and osteoporosis among both men and women in the United States based on data from the Third National Health and Nutrition Survey (NHANES III 1988–1994) (100). Using the World Health Organization (WHO) criteria to define osteopenia and osteoporosis, they derived two different cutoffs for men based on the young normal male and female reference groups. Based on bone density measured by dual x-ray absorptiometry (DXA) at various sites of the proximal femur (femoral neck, trochanter, intertrochanter, and total hip), osteoporosis in men was defined as a BMD value greater than 2.5 sd below the mean of either white men or women aged 20–29 yr (male and female cutoffs, respectively). Osteopenia was defined as a bone density value between 1 and 2.5 sd below the respective male and female young reference means. Using male cutoffs, 3–6% of men over age 50 yr were estimated to have osteoporosis, whereas 28–47% had osteopenia (100). Using female cutoffs, 1–4% had osteoporosis, and 15–33% had osteopenia (100). The prevalence estimates were highest using the femoral neck BMD of all proximal femur sites measured. Based on data from Rochester, Minnesota, if bone density at any of the total hip, spine, or wrist was used, the prevalence of osteoporosis in men over age 50 was estimated to be 19% using male reference ranges, and only 3% if the female reference ranges were used (101). The prevalence estimates also differed if an estimation of volumetric BMD was used instead of areal bone density (101). Although there remains debate over the most appropriate criteria for defining osteoporosis in men, it would appear that using sex-specific reference ranges may provide a better estimate for the proportion of men at risk for an osteoporotic fracture. However, as shown in Fig. 2, even using gender-specific femoral neck BMD T-scores to define osteopenia and osteoporosis, a significantly smaller proportion of nonvertebral and hip fractures occur in men characterized as having osteoporosis, as compared with women (20).
Figure 2.
Percentage of nonvertebral or hip fractures that occurred in men and women with osteoporosis, osteopenia, or normal BMD using gender-specific T-scores. [Reproduced from S. C. E. Schuit et al.: Bone 34:195–202, 2004 (20), with permission from Elsevier.]
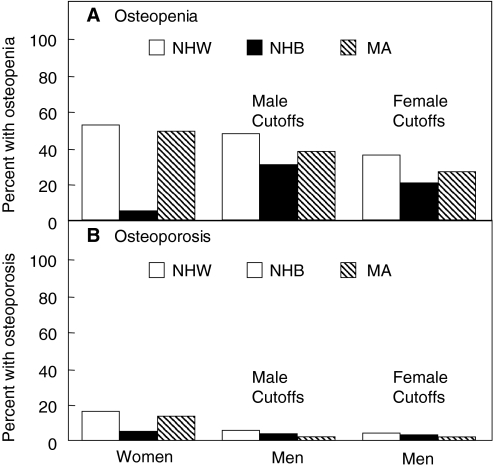
Prevalence estimates of osteoporosis and osteopenia in other ethnic groups and races is limited. Based on data from NHANES III, investigators found the prevalence of osteopenia and osteoporosis in men to be highest for non-Hispanic whites, compared with Mexican-American and non-Hispanic black men (100) (Fig. 3). Others have also shown that white men have lower BMD (based on DXA) than black men at the radius (102), lumbar spine (102,103), and femoral neck (102,103), even after adjustment for body size. These findings are consistent with estimates of hip fracture incidence among different ethnic groups within the United States identifying that white men have higher fracture rates than either Hispanic or black men (104,105,106). Comparative prevalence estimates in the United States among Asian populations have not been studied for men. Although Asian men do tend to have similar or slightly lower bone density values when compared with white men (107), hip fracture incidence among Asian men in the United States is actually lower than white men (104,108). Data on prevalence estimates for osteoporosis in men from other countries are limited.
Figure 3.
Patterns of osteopenia and osteoporosis by race or ethnicity in men using either male or female cutoffs compared with the patterns seen in women. NHW, Non-Hispanic white; NHB, non-Hispanic black; MA, Mexican American. [Reproduced from A. C. Looker et al.: J Bone Miner Res 12:1761–1768, 1997 (100), with permission of the American Society for Bone and Mineral Research.]
III. Pathogenesis of Bone Loss in Men
A. Overview
Male osteoporosis is a heterogeneous entity with multiple underlying causes. Thus, although it is useful to consider each of the possible causes individually for a better understanding of pathogenesis, several different factors may be present in any given individual. Table 1 lists the major causes of bone loss in men, separating these into primary causes (age-related and idiopathic osteoporosis) and secondary causes (those due to clearly identifiable diseases or drugs). Many of these are discussed in more detail in subsequent sections, although an exhaustive discussion of each of the secondary causes of osteoporosis is beyond the scope of this review.
Table 1.
Primary and secondary causes of osteoporosis in men
| Primary osteoporosis |
| Age-related osteoporosis |
| Idiopathic osteoporosis |
| Secondary osteoporosis |
| Alcoholism |
| Glucocorticoid excess (endogenous or exogenous) |
| Hypogonadism (e.g., hormonal suppressive therapy for prostate cancer) |
| Hyperparathyroidism |
| Hyperthyroidism |
| Gastrointestinal disorders |
| Malabsorption syndromes |
| Inflammatory bowel disease, gluten enteropathy |
| Primary biliary cirrhosis |
| Post gastrectomy |
| Hypercalciuria |
| Chronic obstructive pulmonary disease |
| Posttransplant osteoporosis |
| Neuromuscular disorders |
| Systemic illnesses |
| Rheumatoid arthritis |
| Multiple myeloma |
| Other malignancies |
| Masocytosis |
| Medication/drug-related |
| Glucocorticoids |
| Anticonvulsants |
| Thyroid hormone |
| Chemotherapeutics |
| Lifestyle choices |
| Cigarette smoking |
| Sedentary lifestyle |
[Adapted from L. Gennari and J.P. Bilezikian: Endocrinol Metab Clin North Am36:399–419, 2007 (29) with permission from Elsevier.]
B. Age-related bone loss in men
1. Pattern of age-related changes in bone mass in men.
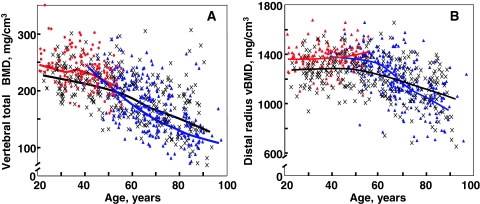
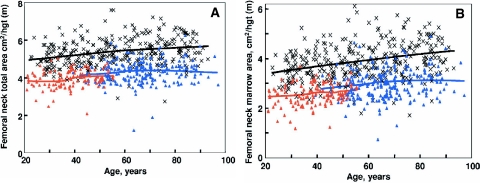
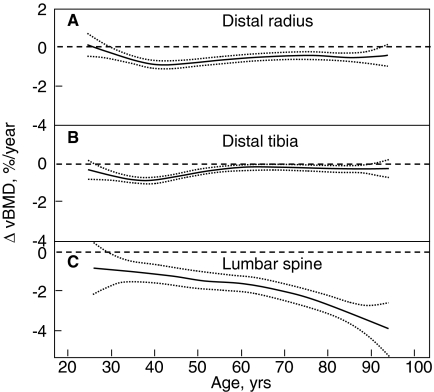
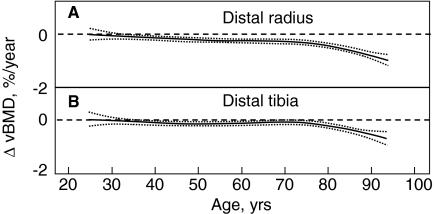
Although DXA is an extremely important clinical tool, its utility is limited by the fact that it cannot clearly separate trabecular from cortical bone or provide information on possible changes in bone size or geometry with age. However, the recent application of central and peripheral quantitative computed tomography (QCT), including high resolution (HR) QCT, has allowed for a better definition of age-associated changes in volumetric BMD (vBMD), geometry, and microstructure. Thus, Riggs et al. (109) used central and peripheral QCT to define age-related changes in these parameters in a population-based sample of men (n = 323) and women (n = 373) aged 20 to 97 yr. As shown in Fig. 4A, there were large decreases in vBMD at the spine (which consists principally of trabecular bone) over life, with a similar pattern of changes in trabecular bone at the femur neck, distal radius, and distal tibia, which seemed to begin even before midlife. These decreases were somewhat smaller in men (approximately 45%) compared with women (approximately 55%; P < 0.001). Even in this cross-sectional study, there was an apparent small midlife acceleration in the slope of the decrease in women that accounted for much of their significantly greater decrease in vertebral vBMD over life compared with men. In contrast to this pattern of changes in trabecular vBMD at the spine, cortical vBMD at the radius showed little change until midlife in either men or women (Fig. 4B), with qualitatively similar changes in cortical vBMD at the distal tibia and femur neck. Thereafter, there were linear decreases in both sexes, but the decreases were greater in women (28%) than in men (18%; P < 0.001). As is also evident, whereas young adult women tended to have higher trabecular vBMD at the spine and cortical vBMD at the radius, due to the greater bone loss with aging in the women compared with the men, this pattern was reversed in the elderly subjects, with older men having higher vBMD at both sites compared with older women. Aging was also associated with increases in bone cross-sectional area at various sites because of continued periosteal apposition throughout life (Fig. 5A). Bone marrow space, however, increased even more because of ongoing bone resorption (Fig. 5B). Thus, because endocortical resorption increased even more than periosteal apposition, there was a net decrease in cortical area and thickness. This process, however, also resulted in outward displacement of the cortex, which increased the strength of bone to bending stresses and partially offset the decrease in bone strength resulting from decreased cortical area. In more recent studies (110), these cross-sectional findings were confirmed by longitudinal data, which showed significant trabecular bone loss at the spine, distal radius, and distal tibia before midlife in men, with an apparent attenuation in rates of trabecular bone loss at the distal radius and tibia, but not at the spine, in older men (Fig. 6, A–C). By contrast, cortical vBMD remained relatively stable until age 65–70 yr, with loss of cortical bone thereafter (Fig. 7, A and B).
Figure 4.
A, Values for vBMD (mg/cm3) of the total vertebral body in a population sample of Rochester, Minnesota, women and men between the ages of 20 and 97 yr. Individual values and smoother lines are given for premenopausal women in red, for postmenopausal women in blue, and for men in black. B, Values for cortical vBMD at the distal radius in the same cohort. Color code is as in panel A. All changes with age were significant (P < 0.05). [Reproduced from B. L. Riggs et al.: J Bone Miner Res 19:1945–1954, 2004 (109), with permission of the American Society for Bone and Mineral Research.]
Figure 5.
A, Values for total area of the femoral neck, adjusted for height, in a population sample between 20 and 97 yr of age. Individual values and smoother lines are given for premenopausal women in red, for postmenopausal women in blue, and for men in black. B, Values for total marrow area, a surrogate for cortical bone loss caused by endocortical resorption, of the femoral neck, adjusted for height in the sample. Color code is as in panel A. [Reproduced from B. L. Riggs et al.: J Bone Miner Res 19:1945–1954, 2004 (109), with permission of the American Society for Bone and Mineral Research.]
Figure 6.
Age-specific rates of change in vBMD at trabecular scanning sites in men at the distal radius (A), distal tibia (B), and lumbar spine (C). Data are shown with a smoothing spline and the 95% confidence interval. [Reproduced from B. L. Riggs et al.: J Bone Miner Res 23:205–214, 2008 (110), with permission of the American Society for Bone and Mineral Research.]
Figure 7.
Age-specific changes in vBMD at cortical scanning sites at distal radius (A) and distal tibia (B) in men. Data are shown with a smoothing spline and the 95% confidence interval. [Reproduced from B. L. Riggs et al.: J Bone Miner Res 23:205–214, 2008 (110), with permission of the American Society for Bone and Mineral Research.]
Data from the MrOs study examining a large (n = 3358) cohort of men aged 65–100 yr (111) have provided considerable insight into changes in femoral neck and shaft dimensions and vBMD in older men. Compared with men aged 65–69 yr, men over the age of 85 yr had 22.1% lower trabecular vBMD but similar cortical vBMD at the femur neck. By contrast, cortical vBMD at the femur shaft was 3.9% lower in the older men. At the femur shaft, bone cross-sectional area increased by 8.9% in the older age group, again consistent with ongoing periosteal apposition with age, but because endocortical area increased by 21.9%, cortical area decreased by 5.9%.
Collectively, these cross-sectional and longitudinal studies have thus established that, in both sexes, trabecular bone loss begins in young adult life, whereas cortical bone loss begins after midlife, with overall decreases in vBMD being smaller in men compared with women. There does appear to be ongoing periosteal apposition with age, with increases in bone cross-sectional area, but because of larger increases in the endocortical area, there is a net decrease in cortical area.
2. Pattern of age-related changes in bone structure in men.
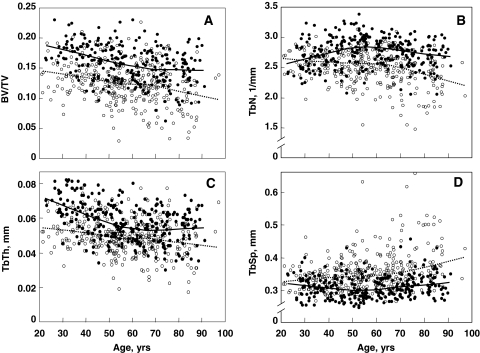
The development and validation of HR peripheral QCT (pQCT) imaging (112,113,114,115) has allowed for population studies of age-related changes in bone microstructure, at least at the radius and tibia. Khosla et al. (116) used this technology in a random sample of men (n = 278) and women (n = 324), ages 21–97 yr, to define sex and age effects on bone microstructure at the wrist. Relative to young women (ages 20–29 yr), young men had greater trabecular bone volume/tissue volume (BV/TV; by 26%, P = 0.001) and trabecular thickness (TbTh; by 28%, P < 0.001) but similar values for trabecular number (TbN) and trabecular separation (TbSp). Between ages 20 and 90 yr, cross-sectional decreases in BV/TV were similar in men (−26%) and in women (−27%) (Fig. 8A), but whereas women had significant decreases in TbN (−13%) and increases in TbSp (+24%), these parameters had little net change over life in men (+7% and −2% for TbN and TbSp, respectively; P < 0.001 vs. women; Fig. 8, B and D). However, TbTh decreased to a greater extent in men (−24%) than in women (−18%; P = 0.010 vs. men; Fig. 8C). These findings using HR-pQCT imaging are, in fact, very similar to earlier studies by Aaron et al. (117) using cadaveric transiliac bone biopsies. These investigators also found parallel decreases in BV/TV with age in men and women, as well as a significant decrease in TbN over life in women but not in men. In addition, similar to the HR-pQCT data, TbTh at the iliac crest was higher in young men compared with age-matched women, but decreased more over life in men compared with women (117). The concordance of age-related changes in trabecular structure at the radius using HR-pQCT with the previous work using bone histomorphometry does suggest that the pattern of changes in trabecular bone may be similar at multiple skeletal sites. Collectively, these data indicate that whereas decreases with age in trabecular BV/TV are similar in men and women, the structural basis for the decrease in trabecular volume is quite different between the sexes. Thus, over life, women undergo loss of trabeculae with an increase in TbSp, whereas men begin young adult life with thicker trabeculae and primarily sustain trabecular thinning, with no net change in TbN or TbSp. Because decreases in TbN have been shown by finite element modeling (118) to have a much greater impact on bone strength compared with decreases in TbTh, these findings may help explain the lower lifelong risk of fractures in men, and specifically, their virtual immunity to age-related increases in distal forearm fractures.
Figure 8.
Age-related changes in trabecular bone microstructural variables at the wrist in Rochester, Minnesota, women and men. A, BV/TV; B, TbN; C, TbTh; and D, TbSp. Individual values and smoother lines are given for women using open circles and dashed lines and for men using closed circles and solid lines. [Reproduced from S. Khosla et al.: J Bone Miner Res 21:124–131, 2006 (116), with permission of the American Society for Bone and Mineral Research.]
3. Role of sex steroids.
Because most men do not develop overt hypogonadism with aging, the prevailing opinion had been that sex steroid deficiency was not a major cause of age-related bone loss in men. It is now clear, however, that the failure of earlier studies to find major decreases in serum levels of total sex steroids was caused by the fact that they did not account for the confounding effect of a greater than 2-fold age-related rise in levels of serum SHBG (119). It is generally believed that circulating sex steroids that are bound to SHBG have restricted access to target tissues, whereas the 1 to 3% fraction that is free and the 35 to 55% fraction that is bound loosely to albumin are readily accessible (120,121). Although there are various methods to assess the bioavailable, or non-SHBG-bound, sex steroids, several groups have reported substantial decreases in serum levels of free or bioavailable sex steroid levels with aging (119,122). Data from a population of 346 men from Rochester, Minnesota (119), are shown in Table 2. Similar findings were reported by Orwoll et al. (123) from the MrOs study, where in a sample of 2623 men over the age of 65 yr, serum free testosterone and free estradiol declined significantly with age, and this was associated with increases in serum SHBG levels. The precise cause of the age-related increase in serum SHBG levels and the failure of the hypothalamic-pituitary-testicular axis to compensate for this and maintain free or bioavailable sex steroids at young normal levels is unclear and is the focus of ongoing studies.
Table 2.
Changes in serum sex steroids and gonadotropins over life in a random sample of 346 Rochester, MN, men aged 23–90 yr
| Hormone | Percent change |
|---|---|
| Bioavailable estrogen | −47a |
| Bioavailable testosterone | −64a |
| SHBG | +124a |
| LH | +285a |
| FSH | +505a |
Based on data in Ref. 119.
P < 0.005.
Although both serum free or bioavailable testosterone and estradiol levels decline with age in men, the traditional notion had been that because testosterone is the major sex steroid in men, it was the decrease in bioavailable testosterone levels that would be associated most closely with bone loss in men. The initial attempts to address this issue came from cross-sectional observational studies in which sex steroid levels were related to areal BMD by DXA at various sites in cohorts of adult men. Slemenda et al. (124) found that BMD at various sites in 93 healthy men over age 55 yr correlated with serum estradiol levels (correlation coefficients, depending on the site, of +0.21 to +0.35; P = 0.01 to 0.05) and, in fact, inversely with serum testosterone levels (correlation coefficients of −0.20 to −0.28; P = 0.03 to 0.10). Subsequent to this report, other similar cross-sectional studies have demonstrated significant positive associations between BMD by DXA and estrogen levels in men (119,122,125,126,127,128,129), particularly circulating bioavailable estradiol levels.
Although these findings are compatible with the hypothesis that estrogen plays an important role in maintaining bone mass in men, they suffer from two potential weaknesses. First, cross-sectional data cannot clearly dissociate the effects of estrogen to maintain or prevent bone loss from the effects of estrogen to achieve peak bone mass. For example, a particular individual with a relatively low bone mass at age 50 yr and low estradiol levels (relative to his age-matched peers) could have had lifelong low estradiol levels going back to childhood. In this case, the low estradiol levels would reflect a deficiency in achieving peak bone mass, not necessarily an effect of estrogen to maintain or prevent bone loss. A second weakness of cross-sectional observational data is that correlation does not prove causality.
To circumvent the first of these problems, Khosla et al. (130) studied, in a longitudinal manner, elderly (60 to 90 yr) men in whom rates of change in BMD using DXA at various sites over 4 yr were related to sex steroid levels. Forearm sites (distal radius and ulna) provided the clearest data, perhaps because of the greater precision of peripheral site measurements as compared with central sites such as the spine or hip. BMD at the forearm sites declined by 0.49 to 0.66% per year in these men, and these decreases were associated more closely with serum bioavailable estradiol levels than with bioavailable testosterone levels. Moreover, further analysis of the data suggested that there may be a threshold bioavailable estradiol level of approximately 40 pmol/liter (11 pg/ml), below which the rate of bone loss in these men clearly was associated with bioavailable estradiol levels. Above this level, there did not appear to be any relationship between the rate of bone loss and bioavailable estradiol levels. In these older men, the bioavailable estradiol level of 40 pmol/liter (11 pg/ml) represented the median bioavailable estradiol level and corresponded to a total estradiol level of approximately 114 pmol/liter (31 pg/ml), which is close to the middle of the reported normal range for estradiol levels in men (10 to 50 pg/ml). Similar findings were reported by Gennari et al. (131) where, in a cohort of elderly Italian men, those subjects with serum free estradiol levels below the median value lost bone over 4 yr at the lumbar spine and femur neck, whereas the men with free estradiol levels above the median did not lose bone. In further studies using QCT at various sites, Khosla et al. (132) found that in elderly men, bioavailable estradiol was the most consistent predictor of vBMD and some of the geometric variables related to bone size, and that the possible “threshold” for skeletal estrogen deficiency was most evident at cortical sites. Moreover, at least in men, serum estradiol levels measured by either a sensitive RIA or by tandem mass spectroscopy provided virtually identical correlations with BMD (133).
Because 85% or more of circulating estrogen levels in men are derived not from direct testicular secretion but rather from peripheral aromatization of testosterone (134), several studies have examined possible relationships between variations in the enzyme aromatase (CYP19) that is responsible for the conversion of androgens to estrogens in the testis and in peripheral tissues and BMD in men (135,136). Thus, Gennari et al. (136) found that males with a high number of TTAA repeat sequences in intron 4 of the CYP19 gene had higher serum estradiol levels and decreased rates of bone loss compared with those with a lower number of repeats, irrespective of serum SHBG or androgen levels. Interestingly, the association between the CYP19 polymorphisms and serum estradiol levels was attenuated with increases in fat mass, consistent with a role for peripheral adipose tissue in contributing to circulating estrogen levels in men and in reducing the impact of genetic variation in the CYP19 enzyme by simply increasing the amount of enzyme present peripherally.
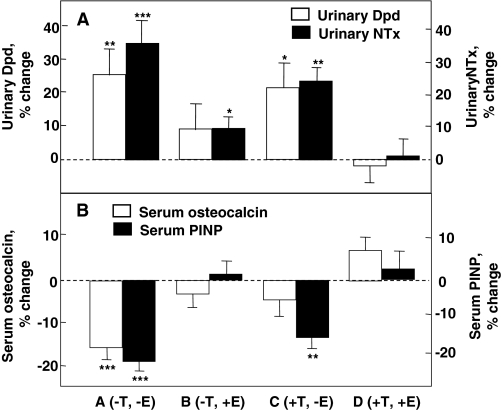
Although these studies helped to establish that estrogen levels are associated with skeletal maintenance in males, they could not definitively establish causal relationships. To address this issue, Falahati-Nini et al. (137) performed a direct interventional study to distinguish between the relative contributions of estrogen vs. testosterone in regulating bone resorption and formation in normal elderly men. Endogenous estrogen and testosterone production were suppressed in 59 elderly men using a combination of a long-acting GnRH agonist and an aromatase inhibitor. Physiological estrogen and testosterone levels were maintained by simultaneously placing the men on estrogen and testosterone patches delivering doses of sex steroids that mimicked circulating estradiol and testosterone levels in this age group. After baseline measurements of bone resorption [urinary deoxypyridinoline (Dpd) and N-telopeptide of type I collagen (NTx)] and bone formation [serum osteocalcin and amino-terminal propeptide of type I collagen (PINP)] markers, the subjects were randomized to one of four groups: group A (−T, −E), discontinued both the testosterone and estrogen patches; group B (−T, +E), discontinued the testosterone patch but continued the estrogen patch; group C (+T, −E), continued the testosterone patch but discontinued the estrogen patch; and group D (+T, +E) continued both patches. Because gonadal and aromatase blockade was continued throughout the 3-wk period, separate effects of estrogen vs. testosterone (in the absence of aromatization to estrogen) on bone metabolism could be delineated.
As shown in Fig. 9A, significant increases in both urinary Dpd and NTx excretion, group A (−T, −E), were prevented completely by continuing testosterone and estrogen replacement [group D (+T, +E)]. Estrogen alone (group B) was almost completely able to prevent the increase in bone resorption, whereas testosterone alone (group C) was much less effective. Using a two-factor ANOVA model, the effects of estrogen on urinary Dpd and NTx excretion were highly significant (P = 0.005 and 0.0002, respectively). Estrogen accounted for 70% or more of the total effect of sex steroids on bone resorption in these older men, whereas testosterone could account for no more than 30% of the effect. Using a somewhat different design, Leder et al. (138) have confirmed an independent effect of testosterone on bone resorption, although the data in the aggregate clearly favor a more prominent effect of estrogen on the control of bone resorption in men.
Figure 9.
Percent changes in bone resorption markers (urinary Dpd and NTx) (A) and bone formation markers [serum osteocalcin and N-terminal extension peptide of type I collagen (PINP)] (B) in a group of elderly men (mean age 68 yr) made acutely hypogonadal and treated with an aromatase inhibitor (group A), estrogen alone (group B), testosterone alone (group C), or both (group D). See text for details. Asterisks indicate significance for change from baseline: *, P < 0.05; **, P < 0.01; ***, P < 0.001. [Adapted from A. Falahati-Nini et al.: J Clin Invest 106:1553–1560, 2000 (137), with permission from the American Society of Clinical Investigation.]
Figure 9B shows the corresponding changes in the bone formation markers, serum osteocalcin and PINP. The reductions in both osteocalcin and PINP levels with the induction of sex steroid deficiency (group A) were prevented with continued estrogen and testosterone replacement (group D). Interestingly, serum osteocalcin, which is a marker of function of the mature osteoblast and osteocyte (139), was maintained by either estrogen or testosterone (ANOVA P values of 0.002 and 0.013, respectively). By contrast, serum PINP, which represents type I collagen synthesis throughout the various stages of osteoblast differentiation (140), was maintained by estrogen (ANOVA P value 0.0001), but not testosterone.
Collectively, these findings provided conclusive proof of an important (and indeed, dominant) role for estrogen in bone metabolism in the mature skeleton of adult men. Similar findings were subsequently reported by Taxel et al. (141) in a study of 15 elderly men treated with an aromatase inhibitor for 9 wk, where suppression of estrogen production resulted in significant increases in bone resorption markers and a suppression of bone formation markers.
Despite the increasing evidence of a more dominant role of estrogens than testosterone on bone metabolism in men, the relative role of estrogens and androgens on fracture risk in men remains understudied. Although some studies have shown an association in men between hypogonadism, or low serum testosterone levels, and fractures (142,143), estradiol levels were not measured in these studies, so it remains possible that low estradiol levels may have accounted for these effects because testosterone is aromatized to estradiol. In a large, population-based study of elderly men from the Rancho-Bernardo Study, low estradiol levels have been associated with vertebral fractures (144). Men in the lowest quintile of total estradiol levels had significantly higher risk for fracture than those in the highest quintile (odds ratio, 4.16; 95% CI, 1.22 to 14.19), whereas men with low testosterone levels compatible with hypogonadism had no significant increased odds for fracture (odds ratio, 1.24; 95% CI, 0.54 to 2.83) (144). In addition, among 793 men from the Framingham Study followed for up to 18 yr, those with low total estradiol levels (≤18 pg/ml) at the beginning of the follow-up period had an increased risk for incident hip fracture (hazard ratio, 3.1; 95% CI, 1.4 to 6.9) when compared with men with high estradiol levels (≥34.1 pg/ml), whereas men with estradiol levels in the midrange had no apparent increased risk for incident hip fracture (hazard ratio, 0.9; 95% CI, 0.2–2.0) (145). This increased risk for hip fracture increased exponentially below serum estradiol levels of approximately 20 pg/ml (74 pmol/liter) (145), further supporting the theory of a threshold effect of low estradiol on bone metabolism (130,146). Although no association was observed between low testosterone levels and hip fracture risk, men with both low estradiol and low testosterone levels had the greatest risk for hip fracture (hazard ratio, 6.5; 95% CI, 2.9 to 14.3) (145) when compared with men with both estradiol and testosterone levels in the mid- to high range. Data from a Swedish cohort of elderly men indicate that free testosterone also independently predicted prevalent fractures in these men; interestingly, the effect of free testosterone was independent of BMD, suggesting that free testosterone may also be a marker for variables other than BMD that may impact on fracture risk, such as propensity to fall or overall general health (147). Similar findings have recently been reported by Meier et al. (148) using data from the Dubbo Osteoporosis Epidemiology study. In this analysis, serum testosterone levels predicted fracture risk in elderly men independent of BMD, again suggesting an important role for testosterone in modulating nonskeletal factors, such as muscle strength, predisposing to fracture risk.
It appears, therefore, that similar to women, declining bioavailable estrogen levels may play a significant role in mediating age-related bone loss and fracture risk in men. However, declining bioavailable testosterone levels may also contribute, because as demonstrated above, testosterone does have some antiresorptive effects and is important for the maintenance of bone formation. Moreover, it provides the substrate for aromatization to estradiol. In addition, at least in rodents, testosterone has been shown to enhance periosteal apposition (149), and studies in young Swedish men indicate that whereas serum free estradiol was a negative predictor, serum free testosterone was a positive predictor of cortical bone size (150). Because larger bones are more resistant to fracture, effects of testosterone on increasing bone size in men may also provide important protection against fracture risk.
These findings in adult men are consistent with reports of a male with homozygous deletion of the ERα gene (151) and aromatase-deficient males (152,153,154,155), all of whom had unfused epiphyses, elevated markers of bone remodeling, and low bone mass, despite normal or elevated testosterone levels. Moreover, the aromatase-deficient males responded to estrogen therapy with marked increases in bone mass by DXA (153,155,156,157), consistent with an “anabolic” effect of estrogen in this setting, in contrast to its predominantly antiresorptive action in postmenopausal women. A more recent study of the response to estrogen therapy in one of the aromatase-deficient males using pQCT found that the increase in bone mass by DXA was largely due to an increase in bone size, rather than changes in trabecular or cortical vBMD, suggesting a potentially important effect of estrogen on bone growth during puberty (154). However, whether this was a direct effect of estrogen on periosteal growth or an indirect effect mediated, for example, by changes in circulating GH or IGF-I levels remains unclear at this point.
4. Potential role of other hormonal factors.
As in aging women, serum PTH levels also increase with age in men (119). However, because the higher sex steroid levels in aging men as compared with elderly women may protect against the bone-resorbing effects of PTH (158), it has been more difficult to demonstrate a direct role for PTH in contributing to age-related bone loss in men (159). Nonetheless, certainly in the presence of vitamin D deficiency or insufficiency, which appears to be fairly prevalent (160), secondary hyperparathyroidism may contribute significantly to bone loss in men. Aging is also associated with decreases in the amplitude and frequency of GH secretion (161), which leads to decreased production of IGF-I by the liver and other tissues. Indeed, serum IGF-I levels decrease markedly with age, and there are also smaller decreases in serum IGF-II levels (162,163). Moreover, at least in men between the ages of 20 and 40 yr, changes in trabecular microstructure, particularly the apparent conversion of thick trabeculae into more numerous, thinner trabeculae, was most closely associated with declining IGF-I levels (164). Because the activity of the IGFs are modulated by IGF binding proteins (IGFBPs) (165), several studies have also examined possible age-related changes in these proteins. Of these proteins, IGFBP-2, which is generally considered an inhibitory binding protein, increases significantly with age in women and men, and is associated inversely with BMD (166) and positively with bone turnover markers (167). Although age-related decreases in circulating IGF-I levels and/or in the activity of the IGF system (due to increases in inhibitory binding proteins, such as IGFBP-2) may contribute to impaired bone formation with aging, these changes may also explain, at least in part, the age-related increase in circulating SHBG levels (119). Thus, IGF-I has been shown to inhibit SHBG production by hepatocytes in vitro (168), and serum SHBG levels are inversely correlated with IGF-I levels in men (169). As such, age-related changes in the GH/IGF system may modulate the activity of sex steroids via changes in circulating SHBG levels.
Other changes in endocrine function with aging appear to make smaller contributions to bone loss. Among the weak adrenal androgens, levels of serum dehydroepiandrosterone (DHEA) and DHEA sulfate decrease by about 80% (170), but the role of these changes in mediating bone loss is unclear. In a recent clinical trial, Nair et al. (171) found small (∼2%) increases in BMD at the femur neck, but not other skeletal sites, after 2 yr of DHEA treatment of elderly men, arguing against an important role for DHEA in age-related bone loss in men.
Finally, whereas the above discussion has focused on age-related changes in hormonal or other growth factors, it is also likely that with aging, there are intrinsic changes in stem or osteoprogenitor cells that result in impairments in bone formation. However, possible changes in these cells with aging have not been clearly defined, and this is an important area for future investigation.
5. Role of other factors, including nutrition and changes in muscle mass.
As noted above, vitamin D deficiency, with or without adequate calcium intake, likely contributes to the age-related increase in serum PTH levels and to bone loss, at least in a subset of aging men (160). In several population-based studies, 25-hydroxyvitamin D, an indicator of vitamin D nutrition, decreased by 30–60% (172). This may be a particularly important problem in housebound individuals with poor nutrition and inadequate exposure to UV radiation, especially populations who reside in countries with higher latitudes, such as Great Britain and France, and where dairy products are not fortified with vitamin D. Other nutritional factors, such as inadequate calcium (173) or protein (174) intake may also play a role in accelerating age-related bone loss in men. In addition to these nutritional factors, Frost has suggested in a number of publications (175,176,177) that the loss of muscle mass with aging is perhaps the principal cause of involutional osteoporosis in both sexes. Indeed, a number of studies have shown high correlations between lean body mass and total body bone mineral (177). Moreover, in a population sample, Proctor et al. (178) found that physical activity declined by 34 and 38% and lean body mass declined by 18 and 17% with aging in women and men, respectively, and decreases in muscle strength have been associated with the risk of osteoporosis in women as well as men (173). Thus, it appears likely that with aging, a number of nutritional and lifestyle factors, particularly declining levels of physical activity and muscle mass, contribute to bone loss as well as risk of falls, ultimately increasing the overall risk of fractures.
C. Idiopathic osteoporosis in men
For the purposes of this discussion, idiopathic osteoporosis in men is defined as the development of osteoporosis and fractures in a male before the age of 60 yr (i.e., generally before the above age-related changes are evident). However, there may well be considerable heterogeneity in the causes of idiopathic osteoporosis in men. Some cases may represent mild, unrecognized variants of osteogenesis imperfecta; others may represent defects in peak bone mass acquisition caused by genetic or environmental factors. A subset of these men appear to have hypercalciuria with or without increased bone resorption (179,180,181). Indeed, idiopathic hypercalciuria in men is associated with low BMD (179), and up to 10% of men with idiopathic osteoporosis have hypercalciuria (182). Despite this heterogeneity in causes of idiopathic osteoporosis, however, there are some interesting similarities to the hormonal abnormalities being uncovered in these patients and those present in elderly men.
Perhaps the most consistent abnormality noted in the albeit small groups of patients studied to date in an increase in serum SHBG levels, leading to decreased free estradiol and androgen indices (183,184,185,186). Circulating total estradiol levels may also be reduced in these patients despite normal testosterone levels, consistent with subtle aromatization defects in at least a subset of these patients (183,187). Interestingly, circulating IGF-I levels may also be reduced in these patients (188), despite a normal GH secretory capacity (189). The reduction in IGF-I levels appears to be associated with a simple sequence repeat in the IGF-I gene (192/192) that is present at an increased frequency in these men (190). As with aging, low IGF-I levels may contribute both to impaired bone formation and to an increase in SHBG levels, the latter resulting in reduced availability of sex steroids. Thus, there are some striking parallels in the hormonal abnormalities present in these younger men with idiopathic osteoporosis and those present in aging men that clearly warrant further investigation.
D. Secondary osteoporosis in men
As shown in Table 1, there are a number of possible secondary causes of osteoporosis in men that may be superimposed on underlying age-related bone loss or idiopathic osteoporosis. Indeed, in some series, secondary causes may account for, or contribute significantly to, up to 40% of the cases of osteoporosis in men (191). The three major causes of secondary osteoporosis in men are alcohol abuse, glucocorticoid excess (either endogenous or, more commonly, chronic glucocorticoid therapy), and overt hypogonadism, with the latter increasingly due to hormonal suppressive therapy for the treatment of prostate cancer (192). Of these, glucocorticoid-induced osteoporosis is the most common cause of secondary osteoporosis in men; however, because this disorder is not unique to men, it is not discussed in detail here, but the reader is referred to excellent recent discussions of this topic (193,194). The other secondary causes listed in Table 1 should be considered and ruled out in the appropriate clinical setting, but a detailed discussion of each of these is beyond the scope of the present discussion.
IV. Diagnostic Criteria
A. Overview
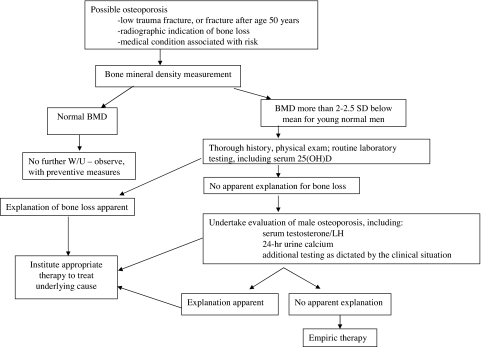
Unfortunately, guidelines for efficient, cost-effective evaluations of patients having, or suspected of having, osteoporosis are poorly validated for either sex. Current practice is based on existing knowledge of the epidemiology and clinical characteristics of osteoporosis (195,196) rather than upon models that have been carefully tested in prospective studies. Nevertheless, existing data allow the formulation of a reasonable approach (Fig. 10).
Figure 10.
A general scheme for the evaluation of men suspected of having increased fracture risk.
B. Which men should be selected for evaluation?
Men are rarely evaluated or treated for osteoporosis (65,197), in part because recognition of the problem is not as widespread as it is for osteoporosis in women. In addition, the clinical situations that should prompt an evaluation are less well defined in men. Although a host of clinical factors have been associated with low BMD or fracture (Table 1), many of these reports involve small numbers of cases and lack adequate study power to provide confident estimates of the degree of risk. Using objective criteria of study design, Espallargues et al. (198), and more recently Liu et al. (199), examined the world’s literature to identify factors associated with both low BMD and fracture in men. Risk factors for fracture include those that appear to be mediated via an association with skeletal fragility as well as those that may act by increasing the risk of falls. Moreover, some of these factors are associated with increased fracture risk independent of BMD—a fact of critical importance in clinical decision-making in men and women because it is the combination of these factors plus BMD that determines fracture risk and thus should determine diagnostic and therapeutic aggressiveness.
C. Measures of bone mineral density
BMD is highly predictive of fracture risk in men. In patients who have conditions associated with low BMD and fracture, the measurement of BMD should be strongly considered. Clinical judgment may also prompt the assessment of bone density in men with the conditions not so clearly related to fracture risk. BMD measurements can be useful in several ways, including contributing to the diagnosis of skeletal fragility, gauging its severity, and guiding decisions concerning therapy. Generalized screening of older men with bone density measures has been recommended in men over 70 yr (200) and is worth further evaluation as a strategy. However, a recent cost-effectiveness analysis supported generalized screening only in those over 80 yr of age, as well as in men over the age of 65 yr who have previously experienced a fracture (201).
DXA is readily available, there are well-developed reference data for its use, low DXA BMD levels are strongly related to fracture risk in men (202,203), and pharmacological therapies appear to be effective in men chosen on the basis of low DXA BMD levels. For all these reasons, DXA must be considered the first choice for assessing bone strength in men. On the other hand, ultrasound measurements are relatively simple and inexpensive, and low ultrasound measures are also associated with increased fracture risk in men (204). Unfortunately, normative data are not well established for ultrasound, and ultrasound criteria for choosing men for pharmacological therapy are not validated. Using ultrasound to determine which men should receive DXA measures is probably not effective (205), and combining ultrasound with DXA seems to offer little additional advantage in identifying fracture risk (204). Finally, whereas QCT is gaining popularity as a research tool to examine changes in trabecular and cortical vBMD with aging and various diseases, it should be noted that diagnostic criteria for QCT-based vBMD measurements have not been established. DXA T-score criteria for diagnosing osteopenia and osteoporosis cannot be used with this modality, and in fact there is not sufficient information concerning the relationship of CT measures and fracture risk to substantiate the use of CT measures in men in the clinical setting.
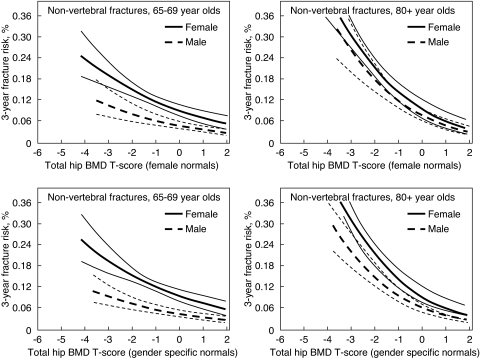
The bone density criteria that should be used to identify men with high fracture risk, and thus in need of intervention, is controversial. Although it is clear that there is an inverse association between DXA BMD and fracture risk, the specifics of the relationship are not as well established in men as in women. Some have suggested that the relationship between the absolute level of bone density and fracture risk is the same in men and women (206), whereas others have noted sex differences (Fig. 11) (202). Interestingly, in the latter studies sex differences were most apparent at younger ages and became less apparent in older men. If true, this offers a potential explanation for the lack of sex differences in studies of hip fractures, which usually occur late in life (206). In addition to these issues, some have been concerned that the use of diagnostic cutoffs in men that are based on reference ranges in women would reduce the number of men identified as at risk (207), a conundrum in view of the frequency of fractures in men. In the absence of well-powered prospective studies involving both sexes, it remains most common to judge BMD results in light of sex-specific reference ranges. Certainly it would be preferable to utilize diagnostic criteria based on absolute fracture risk, a goal being currently addressed by several professional organizations, and models incorporating clinical risk factors with or without BMD to predict 10-yr probabilities of hip or other major osteoporotic fractures in women and men have recently been published (208). Currently, however, T-score-based criteria remain the basis for therapeutic decisions in both sexes. The diagnosis of osteoporosis in men is commonly made at a BMD T-score level of −2.5 or less, but in fact there is no obvious T-score that should dictate clinical decisions about additional evaluation or therapy. Rather, with lower levels of BMD, the clinical concern should be greater. For instance, in men with BMD T-score levels of −1.5 or less, the presence of other risk factors for fracture may trigger additional diagnostic measures or therapeutic intervention. BMD T-score levels below −2 to −2.5 commonly prompt the consideration of pharmacological therapy. Unfortunately, there are no large-scale therapeutic trials in men that allow estimates of the cost effectiveness of treatment based on baseline BMD levels.
Figure 11.
Three-year risk of fracture (and 95% confidence limits) by sex-specific total hip BMD T-score and age in older women and men. T-scores for males using male normal values for the total hip are equivalent to the following BMD values: T-score of −2 = 0.753 g/cm2; T-score of −1 = 0.897 g/cm2; T-score of 0 = 1.041 g/cm2. T-scores for females using female normal values for the total hip are equivalent to the following BMD values: T-score of −2 = 0.698 g/cm2; T-score of −1 = 0.820 g/cm2; T-score of 0 = 0.942 g/cm2. T-scores for both sexes using female normal values for the total hip are equivalent to the following BMD values: T-score of −2 = 0.698 g/cm2; T-score of −1 = 0.820 g/cm2; T-score of 0 = 0.942 g/cm2. [Reproduced from S. R. Cummings et al.: J Bone Miner Res 21:1550–1556, 2006 (202), with permission of the American Society for Bone and Mineral Research.]
D. Laboratory evaluation
The diagnostic yield and cost effectiveness of laboratory studies in men with low bone density is unknown. Nevertheless, in the presence of low BMD it is considered important to determine the cause of the osteopenic disorder. Of particular concern, osteomalacia is estimated to be present in <4% to 47% of men with femoral fractures, with most reports being ≤20% (209,210). Although the exact magnitude of the problem presented by osteomalacia in men is uncertain, the differential diagnosis of low bone mass and fractures in men must include osteomalacia. This becomes particularly imperative because the treatment for osteomalacia differs considerably from that of osteoporosis.
The history and physical examination can provide evidence of genetic, nutritional/environmental, social, medical, or pharmacological factors that contribute to the cause of osteoporosis. Routine laboratory testing should include levels of serum creatinine, calcium, phosphorus, alkaline phosphatase, and liver function tests, as well as a complete blood count. Given the widespread prevalence of vitamin D deficiency (160), serum 25-hydroxyvitamin D levels should also be obtained in patients with primary or secondary male osteoporosis. However, given the potential variability of assays for 25-hydroxyvitamin D levels (211), use of a validated assay (preferably using mass spectroscopy) is important in assessing vitamin D status. If, on the basis of this information, there is evidence for medical conditions associated with bone loss (e.g., hyperparathyroidism, malignancy, Cushing’s syndrome, thyrotoxicosis, malabsorption, etc.), a definitive diagnosis should be pursued with appropriate testing.
In men with reduced bone mass, and in whom no clear pathophysiology is identified by the routine methods above, additional testing might include a 24-h urine calcium and creatinine to identify idiopathic hypercalciuria and serum testosterone and LH. Currently, testosterone is the standard assessment tool for detecting hypogonadism, but serum estradiol levels are more closely associated with BMD than are those of testosterone. As assays for low levels of serum estradiol become more accurate and precise, it may also be useful to measure estradiol concentrations in men. For instance, serum testosterone and estradiol levels are not highly correlated (123), and men who have less aromatase activity could present with low estradiol concentrations without low testosterone levels.
Other measures may be appropriate, depending on the clinical context (e.g., PTH, 24-h urine cortisol, immunological markers of sprue, etc.), but there is little information concerning their usefulness unless there are specific clinical indications for obtaining them. Similarly, higher levels of biochemical markers of bone turnover appear to be related to increased bone loss and fracture (212,213,214), but whether they add practical information over that provided by BMD measures is uncertain.
V. Treatment
A. Overview
Therapy of osteoporosis in men is less well defined than in women. There have been few trials of osteoporosis therapies performed specifically in male populations, the available trials are relatively small, and in most the endpoint has been change in BMD. Thus they lack the power to confidently address drug effects on fracture risk (215). However, for the most common osteoporosis therapies (bisphosphonates and PTH) the effects in men appear very similar to those in women.
B. Bisphosphonates
1. Use of bisphosphonates in men.
Several trials of bisphosphonates in men have shown benefit. For instance, in a trial involving 241 men aged 31–87 yr with low BMD (spine or hip BMD T-score ≤ −2.0), alendronate had positive results on bone mass. Although the trial was not powered for a fracture outcome, the results suggest that therapy reduced the rate of vertebral fracture (216). In similar studies, risedronate increased BMD and appeared to reduce vertebral fracture risk in older men (217,218). In one very small study in men after stroke, risedronate appeared to reduce the risk of hip fracture (219). The increase in BMD resulting from bisphosphonate therapy in men appears to be very similar to that previously reported in postmenopausal women (216,220). Moreover, BMD changes in response to therapy are as great in men with low free testosterone levels as in those with normal levels (216), suggesting that bisphosphonates should be effective in men with hypogonadism.
Bisphosphonates are also effective in men with secondary causes of osteoporosis (221), have positive effects on BMD in men receiving glucocorticoids (222,223,224), and are effective in preventing bone loss in states of immobilization (225), repetitive loading (stress fractures) (226), and in inflammatory conditions (e.g., rheumatoid arthritis) (227).
The effects of newer bisphosphonates (ibandronate, zoledronate) on BMD and fracture risk have not been reported in men, but trials are under way. There is no compelling reason to suspect that their effects will be substantially different than those in women.
2. Bisphosphonate use during androgen deprivation therapy for prostate cancer.
Men who receive antiandrogen therapy for prostate carcinoma are at risk of bone loss and fractures (228,229), and antiresorptive therapy should provide some protection for those patients. In fact, a number of well-designed trials have demonstrated the effectiveness of bisphosphonates in preventing bone loss in these men (192). Although no large trials with a fracture endpoint are available, it is reasonable to utilize bisphosphonates to avoid bone loss in men receiving androgen deprivation therapy, particularly when baseline BMD is low or there are other risk factors for fracture.
C. Parathyroid hormone (teriparatide)
PTH therapy is effective in increasing BMD in men with primary osteoporosis (230,231), and its use is associated with evidence of an early increase in remodeling essentially identical to that seen in women (232). Moreover, therapy appears to reduce the likelihood of vertebral fractures (233). The studies available are of small size and short duration, and thus there is no evidence of nonvertebral fracture reduction. Nevertheless, the similarity of the effects in men with those seen in larger antifracture studies on women strongly suggests that PTH therapy should be useful in both sexes. Simultaneous therapy with bisphosphonate appears to blunt the effects of PTH in men as it does in women (234,235). Although the effectiveness of PTH administration in the prevention of fractures in men, either alone or in concert with other agents, remains unclear, its potential appears similar in men and women (236).
D. Calcitonin
From a theoretical perspective calcitonin should be useful in reducing osteoclastic activity in at least some patients with osteoporosis or in those at risk of continuing bone loss, but there are few data. The available studies are small, and most are not adequately designed. In an uncontrolled, 12-month trial of sc administered cyclical calcitonin (100 IU three times per week for 3 months, followed by 3 months without calcitonin) in men with vertebral osteoporosis, small benefits were noted in spinal and proximal femoral bone density, compared with baseline (237). Intranasal calcitonin decreased biochemical markers of bone turnover and had a beneficial effect on spine BMD (238). Studies of the effectiveness of intranasal calcitonin in men include one open-label study (239) that suggested that therapy reduced the risk of vertebral fracture.
E. Thiazide diuretics
Thiazide administration may have positive effects on bone mass, rates of bone loss, and hip fracture risk in men (240,241). For instance, in case-controlled trials the use of thiazides reduced the rate of loss in calcaneal bone density by 49% compared with controls, and the relative risk of hip fracture was halved by exposure to thiazides for more than 6 yr (242). Similarly, thiazide use in men was associated with an adjusted odds ratio of femur fracture of 0.2 (95% CI, 0.1–0.7) (243). Other diuretics did not seem to impart the same benefits. Unfortunately, none of the available studies has been randomized or controlled, so a confident estimate of the magnitude of the protective effect is not possible. The mechanism for the positive effect is unclear, but it has been postulated to stem from the hypocalciuric effects of thiazides. In fact, one study showed that an increase in BMD resulted from thiazide use in men with hypercalciuria (244). Although not appropriately considered a primary treatment modality, a thiazide is probably the diuretic of choice in osteoporotic patients (other considerations notwithstanding).
F. Strontium ranelate
Strontium ranelate administration has interesting effects on bone remodeling in that it appears to induce an increase in bone formation as well as a reduction in bone resorption and results in improved BMD and reduced fracture risk in women (245). The effects of strontium should not be sex specific, and studies of the usefulness of strontium therapy in men are under way but results are not yet available.
G. Sex steroid therapy
1. Overview.
As reviewed in Section III, sex steroids exert complex effects on bone. Whereas there may be treatment opportunities with both estrogens and androgens, there is very little information concerning the effects of estrogens in the therapeutic context, and most treatment trials for low BMD have involved testosterone.
2. Estrogen.
Although estrogens exert important effects on bone remodeling, there have been few attempts to use estrogen administration to prevent or improve bone mass in men. There is an appropriate reluctance to induce adverse effects (e.g., gynecomastia), and studies of the effects of even low-dose estrogen on bone in men are not available. Two small, short-term trials of raloxifene in older men with low BMD suggested that selective estrogen receptor modulators could have positive effects on bone remodeling (246,247), at least in the subset of men with low endogenous estrogen levels. Of course, treatment with testosterone also results in an increase in estrogen levels via the effects of aromatase, and the effects of testosterone therapy on bone are probably at least in part the result of estrogen action.
3. Testosterone replacement in hypogonadal adult men.
Hypogonadism is associated with increased bone loss and fracture. Testosterone therapy in hypogonadal men positively affects bone mass, at least in most patient groups (248). The increase in bone mass with testosterone therapy can be expected to be modest in the short term (up to 24 months), but Behre et al. (249) noted an increase in spinal trabecular BMD of more than 20% in the first year of testosterone therapy in a group of hypogonadal men and further increases thereafter. The most marked increases were observed in those with the lowest testosterone levels before therapy. Using micro-MRI imaging, Benito et al. (250) noted that trabecular architecture appeared to improve in hypogonadal men treated with testosterone. Most studies of testosterone replacement have included younger men, but there is a suggestion that in older men with hypogonadism the response to therapy can be expected to be similar to that in younger adult patients (249,251).
Despite the generally positive tenor of most studies of the skeletal effects of testosterone replacement, in some patient groups, for instance those with Kleinfelter’s syndrome, the advantage associated with androgen therapy is questionable because the available studies report very mixed results (252). This may be because the level of androgen deficiency in Kleinfelter’s (as in the case of some other causes of hypogonadism) is quite variable. These findings suggest the need to carefully consider the potential benefits of androgen replacement in each patient individually.
In addition to the generally positive effects of androgen replacement therapy on BMD in hypogonadal men, additional benefits may be gained from the increases that have been noted in strength and lean body mass in these patients (253). Because lean body mass and strength have been correlated with bone mass and a reduced propensity to fall, they may further serve to promote bone health and reduce fracture risk. However, thus far therapeutic trials of testosterone have included BMD as the primary endpoint, and the effects of testosterone therapy on fracture risk are unknown.
The most efficacious doses and routes of androgen administration for the prevention/therapy of bone loss in men remain uncertain. The specific testosterone levels necessary for an optimal effect have not been defined. Current recommendations are to attempt to achieve testosterone concentrations similar to those of normal young men.
4. Testosterone replacement in andropause.
There is considerable controversy concerning the use of testosterone replacement therapy in older men, including its usefulness and safety in men at risk for fracture. The Institute of Medicine recommended a series of clinical trials to help determine the efficacy of testosterone for several important outcomes (254). Those trials are being developed and should provide additional information concerning bone. Although available data are few, trials of im testosterone administration in older men with low testosterone levels have suggested that it may result in increased strength and improved body composition (253) and that bone mass and biochemical indices of remodeling may improve (255). Thus far, positive effects on bone density are more apparent in men treated with im testosterone than with transdermal administration (171,256), suggesting that higher testosterone levels may be necessary to achieve these results. Trials to date have selected men who have relatively low testosterone levels, without regard to baseline estradiol concentrations.
The choice of pharmacological therapy in older men with osteoporosis and low sex hormone levels is difficult. Whereas testosterone therapy is indicated in men with symptoms of hypogonadism, the absence of information concerning the effectiveness of testosterone on the prevention of fractures reduces its attractiveness as a primary osteoporosis therapy. The use of testosterone therapy in osteoporotic men is made more problematic by the uncertainties surrounding the practical use of testosterone for skeletal indications. On the other hand, bisphosphonate treatment appears to be effective regardless of gonadal function. Similarly, PTH therapy also induces improvement in BMD in men with low sex steroid levels (230). Thus, in older men with osteoporosis it may be more appropriate to treat with an established osteoporosis drug regardless of gonadal function. It may be that there is a subset of older men with low sex steroid levels who may respond more briskly (e.g., men with low estradiol levels). If such a subset is identified in the future, replacement therapy could be targeted for its benefit.
Selective androgen receptor modulators are being developed and promise to be useful as osteoprotective agents while reducing adverse effects on prostate, lipids, etc. (257). Animal studies suggest that selective estrogen receptor modulators may have encouraging effects in males (258).
5. Testosterone therapy in secondary forms of metabolic bone disease.
A variety of systemic illnesses and medications are associated with lowered testosterone levels, and it has been postulated that relative hypogonadism may contribute to the bone loss that also accompanies many of these conditions. For instance, renal insufficiency, glucocorticoid excess, post transplantation, malnutrition, and alcoholism are all associated with osteopenia and with low testosterone concentrations. Although there is little experience with testosterone supplementation in these patients, there may be advantages to skeletal health as well as to other tissues (muscle, red cells, etc.). In a randomized study of crossover design, Reid et al. (259) reported that testosterone therapy apparently improved bone density and body composition in a small group of men receiving glucocorticoids. In a double-blind, placebo-controlled study, testosterone replacement was shown to have beneficial effects on BMD in glucocorticoid-treated men (260). The number of patients affected by conditions associated with low testosterone levels is potentially quite large, and more information is needed to understand the role of androgen replacement in the prevention/therapy of concomitant bone loss.
6. Unresolved issues concerning testosterone therapy and bone.
Important questions remain unresolved concerning the role of testosterone treatment in the prevention/therapy of osteoporosis in hypogonadal men, including:
The degree of hypogonadism (level of testosterone or estradiol) at which adverse skeletal effects begin to occur is uncertain, and hence it is difficult to choose those patients who would benefit from replacement.
It is unknown whether it is useful to assess estrogen concentrations in the diagnosis of hypogonadal bone disease in men and whether estrogen measurements are useful to monitor the success of testosterone therapy.
In general, available testosterone treatment studies are of relatively short duration, and it is unclear how long any increases in bone mass can be sustained and what eventual treatment effect can be expected.
The increase in bone mass that appears to accompany testosterone therapy is of uncertain usefulness in preventing fractures. To what extent testosterone may reduce fractures via effects on fall risk is unknown.
Whether pretreatment age, duration of hypogonadism, degree of osteopenia, remodeling state, and associated medical conditions affect the therapeutic response to testosterone is relatively unknown.
The potential adverse effects of long-term androgen therapy (e.g., prostate, lipids) are not well delineated.
H. Calcium and vitamin D
Calcium intake is probably important in the achievement of optimal peak bone mass in boys (261), as well as the prevention of bone loss later in life. Certainly vitamin D insufficiency is common in older men, and low vitamin D levels have been linked to lower BMD (262) and an increased risk of falls (263). However, few prospective studies have addressed the specific benefits of calcium and vitamin D supplementation in men. No bone density benefit was observed from calcium/vitamin D supplementation in an already well-nourished population of men (mean dietary calcium intake > 1000 mg/d) (264), and no antifracture benefit was observed in a large trial of vitamin D supplementation in older men and women with relatively high baseline intakes (265). Dietary calcium intake was not found to be related to fracture rate in the men followed as part of the Health Professionals Follow-up Study (266). On the other hand, an improvement in bone density was noted in healthy older men in response to a calcium and vitamin D supplement, whereas placebo-treated men lost bone (267). Calcium and vitamin D3-fortified milk improved femoral bone structure in older men (268), and low dietary calcium intake has been linked to higher fracture risk in other studies (96). Although calcium and vitamin D supplementation have not been demonstrated to reduce fracture risk in men (269,270), on the basis of available information the U.S. Institute of Medicine recently recommended that men should have a calcium intake of 1200 mg/d and a vitamin D intake of 800 IU. Others have recently suggested a vitamin D intake of 1000 IU per day or more (160,271). A reasonable approach, therefore, is to suggest a calcium intake of at least 1200 mg/d in preventative as well as therapeutic situations. A NIH Consensus Development Conference has suggested the somewhat higher calcium intake of 1500 mg/d in men after 65 yr of age (272).
I. Exercise
Whereas an exercise prescription is difficult to generate with currently available information, activity is probably beneficial in several ways. Reductions in strength and coordination contribute to fracture via an increased risk of falling (273). In addition, inactivity is associated with bone loss, and exercise may aid in maintaining bone mass. Specific exercise prescriptions to accomplish these goals have not been confirmed in men or women, although strength can be dramatically increased and risk of falls reduced in the elderly with achievable levels of exercise (274). That fracture rates are lower in elderly men who exercise modestly buttresses this contention (275). Falls and fall prevention strategies have been reviewed (276,277).
VI. Unresolved Issues Concerning Osteoporosis in Men
As is evident from this review, considerable inroads have been made into understanding all aspects of osteoporosis in men. However, there are major unresolved issues that should set the agenda for future research in this area. These include:
The most appropriate definition for osteoporosis in men in the absence of fractures— specifically, whether male or female reference ranges should be used to define T-scores in men.
Based on this definition, a better understanding of the true prevalence of osteoporosis in men in various ethnic groups.
Further understanding of the hormonal and nonhormonal factors causing age-related bone loss in men and specifically, the underlying mechanism(s) for the significant, ongoing trabecular bone loss in men (and women) in young adult life.
Further clarification of the different causes of “idiopathic” osteoporosis in young adult men, which is clearly a heterogeneous entity.
The cost effectiveness of obtaining various laboratory tests in the evaluation of men with osteoporosis.
The potential utility of measuring serum estradiol levels (in addition to serum testosterone levels) using standardized mass spectroscopy assays as part of the evaluation of osteoporosis in men.
The skeletal (and nonskeletal) benefits vs. risks of testosterone treatment of aging men with declining total and bioavailable testosterone levels.
Studies with pharmacological agents directly assessing fracture risk reduction in men, rather than relying on inferences from studies in women.
VII. Summary and Conclusions
With the aging of the population, osteoporosis in men is increasing as a public health problem. Hip, vertebral, and other fractures occur in men at a significant frequency, and both hip and vertebral fractures are associated with increased mortality in men. Although osteoporosis in men is a heterogeneous clinical entity, declining sex steroid levels and, in particular, declining bioavailable estradiol levels, appear to play an important role in mediating age-related bone loss in men. Other hormonal factors, such as age-related increases in serum PTH levels, vitamin D insufficiency, and declining IGF-I levels, may also have a role in pathogenesis. Secondary causes of osteoporosis also contribute significantly to fractures in men. Although there is ongoing debate regarding diagnostic criteria for defining osteoporosis in men, sex-specific reference ranges for BMD by DXA are commonly used. Fortunately, most of the drugs evaluated for the prevention and treatment of osteoporosis in women, particularly bisphosphonates and PTH, also appear to be effective in men. Given the availability of effective therapies for the prevention and treatment of osteoporosis in men, awareness regarding this disorder is critical for the prevention of morbidity and mortality as a consequence of fractures in aging men.
Acknowledgments
The authors thank James Peterson for help with the figures and Amanda Oelkers for secretarial assistance.
Footnotes
This work was supported by National Institutes of Health Grants AG004875, AR027065, DE014386, HL070838, AR049439, AR45647, AG027810, and RR024140.
Disclosure: S.K. has served on an advisory board for Novartis. S.A. has received honoraria from Merck. E.O. has nothing to declare.
First Published Online May 1. 2008
Abbreviations: BV/TV, Bone volume/tissue volume; CI, Confidence interval; DHEA, dehydroepiandrosterone; Dpd, deoxypyridinoline; DXA, dual x-ray absorptiometry; HR, high resolution; IGFBP, IGF binding protein; NTx, N-telopeptide of type I collagen; PINP, amino-terminal propeptide of type I collagen; pQCT, peripheral QCT; pyr, person years at risk; QCT, quantitative computed tomography; TbN, trabecular number; TbSp, trabecular separation; TbTh, trabecular thickness; vBMD, volumetric BMD.
References
- Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PWF, Kiel DP 2000 Risk factors for longitudinal bone loss in elderly men and women: the Framingham osteoporosis study. J Bone Miner Res 15:710–720 [DOI] [PubMed] [Google Scholar]
- Jones G, Nguyen T, Sambrook P, Kelly PJ, Eisman JA 1994 Progressive loss of bone in the femoral neck in elderly people: longitudinal findings from the Dubbo osteoporosis epidemiology study. BMJ 309:691–695 [PMC free article] [PubMed] [Google Scholar]
- Melton LJ, Chrischilles EA, Cooper C, Lane AW, Riggs BL 1992 How many women have osteoporosis. J Bone Miner Res 7:1005–1010 [DOI] [PubMed] [Google Scholar]
- Forsen L, Sogaard AJ, Meyer HE, Edna T, Kopjar B 1999 Survival after hip fracture: short- and long-term excess mortality according to age and gender. Osteoporos Int 10:73–78 [DOI] [PubMed] [Google Scholar]
- Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA 1999 Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 353:878–882 [DOI] [PubMed] [Google Scholar]
- Myers AH, Robinson EG, Van Natta ML, Michelson JD, Collins K, Baker SP 1991 Hip fractures among the elderly: factors associated with in-hospital mortality. Am J Epidemiol 134:1128–1137 [DOI] [PubMed] [Google Scholar]
- Diamond TH, Thornley SW, Sekel R, Smerdely P 1997 Hip fracture in elderly men: prognostic factors and outcomes [see comments]. Med J Aust 167:412–415 [DOI] [PubMed] [Google Scholar]
- Cooper C, Campion G, Melton LJ 1992 Hip fractures in the elderly: a world-wide projection. Osteoporos Int 2:285–289 [DOI] [PubMed] [Google Scholar]
- Johnell O, Kanis JA 2006 An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733 [DOI] [PubMed] [Google Scholar]
- Anonymous 1993 Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 94:646–650 [DOI] [PubMed] [Google Scholar]
- Singer BR, McLauchlan GJ, Robinson CM, Christie J 1998 Epidemiology of fractures in 15,000 adults: the influence of age and gender. J Bone Joint Surg Br 80:243–248 [DOI] [PubMed] [Google Scholar]
- Garraway WM, Stauffer RN, Kurland LT, O'Fallon WM 1979 Limb fractures in a defined population. I. Frequency and distribution. Mayo Clin Proc 54:701–707 [PubMed] [Google Scholar]
- Donaldson LJ, Cook A, Thomson RG 1990 Incidence of fractures in a geographically defined population. J Epidemiol Community Health 44:241–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis JA, Pitt FA 1992 Epidemiology of osteoporosis. Bone 13(Suppl 1):S7–S15 [DOI] [PubMed] [Google Scholar]
- van Staa TP, Dennison EM, Leufkens HG, Cooper C 2001 Epidemiology of fractures in England and Wales. Bone 29:517–522 [DOI] [PubMed] [Google Scholar]
- Court-Brown CM, Caesar B 2006 Epidemiology of adult fractures: a review. Injury 37:691–697 [DOI] [PubMed] [Google Scholar]
- Islam SS, Biswas RS, Nambiar AM, Syamlal G, Velilla AM, Ducatman AM, Doyle EJ 2001 Incidence and risk of work-related fracture injuries: experience of a state-managed workers’ compensation system. J Occup Environ Med 43:140–146 [DOI] [PubMed] [Google Scholar]
- Kanis JA, Johnell O, Oden A, Sernbo I, Redlund-Johnell I, Dawson A, De Laet C, Jonsson B 2000 Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int 11:669–674 [DOI] [PubMed] [Google Scholar]
- Cooley H, Jones G 2001 A population-based study of fracture incidence in southern Tasmania: lifetime fracture risk and evidence for geographic variations within the same country. Osteoporos Int 12:124–130 [DOI] [PubMed] [Google Scholar]
- Schuit SCE, van der Klift M, Weel AEAM, de Laet CEDH, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JPTM, Pols HAP 2004 Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone 34:195–202 [DOI] [PubMed] [Google Scholar]
- Melton III LJ, Crowson CS, O'Fallon WM 1999 Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos Int 9:29–37 [DOI] [PubMed] [Google Scholar]
- Jonsson BY, Siggeirsdottir K, Mogensen B, Sigvaldason H, Sigursson G 2004 Fracture rate in a population-based sample of men in Reykjavik. Acta Orthop Scand 75:195–200 [DOI] [PubMed] [Google Scholar]
- Sattin RW, Lambert Huber DA, DeVito CA, Rodriguez JG, Ros A, Bacchelli S, Stevens JA, Waxweiler RJ 1990 The incidence of fall injury events among the elderly in a defined population. Am J Epidemiol 131:1028–1037 [DOI] [PubMed] [Google Scholar]
- Winner SJ, Morgan CA, Evans JG 1989 Perimenopausal risk of falling and incidence of distal forearm fracture. BMJ 298:1486–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JA, Sogolow ED 2005 Gender differences for non-fatal unintentional fall related injuries among older adults. Inj Prev 11:115–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis JA, Johnell O, De Laet C, Jonsson B, Oden A, Ogelsby AK 2002 International variations in hip fracture probabilities: implications for risk assessment. J Bone Miner Res 17:1237–1244 [DOI] [PubMed] [Google Scholar]
- Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA 1990 Hip fracture incidence among the old and very old: a population-based study of 745435 cases. Am J Public Health 80:871–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AD, Silverthorn KG, Houston CS, Bernhardson S, Wajda A, Roos LL 1991 The incidence of fracture of the proximal femur in two million Canadians from 1972 to 1984: projections for Canada in the year 2006. Clin Orthop Rel Res 266:111–118 [PubMed] [Google Scholar]
- Gennari L, Bilezikian JP 2007 Osteoporosis in men. Endocrinol Metab Clin North Am 36:399–419 [DOI] [PubMed] [Google Scholar]
- Clark P, Lavielle P, Franco-Marina F, Ramirez E, Salmeron J, Kanis JA, Cummings SR 2005 Incidence rates and life-time risk of hip fractures in Mexicans over 50 years of age: a population-based study. Osteoporos Int 16:2025–2030 [DOI] [PubMed] [Google Scholar]
- Schwartz AV, Kelsey JL, Maggi S, Tuttleman M, Ho SC, Jonsson PV, Poor G, Sisson de Castro JA, Xu L, Matkin CC, Nelson LM, Heyse SP 1999 International variation in the incidence of hip fractures: cross-national project on osteoporosis for the World Health Organization Program for Research on Aging. Osteoporos Int 9:242–253 [DOI] [PubMed] [Google Scholar]
- Castro da Rocha FA, Ribeiro AR 2003 Low incidence of hip fractures in an equatorial area. Osteoporos Int 14:496–499 [DOI] [PubMed] [Google Scholar]
- Agnusdei D, Camporeale A, Gerardi D, Rossi S, Bocchi L, Gennari C 1993 Trends in the incidence of hip fracture in Siena, Italy, from 1980 to 1991. Bone 14:S31–S34 [DOI] [PubMed] [Google Scholar]
- Paspati I, Galanos A, Lyritis GP 1998 Hip fracture epidemiology in Greece during 1977–1992. Calcif Tissue Int 62:542–547 [DOI] [PubMed] [Google Scholar]
- Wildner M, Casper W, Bergmann KE 1999 A secular trend in hip fracture incidence in East Germany. Osteoporos Int 9:144–150 [DOI] [PubMed] [Google Scholar]
- Rogmark C, Sernbo I, Johnell O, Nilsson JA 1999 Incidence of hip fractures in Malmo, Sweden, 1992–1995. A trend-break. Acta Orthop Scand 70:19–22 [DOI] [PubMed] [Google Scholar]
- Huusko TM, Karppi P, Avikainen V, Kautiainen H, Sulkava R 1999 The changing picture of hip fractures: dramatic change in age distribution and no change in age-adjusted incidence within 10 years in central Finland. Bone 24:257–259 [DOI] [PubMed] [Google Scholar]
- Kannus P, Niemi S, Parkkari J, Palvanen M, Vuori I, Jarvinen M 1999 Hip fractures in Finland between 1970 and 1997 and predictions for the future [see comments]. Lancet 353:802–805 [DOI] [PubMed] [Google Scholar]
- Ismail AA, Pye SR, Cockerill WC, Lunt M, Silman AJ, Reeve J, Banzer D, Benevolenskaya LI, Bhalla A, Bruges Armas J, Cannata JB, Cooper C, Delmas PD, Dequeker J, Dilsen G, Falch JA, Felsch B, Felsenberg D, Finn JD, Gennari C, Hoszowski K, Jajic I, Janott J, Johnell O, Kanis JA, Kragl G, Lopez Vaz A, Lorenc R, Lyritis G, Marchand F, Masaryk P, Matthis C, Miazgowski T, Naves-Diaz M, Pols HAP, Poor G, Rapado A, Raspe HH, Reid DM, Reisinger W, Scheidt-Nave C, Stepan J, Todd C, Weber K, Woolf AD, O'Neill TW 2002 Incidence of limb fracture across Europe: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int 13:565–571 [DOI] [PubMed] [Google Scholar]
- Giversen IM 2006 Time trends of age-adjusted incidence rates of first hip fractures: a register-based study among older people in Viborg County, Denmark, 1987–1997. Osteoporos Int 17:552–564 [DOI] [PubMed] [Google Scholar]
- Hernandez JL, Olmos JM, Alonso MA, Gonzalez-Fernandez CR, Martinez J, Pajaron M, Llorca J, Gonzalez-Macias J 2006 Trend in hip fracture epidemiology over a 14-year period in a Spanish population. Osteoporos Int 17:464–470 [DOI] [PubMed] [Google Scholar]
- Icks A, Haastert B, Wildner M, Becker C, Meyer G, Trend of hip fracture incidence in Germany 1995–2004: a population-based study. Osteoporos Int, in press [DOI] [PubMed] [Google Scholar]
- Sanders KM, Nicholson GC, Ugoni AM, Pasco JA, Seeman E, Kotowicz MA 1999 Health burden of hip and other fractures in Australia beyond 2000. Projections based on the Geelong Osteoporosis Study [see comments]. Med J Aust 170:467–470 [DOI] [PubMed] [Google Scholar]
- Chang KP, Center JR, Nguyen TV, Eisman JA 2004 Incidence of hip and other osteoporotic fractures in elderly men and women: Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res 19:532–536 [DOI] [PubMed] [Google Scholar]
- Ling X, Lu A, Zhao X, Chen X, Cummings SR 1996 Very low rates of hip fracture in Beijing, People’s Republic of China, the Beijing Osteoporosis Project. Am J Epidemiol 144:901–907 [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Sakata K, Yoshimura N 1997 Epidemiology of osteoporosis in Japan. Osteoporos Int 7:S99–102 [DOI] [PubMed] [Google Scholar]
- Yan L, Zhou B, Prentice A, Wang X, Golden MH 1999 Epidemiological study of hip fracture in Shenyang, People’s Republic of China. Bone 24:151–155 [DOI] [PubMed] [Google Scholar]
- Lau EM, Cooper C, Fung H, Lam D, Tsang KK 1999 Hip fracture in Hong Kong over the last decade—a comparison with the UK. J Public Health Med 21:249–250 [DOI] [PubMed] [Google Scholar]
- Lau EM, Lee JK, Suriwongpaisal P, Saw SM, Das De S, Khir A, Sambrook P 2001 The incidence of hip fracture in four Asian countries: the Asian Osteoporosis Study (AOS). Osteoporos Int 12:239–243 [DOI] [PubMed] [Google Scholar]
- Hagino H, Katagiri H, Okano T, Yamamoto K, Teshima R 2005 Increasing incidence of hip fracture in Tottori Prefecture, Japan: trend from 1986 to 2001. Osteoporos Int 16:1963–1968 [DOI] [PubMed] [Google Scholar]
- Zebaze RMD, Seeman E 2003 Epidemiology of hip and wrist fractures in Cameroon, Africa. Osteoporos Int 14:301–305 [DOI] [PubMed] [Google Scholar]
- El Maghraoui A, Koumba BA, Jroundi I, Achemlal L, Bezza A, Tazi MA 2005 Epidemiology of hip fractures in 2002 in Rabat, Morocco. Osteoporos Int 16:597–602 [DOI] [PubMed] [Google Scholar]
- Maggi S, Kelsey JL, Litvak J, Heyse SP 1991 Incidence of hip fractures in the elderly: a cross-national analysis [see comments]. Osteoporos Int 1:232–241 [DOI] [PubMed] [Google Scholar]
- Memon A, Pospula WM, Tantawy AY, Abdul-Ghafar S, Suresh A, Al-Rowaih A 1998 Incidence of hip fracture in Kuwait. Int J Epidemiol 27:860–865 [DOI] [PubMed] [Google Scholar]
- Johnell O, Borgstrom F, Jonsson B, Kanis J 2007 Latitude, socioeconomic prosperity, mobile phones and hip fracture risk. Osteoporos Int 18:333–337 [DOI] [PubMed] [Google Scholar]
- Solomon L 1968 Osteoporosis and fracture of the femoral neck in the South African Bantu. J Bone Joint Surg 50B:2–13 [PubMed] [Google Scholar]
- Gullberg B, Duppe H, Nilsson B, Redlund-Johnell I, Sernbo I, Obrant K, Johnell O 1993 Incidence of hip fractures in Malmo, Sweden (1950–1991). Bone 14:S23–S29 [DOI] [PubMed] [Google Scholar]
- Hedlund R, Ahlbom A, Lindgren U 1986 Hip fracture incidence in Stockholm 1972–1981. Acta Orthop Scand 57:30–34 [DOI] [PubMed] [Google Scholar]
- Melton III LJ, O'Fallon WM, Riggs BL 1987 Secular trends in the incidence of hip fractures. Calcif Tissue Int 41:57–64 [DOI] [PubMed] [Google Scholar]
- Spector TD, Cooper C, Lewis AF 1990 Trends in admissions for hip fracture in England and Wales, 1968–1985. BMJ 300:1173–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton III LJ, Therneau TM, Larson DR 1998 Long-term trends in hip fracture prevalence: the influence of hip fracture incidence and survival. Osteoporos Int 8:68–74 [DOI] [PubMed] [Google Scholar]
- Zingmond DS, Melton 3rd LJ, Silverman SL 2004 Increasing hip fracture incidence in California Hispanics, 1983 to 2000. Osteoporos Int 15:603–610 [DOI] [PubMed] [Google Scholar]
- Gullberg B, Johnell O, Kanis JA 1997 World-wide projections for hip fracture. Osteoporos Int 7:407–413 [DOI] [PubMed] [Google Scholar]
- Schurch MA, Rizzoli R, Mermillod B, Vasey H, Michel JP, Bonjour JP 1996 A prospective study on socioeconomic aspects of fracture of the proximal femur. J Bone Miner Res 11:1935–1942 [DOI] [PubMed] [Google Scholar]
- Kiebzak GM, Beinart GA, Perser K, Ambrose CG, Siff SJ, Heggeness MH 2002 Undertreatment of osteoporosis in men with hip fracture. Arch Intern Med 162:2217–2222 [DOI] [PubMed] [Google Scholar]
- Cree M, Soskolne CL, Belseck E, Hornig J, McElhaney JE, Brant R, Suarez-Almazor M 2000 Mortality and institutionalization following hip fracture. J Am Geriatr Soc 48:283–288 [DOI] [PubMed] [Google Scholar]
- Chariyalertsak S, Suriyawongpisal P, Thakkinstain A 2001 Mortality after hip fractures in Thailand. Int Orthop 25:294–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetti A, Herrmann F, Hoffmeyer P, Schurch MA, Bonjour JP, Rizzoli R 2002 Survival and potential years of life lost after hip fracture in men and age-matched women. Osteoporos Int 13:731–737 [DOI] [PubMed] [Google Scholar]
- Karagiannis A, Papakitsou E, Dretakis K, Galanos A, Megas P, Lambiris E, Lyritis GP 2006 Mortality rates of patients with a hip fracture in a southwestern district of Greece: ten-year follow-up with reference to the type of fracture. Calcif Tissue Int 78:72–77 [DOI] [PubMed] [Google Scholar]
- Bass E, French DD, Bradham DD, Rubenstein LZ 2007 Risk-adjusted mortality rates of elderly veterans with hip fractures. Ann Epidemiol 17:514–519 [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Suzuki S, Wingstrand H 2007 Risk of mortality following hip fracture in Japan. J Orthop Sci 12:113–117 [DOI] [PubMed] [Google Scholar]
- Poor G, Atkinson EJ, O'Fallon WM, Melton 3rd LJ 1995 Determinants of reduced survival following hip fractures in men. Clin Orthop Relat Res 260–265 [PubMed] [Google Scholar]
- Pande I, Scott DL, O'Neill TW, Pritchard C, Woolf AD, Davis MJ 2006 Quality of life, morbidity, and mortality after low trauma hip fracture in men. Ann Rheum Dis 65:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche JJW, Wenn RT, Sahota O, Moran CG 2005 Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ 331:1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill TW, Felsenberg D, Varlow J, Cooper C, Kanis JA, Silman AJ 1996 The prevalence of vertebral deformity in European men and women: the European vertebral osteoporosis study. J Bone Miner Res 11:1010–1018 [DOI] [PubMed] [Google Scholar]
- Davies KM, Stegman MR, Heaney RP, Recker RR 1996 Prevalence and severity of vertebral fracture: the Saunders County Bone Quality Study. Osteoporos Int 6:160–165 [DOI] [PubMed] [Google Scholar]
- Santavirta S, Konttinen YT, Heliovaara M, Knekt P, Luthje P, Aromaa A 1992 Determinants of osteoporotic thoracic vertebral fracture: screening of 57,000 Finnish women and men. Acta Orthop Scand 63:198–202 [DOI] [PubMed] [Google Scholar]
- Szulc P, Munoz F, Marchand F, Delmas PD 2001 Semiquantitative evaluation of prevalent vertebral deformities in men and their relationship with osteoporosis: the MINOS study. Osteoporos Int 12:302–310 [DOI] [PubMed] [Google Scholar]
- Felsenberg D, Silman AJ, Lunt M, Armbrecht G, Ismail AA, Finn JD, Cockerill WC, Banzer D, Benevolenskaya LI, Bhalla A, Bruges Armas J, Cannata JB, Cooper C, Dequeker J, Eastell R, Felsch B, Gowin W, Havelka S, Hoszowski K, Jajic I, Janott J, Johnell O, Kanis JA, Kragl G, Lopez Vaz A, Lorenc R, Lyritis G, Masaryk P, Matthis C, Miazgowski T, Parisi G, Pols HAP, Poor G, Raspe HH, Reid DM, Reisinger W, Scheidt-Nave C, Stepan JJ, Todd CJ, Weber K, Woolf AD, Yershova OB, Reeve J, O'Neill TW 2002 Incidence of vertebral fracture in Europe: results from the European Prospective Osteoporosis Study (EPOS). J Bone Miner Res 17:716–724 [DOI] [PubMed] [Google Scholar]
- Cooper C, Atkinson EJ, O'Fallon WM, Melton 3rd LJ 1992 Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res 7:221–227 [DOI] [PubMed] [Google Scholar]
- Van Der Klift M, De Laet CEDH, McCloskey EV, Hofman A, Pols HAP 2002 The incidence of vertebral fractures in men and women: the Rotterdam study. J Bone Miner Res 17:1051–1056 [DOI] [PubMed] [Google Scholar]
- Ismail AA, O'Neil TW, Cooper C, Finn JD, Bhalla AK, Cannata JB, Delmas P, Falch JA, Felsch B, Hoszowski K, Johnell O, Diaz-Lopez JB, Lopez Vaz A, Marchand F, Raspe H, Reid DM, Todd C, Weber K, Woolf A, Reeve J, Silman AJ 1998 Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int 8:291–297 [DOI] [PubMed] [Google Scholar]
- Melton III LJ, Atkinson EJ, Cooper C, O'Fallon WM, Riggs BL 1999 Vertebral fractures predict subsequent fractures. Osteoporos Int 10:214–221 [DOI] [PubMed] [Google Scholar]
- Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B 2004 Fracture risk following an osteoporotic fracture. Osteoporos Int 15:175–179 [DOI] [PubMed] [Google Scholar]
- Hasserius R, Karlsson MK, Nilsson BE, Redlund-Johnell I, Johnell O, European Vertebral Osteoporosis S 2003 Prevalent vertebral deformities predict increased mortality and increased fracture rate in both men and women: a 10-year population-based study of 598 individuals from the Swedish cohort in the European Vertebral Osteoporosis Study. Osteoporos Int 14:61–68 [DOI] [PubMed] [Google Scholar]
- Scane AC, Francis RM, Sutcliffe AM, Francis MJ, Rawlings DJ, Chapple CL 1999 Case-control study of the pathogenesis and sequelae of symptomatic vertebral fractures in men. Osteoporos Int 9:91–97 [DOI] [PubMed] [Google Scholar]
- Burger H, Van Daele PLA, Grashuis K, Hofman A, Grobbee DE, Schutte HE, Birkenhager JC, Pols HAP 1997 Vertebral deformities and functional impairment in men and women. J Bone Miner Res 12:152–157 [DOI] [PubMed] [Google Scholar]
- Matthis C, Weber U, O'Neill TW, Raspe H 1998 Health impact associated with vertebral deformities: results from the European Vertebral Osteoporosis Study (EVOS). Osteoporos Int 8:364–372 [DOI] [PubMed] [Google Scholar]
- Delmas PD, Marin F, Marcus R, Misurski DA, Mitlak BH 2007 Beyond hip: importance of other nonspinal fractures. Am J Med 120:381–387 [DOI] [PubMed] [Google Scholar]
- Melton 3rd LJ, Amadio PC, Crowson CS, O'Fallon WM 1998 Long-term trends in the incidence of distal forearm fractures. Osteoporos Int 8:341–348 [DOI] [PubMed] [Google Scholar]
- Wigg AER, Hearn TC, McCaul KA, Anderton SM, Wells VM, Krishnan J 2003 Number, incidence, and projections of distal forearm fractures admitted to hospital in Australia. J Trauma 55:87–93 [DOI] [PubMed] [Google Scholar]
- Mallmin H, Ljunghall S, Persson I, Naessen T, Krusemo UB, Bergstrom R 1993 Fracture of the distal forearm as a forecaster of subsequent hip fracture: a population-based cohort study with 24 years of follow-up. Calcif Tissue Int 52:269–272 [DOI] [PubMed] [Google Scholar]
- Cuddihy MT, Gabriel SE, Crowson CS, O'Fallon WM, Melton 3rd LJ 1999 Forearm fractures as predictors of subsequent osteoporotic fractures. Osteoporos Int 9:469–475 [DOI] [PubMed] [Google Scholar]
- Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M 2002 Increasing number and incidence of low-trauma ankle fractures in elderly people: Finnish statistics during 1970–2000 and projections for the future. Bone 31:430–433 [DOI] [PubMed] [Google Scholar]
- Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B 2004 Mortality after osteoporotic fractures. Osteoporos Int 15:38–42 [DOI] [PubMed] [Google Scholar]
- Center JR, Bliuc D, Nguyen TV, Eisman JA 2007 Risk of subsequent fracture after low-trauma fracture in men and women. JAMA 297:387–394 [DOI] [PubMed] [Google Scholar]
- Nordqvist A, Petersson C 1994 The incidence of fractures of the clavicle. Clin Orthop Relat Res 127–132 [PubMed] [Google Scholar]
- Melton III LJ, Sampson JM, Morrey BJ, Ilstrup DM 1981 Epidemiologic features of pelvic fractures. Clin Orthop 155:43–47 [PubMed] [Google Scholar]
- Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M 2000 Epidemiology of osteoporotic pelvic fractures in elderly people in Finland: sharp increase in 1970–1997 and alarming projections for the new millennium. Osteoporos Int 11:443–448 [DOI] [PubMed] [Google Scholar]
- Looker AC, Orwoll ES, Johnston CC, Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP 1997 Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res 12:1761–1768 [DOI] [PubMed] [Google Scholar]
- Melton III LJ, Atkinson EJ, O'Connor MK, O'Fallon WM, Riggs BL 1998 Bone density and fracture risk in men. J Bone Miner Res 13:1915–1923 [DOI] [PubMed] [Google Scholar]
- Nelson DA, Jacobsen G, Barondess DA, Parfitt AM 1995 Ethnic differences in regional bone density, hip axis length, and lifestyle variables among healthy black and white men. J Bone Miner Res 10:782–787 [DOI] [PubMed] [Google Scholar]
- Bell NH, Gordon L, Stevens J, Shary JR 1995 Demonstration that bone mineral density of the lumbar spine, trochanter, and femoral neck is higher in black than in white young men. Calcif Tissue Int 56:11–13 [DOI] [PubMed] [Google Scholar]
- Silverman SL, Madison RE 1988 Decreased incidence of hip fracture in Hispanics, Asians, and Blacks: California Hospital Discharge Data. Am J Public Health 78:1482–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS, Jacobsen SJ, Furner SE, Levy PS, Brody JA, Goldberg J 1998 Hip fracture incidence among elderly Hispanics. Am J Public Health 88:1245–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer RL 1988 Ethnic differences in hip fracture: a reduced incidence in Mexican Americans. Am J Epidemiol 127:145–149 [DOI] [PubMed] [Google Scholar]
- Dennison E, Yoshimura N, Hashimoto T, Cooper C 1998 Bone loss in Great Britain and Japan: a comparative longitudinal study. Bone 23:379–382 [DOI] [PubMed] [Google Scholar]
- Lauderdale DS, Jacobsen SJ, Furner SE, Levy PS, Brody JA, Goldberg J 1997 Hip fracture incidence among elderly Asian-American populations. Am J Epidemiol 146:502–509 [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton III LJ, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S 2004 Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res 19:1945–1954 [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton III LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S 2008 A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res 23:205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall LM, Lang TF, Lambert LC, Zmuda JM, Ensrud KE, Orwoll ES, Osteoporotic Fractures in Men (MrOS) Research Group 2006 Dimensions and volumetric BMD of the proximal femur and their relation to age among older U.S. men. J Bone Miner Res 21:1197–1206 [DOI] [PubMed] [Google Scholar]
- Muller R, Hildebrand T, Ruegsegger P 1994 Non-invasive bone biopsy: a new method to analyze and display the three-dimensional structure of trabecular bone. Phys Med Biol 39:145–164 [DOI] [PubMed] [Google Scholar]
- Laib A, Hilderbrand T, Hauselmann HJ, Ruegsegger P 1997 Ridge number density: a parameter for in vivo bone structure analysis. Bone 21:541–546 [DOI] [PubMed] [Google Scholar]
- Laib A, Hauselmann HJ, Ruegsegger P 1998 In vivo high resolution 3D-QCT of the human forearm. Technol Health Care 6:329–337 [PubMed] [Google Scholar]
- Laib A, Ruegsegger P 1999 Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone 24:35–39 [DOI] [PubMed] [Google Scholar]
- Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, Peterson JM, Melton III LJ 2006 Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res 21:124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaron JE, Makins NB, Sagreiya K 1987 The microanatomy of trabecular bone loss in normal aging men and women. Clin Orthop 215:260–271 [PubMed] [Google Scholar]
- Silva MJ, Gibson LJ 1997 Modeling the mechanical behavior of vertebral trabecular bone: effects of age-related changes in microstructure. Bone 21:191–199 [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton III LJ, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL 1998 Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 83:2266–2274 [DOI] [PubMed] [Google Scholar]
- Mendel CM 1989 The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev 10:232–274 [DOI] [PubMed] [Google Scholar]
- Hammond GL 2002 Access of reproductive steroids to target tissues. Obstet Gynecol Clin North Am 29:411–423 [DOI] [PubMed] [Google Scholar]
- Greendale GA, Edelstein S, Barrett-Connor E 1997 Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo study. J Bone Miner Res 12:1833–1843 [DOI] [PubMed] [Google Scholar]
- Orwoll E, Lambert LC, Marshall LM, Phipps K, Blank J, Barrett-Connor E, Cauley J, Ensrud K, Cummings S 2006 Testosterone and estradiol among older men. J Clin Endocrinol Metab 91:1336–1344 [DOI] [PubMed] [Google Scholar]
- Slemenda CW, Longcope C, Zhou L, Hui SL, Peacock M, Johnston C 1997 Sex steroids and bone mass in older men: positive associations with serum estrogens and negative associations with androgens. J Clin Invest 100:1755–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center JR, Nguyen TV, Sambrook PN, Eisman JA 1999 Hormonal and biochemical parameters in the determination of osteoporosis in elderly men. J Clin Endocrinol Metab 84:3626–3635 [DOI] [PubMed] [Google Scholar]
- Ongphiphadhanakul B, Rajatanavin R, Chanprasertyothin S, Piaseu N, Chailurkit L 1998 Serum oestradiol and oestrogen-receptor gene polymorphism are associated with bone mineral density independently of serum testosterone in normal males. Clin Endocrinol (Oxf) 49:803–809 [DOI] [PubMed] [Google Scholar]
- van den Beld AW, de Jong FH, Grobbee DE, Pols HAP, Lamberts SWJ 2000 Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab 85:3276–3282 [DOI] [PubMed] [Google Scholar]
- Amin S, Zhang Y, Sawin CT, Evans SR, Hannan MT, Kiel DP, Wilson PWF, Felson DT 2000 Association of hypogonadism and estradiol levels with bone mineral density in elderly men from the Framingham study. Ann Intern Med 133:951–963 [DOI] [PubMed] [Google Scholar]
- Szulc P, Munoz F, Claustrat B, Garnero P, Marchand F 2001 Bioavailable estradiol may be an important determinant of osteoporosis in men: the MINOS study. J Clin Endocrinol Metab 86:192–199 [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton LJ, Atkinson EJ, O'Fallon WM 2001 Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab 86:3555–3561 [DOI] [PubMed] [Google Scholar]
- Gennari L, Merlotti D, Martini G, Gonnelli S, Franci B, Campagna S, Lucani B, Canto ND, Valenti R, Gennari C, Nuti R 2003 Longitudinal association between sex hormone levels, bone loss, and bone turnover in elderly men. J Clin Endocrinol Metab 88:5327–5333 [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton III LJ, Robb RA, Camp JJ, Atkinson EJ, Oberg AL, Rouleau PA, Riggs BL 2005 Relationship of volumetric BMD and structural parameters at different skeletal sites to sex steroid levels in men. J Bone Miner Res 20:730–740 [DOI] [PubMed] [Google Scholar]
- Khosla S, Amin S, Singh RJ, Atkinson EJ, Melton LJI, Riggs BL, Comparison of sex steroid measurements in men by immunoassay versus mass spectroscopy and relationships with cortical and trabecular volumetric bone mineral density. Osteoporos Int, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennari L, Nuti R, Bilezikian JP 2004 Aromatase activity and bone homeostasis in men. J Clin Endocrinol Metab 89:5898–5907 [DOI] [PubMed] [Google Scholar]
- Van Pottelbergh I, Goemaere S, Kaufman JM 2003 Bioavailable estradiol and an aromatase gene polymorphism are determinants of bone mineral density changes in men over 70 years of age. J Clin Endocrinol Metab 88:3075–3081 [DOI] [PubMed] [Google Scholar]
- Gennari L, Masi L, Merlotti D, Picariello L, Falchetti A, Tanini A, Mavilia C, Del Monte F, Gonnelli S, Lucani B, Gennari C, Brandi ML 2004 A polymorphic CYP19 TTTA repeat influences aromatase activity and estrogen levels in elderly men: effects on bone metabolism. J Clin Endocrinol Metab 89:2803–2810 [DOI] [PubMed] [Google Scholar]
- Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S 2000 Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 106:1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder BZ, LeBlanc KM, Schoenfeld DA, Eastell R, Finkelstein JS 2003 Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab 88:204–210 [DOI] [PubMed] [Google Scholar]
- Kasai R, Bianco P, Robey PG, Kahn AJ 1994 Production and characterization of an antibody against the human bone GLA protein (BGP/osteocalcin) propeptide and its use in immunocytochemistry of bone cells. Bone Miner 25:167–182 [DOI] [PubMed] [Google Scholar]
- Lian JB, Stein GS, Canalis E, Gehron Robey P, Boskey AL 1999 Bone formation: osteoblast lineage cells, growth factors, matrix proteins, and the mineralization process. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism 3:14–29 [Google Scholar]
- Taxel P, Kennedy DG, Fall PM, Willard AK, Clive JM, Raisz LG 2001 The effect of aromatase inhibition on sex steroids, gonadotropins, and markers of bone turnover in older men. J Clin Endocrinol Metab 86:2869–2874 [DOI] [PubMed] [Google Scholar]
- Baillie SP, Davison CE, Johnson FJ, Francis RM 1992 Pathogenesis of vertebral crush fractures in men. Age Ageing 21:139–141 [DOI] [PubMed] [Google Scholar]
- Stanley HL, Schmitt BP, Poses RM, Deiss WP 1991 Does hypogonadism contribute to the occurrence of a minimal trauma hip fracture in elderly men? J Am Geriatr Soc 39:766–771 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Mueller JE, von Muhlen DG, Laughlin GA, Schneider DL, Sartoris DJ 2000 Low levels of estradiol are associated with vertebral fractures in older men, but not women: the Rancho Bernardo Study. J Clin Endocrinol Metab 85:219–223 [DOI] [PubMed] [Google Scholar]
- Amin S, Zhang Y, Felson DT, Sawin CT, Hannan MT, Wilson PWF, Kiel DP 2006 Estradiol, testosterone, and the risk for hip fractures in elderly men from the Framingham Study. Am J Med 119:426–433 [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton LJ, Riggs BL 2002 Estrogen and the male skeleton. J Clin Endocrinol Metab 87:1443–1450 [DOI] [PubMed] [Google Scholar]
- Mellstrom D, Johnell O, Ljunggren O, Eriksson AL, Lorentzon M, Mallmin H, Holmberg A, Redlund-Johnell I, Orwoll E, Ohlsson C 2006 Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res 21:529–535 [DOI] [PubMed] [Google Scholar]
- Meier C, Nguyen TV, Handelsman DJ, Schindler C, Kushnir MM, Rockwood AL, Meikle AW, Center JR, Eisman JA, Seibel MJ 2008 Endogenous sex hormones and incident fracture risk in older men. Arch Intern Med 168:47–54 [DOI] [PubMed] [Google Scholar]
- Turner RT, Wakley GK, Hannon KS 1990 Differential effects of androgens on cortical bone histomorphometry in gonadectomized male and female rats. J Orthop Res 8:612–617 [DOI] [PubMed] [Google Scholar]
- Lorentzon M, Swanson C, Andersson N, Mellstrom D, Ohlsson C 2005 Free testosterone is a positive, whereas free estradiol is a negative, predictor of cortical bone size in young Swedish men: the GOOD study. J Bone Miner Res 20:1334–1341 [DOI] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS 1994 Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331:1056–1061 [DOI] [PubMed] [Google Scholar]
- Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K 1995 Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 80:3689–3698 [DOI] [PubMed] [Google Scholar]
- Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER 1997 Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 337:91–95 [DOI] [PubMed] [Google Scholar]
- Bouillon R, Bex M, Vanderschueren D, Boonen S 2004 Estrogens are essential for male pubertal periosteal bone expansion. J Clin Endocrinol Metab 89:6025–6029 [DOI] [PubMed] [Google Scholar]
- Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M, Clyne CD, Davis S, Simpson ER, Carani C 2004 Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab 89:61–70 [DOI] [PubMed] [Google Scholar]
- Rochira V, Faustini-Fustini M, Balestrieri A, Carani C 2000 Estrogen replacement therapy in a man with congenital aromatase deficiency: effects of different doses of transdermal estradiol on bone mineral density and hormonal parameters. J Clin Endocrinol Metab 85:1841–1845 [DOI] [PubMed] [Google Scholar]
- Bilezikian JP, Morishima A, Bell J, Grumbach MM 1998 Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N Engl J Med 339:599–603 [DOI] [PubMed] [Google Scholar]
- Cosman F, Shen V, Xie F, Seibel M, Ratcliffe A, Lindsay R 1993 Estrogen protection against bone resorbing effects of parathyroid hormone infusion. Ann Intern Med 118:337–343 [DOI] [PubMed] [Google Scholar]
- Kennel KA, Riggs BL, Achenbach SJ, Oberg AL, Khosla S 2003 Role of parathyroid hormone in mediating age-related changes in bone resorption in men. Osteoporos Int 14:631–636 [DOI] [PubMed] [Google Scholar]
- Holick MF 2007 Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- Ho KY, Evans WS, Blizzard RM, Veldhuis JD, Merriam GR, Samojlik E, Furlanetto R, Rogol AD, Kaiser DL, Thorner MO 1987 Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab 64:51–58 [DOI] [PubMed] [Google Scholar]
- Bennett A, Wahner HW, Riggs BL, Hintz RL 1984 Insulin-like growth factors I and II, aging and bone density in women. J Clin Endocrinol Metab 59:701–704 [DOI] [PubMed] [Google Scholar]
- Boonen S, Mohan S, Dequeker J, Aerssens J, Vanderschueren D, Verbeke G, Broos P, Bouillon R, Baylink DJ 1999 Down-regulation of the serum stimulatory components of the insulin-like growth factor (IGF) system (IGF-I, IGF-II, IGF binding protein [BP]-3, and IGFBP-5) in age-related (type II) femoral neck osteoporosis. J Bone Miner Res 14:2150–2158 [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton III LJ, Achenbach SJ, Oberg AL, Riggs BL 2006 Hormonal and biochemical determinants of trabecular microstructure at the ultradistal radius in women and men. J Clin Endocrinol Metab 91:885–891 [DOI] [PubMed] [Google Scholar]
- Clemmons DR 1997 Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev 8:45–62 [DOI] [PubMed] [Google Scholar]
- Amin S, Riggs BL, Atkinson EJ, Oberg AL, Melton III LJ, Khosla S 2004 A potentially deleterious role of IGFBP-2 on bone density in aging men and women. J Bone Miner Res 19:1075–1083 [DOI] [PubMed] [Google Scholar]
- Amin S, Riggs BL, Melton III LJ, Achenbach SJ, Atkinson EJ, Khosla S 2007 High serum IGFBP-2 is predictive of increased bone turnover in aging men and women. J Bone Miner Res 22:799–807 [DOI] [PubMed] [Google Scholar]
- Crave JC, Lejeune H, Brebant C, Baret C, Pugeat M 1995 Differential effects of insulin and insulin-like growth factor I on the production of plasma steroid-binding globulins by human hepatoblastoma-derived (Hep G2) cells. J Clin Endocrinol Metab 80:1283–1289 [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Scheidt-Nave C, Leidig-Bruckner G, Woitge H, Blum WF, Wuster C, Haack D, Ziegler R 1996 Relationship between circulating insulin-like growth factor components and sex hormones in a population-based sample of 50- to 80-year-old men and women. J Clin Endocrinol Metab 81:2534–2540 [DOI] [PubMed] [Google Scholar]
- Labrie F, Belanger A, Cusan L, Gomez JL, Candas B 1997 Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab 82:2396–2402 [DOI] [PubMed] [Google Scholar]
- Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton III LJ, Smith GE, Khosla S, Jensen MD 2006 DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355:1647–1659 [DOI] [PubMed] [Google Scholar]
- Eastell R, Riggs BL 1997 Vitamin D and osteoporosis. San Diego: Academic Press; 695–711 [Google Scholar]
- Nguyen TV, Center JR, Eisman JA 2000 Osteoporosis in elderly men and women: effects of dietary calcium, physical activity, and body mass index. J Bone Miner Res 15:322–331 [DOI] [PubMed] [Google Scholar]
- Palacios C 2006 The role of nutrients in bone health, from A to Z. Crit Rev Food Sci Nutr 46:621–628 [DOI] [PubMed] [Google Scholar]
- Frost HM 1997 On our age-related bone loss: insights from a new paradigm. J Bone Miner Res 12:1539–1546 [DOI] [PubMed] [Google Scholar]
- Frost HM 1997 Osteoporoses: their nature and therapeutic targets (insights from a new paradigm). In: Anabolic treatments for osteoporosis. Boca Raton, FL: CRC Press; 1–29 [Google Scholar]
- Frost HM, Schonau E 2000 The “muscle-bone unit” in children and adolescents: a 2000 overview. J Pediatr Endocrinol Metab 13:571–590 [DOI] [PubMed] [Google Scholar]
- Proctor DN, Melton LJ, Khosla S, Crowson CS, O'Connor MK, Riggs BL 2000 Relative influence of physical activity, muscle mass and strength on bone density. Osteoporos Int 11:944–952 [DOI] [PubMed] [Google Scholar]
- Perry III HM, Fallon MD, Bergfeld M, Teitelbaum SL, Avioli LV 1982 Osteoporosis in young men. Arch Intern Med 142:1295–1298 [DOI] [PubMed] [Google Scholar]
- Resch H, Pietschmann P, Woloszczuk W, Krexner E, Bernecker P, Willvonseder R 1992 Bone mass and biochemical parameters of bone metabolism in men with spinal osteoporosis. Eur J Clin Invest 22:542–545 [DOI] [PubMed] [Google Scholar]
- Bilezikian JP 1999 Osteoporosis in men. J Clin Endocrinol Metab 84:3431–3434 [DOI] [PubMed] [Google Scholar]
- Peris P, Guanabens N, Monegal A, Suris X, Alvarez L, Martinez de Osaba MJ, Hernandez MV, Munoz-Gomez J 1995 Aetiology and presenting symptoms in male osteoporosis. Br J Rheumatol 34:936–941 [DOI] [PubMed] [Google Scholar]
- Gillberg P, Johansson AG, Ljunghall S 1999 Decreased estradiol levels and free androgen index and elevated sex hormone-binding globulin levels in male idiopathic osteoporosis. Calcif Tissue Int 64:209–213 [DOI] [PubMed] [Google Scholar]
- Legrand E, Hedde C, Gallois Y, Degasne I, Boux De Casson F, Mathieu E, Basle MF, Chappard D, Audran M 2001 Osteoporosis in men: a potential role for the sex hormone binding globulin. Bone 29:90–95 [DOI] [PubMed] [Google Scholar]
- Evans SF, Davie MW 2002 Low body size and elevated sex-hormone binding globulin distinguish men with idiopathic vertebral fracture. Calcif Tissue Int 70:9–15 [DOI] [PubMed] [Google Scholar]
- Van Pottelbergh I, Goemaere S, Zmierczak H, Kaufman JM 2004 Perturbed sex steroid status in men with idiopathic osteoporosis and their sons. J Clin Endocrinol Metab 89:4949–4953 [DOI] [PubMed] [Google Scholar]
- Carlsen CG, Soerensen TH, Eriksen EF 2000 Prevalence of low serum estradiol levels in male osteoporosis. Osteoporos Int 11:697–701 [DOI] [PubMed] [Google Scholar]
- Kurland ES, Rosen CJ, Cosman F, McMahon D, Chan F, Shane E, Lindsay R, Dempster D, Bilezikian JP 1997 Insulin-like growth factor-I in men with idiopathic osteoporosis. J Clin Endocrinol Metab 82:2799–2805 [DOI] [PubMed] [Google Scholar]
- Kurland ES, Chan FKW, Rosen CJ, Bilezikian JP 1998 Normal growth hormone secretory reserve in men with idiopathic osteoporosis and reduced circulating levels of insulin-like growth factor I. J Clin Endocrinol Metab 83:2576–2579 [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Kurland ES, Vereault D, Adler RA, Rackoff PJ, Craig WY, Witte S, Rogers J, Bilezikian JP 1998 Association between serum insulin growth factor-I (IGF-I) and a simple sequence repeat in IGF-I gene: implications for genetic studies of bone mineral density. J Clin Endocrinol Metab 83:2286–2290 [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ 1986 Medical progress series: involutional osteoporosis. N Engl J Med 314:1676–1686 [DOI] [PubMed] [Google Scholar]
- Smith MR 2007 Androgen deprivation therapy for prostate cancer: new concepts and concerns. Curr Opin Endocrinol Diabetes Obes 14:247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP 2007 Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 18:1319–1328 [DOI] [PubMed] [Google Scholar]
- Berris KK, Repp AL, Kleerekoper M 2007 Glucocorticoid-induced osteoporosis. Curr Opin Endocrinol Diabetes Obes 14:446–450 [DOI] [PubMed] [Google Scholar]
- Orwoll E, Klein R 2008 Osteoporosis in men: epidemiology, pathophysiology, and clinical characterization. In: Marcus R, Feldman D, Nelson D, Rosen C, eds. Osteoporosis. 2nd ed. San Diego: Academic Press; 1055–1094 [Google Scholar]
- Vanderschueren D, Boonen S, Bouillon R 2000 Osteoporosis and osteoporotic fractures in men: a clinical perspective. Baillieres Best Pract Res Clin Endocrinol Metab 14:299–315 [DOI] [PubMed] [Google Scholar]
- Feldstein AC, Nichols G, Orwoll E, Elmer PJ, Smith DH, Herson M, Aikin M 2005 The near absence of osteoporosis treatment in older men with fractures. Osteoporos Int 16:953–962 [DOI] [PubMed] [Google Scholar]
- Espallargues M, Sampietro-Colom L, Estrada MD, Sola M, del Rio L, Setoain J, Granados A 2001 Identifying bone-mass-related risk factors for fracture to guide bone densitometry measurements: a systematic review of the literature. Osteoporos Int 12:811–822 [DOI] [PubMed] [Google Scholar]
- Liu H, Paige N, Goldzweig C, Wong E, Zhou A, Suttorp M, Munjas B, Orwoll E, Shekelle P 2008 Screening for osteoporosis in men: a systematic review and background paper for a guideline of the American College of Physicians. Ann Intern Med 148: 685–701 [DOI] [PubMed] [Google Scholar]
- Writing Group for the IPDC 2004 Diagnosis of osteoporosis in men, premenopausal women, and children. J Clin Densitom 7:17–26 [DOI] [PubMed] [Google Scholar]
- Schousboe JT, Taylor BC, Fink HA, Kane RL, Cummings SR, Orwoll ES, Melton III LJ, Bauer DC, Ensrud KE 2007 Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men. JAMA 298:629–637 [DOI] [PubMed] [Google Scholar]
- Cummings SR, Cawthon PM, Ensrud KE, Cauley JA, Fink HA, Orwoll ES, Osteoporotic Fractures in Men (MrOS) Research Groups; Study of Osteoporotic Fractures Research Groups 2006 BMD and risk of hip and nonvertebral fractures in older men: a prospective study and comparison with older women. J Bone Miner Res 21:1550–1556 [DOI] [PubMed] [Google Scholar]
- Nguyen ND, Pongchaiyakul C, Center JR, Eisman JA, Nguyen TV 2005 Identification of high-risk individuals for hip fracture: a 14-year prospective study. J Bone Miner Res 20:1921–1928 [DOI] [PubMed] [Google Scholar]
- Bauer DC, Ewing SK, Cauley JA, Ensrud KE, Cummings SR, Orwoll ES, Osteoporotic Fractures in Men Research Group 2007 Quantitative ultrasound predicts hip and non-spine fracture in men: the MrOS study. Osteoporos Int 18:771–777 [DOI] [PubMed] [Google Scholar]
- Adler RA, Funkhouser HL, Holt CM 2001 Utility of heel ultrasound bone density in men. J Clin Densitom 4:225–230 [DOI] [PubMed] [Google Scholar]
- Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton III LJ, O'Neill T, Pols H, Reeve J, Silman AJ, Tenenhouse A 2005 Predictive value of BMD for hip and other fractures. J Bone Miner Res 20:1185–1194 [DOI] [PubMed] [Google Scholar]
- Melton LJ, Orwoll ES, Wasnich RD 2001 Does bone density predict fractures comparably in men and women? Osteoporos Int 12:707–709 [DOI] [PubMed] [Google Scholar]
- Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E 2008 FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordon LD, Peacock M 1990 Osteomalacia and osteoporosis in femoral neck fracture. Bone Miner 11:247–259 [DOI] [PubMed] [Google Scholar]
- Wilton TJ, Hosking DJ, Pawley E, Stevens A, Harvey L 1987 Osteomalacia and femoral neck fractures in the elderly patient. J Bone Joint Surg Br 69:388–390 [DOI] [PubMed] [Google Scholar]
- Brinkley N, Krueger DE, Cowgill CS, Plum L, Lake E, Hansen KE, DeLuca HF, Drezner MK 2004 Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab 89:3152–3157 [DOI] [PubMed] [Google Scholar]
- Meier C, Nguyen TV, Center JR, Seibel MJ, Eisman JA 2005 Bone resorption and osteoporotic fractures in elderly men: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res 20:579–587 [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Meier C, Center JR, Eisman JA, Seibel MJ 2007 Bone turnover in elderly men: relationships to change in bone mineral density. BMC Musculoskelet Disord 8:13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier C, Liu PY, Handelsman DJ, Seibel MJ 2005 Endocrine regulation of bone turnover in men. Clin Endocrinol (Oxf) 63:603–616 [DOI] [PubMed] [Google Scholar]
- Maclean C, Newberry S, Maglione M, Ranganath V, Suttorp M, Mojica W, Timmer M, Alexander A, McNamara M, Desai SB, Zhou A, Chen S, Carter J, Tringale C, Valentine D, Johnsen B, Grossman J 2008 Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 148:197–213 [DOI] [PubMed] [Google Scholar]
- Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, Adami S, Weber K, Lorenc R, Pietschmann P, Vandormael K, Lombardi A 2000 Alendronate for the treatment of osteoporosis in men. N Engl J Med 343:604–610 [DOI] [PubMed] [Google Scholar]
- Boonen S, Delmas P, Wenderoth D, Stoner K, Eusebio R, Orwoll E 2006 Risedronate safe and effective in men with osteoporosis: a 2-year double-blind randomized placebo-controlled multicenter study [Abstract]. Osteoporos Int 17:S106–107 [Google Scholar]
- Ringe JD, Faber H, Farahmand P, Dorst A 2006 Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int 26:427–431 [DOI] [PubMed] [Google Scholar]
- Sato Y, Iwamoto J, Kanoko T, Satoh K 2005 Risedronate sodium therapy for prevention of hip fracture in men 65 years or older after stroke. Arch Intern Med 165:1743–1748 [DOI] [PubMed] [Google Scholar]
- Tucci JR, Tonino RP, Emkey RD, Peverly CA, Kher U, Santora AC 1996 Effect of three years of oral alendronate treatment in postmenopausal women with osteoporosis. Am J Med 101:488–501 [DOI] [PubMed] [Google Scholar]
- Ho YV, Frauman AG, Thomson W, Seeman E 2000 Effects of alendronate on bone density in men with primary and secondary osteoporosis. Osteoporos Int 11:98–101 [DOI] [PubMed] [Google Scholar]
- Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, Thamsborg G, Liberman UA, Delmas PD, Malice M, Czachur M, Daifotis AG 1998 Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med 339:292–299 [DOI] [PubMed] [Google Scholar]
- Reid DM, Hughes RA, Laan RF, Sacco-Gibson NA, Wenderoth DH, Adami S, Eusebio RA, Devogelaer JP 2000 Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. J Bone Miner Res 15:1006–1013 [DOI] [PubMed] [Google Scholar]
- Iwamoto J, Takeda T, Sato Y 2007 Effects of antifracture drugs in postmenopausal, male and glucocorticoid-induced osteoporosis—usefulness of alendronate and risedronate. Expert Opin Pharmacother 8:2743–2756 [DOI] [PubMed] [Google Scholar]
- LeBlanc AD, Driscol TB, Shackelford LC, Evans HJ, Rianon NJ, Smith SM 2002 Alendronate as an effective countermeasure to disuse induced bone loss. J Musculoskelet Neuronal Interact 2:335–343 [PubMed] [Google Scholar]
- Milgrom C, Finestone A, Novack V, Pereg D, Goldich Y, Kreiss Y, Zimlichman E, Kaufman S, Liebergall M, Burr D 2004 The effect of prophylactic treatment with risedronate on stress fracture incidence among infantry recruits. Bone 35:418–424 [DOI] [PubMed] [Google Scholar]
- Jarrett SJ, Conaghan PG, Sloan VS, Papanastasiou P, Ortmann CE, O'Connor PJ, Grainger AJ, Emery P 2006 Preliminary evidence for a structural benefit of the new bisphosphonate zoledronic acid in early rheumatoid arthritis. Arthritis Rheum 54:1410–1414 [DOI] [PubMed] [Google Scholar]
- Greenspan SL, Coates PS, Sereika SM, Nelson JB, Trump DL, Resnick NM 2005 Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab 90:6410–6417 [DOI] [PubMed] [Google Scholar]
- Shahinian VB, Kuo YF, Freeman JL, Goodwin JS 2005 Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 352:154–164 [DOI] [PubMed] [Google Scholar]
- Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, Kaufman JM, Clancy AD, Gaich GA 2003 The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18:9–17 [DOI] [PubMed] [Google Scholar]
- Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP 2000 Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 85:3069–3076 [DOI] [PubMed] [Google Scholar]
- Satterwhite J, Melnick K, O'Brien L, Myers S, Heathman M 2001 Men and postmenopausal women with osteoporosis have similar lumbar spine bone mineral density responses to recombinant human parathyroid hormone (1-34) despite pharmacokinetic and biochemical marker differences. Arthritis Rheum 44(Suppl):S255 [Google Scholar]
- Kaufman JM, Vermeulen A 2005 The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 26:833–876 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM 2003 The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349:1216–1226 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Leder BZ, Burnett SAM, Wyland JJ, Lee H, Victoria de la Paz A, Gibson KJ, Neer RM 2006 Effects of teriparatide, alendronate, or both on bone turnover in osteoporotic men. J Clin Endocrinol Metab 91:2882–2887 [DOI] [PubMed] [Google Scholar]
- Girotra M, Rubin MR, Bilezikian JP 2006 The use of parathyroid hormone in the treatment of osteoporosis. Rev Endocr Metab Disord 7:113–121 [DOI] [PubMed] [Google Scholar]
- Erlacher L, Kettenbach J, Kiener H, Graninger W, Kainberger F, Pietschmann P 1997 Salmon calcitonin and calcium in the treatment of male osteoporosis: the effect on bone mineral density. Wien Klin Wochenschr 109:270–274 [PubMed] [Google Scholar]
- Trovas GP, Lyritis GP, Galanos A, Raptou P, Constantelou E 2002 A randomized trial of nasal spray salmon calcitonin in men with idiopathic osteoporosis: effects on bone mineral density and bone markers. J Bone Miner Res 17:521–527 [DOI] [PubMed] [Google Scholar]
- Toth E, Csupor E, Meszaros S, Ferencz V, Nemeth L, McCloskey EV, Horvath C 2005 The effect of intranasal salmon calcitonin therapy on bone mineral density in idiopathic male osteoporosis without vertebral fractures—an open label study. Bone 36:47–51 [DOI] [PubMed] [Google Scholar]
- LaCroix AZ, Wienpahl J, White LR, Wallace RB, Scherr PA, George LK, Cornoni-Huntley J, Ostfeld AM 1990 Thiazide diuretic agents and the incidence of hip fracture. N Engl J Med 322:286–290 [DOI] [PubMed] [Google Scholar]
- Morton DJ, Barrett-Connor EL, Edelstein SL 1994 Thiazides and bone mineral density in elderly men and women. Am J Epidemiol 139:1107–1115 [DOI] [PubMed] [Google Scholar]
- Wasnich R, Davis J, Ross P, Vogel J 1990 Effect of thiazide on rates of bone mineral loss: a longitudinal study. BMJ 301:1301–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herings RMC, Stricker BHC, de Boer A, Bakker A, Sturmans F, Stergachis A 1996 Current use of thiazide diuretics and prevention of femur fractures. J Clin Epidemiol 49:115–119 [DOI] [PubMed] [Google Scholar]
- Adams JS, Song CF, Kantorovich V 1999 Rapid recovery of bone mass in hypercalciuric, osteoporotic men treated with hydrochlorothiazide. Ann Intern Med 130:658–660 [DOI] [PubMed] [Google Scholar]
- O'Donnell S, Cranney A, Wells GA, Adachi JD, Reginster JY 2006 Strontium ranelate for preventing and treating postmenopausal osteoporosis.[Update of Cochrane Database Syst Rev. 2006;3:CD005326]. Cochrane Database Syst Rev:CD005326 [DOI] [PubMed] [Google Scholar]
- Doran PM, Riggs BL, Atkinson EJ, Khosla S 2001 Effects of raloxifene, a selective estrogen receptor modulator, on bone turnover markers and serum sex steroid and lipid levels in elderly men. J Bone Miner Res 16:2118–2125 [DOI] [PubMed] [Google Scholar]
- Smith MR, Fallon MA, Lee H, Finkelstein JS 2004 Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab 89:3841–3846 [DOI] [PubMed] [Google Scholar]
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM 2006 Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society Clinical Practice Guideline. [Erratum (2006) 91:2688]. J Clin Endocrinol Metab 91:1995–2010 [DOI] [PubMed] [Google Scholar]
- Behre HM, Kliesch S, Leifke E, Link TM, Nieschlag E 1997 Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. J Clin Endocrinol Metab 82:2386–2390 [DOI] [PubMed] [Google Scholar]
- Benito M, Vasilic B, Wehrli FW, Bunker B, Wald M, Gomberg BR, Wright AC, Zemel BS, Cucchiara AJ, Snyder PJ 2005 Effect of testosterone replacement on trabecular architecture in hypogonadal men. J Bone Miner Res 20:1785–1791 [DOI] [PubMed] [Google Scholar]
- Morley JE, Perry III HM, Kaiser FE, Kraenzle D, Jensen J, Houston K, Mattammal M, Perry Jr HM 1993 Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc 41:149–152 [DOI] [PubMed] [Google Scholar]
- Wong FHW, Pun KK, Wang C 1993 Loss of bone mass in patients with Klinefelter’s syndrome despite sufficient testosterone replacement. Osteoporos Int 7:281–287 [DOI] [PubMed] [Google Scholar]
- Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL 2005 Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab 90:1502–1510 [DOI] [PubMed] [Google Scholar]
- 2004 Committee on Assessing the Need for Clinical Trials of Testosterone Replacement Therapy. In: Liverman CT, Blazer DG, eds. Testosterone and aging: clinical research directions. Washington, DC: The National Academic Press; 1–10 [Google Scholar]
- Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL 2004 Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab 89:503–510 [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Holmes JH, Dlewati A, Staley J, Santanna J, Kapoor SC, Attie MF, Haddad JG, Strom BL 1999 Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab 84:1966–1972 [DOI] [PubMed] [Google Scholar]
- Negro-Vilar A 1999 Selective androgen receptor modulators (SARMs): a novel approach to androgen therapy for the new millennium. J Clin Endocrinol Metab 84:3459–3462 [DOI] [PubMed] [Google Scholar]
- Gao W, Kearbey JD, Nair VA, Chung K, Parlow AF, Miller DD, Dalton JT 2004 Comparison of the pharmacological effects of a novel selective androgen receptor modulator, the 5α-reductase inhibitor finasteride, and the antiandrogen hydroxyflutamide in intact rats: new approach for benign prostate hyperplasia. Endocrinology 145:5420–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid IR, Wattie DJ, Evans MC, Stapleton JP 1996 Testosterone therapy in glucocorticoid-treated men. Arch Intern Med 156:1173–1177 [PubMed] [Google Scholar]
- Crawford BAL, Liu PY, Kean MT, Bleasel JF, Handelsman DJ 2003 Randomized placebo-controlled trial of androgen effects on muscle and bone in men requiring long-term systemic glucocorticoid treatment. J Clin Endocrinol Metab 88:3167–3176 [DOI] [PubMed] [Google Scholar]
- Johnston CC, Miller JZ, Slemenda CW, Reister TK, Hui S, Christian JC, Peacock M 1992 Calcium supplementation and increases in bone mineral density in children. N Engl J Med 327:82–87 [DOI] [PubMed] [Google Scholar]
- Hannan MT, Litman HJ, Araujo AB, McLennan CE, McLean RR, McKinlay JB, Chen TC, Holick MF 2008 Serum 25-hydroxyvitamins D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab, 93:40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranney A, Horsley T, O'Donnell S, Weiler HA, Puil L, Ooi DS, Atkinson SA, Ward LM, Moher D, Hanley DA, Fang M, Yazdi F, Garritty C, Sampson M, Barrowman N, Tsertsvadze A, Mamaladze V 2007 Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 158:1–235 [PMC free article] [PubMed] [Google Scholar]
- Orwoll ES, Oviatt SK, McClung MR, Deftos LJ, Sexton G 1990 The rate of bone mineral loss in normal men and the effects of calcium and cholecalciferol supplementation. Ann Intern Med 112:29–34 [DOI] [PubMed] [Google Scholar]
- Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM 1996 Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med 124:400–406 [DOI] [PubMed] [Google Scholar]
- Owusu W, Willett WC, Feskanich D, Ascherio A, Spiegelman D, Colditz GA 1997 Calcium intake and the incidence of forearm and hip fractures among men. J Nutr 127:1782–1787 [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Harris SS, Krall EA, Dallal GE 1997 Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 337:670–676 [DOI] [PubMed] [Google Scholar]
- Daly RM, Bass S, Nowson C 2006 Long-term effects of calcium-vitamin-D3-fortified milk on bone geometry and strength in older men. Bone 39:946–953 [DOI] [PubMed] [Google Scholar]
- Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, Anderson FH, Cooper C, Francis RM, Donaldson C, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA 2005 Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet 365:1621–1628 [DOI] [PubMed] [Google Scholar]
- Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C 2007 Effect of annual intramuscular vitamin D on fracture risk in elderly men and women—a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology 46:1852–1857 [DOI] [PubMed] [Google Scholar]
- Cannell J, Hollis B, Zasloff M, Heaney R 2008 Diagnosis and treatment of vitamin D deficiency. Expert Opin Pharmacother 9:107–118 [DOI] [PubMed] [Google Scholar]
- 1994 NIH Consensus statement, Volume 12, Number 4(ed.). Bethesda, MD: National Institutes of Health [Google Scholar]
- Kannus P, Sievanen H, Palvanen M, Jarvinen T, Parkkari J 2005 Prevention of falls and consequent injuries in elderly people. Lancet 366:1885–1893 [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Baker DI, McAvay G, Claus EB, Garrett P, Gottschalk M, Koch ML, Trainor K, Horwitz RI 1994 A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med 331:821–827 [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Chao A, Ross RK, Henderson BE 1991 Exercise and other factors in the prevention of hip fracture: The Leisure World study. Epidemiology 2:16–25 [DOI] [PubMed] [Google Scholar]
- Beck B, Marcus R 1999 Skeletal effects of exercise in men. In: Orwoll ES, ed. Osteoporosis in men: the effects of gender on skeletal health. San Diego: Academic Press; 129–155 [Google Scholar]
- Berry SD, Kiel DP 2008 Falls as risk factors for fracture. In: Marcus R, Feldman D, Nelson D, Rosen C, eds. Osteoporosis. San Diego: Academic Press; 911–917 [Google Scholar]