Abstract
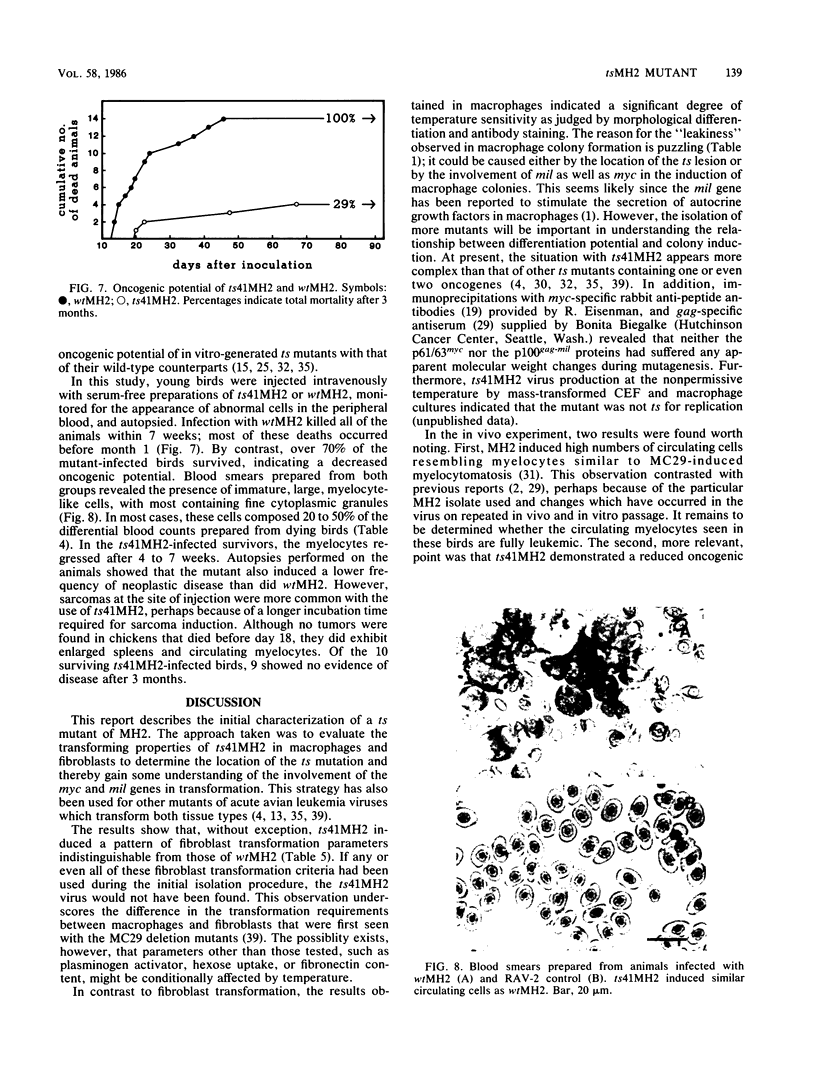
A conditional mutant of the MH2 avian retrovirus, termed ts41MH2, was isolated. Unlike wtMH2, ts41MH2 permitted transformed macrophages to differentiate during a 5- to 7-day temperature shift from 37 to 42 degrees C. Mutant-infected cells incubated at 42 degrees C exhibited a flattened morphology and then fused to form giant multinucleated cells that closely resembled normal macrophage maturation in vitro. These differentiated cells reacted strongly with a myeloid-macrophage-specific monoclonal antibody. The process of differentiation was inhibited when ts41MH2-transformed nonproducer clones were superinfected before the temperature shift with the myc gene-containing MC29 or OK10 viruses. By contrast, no inhibition was observed in clones superinfected with the MH2-PA200 virus that contains only the mil gene. The mutant also demonstrated a reduced oncogenic potential relative to that of wtMH2 when it was inoculated intravenously into young birds. However, in contrast to the results obtained with hematopoietic cells, none of the five fibroblast transformation parameters tested for ts41MH2 were altered from those induced by wtMH2. These results suggest that the mutation in ts41MH2 is located in a region of myc required for macrophage transformation, but not required for fibroblast transformation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Leutz A., Graf T. Autocrine growth induced by src-related oncogenes in transformed chicken myeloid cells. Cell. 1984 Dec;39(3 Pt 2):439–445. doi: 10.1016/0092-8674(84)90451-3. [DOI] [PubMed] [Google Scholar]
- Alexander R. W., Moscovici C., Vogt P. K. Avian oncovirus Mill Hill No. 2: pathogenicity in chickens. J Natl Cancer Inst. 1979 Feb;62(2):359–366. [PubMed] [Google Scholar]
- Bechade C., Calothy G., Pessac B., Martin P., Coll J., Denhez F., Saule S., Ghysdael J., Stéhelin D. Induction of proliferation or transformation of neuroretina cells by the mil and myc viral oncogenes. Nature. 1985 Aug 8;316(6028):559–562. doi: 10.1038/316559a0. [DOI] [PubMed] [Google Scholar]
- Beug H., Leutz A., Kahn P., Graf T. Ts mutants of E26 leukemia virus allow transformed myeloblasts, but not erythroblasts or fibroblasts, to differentiate at the nonpermissive temperature. Cell. 1984 Dec;39(3 Pt 2):579–588. doi: 10.1016/0092-8674(84)90465-3. [DOI] [PubMed] [Google Scholar]
- Beug H., Palmieri S., Freudenstein C., Zentgraf H., Graf T. Hormone-dependent terminal differentiation in vitro of chicken erythroleukemia cells transformed by ts mutants of avian erythroblastosis virus. Cell. 1982 Apr;28(4):907–919. doi: 10.1016/0092-8674(82)90070-8. [DOI] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Bister K., Ramsay G. M., Hayman M. J. Deletions within the transformation-specific RNA sequences of acute leukemia virus MC29 give rise to partially transformation-defective mutants. J Virol. 1982 Mar;41(3):754–766. doi: 10.1128/jvi.41.3.754-766.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll J., Righi M., Taisne C., Dissous C., Gegonne A., Stehelin D. Molecular cloning of the avian acute transforming retrovirus MH2 reveals a novel cell-derived sequence (v-mil) in addition to the myc oncogene. EMBO J. 1983;2(12):2189–2194. doi: 10.1002/j.1460-2075.1983.tb01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner P., Bunte T., Greiser-Wilke I., Moelling K. Decreased DNA-binding ability of purified transformation-specific proteins from deletion mutants of the acute avian leukemia virus MC29. Proc Natl Acad Sci U S A. 1983 May;80(10):2861–2865. doi: 10.1073/pnas.80.10.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner P., Greiser-Wilke I., Moelling K. Nuclear localization and DNA binding of the transforming gene product of avian myelocytomatosis virus. Nature. 1982 Mar 18;296(5854):262–269. doi: 10.1038/296262a0. [DOI] [PubMed] [Google Scholar]
- Eisenman R. N., Tachibana C. Y., Abrams H. D., Hann S. R. V-myc- and c-myc-encoded proteins are associated with the nuclear matrix. Mol Cell Biol. 1985 Jan;5(1):114–126. doi: 10.1128/mcb.5.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enrietto P. J., Hayman M. J., Ramsay G. M., Wyke J. A., Payne L. N. Altered pathogenicity of avian myelocytomatosis (MC29) viruses with mutations in the v-myc gene. Virology. 1983 Jan 15;124(1):164–172. doi: 10.1016/0042-6822(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Frykberg L., Palmieri S., Beug H., Graf T., Hayman M. J., Vennström B. Transforming capacities of avian erythroblastosis virus mutants deleted in the erbA or erbB oncogenes. Cell. 1983 Jan;32(1):227–238. doi: 10.1016/0092-8674(83)90513-5. [DOI] [PubMed] [Google Scholar]
- Graf T., Ade N., Beug H. Temperature-sensitive mutant of avian erythroblastosis virus suggests a block of differentiation as mechanism of leukaemogenesis. Nature. 1978 Oct 12;275(5680):496–501. doi: 10.1038/275496a0. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Graf T., Royer-Pokora B., Schubert G. E., Beug H. Evidence for the multiple oncogenic potential of cloned leukemia virus: in vitro and in vitro studies with avian erythroblastosis virus. Virology. 1976 Jun;71(2):423–433. doi: 10.1016/0042-6822(76)90370-6. [DOI] [PubMed] [Google Scholar]
- Graf T., Stéhelin D. Avian leukemia viruses. Oncogenes and genome structure. Biochim Biophys Acta. 1982 Jun 28;651(4):245–271. doi: 10.1016/0304-419x(82)90014-2. [DOI] [PubMed] [Google Scholar]
- Graf T. Two types of target cells for transformation with avian myelocytomatosis virus. Virology. 1973 Aug;54(2):398–413. doi: 10.1016/0042-6822(73)90152-9. [DOI] [PubMed] [Google Scholar]
- Hann S. R., Abrams H. D., Rohrschneider L. R., Eisenman R. N. Proteins encoded by v-myc and c-myc oncogenes: identification and localization in acute leukemia virus transformants and bursal lymphoma cell lines. Cell. 1983 Oct;34(3):789–798. doi: 10.1016/0092-8674(83)90535-4. [DOI] [PubMed] [Google Scholar]
- Hayman M. J. Avian acute leukemia viruses. Curr Top Microbiol Immunol. 1983;103:109–125. doi: 10.1007/978-3-642-68943-7_5. [DOI] [PubMed] [Google Scholar]
- Jansen H. W., Lurz R., Bister K., Bonner T. I., Mark G. E., Rapp U. R. Homologous cell-derived oncogenes in avian carcinoma virus MH2 and murine sarcoma virus 3611. Nature. 1984 Jan 19;307(5948):281–284. doi: 10.1038/307281a0. [DOI] [PubMed] [Google Scholar]
- Jansen H. W., Patschinsky T., Walther N., Lurz R., Bister K. Molecular and biological properties of MH2D12, a spontaneous mil deletion mutant of avian oncovirus MH2. Virology. 1985 Apr 30;142(2):248–262. doi: 10.1016/0042-6822(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Jansen H. W., Rückert B., Lurz R., Bister K. Two unrelated cell-derived sequences in the genome of avian leukemia and carcinoma inducing retrovirus MH2. EMBO J. 1983;2(11):1969–1975. doi: 10.1002/j.1460-2075.1983.tb01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn P., Nakamura K., Shin S., Smith R. E., Weber M. J. Tumorigenicity of partial transformation mutants of Rous sarcoma virus. J Virol. 1982 May;42(2):602–611. doi: 10.1128/jvi.42.2.602-611.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan N. C., Flordellis C. S., Garon C. F., Duesberg P. H., Papas T. S. Avian carcinoma virus MH2 contains a transformation-specific sequence, mht, and shares the myc sequence with MC29, CMII, and OK10 viruses. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6566–6570. doi: 10.1073/pnas.80.21.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan N. C., Flordellis C. S., Mark G. E., Duesberg P. H., Papas T. S. Nucleotide sequence of avian carcinoma virus MH2: two potential onc genes, one related to avian virus MC29 and the other related to murine sarcoma virus 3611. Proc Natl Acad Sci U S A. 1984 May;81(10):3000–3004. doi: 10.1073/pnas.81.10.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S., Beug H., Doederlein G., Graf T. Detection of avian hematopoietic cell surface antigens with monoclonal antibodies to myeloid cells. Their distribution on normal and leukemic cells of various lineages. Exp Cell Res. 1983 Feb;143(2):383–394. doi: 10.1016/0014-4827(83)90065-4. [DOI] [PubMed] [Google Scholar]
- Linial M. Two retroviruses with similar transforming genes exhibit differences in transforming potential. Virology. 1982 Jun;119(2):382–391. doi: 10.1016/0042-6822(82)90097-6. [DOI] [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- Mladenov Z., Heine U., Beard D., Beard J. W. Strain MC29 avian leukosis virus. Myelocytoma, endothelioma, and renal growths: pathomorphological and ultrastructural aspects. J Natl Cancer Inst. 1967 Mar;38(3):251–285. [PubMed] [Google Scholar]
- Moscovici M. G., Moscovici C. Isolation and characterization of a temperature-sensitive mutant of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1421–1425. doi: 10.1073/pnas.80.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachl C., Biegalke B., Linial M. RNA and protein encoded by MH2 virus: evidence for subgenomic expression of v-myc. J Virol. 1983 Jan;45(1):133–139. doi: 10.1128/jvi.45.1.133-139.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri S., Beug H., Graf T. Isolation and characterization of four new temperature-sensitive mutants of avian erythroblastosis virus (AEV). Virology. 1982 Dec;123(2):296–311. doi: 10.1016/0042-6822(82)90263-x. [DOI] [PubMed] [Google Scholar]
- Palmieri S., Kahn P., Graf T. Quail embryo fibroblasts transformed by four v-myc-containing virus isolates show enhanced proliferation but are non tumorigenic. EMBO J. 1983;2(12):2385–2389. doi: 10.1002/j.1460-2075.1983.tb01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri S. Transformation of erythroid cells by Rous sarcoma virus (RSV). Virology. 1985 Jan 30;140(2):269–280. doi: 10.1016/0042-6822(85)90365-4. [DOI] [PubMed] [Google Scholar]
- Payne L. N., Biggs P. M. Genetic resistance of fowl to MH2 reticuloendothelioma virus. J Gen Virol. 1970 Jun;7(3):177–185. doi: 10.1099/0022-1317-7-3-177. [DOI] [PubMed] [Google Scholar]
- Ramsay G. M., Enrietto P. J., Graf T., Hayman M. J. Recovery of myc-specific sequences by a partially transformation-defective mutant of avian myelocytomatosis virus, MC29, correlates with the restoration of transforming activity. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6885–6889. doi: 10.1073/pnas.79.22.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay G. M., Hayman M. J. Isolation and biochemical characterization of partially transformation-defective mutants of avian myelocytomatosis virus strain MC29: localization of the mutation to the myc domain of the 110,000-dalton gag-myc polyprotein. J Virol. 1982 Mar;41(3):745–753. doi: 10.1128/jvi.41.3.745-753.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay G., Graf T., Hayman M. J. Mutants of avian myelocytomatosis virus with smaller gag gene-related proteins have an altered transforming ability. Nature. 1980 Nov 13;288(5787):170–172. doi: 10.1038/288170a0. [DOI] [PubMed] [Google Scholar]
- Ramsay G., Hayman M. J., Bister K. Phosphorylation of specific sites in the gag-myc polyproteins encoded by MC29-type viruses correlates with their transforming ability. EMBO J. 1982;1(9):1111–1116. doi: 10.1002/j.1460-2075.1982.tb01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer-Pokora B., Beug H., Claviez M., Winkhardt H. J., Friis R. R., Graf T. Transformation parameters in chicken fibroblasts transformed by AEV and MC29 avian leukemia viruses. Cell. 1978 Apr;13(4):751–760. doi: 10.1016/0092-8674(78)90225-8. [DOI] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]