Abstract
The deleterious effects of diabetes mellitus on the retinal, renal, cardiovascular, and peripheral nervous systems are widely acknowledged. Less attention has been given to the effect of diabetes on cognitive function. Both type 1 and type 2 diabetes mellitus have been associated with reduced performance on numerous domains of cognitive function. The exact pathophysiology of cognitive dysfunction in diabetes is not completely understood, but it is likely that hyperglycemia, vascular disease, hypoglycemia, and insulin resistance play significant roles. Modalities to study the effect of diabetes on the brain have evolved over the years, including neurocognitive testing, evoked response potentials, and magnetic resonance imaging. Although much insightful research has examined cognitive dysfunction in patients with diabetes, more needs to be understood about the mechanisms and natural history of this complication in order to develop strategies for prevention and treatment.
I. Introduction
- II. Cognitive Dysfunction in Patients with Diabetes
- A. Type 1 diabetes
- B. Type 2 diabetes
- C. Hypoglycemia and cognitive dysfunction
- D. Section summary
- III. Pathophysiology of Cognitive Dysfunction in Diabetes
- A. The role of hyperglycemia
- B. The role of vascular disease
- C. The role of hypoglycemia
- D. The role of insulin resistance and amyloid
IV. Modalities for Assessment of Cognitive Dysfunction in Patients with Diabetes
V. Future Directions
VI. Conclusion
I. Introduction
DIABETES MELLITUS IS a complex metabolic disease that can have devastating effects on multiple organs in the body. Diabetes is the leading cause of end stage renal disease in the United States (1) and is also a common cause of vision loss, neuropathy, and cardiovascular disease. A less addressed and not as well recognized complication of diabetes is cognitive dysfunction. Patients with type 1 and type 2 diabetes mellitus have been found to have cognitive deficits that can be attributed to their disease. Both hypoglycemia and hyperglycemia have been implicated as causes of cognitive dysfunction, and many patients fear that recurrent hypoglycemia will impair their memory over time. Although much research has been done, the pathophysiology underlying this complication is not well understood, and the most appropriate methods to diagnose, treat, and prevent cognitive dysfunction in diabetes have not yet been defined. In this article, we will review the nature of cognitive dysfunction in type 1 and type 2 diabetes mellitus, the pathophysiology of cognitive dysfunction secondary to diabetes, methodologies used to assess cognitive deficits in patients with diabetes, and potential future directions of research that are needed to advance our understanding of this often overlooked complication of diabetes.
The purpose of this article is to present a comprehensive review of the literature regarding the subject of cognitive dysfunction in diabetes mellitus. To do this, we performed MEDLINE searches for such key words and terms as “diabetes mellitus,” “cognitive function,” “cognition,” “hypoglycemia,” “insulin resistance,” and “Alzheimer’s disease,” among others. We then pursued articles referenced in these sources. Although this is a comprehensive review, it is not exhaustive. In addition, it should be noted that the field of cognitive dysfunction in diabetes is still in its early stages. It must be remembered that although there have been many significant contributions regarding the association of diabetes and cognitive dysfunction and many hypotheses based on this association, the causative mechanisms of diabetes on cognitive dysfunction are still undergoing development.
II. Cognitive Dysfunction in Patients with Diabetes
A. Type 1 diabetes
Cognitive dysfunction in patients with diabetes mellitus was first noted in 1922, when patients with diabetes, who were “free from acidosis but usually not sugar free,” were noted to have impaired memory and attention on cognitive testing compared with controls (2). Since then, there have been many studies designed to better delineate the scope and magnitude of cognitive dysfunction in diabetes (Table 1). The most common cognitive deficits identified in patients with type 1 diabetes are slowing of information processing speed (3,4,5,6) and worsening psychomotor efficiency (3,4,7). However, other deficits have been noted, including deficits in motor speed (5,8,9,10), vocabulary (7,11,12,13), general intelligence (12,14), visuoconstruction (6,12), attention (6), somatosensory examination, motor strength (10), memory (7), and executive function (7,14). Glycemic control appears to play a role in cognitive performance in patients with type 1 diabetes. Functions such as psychomotor efficiency, motor speed (5,15), attention, verbal IQ scores (16,17,18), memory, and academic achievement (17) are improved with better glycemic control. Specifically, an 18-yr follow-up of the Diabetes Control and Complications Trial (DCCT) showed that those patients with type 1 diabetes mellitus with a time weighted mean glycated hemoglobin (HbA1c) less than 7.4% performed significantly better on tests of motor speed and psychomotor efficiency than those subjects whose time weighted mean HbAlc was greater than 8.8% (15). In addition, slowing of all cognitive function, an increased number of mental subtraction errors (19), loss of inhibition and focus (20), impaired speed of information processing, decreased attention, and impaired working memory (21) have all been noted during acute hyperglycemia in patients with type 1 and type 2 diabetes.
Table 1.
Summary of cognitive domains that have been found to be negatively affected by type 1 diabetes mellitus
| Slowing of information processing* |
| Psychomotor efficiency* |
| Attention* |
| Memory |
| Learning |
| Problem solving |
| Motor speed |
| Vocabulary |
| General intelligence |
| Visuoconstruction* |
| Visual perception |
| Somatosensory examination |
| Motor strength |
| Mental flexibility* |
| Executive function |
Domains marked by asterisks have particularly strong supporting data.
A recent meta-analysis included 33 studies examining cognitive function in adult subjects with type 1 diabetes mellitus (22). It found that there were significant reductions in overall cognition, fluid and crystallized intelligence, speed of information processing, psychomotor efficiency, visual and sustained attention, mental flexibility, and visual perception in subjects with type 1 diabetes compared with controls. There was no difference in memory, motor speed, selective attention, and language. All studies included healthy matched control groups and used reliable testing measures at normal blood glucose values. Most studies included in the meta-analysis controlled for depression; however, similar findings were seen in those studies that did not control for depression. It is unclear whether any of these studies controlled for other chronic diseases that could affect cognitive function. Worse cognition was associated with increased diabetes complications, but not with glycemic control in these populations. However, this last finding was confounded by the heterogeneity of how different studies defined “well” vs. “poorly” controlled diabetes (22).
As was demonstrated in the work of Brands et al. (22), cognitive function may be worse in patients with type 1 diabetes who experience other diabetes complications. Deficits in fluid intelligence, information processing, attention, and concentration have been associated with the presence of background retinopathy (23). Proliferative retinopathy, macrovascular complications, hypertension, and duration of diabetes were associated with poorer performance on tests measuring psychomotor speed and visuoconstruction ability in patients with type 1 diabetes (4,5,6). Patients with distal symmetrical polyneuropathy displayed worse cognitive function on most cognitive domains except for memory (5). However, other studies were unable to identify a relationship between impaired cognitive function and diabetic complications (24). Future study will be necessary to determine whether there is a link between complications and alterations in cognition.
Although complications like retinopathy and nephropathy usually require years of diabetes before becoming clinically apparent, the onset of cognitive impairment has been found to occur early in patients with type 1 diabetes. Deficits in cognitive function have been detected as early as 2 yr after diagnosis in children with type 1 diabetes, and these patients experienced less positive changes than controls over time in general intelligence, vocabulary, block design, speed of processing, and learning (12). Six years after diagnosis, these same subjects had impaired IQ, attention, processing speed, long-term memory, and executive function compared with controls (14). The age of onset of type 1 diabetes may also contribute to the presence of cognitive dysfunction, because those who developed type 1 diabetes at less than 4 yr of age had impaired executive skills, attention, and processing speed when compared with those that were diagnosed after 4 yr of age (14). Of note, chronic disease and time away from school (secondary to illness, etc.) were not controlled for in these studies.
Interestingly, several studies have shown patient gender to influence neurocognitive function in patients with type 1 diabetes mellitus. Skenazy and Bigler (10) found that men with type 1 diabetes had reduced performance on oscillation, strength grip, and somatosensory testing compared with male controls, and the magnitude of this difference was greater than that measured between women with type 1 diabetes and their gender-matched controls. In addition, a decline in verbal intelligence was seen in boys with type 1 diabetes between the ages of 7 and 16, which correlated with worse glycemic control. This was not seen in girls of similar ages (13). However, most human studies have not distinguished between genders when describing results of neurocognitive testing, and therefore more controlled analysis should be done before any conclusions are drawn.
Of note, the strength of these neurocognitive studies is variable. Covariates that could affect neurocognitive testing include age, education, sex, history of other chronic illnesses, psychiatric disorders, neurological disorders, substance abuse, absence from school, socioeconomic status, and hypoglycemia/hyperglycemia during testing. The reviewed reports controlled for at least some of these covariates, however most fail to control for all of them. For example, only two studies that have been mentioned (6,24) have reported controlling for hyperglycemia at the time of testing, which has been proven to affect cognitive function (see Section II.A). The cognitive domains that are affected by type 1 diabetes with the best evidence based on our review are indicated in Table 1 with an asterisk.
B. Type 2 diabetes
Patients with type 2 diabetes mellitus have also been found to have cognitive impairment (Table 2). Type 2 diabetes has been associated with decreases in psychomotor speed (25,26), frontal lobe/executive function (26,27,28), verbal memory (29), processing speed (29), complex motor functioning (26), working memory (27,28), immediate recall, delayed recall (30), verbal fluency (26,31), visual retention (32), and attention (33). The impact of these subtle neurocognitive deficits on the daily lives of patients with type 2 diabetes is not clear. Sinclair et al. (34) found that subjects with mini-mental status exam scores less than 23 fared worse on measures of self care and ability to perform activities of daily living. These subjects also displayed an increased need for personal care and increased rates of hospitalization when compared with controls. Patients with diabetes also have been found to have slower walking speed, lack of balance, and increased falls associated with type 2 diabetes, but whether the cerebral affects of diabetes contributed to these abnormalities is debatable (35). Complicating the impact of mild neurocognitive dysfunction secondary to diabetes on daily living is the observation that patients with diabetes are twice as likely to have depression (27,36), which will also negatively affect cognitive function and daily activities. Type 2 patients also have an increased incidence of Alzheimer’s disease (37,38,39,40,41,42,43,44) and increased incidence of vascular dementia (38,42,45). Recently, Bruce et al. (36) found that 17.5% of elderly patients with type 2 diabetes had moderate to severe deficits in activities of daily living, 11.3% had cognitive impairment, and 14.2% had depression.
Table 2.
Summary of cognitive domains that have been found to be negatively affected by type 2 diabetes mellitus
| Memory* |
| Verbal memory |
| Visual retention |
| Working memory |
| Immediate recall |
| Delayed recall |
| Psychomotor speed* |
| Executive function* |
| Processing speed |
| Complex motor function |
| Verbal fluency |
| Attention |
| Depression |
Domains marked by asterisks have particularly strong supporting data.
Glycemic control appears to play a role in determining the degree of cognitive dysfunction detected in patients with type 2 diabetes, although this has not uniformly been observed (46). In a population of nearly 2000 postmenopausal women, Yaffe et al. (47) found that those with a HbA1c of more than 7.0% had a 4-fold increase in developing mild cognitive impairment. Grodstein et al. (30) found that elderly subjects who took oral diabetic medication, unlike those on insulin, had similar scores on general tests for cognition as subjects without diabetes. Other studies have demonstrated an inverse relationship between HbAlc and working memory (27,28), executive functioning (27), learning (26), and complex psychomotor performance (26,48) in patients with type 2 diabetes mellitus, supporting the hypothesis that worsening glucose control leads to worsening cognitive function much like with type 1 diabetes. Also similar to type 1 diabetes is the association between alterations in cognitive function in patients with type 2 diabetes and diabetes complications like peripheral neuropathy (28) and duration of type 2 diabetes (25,33).
Impaired glucose tolerance without diabetes is also a risk factor for cognitive dysfunction. Multiple investigations of patients with impaired glucose tolerance have shown them to have lower mini-mental status exam and long-term memory scores (49), impaired verbal fluency (31), increased Alzheimer’s dementia (39), and increased vascular dementia (38) compared with control subjects. These observations mirror the positive relationship found between hyperglycemia in patients without diabetes and cardiovascular disease (50,51,52). The pathophysiology of this relationship is unclear, and there is evidence that both hyperglycemia and other aspects of insulin resistance could contribute to this, which will be addressed later. Of note, however, not all studies found that patients with impaired glucose tolerance (33,53,54) or type 2 diabetes mellitus (54,55) perform worse than normoglycemic individuals.
However, like neurocognitive studies examining type 1 diabetes, the strength of these neurocognitive studies evaluating type 2 diabetes and impaired glucose tolerance is variable. Although most of these studies controlled for age, there was uneven control for other covariates including education, psychiatric disorders, neurological disorders, hyperglycemia and hypoglycemia during testing, and chronic illness. The cognitive domains that are affected by type 2 diabetes with the best evidence based on our review are indicated in Table 1 with an asterisk.
C. Hypoglycemia and cognitive dysfunction
Repetitive episodes of moderate to severe hypoglycemia have been implicated as one possible etiology of cognitive dysfunction in diabetes. This is significant because the risk of hypoglycemia increases as efforts to achieve the level of glycemia necessary to minimize the risk of developing the microvascular complications of diabetes are intensified (56,57,58). The reason for severe hypoglycemia secondary to intensive insulin management is complex and multifactorial, however the initial intelligence of patients with type 1 diabetes before intensive management does not predispose to more future hypoglycemia episodes, as shown by an analysis of data collected during the DCCT (59). During acute hypoglycemia episodes, it has been shown that performance on immediate verbal memory, immediate visual memory, working memory, delayed memory, visual-motor skills, visual-spatial skills, and global cognitive dysfunction are all impaired (60,61). In a more recent study, prospective memory (that is, “remembering to remember”) and immediate and delayed recall were both impaired secondary to hypoglycemia in patients with type 1 diabetes, suggesting that impairments with both recall and learning/consolidation occur during hypoglycemia (62). Interestingly, in some studies there was no difference in reaction time (63), memory (62), and overall cognitive performance (61) between hypoglycemia aware and unaware patients during hypoglycemic episodes, despite the fact that the glucose level at which the counterregulatory hormone response was elicited was higher in subjects with awareness of hypoglycemia.
Although cognitive impairment may occur during hypoglycemia, the effect of repetitive hypoglycemia on subsequent cognitive function during euglycemia is less clear. Studies have shown impaired verbal IQ scores (14,64), full scale IQ scores (14,20,64), attention (20), verbal skills (11), short-term memory, verbal memory (17), vigilance (65), and visual-spatial memory (8,18) in patients with a history of type 1 diabetes and severe hypoglycemia (defined as being associated with seizures, coma, or the need for external assistance), compared with patients with type 1 diabetes without a history of severe hypoglycemia. However, it is possible that some of these “abnormalities” could be the result of slower, more deliberate completion of the tasks without loss of accuracy (66). More recently, no association between multiple severe episodes of hypoglycemia and impaired cognitive function in patients with type 1 diabetes mellitus was found in an 18-yr follow-up of the DCCT (15). Although the DCCT follow-up has been regarded as a landmark study that provides reassurance to diabetologists and patients, it is important to recognize its limitations, including the fact that it did not randomize patients into a “severe hypoglycemia” group and a control group (because such randomization would be not only logistically impossible but also unethical). A lack of association between severe hypoglycemia and cognitive dysfunction was confirmed by other studies (23,68,69,70,71), as well as with a meta-analysis, which showed no association between hypoglycemia and cognitive function (22). Of note, however, most data analyzing the effects of hypoglycemia look at young to middle-aged patients; data regarding the impact of hypoglycemia on older individuals is lacking.
One possible reason that some studies found an association between frequent hypoglycemia and cognitive dysfunction and others did not is that the positive investigations may have included subjects with diabetes onset earlier in life. Patients with type 1 diabetes diagnosed at less than 5 yr of age may have more severe (often with seizures) and frequent hypoglycemia episodes than those diagnosed at ages older than 5 yr; these younger patients have been found to have worse cognitive dysfunction (9,18,72,73). The severity of the hypoglycemia as well as the susceptibility of young brains to injury may explain the discrepancy (9,74). Another explanation for discrepancy between reports is that subjects with more hypoglycemia may have overall tighter glycemic control, which may offset the neurocognitive damage from hypoglycemia. This was most likely the case in the population studied by Kaufman et al. (17) in which children with more frequent episodes of hypoglycemia (<70 mg/dl) actually had increased memory and verbal scores, as well as overall better academic achievement when compared with less well-controlled children with diabetes.
D. Section summary
Clearly, much research has been done on cognitive dysfunction in patients with type 1 and type 2 diabetes mellitus. Although results are not consistent and many different deficits have been identified, some conclusions can be drawn. In patients with type 1 diabetes mellitus, deficits in speed of information processing, psychomotor efficiency, attention, mental flexibility, and visual perception seem to be present, whereas in patients with type 2 diabetes, an increase in memory deficits, a reduction in psychomotor speed, and reduced frontal lobe/executive function have been identified. Severe hypoglycemic episodes may contribute to cognitive dysfunction in the young; however, as patients age episodes seem to have less of an influence. Finally, improved diabetes control and decreased diabetic complications seem to be associated with less cognitive dysfunction, although this association is clearer in patients with type 2 diabetes than with type 1 diabetes.
However, some questions remained unanswered. First, it is not clear whether cognitive impairments seen in neurocognitive testing result in meaningful deficits either socially or professionally. Given the subjective nature of assessing professional and social activities, it will be difficult to address this question. Second, although the data suggest that hyperglycemia contributes to cognitive impairment, the magnitude of this contribution and how hyperglycemic one must be to experience the ill effects of hyperglycemia on cognition are not clear. Lastly, it is unknown whether mild neurocognitive impairments will progress to overt dementia. Large randomized controlled trials such as the Epidemiology of Diabetes Interventions and Complications (EDIC) study/DCCT and the ongoing Action to Control Cardiovascular Risk in Diabetes (ACCORD) study should hopefully continue to address these last two questions.
III. Pathophysiology of Cognitive Dysfunction in Diabetes
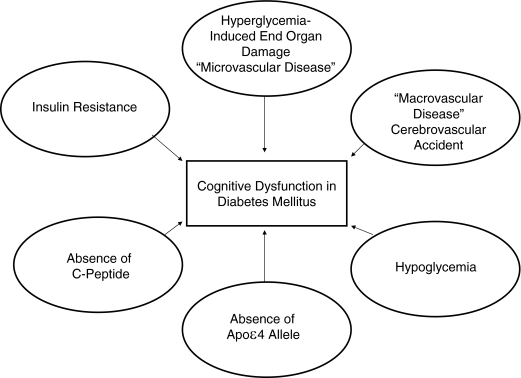
The pathophysiology underlying the development of cognitive dysfunction in patients with diabetes has not been completely elucidated. Many hypotheses with supporting evidence exist, including potential causative roles for hyperglycemia, vascular disease, hypoglycemia, insulin resistance, and amyloid deposition (Fig. 1). Although further research into each of these candidate mechanisms is necessary, it may be that the cause of cognitive dysfunction in patients with diabetes will turn out to be a combination of these factors, depending on the patient’s type of diabetes, comorbidities, age, and type of therapy.
Figure 1.
Summary of possible mechanistic contributors to cognitive dysfunction seen in diabetes mellitus. Not all mechanisms are present in every patient.
A. The role of hyperglycemia
As reviewed in Sections II.A and II.B, hyperglycemia appears to be related to abnormalities in cognitive function in patients with both type 1 and type 2 diabetes. However, the mechanisms through which hyperglycemia might mediate this effect are less than clear. In other organs, hyperglycemia alters function through a variety of mechanisms including polyol pathway activation, increased formation of advanced glycation end products (AGEs), diacylglycerol activation of protein kinase C, and increased glucose shunting in the hexosamine pathway (75,76,77,78). These same mechanisms may be operative in the brain and induce the changes in cognitive function that have been detected in patients with diabetes.
It has long been known that hyperglycemia increases flux through the polyol pathway in nervous tissue. In the streptozotocin-treated rat (glucose concentration 27.4 ± 0.3 mmol/liter vs. 5.9 ± 0.1 mmol/liter in control rats), an increase in sorbitol was measured in cranial nerves, sciatic nerve, cerebral cortex, and retina. This accumulation was reduced significantly when the animals were treated with the aldose reductase inhibitor tolerstat (79). Another study looking at streptozotocin-treated rats (HbA1c 7.9 ± 0.3 vs. 3.3 ± 0.0 in control rats) also found that administering an aldose reductase inhibitor, sorbinil, reduced the accumulation of brain tissue sorbitol and corrected the reduced cognitive function normally seen in rats with hyperglycemia (80). Whether this pathway contributes to neurocognitive dysfunction in humans with diabetes is unknown.
The role of AGEs and receptors for AGE (RAGEs) in the development of cerebral complications of diabetes also remains uncertain. Diabetic mice (32% HbA1c vs. 12% in control mice) with demonstrated cognitive impairment have been found to have increased expression of RAGEs in neurons and glial cells and damage to white matter and myelin (78), suggesting a possible role of RAGEs in the development of cerebral dysfunction (81). In humans, patients with diabetes and Alzheimer’s disease have been found to have greater N-carboxymethyllysine (a type of AGE) staining on brain slices obtained postmortem than patients with Alzheimer’s disease alone (82). However, a second autopsy study failed to find a difference in the quantity of AGE-like glycated protein rich neurofibrillary tangles and senile plaques, like those seen in patients with Alzheimer’s disease, between human subjects with diabetes and controls (83). Experiments performed in animal models provide limited evidence to support the hypothesis that AGE-induced brain injury may be a mechanism through which hyperglycemia and diabetes alter cerebral function. In vitro the addition of AGEs to bovine brain microvascular endothelial cells up-regulates both tissue factor mRNA (which induces blood coagulation) and reactive oxygen species through a mechanism that is reversed with treatment by the free radical scavenger edaravone (84). In addition, in a rat model of focal cerebral ischemia, the infusion of AGEs increased cerebral infarct size, whereas the coadministration of aminoguanidine, an inhibitor of AGE cross-linking, attenuated the infarct volume (85).
Few investigators have examined the role of diacylglycerol activation of protein kinase C and increased glucose shunting in the development of cognitive dysfunction in diabetes. Brain expression of protein kinase C-α was shown to be significantly increased in one untreated diabetic rat model (approximate blood glucose, 15 mmol/liter) compared with the treated diabetic rat and control rats (approximate blood glucose, 6 mmol/liter) (86), but another study found no increase in protein kinase C activity in diabetic rats (approximate blood glucose, 17 mmol/liter) compared with controls (approximate blood glucose, 6.4 mmol/liter) (87). Support for the possible role of the hexosamine pathway comes from the interesting observation that cerebral chitin, an N-acetyl-glucosamine polymer produced via the hexosamine pathway, is increased in human subjects with Alzheimer’s disease on autopsy (88). If hyperglycemia from diabetes shunts glucose toward the production of chitin, it is possible that the accumulation of this molecule could contribute to abnormalities in cognition.
Hyperglycemia has also been proposed to cause end organ damage through increases in reactive oxygen species, in particular superoxide, which could then lead to increased polyol pathway activation, increased formation of AGEs, activation of protein kinase C, and increased glucose shunting in the hexosamine pathway (76). Using streptozotocin to induce diabetes in rats (blood glucose, 20.72 ± 2.25 vs. 6.04 ± 0.64 mmol/liter in control rats), Aragno et al. (89) found that RAGEs, galectin-3 (a proatherogenic molecule), and the polyol pathway activation all were increased in rat brains, whereas activity of the glycolytic enzyme glyceradehyde-3-phosphate dehydrogenase was decreased, indicating elevated superoxide levels. Nuclear factor κB transcription factors, a proinflammatory gene marker up-regulated by AGEs, and S-100 protein, a marker for brain injury that can bind to RAGEs, were both up-regulated in the hippocampus in this animal model, although the effect in other regions was not assessed. These data suggest that oxidative stress may trigger a cascade of events that lead to neuronal damage. Interestingly, dehydroepiandrosterone, an adrenal androgen and antioxidant, significantly reduces these changes, suggesting a potential therapy worthy of more investigation.
In addition to hyperglycemic-induced end organ damage, altered neurotransmitter function has been observed in diabetic models and may also contribute to cognitive dysfunction. In diabetic rats (blood glucose 28.6 ± 1.1 vs. 6.3 ± 0.2 mmol/liter in control rats), there is an impairment of long-term potentiation, defined as activity-dependent prolonged enhancement of synaptic strength, in neurons rich in receptors for the neurotransmitter N-methyl-d-aspartate (NMDA), which could contribute to learning deficits (90). Other, neurochemical changes have been observed, including decreased acetylcholine (91), decreased serotonin turnover, decreased dopamine activity, and increased norepinephrine (86,92) in the brains of animals with diabetes. Interestingly, these changes were all reversed with insulin. One proposed hypothesis is that the alternating high and low glucose levels seen in patients with poorly controlled diabetes may worsen neurotransmitter function (92).
B. The role of vascular disease
Patients with diabetes have a 2- to 6-fold increased risk in thrombotic stroke (41,93), and vascular disease has long been hypothesized to contribute to abnormalities in cognition in such patients. Autopsy studies on patients with long-standing type 1 diabetes have shown changes related to vascular disease, including diffuse brain degeneration, pseudocalcinosis, demyelination of cranial nerves and spinal cord, and nerve fibrosis (94,95). Thickening of capillary basement membranes, the hallmark of diabetic microangiopathy, has been found in the brains of patients with diabetes (96). Patients with diabetes have also been found to have decreased global rates of cerebral blood flow as measured using xenon, and the magnitude of reduction correlates with the duration of the disease. However, blood glucose levels were not controlled during the experiment (range, 3.1–21.2; mean, 8.8 ± 4.74 mmol/liter) (97). Interestingly, the rate of cerebral blood flow in patients with diabetes is similar to that found in Alzheimer’s patients with dementia (92). These observations in humans with diabetes are supported by studies in streptozotocin-treated rats with chronic hyperglycemia (mean plasma glucose, approximately 29 mmol/liter) (98). One can speculate that the decrease in cerebral blood flow, coupled with the stimulation of the thromboxane A2 receptor known to occur in patients with diabetes (92), could contribute to the inability of cerebral vessels to adequately vasodilate, which may in turn increase the likelihood of ischemia. The coexistence of ischemia and hyperglycemia may be particularly detrimental to the brain. Even modestly elevated blood glucose levels (greater than 8.6 mmol/liter) in humans during a cerebrovascular event correlates with poorer clinical recovery (99). One potential mechanism through which hyperglycemia could potentiate ischemic damage is lactate accumulation. Hyperglycemia provides more substrate for lactate to form, causing cellular acidosis and worsening injury (93). Another mechanism is the accumulation of glutamate in the setting of hyperglycemia and ischemia (100). Glutamate, an excitatory amino acid neurotransmitter, has been shown to cause neuronal damage in the brain (101).
Although the exact mechanism is not known, the lack of C-peptide in patients with type 1 diabetes may by itself worsen cognitive impairment through its actions on the endothelium. Evidence for this is suggested by a rat model (blood glucose approximately 23 mmol/liter) in which replacement of C-peptide to normal levels normalized cognitive function and reduced hippocampal apoptosis (102). The relevance to humans is uncertain, however, because humans with type 1 diabetes do not have hippocampal atrophy (103).
C. The role of hypoglycemia
As mentioned in Section II.C, whether repeated episodes of hypoglycemia contribute to cognitive dysfunction is controversial and most likely depends on the age of the patient. However, there is no argument that if severe hypoglycemia lasts for a very long time, brain damage and death can occur (93,104,105,106). Most endocrinologists have personal experience with patients who have experienced severe hypoglycemia (<2 mm) with little or no permanent consequence. This most likely is secondary to inaccuracies of glucometer at low blood glucose levels (107), inadequate time with severe hypoglycemia, or variations in patients’ glycogen stores. That said, it has been shown in animal models that after 30–60 min of blood glucose levels between 0.12 and 1.36 mmol/liter, neuronal necrosis occurs with accompanying extracellular increases in aspartate, alkalemia, and neuronal energy failure, ultimately leading to a flat electroencephalograph (EEG) (105,106). The cortex, basal ganglia, and hippocampus appear to be most vulnerable to hypoglycemia, with laminar necrosis and gliosis found in these regions on autopsies performed in human patients who died of hypoglycemia (104). Other human autopsy studies done after death secondary to hypoglycemia have shown multifocal or diffuse necrosis of the cerebral cortex and chromatolysis of ganglion cells (108). In animal models, hypoglycemia-induced damage seems to be selective to neurons with sparing of astrocytes and oligodendrocytes (92). Although counterintuitive, the time to neuronal death may be asymmetric between hemispheres in severe, prolonged hypoglycemia, making the differentiation of hypoglycemic brain damage from ischemia difficult on a clinical basis (105). Some have hypothesized that hypoglycemia-induced neuronal damage occurs as a result of overactivation of a subtype of the excitatory neurotransmitter NMDA receptor (109). Interestingly, there exists an NMDA receptor antagonist that has been shown to prevent neuronal necrosis, suggesting a potential therapy for hypoglycemia-induced brain damage (110). Such a therapy may be helpful in young children with type 1 diabetes who seem to be particularly susceptible to cerebral complications of hypoglycemia. There may also be a relationship to hypoglycemia during early nocturnal sleep, a time in which consolidation of memories occurs, and cognitive dysfunction. Compared with test outcomes after a night of sleep in euglycemia, human control subjects and subjects with type 1 diabetes exhibited impaired declarative memory (memory of facts) after undergoing a short, relatively mild hypoglycemic clamp (2.2 mmol/liter) during early sleep (111). However, no neurocognitive deficits were seen in several other studies in which nocturnal hypoglycemia was induced later during the sleeping period (112,113).
D. The role of insulin resistance and amyloid
Although the role of insulin on cerebral metabolism and function is still evolving, fascinating research has given us more insight into this field over the last 20 yr. Historically, the brain was thought to be an insulin-independent organ; however, many recent discoveries have questioned that notion. Insulin receptors and mRNA expression have been found to be widely distributed in rat brain using immunohistochemistry and in situ hybridization (114,115), respectively, including in the olfactory bulb, hypothalamus, hippocampus, cerebellum, piriform cortex, cerebral cortex, and amygdala. The insulin-responsive glucose transporter 4 (GLUT4) has also been found in select regions of the rat brain, including the pituitary, hypothalamus, and medulla (116). GLUT8, also known as GLUTx1, is also found in the rat brain, specifically in the hippocampus, hypothalamus, cerebellum, and brainstem (117). GLUT8 has similar properties to GLUT4 and is up-regulated in response to insulin in some (118) but not all murine tissues, including the brain (119). Despite the presence of insulin receptors and insulin-sensitive glucose transporters, the effect of insulin on cerebral glucose metabolism is still uncertain. Many laboratories, including our own, have failed to demonstrate an effect of insulin on cerebral glucose metabolism in humans (120,121,122). However, other laboratories using fluorodeoxyglucose positron emission tomography (PET) have found a significant increase in brain glucose metabolism in the setting of hyperinsulinemia in humans (123), an effect that is reduced in subjects with peripheral insulin resistance (124). Despite the ongoing controversy of the effect of insulin on cerebral glucose metabolism, there is a large and growing body of evidence that insulin resistance, long recognized as a factor contributing to the onset of type 2 diabetes, may play a role in the pathogenesis of Alzheimer’s disease.
The clinical diagnosis of Alzheimer’s disease is made in the presence of a significant gradual and progressive decline in memory with at least one other cognitive, social, or occupational disturbance (125). The incidence of Alzheimer’s disease has been found to be approximately 1.2- to 1.7-fold higher in patients with type 2 diabetes and insulin resistance compared with a control population in most (37,38,39,40,41,42,43,47), but not all (126,127,128), investigations. The reason for the discrepancy could be the populations studied. Data that support a relationship between Alzheimer’s and insulin resistance all examined older subjects ascertained from the general population. The reports that failed to find an association collected data from a more narrowly defined population, such as those with a high incidence of the apolipoprotein E type 4 (APOE-ε4) allele (128) or a high percentage of early-onset Alzheimer’s disease (126). Interestingly, it appears that type 2 diabetes is also more common in populations with Alzheimer’s disease (129). Whether the association between Alzheimer’s disease and patients with type 2 diabetes reflects the impact of poor metabolic control on brain function or the actual effects of insulin and insulin resistance on the brain is unclear. Patients with Alzheimer’s disease and normal glucose tolerance have a more robust insulin secretory response to an oral glucose load than controls, suggesting that they may have increased insulin resistance (130,131). Some have suggested that the insulin resistance occurs in the brain itself and have hypothesized that the desensitization of neuronal insulin receptors plays an important role in the development of sporadic Alzheimer’s disease (132). Such a concept is supported by observations that patients with Alzheimer’s disease have an elevated concentration of insulin in their cerebral spinal fluid under fasting conditions (131), along with an increase in insulin receptor density in the occipital region and a decrease in tyrosine kinase activity (which is downstream to insulin binding) in the temporal and occipital lobes compared with controls (133). However, others have found that patients with Alzheimer’s disease have a decrease in cerebral spinal fluid insulin levels, suggesting that there may be impaired insulin transport across the blood brain barrier or increased insulin catabolism that accounts for the impaired central insulin action (134).
The mechanisms through which insulin resistance might alter cognitive function remain uncertain, but effects on neurotransmission and memory formation have been hypothesized. An impairment in central cholinergic activity is believed to contribute to the pathogenesis of Alzheimer’s disease (135), and interestingly, rats with streptozotocin- induced diabetes have a decrease in the production and release of acetylcholine compared with control rats (91). Mice models in which cholinergic activity is blocked by scopolamine experience amnesia and hyperactivity, a deficit that can be reversed by glucose administration (136,137). Glucose administration with a rise in endogenous insulin levels or insulin administration to patients with Alzheimer’s disease have also been shown to alter behavior, perhaps by enhancing cholinergic activity (138). In these studies, patients with Alzheimer’s demonstrated an improvement in declarative memory during either a hyperglycemic or a hyperinsulinemic euglycemic clamp (138,139). Furthermore, patients with memory impairment and Alzheimer’s disease had improved verbal memory acutely after intranasal insulin administration, which had no effect on peripheral glucose or insulin levels but had previously been shown to increase central nervous system insulin levels (140). In addition to affecting cholinergic activity, diabetes and insulin may affect long-term potentiation in opposing ways. Long-term potentiation is critical to the formation of memories and is induced by NMDA receptor activation, a process that is up-regulated in the presence of insulin (141). However, rats with diabetes, and presumed relative insulin deficiency, have decreased long-term potentiations in the hippocampus as measured by electrophysiology (90). As would be expected if long-term potentiation were reduced, rat hippocampal neurons exposed to insulin exhibited inhibition of spontaneous firing (142). Interestingly, patients with Alzheimer’s disease have a reduced cerebral glucose uptake as measured by PET (143,144,145) and have a reduced number of glucose transporters in the brain microvessels, frontal cortex, hippocampus, caudate nucleus, parietal, occipital, and temporal lobe compared with controls on autopsy studies (146,147). Perhaps the reduction in glucose uptake has a direct effect on how insulin regulates hippocampal function in these patients. Future experiments to identify the relative roles of glucose and insulin in human cognition are necessary to clarify these relationships.
The impact of insulin on cognitive function has also been examined in control subjects without Alzheimer’s disease or diabetes mellitus. In our laboratory, we found that inducing hyperinsulinemia using an insulin infusion in control subjects reduces parietal region P300 amplitude secondary to memory triggers (148). Other clamp studies found improved vigilance, memory, and selective attention in the setting of hyperinsulinemia (149,150), whereas intranasal insulin treatment for 8 wk improved delayed recall, enhanced mood, and self confidence and reduced anger in nondiabetic, nondementia subjects (151). Based on these studies, it is hypothesized that in Alzheimer’s disease, cerebral insulin resistance requires higher levels of insulin to facilitate memory (46,152). Although cerebral insulin is higher in these patients, it may not be enough to compensate for the insulin resistance. However, this does not necessarily prove that hyperinsulinemia directly improves cognitive function. Hyperinsulinemia can stimulate epinephrine release, and both insulin and epinephrine have been shown to increase lactate (153). Lactate can then in turn be used as a source of energy in brain metabolism (154,155), although the benefits of lactate therapy have not been proven to be beneficial yet in Alzheimer’s patients (156).
Insulin resistance and type 2 diabetes mellitus may contribute to cognitive dysfunction through three other indirect mechanisms. First, cognitive dysfunction in patients with type 2 diabetes has been correlated to inflammatory markers, and increased inflammation may contribute to the development of Alzheimer’s or macrovascular disease. In one investigation, patients with the metabolic syndrome, elevated C-reactive protein, and elevated IL-6 were found to have impaired cognitive function, whereas those patients with the metabolic syndrome and normal levels of these inflammatory markers had similar cognition to controls (47). Patients with type 2 diabetes are known to have higher levels of inflammatory markers including C-reactive protein, α- 1-antichymotrypsin, IL-6, and intercellular adhesion molecule 1 than control populations (157). These findings raise the possibility that insulin resistance and Alzheimer’s disease share a common pathophysiology, because patients with Alzheimer’s disease demonstrate increased inflammatory markers as well (158,159).
A second potential mechanism through which insulin resistance and type 2 diabetes could contribute to cognitive dysfunction is through the disruption of the hypothalamic-pituitary adrenal axis. Both animals (160) and humans (161) with type 2 diabetes have an up-regulation of the hypothalamic-pituitary-adrenal axis, with increased serum cortisol compared with controls. In other research, hypercortisolemia has been found to cause cognitive dysfunction. Healthy humans treated with dexamethasone (162), corticosterone (163), and hydrocortisone (164) to mimic stress conditions all performed worse on memory testing. In a study of healthy elderly patients, those with higher serum cortisol levels performed more poorly on memory and attention testing (165). In addition, patients with Cushing’s disease have been found to have worse performance on memory, attention, reasoning, and concept formation testing compared with controls (166), which may be attributed to a significant reduction in cerebral glucose metabolism found on PET scan in those patients with Cushing’s disease (167). Supporting these findings are the animal studies in which glucocorticoids cause structural damage and reduce function of neurons in the hippocampus (168,169,170,171,172). Based on the facts that type 2 diabetes causes an up-regulation of the hypothalamic-pituitary-adrenal axis and hypercortisolemia can cause cognitive dysfunction, it can be hypothesized that the increase in cortisol levels seen in patients with type 2 diabetes may contribute to cognitive dysfunction.
The third potential mechanism through which insulin resistance may indirectly contribute to cognitive dysfunction is by promoting the formation of senile plaques found in Alzheimer’s disease. Intracellular neurofibrillary tangles and extracellular senile plaques composed of β-amyloid are the pathological hallmarks of Alzheimer’s disease (173,174,175). β-Amyloid is formed from the cleavage of amyloid precursor protein (APP), produced in neurons (176), by the enzymes β- and γ-secretase (177). β-Amyloid is eventually degraded by the insulin-degrading enzyme (178,179). Amyloid β- peptides can by themselves bind to RAGEs and bring about microglial and neuronal dysfunction and oxidative stress (81). Interestingly, amyloid β-peptides, AGEs, and RAGEs have all been colocalized in astrocytes using immunohistochemistry in human brain slices (180). In addition, there is a growing body of evidence that insulin and insulin resistance can affect the metabolism of APP and β-amyloid, thus potentially increasing the burden of cerebral senile plaques. The role of insulin resistance in the metabolism of APP and β-amyloid was further clarified by Craft et al. (181). In their experiment, plasma levels of APP, the precursor to β-amyloid, were lower in those subjects with insulin resistance and Alzheimer’s disease when undergoing a hyperinsulinemic-euglycemic clamp. This corresponded with improved memory testing. One potential explanation of this observation is that insulin resistance may cause decreased APP degradation that can be overcome by the elevating serum and presumably tissue insulin levels (181). Similar findings have come from experiments in rat hippocampal neurons, where insulin was found to up-regulate insulin degrading enzyme, thereby increasing β-amyloid degradation (182). However, not all studies have agreed with this hypothesis. In a study using neuroblastoma cell lines by Gasparini et al. (178), insulin was found to decrease intracellular β-amyloid and increase extracellular levels of β-amyloid by both promoting its secretion and inhibiting its degradation via the insulin-degrading enzyme. This would contradict the majority of the evidence that insulin has a protective effect against memory loss. However, whereas it is widely believed that extracellular accumulation of β-amyloid plays a critical role in the development of Alzheimer’s, other evidence is suggesting there is a pathogenic role for intracellular β-amyloid (183,184,185). More research is needed concerning the pathophysiology of β-amyloid and insulin before conclusions can be drawn.
Of interest is the observation that the pancreatic islets in patients with type 2 diabetes mellitus are characterized by β-cell loss and deposition of islet amyloid (186), which is reminiscent of the neuronal loss and β-amyloid deposition seen in Alzheimer’s disease (187). The constitutions of islet and neural β-amyloid are similar (129,188), and both are toxic to islet and neurons, respectively (189,190). In a series of 29 patients in whom both brain and pancreas autopsy specimens were available, all had amyloid detected to some degree in both the brain and pancreas (187). In another study, islet amyloid was more abundantly present on autopsy in patients with Alzheimer’s disease than in those without Alzheimer’s disease (129). Based on the similarity between islet and neural β-amyloid, some have speculated that a shared pathogenesis may be present in patients with type 2 diabetes and patients with Alzheimer’s disease, possibly involving a defect in a chaperone protein (191) that helps intracellular protein trafficking (129). Rat models of type 2 diabetes (BBZDR/Wor), even more so than type 1 (BB/Wor) diabetes, demonstrate an increase in Alzheimer’s pathology, including increased APP, β-amyloid, and β-secretase and loss of neurons (192). Despite this compelling evidence linking insulin resistance and type 2 diabetes mellitus to Alzheimer’s disease and pathology, several autopsy studies performed in humans have failed to identify an increase in senile plaques or neurofibrillary tangles in subjects with diabetes compared with age-matched controls (83,129), although the duration of diabetes did correlate with the density of senile plaques in one of these studies (129).
The relationship between insulin resistance and cognitive dysfunction in Alzheimer’s disease appears to depend on the presence or absence of the APOE-ε4 allele. Curiously, although the presence of the APOE-ε4 allele is associated with an increased incidence of Alzheimer’s disease (193), it seems that insulin resistance is only a significant risk factor for Alzheimer’s disease in those patients without the APOE-ε4 allele (39,134). Patients with Alzheimer’s disease and no APOE-ε4 allele have been observed to have lower glucose disposal rates during a hyperinsulinemic-euglycemic clamp than subjects with Alzheimer’s disease and the APOE-ε4 allele, as well as those subjects without Alzheimer’s disease or the APOE-ε4 allele. Subjects with Alzheimer’s disease without the APOE-ε4 allele also had improved memory scores in the setting of hyperinsulinemia, which was not the case in APOE-ε4 allele-positive subjects (149,181). Based on this information, it seems that insulin resistance/type 2 diabetes and APOE-ε4 allele positivity are distinct and separate risk factors for the development of Alzheimer’s disease, a hypothesis that is supported by the fact that those with diabetes had a low incidence of the APOE-ε4 allele (128). However, there is again a contradiction in the literature; in the Honolulu-Asia Aging Study, those subjects with both type 2 diabetes and the APOE-ε4 allele had an additive increased risk of dementia and Alzheimer’s pathology (43). This study was specific for elderly Japanese-American men, so it seems that additional multiethnic studies are needed to understand this discrepancy better.
The association between insulin resistance and Alzheimer’s disease has been sufficiently compelling for investigators to examine whether peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists can treat Alzheimer’s disease in the absence of diabetes. To date, two trials have demonstrated rosiglitazone to have a beneficial effect on memory in patients with Alzheimer’s disease. In a small randomized study published by Watson et al. (194) in 2005, patients with mild Alzheimer’s disease treated for 6 months with rosiglitazone had better memory and selective attention than controls. A much larger study published in 2006 found that patients with Alzheimer’s disease without the APOE-ε4 allele had improvements in cognitive testing after 6 months of rosiglitazone, whereas those Alzheimer’s disease patients with the APOE-ε4 allele did not have improvements (195). Multiple mechanisms have been proposed to address how PPAR-γ agonists may affect the pathophysiology responsible for Alzheimer’s disease, including reducing serum glucocorticoids (196), decreasing glial inflammation (197,198), protecting against β-amyloid-induced neurodegeneration (199), decreasing β-amyloid production (197), increasing β-amyloid degradation (196,200), and decreasing phosphorylation of tau proteins, the mechanism by which neurofibrillary tangles are formed (201). Interestingly, despite all of these data, it is still not totally clear how rosiglitazone benefited patients with Alzheimer’s disease in clinical trials because it has been shown not to cross the blood-brain barrier (196). These data are compelling enough to warrant further, longer-term studies on the benefits of PPAR-γ agonists in the treatment of Alzheimer’s disease. However, clinicians must weight the benefits against the newly documented cardiovascular risks of these treatments (202,203).
IV. Modalities for Assessment of Cognitive Dysfunction in Patients with Diabetes
Although progress is being made, the difficulty of detecting neurocognitive dysfunction in patients with diabetes in the clinical setting may explain in part why the field of cognitive dysfunction in diabetes has not advanced similarly to other fields dealing with hyperglycemia-associated end organ damage. Neurocognitive testing in which an examiner administers a battery of tests to assess different aspects of cerebral function has long been the gold standard for the assessment of neurocognitive function. Although cumbersome to administer and score, it has been very useful in assessing neurocognition in a variety of disease states, including diabetes, as was demonstrated in Section II. However, such tests have a relatively high rate of intrasubject variability that reduces their ability to identify mild deficits or preclinical disease. Also, many studies examining the effect of diabetes on brain function use multiple neurocognitive tests that assess the same psychological process. When the results of these different tests don’t agree, determining which results to base conclusions on can be confusing (204). In addition, not all neurocognitive tests are created equal. Although many neurocognitive tests are well validated in a diverse population to distinguish between “normal” and “abnormal” results, other tests do not have adequate reliability data, are based on unacceptably small norms, are administered inappropriately, or do not properly distinguish between two or more diagnostic groups (205). Finally, neurocognitive testing is unable to provide specific information about the neural structures responsible for any dysfunction identified. For example, although it appears that white matter function such as processing speed, attention, and visual-spatial processing are particularly affected by diabetes (4), localization of this dysfunction to white or gray matter is not possible using the battery of tests available to assess neurocognition.
Because of the limitations in neurocognitive testing, a number of modalities have been used to assess cognitive function in patients with diabetes (Table 3). One of the oldest modalities has been to measure electrical activity such as evoked response potentials in the brain after the administration of different stimuli. Abnormal evoked response potentials can reveal subclinical sensory nerve conduction deficits that may not otherwise be apparent (206). For example, flash electroretinography has shown decreased potentials from the retina in diabetic subjects before ophthalmoscopic signs of retinopathy were seen (108). In addition, pattern electroretinogram, which looks at the pattern of retinal stimuli originating from the ganglion cells, is also decreased in patients with diabetes (108). The evoked response of nerves involved in sensing auditory stimuli is also abnormal; brainstem auditory-evoked potentials demonstrated acoustic pathway impairment in patients with diabetes (207,208,209). Evoked potentials related to memory may also be affected because auditory P300 event-related potentials had significantly longer latencies in patients with type 2 diabetes compared with controls, which could relate to attention and short-term memory defects (210). Central somatosensory-evoked potentials were found to be prolonged in patients with diabetes as well (211). In another study looking at both type 1 and type 2 diabetes, slowed latency in visual and somatosensory-evoked potentials was observed in patients with type 1 diabetes, whereas patients with type 2 diabetes had slowed latency of visual, somatosensory, and brainstem auditory-evoked potentials (212). In this investigation, increasing HbA1c was related to reduced cognitive performance. Event-related potentials have also helped define brain adaptations to hypoglycemia. During hypoglycemia, normal subjects do not experience a delay in initial perception and precognitive processing, but they do have a delay in central processes such as stimulus selection and selective central motor activation (213). Of note, although all except one of these studies controlled for hypoglycemia during testing (211), none of the studies adequately controlled for hyperglycemia during testing, although Kurita et al. (210) found no difference between those subjects with high and relatively normal blood glucose values.
Table 3.
Summary of modalities for assessment of cognitive dysfunction in patients with diabetes
| Neurocognitive testing |
| Evoked response potentials |
| EEG |
| MRI |
| fMRI |
| SPECT |
| PET |
EEG can also assess spontaneous cerebral electrical activity and has been used in patients with type 1 and type 2 diabetes. Patients with type 2 diabetes have been found to have slowing in the EEG frequency band analysis over the central cortex area and reduction of alpha activity over the parietal area. These findings correlated with reduced visual retention on neurocognitive testing but were not simply related to hyperglycemia because making nondiabetic subjects with hyperglycemia did not reproduce these findings (32). Subjects with type 1 diabetes have also been found to have abnormal EEG results compared with controls, with those patients with a history of having severe hypoglycemia having the most abnormalities (65,214,215).
Magnetic resonance imaging (MRI) has been used in a number of studies to examine cerebral structure in patients with type 1 and type 2 diabetes and has pretty consistently found the brains of such subjects to have leukoariosis, which are hyperintense white matter lesions (207,216). One study found that 69% of middle-aged adults with long-standing type 1 diabetes had abnormal MRI scans, compared with 12% of healthy, aged-matched volunteers, with an increased number of larger, high-signal lesions in the cerebrum, cerebellum, and brain stem being the primary abnormality identified (207). However, this was not confirmed by a more recent published study in which relatively young patients (25–40 yr old) with type 1 diabetes for more than 15 yr did not have a significant difference in white matter hyperintensities compared with healthy controls. In addition, white matter hyperintensities did not correlate with depressive history, retinopathy, severe hypoglycemia, glycemia control, and most neurocognitive tests (with the exception of delayed memory) (7). This is in agreement with previous studies (3,217). The reason for the discrepancy may have been that subjects in the former study had more severe microvascular complications and that differences in cardiovascular risk factors between subjects with diabetes and controls were not controlled for. In patients with type 2 diabetes, these white matter hyperintensities have been noted to correlate with reduced performance on tests of attention, executive function, information processing speed, and memory (216,218). The nature of these white matter lesions is uncertain, but investigators have hypothesized that they could represent demyelination, increased water content, angionecrosis, cystic infarcts, or gliosis (i.e. brain tissue scarring) (92).
MRI has also demonstrated that subjects with type 2 diabetes have hippocampal and amygdala atrophy relative to control subjects (219). The hippocampus and amygdala are responsible for such functions as memory and behavior and, interestingly, are also found to be atrophied in Alzheimer’s patients (219). However, a similar study in subjects with type 1 diabetes failed to identify reductions in hippocampal and amygdala volume, although these subjects did have an increase in cerebrospinal fluid on MRI, suggesting mild global cerebral atrophy (103). Another study compared MRI findings and neuropsychometric testing in patients with an early age of onset of type 1 diabetes (younger than age 7) and a later age of onset (7–17 yr old). Subjects with early onset disease had larger ventricular volumes and more prevalent ventricular atrophy than those with later onset, which corresponded to poorer intellectual and information processing ability (220). Atrophy in subcortical and periventricular areas has also been associated with reduced performance on memory tasks in patients with type 2 diabetes (216). A history of hypoglycemia appears to be related to an increase in cerebral atrophy (70).
Recently, voxel-based morphometry, in which differences in local characteristics of tissue are measured using MRI, has been used to evaluate both the gray and white matter of patients with type 1 diabetes. Musen et al. (221) found that compared with controls, patients with type 1 diabetes had lower gray matter density in certain areas of the temporal lobe, frontal lobe, and thalamus. They also found that higher HbA1c levels correlated with lower gray matter density in areas important for language, memory, and attention, whereas a history of severe hypoglycemia correlated with less gray matter density in the left cerebellar posterior lobe (221). Wessels et al. (222) performed a similar experiment but compared patients with type 1 diabetes and retinopathy, patients with type 1 diabetes and no retinopathy, and controls. They found that patients with type 1 diabetes and proliferative retinopathy had decreased gray matter density in the right inferior gyrus and right occipital lobe compared with those patients with diabetes and no retinopathy, as well as to controls. More recently, Wessels and colleagues applied voxel-based morphometry to white matter volumes. They found that those subjects with type 1 diabetes and proliferative retinopathy had significantly smaller white matter volume than subjects without diabetes, and that smaller white matter volume correlated with worse performances on attention, speed of information processing, and executive function. This was not seen in patients with type 1 diabetes who had no proliferative retinopathy, suggesting a possible common mechanism in the development of retinopathy and cerebral dysfunction (6). Preliminary findings by Perantie et al. (223) have found that a history of severe hypoglycemia in children is associated with decreased gray matter in the left superior temporal region, whereas increased HbAlc levels are associated with reductions of gray matter in the right cuneus and precuneus regions, reductions of white matter in the right posterior parietal region, and increased gray matter in the right prefrontal cortex.
Functional MRI (fMRI) has also been used to assess cerebral function in patients with diabetes. fMRI is based on the fact that increases in cerebral blood flow and metabolism during stimulus-induced neuronal activation are accompanied by a relative reduction in deoxyhemoglobin content of the activated tissue relative to the adjacent unactivated brain. Because deoxyhemoglobin is a paramagnetic molecule, it can be visualized by MRI. Rosenthal et al. (224) applied fMRI to the study of cerebral function during standard neurocognitive testing in subjects with type 1 diabetes subjected to both euglycemia and hypoglycemia. They found that the effect of acute hypoglycemia on cerebral blood flow is task and region specific. For example, during hypoglycemia, the slower finger tapping corresponded to decreased activation of the right premotor cortex, supplementary motor area, and left hippocampus and with increased activation in the left cerebellum and right frontal pole. In addition, during hypoglycemia deterioration of four-choice reaction time correlated with reduced activation in the motor and visual systems but with increased activation of the part of the parietal cortex involved in planning (224). More recently, Wessels et al. (24) applied this methodology to determine whether subjects with type 1 diabetes with known hyperglycemia-induced end organ damage, specifically retinopathy, had differing areas of cerebral activation with cognitive stimuli and hypoglycemia compared with patients with type 1 diabetes and no microvascular complications. Although there was no difference seen in cognitive ability between the two groups, there was an overall increase in activation and less appropriate deactivation of certain brain regions during hypoglycemia in the diabetic group with retinopathy. The investigators hypothesized that regional alterations in activation were secondary to hyperglycemia-induced end organ damage in the central nervous system, causing altered neurovascular coupling or functional microvascular alterations (24). Preliminary findings by Musen et al. (225) have found decreased activation in the superior temporal gyrus and the parahippocampal gyrus in response to lexical and recognition stimuli, respectively, in a group composed of patients with long-standing type 1 diabetes compared with controls.
Other imaging modalities such as single photon emission computed tomography (SPECT) and PET have been used to assess cerebral function in patients with diabetes mellitus. SPECT is particularly good at assessing cerebral perfusion and has demonstrated in an uncontrolled study that patients with type 2 diabetes and dementia have a high incidence of hypoperfusion in at least one area of the brain (226). However, SPECT has also demonstrated that patients with type 1 diabetes have hyperperfusion in the prefrontal and frontal brain regions compared with controls (227). Other investigators found that when the SPECT data are corrected for the increase in cerebral atrophy seen in patients with diabetes, cerebral blood flow and glucose metabolism values were within normal range (228). PET with fluorodeoxyglucose is a technique that can be used to assess glucose metabolism because the compound is taken up and trapped in the cell by phosphorylation. When this method was used in patients with type 1 diabetes and a history of severe hypoglycemia and hypoglycemia unawareness, no differences in glucose metabolism were found relative to controls, although neuropsychological testing was also not significantly different (69). Two pilot studies, one by our own laboratory, have used diffusion tensor imaging, a type of MRI that measures white matter integrity quantitatively by fractional anisotropy (229,230), in patients with diabetes. Preliminary findings show a reduction in white matter integrity in patients with type 1 diabetes that was associated with severity of hyperglycemia (231) and poorer performance on certain neurocognitive tests (67).
In general, assessment modalities to detect cognitive dysfunction associated with diabetes have been disappointing. Neurocognitive testing is cumbersome and lacks pathophysiological insight. Evoked response potentials and EEG seem to be good at detecting sensory/perception deficits but are also cumbersome and do not provide as much information about more complex cognitive functions. Although promising, there have been conflicting results with regard to MRI studies, specifically in patients with type 1 diabetes. The utility of SPECT, PET, and diffusion tensor imaging in monitoring or detecting changes in patients with cognitive dysfunction and diabetes remains to be determined.
V. Future Directions
Although much research has been done on the impact of diabetes mellitus on cognitive function, many questions still remain. It is clear that patients with type 1 and type 2 diabetes have been found to have abnormalities in neurocognitive function, although the natural history and clinical significance of these findings have not yet been clearly defined. For example, we know that there is an increased incidence of dementia in patients with type 2 diabetes (37,38,40,41,42,43,44), but we do not know whether the more subtle changes in memory and in other measures on neurocognitive testing are a precursor to true dementia or represent another process. We also do not know whether the incidence of dementia is increased in patients with type 1 diabetes compared with the rest of the population. In prior decades, this was not an issue because patients with type 1 diabetes died at relatively young ages from other complications of the disease. However, now that patients with type 1 diabetes are living longer and better with the disease, this must be assessed. Finally, it is not clear whether the subtle cognitive deficits identified in many studies truly impact the lives of patients living with diabetes (34). Future studies, perhaps longitudinal in nature or involving better serum/imaging biomarkers, will be of tremendous benefit in providing better understanding of the natural history of this complication.
Future study will also be important in understanding the pathogenesis of cognitive dysfunction secondary to diabetes. Although it seems that hyperglycemia and hyperglycemia-induced end organ damage contribute to this problem, the actual mechanisms through which hyperglycemia alters cerebral structure and function are not clear. Improved glycemic control is likely of therapeutic benefit, as has been suggested by many retrospective studies (5,15,16,17,18,26,27,28,48), but a prospective study is needed to determine whether this is true. In addition, identification of the mechanisms through which hyperglycemia may impair cognitive function in patients with diabetes will stimulate new research into ways to prevent and treat all of the hyperglycemia-associated complications of diabetes.
VI. Conclusion
In conclusion, there have been significant gains in our understanding of the effect of diabetes on cognitive dysfunction. Evidence from neurocognitive testing suggests that cognitive dysfunction should be listed as one of the many complications of diabetes, along with retinopathy, neuropathy, nephropathy, and cardiovascular disease. The pathogenesis of cognitive dysfunction is only partially understood. Although many studies suggest that changes in cerebral structure and function in diabetes are related to hyperglycemia-induced end organ damage, macrovascular disease, hypoglycemia, insulin resistance, and amyloid lesions may play a role in some patients. Greater understanding of the natural history of this diabetes complication and the mechanisms responsible for its development will continue to advance as biochemical and imaging modalities continue to evolve. As new knowledge is gained, it can be applied to develop new and improved ways to prevent and treat all of the hyperglycemia-related complications of diabetes.
Footnotes
This work was supported by NIH Grants NS35192 (to E.R.S.), DK62440 (to E.R.S.), and 2 T32 DK007203–29A1 (to C.T.K.).
Disclosure Statement: E.R.S. has served on advisory boards for Pfizer and Merck. C.T.K. has no disclosures to state.
First Published Online April 24, 2008
Abbreviations: AGE, Advanced glycation end product; APOE-ε4, apolipoprotein E type 4; APP, amyloid precursor protein; DCCT, Diabetes Control and Complications Trial; EEG, electroencephalograph(y); fMRI, functional MRI; GLUT, glucose transporter; HbA1c, glycated hemoglobin; MRI, magnetic resonance imaging; NMDA, N-methyl-d- aspartate; PET, positron emission tomography; PPAR-γ, peroxisome proliferator-activated receptor-γ; SPECT, single photon emission computed tomography.
References
- 2003 U.S. Renal Data System (USRDS) Annual Data Report: Atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases [Google Scholar]
- Miles WR, Root HF 1922 Psychologic tests applied to diabetic patients. Arch Intern Med 30:767–777 [Google Scholar]
- Brands AM, Kessels RP, Hoogma RP, Henselmans JM, van der Beek Boter JW, Kappelle LJ, de Haan EH, Biessels GJ 2006 Cognitive performance, psychological well-being, and brain magnetic resonance imaging in older patients with type 1 diabetes. Diabetes 55:1800–1806 [DOI] [PubMed] [Google Scholar]
- Ryan CM, Geckle MO, Orchard TJ 2003 Cognitive efficiency declines over time in adults with type 1 diabetes: effects of micro- and macrovascular complications. Diabetologia 46:940–948 [DOI] [PubMed] [Google Scholar]
- Ryan CM, Williams TM, Finegold DN, Orchard TJ 1993 Cognitive dysfunction in adults with type 1 (insulin-dependent) diabetes mellitus of long duration: effects of recurrent hypoglycaemia and other chronic complications. Diabetologia 36:329–334 [DOI] [PubMed] [Google Scholar]
- Wessels AM, Rombouts SA, Remijnse PL, Boom Y, Scheltens P, Barkhof F, Heine RJ, Snoek FJ 2007 Cognitive performance in type 1 diabetes patients is associated with cerebral white matter volume. Diabetologia 20:1763–1769 [DOI] [PubMed] [Google Scholar]
- Weinger K, Jacobson AM, Musen G, Lyoo IK, Ryan CM, Jimerson DC, Renshaw PF 2008 The effects of type 1 diabetes on cerebral white matter. Diabetologia 51:417–425 [DOI] [PubMed] [Google Scholar]
- Hershey T, Bhargava N, Sadler M, White NH, Craft S 1999 Conventional versus intensive diabetes therapy in children with type 1 diabetes: effects on memory and motor speed. Diabetes Care 22:1318–1324 [DOI] [PubMed] [Google Scholar]
- Ryan CM 1988 Neurobehavioral complications of type I diabetes. Examination of possible risk factors. Diabetes Care 11:86–93 [DOI] [PubMed] [Google Scholar]
- Skenazy JA, Bigler ED 1984 Neuropsychological findings in diabetes mellitus. J Clin Psychol 40:246–258 [DOI] [PubMed] [Google Scholar]
- Hershey T, Craft S, Bhargava N, White NH 1997 Memory and insulin dependent diabetes mellitus (IDDM): effects of childhood onset and severe hypoglycemia. J Int Neuropsychol Soc 3:509–520 [PubMed] [Google Scholar]
- Northam EA, Anderson PJ, Werther GA, Warne GL, Adler RG, Andrewes D 1998 Neuropsychological complications of IDDM in children 2 years after disease onset. Diabetes Care 21:379–384 [DOI] [PubMed] [Google Scholar]
- Schoenle EJ, Schoenle D, Molinari L, Largo RH 2002 Impaired intellectual development in children with type I diabetes: association with HbA(1c), age at diagnosis and sex. Diabetologia 45: 108–114 [DOI] [PubMed] [Google Scholar]
- Northam EA, Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA 2001 Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care 24:1541–1546 [DOI] [PubMed] [Google Scholar]
- Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W, Harth J 2007 Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 356:1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CS 1986 Neuropsychological profiles in men with insulin-dependent diabetes. J Consult Clin Psychol 54:386–389 [DOI] [PubMed] [Google Scholar]
- Kaufman FR, Epport K, Engilman R, Halvorson M 1999 Neurocognitive functioning in children diagnosed with diabetes before age 10 years. J Diabetes Complications 13:31–38 [DOI] [PubMed] [Google Scholar]
- Perantie DC, Lim A, Wu J, Weaver P, Warren SL, Hayden T, Christ S, Sadler M, White NH, Hershey T 2007 Severe hypoglycemia vs. hyperglycemia: unique effects on cognition in youth with type 1 diabetes mellitus (TIDM). Diabetes 56(Suppl 1):(Abstract 1887) [Google Scholar]
- Cox DJ, Kovatchev BP, Gonder-Frederick LA, Summers KH, McCall A, Grimm KJ, Clarke WL 2005 Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes Care 28:71–77 [DOI] [PubMed] [Google Scholar]
- Rovet J, Alvarez M 1997 Attentional functioning in children and adolescents with IDDM. Diabetes Care 20:803–810 [DOI] [PubMed] [Google Scholar]
- Sommerfield AJ, Deary IJ, Frier BM 2004 Acute hyperglycemia alters mood state and impairs cognitive performance in people with type 2 diabetes. Diabetes Care 27:2335–2340 [DOI] [PubMed] [Google Scholar]
- Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP 2005 The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care 28:726–735 [DOI] [PubMed] [Google Scholar]
- Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJ, Wardlaw J, Deary IJ, Frier BM 2003 Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes 52:149–156 [DOI] [PubMed] [Google Scholar]
- Wessels AM, Rombouts SA, Simsek S, Kuijer JP, Kostense PJ, Barkhof F, Scheltens P, Snoek FJ, Heine RJ 2006 Microvascular disease in type 1 diabetes alters brain activation: a functional magnetic resonance imaging study. Diabetes 55:334–340 [DOI] [PubMed] [Google Scholar]
- Gregg EW, Yaffe K, Cauley JA, Rolka DB, Blackwell TL, Narayan KM, Cummings SR 2000 Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of Osteoporotic Fractures Research Group. Arch Intern Med 160: 174–180 [DOI] [PubMed] [Google Scholar]
- Reaven GM, Thompson LW, Nahum D, Haskins E 1990 Relationship between hyperglycemia and cognitive function in older NIDDM patients. Diabetes Care 13:16–21 [DOI] [PubMed] [Google Scholar]
- Munshi M, Grande L, Hayes M, Ayres D, Suhl E, Capelson R, Lin S, Milberg W, Weinger K 2006 Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care 29:1794–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmuter LC, Hakami MK, Hodgson-Harrington C, Ginsberg J, Katz J, Singer DE, Nathan DM 1984 Decreased cognitive function in aging non-insulin-dependent diabetic patients. Am J Med 77:1043–1048 [DOI] [PubMed] [Google Scholar]
- Messier C 2005 Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobiol Aging 26(Suppl 1):26–30 [DOI] [PubMed] [Google Scholar]
- Grodstein F, Chen J, Wilson RS, Manson JE 2001 Type 2 diabetes and cognitive function in community-dwelling elderly women. Diabetes Care 24:1060–1065 [DOI] [PubMed] [Google Scholar]
- Kanaya AM, Barrett-Connor E, Gildengorin G, Yaffe K 2004 Change in cognitive function by glucose tolerance status in older adults: a 4-year prospective study of the Rancho Bernardo study cohort. Arch Intern Med 164:1327–1333 [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Perryman K, Fitten J, Kavonian GD, Morley JE 1988 Cortical function in elderly non-insulin dependent diabetic patients. Behavioral and electrophysiologic studies. Arch Intern Med 148:2369–2372 [PubMed] [Google Scholar]
- Fontbonne A, Berr C, Ducimetiere P, Alperovitch A 2001 Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 24:366–370 [DOI] [PubMed] [Google Scholar]
- Sinclair AJ, Girling AJ, Bayer AJ 2000 Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self- management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract 50:203–212 [DOI] [PubMed] [Google Scholar]
- Gregg EW, Brown AM 2003 Cognitive and physical disabilities and aging-related complications of diabetes. Clin Diabetes 21:113–118 [Google Scholar]
- Bruce DG, Casey GP, Grange V, Clarnette RC, Almeida OP, Foster JK, Ives FJ, Davis TM 2003 Cognitive impairment, physical disability and depressive symptoms in older diabetic patients: the Fremantle Cognition in Diabetes Study. Diabetes Res Clin Pract 61:59–67 [DOI] [PubMed] [Google Scholar]
- Cukierman T, Gerstein HC, Williamson JD 2005 Cognitive decline and dementia in diabetes–systematic overview of prospective observational studies. Diabetologia 48:2460–2469 [DOI] [PubMed] [Google Scholar]
- Curb JD, Rodriguez BL, Abbott RD, Petrovitch H, Ross GW, Masaki KH, Foley D, Blanchette PL, Harris T, Chen R, White LR 1999 Longitudinal association of vascular and Alzheimer’s dementias, diabetes, and glucose tolerance. Neurology 52:971–975 [DOI] [PubMed] [Google Scholar]
- Kuusisto J, Koivisto K, Mykkanen L, Helkala EL, Vanhanen M, Hanninen T, Kervinen K, Kesaniemi YA, Riekkinen PJ, Laakso M 1997 Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: cross-sectional population based study. BMJ 315:1045–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O'Brien PC, Palumbo PJ 1997 Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol 145:301–308 [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R 2001 Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol 154:635–641 [DOI] [PubMed] [Google Scholar]
- Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, Breteler MM 1996 Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia 39:1392–1397 [DOI] [PubMed] [Google Scholar]
- Peila R, Rodriguez BL, Launer LJ 2002 Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes 51:1256–1262 [DOI] [PubMed] [Google Scholar]
- Sullivan MG, Glycemic control shown to prevent dementia. 10th International Conference on Alzheimer’s Disease and Related Disorders, Madrid, Spain, 2006 [Google Scholar]
- Yoshitake T, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, Ohmori S, Nomiyama K, Kawano H, Ueda K, Sueshi K, Tsuneyoshi M, Fujishima M 1995 Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hisayama Study. Neurology 45:1161–1168 [DOI] [PubMed] [Google Scholar]
- Watson GS, Craft S 2003 The role of insulin resistance in the pathogenesis of Alzheimer’s disease: implications for treatment. CNS Drugs 17:27–45 [DOI] [PubMed] [Google Scholar]
- Yaffe K, Blackwell T, Whitmer RA, Krueger K, Barrett Connor E 2006 Glycosylated hemoglobin level and development of mild cognitive impairment or dementia in older women. J Nutr Health Aging 10:293–295 [PubMed] [Google Scholar]
- Ryan CM, Geckle MO 2000 Circumscribed cognitive dysfunction in middle-aged adults with type 2 diabetes. Diabetes Care 23:1486–1493 [DOI] [PubMed] [Google Scholar]
- Vanhanen M, Koivisto K, Kuusisto J, Mykkanen L, Helkala EL, Hanninen T, Riekkinen Sr P, Soininen H, Laakso M 1998 Cognitive function in an elderly population with persistent impaired glucose tolerance. Diabetes Care 21:398–402 [DOI] [PubMed] [Google Scholar]
- de Vegt F, Dekker JM, Ruhe HG, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ 1999 Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia 42:926–931 [DOI] [PubMed] [Google Scholar]
- Dilley J, Ganesan A, Deepa R, Deepa M, Sharada G, Williams OD, Mohan V 2007 Association of A1C with cardiovascular disease and metabolic syndrome in Asian Indians with normal glucose tolerance. Diabetes Care 30:1527–1532 [DOI] [PubMed] [Google Scholar]
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N 2004 Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 141:413–420 [DOI] [PubMed] [Google Scholar]
- Kumari M, Marmot M 2005 Diabetes and cognitive function in a middle-aged cohort: findings from the Whitehall II study. Neurology 65:1597–1603 [DOI] [PubMed] [Google Scholar]
- Scott RD, Kritz-Silverstein D, Barrett-Connor E, Wiederholt WC 1998 The association of non-insulin-dependent diabetes mellitus and cognitive function in an older cohort. J Am Geriatr Soc 46:1217–1222 [DOI] [PubMed] [Google Scholar]
- Lindeman RD, Romero LJ, LaRue A, Yau CL, Schade DS, Koehler KM, Baumgartner RN, Garry PJ 2001 A biethnic community survey of cognition in participants with type 2 diabetes, impaired glucose tolerance, and normal glucose tolerance: the New Mexico Elder Health Survey. Diabetes Care 24:1567–1572 [DOI] [PubMed] [Google Scholar]
- 1993 The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin- dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 329:977–986 [DOI] [PubMed] [Google Scholar]
- 1998 Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352:837–853 [PubMed] [Google Scholar]
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B 2005 Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin EJ, Deary IJ 1999 Effects of repeated hypoglycemia on cognitive function: a psychometrically validated reanalysis of the Diabetes Control and Complications Trial data. Diabetes Care 22:1273–1277 [DOI] [PubMed] [Google Scholar]
- Sommerfield AJ, Deary IJ, McAulay V, Frier BM 2003 Short-term, delayed, and working memory are impaired during hypoglycemia in individuals with type 1 diabetes. Diabetes Care 26:390–396 [DOI] [PubMed] [Google Scholar]
- Widom B, Simonson DC 1990 Glycemic control and neuropsychologic function during hypoglycemia in patients with insulin-dependent diabetes mellitus. Ann Intern Med 112:904–912 [DOI] [PubMed] [Google Scholar]
- Warren RE, Zammitt NN, Deary IJ, Frier BM 2007 The effects of acute hypoglycaemia on memory acquisition and recall and prospective memory in type 1 diabetes. Diabetologia 50:178–185 [DOI] [PubMed] [Google Scholar]
- Maran A, Lomas J, Macdonald IA, Amiel SA 1995 Lack of preservation of higher brain function during hypoglycaemia in patients with intensively-treated IDDM. Diabetologia 38:1412–1418 [DOI] [PubMed] [Google Scholar]
- Deary IJ, Crawford JR, Hepburn DA, Langan SJ, Blackmore LM, Frier BM 1993 Severe hypoglycemia and intelligence in adult patients with insulin-treated diabetes. Diabetes 42:341–344 [DOI] [PubMed] [Google Scholar]
- Howorka K, Pumprla J, Saletu B, Anderer P, Krieger M, Schabmann A 2000 Decrease of vigilance assessed by EEG-mapping in type I diabetic patients with history of recurrent severe hypoglycaemia. Psychoneuroendocrinology 25:85–105 [DOI] [PubMed] [Google Scholar]
- Wredling R, Levander S, Adamson U, Lins PE 1990 Permanent neuropsychological impairment after recurrent episodes of severe hypoglycaemia in man. Diabetologia 33:152–157 [DOI] [PubMed] [Google Scholar]
- Kodl C, Franc D, Anderson FS, Lim KO, Seaquist ER, Diffusion tensor imaging (DTI) identifies defects in white matter microstructure in subjects with diabetes. American Diabetes Association 67th Scientific Session, Chicago, IL, 2007 [Google Scholar]
- DCCT Research Group1996 Effects of intensive diabetes therapy on neuropsychological function in adults in the Diabetes Control and Complications Trial. Ann Intern Med 124:379–388 [DOI] [PubMed] [Google Scholar]
- Chabriat H, Sachon C, Levasseur M, Grimaldi A, Pappata S, Rougemont D, Masure MC, De Recondo A, Samson Y 1994 Brain metabolism after recurrent insulin induced hypoglycaemic episodes: a PET study. J Neurol Neurosurg Psychiatry 57:1360–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perros P, Deary IJ, Sellar RJ, Best JJ, Frier BM 1997 Brain abnormalities demonstrated by magnetic resonance imaging in adult IDDM patients with and without a history of recurrent severe hypoglycemia. Diabetes Care 20:1013–1018 [DOI] [PubMed] [Google Scholar]
- Reichard P, Britz A, Rosenqvist U 1991 Intensified conventional insulin treatment and neuropsychological impairment. BMJ 303:1439–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ack M, Miller I, Weil Jr WB 1961 Intelligence of children with diabetes mellitus. Pediatrics 28:764–770 [PubMed] [Google Scholar]
- Ryan C, Vega A, Drash A 1985 Cognitive deficits in adolescents who developed diabetes early in life. Pediatrics 75:921–927 [PubMed] [Google Scholar]
- Ryan CM 1997 Effects of diabetes mellitus on neuropsychological functioning: a lifespan perspective. Semin Clin Neuropsychiatry 2:4–14 [DOI] [PubMed] [Google Scholar]
- Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH 2002 Ageing and diabetes: implications for brain function. Eur J Pharmacol 441:1–14 [DOI] [PubMed] [Google Scholar]
- Brownlee M 2005 The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625 [DOI] [PubMed] [Google Scholar]
- Klein JP, Waxman SG 2003 The brain in diabetes: molecular changes in neurons and their implications for end-organ damage. Lancet Neurol 2:548–554 [DOI] [PubMed] [Google Scholar]
- Toth C, Schmidt AM, Tuor UI, Francis G, Foniok T, Brussee V, Kaur J, Yan SF, Martinez JA, Barber PA, Buchan A, Zochodne DW 2006 Diabetes, leukoencephalopathy and rage. Neurobiol Dis 23:445–461 [DOI] [PubMed] [Google Scholar]
- Sredy J, Sawicki DR, Notvest RR 1991 Polyol pathway activity in nervous tissues of diabetic and galactose-fed rats: effect of dietary galactose withdrawal or tolrestat intervention therapy. J Diabet Complications 5:42–47 [DOI] [PubMed] [Google Scholar]
- Malone JI, Hanna SK, Liang X, Saporta S, Park CR, Diamond D 2007 Diabetes increases polyol pathway activity in the brain which is blocked by sorbinil. Diabetes 56(Suppl 1) (Abstract 793) [Google Scholar]
- Yan SD, Stern D, Schmidt AM 1997 What’s the RAGE? The receptor for advanced glycation end products (RAGE) and the dark side of glucose. Eur J Clin Invest 27:179–181 [DOI] [PubMed] [Google Scholar]
- Girones X, Guimera A, Cruz-Sanchez CZ, Ortega A, Sasaki N, Makita Z, Lafuente JV, Kalaria R, Cruz-Sanchez FF 2004 N ε- carboxymethyllysine in brain aging, diabetes mellitus, and Alzheimer’s disease. Free Radic Biol Med 36:1241–1247 [DOI] [PubMed] [Google Scholar]
- Heitner J, Dickson D 1997 Diabetics do not have increased Alzheimer-type pathology compared with age-matched control subjects. A retrospective postmortem immunocytochemical and histofluorescent study. Neurology 49:1306–1311 [DOI] [PubMed] [Google Scholar]
- Niiya Y, Abumiya T, Shichinohe H, Kuroda S, Kikuchi S, Ieko M, Yamagishi S, Takeuchi M, Sato T, Iwasaki Y 2006 Susceptibility of brain microvascular endothelial cells to advanced glycation end products-induced tissue factor upregulation is associated with intracellular reactive oxygen species. Brain Res 1108:179–187 [DOI] [PubMed] [Google Scholar]
- Zimmerman GA, Meistrell 3rd M, Bloom O, Cockroft KM, Bianchi M, Risucci D, Broome J, Farmer P, Cerami A, Vlassara H, Tracey KJ1995 Neurotoxicity of advanced glycation endproducts during focal stroke and neuroprotective effects of aminoguanidine. Proc Natl Acad Sci USA 92:3744–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan R, Sheeladevi R, Suthanthirarajan N 2004 PKC-α mediated alterations of indoleamine contents in diabetic rat brain. Brain Res Bull 64:189–194 [DOI] [PubMed] [Google Scholar]
- Sieber FE, Hurn P, Alkayed NJ, Traystman RJ 2001 Gender-based differences in Na+ -K+ adenosine triphosphatase activity occur in the microcirculation of the diabetic rat brain. Anesthesiology 94:372–375 [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Siedlak SL, Fortino AE, Perry G, Ghetti B, Smith MA 2005 Chitin-like polysaccharides in Alzheimer’s disease brains. Curr Alzheimer Res 2:419–423 [DOI] [PubMed] [Google Scholar]
- Aragno M, Mastrocola R, Medana C, Restivo F, Catalano MG, Pons N, Danni O, Boccuzzi G 2005 Up-regulation of advanced glycated products receptors in the brain of diabetic rats is prevented by antioxidant treatment. Endocrinology 146:5561–5567 [DOI] [PubMed] [Google Scholar]
- Kamal A, Biessels GJ, Urban IJ, Gispen WH 1999 Hippocampal synaptic plasticity in streptozotocin-diabetic rats: impairment of long-term potentiation and facilitation of long-term depression. Neuroscience 90:737–745 [DOI] [PubMed] [Google Scholar]
- Welsh B, Wecker L 1991 Effects of streptozotocin-induced diabetes on acetylcholine metabolism in rat brain. Neurochem Res 16: 453–460 [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Kappelle AC, Bravenboer B, Erkelens DW, Gispen WH 1994 Cerebral function in diabetes mellitus. Diabetologia 37:643–650 [DOI] [PubMed] [Google Scholar]
- McCall AL 1992 The impact of diabetes on the CNS. Diabetes 41:557–570 [DOI] [PubMed] [Google Scholar]
- Reske-Nielsen E, Lundbaek K 1965 Pathological changes in the central and peripheral nervous system of young long-term diabetics. I. Diabetic encephalopathy. Diabetologia 1:233–241 [DOI] [PubMed] [Google Scholar]
- Reske-Nielsen E, Lundbaek K 1968 Pathological changes in the central and peripheral nervous system of young long-term diabetics. II. The spinal cord and peripheral nerves. Diabetologia 4:34–43 [DOI] [PubMed] [Google Scholar]
- Johnson PC, Brendel K, Meezan E 1982 Thickened cerebral cortical capillary basement membranes in diabetics. Arch Pathol Lab Med 106:214–217 [PubMed] [Google Scholar]
- Rodriguez G, Nobili F, Celestino MA, Francione S, Gulli G, Hassan K, Marenco S, Rosadini G, Cordera R 1993 Regional cerebral blood flow and cerebrovascular reactivity in IDDM. Diabetes Care 16:462–468 [DOI] [PubMed] [Google Scholar]
- Duckrow RB, Beard DC, Brennan RW 1987 Regional cerebral blood flow decreases during chronic and acute hyperglycemia. Stroke 18:52–58 [DOI] [PubMed] [Google Scholar]
- Kushner M, Nencini P, Reivich M, Rango M, Jamieson D, Fazekas F, Zimmerman R, Chawluk J, Alavi A, Alves W 1990 Relation of hyperglycemia early in ischemic brain infarction to cerebral anatomy, metabolism, and clinical outcome. Ann Neurol 28:129–135 [DOI] [PubMed] [Google Scholar]
- Li PA, Shuaib A, Miyashita H, He QP, Siesjo BK, Warner DS 2000 Hyperglycemia enhances extracellular glutamate accumulation in rats subjected to forebrain ischemia. Stroke 31:183–192 [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P 1993 Oxidative stress, glutamate, and neurodegenerative disorders. Science 262:689–695 [DOI] [PubMed] [Google Scholar]
- Sima AA, Li ZG 2005 The effect of C-peptide on cognitive dysfunction and hippocampal apoptosis in type 1 diabetic rats. Diabetes 54:1497–1505 [DOI] [PubMed] [Google Scholar]
- Lognig BM, Kromeke O, Optenhostert-Porstt C, Wolf OT 2005 Hippocampal volume and cognitive performance in long-standing type 1 diabetic patients without macrovascular complications. Diabet Med 23:32–39 [DOI] [PubMed] [Google Scholar]
- Patrick AW, Campbell IW 1990 Fatal hypoglycaemia in insulin-treated diabetes mellitus: clinical features and neuropathological changes. Diabet Med 7:349–354 [DOI] [PubMed] [Google Scholar]
- Auer RN 2004 Hypoglycemic brain damage. Metab Brain Dis 19:169–175 [DOI] [PubMed] [Google Scholar]
- Auer RN 2004 Hypoglycemic brain damage. Forensic Sci Int 146:105–110 [DOI] [PubMed] [Google Scholar]
- Elusiyan JB, Adeodu OO, Adejuyigbe EA 2006 Evaluating the validity of a bedside method of detecting hypoglycemia in children. Pediatr Emerg Care 22:488–490 [DOI] [PubMed] [Google Scholar]
- Comi G 1997 Evoked potentials in diabetes mellitus. Clin Neurosci 4:374–379 [PubMed] [Google Scholar]
- Siesjo BK, Bengtsson F 1989 Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: a unifying hypothesis. J Cereb Blood Flow Metab 9:127–140 [DOI] [PubMed] [Google Scholar]
- Wieloch T 1985 Hypoglycemia-induced neuronal damage prevented by an N-methyl-D-aspartate antagonist. Science 230:681–683 [DOI] [PubMed] [Google Scholar]
- Jauch-Chara K, Hallschmid M, Gais S, Schmid SM, Oltmanns KM, Colmorgen C, Born J, Schultes B 2007 Hypoglycemia during sleep impairs consolidation of declarative memory in type 1 diabetic and healthy humans. Diabetes Care 30:2040–2045 [DOI] [PubMed] [Google Scholar]
- Bendtson I, Gade J, Theilgaard A, Binder C 1992 Cognitive function in type 1 (insulin-dependent) diabetic patients after nocturnal hypoglycaemia. Diabetologia 35:898–903 [DOI] [PubMed] [Google Scholar]
- Matyka KA, Wigg L, Pramming S, Stores G, Dunger DB 1999 Cognitive function and mood after profound nocturnal hypoglycaemia in prepubertal children with conventional insulin treatment for diabetes. Arch Dis Child 81:138–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks JL, Porte Jr D, Stahl WL, Baskin DG 1990 Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology 127:3234–3236 [DOI] [PubMed] [Google Scholar]
- Unger J, McNeill TH, Moxley 3rd RT, White M, Moss A, Livingston JN 1989 Distribution of insulin receptor-like immunoreactivity in the rat forebrain. Neuroscience 31:143–157 [DOI] [PubMed] [Google Scholar]
- Brant AM, Jess TJ, Milligan G, Brown CM, Gould GW 1993 Immunological analysis of glucose transporters expressed in different regions of the rat brain and central nervous system. Biochem Biophys Res Commun 192:1297–1302 [DOI] [PubMed] [Google Scholar]
- Ibberson M, Uldry M, Thorens B 2000 GLUTX1, a novel mammalian glucose transporter expressed in the central nervous system and insulin-sensitive tissues. J Biol Chem 275:4607–4612 [DOI] [PubMed] [Google Scholar]
- Carayannopoulos MO, Chi MM, Cui Y, Pingsterhaus JM, McKnight RA, Mueckler M, Devaskar SU, Moley KH 2000 GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc Natl Acad Sci USA 97:7313–7318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan LP, Gorovits N, Hoskin EK, Alves SE, Katz EB, Grillo CA, Piroli GG, McEwen BS, Charron MJ 2001 Localization and regulation of GLUTx1 glucose transporter in the hippocampus of streptozotocin diabetic rats. Proc Natl Acad Sci USA 98:2820–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaquist ER, Damberg GS, Tkac I, Gruetter R 2001 The effect of insulin on in vivo cerebral glucose concentrations and rates of glucose transport/metabolism in humans. Diabetes 50:2203–2209 [DOI] [PubMed] [Google Scholar]
- Hasselbalch SG, Knudsen GM, Videbaek C, Pinborg LH, Schmidt JF, Holm S, Paulson OB 1999 No effect of insulin on glucose blood-brain barrier transport and cerebral metabolism in humans. Diabetes 48:1915–1921 [DOI] [PubMed] [Google Scholar]
- Namba H, Lucignani G, Nehlig A, Patlak C, Pettigrew K, Kennedy C, Sokoloff L 1987 Effects of insulin on hexose transport across blood-brain barrier in normoglycemia. Am J Physiol 252:E299–E303 [DOI] [PubMed] [Google Scholar]
- Bingham EM, Hopkins D, Smith D, Pernet A, Hallett W, Reed L, Marsden PK, Amiel SA 2002 The role of insulin in human brain glucose metabolism: an 18-fluoro-deoxyglucose positron emission tomography study. Diabetes 51:3384–3390 [DOI] [PubMed] [Google Scholar]
- Anthony K, Reed LJ, Dunn JT, Bingham E, Hopkins D, Marsden PK, Amiel SA 2006 Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: the cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes 55:2986–2992 [DOI] [PubMed] [Google Scholar]
- 1994 Diagnostic and statistical manual of mental disorders (DSM-IV). Washington, DC: American Psychiatric Association [Google Scholar]
- Ferini-Strambi L, Smirne S, Garancini P, Pinto P, Franceschi M 1990 Clinical and epidemiological aspects of Alzheimer’s disease with presenile onset: a case control study. Neuroepidemiology 9:39–49 [DOI] [PubMed] [Google Scholar]
- Landin K, Blennow K, Wallin A, Gottfries CG 1993 Low blood pressure and blood glucose levels in Alzheimer’s disease. Evidence for a hypometabolic disorder? J Intern Med 233:357–363 [DOI] [PubMed] [Google Scholar]
- Nielson KA, Nolan JH, Berchtold NC, Sandman CA, Mulnard RA, Cotman CW 1996 Apolipoprotein-E genotyping of diabetic dementia patients: is diabetes rare in Alzheimer’s disease? J Am Geriatr Soc 44:897–904 [DOI] [PubMed] [Google Scholar]
- Janson J, Laedtke T, Parisi JE, O'Brien P, Petersen RC, Butler PC 2004 Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 53:474–481 [DOI] [PubMed] [Google Scholar]
- Bucht G, Adolfsson R, Lithner F, Winblad B 1983 Changes in blood glucose and insulin secretion in patients with senile dementia of Alzheimer type. Acta Med Scand 213:387–392 [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Sasaki K, Akiyama K 1991 Increased insulin levels after OGTT load in peripheral blood and cerebrospinal fluid of patients with dementia of Alzheimer type. Biol Psychiatry 30:1219–1228 [DOI] [PubMed] [Google Scholar]
- Hoyer S 1998 Is sporadic Alzheimer disease the brain type of non-insulin dependent diabetes mellitus? A challenging hypothesis. J Neural Transm 105:415–422 [DOI] [PubMed] [Google Scholar]
- Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, Jellinger K, Riederer P 1998 Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm 105:423–438 [DOI] [PubMed] [Google Scholar]
- Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte Jr D 1998 Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology 50:164–168 [DOI] [PubMed] [Google Scholar]
- Wenk GL 2006 Neuropathologic changes in Alzheimer’s disease: potential targets for treatment. J Clin Psychiatry 67(Suppl 3):3–7; quiz, 23 [PubMed] [Google Scholar]
- Stone WS, Cottrill KL, Walker DL, Gold PE 1988 Blood glucose and brain function: interactions with CNS cholinergic systems. Behav Neural Biol 50:325–334 [DOI] [PubMed] [Google Scholar]
- Stone WS, Croul CE, Gold PE 1988 Attenuation of scopolamine-induced amnesia in mice. Psychopharmacology (Berl) 96:417–420 [DOI] [PubMed] [Google Scholar]
- Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A 1996 Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging 17:123–130 [DOI] [PubMed] [Google Scholar]
- Craft S, Dagogo-Jack SE, Wiethop BV, Murphy C, Nevins RT, Fleischman S, Rice V, Newcomer JW, Cryer PE 1993 Effects of hyperglycemia on memory and hormone levels in dementia of the Alzheimer type: a longitudinal study. Behav Neurosci 107:926–940 [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Frey 2nd WH, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, Cherrier MM, Craft S 2006 Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging 27:451–458 [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV 2001 Insulin promotes rapid delivery of N-methyl-D- aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci USA 98:3561–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palovcik RA, Phillips MI, Kappy MS, Raizada MK 1984 Insulin inhibits pyramidal neurons in hippocampal slices. Brain Res 309:187–191 [DOI] [PubMed] [Google Scholar]
- Mirzaei S, Gelpi E, Booij J, Rodrigues M, Neumann I, Zaknun J, Koehn H, Knoll P 2004 New approaches in nuclear medicine for early diagnosis of Alzheimer’s disease. Curr Alzheimer Res 1:219–229 [DOI] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Li Y, Li J, Zhan J, Tsui WH, Boppana M, Pupi A, de Leon MJ 2006 Visual rating of medial temporal lobe metabolism in mild cognitive impairment and Alzheimer’s disease using FDG-PET. Eur J Nucl Med Mol Imaging 33:210–221 [DOI] [PubMed] [Google Scholar]
- von Borczyskowski D, Wilke F, Martin B, Brenner W, Clausen M, Mester J, Buchert R 2006 Evaluation of a new expert system for fully automated detection of the Alzheimer’s dementia pattern in FDG PET. Nucl Med Commun 27:739–743 [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Harik SI 1989 Abnormalities of the glucose transporter at the blood-brain barrier and in brain in Alzheimer’s disease. Prog Clin Biol Res 317:415–421 [PubMed] [Google Scholar]
- Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P 1994 Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer’s disease. Ann Neurol 35:546–551 [DOI] [PubMed] [Google Scholar]
- Benedict L, Nelson CA, Schunk E, Sullwold K, Seaquist ER 2006 Effect of insulin on the brain activity obtained during visual and memory tasks in healthy human subjects. Neuroendocrinology 83:20–26 [DOI] [PubMed] [Google Scholar]
- Craft S, Asthana S, Schellenberg G, Cherrier M, Baker LD, Newcomer J, Plymate S, Latendresse S, Petrova A, Raskind M, Peskind E, Lofgreen C, Grimwood K 1999 Insulin metabolism in Alzheimer’s disease differs according to apolipoprotein E genotype and gender. Neuroendocrinology 70:146–152 [DOI] [PubMed] [Google Scholar]
- Kern W, Peters A, Fruehwald-Schultes B, Deininger E, Born J, Fehm HL 2001 Improving influence of insulin on cognitive functions in humans. Neuroendocrinology 74:270–280 [DOI] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W 2004 Intranasal insulin improves memory in humans. Psychoneuroendocrinology 29:1326–1334 [DOI] [PubMed] [Google Scholar]
- Craft S, Asthana S, Cook DG, Baker LD, Cherrier M, Purganan K, Wait C, Petrova A, Latendresse S, Watson GS, Newcomer JW, Schellenberg GD, Krohn AJ 2003 Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology 28:809–822 [DOI] [PubMed] [Google Scholar]
- Lockette W, Kirkland K, Farrow S 1996 α2-Adrenergic agonists increase cellular lactate efflux. Hypertension 27:1104–1107 [DOI] [PubMed] [Google Scholar]
- Holloway R, Zhou Z, Harvey HB, Levasseur JE, Rice AC, Sun D, Hamm RJ, Bullock MR 2007 Effect of lactate therapy upon cognitive deficits after traumatic brain injury in the rat. Acta Neurochir (Wien) 149:919–927; discussion, 927 [DOI] [PubMed] [Google Scholar]
- Maran A, Crepaldi C, Trupiani S, Lucca T, Jori E, Macdonald IA, Tiengo A, Avogaro A, Del Prato S 2000 Brain function rescue effect of lactate following hypoglycaemia is not an adaptation process in both normal and type I diabetic subjects. Diabetologia 43:733–741 [DOI] [PubMed] [Google Scholar]
- Kalman J, Palotas A, Bodi N, Kincses TZ, Benedek G, Janka Z, Antal A 2005 Lactate infusion fails to improve semantic categorization in Alzheimer’s disease. Brain Res Bull 65:533–539 [DOI] [PubMed] [Google Scholar]
- Hak AE, Pols HA, Stehouwer CD, Meijer J, Kiliaan AJ, Hofman A, Breteler MM, Witteman JC 2001 Markers of inflammation and cellular adhesion molecules in relation to insulin resistance in nondiabetic elderly: the Rotterdam study. J Clin Endocrinol Metab 86:4398–4405 [DOI] [PubMed] [Google Scholar]
- Hull M, Strauss S, Berger M, Volk B, Bauer J 1996 The participation of interleukin-6, a stress-inducible cytokine, in the pathogenesis of Alzheimer’s disease. Behav Brain Res 78:37–41 [DOI] [PubMed] [Google Scholar]
- Rosler N, Wichart I, Jellinger KA 2001 Intra vitam lumbar and post mortem ventricular cerebrospinal fluid immunoreactive interleukin-6 in Alzheimer’s disease patients. Acta Neurol Scand 103:126–130 [DOI] [PubMed] [Google Scholar]
- Tojo C, Takao T, Nishioka T, Numata Y, Suemaru S, Hashimoto K 1996 Hypothalamic-pituitary-adrenal axis in WBN/Kob rats with non-insulin dependent diabetes mellitus. Endocr J 43:233–239 [DOI] [PubMed] [Google Scholar]
- Lee ZS, Chan JC, Yeung VT, Chow CC, Lau MS, Ko GT, Li JK, Cockram CS, Critchley JA 1999 Plasma insulin, growth hormone, cortisol, and central obesity among young Chinese type 2 diabetic patients. Diabetes Care 22:1450–1457 [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Craft S, Hershey T, Askins K, Bardgett ME 1994 Glucocorticoid-induced impairment in declarative memory performance in adult humans. J Neurosci 14:2047–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C 2000 Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci 3:313–314 [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, Alderson AL 1999 Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry 56:527–533 [DOI] [PubMed] [Google Scholar]
- Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NP, Meaney MJ 1994 Basal cortisol levels and cognitive deficits in human aging. J Neurosci 14:2893–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget H, Lacroix A, Somma M, Cohen H 2000 Cognitive decline in patients with Cushing’s syndrome. J Int Neuropsychol Soc 6:20–29 [DOI] [PubMed] [Google Scholar]
- Brunetti A, Fulham MJ, Aloj L, De Souza B, Nieman L, Oldfield EH, Di Chiro G 1998 Decreased brain glucose utilization in patients with Cushing’s disease. J Nucl Med 39:786–790 [PubMed] [Google Scholar]
- Brown ES, Rush AJ, McEwen BS 1999 Hippocampal remodeling and damage by corticosteroids: implications for mood disorders. Neuropsychopharmacology 21:474–484 [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P 1999 Stress and hippocampal neurogenesis. Biol Psychiatry 46:1472–1479 [DOI] [PubMed] [Google Scholar]
- Joels M, de Kloet ER 1989 Effects of glucocorticoids and norepinephrine on the excitability in the hippocampus. Science 245:1502–1505 [DOI] [PubMed] [Google Scholar]
- Pavlides C, McEwen BS 1999 Effects of mineralocorticoid and glucocorticoid receptors on long-term potentiation in the CA3 hippocampal field. Brain Res 851:204–214 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE 1990 Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci 10:2897–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E 1997 Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 18:351–357 [DOI] [PubMed] [Google Scholar]
- Masters CL, Multhaup G, Simms G, Pottgiesser J, Martins RN, Beyreuther K 1985 Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer’s disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J 4:2757–2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K 1985 Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA 82:4245–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M 1987 Neuronal localization of amyloid β protein precursor mRNA in normal human brain and in Alzheimer’s disease. EMBO J 6:3627–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Lieberburg I 1999 Cellular mechanisms of β-amyloid production and secretion. Proc Natl Acad Sci USA 96:11049–11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini L, Gouras GK, Wang R, Gross RS, Beal MF, Greengard P, Xu H 2001 Stimulation of β-amyloid precursor protein trafficking by insulin reduces intraneuronal β-amyloid and requires mitogen-activated protein kinase signaling. J Neurosci 21:2561–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekrellis K, Ye Z, Qiu WQ, Walsh D, Hartley D, Chesneau V, Rosner MR, Selkoe DJ 2000 Neurons regulate extracellular levels of amyloid β-protein via proteolysis by insulin-degrading enzyme. J Neurosci 20:1657–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Toki S, Chowei H, Saito T, Nakano N, Hayashi Y, Takeuchi M, Makita Z 2001 Immunohistochemical distribution of the receptor for advanced glycation end products in neurons and astrocytes in Alzheimer’s disease. Brain Res 888:256–262 [DOI] [PubMed] [Google Scholar]
- Craft S, Asthana S, Schellenberg G, Baker L, Cherrier M, Boyt AA, Martins RN, Raskind M, Peskind E, Plymate S 2000 Insulin effects on glucose metabolism, memory, and plasma amyloid precursor protein in Alzheimer’s disease differ according to apolipoprotein-E genotype. Ann NY Acad Sci 903:222–228 [DOI] [PubMed] [Google Scholar]
- Zhao L, Teter B, Morihara T, Lim GP, Ambegaokar SS, Ubeda OJ, Frautschy SA, Cole GM 2004 Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer’s disease intervention. J Neurosci 24:11120–11126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR 2000 Intraneuronal Aβ42 accumulation in human brain. Am J Pathol 156:15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S 2007 Intracellular amyloid-β in Alzheimer’s disease. Nat Rev Neurosci 8:499–509 [DOI] [PubMed] [Google Scholar]
- Mochizuki A, Tamaoka A, Shimohata A, Komatsuzaki Y, Shoji S 2000 Aβ42-positive non-pyramidal neurons around amyloid plaques in Alzheimer’s disease. Lancet 355:42–43 [DOI] [PubMed] [Google Scholar]
- Westermark P, Wilander E 1978 The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia 15:417–421 [DOI] [PubMed] [Google Scholar]
- Schwartz P 1965 Senile cerebral, pancreatic insular and cardiac amyloidosis. Trans NY Acad Sci 27:393–413 [DOI] [PubMed] [Google Scholar]
- Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB 1987 Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci USA 84:8628–8632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A, Razzaboni B, Weir GC, Yankner BA 1994 Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature 368:756–760 [DOI] [PubMed] [Google Scholar]
- Schubert D, Behl C, Lesley R, Brack A, Dargusch R, Sagara Y, Kimura H 1995 Amyloid peptides are toxic via a common oxidative mechanism. Proc Natl Acad Sci USA 92:1989–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EA, Weissman JS, Horwich AL 1994 Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell 78:365–372 [DOI] [PubMed] [Google Scholar]
- Li ZG, Zhang W, Sima AA 2007 Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes 56:1817–1824 [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA 1993 Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261:921–923 [DOI] [PubMed] [Google Scholar]
- Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S 2005 Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry 13:950–958 [DOI] [PubMed] [Google Scholar]
- Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses AD 2006 Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J 6:246–254 [DOI] [PubMed] [Google Scholar]
- Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR 2006 Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol 199:265–273 [DOI] [PubMed] [Google Scholar]
- Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O’Banion K, Klockgether T, Van Leuven F, Landreth GE 2005 Acute treatment with the PPARγ agonist pioglitazone and ibuprofen reduces glial inflammation and Aβ1–42 levels in APPV717I transgenic mice. Brain 128:1442–1453 [DOI] [PubMed] [Google Scholar]
- Landreth G 2006 PPARγ agonists as new therapeutic agents for the treatment of Alzheimer’s disease. Exp Neurol 199:245–248 [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Godoy JA, Quintanilla RA, Koenig CS, Bronfman M 2005 Peroxisome proliferator-activated receptor γ is expressed in hippocampal neurons and its activation prevents β-amyloid neurodegeneration: role of Wnt signaling. Exp Cell Res 304:91–104 [DOI] [PubMed] [Google Scholar]
- Camacho IE, Serneels L, Spittaels K, Merchiers P, Dominguez D, De Strooper B 2004 Peroxisome-proliferator-activated receptor γ induces a clearance mechanism for the amyloid-β peptide. J Neurosci 24:10908–10917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Abramo C, Ricciarelli R, Pronzato MA, Davies P 2006 Troglitazone, a peroxisome proliferator-activated receptor-γ agonist, decreases tau phosphorylation in CHOtau4R cells. J Neurochem 98:1068–1077 [DOI] [PubMed] [Google Scholar]
- Lago RM, Singh PP, Nesto RW 2007 Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet 370:1129–1136 [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K 2007 Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356:2457–2471 [DOI] [PubMed] [Google Scholar]
- Ryan CM 1999 Memory and metabolic control in children. Diabetes Care 22:1239–1241 [DOI] [PubMed] [Google Scholar]
- Williams AD 2001 Psychometric concerns in neuropsychological testing. NeuroRehabilitation 16:221–224 [PubMed] [Google Scholar]
- Walsh P, Kane N, Butler S 2005 The clinical role of evoked potentials. J Neurol Neurosurg Psychiatry 76(Suppl 2):ii16–ii22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejgaard A, Gade A, Larsson H, Balle V, Parving A, Parving HH 1991 Evidence for diabetic encephalopathy. Diabet Med 8:162–167 [DOI] [PubMed] [Google Scholar]
- Martini A, Comacchio F, Fedele D, Crepaldi G, Sala O 1987 Auditory brainstem evoked responses in the clinical evaluation and follow-up of insulin-dependent diabetic subjects. Acta Otolaryngol 103:620–627 [PubMed] [Google Scholar]
- Parving A, Elberling C, Balle V, Parbo J, Dejgaard A, Parving HH 1990 Hearing disorders in patients with insulin-dependent diabetes mellitus. Audiology 29:113–121 [DOI] [PubMed] [Google Scholar]
- Kurita A, Katayama K, Mochio S 1996 Neurophysiological evidence for altered higher brain functions in NIDDM. Diabetes Care 19:360–364 [DOI] [PubMed] [Google Scholar]
- Gupta PR, Dorfman LJ 1981 Spinal somatosensory conduction in diabetes. Neurology 31:841–845 [DOI] [PubMed] [Google Scholar]
- Pozzessere G, Rizzo PA, Valle E, Mollica MA, Meccia A, Morano S, Di Mario U, Andreani D, Morocutti C 1988 Early detection of neurological involvement in IDDM and NIDDM. Multimodal evoked potentials versus metabolic control. Diabetes Care 11:473–480 [DOI] [PubMed] [Google Scholar]
- Lobmann R, Smid HG, Pottag G, Wagner K, Heinze HJ, Lehnert H 2000 Impairment and recovery of elementary cognitive function induced by hypoglycemia in type-1 diabetic patients and healthy controls. J Clin Endocrinol Metab 85:2758–2766 [DOI] [PubMed] [Google Scholar]
- Amiel SA, Pottinger RC, Archibald HR, Chusney G, Cunnah DT, Prior PF, Gale EA 1991 Effect of antecedent glucose control on cerebral function during hypoglycemia. Diabetes Care 14:109–118 [DOI] [PubMed] [Google Scholar]
- Haumont D, Dorchy H, Pelc S 1979 EEG abnormalities in diabetic children: influence of hypoglycemia and vascular complications. Clin Pediatr (Phila) 18:750–753 [DOI] [PubMed] [Google Scholar]
- Akisaki T, Sakurai T, Takata T, Umegaki H, Araki A, Mizuno S, Tanaka S, Ohashi Y, Iguchi A, Yokono K, Ito H 2006 Cognitive dysfunction associates with white matter hyperintensities and subcortical atrophy on magnetic resonance imaging of the elderly diabetes mellitus Japanese elderly diabetes intervention trial (J-EDIT). Diabetes Metab Res Rev 22:376–384 [DOI] [PubMed] [Google Scholar]
- Yousem DM, Tasman WS, Grossman RI 1991 Proliferative retinopathy: absence of white matter lesions at MR imaging. Radiology 179:229–230 [DOI] [PubMed] [Google Scholar]
- Manschot SM, Brands AM, van der Grond J, Kessels RP, Algra A, Kappelle LJ, Biessels GJ 2006 Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes 55:1106–1113 [DOI] [PubMed] [Google Scholar]
- den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, Breteler MM 2003 Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia 46:1604–1610 [DOI] [PubMed] [Google Scholar]
- Ferguson SC, Blane A, Wardlaw J, Frier BM, Perros P, McCrimmon RJ, Deary IJ 2005 Influence of an early-onset age of type 1 diabetes on cerebral structure and cognitive function. Diabetes Care 28:1431–1437 [DOI] [PubMed] [Google Scholar]
- Musen G, Lyoo IK, Sparks CR, Weinger K, Hwang J, Ryan CM, Jimerson DC, Hennen J, Renshaw PF, Jacobson AM 2006 Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes 55:326–333 [DOI] [PubMed] [Google Scholar]
- Wessels AM, Simsek S, Remijnse PL, Veltman DJ, Biessels GJ, Barkhof F, Scheltens P, Snoek FJ, Heine RJ, Rombouts SA 2006 Voxel-based morphometry demonstrates reduced grey matter density on brain MRI in patients with diabetic retinopathy. Diabetologia 49:2474–2480 [DOI] [PubMed] [Google Scholar]
- Perantie DC, Wu J, Koller JM, Lim A, Warren SL, Black KJ, Sadler M, White NH, Hershey T 2007 Voxel-based morthometry detects brain volume changes due to hypo- and hyperglycemia in youth with type 1 diabetes mellitus (TIDM). Diabetes 56(Suppl 1) (Abstract 1886) [Google Scholar]
- Rosenthal JM, Amiel SA, Yaguez L, Bullmore E, Hopkins D, Evans M, Pernet A, Reid H, Giampietro V, Andrew CM, Suckling J, Simmons A, Williams SC 2001 The effect of acute hypoglycemia on brain function and activation: a functional magnetic resonance imaging study. Diabetes 50:1618–1626 [DOI] [PubMed] [Google Scholar]
- Musen G, Bolo N, Jacobson A, Southwick A 2007 Brain functional correlates of gray matter changes in type 1 diabetes. Diabetes 56(Suppl 1) (Abstract 792) [Google Scholar]
- Niwa H, Koumoto C, Shiga T, Takeuchi J, Mishima S, Segawa T, Atsumi T, Shimizu C, Koike T, Yoshioka N 2006 Clinical analysis of cognitive function in diabetic patients by MMSE and SPECT. Diabetes Res Clin Pract 72:142–147 [DOI] [PubMed] [Google Scholar]
- Keymeulen B, de Metz K, Cluydts R, Bossuyt A, Somers G 1996 Technetium-99m hexamethylpropylene amine oxime single- photon emission tomography of regional cerebral blood flow in insulin-dependent diabetes. Eur J Nucl Med 23:163–168 [DOI] [PubMed] [Google Scholar]
- Sabri O, Hellwig D, Schreckenberger M, Schneider R, Kaiser HJ, Wagenknecht G, Mull M, Buell U 2000 Influence of diabetes mellitus on regional cerebral glucose metabolism and regional cerebral blood flow. Nucl Med Commun 21:19–29 [DOI] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP 2002 Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry 51:890–895 [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Chang PN, Muetzel RL, Caros L, Lim KO 2006 Diffusion tensor imaging in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 30:1799–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Perantie DC, Sadler M, White NH, Hershey T 2007 Pilot diffusion tensor imaging study in youth with type 1 diabetes mellitus reveals change in white matter integrity associated with chronic hyperglycemia. Diabetes 56(Suppl 1) (Abstract 1889) [Google Scholar]