Abstract
Ketosis-prone diabetes (KPD) is a widespread, emerging, heterogeneous syndrome characterized by patients who present with diabetic ketoacidosis or unprovoked ketosis but do not necessarily have the typical phenotype of autoimmune type 1 diabetes. Multiple, severe forms of β-cell dysfunction appear to underlie the pathophysiology of KPD. Until recently, the syndrome has lacked an accurate, clinically relevant and etiologically useful classification scheme. We have utilized a large, longitudinally followed, heterogeneous, multiethnic cohort of KPD patients to identify four clinically and pathophysiologically distinct subgroups that are separable by the presence or absence of β-cell autoimmunity and the presence or absence of β-cell functional reserve. The resulting “Aβ” classification system of KPD has proven to be highly accurate and predictive of such clinically important outcomes as glycemic control and insulin dependence, as well as an aid to biochemical and molecular investigations into novel causes of β-cell dysfunction. In this review, we describe the current state of knowledge in regard to the natural history, pathophysiology, and treatment of the subgroups of KPD, with an emphasis on recent advances in understanding their immunological and genetic bases.
I. Introduction
- II. Case Reports
- A. Case 1
- B. Case 2
- C. Case 3
III. History of KPD
IV. Classification of KPD
- V. Natural History and Characteristics of KPD Syndromes
- A. Houston
- B. Atlanta
- C. Paris
- D. Dallas
- VI. Pathophysiology of KPD Syndromes
- A. A+β− and A+β+
- B. A−β−
- C. A−β+
- VII. Management of KPD
- A. Management of DKA
- B. Management in the first 2–10 wk following resolution of DKA
- C. Long-term management
- D. Special considerations
VIII. Conclusion and Prospects
I. Introduction
FORMS OF DIABETES that do not fit the traditional categories defined by the American Diabetes Association (ADA) have been emerging in recent decades. Such forms were first noted and may be particularly widespread among persons of non-Caucasian ethnicity, but their prevalence appears to be increasing worldwide. Recent data from longitudinally followed cohorts have clarified the clinical features and taxonomy of some of these syndromes that are “intermediate” between type 1 and type 2 diabetes. Their recognition coincides with the emergence of the concept that early β-cell dysfunction is likely to be a primary defect in the pathophysiology of diabetes, regardless of “type”. Since many of them have been recognized because of the initial presentation of the patients with diabetic ketoacidosis (DKA) or unprovoked ketosis despite lacking the classic phenotype of autoimmune type 1 diabetes, investigation of these forms of diabetes could be of great value in uncovering novel mechanisms of β-cell dysfunction.
Several investigators have taken advantage of the striking and unequivocal presentation of DKA in “atypical” patients to define syndromes of ketosis-prone diabetes (KPD). We begin this chapter with brief case reports of three patients in our longitudinal cohort who represent syndromes of KPD that are clearly distinguishable from autoimmune type 1 diabetes.
II. Case Reports
A. Case 1
1. Clinical presentation.
Patient 1, a 17-yr-old Hispanic woman, presented in October 2000 to the Ben Taub General Hospital (Houston, TX) with DKA resulting from discontinuation of insulin therapy. She had been diagnosed with diabetes 10 yr previously, had always required multiple daily injections of insulin, and had experienced several previous episodes of DKA. The past medical history was otherwise unremarkable. There was a history of diabetes in her maternal grandmother and mother. The mother had been diagnosed with diabetes in her late 30s, had become insulin-dependent shortly after diagnosis, and also had been admitted to our hospital with DKA in the past. There was no history of smoking, alcohol, or illicit drug use. Her only medication was insulin. Menses were regular. Her weight at presentation was 133 lb, height 5 ft-3 in, and body mass index (BMI) 24.3 kg/m2. The physical examination was unremarkable except for signs of volume depletion; specifically, she had no lesions of acanthosis nigricans, and the ocular and neurological examinations were normal. Laboratory tests revealed no evidence of acute infection, cardiac ischemia, cerebrovascular disease, renal or liver dysfunction, or recent alcohol use. The arterial pH was acidemic, anion gap 24, serum bicarbonate 12 mmol/liter, and serum glucose 344 mg/dl. The patient was admitted to the hospital and received standard treatment for DKA with iv fluids and insulin. She recovered uneventfully and was discharged on the third hospital day on a regimen of NPH insulin twice daily with insulin lispro before meals. She was followed closely in our KPD research clinic thereafter.
2. Baseline biochemistry and serology.
Glycosylated hemoglobin (HbA1c) at presentation was 13.8%. Autoantibodies to β-cell antigens were measured at the Robert H. Williams Laboratory (University of Washington, Seattle, WA) (1), utilizing highly sensitive and specific assays in which the upper limit of normal was defined as the ethnic-specific value at the 99th percentile of the levels measured in large groups of Caucasians, Hispanics, and African-Americans living in Houston. Autoantibodies to glutamic acid decarboxylase (GAD) 65 and IA-2 were absent in the serum. β-Cell functional reserve was assessed 1 wk after resolution of DKA by serum C-peptide levels after an overnight fast and after stimulation with glucagon. The fasting C-peptide level was undetectable (<0.5 ng/ml) at baseline and did not change after glucagon stimulation.
3. Evolution of β-cell function.
β-Cell functional reserve was assessed annually in our clinic. C-peptide secretion was consistently absent, both at baseline and after glucagon stimulation.
4. Clinical course.
he patient was initially treated with a basal (ultralente)/bolus (regular) insulin regimen, which was later changed to insulin glargine (∼55 U/d) with insulin lispro (∼10 U) before meals. She suffered a miscarriage in the first trimester with a relapse of DKA in November 2002 but completed an uncomplicated term pregnancy with continuous sc insulin infusion therapy in 2003. She developed peripheral sensory neuropathy but has been free of other microvascular or macrovascular complications. There have been no episodes of DKA since 2002. Nonpregnant HbA1c levels have ranged from 7.9 to 11.2%, and nonpregnant weight has ranged from 132 to 144 lb. She adheres to a diabetic diet intermittently and does not exercise. Blood pressure and serum lipid levels are at desired goals without medications. She remains on continuous sc insulin infusion therapy.
5. Conclusion.
This young, lean Hispanic patient with childhood-onset diabetes and a family history of relatively early-onset diabetes has no evidence of β-cell autoimmunity, was unable to come off insulin therapy without relapse of ketoacidosis, and has had difficulty achieving optimal glycemic control. Shortly after the index episode of DKA, she had virtually no β-cell functional reserve, and this has not improved over time with intensive insulin therapy. She has the phenotype of what we have recently defined as “A−β−” KPD (1,2).
B. Case 2
1. Clinical presentation.
Patient 2, a 56-yr-old African-American man with no prior history of diabetes, presented with DKA in January 2000. He complained of polyuria, polydipsia, blurred vision, fatigue, and a 25-lb weight loss developing over the preceding 2 months. Evaluation in the emergency center revealed DKA with no clinical evidence of other precipitating illnesses or stressful events.
The past medical history was significant for hypertension. One brother was known to have diabetes and was being treated with a oral medication. The patient did not smoke or abuse alcohol.
His weight was 198 lb, height 5 ft-11 in, and BMI 28 kg/m2. Mild acanthosis nigricans was present on the neck. The remainder of the physical examination was unremarkable except for increased abdominal girth. There was no clinical evidence of diabetic retinopathy, neuropathy, or nephropathy. Laboratory tests revealed no evidence of acute infection, cardiac ischemia, or cerebrovascular disease, renal or liver dysfunction, or recent alcohol use. The arterial pH was 7.22, anion gap 35, bicarbonate 12 mmol/liter, and glucose 765 mg/dl. The patient was admitted to the hospital and received standard treatment for DKA with iv fluids and insulin. He recovered uneventfully and was discharged on the third hospital day on a regimen of NPH insulin twice daily with insulin lispro before meals. He was followed closely in our KPD research clinic thereafter.
2. Baseline biochemistry and serology.
HbA1c at the time of hospital admission was 12%. Evaluation of β-cell autoantibodies as described above revealed GAD65 autoantibodies in high titer, but not IA-2 autoantibodies. β-Cell functional reserve assessed 2 wk after the acute episode of DKA revealed a fasting C-peptide level of 1.15 ng/ml and a peak C-peptide level after glucagon stimulation of 1.62 ng/ml.
3. Evolution of β-cell function.
Fasting C-peptide levels and peak responses to glucagon increased over time, then reached a plateau. In early 2007, the fasting C-peptide was 2.6 ng/ml, and the peak postglucagon level was 5.2 ng/ml.
4. Clinical course.
Following a protocol described previously (1), we gradually withdrew insulin therapy and substituted oral diabetic medications with close monitoring for recurrence of ketoacidosis or decline in glycemic control. The patient tolerated the withdrawal without mishap, and insulin was totally discontinued by the end of 2000. Pioglitazone was added to the regime in May 2000. He has had no recurrence of DKA. HbA1c levels since 2001 have ranged between 6.2 and 7.8%, and the most recent in 2007 is 7.4%. His weight has ranged between 218 and 232 lb, and the current BMI is 30.8 kg/m2. Blood pressure and fasting lipid levels are within desired ranges. His current medications are pioglitazone 15 mg/d, glipizide XL 10 mg/d, metformin 1000 mg twice daily, simvastatin 40 mg/d, metoprolol XL 50 mg/d, candesartan 32 mg/d, hydrochlorothiazide 25 mg/d, and aspirin 81 mg/d.
5. Conclusion.
This middle-aged, overweight African-American patient, presenting with DKA as the first manifestation of diabetes, has evidence of β-cell autoimmunity, is able to come off insulin therapy without relapse of ketoacidosis, and has experienced significant glycemic improvement. Shortly after the acute episode of DKA, he had partially preserved β-cell functional reserve, and this improved further while he was being treated with oral antidiabetic agents. He has the phenotype of A+β+ KPD (1,2).
C. Case 3
1. Clinical presentation.
Patient 3, a 44-yr-old Hispanic man, presented with DKA in June 2004. He had experienced polyuria, polydipsia, and fatigue for 1 month preceding the DKA episode and had lost 30 lb of weight over 3 to 4 months. These symptoms prompted him to consult a physician 3 wk before presentation to the emergency center, and he was placed on an oral antidiabetic agent whose name he was unable to recall. There was no evidence of acute illness or severe stress provoking the DKA. There was no significant past medical history. Both parents and a sister had type 2 diabetes. He denied smoking or illicit drug use, and used alcohol occasionally. His weight was 252 lb, height 5 ft-10 in, and BMI 36 kg/ m2. Examination revealed obesity with increased abdominal girth but was otherwise unremarkable.
Laboratory tests revealed no evidence of acute infection, cardiac ischemia, or cerebrovascular disease, renal or liver dysfunction, or recent alcohol use. The arterial pH was 7.31, anion gap 21, bicarbonate 14 mmol/liter, and glucose 359 mg/dl. The patient was admitted to the hospital and received standard treatment for DKA with iv fluids and insulin. He recovered uneventfully and was discharged on the second hospital day on a regimen of NPH insulin twice daily with regular insulin before meals. He was followed closely in our KPD research clinic thereafter.
2. Baseline biochemistry and serology.
HbA1c at presentation with DKA was 12.4%. β-Cell autoantibodies were absent in the serum. The fasting C-peptide level measured 2 wk after recovery from DKA was 3.6 ng/ml, and peak C-peptide after glucagon stimulation was 6.6 ng/ml.
3. Evolution of β-cell function.
β-Cell functional reserve was assessed at 6-month intervals in our clinic. Fasting C-peptide levels remained stable. In February 2006, the fasting C-peptide level was 3.2 ng/ml.
4. Clinical course.
We gradually withdrew insulin therapy and substituted oral antidiabetic medications with close monitoring for recurrence of ketoacidosis or decline in glycemic control. The patient tolerated the withdrawal without mishap, and insulin was totally discontinued by March 2006. He has maintained excellent glycemic control, with HbA1c levels ranging between 5.2 and 6.1%, no recurrence of ketoacidosis, and no episodes of hypoglycemia. He has been treated with metformin since September 2006. Blood pressure has remained at the desired goal without specific medications. The weight has ranged from 248 to 272 lb, and the current BMI is 38 kg/m2. His current medications are metformin 500 mg twice daily, simvastatin 40 mg/d, slow release niacin 500 mg/d, and aspirin 81 mg/d.
5. Conclusion.
This middle-aged, obese Hispanic patient, presenting with unprovoked DKA very soon after initial diagnosis of type 2 diabetes, has no evidence of β-cell autoimmunity, was able to come off insulin therapy without relapse of ketoacidosis, and has achieved sustained euglycemia. Shortly after the acute episode of DKA, he had partially preserved β-cell functional reserve, which has remained stable over time with oral antidiabetic therapy. He has the phenotype of A−β+ KPD (1,2).
III. History of KPD
Occasional reports of African or African-American patients whose clinical features seemed to be intermediate between those of type 1 and type 2 diabetes have appeared since the late 1960s. In a review of tropical diabetes published in 1967, Dodu (3) noted that some patients required revision of their type of diabetes over time. In 1968, Adadevoh (4) described a few Nigerian patients with “reversible” diabetes who displayed only transient insulin dependence. A decade later, Oli (5) described a series of seven Nigerian patients who presented with ketosis and initially required insulin therapy but later experienced “remission” of diabetes. In a classic paper in 1987, Winter et al. (6) described a cohort of obese African-American children who were atypical because they lacked islet cell autoantibodies, presented with DKA as the initial manifestation of diabetes, and became insulin independent over time. The elegant studies of Banerji et al. (7) in 1994 described a somewhat different atypical syndrome in overweight, adult Afro-Caribbean patients who had clinical characteristics of type 2 diabetes but presented with DKA; the term Flatbush diabetes entered the literature at this point. The following year, Umpierrez et al. (8) carefully characterized obese African-American patients in Atlanta, Georgia, who had late-onset diabetes presenting with DKA. They noted that β-cell functional reserve was higher at baseline in these obese patients than in typical lean patients who developed DKA, and that it improved further after 12 wk of treatment. These investigators introduced the concept of BMI as a means to distinguish two phenotypes (obese or lean) of patients presenting with DKA, based on their immunological and β-cell functional differences (9).
These earlier studies suggested that such atypical forms of diabetes were uncommon and perhaps restricted to persons of African ancestry. However, from 1995 to 2003, case series and retrospective reviews reported (often large) numbers of patients from a wide array of geographic areas and ethnic backgrounds, with the common themes of absent islet cell autoantibodies, presentation with unprovoked ketosis or ketoacidosis, and frequent evolution to insulin independence. These included reports of Japanese patients (10); Apache Indians (11); African-Americans in Ohio (12); multiethnic U.S. populations including Hispanics, Caucasians, and Native Americans (13,14,15); Europeans (16); Pakistanis (17); and Chinese (18). The term “ketosis-prone diabetes” was introduced in 2002 by Sobngwi et al. in a review of diabetes in West Africans (19).
In 2003, Maldonado et al. (1) reported a large, longitudinal, prospective study of multiethnic patients with four different forms of KPD in Houston, Texas, and introduced a classification scheme based on two criteria: autoantibodies and β-cell functional reserve. In 2004, Mauvais-Jarvis et al. (20) published a 10-yr longitudinal study of KPD in immigrants from sub-Saharan Africa living in Paris. These authors classified autoantibody-negative KPD patients according to insulin dependence and contrasted their natural history to that of patients with typical type 1 and type 2 diabetes. In 2006, Ramos-Roman et al. (21) reported 4-yr outcomes in patients with atypical diabetes compared with those in patients with typical type 1 and type 2 diabetes in Dallas, Texas.
IV. Classification of KPD
To date, attempts to differentiate patients with KPD into clinically distinct and relevant subgroups have resulted in four different classification schemes: the ADA classification, a BMI-based system, a modified ADA classification, and the Aβ system.
The first is contained within the ADA’s most recent classification of diabetes in general (15) and has been adopted by investigators at the University of Texas Southwestern Medical School (Dallas, TX). All patients who experience DKA are defined as having type 1 diabetes, and among this group those who lack autoantibodies are referred to as “idiopathic type 1” or “type 1b.” Strictly interpreted, the ADA scheme would define patients with both type 1a and type 1b diabetes as insulin dependent, because it does not mention possible reversion to insulin independence in either category; however, the Dallas group considers patients with type 1b to behave more like patients with type 2 diabetes, with some becoming insulin-independent. A second scheme is that developed by investigators at Emory University (Atlanta, GA) who separate KPD patients into lean or obese (9). “Lean KPD” patients are those with clinical characteristics of type 1 diabetes with low β-cell function, whereas “obese KPD” patients are those with clinical characteristics of type 2 diabetes with some preservation of β-cell function. A modification of the ADA scheme is used by investigators at the University of Paris who divide KPD patients into three groups (20). Patients with β-cell autoantibodies are classified as type 1a just as in the ADA scheme, whereas those who lack autoantibodies are distinguished retroactively, based on long-term insulin dependence, into “KPD insulin-dependent” (KPD-ID) and “KPD non-insulin dependent” (KPD-NID). Both type 1a and KPD-ID patients have clinical characteristics of type 1 diabetes with poor β-cell function, whereas subjects with KPD-NID have clinical characteristics of type 2 diabetes with preserved β-cell function for a prolonged duration.
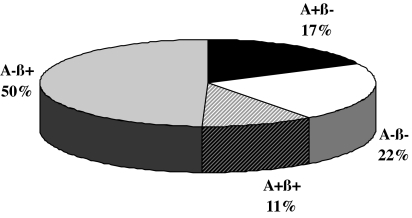
Our collaborative group at Baylor College of Medicine and the University of Washington has used a classification system that distinguishes four KPD subgroups based on the presence or absence of autoantibodies and the presence or absence of β-cell functional reserve (Aβ classification) (1). The four subgroups are: A+β− (patients with autoantibodies and absent β-cell function); A+β+ (those with autoantibodies but preserved β-cell functional reserve); A−β− (those without autoantibodies but absent β-cell function); and A−β+ (those without autoantibodies and preserved β-cell functional reserve). A+β− and A−β− patients are immunologically and genetically distinct from each other but share clinical characteristics of type 1 diabetes with very low β-cell function, whereas A+β+ and A−β+ patients are immunologically and genetically distinct from each other but share clinical characteristics of type 2 diabetes with preserved β-cell functional reserve (Fig. 1 and Table 1).
Figure 1.
Frequency distribution of patients in the four Aβ groups in a multiethnic adult U.S. urban population. [Reproduced with permission from M. Maldonado et al.: J Clin Endocrinol Metab 88:5090–5098, 2003 (1). Copyright The Endocrine Society.]
Table 1.
″Aβ″ groups: clinical characteristics
| A+β− | A−β− | A+β+ | A−β+ | P | |
|---|---|---|---|---|---|
| n | 18 (17) | 23 (22) | 11 (11) | 51 (50) | |
| Age (yr) | 34 ± 17 | 38 ± 15 | 43 ± 14 | 42 ± 13 | 0.1 |
| Age at diagnosis (yr) | 25 ± 17 | 26 ± 12 | 42 ± 12 | 39 ± 12 | <0.0001 |
| Years with diabetes | 9.1 ± 10.4 | 9.8 ± 8.7 | 0.9 ± 3.0 | 3.0 ± 4.8 | <0.0001 |
| Family history of diabetes | 9 (50) | 19 (83) | 9 (82) | 45 (88) | 0.01 |
| BMI (kg/m2) | 24.5 ± 3.9 | 23.0 ± 2.8 | 30.6 ± 7.6 | 29.4 ± 8.3 | 0.0003 |
| Weight category | <0.0001 | ||||
| Lean | 11 (61) | 17 (74) | 2 (18) | 17 (33) | |
| Overweight | 5 (28) | 6 (26) | 4 (36) | 13 (26) | |
| Obese | 2 (11) | 0 | 5 (46) | 21(41) | |
| New onset diabetes | 3 (17) | 2 (9) | 10 (91) | 26 (51) | <0.0001 |
| Male:female ratio | 1:1 | 1.3:1 | 0.6:1 | 1.7:1 | 0.4 |
| Ethnicity | 0.02 | ||||
| African-American | 13 (72) | 9 (39) | 4 (36.4) | 14 (27) | |
| Hispanic-American | 2 (11) | 9 (39) | 4 (36.4) | 30 (59) | |
| Caucasian-American | 2 (11) | 5 (22) | 3 (27.2) | 6 (12) | |
| Asian-American | 1 (6) | 0 | 0 | 1 (2) | |
| Recurrent DKA episodes | 7 (39) | 7 (30) | 1 (9) | 1 (2) | <0.0001 |
| Insulin discontinued by 6 months | 0 (0) | 0 (0) | 5 (45) | 26 (51) | <0.0001 |
| Antidiabetic regimen at 12 months | <0.0001 | ||||
| Insulin only | 17 (94) | 23 (100) | 3 (27) | 17 (33) | |
| Insulin + oral hypoglycemics | 1 (6) | 0 | 3 (27) | 8 (16) | |
| Oral hypoglycemics only | 0 | 0 | 3 (27) | 21 (41) | |
| Diet and exercise only | 0 | 0 | 2 (19) | 5 (10) |
Percentages are shown in parentheses.
The value of a nosological system depends on the accuracy with which it predicts clinical behavior or distinguishes pathophysiological mechanisms. In the case of KPD, a key determinant of clinical behavior is long-term β-cell functional reserve, which is necessary for such important outcomes as glycemic control (22) and insulin dependence (23). To specify the most accurate classification method, we compared the ability of the four existing systems to predict long-term β-cell functional reserve in a large, multiethnic cohort of 294 KPD patients (138 new onset), followed for at least 12 months and tested repeatedly (2). The Aβ system was the most accurate in predicting preserved β-cell function 12 months after the index DKA, with 99.4% sensitivity and 95.9% specificity; positive and negative predictive values of 97.1 and 99.2%, respectively; positive and negative likelihood ratios of 24.55 and 0.01, respectively; and area under the receiver operator characteristic (ROC) curve of 0.972 (2,24). The modified ADA system had slightly greater specificity, positive predictive value and positive likelihood ratio, but significantly lower sensitivity, negative predictive value, negative likelihood ratio, and ROC area under the curve (AUC). The other two schemes were generally less accurate, with the ADA system being the least reliable.
Recently, the term “ketosis-prone type 2 diabetes” has entered the literature. In general, this term refers to the A−β+ KPD subgroup, or in some instances, even further restricted to those A−β+ patients who present with “unprovoked” DKA or ketosis and new onset diabetes (25,26). A−β+ patients comprise the largest subgroup of KPD patients and are the ones who most commonly come to the notice of physicians because they present with DKA yet have all the clinical features and subsequent behavior of type 2 diabetes; hence, ketosis-prone type 2 diabetes is certainly a fitting description for them. However, we believe that from a heuristic standpoint, in the interest of defining and investigating novel syndromes of β-cell dysfunction, the broader terminology of ketosis-prone diabetes with its four subgroups subsumed under the Aβ classification is more useful and unbiased, because it does not presume to define a syndrome a priori. Moreover, in cohorts of clinically heterogeneous, multiethnic patients, the four subgroups of KPD are useful as controls for one another in investigations of etiology and natural history.
V. Natural History and Characteristics of KPD Syndromes
Several small series and retrospective reports have described patients with different phenotypes of KPD, as detailed earlier (11,12,14). However, the natural histories of these syndromes are best detailed in studies utilizing large cohorts with longitudinal follow-up. Data from four such cohorts (in Houston, Atlanta, Paris, and Dallas) have been published.
A. Houston
The longitudinal Houston cohort comprises over 500 multiethnic patients (44% African-American, 40% Hispanic, 15% Caucasian, 1% Asian), including 185 patients who have been followed for a mean of 5.5 yr (27). A comprehensive database compiles information on the natural history of all four groups of KPD. A+β− KPD, identical to early-onset, autoimmune type 1 diabetes, displays a typical course of complete insulin dependence and difficulty in attaining and achieving excellent long-term glycemic control. A−β− KPD patients follow a very similar course. The more novel aspects of the natural history of KPD are revealed in the two β+ groups.
The majority of A+β+ KPD patients have new onset diabetes and follow one of two courses. About 50% are able to maintain long-term β-cell functional reserve and come off insulin therapy successfully shortly after resolution of DKA, whereas the others remain insulin dependent. The relative level of initial β-cell functional reserve by fasting C-peptide measurement or C-peptide response to glucagon does not predict which A+β+ KPD patients will become insulin dependent and which ones will not. However, two immunological markers offer significant predictive value. The first is epitope specificity of the GAD65 autoantibody (88% of A+β+ KPD patients are GAD Ab positive). Patients whose GAD65 Ab is directed toward an amino-terminal epitope in GAD defined by monoclonal antibody DPD (28) have greater β-cell functional reserve than those who do not have this characteristic, both initially and upon 12-month follow-up. These patients are also significantly more likely to be insulin independent after 2 yr. The other marker lies in the pattern of histocompatibility locus antigen (HLA) alleles associated with susceptibility or resistance to autoimmune type 1 diabetes. New-onset A+β+ KPD patients whose β-cell function deteriorates over 2 yr tend to possess one or more susceptibility alleles (DQB1*02, DRB1*03, DRB1*04, DQB1*0302), whereas those with preserved β-cell functional reserve have a lower frequency of this pattern. Longer follow-up of A+β+ KPD patients will be necessary to delineate precisely their natural history with respect to glycemic control and β-cell function.
A−β+ KPD patients comprise the largest subgroup of KPD; approximately 50% of these patients have new-onset diabetes and develop DKA without a clinically evident precipitating factor (unprovoked A−β+ KPD), whereas the remainder have had long-standing diabetes before presentation with DKA and develop ketoacidosis in association with acute illness or noncompliance with antidiabetic treatment (provoked A−β+ KPD). Unprovoked A−β+ KPD patients display a striking male predominance (2.6:1, male:female) which is quite distinct from provoked A−β+ KPD patients (0.7:1); this gender imbalance has been noted also in patients with the unprovoked A−β+ KPD phenotype in the Atlanta, Paris, and Dallas cohorts (8,20,29). Longitudinal, prospective assessment of 113 unprovoked and 103 provoked A−β+ KPD patients revealed that despite equivalent degrees of hyperglycemia and β-cell functional reserve at initial testing after the index DKA episode, the former group had significantly greater improvement in β-cell function after 12 months of treatment (2-fold greater), associated with significantly better glycemic control (twice the frequency of attaining HbA1c < 7%) and twice the rate of insulin discontinuation. Differences in β-cell function at 12 months and rates of insulin discontinuation persisted in a subgroup analysis of patients who achieved excellent glycemic control. These data suggest that patients with unprovoked or provoked A−β+ KPD may have distinct underlying mechanisms of β-cell dysfunction.
B. Atlanta
Umpierrez et al. (8) originally reported a short-term longitudinal study of 35 obese patients who presented with new-onset, unprovoked DKA, comparing their clinical characteristics and course to 10 lean patients with DKA, 22 obese nonketotic hyperglycemic patients, and 10 obese nondiabetic patients. β-Cell functional reserve was preserved shortly after the index DKA episode in the obese DKA patients (although significantly less than in the other two obese groups), but not in the lean DKA patients. After 12 wk of intensive glycemic management with insulin, β-cell functional reserve improved significantly in the obese DKA patients (to the extent that insulin therapy could be withdrawn in a majority), but not in the lean DKA patients. The baseline group differences in β-cell function were confirmed in a larger set of similar patients in a subsequent study (9). In this study, 17% of the obese DKA patients had autoantibodies, compared with 48% of the lean DKA patients, suggesting that the obese DKA patients probably represented a mixture of the A−β+ and A+β+ KPD phenotypes, whereas the lean DKA patients probably represented a mixture of A+β− and A−β− KPD phenotypes.
C. Paris
Mauvais-Jarvis et al. (20) reported long-term follow-up of 111 patients of West African origin who presented with DKA or unprovoked ketosis without a prior history of diabetes. Over 10 yr, 84 patients were found retrospectively to have a phenotype corresponding to A−β+ KPD (designated KPD-NID by the investigators), whereas 27 were found to have a phenotype corresponding to A−β− KPD (designated KPD-ID). Their natural history was compared with that of 21 patients with newly diagnosed autoimmune type 1 diabetes and 88 patients with type 2 diabetes. During the observation period, KPD patients with the probable A−β+ phenotype became insulin independent at a mean of 14 wk after presentation with ketosis or ketoacidosis and achieved significant glycemic control with oral antidiabetic agents. The mean duration until relapse to insulin dependence was 40 months, but 40% were still insulin independent at the end of the follow-up period. Relapse to insulin dependence was heralded by ketosis, usually preceded by breakthrough hyperglycemia, and some patients experienced a second remission to insulin independence. This description of relapsing and remitting ketosis and insulin requirement, with ultimate insulin dependence in a little over half the cases, represents the longest follow-up of patients with a probable phenotype of unprovoked A−β+ KPD.
D. Dallas
Piñero-Piloña et al. (15) reported 5-yr clinical follow-up of 54 multiethnic patients (65% African-American, 30% Hispanic, 5% Native-American) who presented with DKA and lacked β-cell autoantibodies (idiopathic type 1b diabetes according to the ADA classification system). β-Cell function was not measured, but insulin could be successfully withdrawn over time in 21 patients. The investigators reported a second longitudinal study (4-yr follow-up) of insulin sensitivity and β-cell function in 12 patients with idiopathic type 1b diabetes compared with 10 patients with autoimmune type 1a diabetes (21). The idiopathic type 1b patients were reported to be less insulin-sensitive and have higher degrees of β-cell function than the type 1a patients. It is probable that the idiopathic type 1b patients represented a mixture of the A−β− and A−β+ KPD phenotypes.
VI. Pathophysiology of KPD Syndromes
A. A+β− and A+β+
Inclusion of patients with islet cell autoantibodies in a longitudinal cohort analysis of patients with KPD permits identification of distinct phenotypes among patients with a presumed autoimmune basis for severe β-cell dysfunction. There is clearly a spectrum of clinical phenotypes among patients with islet autoantibodies who do not present with ketosis, including those termed “latent autoimmune diabetes in adults” (LADA) (30), “type 1.5 diabetes” (31,32,33), and “slowly progressing type 1 diabetes” (34). A similar spectrum exists in KPD that includes the very different phenotypes of A+β− and A+β+ KPD. A+β− KPD is synonymous with classic, early onset autoimmune type 1 diabetes; A+β+ KPD may overlap with LADA. However, there are differences between LADA, as recently defined by the Immunology of Diabetes Society, and A+β+ KPD patients; most importantly, the definition of LADA excludes patients who require insulin within the first 6 months after diagnosis, whereas the majority (90%) of A+β+ KPD patients present with DKA as the first manifestation of diabetes and therefore require insulin at the start. Regardless of these distinctions, distinguishing A+β− from A+β+ KPD permits investigators to explore different autoimmune pathways leading to clinically distinct patterns of β-cell loss, such as different latencies and variable degrees of β-cell destruction.
Hampe et al. (28) investigated the role of epitope-specific autoantibodies to GAD65 in specifying the clinical phenotypes of longitudinally followed A+β− and A+β+ KPD patients. Five GAD65-specific Fab fragments were used to characterize the specificity of the titers of epitope-specific antibodies in relation to longitudinal measures of β-cell function and clinical outcomes. A specific amino-terminal epitope defined by monoclonal antibody DPD correlated strongly with higher β-cell functional reserve, both at baseline and after 1 yr of follow-up, and was associated with A+β+ KPD rather than A+β− KPD. Hence, the later onset and more moderate clinical course (ability to discontinue insulin for over 2 yr after the index episode of DKA in 50% of the patients) of A+β+ KPD compared with A+β− KPD appears to be associated with a specific GAD65Ab epitope pattern. The mechanisms that result in this autoantibody specificity and give rise to variable β-cell functional reserve remain to be elucidated.
B. A−β−
In the Houston cohort, only 10% of these patients had new-onset diabetes when identified at presentation with DKA; the majority had had insulin-dependent diabetes for many years previously (1). Furthermore, Zhang et al. (35) have noted that about 6% of patients meeting the ADA criteria for type 1b diabetes (ketosis-prone, GAD65/IA-2 autoantibody-negative, likely with phenotypes of A−β− or A−β+ KPD) have circulating antibodies against SOX-13 (SRY-related high mobility group box antigen 13), another presumed marker of islet cell autoimmunity. Also, these patients have not been tested for antibodies to the cation efflux transporter ZnT8, a recently identified islet autoantibody present in a small percentage of patients with type 1 diabetes who lack GAD65 and IA-2 antibodies (36). These data raise the concern of whether some A−β− KPD patients are misclassified as A− because of a decline in autoantibody titers over time, or because they possess untested autoantibodies. Although it may be impossible to completely rule out an autoimmune component to A−β− KPD, this is probably not a significant issue in characterizing patients accurately. In the first place, GAD autoantibodies are quite durable, with only 10–20% of patients showing declines in titer to undetectable levels over 10 yr of follow-up (37,38,39). IA-2 autoantibodies are less durable; about 50% of patients who test positive for IA-2-Ab at the time of diagnosis have undetectable levels after 10 yr (37,38). GAD65Abs do not have a relationship to age at diagnosis; in contrast, presence of IA-2-Ab correlates negatively with age at diagnosis (40), possibly explaining our finding of only 36% IA-2-Ab-positive adult patients (only 25% exclusively IA-2-Ab-positive) in the A+ KPD subgroups. Hence, absence of GAD65 or IA-2 Abs, even in patients with long-standing diabetes, is probably quite reliable in classifying them as A−. To limit further the possibility of misclassifying A+β− KPD patients as A−β−, we have performed extensive HLA typing (1,27) and have found that the frequencies of major class II alleles associated with susceptibility to autoimmune type 1 diabetes are not significantly higher in A−β− KPD patients than in ethnic-matched population controls, whereas they are significantly higher in A+β− KPD patients.
The strong family history of diabetes in near relatives of A−β− KPD patients also suggests that there is a familial trait and that variants in genes required for β-cell development, regeneration, or function may contribute to the phenotype. Preliminary data suggest that potentially significant variants in TCF1 and PDX-1, encoding the key β-cell transcription factors HNF1α (hepatocyte nuclear factor-1α) and PDX-1 (pancreas-duodenum homeobox-1), are enriched in A−β− KPD patients compared with ethnic-specific population controls (W. Haaland, D. Mansouri, A. Balasubramanyam, M. Metzker, unpublished data).
C. A−β+
Umpierrez et al. (41) have examined the roles of glucotoxicity and lipotoxicity in inducing the severe but partially reversible β-cell functional defect in an obese African-American patient with the phenotype of unprovoked A−β+ KPD shortly after resolution of the index episode of DKA. The investigators measured the effects of exposure to 20 h of hyperglycemia and 48 h of hyperlipidemia (by lipid infusion) on C-peptide secretion. Acute hyperglycemia but not acute hyperlipidemia caused severe blunting of the C-peptide response to glucose stimulation, and chronic hyperglycemia was associated with reduced expression and insulin-stimulated threonine-308 phosphorylation of Akt2 in skeletal muscle. These data suggest that severe glucotoxic blunting of an intracellular pathway leading to insulin secretion may contribute to the reversible β-cell dysfunction characteristic of A−β+ KPD patients, and that hyperglycemia may be exacerbated by defects in skeletal muscle glucose uptake resulting from glucotoxic down-regulation of skeletal muscle insulin signaling. One mechanism of glucotoxic β-cell dysfunction is increased oxidant stress in the islets. Sobngwi et al. (42) investigated the possibility of X-linked glucose-6-phosphate dehydrogenase (G6PD) deficiency as a genetic basis for the male-predominant A−β+ KPD phenotype in West African patients. They found a higher prevalence of functional G6PD deficiency in the KPD patients compared with patients with type 2 diabetes and a relationship between β-cell functional reserve and erythrocyte G6PD activity; however, the functional G6PD deficiency was not matched by a higher prevalence of G6PD gene mutations. Hence, G6PD dysfunction may contribute to depressed β-cell defense against oxidant stress in the face of acute hyperglycemia, but its cause does not appear to be a genetic mutation.
Variants in key β-cell developmental genes may also contribute to the phenotype of A−β+ KPD. Mauvais-Jarvis et al. (43) found high frequency of a polymorphism leading to an amino acid substitution (R133W) in PAX4, a transcription factor essential for islet morphogenesis β-cell development, among patients with phenotypes of A−β+ or A−β− KPD. Because this variant is found in a high percentage of West Africans and African-Americans with and without type 2 diabetes, but not in Caucasians, its pathophysiological significance in the specific context of KPD is unclear.
VII. Management of KPD
Clinical management of KPD includes: 1) acute management of DKA; 2) outpatient management shortly after resolution of DKA, including classification of the patient according to KPD subgroup and evaluation of predictive factors; and 3) long-term management.
A. Management of DKA
All patients who present with DKA should be treated according to established principles of acute management of the metabolic decompensation. Standard inpatient hospital protocols requiring aggressive fluid replacement; continuous insulin therapy; assessment for and treatment of precipitating factors; monitoring for resolution of hyperglycemia, ketoacidosis, and electrolyte disorders; and transition from iv insulin to sc insulin regimens have been well described (26,44). It is important to note that inpatient treatment during the episode of DKA should be the same regardless of the apparent phenotype of the KPD patient, and that all KPD patients should be discharged from the hospital on a regimen that provides 24-h insulin coverage. Any attempt to withdraw insulin treatment should be based on precise classification of the KPD subgroup and assessment of the predictive factors, which should be performed at the first outpatient visit 1–3 wk after hospital discharge.
B. Management in the first 2–10 wk following resolution of DKA
Assessment of β-cell secretory reserve and β-cell autoimmunity should be performed after complete resolution of DKA to minimize any acute effects of glucose toxicity or desensitization on β-cell function, generally 1–3 wk after resolution of ketoacidosis. The methods for performing these tests, as well as receiver-operator curve analysis to establish the C-peptide cutoffs that distinguish β− from β+ status, have been previously established and published (1).
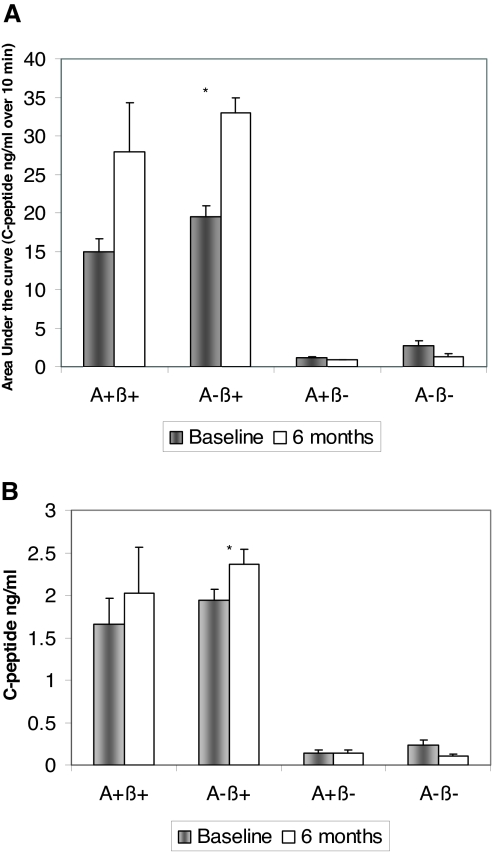
Patients are classified as β− if the fasting serum C-peptide concentration is less than 1 ng/ml (0.33 nmol/liter) and the peak serum C-peptide response to glucagon (measured at 5 and 10 min after iv injection of 1 mg glucagon) is less than 1.5 ng/ml (0.5 nmol/liter), and they are classified as β+ if the fasting serum C-peptide concentration is at least 1 ng/ml (0.33 nmol/liter) or the peak serum C-peptide response to glucagon is at least 1.5 ng/ml (0.5 nmol/liter) (ROC AUC for peak serum C-peptide concentration after glucagon stimulation = 0.96751, ROC AUC for fasting C-peptide = 0.97776, and ROC AUC for C-peptide/glucose ratio = 0.96089). These cutoffs accurately predict β-cell function after 6 months (Fig. 2) and 1 yr (2). They also predict glycemic control after 1 yr (22). Fasting C-peptide levels are also associated with insulin discontinuation in KPD patients (45), and although the cutoffs noted above do not independently predict the potential for successful and safe withdrawal of insulin, a high ratio (>11) of fasting C-peptide (in nanomoles per liter) to glucose (in millimoles per liter) predicts such a course among β+ patients (23). Although glucagon is only one of many physiological β-cell secretagogues, it has proven to be very useful as well as clinically simple in distinguishing the phenotypes of KPD. Future studies may reveal whether testing with additional secretagogues (glucose, arginine) can further refine the mechanisms of β-cell dysfunction in KPD patients.
Figure 2.
A, Group comparison of β-cell functional reserve, as measured by AUC of C-peptide response to glucagon stimulation, at baseline and after 6 months of follow-up and treatment in a dedicated clinic. Values are mean ± sd. There were significant group differences at both time points between the β+ and β− groups (P < 0.0001). *, P < 0.0001 comparing baseline to 6 months in the A−β + group. B, Group comparison of β-cell functional reserve, as measured by fasting C-peptide levels, at baseline and after 6 months of follow-up and treatment in a dedicated clinic. Values are mean ± sd. There were significant group differences at both time points between the β+ and β− groups (P < 0.0001). *, P = 0.01 comparing baseline to 6 months in the A−β + group. [Reproduced with permission from M. Maldonado et al.: J Clin Endocrinol Metab 88:5090–5098, 2003 (1). Copyright The Endocrine Society.]
1. Assessment of β-cell autoimmunity.
Although β-cell functional reserve provides most of the information a clinician requires to predict the patient’s clinical course, careful quantitative assessment of β-cell autoantibodies is also clinically useful, specifically for patients with the A+β+ phenotype. Patients belonging to this predominantly new-onset KPD group follow one of two markedly divergent clinical courses within the first 2 yr of diagnosis; approximately 50% in our cohort maintain stable β-cell function and remain insulin independent, whereas the others experience a change in functional status to β− and become insulin dependent. Hence identification of patients as A+β+ rather than simply β+ alerts the clinician to follow them closely, even if initial β-cell functional reserve is substantial and the person can come off insulin within the first few months; furthermore, these are the patients for whom HLA genotyping might be recommend to provide additional prognostic markers of clinical behavior, because the presence of specific autoimmune type 1 diabetes susceptibility alleles such as HLA DQB1*02 is associated with higher risk of progressive β-cell functional loss. GAD65 and IA-2 autoantibody titers should be measured in the patients’ sera by highly sensitive and specific assays, with care taken to establish the upper limits of the normal range for the autoantibody levels specifically for each regional ethnic group (1). Anti-insulin antibodies are probably not useful because many patients would have previously received insulin therapy. Patients may be classified as A+ if the autoantibody index for either of the autoantibodies exceeds the ethnic-specific 99th percentile or A− if the indices for both are below the 99th percentile.
2. Glycemic management in the first 2–10 wk after discharge.
Insulin should never be discontinued in patients who are classified as β−, regardless of whether they are A+ or A−, because these patients invariably require multiple daily insulin injections to avoid ketosis. Evidence from the Houston longitudinal cohort shows clearly that no patients initially classified as β− by the above protocol have recovered β-cell function sufficiently to warrant a trial of insulin withdrawal.
We assess future insulin dependence or independence in all patients who are initially categorized as β+ as follows. All patients are placed on twice daily NPH insulin at the time of hospital discharge. The dose is determined by the mean daily insulin requirement during the previous two hospital days. Patients with known diabetes may be given insulin at the dose they were receiving before the onset of DKA. In previously insulin-naive patients, an alternative approach would be multidose insulin at a dose of 0.6–0.8 U·kg−1·d−1 (46,47), with 50% as regular or rapid-acting insulin (in three divided doses before meals) and 50% as basal insulin. Dosage can be titrated with frequent glucose monitoring until an optimal dose is established. The first clinic visit is within 2 wk of discharge from the hospital. Subsequent visits are scheduled at 1- to 4-wk intervals as indicated.
If capillary blood glucose values before each meal and at bedtime during a 2-wk period attain ADA goals for fasting/preprandial (90–130 mg/dl) and bedtime/peak postprandial plasma glucose levels (180 mg/dl), the insulin dose is reduced by 50% and the patient is reassessed in the clinic 1 wk later. If the mean blood glucose values remain at ADA goals at two consecutive clinic visits, insulin is discontinued and the patient is monitored closely (by telephone contact). Patients are advised to use urine ketone strips or a blood ketone testing meter to check for significant ketosis if the blood glucose level rises above 200 mg/dl. If blood glucose values remain at goal, patients are instructed to continue with lifestyle modification and are monitored without pharmacological therapy. If blood glucose values increase without development of ketosis, the patients are placed on oral hypoglycemic agents. Conversely, if the patient develops ketosis on decreasing the insulin dose, the insulin regimen is intensified and no further attempts are made to discontinue insulin. The duration of this process of insulin withdrawal is variable and may range from 10 to 14 wk or longer.
C. Long-term management
Once the patient has been classified for KPD type, assessed for predictive factors, and begun an appropriate treatment course, ADA standards of diabetes management should be followed. In addition, we recommend routine reevaluation of β-cell function every 6 months to track its evolution. Patients should be counseled by a nutritionist and diabetic educator periodically. Physical activity for at least 150 min/wk should be recommended, and weight loss may be advised for obese β+ patients. Smoking cessation should be reinforced. Screening and treatment for microvascular and macrovascular complications of diabetes should be advised according to ADA recommendations.
D. Special considerations
1. Subgroups.
As described earlier, A−β + KPD patients may be divided into a mainly new-onset unprovoked group (presenting with DKA in the absence of significant stress) and a previously diagnosed provoked group (DKA associated with significant stress). Patients in the former subgroup have a significantly greater rate of insulin discontinuation and better long-term glycemic control than the latter. New onset diabetes, later onset of diabetes and high levels of β-cell functional reserve (fasting C-peptide to glucose ratio > 11) may be used as reliable predictors of insulin discontinuation in β+ patients (23). New onset diabetes and β-cell functional reserve are especially robust predictors because their significance is retained in a multivariate proportional hazard analysis. Similar predictors of insulin discontinuation have been noted by Sobngwi et al. (45) in West African patients and Hsin et al. (48) in Taiwanese patients. The study of Rasouli and Elbein (49) also rigorously demonstrates that improvement in insulin secretory function (not insulin sensitivity) predicts insulin discontinuation in KPD patients.
2. β-Cell autoantibodies.
The presence of β-cell autoantibodies is a determinant of future β-cell function. In analyses that do not differentiate the four Aβ subgroups, KPD patients with autoantibodies tend to have lower β-cell function both shortly after the correction of the acidosis and on long-term follow-up (15,20). However, this is not an infallible criterion because a significant proportion of A+β+ KPD patients maintain long-term β-cell functional reserve. In fact, most A+β+ KPD patients are able to come off insulin therapy initially, but they require close monitoring for at least 2 yr, because the evolution of their β-cell function is the least predictable of the KPD groups. The presence of specific HLA susceptibility alleles associated with autoimmune type 1 diabetes may predict a more aggressive course and insulin dependence within 1–2 yr (1). Hence, HLA typing may play a useful role in the management of this group of KPD patients, because it may help to identify those who are likely to experience a more aggressive course or may be candidates for future immunomodulatory therapy.
Upon successful insulin withdrawal in A−β+ patients, we frequently use insulin-sensitizing agents such as metformin or a thiazolidinedione because these patients have the highest frequency of the metabolic syndrome among KPD groups (50). If blood glucose levels do not achieve therapeutic targets within 8 wk, we add low doses of a sulfonylurea, or a meglitinide or α-glucosidase inhibitor. We have very limited experience with newer agents such as glucagon-like peptide-1 mimetics or inhibitors of dipeptidyl peptidase-IV, but they are of potential interest because of their β-cell trophic properties and ability to reduce circulating glucagon levels. Aggressive management of the metabolic syndrome (including strategies to decrease intraabdominal fat) and cardiovascular risks are important in all subgroups of KPD patients.
In summary, management of KPD patients requires some special considerations. Because these patients are heterogeneous and the type of diabetes is unclear at presentation with DKA, they should all be maintained on insulin initially. Further management can be guided rationally by accurate classification based on assessment of β-cell functional reserve, β-cell autoantibodies, and in some instances HLA allelotyping. It is also instructive to note that in urban, indigent populations, treatment of KPD patients carries a significant financial cost, due in significant part to misunderstanding regarding the type of diabetes in a patient who presents with DKA and subsequent confusion in management (51,52). We have found that an informed, systematic approach, utilizing the principles outlined above and delivered through a dedicated program, reduces the burden of cost and improves clinical outcomes in KPD patients (53,54).
VIII. Conclusion and Prospects
Although proper epidemiological surveys remain to be conducted, syndromes of KPD appear to be increasingly recognized worldwide, especially among urban, multiethnic populations. They offer challenges to both clinicians and researchers, but also offer the exciting prospect of revealing novel mechanisms of β-cell dysfunction in common forms of type 2 diabetes. It is our view that KPD patients (especially those with A− forms of KPD) represent only the “tip of the iceberg”; below the surface is likely to be a much larger pool of patients who have early or primary β-cell defects in development, expansion in the face of insulin resistance, regeneration in response to injury, or insulin secretion.
Molecular investigations into KPD syndromes should take advantage of clinical samples and databases derived from existing patient cohorts and utilize multiple approaches (genomic, metabolomic, proteomic) to generate etiological hypotheses. Animal models that recapitulate the characteristic β-cell defects of some of the syndromes would be extremely valuable. In the clinical arena, pressing questions include how to classify similar syndromes in children, how to screen asymptomatic patients, and how best to apply new and emerging β-cell trophic therapies to patients with the different KPD subgroups. It is clear that recognition of KPD syndromes and careful phenotypic classification of the patients will facilitate investigations of pathophysiology, the results of which could be relevant to the screening, early diagnosis, and rational treatment of a broader group of patients with nonketotic early β-cell dysfunction.
Supplementary Material
Acknowledgments
The authors acknowledge the invaluable collaborative efforts of the following members of the KPD investigative team at Baylor College of Medicine (Houston, TX) and the University of Washington (Seattle, WA): Drs. Lakshmi Gaur, Ake Lernmark, Dinakar Iyer, Michael Metzker, and Wade Haaland, Ms. Lucille Rodriguez, and Mr. Jesus Villanueva.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Grant Support: RO1 HL73969 and the Alkek Foundation (to A.B.).
First Published Online February 21, 2008
Abbreviations: Ab, Autoantibody; AUC, area under the curve; BMI, body mass index; DKA, diabetic ketoacidosis; GAD, glutamic acid decarboxylase; G6PD, glucose-6-phosphate dehydrogenase; HbA1c, glycosylated hemoglobin; HLA, histocompatibility locus antigen; IA-2, tyrosine phosphatase-like protein IA-2; KPD, ketosis-prone diabetes; KPD-ID, KPD insulin dependent; KPD-NID, KPD non-insulin dependent; LADA, latent autoimmune diabetes in adults; ROC, receiver operator characteristic.
References
- Maldonado M, Hampe CS, Gaur LK, D’Amico S, Iyer D, Hammerle LP, Bolgiano D, Rodriguez L, Rajan A, Lernmark A, Balasubramanyam A 2003 Ketosis-prone diabetes: dissection of a heterogeneous syndrome using an immunogenetic and β-cell functional classification, prospective analysis, and clinical outcomes. J Clin Endocrinol Metab 88:5090–5098 [DOI] [PubMed] [Google Scholar]
- Balasubramanyam A, Garza G, Rodriguez L, Hampe CS, Gaur L, Lernmark A, Maldonado MR 2006 Accuracy and predictive value of classification schemes for ketosis-prone diabetes. Diabetes Care 29:2575–2579 [DOI] [PubMed] [Google Scholar]
- Dodu SR 1967 Diabetes in the tropics. Br Med J 2:747–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adadevoh BK 1968 “Temporary diabetes” in adult Nigerians. Trans R Soc Trop Med Hyg 62:528–530 [DOI] [PubMed] [Google Scholar]
- Oli JM 1978 Remittent diabetes mellitus in Nigeria. Trop Geogr Med 30:57–62 [PubMed] [Google Scholar]
- Winter WE, Maclaren NK, Riley WJ, Clarke DW, Kappy MS, Spillar RP 1987 Maturity-onset diabetes of youth in black Americans. N Engl J Med 316:285–291 [DOI] [PubMed] [Google Scholar]
- Banerji MA, Chaiken RL, Huey H, Tuomi T, Norin AJ, Mackay IR, Rowley MJ, Zimmet PZ, Lebovitz HE 1994 GAD antibody negative NIDDM in adult black subjects with diabetic ketoacidosis and increased frequency of human leukocyte antigen DR3 and DR4. Flatbush diabetes. Diabetes 43:741–745 [DOI] [PubMed] [Google Scholar]
- Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS 1995 Diabetic ketoacidosis in obese African-Americans. Diabetes 44:790–795 [DOI] [PubMed] [Google Scholar]
- Umpierrez GE, Woo W, Hagopian WA, Isaacs SD, Palmer JP, Gaur LK, Nepom GT, Clark WS, Mixon PS, Kitabchi AE 1999 Immunogenetic analysis suggests different pathogenesis for obese and lean African-Americans with diabetic ketoacidosis. Diabetes Care 22:1517–1523 [DOI] [PubMed] [Google Scholar]
- Aizawa T, Katakura M, Taguchi N, Kobayashi H, Aoyagi E, Hashizume K, Yoshizawa K 1995 Ketoacidosis-onset noninsulin dependent diabetes in Japanese subjects. Am J Med Sci 310:198–201 [DOI] [PubMed] [Google Scholar]
- Wilson C, Krakoff J, Gohdes D 1997 Ketoacidosis in Apache Indians with non-insulin-dependent diabetes mellitus. Arch Intern Med 157:2098–2100 [PubMed] [Google Scholar]
- Pinhas-Hamiel O, Dolan LM, Zeitler PS 1997 Diabetic ketoacidosis among obese African-American adolescents with NIDDM. Diabetes Care 20:484–486 [DOI] [PubMed] [Google Scholar]
- Balasubramanyam A, Zern JW, Hyman DJ, Pavlik V 1999 New profiles of diabetic ketoacidosis: type 1 vs type 2 diabetes and the effect of ethnicity. Arch Intern Med 159:2317–2322 [DOI] [PubMed] [Google Scholar]
- Westphal SA 1996 The occurrence of diabetic ketoacidosis in non-insulin-dependent diabetes and newly diagnosed diabetic adults. Am J Med 101:19–24 [DOI] [PubMed] [Google Scholar]
- Pinero-Pilona A, Litonjua P, Aviles-Santa L, Raskin P 2001 Idiopathic type 1 diabetes in Dallas, Texas: a 5-year experience. Diabetes Care 24:1014–1018 [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Philippe J 2000 Characteristics of Caucasian type 2 diabetic patients during ketoacidosis and at follow-up. Schweiz Med Wochenschr 130:576–582 [PubMed] [Google Scholar]
- Jabbar A, Farooqui K, Habib A, Islam N, Haque N, Akhter J 2004 Clinical characteristics and outcomes of diabetic ketoacidosis in Pakistani adults with type 2 diabetes mellitus. Diabet Med 21:920–923 [DOI] [PubMed] [Google Scholar]
- Tan KC, Mackay IR, Zimmet PZ, Hawkins BR, Lam KS 2000 Metabolic and immunologic features of Chinese patients with atypical diabetes mellitus. Diabetes Care 23:335–338 [DOI] [PubMed] [Google Scholar]
- Sobngwi E, Mauvais-Jarvis F, Vexiau P, Mbanya JC, Gautier JF 2002 Diabetes in Africans. Part 2: Ketosis-prone atypical diabetes mellitus. Diabetes Metab 28:5–12 [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Sobngwi E, Porcher R, Riveline JP, Kevorkian JP, Vaisse C, Charpentier G, Guillausseau PJ, Vexiau P, Gautier JF 2004 Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of β-cell dysfunction and insulin resistance. Diabetes 53:645–653 [DOI] [PubMed] [Google Scholar]
- Ramos-Roman MA, Pinero-Pilona A, Adams-Huet B, Raskin P 2006 Comparison of type 1, type 2, and atypical ketosis-prone diabetes at 4 years of diabetes duration. J Diabetes Complications 20:137–144 [DOI] [PubMed] [Google Scholar]
- Maldonado M, D’Amico S, Otiniano M, Balasubramanyam A, Rodriguez L, Cuevas E 2005 Predictors of glycaemic control in indigent patients presenting with diabetic ketoacidosis. Diabetes Obes Metab 7:282–289 [DOI] [PubMed] [Google Scholar]
- Maldonado MR, Otiniano ME, Cheema F, Rodriguez L, Balasubramanyam A 2005 Factors associated with insulin discontinuation in subjects with ketosis-prone diabetes but preserved β-cell function. Diabet Med 22:1744–1750 [DOI] [PubMed] [Google Scholar]
- Banerji MA, Dham S 2007 A comparison of classification schemes for ketosis-prone diabetes. Nat Clin Pract Endocrinol Metab 3:506–507 [DOI] [PubMed] [Google Scholar]
- Umpierrez GE 2006 Ketosis-prone type 2 diabetes: time to revise the classification of diabetes. Diabetes Care 29:2755–2757 [DOI] [PubMed] [Google Scholar]
- Umpierrez GE, Smiley D, Kitabchi AE 2006 Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med 144:350–357 [DOI] [PubMed] [Google Scholar]
- Nalini R, Gaur L, Maldonado M, Hampe C, Rodriguez L, Garza G, Lernmark A, Balasubramanyam A 3 March 2008 HLA class II alleles specify phenotypes of ketosis-prone diabetes (KPD). Diabetes Care 10.2337/dc07-1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampe CS, Nalini R, Maldonado MR, Hall TR, Garza G, Iyer D, Balasubramanyam A 2007 Association of amino-terminal-specific antiglutamate decarboxylase (GAD65) autoantibodies with β-cell functional reserve and a milder clinical phenotype in patients with GAD65 antibodies and ketosis-prone diabetes mellitus. J Clin Endocrinol Metab 92:462–467 [DOI] [PubMed] [Google Scholar]
- Pinero-Pilona A, Raskin P 2001 Idiopathic type 1 diabetes. J Diabetes Complications 15:328–335 [DOI] [PubMed] [Google Scholar]
- Tuomi T, Groop LC, Zimmet PZ, Rowley MJ, Knowles W, Mackay IR 1993 Antibodies to glutamic acid decarboxylase reveal latent autoimmune diabetes mellitus in adults with a non-insulin-dependent onset of disease. Diabetes 42:359–362 [DOI] [PubMed] [Google Scholar]
- Pietropaolo M, Barinas-Mitchell E, Pietropaolo SL, Kuller LH, Trucco M 2000 Evidence of islet cell autoimmunity in elderly patients with type 2 diabetes. Diabetes 49:32–38 [DOI] [PubMed] [Google Scholar]
- Groop L, Groop PH, Koskimies S 1986 Relationship between B-cell function and HLA antigens in patients with type 2 (non-insulin-dependent) diabetes. Diabetologia 29:757–760 [DOI] [PubMed] [Google Scholar]
- Naik RG, Palmer JP 2003 Latent autoimmune diabetes in adults (LADA). Rev Endocr Metab Disord 4:233–241 [DOI] [PubMed] [Google Scholar]
- Kobayashi T 1994 Subtype of insulin-dependent diabetes mellitus (IDDM) in Japan: slowly progressive IDDM–the clinical characteristics and pathogenesis of the syndrome. Diabetes Res Clin Pract 24 Suppl:S95–S99 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Apse K, Pang J, Stanton RC 2000 High glucose inhibits glucose-6-phosphate dehydrogenase via cAMP in aortic endothelial cells. J Biol Chem 275:40042–40047 [DOI] [PubMed] [Google Scholar]
- Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC 2007 The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA 104:17040–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decochez K, Tits J, Coolens JL, Van Gaal L, Krzentowski G, Winnock F, Anckaert E, Weets I, Pipeleers DG, Gorus FK 2000 High frequency of persisting or increasing islet-specific autoantibody levels after diagnosis of type 1 diabetes presenting before 40 years of age. The Belgian Diabetes Registry. Diabetes Care 23:838–844 [DOI] [PubMed] [Google Scholar]
- Borg H, Gottsater A, Fernlund P, Sundkvist G 2002 A 12-year prospective study of the relationship between islet antibodies and β-cell function at and after the diagnosis in patients with adult-onset diabetes. Diabetes 51:1754–1762 [DOI] [PubMed] [Google Scholar]
- Rowley MJ, Mackay IR, Chen QY, Knowles WJ, Zimmet PZ 1992 Antibodies to glutamic acid decarboxylase discriminate major types of diabetes mellitus. Diabetes 41:548–551 [DOI] [PubMed] [Google Scholar]
- Graham J, Hagopian WA, Kockum I, Li LS, Sanjeevi CB, Lowe RM, Schaefer JB, Zarghami M, Day HL, Landin-Olsson M, Palmer JP, Janer-Villanueva M, Hood L, Sundkvist G, Lernmark A, Breslow N, Dahlquist G, Blohme G 2002 Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes 51:1346–1355 [DOI] [PubMed] [Google Scholar]
- Umpierrez GE, Smiley D, Gosmanov A, Thomason D 2007 Ketosis-prone type 2 diabetes: effect of hyperglycemia on β-cell function and skeletal muscle insulin signaling. Endocr Pract 13:283–290 [DOI] [PubMed] [Google Scholar]
- Sobngwi E, Gautier JF, Kevorkian JP, Villette JM, Riveline JP, Zhang S, Vexiau P, Leal SM, Vaisse C, Mauvais-Jarvis F 2005 High prevalence of glucose-6-phosphate dehydrogenase deficiency without gene mutation suggests a novel genetic mechanism predisposing to ketosis-prone diabetes. J Clin Endocrinol Metab 90:4446–4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Smith SB, Le May C, Leal SM, Gautier JF, Molokhia M, Riveline JP, Rajan AS, Kevorkian JP, Zhang S, Vexiau P, German MS, Vaisse C 2004 PAX4 gene variations predispose to ketosis-prone diabetes. Hum Mol Genet 13:3151–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabchi AE, Umpierrez GE, Murphy MB, Barrett EJ, Kreisberg RA, Malone JI, Wall BM 2001 Management of hyperglycemic crises in patients with diabetes. Diabetes Care 24:131–153 [DOI] [PubMed] [Google Scholar]
- Sobngwi E, Vexiau P, Levy V, Lepage V, Mauvais-Jarvis F, Leblanc H, Mbanya JC, Gautier JF 2002 Metabolic and immunogenetic prediction of long-term insulin remission in African patients with atypical diabetes. Diabet Med 19:832–835 [DOI] [PubMed] [Google Scholar]
- Umpierrez GE, Smiley D, Zisman A, Prieto LM, Palacio A, Ceron M, Puig A, Mejia R 2007 Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 30:2181–2186 [DOI] [PubMed] [Google Scholar]
- Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA 2006 Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 29:2739–2748 [DOI] [PubMed] [Google Scholar]
- Hsin Y, Guo H, Wu T 2001 Factors associated with discontinuing insulin therapy after diabetic ketoacidosis in adult diabetic patients. Diabet Med 18:895–899 [DOI] [PubMed] [Google Scholar]
- Rasouli N, Elbein SC 2004 Improved glycemic control in subjects with atypical diabetes results from restored insulin secretion, but not improved insulin sensitivity. J Clin Endocrinol Metab 89:6331–6335 [DOI] [PubMed] [Google Scholar]
- Otiniano ME, Balasubramanyam A, Maldonado M 2005 Presence of the metabolic syndrome distinguishes patients with ketosis-prone diabetes who have a type 2 diabetic phenotype. J Diabetes Complications 19:313–318 [DOI] [PubMed] [Google Scholar]
- Maldonado MR, Chong ER, Oehl MA, Balasubramanyam A 2003 Economic impact of diabetic ketoacidosis in a multiethnic indigent population: analysis of costs based on the precipitating cause. Diabetes Care 26:1265–1269 [DOI] [PubMed] [Google Scholar]
- Javor KA, Kotsanos JG, McDonald RC, Baron AD, Kesterson JG, Tierney WM 1997 Diabetic ketoacidosis charges relative to medical charges of adult patients with type I diabetes. Diabetes Care 20:349–354 [DOI] [PubMed] [Google Scholar]
- Maldonado MR, D’Amico S, Rodriguez L, Iyer D, Balasubramanyam A 2003 Improved outcomes in indigent patients with ketosis-prone diabetes: effect of a dedicated diabetes treatment unit. Endocr Pract 9:26–32 [DOI] [PubMed] [Google Scholar]
- Levetan CS, Passaro MD, Jablonski KA, Ratner RE 1999 Effect of physician specialty on outcomes in diabetic ketoacidosis. Diabetes Care 22:1790–1795 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.