Abstract
Progressive insulin secretory defects, due to either functional abnormalities of the pancreatic β-cells or a reduction in β-cell mass, are the cornerstone of type 2 diabetes. Incretin-based drugs hold the potential to improve glucose tolerance by immediate favorable effect on β-cell physiology as well as by expanding or at least maintaining β-cell mass, which may delay the progression of the disease. Long-term studies in humans are needed to elaborate on these effects.
I. Introduction
- II. The Incretin Effect and Its Mediators
- A. Definitions and overview
- B. Glucose-dependent insulinotropic polypeptide
- C. Glucagon-like peptide 1
- D. Similarities and differences between GIP and GLP-1
- E. The incretins in diabetes
- III. Effects of the Incretins on β-Cell Mass
- A. Effects of GLP-1 receptor signaling
- B. Effects of GIP receptor signaling
- IV. Drugs Based on the Incretins and Their Effects in Diabetic Patients
- A. GLP-1 receptor agonists
- B. Dipeptidyl peptidase inhibitors
- C. Potential of incretin-based drugs to affect β-cell mass in humans
V. Summary
I. Introduction
THE LAST TWO DECADES have seen a major shift in the consensus on the pathogenesis of type 2 diabetes mellitus (T2DM). Although it is clear that resistance of peripheral tissues to insulin is an abnormality common to almost all patients with T2DM, the centrality of β-cell dysfunction as an essential component necessary for development of diabetic glucose tolerance is now widely accepted (1). One cornerstone of this revised model of diabetes is the recognition that the normal β-cell response to insulin resistance is a compensatory increase in insulin secretion sufficient to maintain glucose homeostasis (2,3). Failure of the β-cells to adapt to systemic insulin needs leads to a decrement in insulin- mediated glucose metabolism that causes hyperglycemia (4,5). A second observation underpinning the core role of the β-cell in the genesis of diabetes is the parallel course of insulin secretion and glucose homeostasis. There is evidence that a relative defect in β-cell function, either constitutive or acquired, is present before clinically apparent diabetes (6), suggesting that abnormal insulin secretion is a proximal defect in the process of glucose intolerance. Moreover, progressive β-cell failure is the unfortunate eventuality for most people with established T2DM, and the decline of insulin secretion is associated with worsening metabolic control and the need for more intensive treatment (7). These important clinical observations—that β-cell function can adapt to increased demand, and that β-cell dysfunction is proportional to the degree of glucose intolerance—have led to a currently accepted view that in most people islet function is fluid and amenable to adaptation, but that genetic or environmental factors exist in others that compromise matching of insulin secretion to insulin needs contributing to abnormal glucose metabolism.
Comparisons of insulin secretion in diabetic and nondiabetic humans have identified a number of characteristic abnormalities of β-cell function. That patients with T2DM have impaired insulin responsiveness to stimulation by glucose has long been recognized (8,9,10). This characteristic of diabetic islet function is manifest as the classic absence of first-phase insulin release, an apparent loss of β-cell sensitivity to glucose (11), and delayed insulin secretion in response to oral glucose (8,10). This latter response may be due in part to a decrease in the actions of gastrointestinal (GI) hormones and neural signals engaged after meals to potentiate the insulin response (12). Diabetic patients also have disruption of normal pulsatile insulin secretion (9), abnormal potentiation of nonglucose secretagogues, and a decreased maximal secretory capacity (13). Although the specific molecular mechanisms underlying these abnormalities of β-cell function in T2DM are still under study, taken together they result in delayed insulin responses that are inappropriately low for the degree of plasma glycemia.
Beyond functional defects of insulin secretion, it is also clear that patients with T2DM have anatomical abnormalities of the pancreatic islets. Pathological studies of diabetic and nondiabetic patients have demonstrated decreased islet size and decreased numbers of insulin-producing cells (14,15,16). This has been attributed to the formation of islet amyloid in diabetic islets (16,17,18), with replacement of β-cells, and to increased β-cell apoptosis (16). The reduction in β-cell number seems to precede diabetes, because subjects with impaired glucose tolerance have approximately 50% of the islet mass of weight-matched controls with normal glucose tolerance (16). Islet mass has been shown to correlate with fasting plasma glucose levels (19), suggesting that the number of β-cells, as well as their function, may play a role in physiological regulation. This observation is supported by data from subjects with autologous islet transplants after pancreatectomy, demonstrating that insulin secretory capacity is proportional to the size of the islet graft (20). The accumulated information supporting reduced islet mass in diabetic patients and evidence that processes inherent to the diabetic state such as islet amyloidosis (17,18), glucotoxicity (21), and inflammation (22) cause ongoing β-cell death have led to the widespread belief that the progressive nature of T2DM is due to a steady reduction of insulin secretory capacity.
There has been a general tendency to dichotomize abnormalities in insulin secretion and reduced β-cell mass when considering the role of islet dysfunction in T2DM. This is due in part to studies supporting distinct signaling pathways for the regulation of acute processes like exocytosis of insulin-containing granules and more chronic processes such as cellular hypertrophy, division, and apoptosis (23). In addition, studies of the effect of reduced β-cell mass have often made use of techniques such as partial pancreatectomy of healthy animals with functionally normal islets (24). However, it is plausible and, in fact, seems likely that functional defects in β-cell secretion and reduced numbers of β-cells are linked (25,26). Reduced numbers of β-cells have increased relative demands for insulin secretion and may develop functional defects comparable to diabetic islet function (24,27). Therefore, it can be argued that diminished islet mass has an impact on insulin output beyond simply lowering the overall capacity for insulin production.
Studies in animals suggest that islets hypertrophy and involute as necessary for varying states of insulin sensitivity (28). Examples include pregnancy (29), insulin resistance (30), and the response to pancreatectomy (31). These studies form the basis for considering β-cell mass as a regulable parameter. In this review, we will consider the potential of pharmacological interventions to promote β-cell mass as a means of treating T2DM. The focus of our discussion will be recently developed drugs that act through the incretin signaling pathways. The incretins are insulinotropic hormones derived from the GI tract that link nutrient absorption with β-cell secretion; the major incretins are glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). The incretins activate specific receptors on the β-cell that stimulate insulin release but also activate β-cell growth and inhibit apoptosis in vitro, and chronic stimulation of these pathways in rodents enhances β-cell mass. We will provide an overview of the incretin system and discuss the effects of GLP-1 and GIP to regulate β-cell proliferation, growth, and death. We will also discuss the effects of drugs that work through the incretin pathways in T2DM patients and whether any of these therapeutic effects can be explained by changes in β-cell mass.
II. The Incretin Effect and Its Mediators
A. Definitions and overview
It has long been known that the assimilation of glucose is more efficient when it is given orally than when it is given iv (32). After the development of the RIA and the ability to measure insulin in circulating plasma, investigators noted significantly greater insulin concentrations when glucose was ingested compared with it was given iv (33). The primary explanation for this observation is that factors released from the GI tract regulate insulin secretion and glucose homeostasis. These hormones have been termed incretins, and the relatively greater insulin secretion observed with oral compared with iv glucose is termed the incretin effect. There are two known peptides that act as incretins, GIP and GLP-1. The incretins are produced by specialized cells in the intestinal mucosa and secreted primarily in response to carbohydrate- and lipid-containing meals. There are specific receptors for GIP and for GLP-1 that are expressed on islet cells, as well as in other tissues, and deletion of these receptors in mouse models leads to glucose intolerance (34). These data, and corroborating studies using GIP and GLP-1 receptor antagonists, have demonstrated that so-called “enteroinsular” signaling is a physiological process that is necessary for normal glucose homeostasis.
B. Glucose-dependent insulinotropic polypeptide
GIP (originally referred to as gastric inhibitory polypeptide) is a highly conserved 42-amino acid peptide produced by enteroendocrine K cells in the duodenum and jejunum (35). GIP secretion is stimulated by enteral glucose and lipids in a dose dependent manner (36), and increased plasma levels augment glucose-stimulated insulin secretion (32). Consistent with the incretin effect, GIP release is directly regulated by the products of meal digestion and acts as a feed-forward mechanism to signal the endocrine pancreas of impending substrate fluxes from the gut (37).
After release into the circulation, GIP is rapidly metabolized by the enzyme dipeptidyl peptidase IV (DPP-IV), a ubiquitous protease located on capillary endothelial cells as well as in the circulation. DPP-IV cleaves specifically between residues 2 and 3 leaving GIP(3-42) (35,38). This conversion occurs rapidly, so that the circulating half-life of full-length GIP is only 5–7 min in mammals. Metabolism by DPP-IV inactivates GIP because GIP(3-42) does not stimulate insulin secretion.
A single GIP receptor (GIPr) has been identified and is currently believed to mediate all of the physiological effects of the peptide (39). The GIPr has substantial homology with the secretin-vasoactive intestinal peptide receptor family of seven-transmembrane G protein-coupled receptors. The GIPr is expressed in β-cells of the pancreatic islet, the upper GI tract, adipocytes, adrenal cortex, bone, pituitary, and a variety of brain regions (40,41). Binding of GIP to its receptor on the β-cell is insulinotropic only when ambient glucose concentrations are elevated, typically to greater than 5–6 mm (42). This action has been demonstrated uniformly in a wide range of experimental settings including cultured β-cells, animal models, and humans. In humans, both porcine and synthetic human GIP stimulate insulin secretion when infused to concentrations mimicking those occurring after meals as long as some degree of hyperglycemia is present.
The incretin role of GIP has been demonstrated by several experimental techniques such as immunoneutralization of circulating GIP and by administration of competitive antagonists of the GIP receptor, both of which cause glucose intolerance (43,44). Similarly, targeted gene deletion of the GIP receptor in a line of mice resulted in animals with normal glucose levels in the fasting state and in response to ip glucose loads (45). However, after oral glucose loading GIP receptor knockout mice had significant glucose intolerance and impaired insulin secretory responses. Taken together these studies in rodents indicate that the incretin action of GIP is necessary for normal glucose tolerance.
C. Glucagon-like peptide 1
GLP-1 is cleaved from proglucagon in specific intestinal mucosal cells termed L cells and secreted primarily as an amidated 30-amino acid peptide GLP-1(7-36)NH2 (46). The distribution of the L cells that produce GLP-1 is greatest in the distal small intestine and colon. Similar to GIP, GLP-1 is also released in response to nutrient carbohydrate and fat. Plasma levels peak in the first 60 min after eating (36) and are proportional to meal size (47). Upon reaching the bloodstream, GLP-1, like GIP, is metabolized by DPP-IV, which cleaves the N-terminal dipeptide His-Ala leaving the circulating congener GLP-1(9-36)NH2 (38,48). This rapid metabolism of bioactive GLP-1 results in a plasma half-life of 1–2 min and leaves inactive GLP-1(9-36)NH2, which does not affect insulin secretion or glucose tolerance in humans at physiological concentrations (49).
GLP-1 binds to a specific GLP-1 receptor (GLP-1r) that is expressed on β-cells, as well as the GI tract, heart, lung, kidney, and neurons in the hypothalamus, hindbrain and several other brain regions, and the vagus nerve (50,51,52); controversy exists over whether the GLP-1r is also expressed by pancreatic α-cells. In β-cells the classic action of GLP-1 is to augment glucose-stimulated insulin secretion (23,53). Like GIP, GLP-1 stimulates insulin release in cultured β-cells, animals, and humans in a glucose-dependent manner. However, beyond acting as an acute insulin secretagogue, GLP-1 increases the biosynthesis of important β-cell products including insulin, glucokinase, and the GLUT 2 glucose transporter (54).
The important effects of GLP-1 on β-cell function have been elegantly demonstrated in studies of mice with a targeted deletion of the GLP-1r gene. These mice have glucose intolerance and delayed and diminished insulin secretion (55). Islets from GLP-1r −/− mice have a slight reduction in size, with a relative increase in the percentage of α-cells (56). In addition, the islets of these mice are more susceptible to the toxic effects of streptozotocin than control mice (57). Finally, absence of the GLP-1r blunts the compensatory growth after partial pancreatectomy (58). In sum, studies with GLP-1r −/− mice demonstrate broad effects of GLP-1 signaling on β-cell function, including effects on insulin secretion and growth/death.
D. Similarities and differences between GIP and GLP-1
The incretins share a number of similarities. GIP and GLP-1 have modest degrees of sequence homology as do their receptors. Both peptides are released in response to meal consumption with a dose-dependent relationship to meal size. Both GIP and GLP-1 are metabolized, and inactivated as incretins, by DPP-IV. Not surprising given the general similarities of the GIPr and GLP-1r, early intracellular signaling events after ligand binding are comparable. In the β-cell this causes enhanced insulin secretion during periods when blood glucose is elevated. The general commonalities shared by GIP and GLP-1 suggest the evolution of redundant systems to mediate the incretin effect. In fact, there is evidence that one system compensates in the absence of normal functioning of the other incretin (59,60).
Despite the many similarities between the incretins, there are several key points of distinction, including site of synthesis and the manner in which nutrient stimuli is coupled to secretion, mechanism of action, and extra-incretin effects. GIP is produced primarily in the upper intestine, and there is convincing data connecting K cell secretion of GIP with the digestion and absorption of lipid and carbohydrate (36,61,62). GLP-1 is made throughout the small intestine, but the highest concentrations of L cells are in the distal gut (63). In rats, the case has been made that stimulation of GLP-1 secretion involves signaling through vagal pathways (64,65), although it is not clear that there is a neural component to GLP-1 release in humans. GIP has characteristics more typical of a hormone, with a 5- to 10-fold increase in circulating concentrations after meals and a longer half-life comparable with other peptides that have endocrine actions. In contrast, GLP-1 circulates in lower molar amounts than GIP and increases only 1.5- to 2-fold after meal stimuli (66). The enhanced susceptibility of GLP-1 to DPP-IV and its abbreviated half-life raises the question of whether significant amounts of intact, bioactive, peptide actually reach the arterial circulation and target tissues (67). Therefore some controversy remains over how GLP-1 mediates its actions. Recent evidence supports a neural mechanism initiated by sensors in the hepatic portal vein that would have access to relatively high and dynamic concentrations of GLP-1 (52,56). Finally, beyond acting as an incretin, the only other prominent actions of GIP seem to be stimulus of glucose uptake and triglyceride storage in adipocytes (68,69), and possibly alteration of bone mass and bone turnover (70,71). In contrast, GLP-1 has a variety of effects that all seem to contribute to minimizing elevations in blood glucose. For example, GLP-1 reduces glucagon secretion (72), slows gastric emptying (73,74), induces satiety (75), suppresses hepatic glucose production (76), and promotes insulin-independent glucose disappearance (77,78). Research on the incretins over the last decade has demonstrated the range of actions that these peptides have on postprandial metabolism and supports the notion that GIP and GLP-1 have physiological roles that are distinct and complementary.
E. The incretins in diabetes
The incretin effect is impaired in persons with T2DM (8,12). This has raised the question of the sufficiency of circulating incretins in diabetic individuals. In fact, there is evidence from several groups that persons with type 2 diabetes have increased plasma concentrations of GIP after glucose or meal ingestion, compared with nondiabetic individuals (37,79). Although this finding has not been universal, there is no evidence for reduced GIP secretion as an explanation for a reduced incretin effect in diabetes. The case for GLP-1 is more complex. Different studies have reported that plasma GLP-1 levels are increased (80,81), decreased (47,66,82,83), and similar (84,85,86) in type 2 diabetic and nondiabetic subjects. These studies have used different assays of plasma GLP-1 so that the lack of consensus may be due to technical discrepancies. However, it seems more likely that these differences are the result of the relatively small sample sizes studied with distinct characteristics that could independently affect the results. Regardless, in studies where GLP-1 secretion has been reported as reduced in diabetic subjects, the relative decrease has been modest, suggesting that GLP-1 deficiency probably does not contribute significantly to β-cell dysfunction in T2DM.
Another major difference between GLP-1 and GIP is their activity in persons with diabetes. GIP is characteristic of a number of other GI peptides as being an insulin secretagogue in healthy subjects but relatively inactive in patients with type 2 diabetes. Thus, a number of studies have shown that diabetic subjects given iv GIP during a hyperglycemic stimulus have only minimal augmentation of insulin release (87,88,89). Several recent studies have suggested that the reduction in the GIP response in T2DM patients is not a specific defect but is proportional to other abnormalities in β-cell function (90,91). Nonetheless, it appears that in diabetic subjects with moderate glycemic control, acute infusions of GIP are not likely to be effective for reducing blood glucose.
The case with GLP-1 is completely different. Administration of iv GLP-1 to hyperglycemic T2DM subjects decreased blood glucose levels to nondiabetic values over 3–4 h (88). This effect was at least partly the result of increased insulin secretion and reductions in circulating glucagon. Similarly, continuous infusions of GLP-1 normalized fasting blood glucose overnight and caused postprandial levels to be near-normal as well (92). Administration of GLP-1 caused partial restitution of first-phase insulin release in subjects with established T2DM (76,93), demonstrating that this peptide reverses one of the hallmark lesions of diabetes. And although there is evidence that the β-cell sensitivity to GLP-1 is reduced in T2DM compared with nondiabetic subjects (94), pharmacological amounts of GLP-1 cause a robust improvement in stimulated insulin secretion (87,88,95). The potent effects of acute administration of GLP-1 to correct hyperglycemia and β-cell function in T2DM are persistent because 6 wk of continuous sc infusion caused significant improvements in these parameters in diabetic subjects (96). These findings and numerous other studies have shown that GLP-1 is unique among the insulinotropic gut hormones in maintaining potent effects on the diabetic islet, and for being able to correct hyperglycemia.
III. Effects of the Incretins on β-Cell Mass
A. Effects of GLP-1 receptor signaling
There is thought to be some remodeling of size and function of islet β-cells occurring throughout life that plays a role in the maintenance of normal glucose tolerance (28). Maintenance of islet cell mass, primarily through changes in numbers and size of β-cells, is generally thought to rely on the interplay of division and growth of established β-cells and on rates of apoptosis and β-cell loss (39). The role of neogenesis of endocrine precursors from pancreatic duct cells has been called into question as a significant mechanism for changes in β-cell mass in vivo by several recent studies (97,98,99). Although the role of these processes in normal physiology is still debated, especially with regard to human islets, there is now strong evidence that GLP-1 affects all three, at least in cultured cells and in rodent models. It has been established for some time that GLP-1 signaling in β-cells increases the transcription of several key genes related to glucose sensing and insulin secretion (54,100,101). However, more recent work has demonstrated that activation of the GLP-1r enhances β-cell replication and diminishes β-cell apoptosis, leading to an overall increase in β-cell mass. There is also evidence that GLP-1 can increase neogenesis of β-cells from precursor cells in the pancreas, although the significance of this as a developmental or adaptive mechanism is less clear. Although basic research in this area has proceeded with great speed over the past several years, translation of this work to humans has not yet been accomplished. At this point in time the work on GLP-1 and islet mass is strictly preclinical.
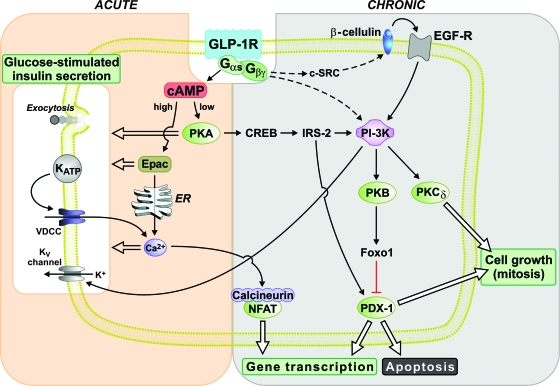
Activation of the GLP-1r in β-cells initiates signaling through the generation of cAMP (100) and activation of phosphatidylinositol-3 kinase (PI-3K) (Fig. 1). There are some data to suggest involvement of the epidermal growth factor receptor as a mediator of GLP-1-induced PI-3K activity (57). Acute effects of GLP-1 on insulin secretion, including enhanced intracellular calcium, membrane depolarization, and insulin exocytosis, are largely mediated through cAMP and subsequent activation of either protein kinase A (PKA) (102) or cAMP-regulated guanine nucleotide exchange factor II (Epac 2) (103). This pathway also affects gene expression through the actions of cAMP response element-binding protein. However, chronic effects of GLP-1, such as gene transcription, activation of cellular growth, and inhibition of apoptosis, can also be attributed to PI-3K downstream pathways, such as protein kinase B and protein kinase C (104,105). Besides cAMP response element-binding protein, the key intermediaries of GLP-1 signaling that mediate more chronic β-cell effects include insulin receptor substrate 2 and the transcription factor pancreatic duodenal homeobox factor-1 (PDX-1). In particular, GLP-1-mediated stimulation of β-cell gene transcripts, such as proinsulin, requires PDX-1 (106,107), although recently support for involvement of the transcription factor NFAT (nuclear factor of activated T cells) has been presented (108,109).
Figure 1.
Signaling pathways triggered in response to GLP-1 receptor activation. The actions of GLP-1 signaling have been subdivided into those mediating primarily acute responses, such as potentiation of insulin secretion, and those mediating chronic responses, such as gene transcription and cell replication. Although a general distinction between acute and chronic effects can be made, there is some degree of overlap and interaction between these signaling pathways.
Several lines of evidence suggest that GLP-1 has a role in β-cell and islet development. For example, activation of GLP-1r signaling promotes conversion of human or rat pancreatic ductal cell lines to islet-like cells that produce insulin in a glucose-dependent fashion (69,110,111). Moreover, GLP-1 stimulates the differentiation and maturation of fetal islet cells to insulin-producing islet cells (112). The effect of GLP-1 on β-cell differentiation involves the transcription factors PDX-1 and its upstream regulator Foxa2 (formerly HNF3β) (69,110,111) and appears to be mediated by MAPKs (69,104), including ERK (113), and an isoform of protein kinase C (104).
GLP-1 also seems to stimulate the proliferation of developed β-cells (104,114,115). Moreover, GLP-1 and GLP-1r agonists reduce apoptosis in primary rodent islets or islet cell lines exposed to cytotoxic agents, such as cytokines, free fatty acids, hydrogen peroxide, or streptozotocin (57,107,116,117). Activation of PDX-1 appears to be a shared component in all molecular pathways involved in cytoprotective effects of GLP-1. Enhanced PI-3K activity as a result of insulin receptor substrate 2 phosphorylation or epidermal growth factor receptor transactivation leads to the up-regulation of PDX-1 via nuclear exclusion of Foxo1 (118,119).
The overall effect of GLP-1r signaling on β-cell proliferation and apoptosis results in increased islet mass (105) and has demonstrable benefits in rodent models of β-cell insufficiency. Treatment with GLP-1r agonists has caused parallel increases in β-cell growth and glucose control in several rodent models of diabetes (115,120,121,122). A similar response has been attributed, at least partially, to endogenous GLP-1 protected from metabolism by DPP-IV inhibitors (117,119). Administration of the GLP-1r agonist exendin-4 during the neonatal period preserves β-cell mass and prevents the development of diabetes in rats with experimentally induced intrauterine growth retardation (58) or exposure to streptozotocin (123). Finally, exendin-4 treatment improves outcomes in diabetic mouse recipients of transplanted rat islets (124,125), as does treatment of mouse islets with adenoviral vectors to promote GLP-1 production by α-cells (126). These studies raise the interesting possibility that activation of the GLP-1r could increase β-cell mass, a novel and potentially effective approach to the treatment of diabetes. Table 1 summarizes the studies in rodents treated with compounds that increase GLP-1r activity that have assessed parameters of β-cell proliferation, apoptosis, or islet mass.
Table 1.
Studies in rodents demonstrating effects of GLP-1r agonists and DPP-IV inhibitors on parameters of islet mass
| Incretin-based drug | Method of administration | β-Cell function | β-Cell histology/mass | Apoptosis |
|---|---|---|---|---|
| GLP-1 | Continuous infusion for 5 d in old and young Wistar rats (121) | Improved glucose tolerance | Increased PDX-1 expression [reversed with infusing Ex-(9-39)], proliferation, and neogenesis. | NA |
| Continuous infusion for 2 d in diabetic Zucker rats (120) | Improved glucose tolerance | Increased proliferation | ↓ | |
| Continuous infusion for 4–8 wk in 8-wk-old female NOD mice (158) | Improved glucose tolerance (lasted for 2–5 wk from the last dose of GLP-1 depending on the duration of treatment) | Increased proliferation and neogenesis | ↓ | |
| Diabetes did not occur in those treated for 8 wk by 21 wk of age (termination of study) and diabetes onset was delayed in rats treated for 4 wk. | ||||
| Increased GLP-1 expression in β−cells of Goto-Kakizaki type 2 diabetic rats secondary to ileal transposition (159) | Improved glucose tolerance | Improved morphology, absence of islet cell degeneration and interstitial fibrosis, and increased neogenesis. | NA | |
| Enhanced GLP-1 expression by im plasmid-based gene transfer in STZ-treated mice (122) | Improved glucose tolerance | Increased β-cell mass with improved morphology | NA | |
| Exendin-4 | Once daily for 10 d in diabetic rats with partial pancreatectomy (115) | Improved glucose tolerance in diabetic rats | Increased replication, neogenesis, and β-cell mass. | NA |
| Once daily for 7–12 d in streptozocin-induced diabetic rats (160) | Improved glucose tolerance lasted 2 wk from discontinuing Ex-4 | Increased expression of IDX-1 in the pancreatic ducts [reversed with infusing Ex-(9-39)], differentiation, and neogenesis | ↓ | |
| Daily for 2 wk in db/db mice (114) | Improved glucose tolerance | NA | ||
| Once daily for 1 wk in diabetic mouse recipient of transplanted rat islets, which were cultured with exendin-4 (124) | Diabetes was cured in 80% of treated animals vs. 10% of controls at 4 wk. | Increased insulin-stained cells in the grafts of treated vs. untreated animals. | NA | |
| Once daily for 11 d (started on d 2) in diabetic mouse recipients of fetal islet-like cell cluster transplant (125) | Improved glucose tolerance in treated animals at 3-month follow-up | Enhanced maturation and differentiation of transplanted fetal islet cells into insulin-producing β-cells | NA | |
| Twice daily for 6 wk in fa/fa Zucker rats (161) | Improved insulin secretion and insulin sensitivity, assessed by euglycemic clamp | Increased β-cell mass*insulin sensitivity index, suggestive of trophic effect of GLP-1 | NA | |
| DPP-IV inhibitors | Des-fluoro-sitagliptin once daily for 11 wk in high-fat diet, streptozocin-induced diabetic mice (162) | Improved glucose tolerance. | Increased proliferation and neogenesis to restore β-cell mass to normal size. Enhanced pancreatic insulin content | NA |
| Enhanced pancreatic insulin secretion | ||||
| P32/98 twice daily for 7 wk in STZ-treated Wistar rats (117) | Improved glucose tolerance and insulin secretion | Enhanced proliferation and β-cell mass by 120% (intervention group gained weight by 230%) | NA | |
| NVP DPP728 for 8 wk in C57BL/6J mice fed normal or high-fat food in comparison with controls in each group (163) | Improved glucose tolerance and insulin secretion in both high-fat and normal food groups receiving DPP-IV inhibitors compared with that in their counterpart controls. | No changes in islet morphology in either group, although islet cell size was significantly reduced in high-fat mice compared with that in their controls | NA |
↓, Decreased; NA, not addressed.
It is important to note that despite the accumulation of an impressive body of evidence that the GLP-1r signaling promotes the expansion or protection of β-cell mass, this information is limited to studies of cultured cells or rodent models. There are important differences in rates and capacity for islet cell turnover and growth between rodents and humans (127), such that extension of findings from animal studies cannot be assumed. Because it is not currently possible to evaluate β-cell mass noninvasively, the question of GLP-1 effects on β-cell proliferation and apoptosis in human subjects cannot be reliably addressed in longitudinal or intervention studies. Rather, the best extensions of the interesting findings in rats and mice to humans are inferential and based on functional studies. For example, administration of exenatide for 3 months in a group of C-peptide-positive islet cell transplant recipients with uncontrolled glycemia resulted in increased insulin secretion and improved meal tolerance, which was sustained for 1 month after the last dose of exenatide (128). Although this finding is compatible with effects of GLP-1r signaling on growth and viability of the islet grafts, it could also be explained by indirect effect on glycemic control or other aspects of metabolism. Thus, whereas preclinical research has provided hope and excitement for GLP-1 based drugs, the critical evidence from clinical studies is not yet available.
B. Effects of GIP receptor signaling
GIP signaling in the β-cell shares many similarities to GLP-1. GIP binds to a specific G protein-coupled receptor that is related to the GLP-1r (129) and enhances glucose-stimulated insulin secretion. The general molecular mechanisms of GIP activation of the β-cell are similar to those of GLP-1, including production of cAMP and activation of PKA, increased intracellular Ca2+, and closure of KATP channels, causing insulin exocytosis (53). However, like GLP-1, a significant component of GIP signaling is independent of PKA and acts through the Epac 2 pathway (130). Administration of GIPr antagonists (44) or targeted deletion of the GIPr gene (45,59) causes a deterioration of glucose tolerance and reduced glucose-stimulated insulin secretion in rodents. Importantly, islet cells from GIPR −/− mice exhibit reduced proinsulin gene expression and insulin content compared with wild-type mice (59), indicating that GIP does more than simply stimulate insulin release during hyperglycemia.
GIP also has proliferative and antiapoptotic effects on β-cells. GIP enhances β-cell proliferation synergistically with glucose (131) through pleiotropic mechanisms that include PKA activation, PI-3K/protein kinase B activity, and p38 MAPK. GIPr signaling protects β-cell lines from the cytotoxicity of streptozotocin (132), glucolipotoxicity (133), and starvation from glucose or serum (134). The cytoprotective effects of GIP are at least partly mediated by a suppression of caspase-3, a mediator of apoptosis (132).
Several studies in animal models have demonstrated the potential importance of GIPr signaling in maintaining islet cell mass. Administration of GIP-antagonist for 11 d to ob/ob mice partially reversed islet hypertrophy with a concurrent improvement of hyperinsulinemia and insulin resistance (68). Compatible with this finding, continuous administration of GIP to Zucker diabetic fatty rats for 2 wk diminished β-cell apoptosis (133). There are as yet no studies of chronic effects of GIPr agonists in humans, so the intriguing results of these animal studies have not been extended to possible clinical applications. However, it is important to note that new drugs that act by blocking the action of DPP-IV increase levels of GIP as well as GLP-1. Therefore, manipulations that increase islet mass in rodents by reducing DPP-IV action are potentially due to actions of both incretins (117,135).
IV. Drugs Based on the Incretins and Their Effects in Diabetic Patients
The last several years have seen the introduction of two classes of antidiabetic drugs based on incretin signaling for clinical use. These classes include GLP-1r agonists and DPP-IV inhibitors, which increase endogenous levels of GLP-1 and GIP (136). With the progress of these compounds to market and their use by large numbers of patients with diabetes, it has been possible to gain insights into the effects of chronic stimulation of the incretin pathways on β-cell function (137). The results of clinical studies of incretin-based drugs has engendered considerable discussion over their potential effects to promote β-cell mass. However, given that these studies have been either randomized trials with clinical outcomes or short-term physiological experiments with functional measures, the matter remains mostly conjectural at this point.
A. GLP-1 receptor agonists
There are a number of compounds developed to activate the GLP-1r that are resistant to DPP-IV and so have extended activity. The most established of these new drugs are synthetic exendin-4 (exenatide; Amylin Pharmaceuticals Inc., La Jolla, CA), and liraglutide (formerly NN2211, Novo Nordisk, Copenhagen, Denmark). Exenatide has been available to treat patients since 2005, and several hundred thousand patients have been treated with this drug to date; liraglutide is in advanced clinical trials.
Exendin-4 is a naturally occurring reptilian peptide of 39 amino acids with considerable homology to GLP-1 (136), which mimics to a great extent the actions of GLP-1 (138). Exenatide has a half-life of 2–3 h in humans after sc injection (139), and so provides a relatively extended activation of the GLP-1r. When administered iv to healthy humans, exenatide affects fasting glucose and insulin levels minimally, demonstrating the glucose dependence that is a noteworthy component of GLP-1 action (140). When administered during the consumption of a standard meal, exenatide infusions reduce the glycemic excursion significantly, at least partially due to enhanced β-cell function. The effects of exendin-4 on insulin secretion persist for up to 3 h after administration (141) and do not affect the normal endocrine responses to hypoglycemia (142).
In diabetic subjects, administration of exenatide stimulates insulin secretion, reduces glucagon levels, reduces body weight and presumably insulin resistance, and delays gastric emptying (139,143), all actions that would tend to mitigate postprandial hyperglycemia. Exenatide partially restores first-phase insulin secretion in T2DM subjects (144), correcting one of the classic abnormalities of diabetes. In type 2 diabetic patients participating in clinical trials, generally subjects under moderate glycemic control with conventional therapies, exenatide improved glycemic control as reflected in reduced hemoglobin A1c levels (133,145,146,147). These findings indicate that the effect of GLP-1r signaling to enhance insulin secretion in acute studies of diabetic subjects persists with chronic intermittent administration of exenatide. However, these results do not necessarily support chronic effects of GLP-1r signaling, such as gene transcription, increased β-cell replication, or reduced apoptosis, which cannot be readily assessed in human studies. In a recent examination of subjects treated with exenatide for 2 yr, the reduction of hemoglobin A1c levels was maintained at a stable level (148). Because of the known progression of type 2 diabetes over time (7), with a general tendency for hemoglobin A1c to worsen with fixed treatment, these results raise the possibility that treatment with exenatide can stabilize the progressive course of T2DM. Such results are consistent with, but do not prove, an effect of exenatide on β-cell function beyond that of an acute secretagogue. However, the 2-yr duration of follow-up is in actuality relatively short to comment on effects to change the natural course of diabetes. In addition, because this was an open-label trial of subjects continuing participation from randomized trials of exenatide, it lacks the proper controls to make a firm conclusion about effects on diabetes progression.
The other GLP-1 mimetic in advanced trials is liraglutide. This compound is a modified GLP-1 molecule that includes a C-16 fatty acyl derivative that promotes binding to albumin. Liraglutide is resistant to metabolism by DPP-IV, is absorbed gradually from the sc space after injection, and reaches peak levels 12 h after administration (149), with an elimination half-life of 12 h (150). Similar to GLP-1, liraglutide improves the sensitivity of the β-cell to glucose in persons with type 2 diabetes (151) and partially restores first-phase insulin release (142). The clinical trials with liraglutide that have been reported are relatively short (138,152), so there is insufficient information to make conclusions about chronic effects of this compound.
A long-acting microsphere-based preparation of exenatide (long-acting release exenatide, LAR-exenatide, Amylin Pharmaceuticals Inc.) has been developed to extend the half life of exendin-4. Administration of 2 mg of LAR-exenatide in 45 patients with poorly controlled type 2 diabetes who were on metformin or lifestyle intervention lowered hemoglobin A1c levels by 1.7%, relative to placebo-treated patients, and body weight by 3.7 kg over a 15-wk period (153). The advent of an extended-release form of exenatide is a view into the future of this class of drugs. There has been a broad effort to develop GLP-1 mimetics that are released into the circulation slowly after injection and have activity that extends over days to weeks. The recent findings with LAR-exenatide provide a proof-of-concept for this approach. Further work will be needed to determine the ultimate efficacy of drugs that provide protracted exposure of GLP-1r to ligand, and whether they provide other advantages to diabetic patients.
B. Dipeptidyl peptidase inhibitors
The action of DPP-IV provides a robust constraint on the incretin effect because it rapidly inactivates GLP-1 and GIP. Several compounds are currently available that provide nearly complete and long-lasting inhibition of DPP-IV, which increases the proportion of active GLP-1 and GIP from 20–30% of the total circulating incretins to 75–90% (154). One DPP-IV inhibitor, sitagliptin (Merck, Rahway, NJ), is now available to treat type 2 diabetic patients, with a number of other compounds in development; of these vildagliptin (Novartis, Basel, Switzerland) has been the subject of most research published on these drugs.
Sitagliptin is an orally available drug that can lower the measurable activity of DPP-IV by more than 95% for 12 h (154). Sitagliptin treatment causes a greater than 2-fold elevation of active GIP and GLP-1, and these are associated with increased insulin secretion, reduced glucagon levels, and improvements in both fasting and postprandial hyperglycemia. In randomized, placebo-controlled studies of sitagliptin in type 2 diabetic patients, glycemic control is improved, as reflected by decreased hemoglobin A1c by 0.5–1%, after several months of monotherapy or addition to other oral agents (154,155,156). The effects of sitagliptin have been ascribed primarily to effects on islet cell function. This drug does not cause weight loss and presumably has minimal effects on insulin sensitivity.
Vildagliptin seems to be comparable to sitagliptin as an inhibitor of DPP-IV activity and has similar effects on incretin levels and glucose control in moderately controlled type 2 diabetic patients (136). Vildagliptin improves insulin secretion and reduces plasma glucagon. Similar to exenatide, the effects of vildagliptin on glycemic control have been followed in studies of up to 52 wk. Compared with patients taking metformin who had a gradual increase in hemoglobin A1c over the course of the trial, those taking the DPP-IV inhibitor had a stable and durable reduction in average plasma glucose (136).
C. Potential of incretin-based drugs to affect β-cell mass in humans
Based on current clinical and pharmacological studies, it appears that the effects of GLP-1r agonists in humans can be extrapolated from what is known about the basic biology of the GLP-1 system. Thus, administration of exenatide, which results in the equivalence of very high levels of GLP-1, recapitulates many of the known pharmacological effects of this hormone- increased insulin secretion, decreased glucagon output, delayed gastric emptying, and reduced food intake. In addition to these positive effects, high levels of GLP-1r action can also cause the nausea that is a prominent side effect of GLP-1r agonists. In contrast, DPP-IV inhibitors only increase plasma GLP-1 and GIP into the high normal or supraphysiological range. Because DPP-IV inhibitors do not share the effects of exenatide on food intake, gastric emptying, or nausea, it has been assumed that these responses are the result of pharmacological amounts of GLP1–1r signaling. Despite significant differences in the relative amount of circulating GLP-1 activity caused by the administration of exenatide or DPP-IV inhibitors, these drugs appear to be equally efficacious for glucose lowering (137). This interesting observation suggests one of two possibilities. Either the physiological mechanisms activated by exenatide and DPP-IV inhibitors that account for improved glycemic control are not a strict function of the amount GLP-1r agonism, or DPP-IV inhibitors act by protecting factors beyond GLP-1, such as GIP.
It is not clear from animal studies whether the effects of GLP-1r signaling to promote expansion or protection of islet cell mass are dose dependent; for the most part these studies used a single dose of exendin-4. However, a single study in a mouse model of diabetes reported a graded response of islet mass and β-cell number to increasing doses of sitagliptin (119). Based on these limited findings, it is not possible to conclude whether the effects of incretin-based drugs to increase islet mass in animal models is dependent on the relative dose of GLP-1r signaling, chronic physiological increases of GLP-1 and GIP, or both.
There are no direct measures currently available to determine whether an antidiabetes drug has the ability to alter the course of T2DM by increasing β-cell mass or attenuating β-cell apoptosis. The progressive nature of diabetes in humans has been inferred from autopsy studies (16), longitudinal monitoring of glycemia (7), and functional measures of insulin secretion (13). Noninvasive imaging techniques that can assess islet mass are currently being explored but have not yet reached the sensitivity that is required for use in humans (26), and pancreatic tissue for histological examination cannot be ethically procured for research purposes only. This leaves no direct means for testing direct effects of incretin-based drugs on β-cell mass in diabetic patients. Given this limitation, the best alternative currently available to gain insights into the effects of GLP-1r agonists and DPP-IV inhibitors on the course of β-cell function in diabetes may be careful natural history studies. An excellent example of this is the recently published A Diabetes Outcome Progression Trial study (157). In this trial, patients with relatively early stage T2DM were randomized to receive one of three conventional oral agents and followed for 4 yr to assess the relative rate of decline in glycemic control. The results demonstrated that patients receiving glyburide had the most rapid decline in glycemic control, those allocated to rosiglitazone the slowest, with the group getting metformin intermediate between these two. Given the current view that progression of diabetes, as reflected in worsened glycemic control on stable therapy, is thought to be at least partially the result of loss of islet mass, these results are compatible with different levels of β-cell protection among different therapeutic agents. Therefore, the first step in ascertaining whether incretin-based drugs can affect islet mass in humans is likely to come from clinical trials such as ADOPT. Comparison of the course of diabetes in patients treated with GLP-1r agonists or DPP-IV inhibitors, using durability of effect on glycemic control as the primary outcome, would provide some insight into whether these agents have chronic effects on β-cell health. Although such studies cannot prove that the effects of incretin-based stimulation of islet mass in animals also pertain in humans, they would go a long way toward establishing the optimal role for these drugs in clinical medicine.
V. Summary
The progressive nature of type 2 diabetes is one of the major challenges in the treatment of affected patients, and agents that could alter the natural history of this condition would add greatly to current treatment approaches. The incretins, GLP-1 and GIP, appear to stimulate islet growth and protect β-cells from a number of stressors. In rodent models of diabetes, GLP-1r agonists and DPP-IV inhibitors increase islet mass and preserve β-cell function. The recent availability of these drugs in the clinic has generated excitement that the beneficial effects seen in preclinical studies will also be conferred to diabetic patients using them. Exenatide and DPP-IV inhibitors are effective for lowering blood glucose in treated patients but the mechanisms by which they work in humans are not completely understood. Although the promise of in vitro and animal studies certainly warrants continued investigation into the effects of incretin-based drugs on islet mass, at present there is not strong evidence that they alter the course of β-cell function in human diabetes.
Supplementary Material
Footnotes
Disclosure Statement: M.S. and B.A.A. have nothing to declare. D.A.D. is a consultant for Merck and Novartis and has received research grants from Amylin and Lilly.
First Published Online February 21, 2008
Abbreviations: DPP-IV, Dipeptidyl peptidase IV; GI, gastrointestinal; GIP, glucose-dependent insulinotropic polypeptide; GIPr, GIP receptor; GLP-1, glucagon-like peptide 1; PDX-1, pancreatic duodenal homeobox factor-1; PI-3K, phosphatidylinositol-3 kinase; PKA, protein kinase A; T2DM, type 2 diabetes mellitus.
References
- Porte Jr D 1991 Banting lecture 1990. β-Cells in type II diabetes mellitus. Diabetes 40:166–180 [DOI] [PubMed] [Google Scholar]
- Bergman RN, Phillips LS, Cobelli C 1981 Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and β-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68:1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, Porte Jr D 1993 Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42:1663–1672 [DOI] [PubMed] [Google Scholar]
- Kahn SE 2001 Clinical review 135: the importance of β-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 86:4047–4058 [DOI] [PubMed] [Google Scholar]
- Bell GI, Polonsky KS 2001 Diabetes mellitus and genetically programmed defects in β-cell function. Nature 414:788–791 [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA 2005 β-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 90:493–500 [DOI] [PubMed] [Google Scholar]
- 1995 U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes 44:1249–1258 [PubMed] [Google Scholar]
- Perley MJ, Kipnis DM 1967 Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects. J Clin Invest 46:1954–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky KS, Given BD, Hirsch LJ, Tillil H, Shapiro ET, Beebe C, Frank BH, Galloway JA, Van Cauter E 1988 Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. N Engl J Med 318:1231–1239 [DOI] [PubMed] [Google Scholar]
- Seltzer HS, Allen EW, Herron Jr AL, Brennan MT 1967 Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest 46:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne MM, Sturis J, Sobel RJ, Polonsky KS 1996 Elevated plasma glucose 2 h postchallenge predicts defects in β-cell function. Am J Physiol 270:E572–E579 [DOI] [PubMed] [Google Scholar]
- Nauck M, Stockmann F, Ebert R, Creutzfeldt W 1986 Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 29:46–52 [DOI] [PubMed] [Google Scholar]
- Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte Jr D 1984 Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 74:1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S 2002 Reduced β-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 45:85–96 [DOI] [PubMed] [Google Scholar]
- Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S 2003 Selective β-cell loss and α-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 88:2300–2308 [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC 2003 β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110 [DOI] [PubMed] [Google Scholar]
- Matveyenko AV, Butler PC 2006 β-Cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes 55:2106–2114 [DOI] [PubMed] [Google Scholar]
- Kahn SE, Andrikopoulos S, Verchere CB 1999 Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes 48:241–253 [DOI] [PubMed] [Google Scholar]
- Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC 2006 Relationship between β-cell mass and fasting blood glucose concentration in humans. Diabetes Care 29:717–718 [DOI] [PubMed] [Google Scholar]
- Robertson RP, Lanz KJ, Sutherland DE, Kendall DM 2001 Prevention of diabetes for up to 13 years by autoislet transplantation after pancreatectomy for chronic pancreatitis. Diabetes 50:47–50 [DOI] [PubMed] [Google Scholar]
- Rossetti L, Giaccari A, DeFronzo RA 1990 Glucose toxicity. Diabetes Care 13:610–630 [DOI] [PubMed] [Google Scholar]
- Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY 2002 Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB 2002 The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 51(Suppl 3):S434–S442 [DOI] [PubMed] [Google Scholar]
- Leahy JL, Bonner-Weir S, Weir GC 1988 Minimal chronic hyperglycemia is a critical determinant of impaired insulin secretion after an incomplete pancreatectomy. J Clin Invest 81:1407–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath MY, Halban PA 2004 Decreased β-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia 47:581–589 [DOI] [PubMed] [Google Scholar]
- Paty BW, Bonner-Weir S, Laughlin MR, McEwan AJ, Shapiro AM 2004 Toward development of imaging modalities for islets after transplantation: insights from the National Institutes of Health Workshop on β Cell Imaging. Transplantation 77:1133–1137 [DOI] [PubMed] [Google Scholar]
- Hosokawa YA, Hosokawa H, Chen C, Leahy JL 1996 Mechanism of impaired glucose-potentiated insulin secretion in diabetic 90% pancreatectomy rats. Study using glucagonlike peptide-1 (7-37). J Clin Invest 97:180–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S 2000 Islet growth and development in the adult. J Mol Endocrinol 24:297–302 [DOI] [PubMed] [Google Scholar]
- Sorenson RL, Brelje TC, Hegre OD, Marshall S, Anaya P, Sheridan JD 1987 Prolactin (in vitro) decreases the glucose stimulation threshold, enhances insulin secretion, and increases dye coupling among islet B cells. Endocrinology 121:1447–1453 [DOI] [PubMed] [Google Scholar]
- Bruning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR 1997 Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 88:561–572 [DOI] [PubMed] [Google Scholar]
- Lee HC, Bonner-Weir S, Weir GC, Leahy JL 1989 Compensatory adaption to partial pancreatectomy in the rat. Endocrinology 124:1571–1575 [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W, Ebert R 1985 New developments in the incretin concept. Diabetologia 28:565–573 [DOI] [PubMed] [Google Scholar]
- McIntyre N, Holdsworth CD, Turner DS 1964 New interpretation of oral glucose tolerance. Lancet 41:20–21 [DOI] [PubMed] [Google Scholar]
- D'Alessio DA 2002 Incretins: glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1. In: Porte Jr D, Sherwin RS, Baron A, ed. Ellenberg, Rifkins' diabetes mellitus. 6th ed. New York: McGraw-Hill; 85–96 [Google Scholar]
- Wolfe MM, Boylan MO, Kieffer TJ, Tseng C-C 1999 Glucose-dependent insulinotropic polypeptide (GIP): incretin vs enterogastrone. In: Greeley GH, ed. Gastrointestinal endocrinology. Totowa, NJ: Humana Press; 439 [Google Scholar]
- Schirra J, Katschinski M, Weidmann C, Schafer T, Wank U, Arnold R, Goke B 1996 Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 97:92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert R, Creutzfeldt W 1987 Gastrointestinal peptides and insulin secretion. Diabetes Metab Rev 3:1–26 [DOI] [PubMed] [Google Scholar]
- Kieffer TJ, McIntosh CH, Pederson RA 1995 Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 136:3585–3596 [DOI] [PubMed] [Google Scholar]
- Finegood DT, Scaglia L, Bonner-Weir S 1995 Dynamics of β-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 44:249–256 [DOI] [PubMed] [Google Scholar]
- Drucker DJ 2006 The biology of incretin hormones. Cell Metab 3:153–165 [DOI] [PubMed] [Google Scholar]
- Bollag RJ, Zhong Q, Phillips P, Min L, Zhong L, Cameron R, Mulloy AL, Rasmussen H, Qin F, Ding KH, Isales CM 2000 Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology 141:1228–1235 [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W, Nauck M 1992 Gut hormones and diabetes mellitus. Diabetes Metab Rev 8:149–177 [DOI] [PubMed] [Google Scholar]
- Irwin N, Gault VA, Green BD, Greer B, McCluskey JT, Harriott P, O'Harte FP, Flatt PR 2004 Effects of short-term chemical ablation of the GIP receptor on insulin secretion, islet morphology and glucose homeostasis in mice. Biol Chem 385:845–852 [DOI] [PubMed] [Google Scholar]
- Tseng CC, Kieffer TJ, Jarboe LA, Usdin TB, Wolfe MM 1996 Postprandial stimulation of insulin release by glucose-dependent insulinotropic polypeptide (GIP). Effect of a specific glucose-dependent insulinotropic polypeptide receptor antagonist in the rat. J Clin Invest 98:2440–2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki K, Yamada Y, Yano H, Niwa H, Ban N, Ihara Y, Kubota A, Fujimoto S, Kajikawa M, Kuroe A, Tsuda K, Hashimoto H, Yamashita T, Jomori T, Tashiro F, Miyazaki J, Seino Y 1999 Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci USA 96:14843–14847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer TJ, Habener JF 1999 The glucagon-like peptides. Endocr Rev 20:876–913 [DOI] [PubMed] [Google Scholar]
- Vilsboll T, Krarup T, Sonne J, Madsbad S, Volund A, Juul AG, Holst JJ 2003 Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab 88:2706–2713 [DOI] [PubMed] [Google Scholar]
- Mentlein R, Gallwitz B, Schmidt WE 1993 Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1 (7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem 214:829–835 [DOI] [PubMed] [Google Scholar]
- Quddusi S, Vahl TP, Hanson K, Prigeon RL, D'Alessio DA 2003 Differential effects of acute and extended infusions of glucagon-like peptide-1 on first- and second-phase insulin secretion in diabetic and nondiabetic humans. Diabetes Care 26:791–798 [DOI] [PubMed] [Google Scholar]
- Bullock BP, Heller RS, Habener JF 1996 Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide- 1 receptor. Endocrinology 137:2968–2978 [DOI] [PubMed] [Google Scholar]
- Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K 2004 Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci 110:36–43 [DOI] [PubMed] [Google Scholar]
- Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D'Alessio DA 2007 GLP-1 receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148:4965–4973 [DOI] [PubMed] [Google Scholar]
- Gromada J, Bokvist K, Ding WG, Holst JJ, Nielsen JH, Rorsman P 1998 Glucagon-like peptide 1 (7-36) amide stimulates exocytosis in human pancreatic β-cells by both proximal and distal regulatory steps in stimulus-secretion coupling. Diabetes 47:57–65 [DOI] [PubMed] [Google Scholar]
- Wang Y, Perfetti R, Greig NH, Holloway HW, DeOre KA, Montrose-Rafizadeh C, Elahi D, Egan JM 1997 Glucagon-like peptide-1 can reverse the age-related decline in glucose tolerance in rats. J Clin Invest 99:2883–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ 1996 Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 2:1254–1258 [DOI] [PubMed] [Google Scholar]
- Burcelin R, Da Costa A, Drucker D, Thorens B 2001 Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes 50:1720–1728 [DOI] [PubMed] [Google Scholar]
- Buteau J, Foisy S, Joly E, Prentki M 2003 Glucagon-like peptide 1 induces pancreatic β-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes 52:124–132 [DOI] [PubMed] [Google Scholar]
- De Leon DD, Deng S, Madani R, Ahima RS, Drucker DJ, Stoffers DA 2003 Role of endogenous glucagon-like peptide-1 in islet regeneration after partial pancreatectomy. Diabetes 52:365–371 [DOI] [PubMed] [Google Scholar]
- Pamir N, Lynn FC, Buchan AM, Ehses J, Hinke SA, Pospisilik JA, Miyawaki K, Yamada Y, Seino Y, McIntosh CH, Pederson RA 2003 Glucose-dependent insulinotropic polypeptide receptor null mice exhibit compensatory changes in the enteroinsular axis. Am J Physiol Endocrinol Metab 284:E931–E939 [DOI] [PubMed] [Google Scholar]
- Pederson RA, Satkunarajah M, McIntosh CH, Scrocchi LA, Flamez D, Schuit F, Drucker DJ, Wheeler MB 1998 Enhanced glucose-dependent insulinotropic polypeptide secretion and insulinotropic action in glucagon-like peptide 1 receptor −/− mice. Diabetes 47:1046–1052 [DOI] [PubMed] [Google Scholar]
- Falko JM, Crockett SE, Cataland S, Mazzaferri EL 1975 Gastric inhibitory polypeptide (GIP) stimulated by fat ingestion in man. J Clin Endocrinol Metab 41:260–265 [DOI] [PubMed] [Google Scholar]
- Ross SA, Dupre J 1978 Effects of ingestion of triglyceride or galactose on secretion of gastric inhibitory polypeptide and on responses to intravenous glucose in normal and diabetic subjects. Diabetes 27:327–333 [DOI] [PubMed] [Google Scholar]
- Eissele R, Goke R, Willemer S, Harthus HP, Vermeer H, Arnold R, Goke B 1992 Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest 22:283–291 [DOI] [PubMed] [Google Scholar]
- Hansen L, Lampert S, Mineo H, Holst JJ 2004 Neural regulation of glucagon-like peptide-1 secretion in pigs. Am J Physiol Endocrinol Metab 287:E939–E947 [DOI] [PubMed] [Google Scholar]
- Rocca AS, Brubaker PL 1999 Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 140:1687–1694 [DOI] [PubMed] [Google Scholar]
- Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ 2001 Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 50:609–613 [DOI] [PubMed] [Google Scholar]
- Hansen L, Deacon CF, Orskov C, Holst JJ 1999 Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 140:5356–5363 [DOI] [PubMed] [Google Scholar]
- Gault VA, Irwin N, Green BD, McCluskey JT, Greer B, Bailey CJ, Harriott P, O'Harte FP, Flatt PR 2005 Chemical ablation of gastric inhibitory polypeptide receptor action by daily (Pro3)GIP administration improves glucose tolerance and ameliorates insulin resistance and abnormalities of islet structure in obesity-related diabetes. Diabetes 54:2436–2446 [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, Hiai H, Mizunoya W, Fushiki T, Holst JJ, Makino M, Tashita A, Kobara Y, Tsubamoto Y, Jinnouchi T, Jomori T, Seino Y 2002 Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 8:738–742 [DOI] [PubMed] [Google Scholar]
- Tsukiyama K, Yamada Y, Yamada C, Harada N, Kawasaki Y, Ogura M, Bessho K, Li M, Amizuka N, Sato M, Udagawa N, Takahashi N, Tanaka K, Oiso Y, Seino Y 2006 Gastric inhibitory polypeptide as an endogenous factor promoting new bone formation after food ingestion. Mol Endocrinol 20:1644–1651 [DOI] [PubMed] [Google Scholar]
- Xie D, Zhong Q, Ding KH, Cheng H, Williams S, Correa D, Bollag WB, Bollag RJ, Insogna K, Troiano N, Coady C, Hamrick M, Isales CM 2007 Glucose-dependent insulinotropic peptide-overexpressing transgenic mice have increased bone mass. Bone 40:1352–1360 [DOI] [PubMed] [Google Scholar]
- Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ, Goke B 2006 Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut 55:243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, Schmiegel WH 1997 Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol 273:E981–E988 [DOI] [PubMed] [Google Scholar]
- Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ 1993 Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci 38:665–673 [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Astrup A, Holst JJ 1998 Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 101:515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigeon RL, Quddusi S, Paty B, D'Alessio DA 2003 Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab 285:E701–E707 [DOI] [PubMed] [Google Scholar]
- D'Alessio DA, Kahn SE, Leusner CR, Ensinck JW 1994 Glucagon-like peptide 1 enhances glucose tolerance both by stimulation of insulin release and by increasing insulin-independent glucose disposal. J Clin Invest 93:2263–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan JM, Meneilly GS, Habener JF, Elahi D 2002 Glucagon-like peptide-1 augments insulin-mediated glucose uptake in the obese state. J Clin Endocrinol Metab 87:3768–3773 [DOI] [PubMed] [Google Scholar]
- Krarup T 1988 Immunoreactive gastric inhibitory polypeptide. Endocr Rev 9:122–134 [DOI] [PubMed] [Google Scholar]
- Orskov C, Jeppesen J, Madsbad S, Holst JJ 1991 Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest 87:415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukase N, Manaka H, Sugiyama K, Takahashi H, Igarashi M, Daimon M, Yamatani K, Tominaga M, Sasaki H 1995 Response of truncated glucagon-like peptide-1 and gastric inhibitory polypeptide to glucose ingestion in non-insulin dependent diabetes mellitus. Effect of sulfonylurea therapy. Acta Diabetol 32:165–169 [DOI] [PubMed] [Google Scholar]
- Vaag AA, Holst JJ, Volund A, Beck-Nielsen HB 1996 Gut incretin hormones in identical twins discordant for non-insulin-dependent diabetes mellitus (NIDDM)–evidence for decreased glucagon-like peptide 1 secretion during oral glucose ingestion in NIDDM twins. Eur J Endocrinol 135:425–432 [DOI] [PubMed] [Google Scholar]
- Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ 2001 Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 86:3717–3723 [DOI] [PubMed] [Google Scholar]
- Knop FK, Vilsboll T, Hojberg PV, Larsen S, Madsbad S, Volund A, Holst JJ, Krarup T 2007 Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 56:1951–1959 [DOI] [PubMed] [Google Scholar]
- Ryskjaer J, Deacon CF, Carr RD, Krarup T, Madsbad S, Holst J, Vilsboll T 2006 Plasma dipeptidyl peptidase-IV activity in patients with type-2 diabetes mellitus correlates positively with HbAlc levels, but is not acutely affected by food intake. Eur J Endocrinol 155:485–493 [DOI] [PubMed] [Google Scholar]
- O'Donovan DG, Doran S, Feinle-Bisset C, Jones KL, Meyer JH, Wishart JM, Morris HA, Horowitz M 2004 Effect of variations in small intestinal glucose delivery on plasma glucose, insulin, and incretin hormones in healthy subjects and type 2 diabetes. J Clin Endocrinol Metab 89:3431–3435 [DOI] [PubMed] [Google Scholar]
- Elahi D, McAloon-Dyke M, Fukagawa NK, Meneilly GS, Sclater AL, Minaker KL, Habener JF, Andersen DK 1994 The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7-37) in normal and diabetic subjects. Regul Pept 51:63–74 [DOI] [PubMed] [Google Scholar]
- Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W 1993 Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 91:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krarup T, Saurbrey N, Moody AJ, Kuhl C, Madsbad S 1987 Effect of porcine gastric inhibitory polypeptide on β-cell function in type I and type II diabetes mellitus. Metabolism 36:677–682 [DOI] [PubMed] [Google Scholar]
- Meier JJ, Gallwitz B, Askenas M, Vollmer K, Deacon CF, Holst JJ, Schmidt WE, Nauck MA 2005 Secretion of incretin hormones and the insulinotropic effect of gastric inhibitory polypeptide in women with a history of gestational diabetes. Diabetologia 48:1872–1881 [DOI] [PubMed] [Google Scholar]
- Meier JJ, Gallwitz B, Kask B, Deacon CF, Holst JJ, Schmidt WE, Nauck MA 2004 Stimulation of insulin secretion by intravenous bolus injection and continuous infusion of gastric inhibitory polypeptide in patients with type 2 diabetes and healthy control subjects. Diabetes 53(Suppl 3):S220–S224 [DOI] [PubMed] [Google Scholar]
- Rachman J, Gribble FM, Barrow BA, Levy JC, Buchanan KD, Turner RC 1996 Normalization of insulin responses to glucose by overnight infusion of glucagon-like peptide 1 (7-36) amide in patients with NIDDM. Diabetes 45:1524–1530 [DOI] [PubMed] [Google Scholar]
- Rachman J, Barrow BA, Levy JC, Turner RC 1997 Near-normalisation of diurnal glucose concentrations by continuous administration of glucagon-like peptide-1 (GLP-1) in subjects with NIDDM. Diabetologia 40:205–211 [DOI] [PubMed] [Google Scholar]
- Kjems LL, Holst JJ, Volund A, Madsbad S 2003 The influence of GLP-1 on glucose-stimulated insulin secretion: effects on β-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 52:380–386 [DOI] [PubMed] [Google Scholar]
- Ahren B, Larsson H, Holst JJ 1997 Effects of glucagon-like peptide-1 on islet function and insulin sensitivity in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 82:473–478 [DOI] [PubMed] [Google Scholar]
- Zander M, Madsbad S, Madsen JL, Holst JJ 2002 Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: a parallel-group study. Lancet 359:824–830 [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA 2004 Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429:41–46 [DOI] [PubMed] [Google Scholar]
- Dor Y 2006 β-Cell proliferation is the major source of new pancreatic β cells. Nat Clin Pract Endocrinol Metab 2:242–243 [DOI] [PubMed] [Google Scholar]
- Teta M, Rankin MM, Long SY, Stein GM, Kushner JA 2007 Growth and regeneration of adult β cells does not involve specialized progenitors. Dev Cell 12:817–826 [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF 1987 Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci USA 84:3434–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglund G, Hussain MA, Holz GG 2000 Glucagon-like peptide 1 stimulates insulin gene promoter activity by protein kinase A-independent activation of the rat insulin I gene cAMP response element. Diabetes 49:1156–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhou J, Doyle ME, Egan JM 2001 Glucagon-like peptide-1 causes pancreatic duodenal homeobox-1 protein translocation from the cytoplasm to the nucleus of pancreatic β-cells by a cyclic adenosine monophosphate/protein kinase A-dependent mechanism. Endocrinology 142:1820–1827 [DOI] [PubMed] [Google Scholar]
- Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG 2006 Cell physiology of cAMP sensor Epac. J Physiol 577:5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, Prentki M 2001 Protein kinase Cζ activation mediates glucagon-like peptide-1-induced pancreatic β-cell proliferation. Diabetes 50:2237–2243 [DOI] [PubMed] [Google Scholar]
- Wang Q, Brubaker PL 2002 Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia 45:1263–1273 [DOI] [PubMed] [Google Scholar]
- Wang H, Iezzi M, Theander S, Antinozzi PA, Gauthier BR, Halban PA, Wollheim CB 2005 Suppression of Pdx-1 perturbs proinsulin processing, insulin secretion and GLP-1 signalling in INS-1 cells. Diabetologia 48:720–731 [DOI] [PubMed] [Google Scholar]
- Bregenholt S, Moldrup A, Blume N, Karlsen AE, Nissen Friedrichsen B, Tornhave D, Knudsen LB, Petersen JS 2005 The long-acting glucagon-like peptide-1 analogue, liraglutide, inhibits β-cell apoptosis in vitro. Biochem Biophys Res Commun 330:577–584 [DOI] [PubMed] [Google Scholar]
- Lawrence MC, Bhatt HS, Easom RA 2002 NFAT regulates insulin gene promoter activity in response to synergistic pathways induced by glucose and glucagon-like peptide-1. Diabetes 51:691–698 [DOI] [PubMed] [Google Scholar]
- Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, Crabtree GR, Kim SK 2006 Calcineurin/NFAT signalling regulates pancreatic β-cell growth and function. Nature 443:345–349 [DOI] [PubMed] [Google Scholar]
- Hui H, Wright C, Perfetti R 2001 Glucagon-like peptide 1 induces differentiation of islet duodenal homeobox-1-positive pancreatic ductal cells into insulin-secreting cells. Diabetes 50:785–796 [DOI] [PubMed] [Google Scholar]
- Bulotta A, Hui H, Anastasi E, Bertolotto C, Boros LG, Di Mario U, Perfetti R 2002 Cultured pancreatic ductal cells undergo cell cycle re-distribution and β-cell-like differentiation in response to glucagon-like peptide-1. J Mol Endocrinol 29:347–360 [DOI] [PubMed] [Google Scholar]
- Abraham EJ, Leech CA, Lin JC, Zulewski H, Habener JF 2002 Insulinotropic hormone glucagon-like peptide-1 differentiation of human pancreatic islet-derived progenitor cells into insulin- producing cells. Endocrinology 143:3152–3161 [DOI] [PubMed] [Google Scholar]
- Gomez E, Pritchard C, Herbert TP 2002 cAMP-dependent protein kinase and Ca2+ influx through L-type voltage-gated calcium channels mediate Raf-independent activation of extracellular regulated kinase in response to glucagon-like peptide-1 in pancreatic β-cells. J Biol Chem 277:48146–48151 [DOI] [PubMed] [Google Scholar]
- Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, Egan JM 2000 Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes 49:741–748 [DOI] [PubMed] [Google Scholar]
- Xu G, Stoffers DA, Habener JF, Bonner-Weir S 1999 Exendin-4 stimulates both β-cell replication and neogenesis, resulting in increased β-cell mass and improved glucose tolerance in diabetic rats. Diabetes 48:2270–2276 [DOI] [PubMed] [Google Scholar]
- Hui H, Nourparvar A, Zhao X, Perfetti R 2003 Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting cells via a cyclic 5′-adenosine monophosphate-dependent protein kinase A- and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology 144:1444–1455 [DOI] [PubMed] [Google Scholar]
- Pospisilik JA, Stafford SG, Demuth HU, Brownsey R, Parkhouse W, Finegood DT, McIntosh CH, Pederson RA 2002 Long-term treatment with the dipeptidyl peptidase IV inhibitor P32/98 causes sustained improvements in glucose tolerance, insulin sensitivity, hyperinsulinemia, and β-cell glucose responsiveness in VDF (fa/fa) Zucker rats. Diabetes 51:943–950 [DOI] [PubMed] [Google Scholar]
- Doyle ME, Egan JM 2007 Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther 113:546–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buteau J, Spatz ML, Accili D 2006 Transcription factor FoxO1 mediates glucagon-like peptide-1 effects on pancreatic β-cell mass. Diabetes 55:1190–1196 [DOI] [PubMed] [Google Scholar]
- Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, Perfetti R 2002 Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology 143:4397–4408 [DOI] [PubMed] [Google Scholar]
- Perfetti R, Zhou J, Doyle ME, Egan JM 2000 Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology 141:4600–4605 [DOI] [PubMed] [Google Scholar]
- Soltani N, Kumar M, Glinka Y, Prud'homme GJ, Wang Q 2007 In vivo expression of GLP-1/IgG-Fc fusion protein enhances β-cell mass and protects against streptozotocin-induced diabetes. Gene Ther 14:981–988 [DOI] [PubMed] [Google Scholar]
- Tourrel C, Bailbe D, Meile MJ, Kergoat M, Portha B 2001 Glucagon-like peptide-1 and exendin-4 stimulate β-cell neogenesis in streptozotocin-treated newborn rats resulting in persistently improved glucose homeostasis at adult age. Diabetes 50:1562–1570 [DOI] [PubMed] [Google Scholar]
- Sharma A, Sorenby A, Wernerson A, Efendic S, Kumagai-Braesch M, Tibell A 2006 Exendin-4 treatment improves metabolic control after rat islet transplantation to athymic mice with streptozotocin-induced diabetes. Diabetologia 49:1247–1253 [DOI] [PubMed] [Google Scholar]
- Suen PM, Li K, Chan JC, Leung PS 2006 In vivo treatment with glucagon-like peptide 1 promotes the graft function of fetal islet-like cell clusters in transplanted mice. Int J Biochem Cell Biol 38:951–960 [DOI] [PubMed] [Google Scholar]
- Wideman RD, Yu IL, Webber TD, Verchere CB, Johnson JD, Cheung AT, Kieffer TJ 2006 Improving function and survival of pancreatic islets by endogenous production of glucagon-like peptide 1 (GLP-1). Proc Natl Acad Sci USA 103:13468–13473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PC, Meier JJ, Butler AE, Bhushan A 2007 The replication of β cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab 3:758–768 [DOI] [PubMed] [Google Scholar]
- Ghofaili KA, Fung M, Ao Z, Meloche M, Shapiro RJ, Warnock GL, Elahi D, Meneilly GS, Thompson DM 2007 Effect of exenatide on β cell function after islet transplantation in type 1 diabetes. Transplantation 83:24–28 [DOI] [PubMed] [Google Scholar]
- Amiranoff B, Vauclin-Jacques N, Laburthe M 1984 Functional GIP receptors in a hamster pancreatic β cell line, In 111: specific binding and biological effects. Biochem Biophys Res Commun 123:671–676 [DOI] [PubMed] [Google Scholar]
- Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H, Seino S 2001 Critical role of cAMP-GEFII–Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem 276:46046–46053 [DOI] [PubMed] [Google Scholar]
- Trumper A, Trumper K, Trusheim H, Arnold R, Goke B, Horsch D 2001 Glucose-dependent insulinotropic polypeptide is a growth factor for β (INS-1) cells by pleiotropic signaling. Mol Endocrinol 15:1559–1570 [DOI] [PubMed] [Google Scholar]
- Ehses JA, Casilla VR, Doty T, Pospisilik JA, Winter KD, Demuth HU, Pederson RA, McIntosh CH 2003 Glucose-dependent insulinotropic polypeptide promotes β-(INS-1) cell survival via cyclic adenosine monophosphate-mediated caspase-3 inhibition and regulation of p38 mitogen-activated protein kinase. Endocrinology 144:4433–4445 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD 2005 Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 28:1092–1100 [DOI] [PubMed] [Google Scholar]
- Trumper A, Trumper K, Horsch D 2002 Mechanisms of mitogenic and anti-apoptotic signaling by glucose-dependent insulinotropic polypeptide in β (INS-1)-cells. J Endocrinol 174:233–246 [DOI] [PubMed] [Google Scholar]
- Conarello SL, Li Z, Ronan J, Roy RS, Zhu L, Jiang G, Liu F, Woods J, Zycband E, Moller DE, Thornberry NA, Zhang BB 2003 Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci USA 100:6825–6830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahren B, Gomis R, Standl E, Mills D, Schweizer A 2004 Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care 27:2874–2880 [DOI] [PubMed] [Google Scholar]
- Amori RE, Lau J, Pittas AG 2007 Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 298:194–206 [DOI] [PubMed] [Google Scholar]
- Harder H, Nielsen L, Tu DT, Astrup A 2004 The effect of liraglutide, a long-acting glucagon-like peptide 1 derivative, on glycemic control, body composition, and 24-h energy expenditure in patients with type 2 diabetes. Diabetes Care 27:1915–1921 [DOI] [PubMed] [Google Scholar]
- Kolterman OG, Kim DD, Shen L, Ruggles JA, Nielsen LL, Fineman MS, Baron AD 2005 Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm 62:173–181 [DOI] [PubMed] [Google Scholar]
- Edwards CM, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, Ghatei MA, Bloom SR 2001 Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 281:E155–E161 [DOI] [PubMed] [Google Scholar]
- Egan JM, Clocquet AR, Elahi D 2002 The insulinotropic effect of acute exendin-4 administered to humans: comparison of nondiabetic state to type 2 diabetes. J Clin Endocrinol Metab 87:1282–1290 [DOI] [PubMed] [Google Scholar]
- Degn KB, Brock B, Juhl CB, Djurhuus CB, Grubert J, Kim D, Han J, Taylor K, Fineman M, Schmitz O 2004 Effect of intravenous infusion of exenatide (synthetic exendin-4) on glucose-dependent insulin secretion and counterregulation during hypoglycemia. Diabetes 53:2397–2403 [DOI] [PubMed] [Google Scholar]
- Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, Taylor K, Kim D, Aisporna M, Wang Y, Baron AD 2003 Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 88:3082–3089 [DOI] [PubMed] [Google Scholar]
- Fehse F, Trautmann M, Holst JJ, Halseth AE, Nanayakkara N, Nielsen LL, Fineman MS, Kim DD, Nauck MA 2005 Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 90:5991–5997 [DOI] [PubMed] [Google Scholar]
- Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD 2004 Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 27:2628–2635 [DOI] [PubMed] [Google Scholar]
- Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD 2005 Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 28:1083–1091 [DOI] [PubMed] [Google Scholar]
- Zinman B, Hoogwerf BJ, Duran Garcia S, Milton DR, Giaconia JM, Kim DD, Trautmann ME, Brodows RG 2007 The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 146:477–485 [DOI] [PubMed] [Google Scholar]
- Buse JB, Klonoff DC, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Maggs DG, Wintle ME 2007 Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther 29:139–153 [DOI] [PubMed] [Google Scholar]
- Agerso H, Jensen LB, Elbrond B, Rolan P, Zdravkovic M 2002 The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia 45:195–202 [DOI] [PubMed] [Google Scholar]
- Juhl CB, Hollingdal M, Sturis J, Jakobsen G, Agerso H, Veldhuis J, Porksen N, Schmitz O 2002 Bedtime administration of NN2211, a long-acting GLP-1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes 51:424–429 [DOI] [PubMed] [Google Scholar]
- Chang AM, Jakobsen G, Sturis J, Smith MJ, Bloem CJ, An B, Galecki A, Halter JB 2003 The GLP-1 derivative NN2211 restores β-cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes 52:1786–1791 [DOI] [PubMed] [Google Scholar]
- Madsbad S, Schmitz O, Ranstam J, Jakobsen G, Matthews DR 2004 Improved glycemic control with no weight increase in patients with type 2 diabetes after once-daily treatment with the long-acting glucagon-like peptide 1 analog liraglutide (NN2211): a 12-week, double-blind, randomized, controlled trial. Diabetes Care 27:1335–1342 [DOI] [PubMed] [Google Scholar]
- Kim D, MacConell L, Zhuang D, Kothare PA, Trautmann M, Fineman M, Taylor K 2007 Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 30:1487–1493 [DOI] [PubMed] [Google Scholar]
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE 2006 Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 29:2632–2637 [DOI] [PubMed] [Google Scholar]
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G 2006 Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 29:2638–2643 [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P 2006 Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 28:1556–1568 [DOI] [PubMed] [Google Scholar]
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G 2006 Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443 [DOI] [PubMed] [Google Scholar]
- Zhang J, Tokui Y, Yamagata K, Kozawa J, Sayama K, Iwahashi H, Okita K, Miuchi M, Konya H, Hamaguchi T, Namba M, Shimomura I, Miyagawa JI 2007 Continuous stimulation of human glucagon-like peptide-1 (7-36) amide in a mouse model (NOD) delays onset of autoimmune type 1 diabetes. Diabetologia 50:1900–1909 [DOI] [PubMed] [Google Scholar]
- Patriti A, Aisa MC, Annetti C, Sidoni A, Galli F, Ferri I, Gulla N, Donini A 2007 How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and β-cell function in Goto-kakizaki rats through an enhanced proglucagon gene expression and L-cell number. Surgery 142:74–85 [DOI] [PubMed] [Google Scholar]
- Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ 2003 Glucagon-like peptide-1 receptor signaling modulates β cell apoptosis. J Biol Chem 278:471–478 [DOI] [PubMed] [Google Scholar]
- Gedulin BR, Nikoulina SE, Smith PA, Gedulin G, Nielsen LL, Baron AD, Parkes DG, Young AA 2005 Exenatide (exendin-4) improves insulin sensitivity and β-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology 146:2069–2076 [DOI] [PubMed] [Google Scholar]
- Mu J, Woods J, Zhou YP, Roy RS, Li Z, Zycband E, Feng Y, Zhu L, Li C, Howard AD, Moller DE, Thornberry NA, Zhang BB 2006 Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic β-cell mass and function in a rodent model of type 2 diabetes. Diabetes 55:1695–1704 [DOI] [PubMed] [Google Scholar]
- Reimer MK, Holst JJ, Ahren B 2002 Long-term inhibition of dipeptidyl peptidase IV improves glucose tolerance and preserves islet function in mice. Eur J Endocrinol 146:717–727 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.