Abstract
Glucotoxicity, lipotoxicity, and glucolipotoxicity are secondary phenomena that are proposed to play a role in all forms of type 2 diabetes. The underlying concept is that once the primary pathogenesis of diabetes is established, probably involving both genetic and environmental forces, hyperglycemia and very commonly hyperlipidemia ensue and thereafter exert additional damaging or toxic effects on the β-cell. In addition to their contribution to the deterioration of β-cell function after the onset of the disease, elevations of plasma fatty acid levels that often accompany insulin resistance may, as glucose levels begin to rise outside of the normal range, also play a pathogenic role in the early stages of the disease. Because hyperglycemia is a prerequisite for lipotoxicity to occur, the term glucolipotoxicity, rather than lipotoxicity, is more appropriate to describe deleterious effects of lipids on β-cell function. In vitro and in vivo evidence supporting the concept of glucotoxicity is presented first, as well as a description of the underlying mechanisms with an emphasis on the role of oxidative stress. Second, we discuss the functional manifestations of glucolipotoxicity on insulin secretion, insulin gene expression, and β-cell death, and the role of glucose in the mechanisms of glucolipotoxicity. Finally, we attempt to define the role of these phenomena in the natural history of β-cell compensation, decompensation, and failure during the course of type 2 diabetes.
I. Glucotoxicity, Lipotoxicity, and Glucolipotoxicity: Definitions and Concepts
- II. Glucotoxicity and Its Linkage to Oxidative Stress
- A. In vitro evidence
- B. In vivo evidence
- C. Mechanisms for reactive oxygen species formation by glucose and for associated loss of insulin promoter transcription factors
- D. Clinical correlations between oxidative stress and glucotoxicity of the β-cell
- III. Glucolipotoxicity
- A. Functional manifestations of glucolipotoxicity in vitro
- B. In vivo evidence for glucolipotoxicity
- C. Studies in humans
- D. The influence of glucose on lipid metabolism and its consequences for the mechanisms of glucolipotoxicity
- IV. The Glucolipoadaptation-Glucolipotoxicity Spectrum and the Implications for the Pathogenesis of β-Cell Dysfunction in Type 2 Diabetes
- A. β-Cell compensation for insulin resistance
- B. β-Cell decompensation and failure
I. Glucotoxicity, Lipotoxicity, and Glucolipotoxicity: Definitions and Concepts
TYPE 2 DIABETES is considered to be a complex syndrome of polygenic nature (1). Several genome-wide scans recently identified risk loci for type 2 diabetes that include gene controlling β-cell development or function, supporting the concept that the genetic susceptibility of the β-cell determines the risk of developing the disease (2,3,4,5,6,7).
Glucotoxicity, lipotoxicity, and glucolipotoxicity are secondary phenomena that are proposed to play a role in all forms of type 2 diabetes. The underlying concept is that once the primary pathogenesis of diabetes is established, probably involving both genetic and environmental forces, hyperglycemia and very commonly hyperlipidemia ensue and thereafter exert additional damaging or toxic effects on the β-cell. In addition to their contribution to the deterioration of β-cell function after the onset of the disease, elevations of plasma fatty acid levels that often accompany insulin resistance (8,9) may, as glucose levels begin to rise outside of the normal range, also play a pathogenic role in the early stages of the disease (10).
The words “glucotoxicity” and “lipotoxicity,” as well as their combination form, “glucolipotoxicity,” are best described as medical jargon rather than scientific terms that can be precisely defined. They represent currently popular concepts that connote adverse or toxic influences on pancreatic β-cell function caused by excessive glucose and/or lipids. In a sense, these are paradoxical concepts because physiological levels of glucose and lipids are not toxic but instead are essential to normal β-cell function. Thus, a spectrum exists going from normoglycemic and normolipidemic conditions to hyperglycemic and hyperlipidemic abnormalities that must be taken into account. The root of these words, “toxicity,” implies damage and leads to the consideration that in certain instances glucose and lipid levels entering or synthesized within tissues might become so elevated that they can cause pathology.
Unger and colleagues first introduced the concepts of glucotoxicity (11) and lipotoxicity (12). In their initial glucose toxicity paper, they put forward the concept that continuous overstimulation of the β-cell by glucose could eventually lead to depletion of insulin stores, worsening of hyperglycemia, and finally deterioration of β-cell function. Exposure of the β-cell to excessive levels of lipids was also hypothesized to be a cause of worsening β-cell function. In this view, failure to correct hyperglycemia and hyperlipidemia dooms the β-cell to a continual onslaught of glucotoxicity and lipotoxicity that perpetuates the downward spiral to β-cell dysfunction and death.
The term glucolipotoxicity has a more recent origin (13) and was coined in recognition of the fact that the alterations in intracellular lipid partitioning underlying the mechanisms of lipotoxicity are dependent upon elevated glucose levels. Consequently, it is our view that hyperglycemia is a prerequisite for lipotoxicity to occur, and that therefore the term glucolipotoxicity, rather than lipotoxicity, is more appropriate to describe deleterious effects of lipids on β-cell function.
This review of the current literature and studies performed in our laboratories will first present experimental evidence in support of glucotoxicity and glucolipotoxicity. We will then propose the existence of a spectrum from glucolipoadaptation to glucolipotoxicity and attempt to define the role of these phenomena in the natural history of β-cell compensation, decompensation, and failure during the course of type 2 diabetes. In accordance with editorial guidelines for this Recent Progress in Hormone Research (RPHR) edition, this review mostly focuses on the work performed in our own laboratories. Although we have attempted to quote the relevant literature from other groups, we could not, due to space limitations, exhaustively cite the work of all other investigators who contributed to this field.
II. Glucotoxicity and Its Linkage to Oxidative Stress
A. In vitro evidence
1. β-Cell lines.
Most of the earliest work demonstrating the paradoxical ability of glucose to diminish β-cell function was reported from experiments using β-cell lines. A key design feature in these experiments was to expose cells to media containing high concentrations of glucose for a protracted period of time, as long as 3–12 months. For example, using HIT-T15 cells and glucose concentrations of 0.8 and 11.1 mm, we observed that prolonged culturing of cells in RPMI 1640 medium containing the lower glucose concentration maintained insulin mRNA levels, insulin content, and glucose-induced insulin secretion. On the other hand, insulin gene expression, insulin content, and glucose-induced insulin secretion were progressively and drastically compromised over time when the higher glucose concentration was used in the media (14). Subsequent observations revealed that culturing in media with high glucose concentrations also caused deterioration in insulin promoter activity as well as pancreas-duodenum homeobox-1 (PDX-1) and MafA binding activity (15,16,17). It is noteworthy that loss of MafA binding occurred much earlier than the loss of PDX-1 binding and that the decrease in insulin content correlated in time much closer to the loss of MafA binding (18,19). The decreased levels of insulin mRNA, insulin content, and insulin release were taken as evidence that chronic exposure to high glucose concentrations caused glucotoxic effects on the β-cells. In later experiments it was shown that the diminution in β-cell function observed in cultures containing high glucose could be reversed by switching to low glucose; however, the efficacy of this intervention was time-dependent. β-Cell function returned when the intervention was performed 5 or 10 wk after glucotoxic defects were present, but not if a longer period of time had elapsed (19). Differentiation of glucotoxic effects from β-cell exhaustion was provided by experiments in which HIT-T15 cells were cultured for long periods of time in media containing 0.8 or 11.1 mm glucose with and without somatostatin, an inhibitor of insulin secretion. The cells cultured with somatostatin had dramatically less insulin in the culture media, demonstrating β-cell rest. Yet, the cells exposed to high glucose and somatostatin still experienced glucotoxic effects on insulin gene expression, content, and glucose-stimulated insulin secretion, thereby eliminating β-cell exhaustion as a cause (20).
Another β-cell line, the INS-1 cell, was also used in similar experiments. In this case, however, the paradoxical effect of a high glucose concentration to decrease insulin mRNA was seen within 24 h and was quickly reversible after culturing the cells in a low glucose concentration (21,22). This abbreviated time course for establishing adverse effects of a high glucose concentration and their ready reversal is more reminiscent of a glucose desensitization phenomenon than glucose toxicity. More recent work using INS-1 cells suggests that glucotoxic β-cells may have additional, more distal defects in the exocytotic pathway (23). A third β-cell line, the βTC-6 cell, behaved very similarly to HIT-T15 cells when cultured under conditions of high and low glucose, with the exception that only MafA binding and not PDX-1 binding to DNA was affected (24). This finding presaged our later reports (18,19) indicating that a decline in MafA alone is sufficient to cause loss of insulin gene expression in glucotoxic states.
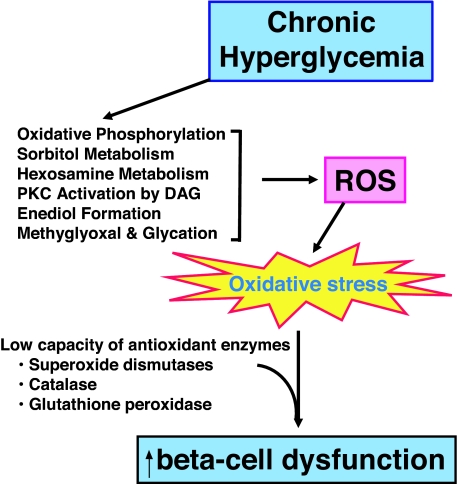
Evidence that glucotoxicity might be related to oxidative stress (Fig. 1) can be found in early reports that N-acetylcysteine and aminoguanidine, both antioxidants, can protect HIT-T15 cells and isolated islets from the adverse effects of prolonged culturing with media containing high glucose concentrations (25,26). We asked whether the decreased PDX-1 binding and decreased insulin mRNA levels in HIT-T15 cells that we observed might also be related to oxidative stress. Using either N-acetylcysteine or aminoguanidine in cells cultured for many passages under high glucose conditions, we observed an antioxidant drug concentration-related preservation of insulin promoter activity, PDX-1 binding, and levels of insulin mRNA (27).
Figure 1.
Biochemical pathways through which elevated levels of glucose can form excessive levels of reactive oxygen species (ROS), which cause oxidative stress and lead to β-cell dysfunction. Under normoglycemic conditions, glucose metabolites flow primarily through oxidative phosphorylation, but during exposure to excessive glucose levels, metabolites also overflow into alternative pathways. The β-cell contains very low levels of antioxidant enzymes and consequently is exquisitely sensitive to ROS. This is consistent with physiological regulation of β-cell function when ROS levels are low, but deleterious effects on the β-cell when ROS levels are abnormally high.
2. Isolated islets.
Chronically culturing isolated pancreatic islets is a major laboratory challenge. Nonetheless, Briaud et al. (28) were successful in culturing rat islets for up to 6 wk in media containing either 5.6 or 16.7 mm glucose and found at 6 wk that insulin mRNA levels were 50% decreased by the higher glucose concentration. In contrast, no changes were observed in mRNA levels for glucose transporter-2 or glucokinase. Adverse effects of chronically elevated levels of glucose were also studied earlier by Montana et al. (29) who transplanted isolated C57BL/6 rat islets under the kidney capsule of syngeneic recipients previously rendered diabetic by streptozotocin. Their strategy was to transplant 150 islets, a number that was insufficient for normalization of glycemia, so that they would be exposed to hyperglycemic conditions after transplant. Control animals were transplanted with 300 islets, which restored and maintained normoglycemia. β-Cell mass in the grafts decreased in the former group but not the latter group. Other related work by this laboratory regarding the adverse effects of elevated glucose levels on β-cell mass and function has been extensively reviewed by Leahy et al. (30).
Reports that β-cells have very low levels of antioxidant enzymes compared with other tissues (Fig. 1) suggest that the β-cell is particularly at risk for oxidative stress (31,32,33). This observation led to many efforts to determine whether overexpression of antioxidant enzymes would protect β-cells against oxidative stress in rodents (34,35,36,37,38,39,40). Tanaka et al. (41) in our group performed studies to determine whether enhancing β-cell antioxidant levels would protect against ribose-induced oxidative stress. Ribose is a much stronger oxidant than glucose and provides a strategy to examine consequences of oxidative stress on islets that circumvents the requirement of culturing islets over many months under high glucose conditions. Adenovirally induced overexpression of glutathione peroxidase, a critical antioxidant enzyme, was used in the experimental approach. Although other antioxidant enzymes, such as superoxide dismutases and catalase, are present in the islet (but also in low concentrations), superoxide dismutase forms hydrogen peroxide whereas catalase, although capable of metabolizing hydrogen peroxide, does not catabolize lipid peroxides. Glutathione peroxidase, on the other hand, catabolizes both hydrogen peroxide and lipid peroxides to form the two nontoxic substances, oxygen and water. Adenoviral overexpression of glutathione peroxidase increased islet levels of this enzyme 6-fold or approximately to the levels found normally in liver. This overexpression protected islets from ribose-induced losses in insulin mRNA, insulin content, and glucose-stimulated insulin secretion (41). Tran et al. (42) in similar studies examined the protective effect of overexpressing the glutamylcysteine ligase catalytic subunit, which regulates the use of cysteine as the rate-limiting substrate to form glutathione, the primary endogenous antioxidant substance. Adenoviral overexpression of this enzyme augmented the level of reduced glutathione in islets and prevented the adverse effects of IL-1-β on glucose-induced insulin secretion. Kaneto et al. (43) observed that adenoviral overexpression of a dominant-negative (DN) mutant of c-Jun N-terminal kinase (JNK) preserved PDX-1 DNA binding in islets exposed to H2O2-induced oxidative stress. They also reported that rat islets infected with DN-JNK-expressing adenovirus and transplanted under renal capsules of streptozotocin-induced diabetic nude mice maintained insulin gene expression in the grafts. However, despite the repeated observation that overexpression of antioxidant enzymes in rodent models protects against oxidative stress, to date no one has reported whether overexpression of antioxidant enzymes is protective against type 2 diabetes mellitus in an animal model. Counter to the concept that oxygen radicals are damaging to islets, Pi et al. (44) performed studies with INS-1 cells and isolated mouse islets and reported evidence that physiological levels of reactive oxygen species (ROS) may be required to support physiological β-cell function. This supports the concept that ROS in low levels are contributory to, but high levels may be harmful for, β-cell function (45).
B. In vivo evidence
1. Zucker diabetic fatty (ZDF) rats.
The ZDF rat, a well-accepted model of type 2 diabetes, has been used extensively to examine the adverse effects of glucotoxicity and lipotoxicity. This animal harbors a mutation in the leptin receptor gene and becomes obese and diabetic as it ages, but it is not dependent on exogenous insulin to live. Typically, the β-cells in ZDF rats initially compensate for the development of obesity and associated insulin resistance by increasing insulin mRNA levels, insulin content, and insulin secretion, but eventually they cannot keep up with this challenge and the animal becomes hyperglycemic. Early studies by Unger's group (46) demonstrated that troglitazone, an insulin-sensitizing drug, could be used to control hyperlipemia and hyperglycemia in this animal, which led to preserved β-cell function and glycemic control. Using this model, we demonstrated that troglitazone treatment to prevent hyperglycemia also had beneficial effects on maintaining both PDX-1 binding to DNA and levels of insulin gene expression (47). We also used isolated islets from ZDF rats to examine whether treatment with antioxidants, either N-acetylcysteine or aminoguanidine, beginning at 6 wk of age would decrease markers of oxidative stress and have beneficial effects on glycemia and levels of insulin mRNA and PDX-1 binding (27). We found that placebo-treated animals developed elevated levels of circulating markers of oxidative stress as they developed hyperglycemia. Treatment with either N-acetylcysteine or aminoguanidine prevented the rise in plasma ROS, greatly attenuated the degree of hyperglycemia, and minimized glycosuria (27). Islets isolated from antioxidant-treated animals exhibited much higher PDX-1 and MafA binding activities, as well as markedly higher levels of insulin mRNA and insulin content. On the other hand, treatment with an inhibitor of nitric oxide synthesis had no beneficial effect.
2. db/db Mice.
Kaneto et al. (48) reported that N-acetylcysteine was effective in enhancing ip glucose tolerance in db/db mice, another well-accepted rodent model of type 2 diabetes carrying a mutation in the leptin receptor gene. They observed that this drug enhanced insulin secretion, moderately decreased blood glucose levels, and led to greater β-cell mass and less apoptosis in the diabetic mice. The authors also reported preserved amounts of insulin content, insulin mRNA, and PDX-1 protein by immunoblotting. Other successful attempts to ameliorate diabetes and oxidative stress in animal models have involved the use of vitamin E in Goto-Kakizaki rats (49) and gliclazide (50), an oral hypoglycemic agent that also has antioxidant properties.
C. Mechanisms for reactive oxygen species formation by glucose and for associated loss of insulin promoter transcription factors
1. ROS formation from glucose metabolism.
Once glucose enters cells, it is primarily and progressively metabolized to glyceraldehyde-3-phosphate, 1:3 bis-P-glycerate, glyceraldehyde-3-phosphate, and pyruvate. Pyruvate then enters the tricarboxylic acid cycle to undergo oxidative phosphorylation, during which formation of ATP and ROS occurs. However, when excess glucose is available to the cell, alternative pathways exist through which excess glucose can be shunted and ROS can be formed from glucose (51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66). Among these are glyceraldehyde autooxidation to methylglyoxal and glycation, enediol and α-ketoaldehyde formation, dihydroxyacetone and diacylglycerol formation with protein kinase C activation, glucosamine and hexosamine metabolism, and sorbitol metabolism (Fig. 1 and reviewed in Ref. 67).
Direct demonstration that human islets can form ROS when they are exposed to high concentrations of glucose was provided by Tanaka et al. (41). To assess the concept that overflow of excess levels of glucose metabolites might induce oxidative stress through alternative pathways, Takahashi et al. (68) examined the consequences of exposing increasing concentrations of d-glyceraldehyde to rat islets. Formation of ROS was observed when 2 mm d-glyceraldehyde was present for 24 h and this was associated with decreased insulin content and inhibition of glucose-stimulated insulin secretion. The adverse effects of this increase in intraislet ROS on β-cell function was prevented by N-acetylcysteine but not by rotenone or myxothiazol, both inhibitors of oxidative phosphorylation. Moreover, pretreatment of the islets with koningic acid, a specific glyceraldehyde-3-phosphate dehydrogenase inhibitor, was used as a strategy to increase endogenous glyceraldehyde levels. Koningic acid, in the presence of 11.1 mm glucose, increased intracellular peroxide levels and inhibited glucose-stimulated insulin secretion. In assessing the pertinence of these studies, it is important to consider whether 2 mm d-glyceraldehyde is a physiologically relevant concentration. Taniguchi et al. (69) reported that islets exposed to 2.8 and 20 mm glucose accumulated approximately 0 and 0.025 pmol/islet d-glyceraldehyde, respectively, whereas exposure to 10 mm d-glyceraldehyde caused an accumulation of 0.12 pmol/islet d-glyceraldehyde. Because we used one fifth of this concentration of d-glyceraldehyde in our experiments, we estimate that it caused intraislet levels of approximately 0.025 pmol/islet, a concentration similar to that observed by Taniguchi et al. using 20 mm glucose. In our work, exposure of rat islets to koningic acid to increase d-glyceraldehyde levels caused an increase in intracellular ROS under 11.1 but not 3.0 mm glucose conditions. This increase in ROS was not significantly different from that observed with 2 mm d-glyceraldehyde. It has also been reported that long-term exposure to high glucose concentrations decreases glyceraldehyde-3-phosphate dehydrogenase activity in islets (52), which could lead to excess d-glyceraldehyde accumulation. It is important to note, however, that ROS are also involved in the mechanisms of insulin resistance (70), which has major implications for consequent β-cell stress (71).
2. Decreased PDX-1 mRNA expression.
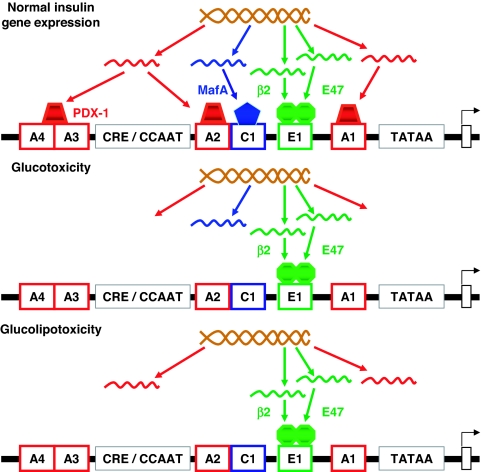
As pointed out in Sections II.A and II.B, the decrease in PDX-1 binding to DNA in HIT-T15 cells and islets from diabetic animals was preventable by antioxidants. To determine the site of action of this oxidative stress-mediated loss of PDX-1, studies were performed using nuclear run-on analyses of PDX-1 gene transcription and both Northern and Western analyses for PDX-1. We observed normal rates of PDX-1 gene transcription, but progressively diminished amounts of PDX-1 mRNA, PDX-1 protein, insulin mRNA, and insulin content as HIT-T15 cell cultures in high glucose concentrations were extended over many weeks (14,15,16,18). These results indicate that oxidative stress had induced a posttranscriptional loss of PDX-1 mRNA as the primary mechanism of action for loss of PDX-1 protein, a primary mechanism for decreased levels of insulin mRNA (Fig. 2).
Figure 2.
Molecular mechanisms of action for glucotoxicity and glucolipotoxicity at the level of insulin gene expression. Under physiological conditions, MafA and PDX-1 are two critically important regulators of the insulin promoter, and respectively bind to the C elements and the A boxes (upper panel). Glucotoxicity greatly diminishes protein levels of PDX-1 and MafA, the former through a posttranscriptional mechanism and latter through a posttranslational mechanism. These abnormalities lead to decreased insulin mRNA, insulin content, and glucose-induced insulin secretion, and are reversible only in the early stages of glucose toxicity (middle panel). Under glucolipotoxic conditions, MafA expression is inhibited, whereas PDX-1 is affected at the post posttranslational level in its ability to translocate to the nucleus. This results in decreased insulin gene expression (lower panel).
3. Decreased MafA protein expression.
As with PDX-1, loss of MafA binding to DNA from glucotoxic cell lines and islets undergoing culture with high glucose conditions could also be prevented by antioxidant treatment (15,16,17,18,19) (pointed out in Sections II.A and II.B). However, in contrast to the findings with our PDX-1 studies, loss of MafA binding was associated with normal levels of MafA mRNA (72). The abnormality identified was the absence of MafA protein, which indicated that a posttranslational, rather than a posttranscriptional, loss of MafA is the mechanism of action (Fig. 2). It is of interest that the loss of MafA protein from glucotoxic HIT-T15 cells occurred earlier and corresponded more closely in time with the loss of insulin content and glucose-stimulated insulin secretion compared with the loss of PDX-1 protein (18,19). Thus, although mutation of either the PDX-1 or the MafA binding sites on β-cell DNA decreases insulin promoter activity approximately 90% (15,16,17,24), the earlier loss of MafA protein in the course of the development of glucotoxicity points to this transcription factor as the more dominant mechanism of action for loss of insulin gene expression in glucotoxic β-cells. However, the relationship between these two transcription factors is complicated by the fact that they appear to regulate the expression of each other (73,74). Importantly, Kitamura et al. (75) recently reported decreased levels of MafA in several murine models of diabetes and demonstrated that transgenic expression of constitutively nuclear Fox01 prevented this loss of MafA.
4. Apoptosis.
The pathogenesis of type 2 diabetes has been reported to be associated with a variety of proapoptotic mechanisms (76), including glucose-induced synthesis of IL-1β (77) and as described by Donath et al. (196) in this issue of Endocrine Reviews, as well as endoplasmic reticulum (ER) stress, described by Scheuner and Kaufman (197) in this issue of Endocrine Reviews. The pathogenic concept of an imbalance of β-cell regeneration and apoptosis was addressed by Butler et al. (78) and described by Haataja et al. (198) in this issue of Endocrine Reviews in a study in which pancreatic tissue from patients with type 2 diabetes mellitus and control subjects was obtained from 124 autopsies. The frequency of β-cell replication was very low in all cases, with no difference between diabetic and control groups. However, the frequency of β-cell apoptosis was increased 10-fold in the lean and 3-fold in the obese cases of type 2 diabetes. In support of this association, increased apoptosis was observed when islets were cultured in 16.7 mm glucose compared with islets cultured in 5.5 mm glucose or 11 mm mannitol plus 5 mm glucose (79). The proapoptotic genes Bad, Bid, and Bik were overexpressed in islets maintained in high glucose concentrations. The antiapoptotic gene Bcl-2 was unaffected by these conditions whereas Bcl-xl was reduced. Expansion of β-cell mass in response to insulin resistance and insulin secretory defects in ZDF rats have been reported to occur, but this was inadequate to maintain normoglycemia (80). These studies also suggested that an increased rate of cell death by apoptosis was responsible for evolution of the diabetic state. Loss of β-cell mass resulting from an increase in β-cell death was reported to be an important contributor to the evolution of the diabetic state in ZDF rats (81). We (M. Bogdani, J. S. Harmon, and R. P. Robertson, unpublished observations) have recently completed studies examining HIT-T15 cells cultured in media containing low vs. high concentrations of glucose over 45 passages and have not observed increased apoptosis, although we observed the time-dependent loss of MafA and PDX-1 protein. Thus, the loss of these two important insulin gene transcription factors appears to be more dominantly related to the posttranslational and posttranscriptional, respectively, mechanisms of action described above rather than apoptosis.
D. Clinical correlations between oxidative stress and glucotoxicity of the β-cell
The preclinical and clinical literature suggesting that diabetes is associated with oxidative stress and that antioxidants might provide valuable ancillary treatment is vast. A complete review of these reports is beyond the scope of this manuscript, which is designed to deal primarily with in vitro and preclinical in vivo data. Nonetheless, this review would be incomplete without at least a brief mention of clinical reports. Focusing on the decade of the 1990s, several groups reported that measurements of oxidative stress markers are elevated in diabetic subjects. For example, using serum and HPLC, Shin et al. (82) found that levels of 8-OH-guanine were five times higher than normal in type 2 diabetic subjects. Gopaul et al. (83) found that 8-epi-PGF2α levels measured by gas chromatography/mass spectroscopy were elevated in plasma. Hydroperoxides were also measured in plasma by HPLC and were reported as elevated by Nourooz-Zadeh et al. (84). DNA base oxidation measured by gas chromatography/mass spectroscopy in white blood cells was reported to be elevated by Rehman et al. (85). Ghiselli et al. (86) reported that salicylate hydroxylation is an early marker for oxidative stress in diabetic patients. Using pancreatic tissue and immunocytochemistry, Sakuraba et al. (87) found reduced β-cell mass and elevated levels of 8-OH-deoxyguanine and 4-OH-2-nonenal proteins. Yoshida et al. (88) reported that the activity of γ-glutamylcysteine synthetase, glutathione concentration, and thiol transport were 77, 77, and 69% of normal controls, respectively, in erythrocytes from diabetic patients, and that treatment with an antidiabetic agent for 6 months resulted in the restoration of γ-glutamylcysteine synthetase activity, glutathione, and thiol transport.
More recently, Del Guerra et al. (89) examined islets isolated from pancreata of 13 type 2 diabetic cadaveric organ donors and observed high levels of oxidative stress markers as well as low levels of glucose-induced insulin secretion, reduced insulin mRNA, but increased levels of PDX-1 and FOX01 mRNAs. Exogenous glutathione improved these abnormalities. In a preliminary study, we found that arginine-induced insulin secretion was improved in a type 2 diabetic patient following 28 d of oral N-acetylcysteine treatment (90). On the other hand, an earlier study of vitamin E treatment for oxidative stress in type 2 diabetic patients failed to show improvement of insulin secretion (91). However, there have been no reports of placebo-controlled, double-blind trials over extended periods of time designed to ascertain whether treatment of diabetic subjects with potent antioxidants for impaired glucose tolerance or manifest type 2 diabetes improves β-cell function.
III. Glucolipotoxicity
A number of in vitro studies, using insulin-secreting cells and isolated islets, have attempted to identify the mechanisms of glucolipotoxicity. In vitro, prolonged exposure of isolated islets or insulin-secreting cells to elevated levels of fatty acids is associated with inhibition of glucose-induced insulin secretion (92,93,94,95), impairment of insulin gene expression (96,97,98,99,100,101), and induction of cell death by apoptosis (80,102,103,104,105,106,107,108,109,110). Importantly, in vitro (98,99,110) and in vivo (111,112) studies have provided evidence that lipotoxicity only occurs in the presence of concomitantly elevated glucose levels. Although this notion has been challenged in isolated human islets (113), one might argue that the severe stress induced by the isolation procedure of human pancreas might sensitize the islets to harmful effects of fatty acids (109). In recent years, a number of studies have contributed to a better understanding of the biochemical and molecular mechanisms of glucolipotoxicity in the β-cell. Perhaps the most significant advance of these past years is the realization that the mechanisms whereby fatty acids affect various aspects of pancreatic β-cell function (namely insulin secretion, insulin gene expression, and cell survival) are distinct.
A. Functional manifestations of glucolipotoxicity in vitro
1. Fatty-acid impairment of insulin secretion.
Prolonged exposure of β -cells to fatty acids in vitro increases basal insulin release and inhibits glucose-stimulated insulin secretion (92,93,94,95), a phenomenon that has also been observed in vivo in rats (114) and humans (115). These two effects have different time frames, and probably distinct mechanisms. The increase in basal insulin release involves enhanced low Km glucose usage (116,117). Culturing islets in the presence of fatty acids for 24 h decreases the activity of citrate synthase, resulting in lowered citrate levels and increased phosphofructokinase activity (117,118). This in turn reduces glucose-6-phosphate levels, disinhibits hexokinase, and increases glucose usage at low glucose concentrations. In vivo, fatty acids also decrease sympathetic nervous system activity, thus enhancing insulin secretion (119). The mechanisms underlying the decrease in glucose-stimulated insulin secretion by fatty acids are less clear, although as discussed below recent studies have provided important insights into potential candidates.
The G protein-coupled receptor GPR40 is specifically expressed in pancreatic β-cells and is activated by long-chain fatty acids (120,121). A role for GPR40 in mediating fatty-acid inhibition of insulin secretion has been suggested by the observation that islets from GPR40 KO mice are insensitive to the inhibitory effects of prolonged fatty acids (122). Using a different line of GPR40 KO mice, we were unable to reproduce these findings and found that deletion of the receptor does not protect islets from fatty-acid inhibition of glucose-induced insulin secretion (123). We therefore do not favor the view that GPR40 plays a major role in the deleterious effects of fatty acids on β-cell function, although further investigation is required to clarify these discrepancies.
Uncoupling protein-2 (UCP-2) is a ubiquitously expressed mitochondrial carrier that has been suggested to uncouple the respiratory chain from ATP synthesis (124), although its biological functions are still unclear. Evidence suggests that UCP-2 modulates insulin secretion and plays a role in glucolipotoxicity. First, increasing UCP-2 expression in β-cells impairs insulin secretion (125,126). Second, UCP-2 KO animals have increased circulating insulin levels and are protected from genetic (124) or nutritional (127) diabetes. Third, UCP-2 expression is increased in islets after high-fat feeding in rodents (112,126) or exposure to fatty acids in vitro (128,129). Fourth, oleic acid activates the UCP-2 promoter in INS-1 cells, an effect mediated directly by sterol regulatory element-binding protein-1c (SREBP1c) (130) and indirectly by peroxisome proliferator-activated receptor-γ (131). Finally, islets isolated from UCP-2 KO animals are protected from lipotoxicity (127). These observations suggested that fatty acids activate the expression of UCP-2 in β-cells, perhaps resulting in mitochondrial uncoupling. Because glucose-induced insulin secretion depends upon ATP generation from glucose metabolism, such uncoupling is predicted to impair insulin secretion. However, the precise contribution of UCP-2 to the mechanisms of glucolipotoxicity awaits the unequivocal demonstration that it acts as an uncoupling protein under physiological or pathophysiological conditions in β-cells. In fact, a recent study demonstrated that transgenic overexpression of UCP-2 did not alter mitochondrial function or glucose-induced insulin secretion. It was, however, associated with decreased production of ROS (132). Therefore, the increase in UCP-2 activity in response to fatty acids may be a cellular defense mechanism against fuel overload and oxidative stress rather than a deleterious response.
A previously unrecognized role for intracellular cholesterol metabolism in the mechanisms of glucolipotoxicity has been suggested by the observation that β-cell specific KO of the ATP-binding cassette transporter subfamily A member 1 (ABCA1), which mediates reverse cholesterol efflux, results in increased cellular cholesterol content and impaired insulin secretion (133). The authors further showed that the ability of rosiglitazone to improve glucose tolerance in mice fed a high-fat diet requires a functional ABCA1 in β-cells (133). The insulin secretory defect in ABCA1 KO β-cells appears to lie downstream of glucose metabolism, probably at the level of insulin exocytosis (133). Consistent with this possibility, Kato et al. (134) have shown that expression of granuphilin, an effector of the small GTP-binding protein Rab27a, which plays a key role in the docking of insulin secretory granules to the plasma membrane, is increased in islets exposed to palmitate as a consequence of augmented expression of SREBP1c, which inhibits insulin secretion in response to fuel and non-fuel stimuli. Recently, Olofsson et al. (135) demonstrated that prolonged exposure of mouse islets to glucose and fatty acids inhibited insulin secretion at a very late stage of exocytosis by interfering with the release of insulin at the fusion pore. These recent findings therefore indicate that the mechanisms by which fatty acids affect insulin secretion might, at least in part, lie at the level of the exocytotic machinery and, consequently, inhibit insulin secretion in response not only to glucose but also to other secretagogues. The clinical implication of these findings is that therapeutic strategies aimed at enhancing insulin secretion in type 2 diabetes might fail in a glucolipotoxic environment.
2. Fatty-acid impairment of insulin gene expression.
Prolonged exposure to fatty acids impairs insulin gene expression in the presence of high glucose (96,97,98,99,100). Studies performed in our laboratory indicate that the mechanisms whereby fatty acids affect insulin secretion are distinct from those by which they impair insulin gene expression. First, whereas both palmitate and oleate inhibit insulin secretion, only palmitate affects the insulin gene (136). Because only palmitate can serve as a substrate for de novo ceramide synthesis, we examined whether ceramide generation might mediate palmitate inhibition of the insulin gene. We observed that a 72-h culture of isolated islets in the presence of palmitate is associated with an increase in ceramide content that can be largely blocked by inhibitors of de novo ceramide synthesis, and that inhibition of ceramide generation prevents the decrease in insulin mRNA levels upon exposure to palmitate (100). Furthermore, palmitate inhibition of insulin mRNA levels is not due to changes in mRNA stability, but to direct inhibition of glucose-induced insulin promoter activity in primary islets (100). More recently, we have observed that palmitate inhibition of insulin gene transcription is due to decreased binding activity of PDX-1 and MafA (101). PDX-1 appears to be affected in its ability to translocate to the nucleus, whereas MafA is affected at the level of its expression (101) (Fig. 2). Interestingly, this appears to contrast with the mechanisms of glucotoxicity, which have been shown to involve posttranslational modifications of MafA (72). The mechanisms whereby ceramide generation from palmitate impairs PDX-1 subcellular localization and MafA expression are unknown, although hypotheses can be proposed based on known targets of ceramide. Two major signaling pathways known to be modulated by ceramide, the MAPK and phosphatidylinositol 3 kinase pathways, regulate insulin gene transcription by influencing transcription factor activity. JNK is a direct target of ceramide in many cell systems (reviewed in Ref. 137). In β-cells, JNK has been shown to repress insulin gene transcription both via c-jun-dependent inhibition of E1-mediated transcription (138,139) and c-jun independent inhibition of PDX-1 binding (43). Recently, Solinas et al. (140) have shown that palmitate activates JNK in β-cells and that the resulting phosphorylation of insulin receptor substrates 1 and 2 impairs insulin signaling and decreases insulin gene transcription. In insulin-sensitive tissues, ceramide formation from palmitate inhibits protein kinase B (PKB or Akt) activity (141,142,143), perhaps via activation of protein kinase C ζ (144), which then negatively regulates PKB (145). A decrease in PKB activity alleviates the inhibition of the transcription factor Fox01, which as a result translocates to the nucleus and represses its target genes (146). In β-cells, PKB is expressed and activated by glucose (106). Expression of a constitutively active mutant of Fox01 results in a complete lack of PDX-1 expression, indicating that PDX-1 and Fox01 exhibit a mutually exclusive pattern of nuclear localization (147). In addition, a gain-of-function of Fox01 in liver and β-cells induces diabetes, due to increased hepatic glucose production and failure of β-cell compensation, the latter being associated with decreased PDX-1 expression (148). On the other hand, Fox01 was recently shown to positively regulate MafA expression and thereby protect β-cells against oxidative damage under glucotoxic conditions (75). Thus, it is likely that modulation of the PKB/Fox01 pathway is involved in fatty-acid inhibition of insulin gene expression. However, the net effect of this pathway on transcription factor function and insulin gene expression under glucolipotoxic conditions remains to be established.
3. Fatty-acid induction of β-cell death.
A number of studies have shown that fatty acids can induce β-cell death by apoptosis in the presence of high glucose (110). In vitro, saturated fatty acids induce β-cell apoptosis (104,108,110), whereas unsaturated fatty acids are usually protective (103,104,110). This difference in the proapoptotic effects of fatty acids is proposed to be due to the greater ability of unsaturated fatty acids to form intracellular triglycerides (103,149). Busch et al. (150) have also shown that the level of expression of the enzyme stearoyl coenzyme A (CoA) desaturase correlates with the resistance of β-cells to the proapoptotic effect of palmitate, indicating that the capability of a cell to desaturate fatty acids protects from glucolipotoxicity.
Several mechanisms have been proposed to mediate fatty acid-induced apoptosis in β-cells, including ceramide formation (102,105,108,151), altered lipid partitioning (110,152,153), and the generation of oxidative stress (107,109,154,155). A potential role for inflammatory mechanisms has been suggested by expression profiling of MIN6 cells exposed to fatty acids, showing a marked increase in the expression of the chemokine monocyte chemoattractant protein-1 (156), although Kharroubi et al. (157) showed that fatty acids do not activate the nuclear factor κB pathway in islets. More recently, a potential role of ER stress and the unfolded protein response has received experimental support (157). In insulin-secreting cells, palmitate, but not oleate, induces markers of ER stress (157,158,159) and causes marked alterations of ER morphology (158). Markers of ER stress are also increased in islets from db/db mice and pancreatic sections of type 2 diabetic patients (159).
The effects of fatty acids on β-cell apoptosis in vitro are difficult to interpret for several reasons. First, significant differences exist between clonal cells and primary β-cells in their sensitivity to the cytotoxic effects of fatty acids. Second, there are species-related differences in the ability of fatty acids to cause cell death. For instance, whereas a 24-h exposure of human islets to elevated glucose and palmitate is sufficient to observe apoptosis (110), we have not detected any cell death in rat islets after 72 h of culture under similar conditions (100,136). Third, the concentrations of fatty acids used in vitro vary among publications. The key determinant of fatty acid potency is the fraction that is unbound to BSA, which depends on the molar ratio of fatty acids to albumin as well as the mode of preparation. Using a fluorescent probe that specifically measures the unbound fraction of fatty acids (160), we observed that when palmitate at a total concentration of 0.5 mm was precomplexed to BSA with a molar ratio of fatty acid:albumin of 5:1, the unbound concentration is in the 200 nm range (V. Poitout, unpublished data), which represents approximately three times the unbound concentration measured in the plasma of lean individuals by the same method (161). Thus, the concentration of fatty acids should be interpreted in the context of the concomitant albumin concentration. Finally, the concentrations of fatty acids in the vicinity of the β-cells in vivo are unknown and are determined by many different factors, including the activity of lipoprotein lipase, which accounts for some of the local delivery of fatty acids to the cells (162). Therefore, the results of in vitro experiments using fatty acids should be interpreted with caution, particularly when marked cytotoxicity is observed.
B. In vivo evidence for glucolipotoxicity
Experiments performed in the ZDF rat were instrumental in establishing the concept of lipotoxicity and identifying some of its basic mechanisms (reviewed in Ref. 163). The limitations of the ZDF model lie in the fact that the effects of chronic hyperglycemia (glucotoxicity) are difficult to separate from those of chronic hyperlipidemia (glucolipotoxicity). In fact, lowering blood glucose levels, but not lipid levels, is sufficient to normalize insulin gene expression in ZDF (111). The fa mutation underlying the ZDF phenotype results in defective leptin signaling and therefore profoundly perturbs intracellular fatty-acid metabolism, thereby limiting the relevance of this model to human type 2 diabetes, in which leptin receptor mutations are extremely rare (164). Considering these limitations, examining whether or not glucolipotoxicity occurs in vivo, and testing mechanistic hypotheses in other, nongenetic animal models is important. Mason et al. (114) have demonstrated that a 48-h perfusion of Intralipid or oleate impairs glucose-induced insulin secretion in normal rats. The influence of genetic predisposition on the insulin secretory response to excessive fatty acid levels is also illustrated by the recent observation that insulin secretion is impaired to a greater extent in heterozygous lean ZDF rats than in Wistar rats after Intralipid infusion (165). To examine whether the fatty-acid inhibition of insulin gene expression previously observed in isolated islets (100,101) was also operative in vivo, we have recently performed a study in which normal Wistar rats were infused alternatively with glucose for 4 h and Intralipid plus heparin for 4 h, for a total of 72 h. In islets isolated at the end of the perfusion from animals infused alternatively with glucose and saline, insulin mRNA levels, PDX-1 nuclear localization, and PDX-1 binding to the endogenous insulin gene promoter were increased. In contrast, in islets from animals perfused with glucose and Intralipid, insulin mRNA levels were reduced, PDX-1 localization was shifted toward the cytosol, and occupancy of the endogenous insulin promoter by PDX-1 was markedly diminished (D. K. Hagman and V. Poitout, manuscript in preparation). These results demonstrate that fatty-acid inhibition of the insulin gene also occurs in vivo.
C. Studies in humans
Studies examining the effects of prolonged fatty acids on insulin secretion in humans have led to conflicting results. Initial reports from Boden and colleagues indicated that a 48-h lipid infusion induces an appropriate insulin secretory response in healthy subjects (166) but is defective in type 2 diabetic patients (167). In contrast, Carpentier et al. (168) have shown that the increase in insulin secretion observed in response to an acute (90-min) lipid infusion in healthy subjects disappears when the infusion is prolonged to 48 h. The loss of insulin secretion is specific to the response to glucose, because the response to arginine remains normal (169). The same group further showed that obese, but not diabetic, subjects are susceptible to the inhibitory effect of lipids on glucose-induced insulin secretion (170). Importantly, the augmentation of the disposition index observed in nondiabetic subjects in response to a 24-h glucose infusion does not occur if lipids are infused simultaneously with glucose (171). Finally, the group of Cusi and De Fronzo (172) has carried out a series of studies in nondiabetic subjects with a family history (FH+) of type 2 diabetes and without. They showed that a 4-d Intralipid infusion enhances insulin secretion (normalized for insulin sensitivity) in control subjects but inhibits glucose-induced insulin secretion in FH+ individuals (172), suggesting that perhaps part of the genetic predisposition to type 2 diabetes is related to the ability of the β-cell to increase insulin secretion in response to elevated fatty acid levels. Importantly, reducing circulating fatty acid levels with Acipimox ameliorates insulin secretion in FH+ subjects (173).
D. The influence of glucose on lipid metabolism and its consequences for the mechanisms of glucolipotoxicity
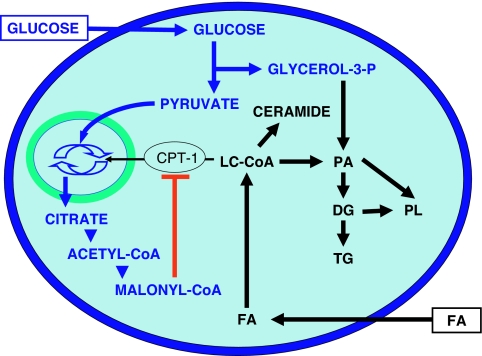
As detailed in Section III.A, experimental evidence strongly supports the notion that deleterious effects of fatty acids on β-cell function only occur in the presence of elevated glucose levels. This requirement for glucose has a biochemical basis (13,174). As first proposed by Prentki and Corkey (175), glucose is a chief determinant of fatty acid partitioning in β-cells (Fig. 3). When glucose concentrations are in the normal range, fatty acids are transported into the mitochondria via the enzyme carnitine-palmitoyl transferase-1 (CPT-1) and undergo β-oxidation with little, if any, functional consequences. In contrast, when both glucose and fatty acid concentrations are elevated, glucose metabolism in the TCA cycle generates cataplerotic signals, such as citrate, which lead to the formation of malonyl-CoA in the cytosol. In the β-cell, malonyl-CoA primarily serves to inhibit CPT-1 activity, thereby blocking fatty acid oxidation and resulting in the accumulation of long-chain acyl-CoA esters (LC-CoA) in the cytosol (175). In turn, accumulation of cytosolic LC-CoA, either directly or via generation of lipid-derived signals, adversely affects β-cell function (176). In addition to its metabolic effects directing fatty-acid partitioning into esterification, glucose coordinately activates the expression of genes involved in lipogenesis (177). Increasing evidence suggests that the enzyme AMP-activated protein kinase (AMPK) acts as a metabolic sensor that detects changes in the cellular energy state and directs the β-cell into a “storage mode” in the face of nutrient oversupply (153), similar to its role in muscle and liver (178). AMPK activity is inversely correlated with the glucose concentration (179) and is stimulated by palmitate (180) in β-cells. Downstream of AMPK, the transcription factor SREBP1c, which transactivates genes involved in fatty acid synthesis (181), appears to act as an effector translating the metabolic signal sensed by AMPK into changes in gene expression, leading to increased lipogenesis and triglyceride accumulation. Glucose also increases the expression of the liver X receptor, which in turn enhances SREBP1c expression and lipid synthesis (182).
Figure 3.
Effects of glucose on lipid partitioning in the β-cell. In the presence of elevated glucose and fatty-acid (FA) levels, the increase in cytosolic malonyl-CoA resulting from glucose metabolism inhibits the enzyme CPT-1. Transport of LC-CoA in the mitochondria is reduced, and the esterification pathway is activated, leading to cytosolic accumulation of lipid-derived signaling molecules such as ceramide, diglycerides (DG), phosphatidic acid (PA), phospholipids (PL), and triglycerides (TG).
Triglyceride accumulation was initially proposed as a mechanism underlying β-cell failure in the ZDF rat (183,184). In isolated islets, prolonged exposure to simultaneously elevated levels of glucose and palmitate is associated with a marked increase in triglyceride content (99), and forcing triglyceride synthesis in islets impairs insulin secretion (185). It is, however, unlikely that triglyceride stores are by themselves toxic, because they are usually seen as a process of fat storage in a relatively inert form that can actually protect against lipotoxicity (149). Thus, triglyceride accumulation is likely a marker of lipotoxic conditions that signals an increase in the flux through the esterification pathway, rather than a bona fide causal mechanism of glucolipotoxicity. The lipid-derived molecules directly responsible for the impairment of β-cell function are, for the most part, still unknown, although the role of intermediates of the esterification pathway (e.g., lysophosphatidic acid, phosphatidic acid, diacylglycerols) has been suggested (186). De novo synthesis of ceramide has been demonstrated to play a role in both fatty acid-induced β-cell death (151) and fatty-acid inhibition of insulin gene expression (100). Interestingly, ceramide is also implicated in the mechanisms of insulin resistance (187). Alternatively, Boucher et al. (188) have provided evidence that glucolipotoxicity was associated with a reduction in the glucose-induced exchange of pyruvate with intermediates of the Krebs cycle (188).
Most studies on the mechanisms of glucolipotoxicity in the β-cell have focused on fatty acid and triglyceride metabolism. Cholesterol metabolism has received less attention, although recent findings suggest that it may play an important role. Exposure of β-cells to oxidized low-density lipoproteins induces apoptosis (189) and decreases insulin gene expression (190), whereas native low-density lipoprotein particles have no effect and high-density lipoproteins are protective. Mice with β-cell-specific deletion of ABCA1, which mediates reverse cholesterol efflux, have increased intracellular cholesterol content in islets and impaired insulin secretion (133). Because liver X receptor regulates ABCA1 expression (133) and has been recently shown to be directly regulated by glucose (191), it appears that glucose coordinately enhances fatty acid esterification and intracellular cholesterol synthesis.
Therefore, chronically elevated glucose directs fatty acid partitioning away from oxidation and toward cellular lipid synthesis. The nature of lipid-derived signals that directly cause β-cell dysfunction is, for the most part, still unknown. In addition to the esterification pathway and the generation of ceramide from fatty acids, recent studies provide evidence that lipoprotein delivery and cholesterol metabolism are involved in the mechanisms of glucolipotoxicity.
IV. The Glucolipoadaptation-Glucolipotoxicity Spectrum and the Implications for the Pathogenesis of β-Cell Dysfunction in Type 2 Diabetes
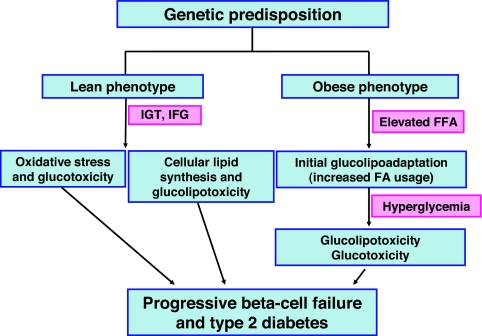
The physiological or pathological significance of the effects of fatty acids and glucose on pancreatic β-cell function is a matter of debate, and it has been further confounded by the various interpretations given to the suffix “-toxicity.” Although one can reasonably assert that fatty acid-induced β-cell death is clearly a toxic manifestation, their effects on functional parameters such as insulin secretion or gene expression are more difficult to categorize as either beneficial or deleterious responses in a short time frame, although they are clearly deleterious in the long run. From the above review of the recent literature we propose the working model illustrated in Fig. 4.
Figure 4.
Contribution of glucotoxicity and glucolipotoxicity to the development of type 2 diabetes. In this hypothesis, lean and obese individuals who develop type 2 diabetes primarily have polygenic defects that predispose them to the disease. Because of genetic abnormalities the lean phenotype develops impaired glucose tolerance (IGT) and/or impaired fasting glucose (IFG), which exposes the β-cell to chronic hyperglycemia. Hyperglycemia generates ROS which cause oxidative stress and worsened β-cell dysfunction. Alternatively but not exclusively, abnormally high blood glucose concentrations cause increased cellular lipid synthesis and glucolipotoxicity, which also causes deterioration in β-cell function. The obese phenotype has intrinsically elevated blood free fatty acid (FFA) levels which causes the β-cell to switch to preferential fatty-acid metabolism (glucolipoadaptation). Over time, however, the β-cell can no longer adapt and glucolipoadaptation evolves toward glucolipotoxicity. This in turn leads to hyperglycemia, which eventually overwhelms the β-cell and leads to its frank dysfunction. FA, Fatty acid.
A. β-Cell compensation for insulin resistance
In normoglycemic individuals experiencing weight gain, the β-cell mounts a compensatory response to counter insulin resistance associated with obesity. This response involves coordinated increases in β-cell mass, insulin biosynthesis, and insulin secretion. Although the molecular signals that trigger functional adaptation of the β-cell are unknown, experimental evidence suggests that these may involve an increased response to fatty acids (192,193). According to this concept, the β-cell becomes sensitized to fatty acids and preferentially metabolizes fatty acids rather than glucose as a fuel. This hypothesis may explain the observed decrease in glucose-induced insulin secretion classically observed in isolated islets after prolonged exposure to fatty acids: if the β-cell has adjusted its intracellular metabolism and signaling toward preferential utilization of fatty acids, it is not unexpected that a subsequent exposure to glucose, in the absence of fatty acids, will trigger a lower secretory response than in islets not previously exposed to fatty acids. In fact, it has been shown that after exposure to fatty acids, the glucose response is reduced but the response to fatty acids is enhanced (156). This switch from glucose to fatty acids as the primary β-cell nutrient might be viewed in this context as an adaptation that should probably not be referred to as “glucolipotoxicity” but rather “glucolipoadaptation” (186), because it contributes to β-cell compensation for insulin resistance and is therefore beneficial in maintaining normal glucose homeostasis.
The magnitude of the compensatory β-cell response is probably genetically determined and, in turn, is a major determinant of the long-term ability of an individual to maintain glucose homeostasis in the face of insulin resistance. In other words, in individuals not predisposed to develop diabetes, the β-cell is capable of mounting a sustained response that adequately compensates for insulin resistance, and normoglycemia is preserved. In contrast, in individuals genetically susceptible to diabetes, the compensatory response is insufficient and β-cell decompensation ensues. A strong experimental argument in favor of this view is provided by the observation that insulin secretion during a hyperglycemic clamp is increased after lipid infusion in control subjects but markedly decreased in individuals with a family history of diabetes (172). These observations indicate that the β-cell response to prolonged lipid exposure is genetically determined and suggests that this might play a role in the subsequent development of diabetes.
B. β-Cell decompensation and failure
In genetically predisposed individuals, β-cell compensation eventually becomes insufficient and the β-cell is no longer able to sustain a secretory response that matches the demand imposed by insulin resistance. In the early stages of this decompensation phase, it is likely that the disturbances of glucose homeostasis are first evidenced by postprandial hyperglycemia. At the level of the β-cell, we postulate that even transient episodes of hyperglycemic excursions are sufficient to alter intracellular metabolism and enable glucolipotoxicity to occur when the intracellular flux of fatty acids rises. We propose that it is during this decompensation phase that glucolipotoxicity plays a major role, in that hyperglycemia is the triggering factor by which elevated fatty acids affect β-cell function. At the level of insulin gene expression, elevated levels of lipids at this stage prevent the compensatory mechanisms that the β-cell attempts to mount in response to hyperglycemia, as illustrated in our recent in vivo studies described in Section III.B. Notably in these studies, insulin gene expression was impaired by combined glucose and lipid infusions before any detectable decrease in insulin secretion, suggesting that defective insulin biosynthesis might be an early defect in glucolipotoxicity, which contributes to eventual β-cell failure, inasmuch as maintenance of adequate intracellular stores of insulin is necessary to sustain increased secretory demand (194).
β-Cell decompensation evolves toward β-cell failure when fasting hyperglycemia occurs. At this stage, it is likely that both glucotoxicity and glucolipotoxicity contribute to the decline in insulin secretion observed over time during the years following diagnosis of type 2 diabetes (195). Because hyperglycemia at this stage is permanent, the effects of glucotoxicity are likely to be fully operative. Similarly, the effects of glucolipotoxicity, perhaps including actual cytotoxicity, probably contribute to the inexorable demise of the β-cell. The therapeutic implications of this model, if correct, are that normalization of blood glucose levels in the early stages of the disease should be sufficient to avoid the deleterious effects of glucotoxicity and glucolipotoxicity.
It should be noted, in conclusion, that the proposed model described above (Fig. 4) is based on extensive experimental evidence in vitro and in rodents, but has not been fully validated in humans. The precise role of elevated glucose and lipid levels on β-cell function in humans and the contribution of these effects to the pathogenesis of type 2 diabetes remain to be established.
Supplementary Material
Footnotes
Studies performed in our laboratories were supported by the National Institutes of Health Grants R01-DK58096 (to V.P.) and R01-DK38325 (to R.P.R.). R.P.R. is the recipient of an American Diabetes Association Mentor-based Fellowship Award. V.P. was the recipient of the 2003 Thomas R. Lee Career Development Award from the American Diabetes Association and holds the Canada Research Chair in Diabetes and Pancreatic β-Cell Function.
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 29, 2007
Abbreviations: AMPK, AMP-activated protein kinase; CoA, coenzyme A; CPT-1, carnitine-palmitoyl transferase-1; ER, endoplasmic reticulum; FH+, with a family history; JNK, c-Jun N-terminal kinase; LC-CoA, long-chain acyl-CoA esters; PDX-1, pancreas-duodenum homeobox-1; PKB, protein kinase B; ROS, reactive oxygen species; SREBP1c, sterol regulatory element-binding protein-1c; UCP-2, uncoupling protein-2; ZDF, Zucker diabetic fatty (rats).
References
- DeFronzo RA 1999 Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev 5:177–269 [Google Scholar]
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P 2007 A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445:881–885 [DOI] [PubMed] [Google Scholar]
- 2007 Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447:661–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Bostrom K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjogren M, Sterner M, Surti A, Svensson M, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S 2007 Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316:1331–1336 [DOI] [PubMed] [Google Scholar]
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M 2007 A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316:1341–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinthorsdottir V, Thorleifsson G, Reynisdottir I, Benediktsson R, Jonsdottir T, Walters GB, Styrkarsdottir U, Gretarsdottir S, Emilsson V, Ghosh S, Baker A, Snorradottir S, Bjarnason H, Ng MC, Hansen T, Bagger Y, Wilensky RL, Reilly MP, Adeyemo A, Chen Y, Zhou J, Gudnason V, Chen G, Huang H, Lashley K, Doumatey A, So WY, Ma RC, Andersen G, Borch-Johnsen K, Jorgensen T, van Vliet-Ostaptchouk JV, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Rotimi C, Gurney M, Chan JC, Pedersen O, Sigurdsson G, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K 2007 A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet 39:770–775 [DOI] [PubMed] [Google Scholar]
- Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT 2007 Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316:1336–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafrir E, Gutman A 1965 Patterns of decrease of free fatty acids during glucose tolerance tests. Diabetes 14:77–83 [DOI] [PubMed] [Google Scholar]
- Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD 1988 Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes 37:1020–1024 [DOI] [PubMed] [Google Scholar]
- Paolisso G, Tataranni PA, Foley JE, Bogardus C, Howard BV, Ravussin E 1995 A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia 38:1213–1217 [DOI] [PubMed] [Google Scholar]
- Unger RH, Grundy S 1985 Hyperglycemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia 28:119–121 [DOI] [PubMed] [Google Scholar]
- Unger RH 1995 Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes 44:863–870 [DOI] [PubMed] [Google Scholar]
- Prentki M, Joly E, El-Assaad W, Roduit R 2002 Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: role in β-cell adaptation and failure in the etiology of diabetes. Diabetes 51(Suppl 3):S405–S413 [DOI] [PubMed] [Google Scholar]
- Robertson RP, Zhang HJ, Pyzdrowski KL, Walseth TF 1992 Preservation of insulin mRNA levels and insulin secretion in HIT cells by avoidance of chronic exposure to high glucose concentrations. J Clin Invest 90:320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LK, Redmon JB, Towle HC, Robertson RP 1993 Chronic exposure of HIT cells to high glucose concentrations paradoxically decreases insulin gene transcription and alters binding of insulin gene regulatory protein. J Clin Invest 92:514–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LK, Sharma A, Peshavaria M, Wright CVE, Towle HC, Robertson RP, Stein R 1995 Reduction of insulin gene transcription in HIT-T15 cells chronically exposed to a supraphysiologic glucose concentration is associated with loss of STF-1 transcription factor expression. Proc Natl Acad Sci USA 92:9127–9131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Olson LK, Robertson RP, Stein R 1995 The reduction of insulin gene transcription in HIT-T15 β-cells chronically exposed to high glucose concentration is associated with the loss of RIPE3b1 and STF-1 transcription factor expression. Mol Endocrinol 9:1127–1134 [DOI] [PubMed] [Google Scholar]
- Harmon JS, Tanaka Y, Olson LK, Robertson RP 1998 Reconstitution of glucotoxic HIT-T15 cells with somatostatin transcription factor-1 partially restores insulin promoter activity. Diabetes 47:900–904 [DOI] [PubMed] [Google Scholar]
- Gleason CE, Gonzalez M, Harmon JS, Robertson RP 2000 Determinants of glucose toxicity and its reversibility in the pancreatic islet β-cell line, HIT-T15. Am J Physiol Endocrinol Metab 279:E997–E1002 [DOI] [PubMed] [Google Scholar]
- Moran A, Zhang H-J, Olson LK, Harmon JS, Poitout V, Robertson RP 1997 Differentiation of glucose toxicity from β-cell exhaustion during the evolution of defective insulin gene expression in the pancreatic islet cell line, HIT-T15. J Clin Invest 99:534–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LK, Qian J, Poitout V 1998 Glucose rapidly and reversibly decreases insulin gene transcription in INS-1 cells via decrements in binding activity of STF-1 and C1 activator. Mol Endocrinol 12:207–219 [DOI] [PubMed] [Google Scholar]
- Pino MF, Ye DZ, Linning KD, Green CD, Wicksteed B, Poitout V, Olson LK 2005 Elevated glucose attenuates human insulin gene promoter activity in INS-1 pancreatic β-cells via reduced nuclear factor binding to the A5/core and Z element. Mol Endocrinol 19:1343–1360 [DOI] [PubMed] [Google Scholar]
- Dubois M, Vacher P, Roger B, Huyghe D, Vandewalle B, Kerr-Conte J, Pattou F, Moustaid-Moussa N, Lang J 2007 Glucotoxicity inhibits late steps of insulin exocytosis. Endocrinology 148:1605–1614 [DOI] [PubMed] [Google Scholar]
- Poitout V, Olson LK, Robertson RP 1996 Chronic exposure of βTC-6 cells to supraphysiologic concentrations of glucose decreases binding of the RIPE-3b1 insulin gene transcription activator. J Clin Invest 97:1041–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneto H, Fujii J, Myint T, Miyazawa N, Islam KN, Kawasaki Y, Suzuki K, Makamura M, Tatsumi H, Yamasaki Y, Taniguchi N 1996 Reducing sugars trigger oxidative modification and apoptosis in pancreatic β-cells by provoking oxidative stress through the glycation reaction. Biochem J 320:855–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri Y, Moller C, Grill V 1997 Long term effects of aminoguanidine on insulin release and biosynthesis: evidence that the formation of advanced glycosylation end products inhibits β-cell function. Endocrinology 138:273–280 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Gleason CE, Tran POT, Harmon JS, Robertson RP 1999 Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci USA 96:10857–10862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briaud I, Rouault C, Reach G, Poitout V 1999 Long-term exposure of isolated rat islets of Langerhans to supraphysiologic glucose concentrations decreases insulin mRNA levels. Metabolism 48:319–323 [DOI] [PubMed] [Google Scholar]
- Montana E, Bonner-Weir S, Weir GC 1993 β-Cell mass and growth after syngeneic islet cell transplantation in normal and streptozotocin diabetic C57BL/6 mice. J Clin Invest 91:780–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy JL, Bonner-Weir S, Weir G 1992 β-Cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care 15:442–455 [DOI] [PubMed] [Google Scholar]
- Grankvist K, Marklund SL, Taljedal IB 1981 CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J 199:393–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedge M, Lortz S, Drinkgern J, Lenzen S 1997 Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 46:1733–1742 [DOI] [PubMed] [Google Scholar]
- Welsh N, Margulis B, Borg LA, Wiklund HJ, Saldeen J, Flodstrom M, Mello MA, Andersson A, Pipeleers DG, Hellerstrom C, Eizirik DL 1995 Differences in the expression of heat-shock proteins and antioxidant enzymes between human and rodent pancreatic islets: implications for the pathogenesis of insulin-dependent diabetes mellitus. Mol Med 1:806–820 [PMC free article] [PubMed] [Google Scholar]
- Grankvist K, Marklund S, Taljedal IB 1981 Superoxide dismutase is a prophylactic against alloxan diabetes. Nature 294:158–160 [DOI] [PubMed] [Google Scholar]
- Kubisch HM, Wang J, Bray TM, Phillips JP 1997 Targeted overexpression of Cu/Zn superoxide dismutase protects pancreatic β-cells against oxidative stress. Diabetes 46:1563–1566 [DOI] [PubMed] [Google Scholar]
- Tiedge M, Lortz S, Munday R, Lenzen S 1998 Complementary action of antioxidant enzymes in the protection of bioengineered insulin-producing RINm5F cells against the toxicity of reactive oxygen species. Diabetes 47:1578–1585 [DOI] [PubMed] [Google Scholar]
- Xu B, Moritz JT, Epstein PN 1999 Overexpression of catalase provides partial protection to transgenic mouse β cells. Free Radic Biol Med 27:830–837 [DOI] [PubMed] [Google Scholar]
- Benhamou PY, Moriscot C, Richard MJ, Beatrix O, Badet L, Pattou F, Kerr-Conte J, Chroboczek J, Lemarchand P, Halimi S 1998 Adenovirus-mediated catalase gene transfer reduces oxidant stress in human, porcine and rat pancreatic islets. Diabetologia 41:1093–1100 [DOI] [PubMed] [Google Scholar]
- Moriscot C, Pattou F, Kerr-Conte J, Richard MJ, Lemarchand P, Benhamou PY 2000 Contribution of adenoviral-mediated superoxide dismutase gene transfer to the reduction in nitric oxide-induced cytotoxicity on human islets and INS-1 insulin-secreting cells. Diabetologia 43:625–631 [DOI] [PubMed] [Google Scholar]
- Hohmeier HE, Thigpen A, Tran VV, Davis R, Newgard CB 1998 Stable expression of manganese superoxide dismutase (MnSOD) in insulinoma cells prevents IL-1β-induced cytotoxicity and reduces nitric oxide production. J Clin Invest 101:1811–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Tran PO, Harmon J, Robertson RP 2002 A role for glutathione peroxidase in protecting pancreatic β cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci USA 99:12363–12368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PO, Parker SM, LeRoy E, Franklin CC, Kavanagh TJ, Zhang T, Zhou H, Vliet P, Oseid E, Harmon JS, Robertson RP 2004 Adenoviral overexpression of the glutamylcysteine ligase catalytic subunit protects pancreatic islets against oxidative stress. J Biol Chem 279:53988–53993 [DOI] [PubMed] [Google Scholar]
- Kaneto H, Xu G, Fujii N, Kim S, Bonner-Weir S, Weir GC 2002 Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J Biol Chem 277:30010–30018 [DOI] [PubMed] [Google Scholar]
- Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S 2007 Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 56:1783–1791 [DOI] [PubMed] [Google Scholar]
- Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H 2003 Glucose toxicity in β-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 52:581–587 [DOI] [PubMed] [Google Scholar]
- Higa M, Zhou YT, Ravazzola M, Baetens D, Orci L, Unger RH 1999 Troglitazone prevents mitochondrial alterations, β cell destruction, and diabetes in obese prediabetic rats. Proc Natl Acad Sci USA 96:11513–11518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon JS, Gleason CE, Tanaka Y, Oseid EA, Hunter-Berger KK, Robertson RP 1999 In vivo prevention of hyperglycemia also prevents glucotoxic effects on PDX-1 and insulin gene expression. Diabetes 48:1995–2000 [DOI] [PubMed] [Google Scholar]
- Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki Y, Hori M 1999 Beneficial effects of antioxidants in diabetes: possible protection of pancreatic β-cells against glucose toxicity. Diabetes 48:2398–2406 [DOI] [PubMed] [Google Scholar]
- Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, Hiai H, Seino Y, Yamada Y 1999 Hyperglycemia causes oxidative stress in pancreatic β-cells of GK rats, a model of type 2 diabetes. Diabetes 48:927–932 [DOI] [PubMed] [Google Scholar]
- Kimoto K, Suzuki K, Kizaki T, Hitomi Y, Ishida H, Katsuta H, Itoh E, Ookawara T, Suzuki K, Honke K, Ohno H 2003 Gliclazide protects pancreatic β-cells from damage by hydrogen peroxide. Biochem Biophys Res Commun 303:112–119 [DOI] [PubMed] [Google Scholar]
- Wolff SP, Dean RT 1988 Aldehydes and dicarbonyls in non-enzymic glycosylation of proteins. Biochem J 249:618–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Matsumoto K, Nishikawa T, Suefuji M, Nakamaru K, Hirashima Y, Kawashima J, Shirotani T, Ichinose K, Brownlee M, Araki E 2003 Mitochondrial reactive oxygen species reduce insulin secretion by pancreatic β-cells. Biochem Biophys Res Commun 300:216–222 [DOI] [PubMed] [Google Scholar]
- Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabo C, Brownlee M 2003 Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 112:1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M 2001 Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820 [DOI] [PubMed] [Google Scholar]
- Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL 1992 Preferential elevation of protein kinase C isoform β II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci USA 89:11059–11063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL 1996 Amelioration of vascular dysfunctions in diabetic rats by an oral PKC β inhibitor. Science 272:728–731 [DOI] [PubMed] [Google Scholar]
- McLellan AC, Thornalley PJ, Benn J, Sonksen PH 1994 Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin Sci (Lond) 87:21–29 [DOI] [PubMed] [Google Scholar]
- Shinohara M, Thornalley PJ, Giardino I, Beisswenger P, Thorpe SR, Onorato J, Brownlee M 1998 Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest 101:1142–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornalley PJ, Langborg A, Minhas HS 1999 Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J 344:109–116 [PMC free article] [PubMed] [Google Scholar]
- Wells-Knecht MC, Thorpe SR, Baynes JW 1995 Pathways of formation of glycoxidation products during glycation of collagen. Biochemistry 34:15134–15141 [DOI] [PubMed] [Google Scholar]
- Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW 1995 Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry 34:3702–3709 [DOI] [PubMed] [Google Scholar]
- Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M 2000 Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA 97:12222–12226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S, Bacote V, Traxinger RR 1991 Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem 266:4706–4712 [PubMed] [Google Scholar]
- Monauni T, Zenti MG, Cretti A, Daniels MC, Targher G, Caruso B, Caputo M, McClain D, Del Prato S, Giaccari A, Muggeo M, Bonora E, Bonadonna RC 2000 Effects of glucosamine infusion on insulin secretion and insulin action in humans. Diabetes 49:926–935 [DOI] [PubMed] [Google Scholar]
- Green K, Brand MD, Murphy MP 2004 Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes 53(Suppl 1):S110–S118 [DOI] [PubMed] [Google Scholar]
- Maechler P, Jornot L, Wollheim CB 1999 Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic β cells. J Biol Chem 274:27905–27913 [DOI] [PubMed] [Google Scholar]
- Robertson RP 2004 Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet β cells in diabetes. J Biol Chem 279:42351–42354 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Tran PO, LeRoy E, Harmon JS, Tanaka Y, Robertson RP 2004 d-Glyceraldehyde causes production of intracellular peroxide in pancreatic islets, oxidative stress, and defective β cell function via non-mitochondrial pathways. J Biol Chem 279:37316–37323 [DOI] [PubMed] [Google Scholar]
- Taniguchi S, Okinaka M, Tanigawa K, Miwa I 2000 Difference in mechanism between glyceraldehyde- and glucose-induced insulin secretion from isolated rat pancreatic islets. J Biochem (Tokyo) 127:289–295 [DOI] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES 2006 Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440:944–948 [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM 2002 Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23:599–622 [DOI] [PubMed] [Google Scholar]
- Harmon JS, Stein R, Robertson RP 2005 Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic β cells. J Biol Chem 280:11107–11113 [DOI] [PubMed] [Google Scholar]
- Wang H, Brun T, Kataoka K, Sharma AJ, Wollheim CB 2007 MAFA controls genes implicated in insulin biosynthesis and secretion. Diabetologia 50:348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raum JC, Gerrish K, Artner I, Henderson E, Guo M, Sussel L, Schisler JC, Newgard CB, Stein R 2006 FoxA2, Nkx2.2, and PDX-1 regulate islet β-cell-specific MafA expression through conserved sequences located between base pairs -8118 and -7750 upstream from the transcription start site. Mol Cell Biol 26:5735–5743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D 2005 FoxO1 protects against pancreatic β cell failure through NeuroD and MafA induction. Cell Metab 2:153–163 [DOI] [PubMed] [Google Scholar]
- Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, Eppler E, Reinecke M 2005 Mechanisms of β-cell death in type 2 diabetes. Diabetes 54(Suppl 2):S108–S113 [DOI] [PubMed] [Google Scholar]
- Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY 2002 Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC 2003 β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110 [DOI] [PubMed] [Google Scholar]
- Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z, Perego C, Usellini L, Nano R, Bonini P, Bertuzzi F, Marlier LN, Davalli AM, Carandente O, Pontiroli AE, Melino G, Marchetti P, Lauro R, Sesti G, Folli F 2001 High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes 50:1290–1301 [DOI] [PubMed] [Google Scholar]
- Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, Polonsky K 1998 Role of apoptosis in failure of β-cell mass compensation for insulin resistance and β-cell defects in the male Zucker diabetes fatty rat. Diabetes 47:358–364 [DOI] [PubMed] [Google Scholar]
- Finegood DT, McArthur MD, Kojwang D, Thomas MJ, Topp BG, Leonard T, Buckingham RE 2001 β-Cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes 50:1021–1029 [DOI] [PubMed] [Google Scholar]
- Shin CS, Moon BS, Park KS, Kim SY, Park SJ, Chung MH, Lee HK 2001 Serum 8-hydroxy-guanine levels are increased in diabetic patients. Diabetes Care 24:733–737 [DOI] [PubMed] [Google Scholar]
- Gopaul NK, Anggard EE, Mallet AI, Betteridge DJ, Wolff SP, Nourooz-Zadeh J 1995 Plasma 8-epi-PGF2 α levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett 368:225–229 [DOI] [PubMed] [Google Scholar]
- Nourooz-Zadeh J, Tajaddini-Sarmadi J, McCarthy S, Betteridge DJ, Wolff SP 1995 Elevated levels of authentic plasma hydroperoxides in NIDDM. Diabetes 44:1054–1058 [DOI] [PubMed] [Google Scholar]
- Rehman A, Nourooz-Zadeh J, Moller W, Tritschler H, Pereira P, Halliwell B 1999 Increased oxidative damage to all DNA bases in patients with type II diabetes mellitus. FEBS Lett 448:120–122 [DOI] [PubMed] [Google Scholar]
- Ghiselli A, Laurenti O, De Mattia G, Maiani G, Ferro-Luzzi A 1992 Salicylate hydroxylation as an early marker of in vivo oxidative stress in diabetic patients. Free Radic Biol Med 13:621–626 [DOI] [PubMed] [Google Scholar]
- Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S 2002 Reduced β-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese type II diabetic patients. Diabetologia 45:85–96 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Hirokawa J, Tagami S, Kawakami Y, Urata Y, Kondo T 1995 Weakened cellular scavenging activity against oxidative stress in diabetes mellitus: regulation of glutathione synthesis and efflux. Diabetologia 38:201–210 [DOI] [PubMed] [Google Scholar]
- Del Guerra S, Lupi R, Marselli L, Masini M, Bugliani M, Sbrana S, Torri S, Pollera M, Boggi U, Mosca F, Del Prato S, Marchetti P 2005 Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes 54:727–735 [DOI] [PubMed] [Google Scholar]
- Robertson RP, Zhou H, Zhang T, Harmon JS, Chronic oxidative stress as a mechanism for glucose toxicity of the β-cell in type 2 diabetes. Cell Biochem Biophys, in press [DOI] [PubMed] [Google Scholar]
- Paolisso G, D'Amore A, Galzerano D, Balbi V, Giugliano D, Varricchio M, D'Onofrio F 1993 Daily vitamin E supplements improve metabolic control but not insulin secretion in elderly type II diabetic patients. Diabetes Care 16:1433–1437 [DOI] [PubMed] [Google Scholar]
- Sako Y, Grill VE 1990 A 48-hour lipid infusion in the rat time-dependently inhibits glucose-induced insulin secretion and β-cell oxidation through a process likely coupled to fatty acid oxidation. Endocrinology 127:1580–1589 [DOI] [PubMed] [Google Scholar]
- Elks ML 1993 Chronic perifusion of rat islets with palmitate suppresses glucose-stimulated insulin release. Endocrinology 133:208–214 [DOI] [PubMed] [Google Scholar]
- Zhou Y-P, Grill V 1995 Long-term exposure to fatty acids and ketones inhibits β-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab 80:1584–1590 [DOI] [PubMed] [Google Scholar]
- Zhou YP, Grill VE 1994 Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose-fatty acid cycle. J Clin Invest 93:870–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremlich S, Bonny C, Waeber G, Thorens B 1997 Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. J Biol Chem 272:30261–30269 [DOI] [PubMed] [Google Scholar]
- Ritz-Laser B, Meda P, Constant I, Klages N, Charollais A, Morales A, Magnan C, Ktorza A, Philippe J 1999 Glucose-induced preproinsulin gene expression is inhibited by the free-fatty acid palmitate. Endocrinology 140:4005–4014 [DOI] [PubMed] [Google Scholar]
- Jacqueminet S, Briaud I, Rouault C, Reach G, Poitout V 2000 Inhibition of insulin gene expression by long-term exposure of pancreatic β-cells to palmitate is dependent upon the presence of a stimulatory glucose concentration. Metabolism 49:532–536 [DOI] [PubMed] [Google Scholar]
- Briaud I, Harmon JS, Kelpe CL, Segu VB, Poitout V 2001 Lipotoxicity of the pancreatic β-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes 50:315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V 2003 Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem 278:30015–30021 [DOI] [PubMed] [Google Scholar]
- Hagman DK, Hays LB, Parazzoli SD, Poitout V 2005 Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J Biol Chem 280:32413–32418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro M, Zhou Y-T, Levi M, Unger RH 1998 Fatty-acid-induced β-cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA 95:2498–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Hannaert JC, Hoorens A, Eizirik DL, Pipeleers DG 2001 Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes 50:1771–1777 [DOI] [PubMed] [Google Scholar]
- Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY 2001 Distinct effects of saturated and monounsaturated fatty acids on β-cell turnover and function. Diabetes 50:69–76 [DOI] [PubMed] [Google Scholar]
- Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, Bergamini E, Mosca F, Di Mario U, Del Prato S, Marchetti P 2002 Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that β-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes 51:1437–1442 [DOI] [PubMed] [Google Scholar]
- Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ 2002 Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic β-cells (INS-1). J Biol Chem 277:49676–49684 [DOI] [PubMed] [Google Scholar]
- Piro S, Anello M, Di Pietro C, Lizzio MN, Patane G, Rabuazzo AM, Vigneri R, Purrello M, Purrello F 2002 Chronic exposure to free fatty acids or high glucose induces apoptosis in rat pancreatic islets: possible role of oxidative stress. Metabolism 51:1340–1347 [DOI] [PubMed] [Google Scholar]
- Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY 2003 Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic β-cell turnover and function. Diabetes 52:726–733 [DOI] [PubMed] [Google Scholar]
- Maestre I, Jordan J, Calvo S, Reig JA, Cena V, Soria B, Prentki M, Roche E 2003 Mitochondrial dysfunction is involved in apoptosis induced by serum withdrawal and fatty acids in the β-cell line INS-1. Endocrinology 144:335–345 [DOI] [PubMed] [Google Scholar]
- El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, Joly E, Dbaibo G, Rosenberg L, Prentki M 2003 Saturated fatty acids synergize with elevated glucose to cause pancreatic β-cell death. Endocrinology 144:4154–4163 [DOI] [PubMed] [Google Scholar]
- Harmon JS, Gleason CE, Tanaka Y, Poitout V, Robertson RP 2001 Antecedent hyperglycemia, not hyperllipidemia, is associated with increased islet triacylglycerol content and decreased insulin gene mRNA level in Zucker diabetic fatty rats. Diabetes 50:2481–2486 [DOI] [PubMed] [Google Scholar]
- Briaud I, Kelpe CL, Johnson LM, Tran POT, Poitout V 2002 Differential effects of hyperlipidemia on insulin secretion in islets of Langerhans from hyperglycemic vs. normoglycemic rats. Diabetes 51:662–668 [DOI] [PubMed] [Google Scholar]
- Dubois M, Kerr-Conte J, Gmyr V, Bouckenooghe T, Muharram G, D'Herbomez M, Martin-Ponthieu A, Vantyghem MC, Vandewalle B, Pattou F 2004 Non-esterified fatty acids are deleterious for human pancreatic islet function at physiological glucose concentration. Diabetologia 47:463–469 [DOI] [PubMed] [Google Scholar]
- Mason TM, Goh T, Tchipashvili V, Sandhu H, Gupta N, Lewis GF, Giacca A 1999 Prolonged elevation of plasma free fatty acids desensitizes the insulin secretory response to glucose in vivo in rats. Diabetes 48:524–530 [DOI] [PubMed] [Google Scholar]
- Paolisso G, Gambardella A, Amato L, Tortoriello R, D'Amore A, Varrichio M, D'Onofrio F 1995 Opposite effects of short- and long-term fatty acid infusion on insulin secretion in healthy subjects. Diabetologia 38:1295–1299 [DOI] [PubMed] [Google Scholar]
- Hosokawa H, Corkey BE, Leahy JL 1997 β-Cell hypersensitivity to glucose following 24-h exposure of rat islets to fatty acids. Diabetologia 40:392–397 [DOI] [PubMed] [Google Scholar]
- Liu YQ, Tornheim K, Leahy JL 1998 Fatty-acid induced β-cell hypersensitivity to glucose. Increased phosphofructokinase activity and lowered glucose-6-phosphate content. J Clin Invest 101:1870–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YQ, Tornheim K, Leahy JL 1998 Shared biochemical properties of glucotoxicity and lipotoxicity in islets decrease citrate synthase activity and increase phosphofructokinase activity. Diabetes 47:1889–1893 [DOI] [PubMed] [Google Scholar]
- Magnan C, Collins S, Berthault MF, Kassis N, Vincent M, Gilbert M, Penicaud L, Ktorza A, Assimacopoulos-Jeannet F 1999 Lipid infusion lowers sympathetic nervous activity and leads to increased β-cell responsiveness to glucose. J Clin Invest 103:413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls Jr HR, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI 2003 The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 278:11303–11311 [DOI] [PubMed] [Google Scholar]
- Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M 2003 Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 422:173–176 [DOI] [PubMed] [Google Scholar]
- Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H 2005 The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab 1:245–258 [DOI] [PubMed] [Google Scholar]
- Latour MG, Alquier T, Oseid E, Tremblay C, Jetton TL, Luo J, Lin DC, Poitout V 2007 GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes 56:1087–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C-Y, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig A-J, Boss O, Kim Y-B, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB 2001 Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, β-cell dysfunction, and type 2 diabetes. Cell 105:745–755 [DOI] [PubMed] [Google Scholar]
- Chan CB, MacDonald PE, Saleh MC, Johns DC, Marban E, Wheeler MB 1999 Overexpression of uncoupling protein 2 inhibits glucose-stimulated insulin secretion from rat islets. Diabetes 48:1482–1486 [DOI] [PubMed] [Google Scholar]
- Chan CB, De Leo D, Joseph JW, McQuaid TS, Ha XF, Xu F, Tsushima RG, Pennefather PS, Salapatek AM, Wheeler MB 2001 Increased uncoupling protein-2 levels in β-cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes 50:1302–1310 [DOI] [PubMed] [Google Scholar]
- Joseph JW, Koshkin V, Zhang CY, Wang J, Lowell BB, Chan CB, Wheeler MB 2002 Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes 51:3211–3219 [DOI] [PubMed] [Google Scholar]
- Lameloise N, Muzzin P, Prentki M, Assimacopoulos-Jeannet F 2001 Uncoupling protein 2: a possible link between fatty acid excess and impaired glucose-induced insulin secretion? Diabetes 50:803–809 [DOI] [PubMed] [Google Scholar]
- Patane G, Anello M, Piro S, Vigneri R, Purrello F, Rabuazzo AM 2002 Role of ATP production and uncoupling protein-2 in the insulin secretory defect induced by chronic exposure to high glucose or free fatty acids and effects of peroxisome proliferator-activated receptor-γ inhibition. Diabetes 51:2749–2756 [DOI] [PubMed] [Google Scholar]
- Medvedev AV, Robidoux J, Bai X, Cao W, Floering LM, Daniel KW, Collins S 2002 Regulation of the uncoupling protein-2 gene in INS-1 β-cells by oleic acid. J Biol Chem 277:42639–42644 [DOI] [PubMed] [Google Scholar]
- Medvedev AV, Snedden SK, Raimbault S, Ricquier D, Collins S 2001 Transcriptional regulation of the mouse uncoupling protein-2 gene. Double E-box motif is required for peroxisome proliferator-activated receptor-γ-dependent activation. J Biol Chem 276:10817–10823 [DOI] [PubMed] [Google Scholar]
- Produit-Zengaffinen N, Davis-Lameloise N, Perreten H, Becard D, Gjinovci A, Keller PA, Wollheim CB, Herrera P, Muzzin P, Assimacopoulos-Jeannet F 2007 Increasing uncoupling protein-2 in pancreatic β cells does not alter glucose-induced insulin secretion but decreases production of reactive oxygen species. Diabetologia 50:84–93 [DOI] [PubMed] [Google Scholar]
- Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, Marsh BJ, Rodrigues B, Johnson JD, Parks JS, Verchere CB, Hayden MR 2007 β-Cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med 13:340–347 [DOI] [PubMed] [Google Scholar]
- Kato T, Shimano H, Yamamoto T, Yokoo T, Endo Y, Ishikawa M, Matsuzaka T, Nakagawa Y, Kumadaki S, Yahagi N, Takahashi A, Sone H, Suzuki H, Toyoshima H, Hasty AH, Takahashi S, Gomi H, Izumi T, Yamada N 2006 Granuphilin is activated by SREBP-1c and involved in impaired insulin secretion in diabetic mice. Cell Metab 4:143–154 [DOI] [PubMed] [Google Scholar]
- Olofsson CS, Collins S, Bengtsson M, Eliasson L, Salehi A, Shimomura K, Tarasov A, Holm C, Ashcroft F, Rorsman P 2007 Long-term exposure to glucose and lipids inhibits glucose-induced insulin secretion downstream of granule fusion with plasma membrane. Diabetes 56:1888–1897 [DOI] [PubMed] [Google Scholar]
- Moore PC, Ugas MA, Hagman DK, Parazzoli SD, Poitout V 2004 Evidence against the involvement of oxidative stress in fatty acid inhibition of insulin secretion. Diabetes 53:2610–2616 [DOI] [PubMed] [Google Scholar]
- Mathias S, Pena LA, Kolesnick RN 1998 Signal transduction of stress via ceramide. Biochem J 335:465–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E, Stein R 1994 c-Jun inhibits transcriptional activation by the insulin enhancer, and the insulin control element is the target of control. Mol Cell Biol 14:655–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GL, Henderson E, Massari ME, Murre C, Stein R 1995 c-Jun inhibits insulin control element-mediated transcription by affecting the transactivation potential of the E2A gene products. Mol Cell Biol 15:1398–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas G, Naugler W, Galimi F, Lee MS, Karin M 2006 Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proc Natl Acad Sci USA 103:16454–16459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers SA, Garza LA, Zhou H, Birnbaum MJ 1998 Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol 18:5457–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Summers SA, Birnbaum MJ, Pittman RN 1998 Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J Biol Chem 273:16568–16575 [DOI] [PubMed] [Google Scholar]
- Schmitz-Peiffer C, Craig DL, Biden TJ 1999 Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 274:24202–24210 [DOI] [PubMed] [Google Scholar]
- Bourbon NA, Yun J, Kester M 2000 Ceramide directly activates protein kinase C ζ to regulate a stress-activated protein kinase signaling complex. J Biol Chem 275:35617–35623 [DOI] [PubMed] [Google Scholar]
- Doornbos RP, Theelen M, van der Hoeven PC, van Blitterswijk WJ, Verkleij AJ, van Bergen en Henegouwen PM 1999 Protein kinase Cζ is a negative regulator of protein kinase B activity. J Biol Chem 274:8589–8596 [DOI] [PubMed] [Google Scholar]
- Whiteman EL, Cho H, Birnbaum MJ 2002 Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab 13:444–451 [DOI] [PubMed] [Google Scholar]
- Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs 3rd WH, Wright CV, White MF, Arden KC, Accili D 2002 The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J Clin Invest 110:1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae J, Biggs 3rd WH, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D 2002 Regulation of insulin action and pancreatic β-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet 32:245–253 [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese Jr RV, Ory DS, Schaffer JE 2003 Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 100:3077–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch AK, Gurisik E, Cordery DV, Sudlow M, Denyer GS, Laybutt DR, Hughes WE, Biden TJ 2005 Increased fatty acid desaturation and enhanced expression of stearoyl coenzyme A desaturase protects pancreatic β-cells from lipoapoptosis. Diabetes 54:2917–2924 [DOI] [PubMed] [Google Scholar]
- Shimabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH 1998 Lipoapoptosis in β-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem 273:32487–32490 [DOI] [PubMed] [Google Scholar]
- Roduit R, Morin J, Masse F, Segall L, Roche E, Newgard CB, Assimacopoulos-Jeannet F, Prentki M 2000 Glucose down-regulates the expression of the peroxisome proliferator-activated receptor-α gene in the pancreatic β-cell. J Biol Chem 275:35799–35806 [DOI] [PubMed] [Google Scholar]
- Ruderman N, Prentki M 2004 AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov 3:340–351 [DOI] [PubMed] [Google Scholar]
- Wang X, Li H, De Leo D, Guo W, Koshkin V, Fantus IG, Giacca A, Chan CB, Der S, Wheeler MB 2004 Gene and protein kinase expression profiling of reactive oxygen species-associated lipotoxicity in the pancreatic β-cell line MIN6. Diabetes 53:129–140 [DOI] [PubMed] [Google Scholar]
- Morgan D, Oliveira-Emilio HR, Keane D, Hirata AE, Santos da Rocha M, Bordin S, Curi R, Newsholme P, Carpinelli AR 2007 Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal β cell line. Diabetologia 50:359–369 [DOI] [PubMed] [Google Scholar]
- Busch AK, Cordery D, Denyer GS, Biden TJ 2002 Expression profiling of palmitate- and oleate-regulated genes provides novel insights into the effects of chronic lipid exposure on pancreatic β-cell function. Diabetes 51:977–987 [DOI] [PubMed] [Google Scholar]
- Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL 2004 Free fatty acids and cytokines induce pancreatic β-cell apoptosis by different mechanisms: role of nuclear factor-κB and endoplasmic reticulum stress. Endocrinology 145:5087–5096 [DOI] [PubMed] [Google Scholar]
- Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A 2006 Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic β-cell apoptosis. Endocrinology 147:3398–3407 [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ 2007 Endoplasmic reticulum stress contributes to β cell apoptosis in type 2 diabetes. Diabetologia 50:752–763 [DOI] [PubMed] [Google Scholar]
- Richieri GV, Anel A, Kleinfeld AM 1993 Interactions of long-chain fatty acids and albumin: determination of free fatty acid levels using the fluorescent probe ADIFAB. Biochemistry 32:7574–7580 [DOI] [PubMed] [Google Scholar]
- Richieri GV, Kleinfeld AM 1995 Unbound free fatty acid levels in human serum. J Lipid Res 36:229–240 [PubMed] [Google Scholar]
- Cruz WS, Kwon G, Marshall CA, McDaniel ML, Semenkovich CF 2001 Glucose and insulin stimulate heparin-releasable lipoprotein lipase activity in mouse islets and INS-1 cells. A potential link between insulin resistance and β-cell dysfunction. J Biol Chem 276:12162–12168 [DOI] [PubMed] [Google Scholar]
- Unger RH 2003 Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology 144:5159–5165 [DOI] [PubMed] [Google Scholar]
- Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Gtand B 1998 A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392:398–401 [DOI] [PubMed] [Google Scholar]
- Goh TT, Mason TM, Gupta N, So A, Lam TK, Lam L, Lewis GF, Mari A, Giacca A 2007 Lipid-induced β-cell dysfunction in vivo in models of progressive β-cell failure. Am J Physiol Endocrinol Metab 292:E549–E560 [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X, Rosner J, Barton M 1995 Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes 44:1239–1242 [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X 1999 Effects of fatty acids and ketone bodies on basal insulin secretion in type 2 diabetes. Diabetes 48:577–583 [DOI] [PubMed] [Google Scholar]
- Carpentier A, Mittelman S, Lamarche B, Bergman R, Giacca A, Lewis G 1999 Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. Am J Physiol 276:E1055–E1066 [DOI] [PubMed] [Google Scholar]
- Carpentier A, Giacca A, Lewis GF 2001 Effect of increased plasma non-esterified fatty acids (NEFAs) on arginine-stimulated insulin secretion in obese humans. Diabetologia 44:1989–1997 [DOI] [PubMed] [Google Scholar]
- Carpentier A, Mittelman SD, Bergman RN, Giacca A, Lewis GF 2000 Prolonged elevation of plasma free fatty acids impairs pancreatic β-cell function in obese nondiabetic humans but not in individuals with type 2 diabetes. Diabetes 49:399–408 [DOI] [PubMed] [Google Scholar]
- Leung N, Sakaue T, Carpentier A, Uffelman K, Giacca A, Lewis GF 2004 Prolonged increase of plasma non-esterified fatty acids fully abolishes the stimulatory effect of 24 hours of moderate hyperglycaemia on insulin sensitivity and pancreatic β-cell function in obese men. Diabetologia 47:204–213 [DOI] [PubMed] [Google Scholar]
- Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K 2003 A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 52:2461–2474 [DOI] [PubMed] [Google Scholar]
- Cusi K, Kashyap S, Gastaldelli A, Bajaj M, Cersosimo E 2007 Effects on insulin secretion and insulin action of a 48-h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Endocrinol Metab 292:E1775–E1781 [DOI] [PubMed] [Google Scholar]
- Poitout V 2002 Lipid partitioning in the pancreatic β-cell: physiologic and pathophysiologic implications. Curr Opin Endocrinol Diabetes 9:152–159 [Google Scholar]
- Prentki M, Corkey BE 1996 Are the β-cell signaling molecules malonyl-CoA and cytosolic long-chain acyl-CoA implicated in multiple tissue defects of obesity and NIDDM? Diabetes 45:273–283 [DOI] [PubMed] [Google Scholar]
- Poitout V, Robertson RP 2002 Minireview: secondary β-cell failure in type 2 diabetes—a convergence of glucotoxicity and lipotoxicity. Endocrinology 143:339–342 [DOI] [PubMed] [Google Scholar]
- Roche E, Farfari S, Witters LA, Assimacopoulos-Jeannet F, Thumelin S, Brun T, Corkey BE, Saha AK, Prentki M 1998 Long-term exposure of β-INS cells to high glucose concentrations increases anaplerosis, lipogenesis, and lipogenic gene expression. Diabetes 47:1086–1094 [DOI] [PubMed] [Google Scholar]
- Hardie DG 2003 Minireview: the AMP-activated protein kinase cascade—the key sensor of cellular energy status. Endocrinology 144:5179–5183 [DOI] [PubMed] [Google Scholar]
- Salt IP, Johnson G, Ashcroft SJ, Hardie DG 1998 AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic β-cells, and may regulate insulin release. Biochem J 335:533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhou L, Li G, Luo T, Gu Y, Qian L, Fu X, Li F, Li J, Luo M 2007 Palmitate activates AMP-activated protein kinase and regulates insulin secretion from β cells. Biochem Biophys Res Commun 352:463–468 [DOI] [PubMed] [Google Scholar]
- Foufelle F, Ferre P 2002 New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Biochem J 366:377–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe SS, Choi AH, Lee JW, Kim KH, Chung JJ, Park J, Lee KM, Park KG, Lee IK, Kim JB 2007 Chronic activation of liver X receptor induces β-cell apoptosis through hyperactivation of lipogenesis: liver X receptor-mediated lipotoxicity in pancreatic β-cells. Diabetes 56:1534–1543 [DOI] [PubMed] [Google Scholar]
- Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH 1994 β-Cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-β-cell relationships. Proc Natl Acad Sci USA 91:10878–10882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Hirose H, Zhou Y-T, Esser V, McGarry JD, Unger RH 1997 Increased lipogenic capacity of the islets of obese rats. A role in the pathogenesis of NIDDM. Diabetes 46:408–413 [DOI] [PubMed] [Google Scholar]
- Kelpe CL, Johnson LM, Poitout V 2002 Increasing triglyceride synthesis inhibits glucose-induced insulin secretion in isolated rat islets of Langerhans. A study using adenoviral expression of diacylglycerol acyltransferase. Endocrinology 143:3326–3332 [DOI] [PubMed] [Google Scholar]
- Prentki M, Nolan CJ 2006 Islet β cell failure in type 2 diabetes. J Clin Invest 116:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA 2007 Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5:167–179 [DOI] [PubMed] [Google Scholar]
- Boucher A, Lu D, Burgess SC, Telemaque-Potts S, Jensen MV, Mulder H, Wang MY, Unger RH, Sherry AD, Newgard CB 2004 Biochemical mechanism of lipid-induced impairment of glucose-stimulated insulin secretion and reversal with a malate analogue. J Biol Chem 279:27263–27271 [DOI] [PubMed] [Google Scholar]
- Cnop M, Hannaert JC, Grupping AY, Pipeleers DG 2002 Low density lipoprotein can cause death of islet β-cells by its cellular uptake and oxidative modification. Endocrinology 143:3449–3453 [DOI] [PubMed] [Google Scholar]
- Abderrahmani A, Niederhauser G, Favre D, Abdelli S, Ferdaoussi M, Yang JY, Regazzi R, Widmann C, Waeber G 2007 Human high-density lipoprotein particles prevent activation of the JNK pathway induced by human oxidised low-density lipoprotein particles in pancreatic β cells. Diabetologia 50:1304–1314 [DOI] [PubMed] [Google Scholar]
- Mitro N, Mak PA, Vargas L, Godio C, Hampton E, Molteni V, Kreusch A, Saez E 2007 The nuclear receptor LXR is a glucose sensor. Nature 445:219–223 [DOI] [PubMed] [Google Scholar]
- Fex M, Nitert MD, Wierup N, Sundler F, Ling C, Mulder H 2006 Enhanced mitochondrial metabolism may account for the adaptation to insulin resistance in islets from C57BL/6J mice fed a high-fat diet. Diabetologia 50:74–83 [DOI] [PubMed] [Google Scholar]
- Nolan CJ, Leahy JL, Delghingaro-Augusto V, Moibi J, Soni K, Peyot ML, Fortier M, Guay C, Lamontagne J, Barbeau A, Przybytkowski E, Joly E, Masiello P, Wang S, Mitchell GA, Prentki M 2006 β Cell compensation for insulin resistance in Zucker fatty rats: increased lipolysis and fatty acid signalling. Diabetologia 49:2120–2130 [DOI] [PubMed] [Google Scholar]
- Leibowitz G, Uckaya G, Oprescu AI, Cerasi E, Gross DJ, Kaiser N 2002 Glucose-regulated proinsulin gene expression is required for adequate insulin production during chronic glucose exposure. Endocrinology 143:3214–3220 [DOI] [PubMed] [Google Scholar]
- 1995 U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes 44:1249–1258 [PubMed] [Google Scholar]
- Donath M, Storling J, Berchtold LA, Billestrup N, Mandrup-Poulsen T 2008 Cytokines and β-cell biology: from concept to clinical translation. Endocr Rev 29:334–350 [DOI] [PubMed] [Google Scholar]
- Scheuner D, Kaufman RJ 2008 The unfolded protein response: a pathway that links insulin demand with β-cell failure and diabetes. Endocr Rev 29:317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haataja L, Gurlo T, Huang CJ, Butler PC 2008 Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev 29:303–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.