Abstract
The endoplasmic reticulum (ER) is the entry site into the secretory pathway for newly synthesized proteins destined for the cell surface or released into the extracellular milieu. The study of protein folding and trafficking within the ER is an extremely active area of research that has provided novel insights into many disease processes. Cells have evolved mechanisms to modulate the capacity and quality of the ER protein-folding machinery to prevent the accumulation of unfolded or misfolded proteins. These signaling pathways are collectively termed the unfolded protein response (UPR). The UPR sensors signal a transcriptional response to expand the ER folding capacity, increase degredation of malfolded proteins, and limit the rate of mRNA translation to reduce the client protein load. Recent genetic and biochemical evidence in both humans and mice supports a requirement for the UPR to preserve ER homeostasis and prevent the β-cell failure that may be fundamental in the etiology of diabetes. Chronic or overwhelming ER stress stimuli associated with metabolic syndrome can disrupt protein folding in the ER, reduce insulin secretion, invoke oxidative stress, and activate cell death pathways. Therapeutic interventions to prevent polypeptide-misfolding, oxidative damage, and/or UPR-induced cell death have the potential to improve β-cell function and/or survival in the treatment of diabetes.
I. Introduction
II. The Endoplasmic Reticulum
- III. ER Transmembrane Sensors of the UPR: IRE1α, ATF6, and PERK
- A. IRE1α initiates splicing of Xbp1 mRNA
- B. ATF6α cleavage regulates UPR transcription
- C. PERK-mediated eIF2α phosphorylation regulates mRNA translation
- IV. ER Stress-Induced Apoptosis: Multiple Pathways to Death
- A. Chop deletion protects from ER stress-induced death
- B. IRE1α mediates JNK activation
- V. ER Stress Stimuli and Physiological Regulation of the UPR
- A. IAPP induces the UPR
- B. Oxidative protein folding, oxidative stress, and activation of the UPR are closely linked events
- C. Glucose regulates protein folding and oxidative stress
- D. Fatty acids and cytokines activate UPR signaling
- VI. The UPR and Diabetes—A Causal Role of ER Stress in Diabetes of Men and Mice
- A. UPR markers are detectable in islets from diabetic men and mice
- B. Mutant proinsulin is sufficient to induce β-cell failure and diabetes
- C. Gene mutations in the PERK/ eIF2α pathway demonstrate that reduced UPR signaling is sufficient to cause diabetes in humans and mice
- D. Deletion of the putative ER co-chaperone gene p58IPK causes destruction of islet mass and diabetes
- E. Wolfram syndrome is a genetic disease that results in ER dysfunction and β-cell failure
- F. What will we learn from deletion of the IRE1α and ATF6 UPR signaling pathways in mice?
- VII. How Can the Accumulation of Misfolded Protein and ER Stress Be Managed to Prevent β-Cell Failure?
- A. Intervention to improve protein folding
- B. Intervention to inhibit cell death signals
VIII. Conclusion
I. Introduction
IN EUKARYOTIC CELLS, protein synthesis and secretion are precisely coupled with the capacity of the endoplasmic reticulum (ER) to fold, process, and traffic proteins to the cell surface. These processes are coupled through several signal transduction pathways collectively known as the unfolded protein response (UPR). The UPR functions to reduce the amount of nascent protein that enters the ER lumen, to increase the ER capacity to fold protein through transcriptional up-regulation of ER chaperones and folding catalysts, and to induce degradation of misfolded and aggregated protein. The presence of unfolded proteins in the ER lumen is sensed through the transmembrane signaling proteins dsRNA-activated protein kinase (PKR)-like ER kinase; (PERK), inositol requiring protein 1α (IRE1α), and activating transcription factor 6 (ATF6). Human genetic diseases and murine genetic models definitively demonstrate that the capacity for insulin production in β-cells is coupled with their survival and requires the protein kinase activity of the UPR sensor PERK and phosphorylation of its major physiological substrate, eukaryotic initiation factor 2, on the α-subunit (eIF2α). PERK senses the biosynthetic protein-folding load on the ER and, through phosphorylation of eIF2α, attenuates and thereby controls the rate of mRNA translation initiation to limit new protein synthesis when the unfolded protein load exceeds the capacity for protein folding. This signaling pathway is essential to prevent accumulation of unfolded polypeptides in the ER lumen, a condition that leads to cell death.
Here, we consider how physiological stimuli can cause accumulation of unfolded protein and activate UPR signaling through IRE1α, PERK, and ATF6. The signaling reactions initiated by these sensors are required to maintain a hospitable environment for proinsulin polypeptide folding, especially under conditions of hyperglycemia or insulin resistance when the demand for insulin production increases dramatically. Furthermore, as the functional β-cell mass decreases in either type 1 or type 2 diabetes, it may be necessary for the remaining functional β-cells to compensate and increase their insulin production, a condition that would further increase the protein-folding demand on the ER.
Multiple studies have demonstrated that genetic disruption of UPR signaling pathways or modifications that produce an excessive protein-folding load on the ER impair protein folding and lead to β-cell death. The mechanisms that cause β-cells to fail upon ER stress are not well understood; however, recent studies suggest that induction of the proapoptotic gene Chop and production of reactive oxygen species (ROS) may be fundamental in the etiology of β-cell failure in diabetes. There is increasing evidence to encourage the development of small molecules that facilitate protein folding and/or inhibit ER stress-induced cell death for therapeutic use in the treatment of diabetes.
II. The Endoplasmic Reticulum
The ER is a membrane-bound organelle that supports the biosynthesis of approximately one third of the cellular protein in a eukaryotic cell (1). The ER provides a unique environment for oxidative protein folding and posttranslational modification of polypeptides destined for the plasma membrane, intracellular organelles, or the extracellular environment. Protein folding in the ER is facilitated through molecular chaperones and folding catalysts that maintain protein solubility, increase the rate of protein folding, and ensure that only properly folded proteins successfully traffic to the Golgi compartment. Protein folding initiates as the nascent polypeptide is translocated into the ER lumen, i.e., cotranslational, and is assisted by molecular chaperones including binding Ig protein (BiP), as well as additional mediators of protein folding including oxidoreductases that catalyze proper disulfide bond formation and rearrangement (2,3,4,5). Successfully folded polypeptides enter the anterograde trafficking pathway due to their interaction with specific cargo receptors and components of the ER-budding vesicles that contain COPII assembly proteins (6,7). However, the mechanisms that distinguish properly folded proteins that are suitable for traffic to the Golgi compartment and those that are improperly folded and subject to retention in the ER lumen for eventual destruction are very complex and poorly understood (8,9).
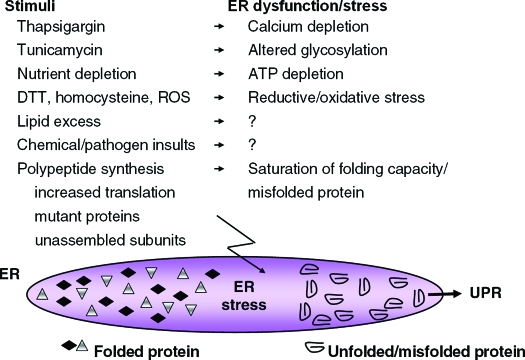
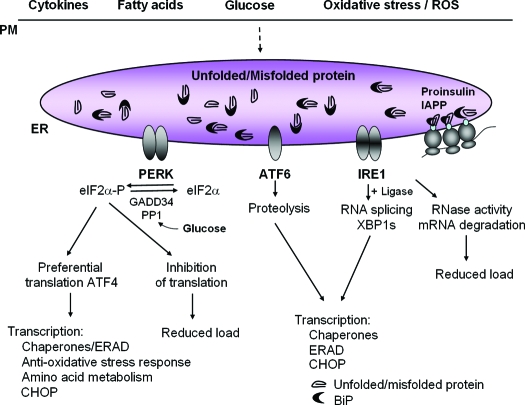
The composition of the ER lumen and the number and size of ER cisternae can vary depending upon cell type and extracellular stimuli. Secretory cells such as Ig-secreting B lymphocytes and pancreatic acinar cells are extensively laden with thin ER cisternae that can support the proper folding and processing of large amounts of secretory protein (10,11). The ER of the pancreatic β-cell produces almost 1 million molecules of insulin per minute. However, conditions that disrupt metabolic homeostasis and protein folding cause distension of the ER cisternae that is considered unfunctional for protein-folding and processing. ER distension has become a hallmark for cells that have defective protein-folding in the ER lumen and is observed in response to pharmacological induction of ER stress, genetically impaired N-linked glycosylation, enhanced mRNA translation, or expression of proteins that are subject to misfolding (Fig. 1) (12,13,14,15,16,17,18). IRE1, PERK, and ATF6 are the proximal sensors of unfolded protein accumulation in the ER and signal collectively to control the load of nascent polypeptides entering the ER lumen, the concentration of chaperones and catalysts of disulfide bond formation within the lumen, and the machinery for degradation of misfolded protein (Fig. 2).
Figure 1.
Pharmacological and physiological stimuli that cause ER stress and activate the UPR. Thapsigargin, tunicamycin, and dithiothreitol (DTT) are the most common pharmacological agents used to induce ER stress and misfolded protein within the ER lumen in vitro. Physiological stimuli that activate the UPR include nutrient deprivation, elevated lipids or cholesterol, homocysteine (169), numerous chemical insults such as ethanol and nonsteroidal antiinflammatory agents (170,171), viral and bacterial pathogens, and ROS. In addition, increased synthesis of proteins that transit the ER, expression of mutant inherently misfolded proteins, expression of difficult-to-fold polypeptides, or unbalanced expression of subunits of multimeric complexes can cause ER stress, accumulation of unfolded protein, and activation of the UPR. Question marks indicate that the mechanism by which ER stress is generated remains to be elucidated.
Figure 2.
The UPR sensors PERK, IRE1α, and ATF6 control mRNA translation and transcriptional induction of UPR-regulated genes. Interaction of BiP with each UPR sensor prevents UPR signaling. Upon accumulation of unfolded protein, BiP is released from each sensor, leading to its activation. The ER protein kinase PERK is activated by homodimerization and autophosphorylation to phosphorylate eIF2α, thereby reducing the rate of mRNA translation and the biosynthetic protein-folding load on the ER. eIF2α phosphorylation paradoxically increases translation of Atf4 mRNA to produce a transcription factor that activates expression of genes encoding protein chaperones, ERAD machinery, enzymes that reduce oxidative stress, and functions in amino acid biosynthesis and transport. Dimerization of the ER protein kinase IRE1α triggers its endoribonuclease activity to induce cleavage of Xbp1 mRNA. Xbp1 mRNA is then ligated by an uncharacterized RNA ligase and translated to produce XBP1s. Concurrently, ATF6 released from BiP transits to the Golgi where it is cleaved to release a transcriptionally active fragment. Cleaved ATF6 acts in concert with XBP1s to induce expression of genes encoding protein chaperones and ERAD machinery. The RNase activity of IRE1α also degrades selective cellular mRNAs to reduce the client protein load upon the ER. Physiological stimuli that can activate the UPR in the β-cell include expression of misfolded proinsulin or IAPP, oxidative stress (ROS), and increases in the extracellular concentrations of glucose, fatty acids, or cytokines.
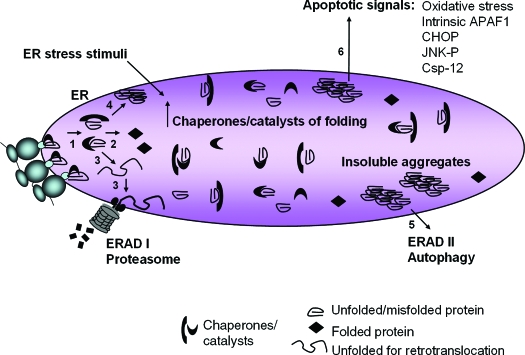
ER-associated degradation (ERAD) of misfolded protein is a crucial function needed to preserve the functionality of the ER. The lumen of the ER has a very high concentration of protein (>100 mg/ml) and an abundance of chaperones and catalysts to favor proper folding of nascent polypeptide chains. However, accumulation of misfolded protein impairs further folding of other nascent polypeptides and compromises cellular function in a number of ways including: 1) sequestration of chaperones with misfolded protein; 2) depletion of energy due to multiple interactions of an unfolded protein with chaperones having ATPase activity; and 3) depletion of reducing equivalents and generation of ROS due to repeated cycles of disulfide bond reduction and reformation. Misfolded, yet soluble, lumenal and membrane proteins undergo retrotranslocation into the cytosol for ubiquitinylation and proteasome-mediated degradation (ERAD I), whereas misfolded proteins that cannot maintain solubility aggregate and are eliminated by autophagy (ERAD II) (Fig. 3) (19,20,21). Interestingly, it was recently reported that hyperglycemia in vitro and in vivo causes proteins to form aggregates that are cleared from β-cells by an autophagy-dependent mechanism (22).
Figure 3.
Pathways of protein misfolding that lead to cell death. Nascent unfolded polypeptides enter the ER and interact with chaperones and catalysts of protein folding to mature into compact, thermodynamically favorable structures, (1) and (2). Failure of this process results in persistence of misfolded polypeptide-chaperone complexes (1) or extraction of soluble, misfolded protein from the ER and degradation through ERAD I (3). Formation of insoluble protein aggregates (4) requires clearance by autophagy (5). ER stress stimuli impair polypeptide folding and induce adaptive increases in chaperones and catalysts within the ER lumen through UPR sensor activation. Chronic or overwhelming stimuli elicit a number of apoptotic signals including oxidative stress, JNK activation, CHOP expression, cleavage of caspase 12, and activation of the intrinsic mitochondrial-dependent cell death pathway (6).
III. ER Transmembrane Sensors of the UPR: IRE1α, ATF6, and PERK
The three transducers of ER stress signaling IRE1α, ATF6, and PERK together emanate a transcriptional and translational program when unfolded proteins accumulate within the ER lumen (Fig. 2) (23,24,25). How does the accumulation of misfolded proteins in the ER lumen activate the UPR sensors? All three sensors (IREα, PERK, and ATF6) are maintained in an inactivate state through interaction with the protein chaperone BiP (26,27). As unfolded proteins accumulate in the ER, release of the protein kinases IRE1α and PERK from BiP permits their dimerization and activation. ATF6 released from BiP transits forward to the Golgi compartment where it is activated by proteolytic processing (28).
An important role for BiP in maintaining the protein-folding environment of the ER is highlighted by a recent report that deletion of the KDEL ER-retention signal of BiP yields a neonatal lethal phenotype with diminished synthesis and membrane localization of surfactant protein (29). Although it is not known how this mutation disrupts ER function to produce this phenotype, analysis of mouse embryonic fibroblasts (MEFs) from these mice suggested that increased secretion of KDEL-deleted BiP reduces BiP levels within the ER lumen. Several UPR genes were up-regulated in these mutant cells under basal conditions, suggesting that expression of KDEL-deleted BiP causes chronic ER stress. Future studies of BiP and the control of ER stress in β-cells should clarify the role of this chaperone/UPR regulator in insulin production.
The particular genes induced upon ER stress vary in different cell types depending upon the ER stress sensors that are activated, tissue-specific transcription factors, and the particular type, degree, and duration of ER stress (30,31,32). Acute or low-level chronic activation of the UPR serves a protective and adaptive function through chaperone induction; however, severe/chronic ER stress that cannot be resolved by the UPR leads to apoptotic cell death (Fig. 3) (13,33). The molecular components of these pathways have been well described and will be discussed briefly.
A. IRE1α initiates splicing of Xbp1 mRNA
The ER transmembrane protein kinase IRE1α is the most fundamental ER stress sensor because it is conserved in all eukaryotic cells (34). The IRE1α ER lumenal domain that regulates kinase activation is homologous to the lumenal domain of the second ER stress-sensing kinase PERK. Uniquely, IRE1α activation of its kinase subdomain elicits an endogenous endoribonuclease activity that specifically cleaves the mRNA encoding the basic leucine zipper-containing transcription factor X-box binding protein 1 (XBP1) to initiate an unconventional splicing reaction required for translation of transcriptionally active XBP1 (XBP1s) (Fig. 2) (35,36,37). IRE1α/XBP1 signaling activates transcription of a subset of UPR genes encoding chaperones, catalysts of folding, and ERAD degradation machinery including EDEM, EDEM2, EDEM3, Derlins-1–3, and HRD1 (38,39,40,41). Cells deleted in IRE1α or XBP1 are defective in ERAD, and it is now evident that the secretory capacity of the cell and survival to ER stress are linked to XBP1 signaling and ERAD function (42).
The RNase activity of IRE1α also degrades mRNAs encoding proteins that are translocated into the ER lumen to reduce the load of newly synthesized proteins that require folding (43). This finding was extended to analysis of ER stress and insulin mRNA levels in the β-cell (44). The findings demonstrated that pharmacologically induced calcium release from the ER induces ER stress and reduces insulin mRNA levels in INS-1E cells and in primary β-cells. ER stress in these studies was accompanied by a rapid initial decrease in the stability of Ins1 and Ins2 mRNAs. Future studies directed to evaluate whether degradation of Ins mRNA is mediated by RNase activity of IRE1α will provide mechanistic insight into how the protein-folding load in the ER regulates proinsulin expression in β-cells.
B. ATF6α cleavage regulates UPR transcription
There are two Atf6 genes in the mammalian genome, Atf6α and Atf6β, and both are expressed in all cell types. ATF6α and ATF6β are type II transmembrane proteins that have a basic leucine zipper and transcriptional activation domain in their cytosolic N-terminal region. The molecular mechanism of activation and functional role of ATF6α have been extensively studied. Accumulation of unfolded protein in the ER lumen induces release of ATF6α from the ER chaperone BiP and reduction of disulfide bridges in the lumenal domain of ATF6α to permit anterograde transit of ATF6α to the Golgi compartment where S1P (site 1 protease)- and S2P (site 2 protease)-mediated proteolytic cleavages produce a transcriptionally active cytosolic fragment (28,45,46). ATF6α acts to enhance induction of UPR genes encoding catalysts of protein folding and degradation (Fig. 2) (47,48). ATF6α is a coactivator of the UPR that interacts with nuclear factor-Y and XBP1 and is capable of binding all three ER-stress response elements, ERSE, UPRE, and ERSE-II in the promoters of UPR-responsive genes (49). Recently, Atf6α-null and Atf6β-null mice were produced and shown to have no significant phenotype. Atf6α-null MEFs isolated from these animals have a defective response to chronic ER stress, including impaired chaperone gene induction and reduced ERAD (47,48). Gene expression analysis identified a subpopulation of approximately 20% of UPR-regulated genes that require ATF6α for maximal induction upon ER stress, whereas approximately 10% of UPR-induced genes are entirely dependent on ATF6α for induction (47). These significant alterations in gene expression profile and resultant ER function upon ER stress suggest that there is a unique and crucial role for ATF6α signaling in support of protein folding, ERAD, and general ER function. In contrast, Atf6β-null MEFs did not have any detectable alterations in UPR gene induction. However, mice with null mutation in both Atf6α and Atf6β died in early murine embryonic development (48). This finding would suggest that ATF6α and ATF6β provide some complementary functions in early development.
C. PERK-mediated eIF2α phosphorylation regulates mRNA translation
Upon accumulation of unfolded protein in the ER lumen, the ER stress sensor PERK mediates phosphorylation of eukaryotic translation initiation factor 2 at Ser51 on the α-subunit (eIF2α) to inhibit mRNA translation, and thereby reduce the protein-folding load on the ER (Fig. 2) (50,51,52). However, there are several mRNAs that actually require eIF2α phosphorylation for translation. One well-studied example of an mRNA that requires eIF2α phosphorylation for translation encodes ATF4. ATF4 is a transcription factor that induces expression of a subset of UPR-regulated genes encoding 1) ER chaperones and ERAD machinery; 2) genes encoding an antioxidative stress response; and 3) genes that activate amino acid biosynthesis and transport (53,54,55,56). ATF4 also induces transcription of the C/EBP homologous protein CHOP (GADD153), a transcription factor that activates downstream genes encoding proapoptotic functions. PERK activation and eIF2α phosphorylation contribute significantly to the transcriptional induction of UPR-regulated genes in higher eukaryotes (54,55,57). Analysis of cells having PERK deletion or having mutation at the regulatory phosphorylation site in eIF2α demonstrated that PERK-mediated phosphorylation of eIF2α is required to resolve ER stress and for cell survival (53,54).
IV. ER Stress-Induced Apoptosis: Multiple Pathways to Death
A paradox of the UPR is that the response leads to the simultaneous activation of both adaptive and proapoptotic pathways. If the adaptive responses of the UPR cannot resolve ER stress, the cells enter an apoptotic death (Fig. 3). Both mitochondrial-dependent and -independent cell death pathways trigger apoptosis in response to ER stress. The ER might actually serve as a site where apoptotic signals are generated and integrated to elicit the death response. Apoptotic signals are produced at the ER by several mechanisms, including: PERK/eIF2α-dependent induction of the proapoptotic transcription factor CHOP; BAK/BAX-regulated Ca2+ release from the ER; IRE1α-mediated activation of apoptosis signal-regulating kinase 1 (ASK1)/c-Jun amino-terminal kinase (JNK); and cleavage and activation of procaspase 12.
A. Chop deletion protects from ER stress-induced death
The best characterized of these proapoptotic pathways is production of the CHOP/GADD153 transcription factor that is regulated by ATF4, and possibly ATF6 (57,58,59). Deletion of Chop partially protects both cells and mice from ER stress-mediated cell death (60). In vivo, Chop deletion protects mice from renal toxicity due to pharmacological induction of ER stress by tunicamycin, neuronal apoptosis induced by ischemia, and neuronal oxidative injury in a model of Parkinson’s disease (60,61,62,63). Chop deletion protects murine β-cells from death that results from either accumulation of misfolded mutant proinsulin or exposure to nitric oxide (64,65).
It is possible that CHOP mediates its proapoptotic effects through transcriptional activation of genes that regulate apoptosis and/or repression of genes that encode protective functions. CHOP activates transcription of Gadd34, a gene that encodes a targeting subunit of protein phosphatase-1. This phosphatase dephosphorylates eIF2α and restores mRNA translation upon recovery from ER stress (66,67,68). It was proposed that premature recovery of mRNA translation before resolution of the ER stress condition could be detrimental to the cell through generation of ROS (61). CHOP induces expression of additional genes that encode functions in apoptosis, including death receptor 5 DR5 (69), tribbles 3 TRB3 (70), and the BCL2 homology 3 (BH3)-only containing B cell lymphoma 2 (BCL2) family member BCL2 interacting mediator of cell death (BIM) (71). CHOP expression also leads to the induction of the downstream of Chop genes, Doc1, carbonic anhydrase CA-VI; Doc4, a homolog of Tenm/Odz; and Doc6, a villin and gelsolin homolog (72,73). Additionally, CHOP was reported to reduce expression of the antiapoptotic factor BCL2 and to deplete cellular glutathione (74). A greater understanding of the role of each of these gene products in the ER stress response and in apoptosis should shed light into the role of UPR signaling in normal physiology and in β-cell failure.
B. IRE1α mediates JNK activation
ER stress-induced apoptosis can also be signaled through IRE1α-dependent activation of the MAPK cascade. The IRE1α cytoplasmic domain interacts with the adaptor protein TNF receptor-associated factor (TRAF) 2. TRAF2 is an adapter that signals ligation of the TNF receptor 1 (TNFR1) to mediate apoptosis through the MAPKs JNK and p38. In a similar manner, IRE1α and TRAF2 interact with the MAPK ASK1, that subsequently phosphorylates JNK (75,76). Thioredoxin (TDX) is a redox-sensitive inhibitor of ASK1 (77). ROS can oxidize TDX to cause its dissociation from ASK1, leading to ASK1-dependent activation of JNK and p38 MAPK, thereby inducing cell death (78,79). Thus, oxidative stress and ER stress may induce cell death by using the same molecular complex consisting of TRAF2/ASK1/TDX.
IRE1α can also interact with TNFR1 to form a complex with TRAF2 and ASK1 and activate JNK (80,81). Although IRE1α and TNFR1 are in different cellular compartments, ER stress increases the abundance of ER-localized TNFR1 (80,81). The activation of JNK by ER stress is impaired in Tnfr1−/− cells, and the expression of TNFα is up-regulated by the IRE1α pathway during ER stress (80,81). In addition, IRE1α/TRAF2 can also interact with the inhibitor of κB kinase (IKK) to mediate activation of nuclear factor κB, which can promote apoptosis in response to ER stress (81). Finally, TNFα can activate the UPR in a ROS-dependent manner (82). These findings indicate that an intricate relationship exists between death receptor signaling, oxidative stress, and activation of the UPR.
In addition to the IRE1α and PERK-dependent UPR apoptotic pathways, ER stress can initiate other proapoptotic events as well, including relocalization of BCL-2 family members, cleavage of ER-specific caspases, p53 activation, and disruption of cellular calcium homeostasis (33,83). In general, preventing the initiation of these events singly by genetic ablation confers to cells a degree of protection from ER stress.
V. ER Stress Stimuli and Physiological Regulation of the UPR
Generally, most investigations on the UPR use pharmacological treatments such as thapsigargin or tunicamycin, which deplete ER calcium stores and inhibit N-linked glycosylation, respectively (Fig. 1). Reduction of disulfide bonds through dithiothreitol treatment also disrupts protein folding and has the advantage that it is reversible upon removal from the medium. Unfortunately, these treatments are not physiological and do not provide great insight into the types of protein-folding stress that cells experience in vivo. In vivo physiological stimuli that activate the UPR could include alterations in ER calcium homeostasis, nutrient deprivation or excess, mitochondrial dysfunction, oxidative stress, pathogenic infections, or a variety of chemical insults. The UPR could also be induced by physiological increases in mRNA translation, expression of difficult-to-fold proteins, unbalanced expression and accumulation of unassembled subunits of protein complexes, or translation of mutant proteins with intrinsic folding defects (Fig. 1). The identification of conditions and/or stimuli that cause accumulation of misfolded proteins in the ER lumen of the β-cell should provide fundamental insight into how ER stress is generated and suggest novel approaches to prevent β-cell failure and apoptosis.
A. IAPP induces the UPR
The study of islet amyloid protein (IAPP) has led to the conclusion that altered tertiary structure, self-association, and deposition of aggregated IAPP in the islet is a major pathology associated with human type 2 diabetes (84). In contrast to murine IAPP, the human, feline, and nonhuman primate forms of the IAPP molecule are amyloidogenic. Therefore, to study the role of IAPP amyloidogenesis in β-cell failure, rodent transgenic models have been created that express human IAPP (hIAPP) (85,86,87). When hIAPP is expressed at physiological levels in murine β-cells, cellular aggregates of hIAPP accumulate, the UPR is robustly induced, and ubiquitinylation of hIAPP occurs, suggesting that a substantial amount of misfolded hIAPP is targeted for proteasomal degradation (88). These findings support the hypothesis that IAPP aggregation is a major stimulus that activates the UPR in β-cells. UPR activation may be essential to maintain ER homeostasis by degrading misfolded hIAPP and, therefore, limiting hIAPP oligomerization. Furthermore, an inadequate UPR may predispose to greater hIAPP oligomer accumulation, thereby accentuating amyloid formation and toxicity (89). Resolution or prevention of this stress may be crucial in controlling β-cell failure in human type 2 diabetes.
B. Oxidative protein folding, oxidative stress, and activation of the UPR are closely linked events
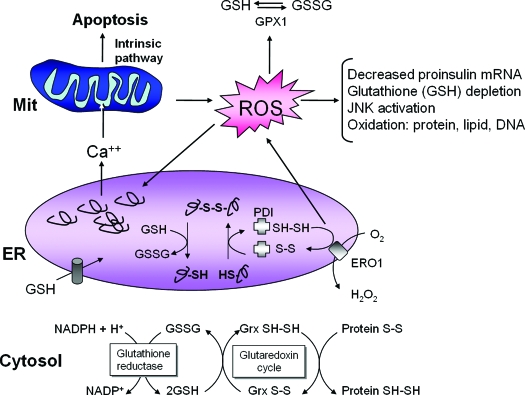
Because genetic studies in mice revealed a crucial role for PERK/eIF2α signaling in β-cell function and survival, a hypothesis is required to explain the unique requirement for PERK/eIF2α in β-cells (15,16,54,90,91). Previous studies suggested that the PERK/eIF2α subpathway reduces the production of ROS by attenuating protein synthesis when protein-folding reactions in the ER are compromised. Proinsulin is a major biosynthetic product of β-cells that requires disulfide bond formation for correct folding. The ER contains many members of the thiol-disulfide oxidoreductase family. These oxidoreductases catalyze substrate protein oxidation and isomerization (Fig. 4). After catalysis of disulfide bond formation and isomerization within a substrate, the active site cysteine residues of protein disulfide isomerase, PDI, must be regenerated by oxidation through a thiol reductase, such as ERO1. In this reaction, ERO1 uses flavin adenine dinucleotide to transfer electrons from PDI to molecular oxygen and generates ROS in the process (20,55,92,93). Either suboptimal folding conditions or a high biosynthetic load could cause unproductive and repeated cycles of protein oxidation and reduction and thereby increased ROS production. Furthermore, there are additional events whereby ER stress accentuates ROS production. Unresolved accumulation of unfolded protein in the ER causes calcium leak from the ER lumen and uptake into the mitochondrial matrix. Calcium loading in the mitochondrial matrix increases mitochondrial production of ROS and causes outer mitochondrial membrane permeability transition leading to apoptotic cell death (94,95,96). Not only can oxidative protein folding in the ER generate ROS, but ROS can, in turn, impede protein folding through direct protein modification, chaperone inactivation, and/or depletion of cellular glutathione, thereby creating a vicious cycle of ER stress and oxidative stress. β-Cells express low levels of enzymes that detoxify ROS, i.e., catalase and glutathione peroxidase; thus, they are particularly sensitive to ROS production that leads to oxidation of proteins, lipids, and nucleic acids (97). Therefore, β-cells may selectively require PERK/eIF2α signaling to limit mRNA translation so that protein synthesis does not exceed the chaperone capacity for folding and thereby limit ROS production.
Figure 4.
ER stress, protein misfolding, and oxidative stress are intimately interrelated. Protein folding within the ER lumen is ushered by a family of oxidoreductases that catalyze disulfide bond formation and isomerization. ER stress causes an increase in the formation of incorrect intermolecular and/or intramolecular disulfide bonds that require breakage and reformation for proteins to attain the appropriate folded conformation. PDI catalyzes disulfide bond formation and isomerization, whereas glutathione transported into the ER reduces improperly paired disulfide bonds. Reoxidation of PDI is mediated by ERO1; however, ROS are produced in the process. Cellular ROS can deplete glutathione and increase the misfolded protein load in the ER. In turn, ROS can also cause ER stress through modification of proteins and lipids that are necessary to maintain ER homeostasis. Consumption of excessive cellular glutathione due to ER stress could inhibit glutaredoxin reduction and cause accumulation of oxidized cytosolic proteins. ER stress also causes calcium leak from the ER for accumulation in the inner mitochondrial matrix. This calcium loading in the mitochondria can generate additional ROS through disruption of electron transport and opening of the mitochondrial permeability pore. Thus, accumulation of misfolded protein in the ER increases ROS production that can further amplify ER stress, disrupt insulin production, and cause cell death.
C. Glucose regulates protein folding and oxidative stress
1. Glucose controls mRNA translation and UPR signaling.
β-Cells are specialized in their coupling of acute increases in extracellular glucose and free fatty acid with increases in proinsulin mRNA translation and insulin secretion (98,99,100,101,102). This coupling likely ensures that insulin is produced and packaged into secretory granules to meet the metabolic demands for insulin in the organism. However, in most cell types the rate of mRNA translation does not respond to the extracellular glucose concentration. The periodic increases in proinsulin mRNA translation may generate a biosynthetic load that is a stimulus for UPR activation in β-cells.
The hypothesis that β-cells have a high protein-folding load is supported by the observation that proinsulin represents up to 20% of the total mRNA and 30–50% of the total protein synthesis in the β-cell (99,103,104). In addition, the percentage of proinsulin mRNA translation increases with increases in the extracellular glucose concentration. Concomitant with the glucose-stimulated increase in proinsulin translation (101), the activity of cellular initiation factors is enhanced to support increased proinsulin synthesis (105,106,107). The rate of glucose-stimulated proinsulin mRNA translation in β-cells approaches 1 million molecules of proinsulin per minute per cell. The high biosynthetic burden that proinsulin imposes on the ER of β-cells could be a consequence of the excessive protein-folding load imposed on the ER folding machinery, as well as the three disulfide bonds in each proinsulin molecule that are essential for correct tertiary structure (see Section VI.B).
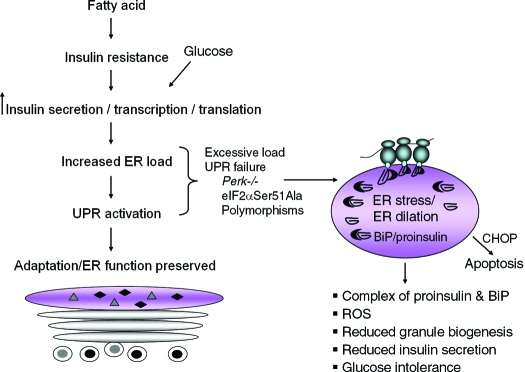
Mice engineered with reduced capacity for eIF2α phosphorylation through heterozygous Ser51Ala mutation at the PERK phosphorylation site have no phenotype on normal chow diet. However, they develop obesity and β-cell failure when fed a high-fat diet. This mutation generates a modestly higher rate of glucose-stimulated translation in the β-cells from mice fed a high-fat diet (Fig. 5) (16). Under these conditions, β-cell failure was associated with impaired proinsulin folding and processing, suggesting that the folding capacity of the β-cell was overwhelmed by enhanced proinsulin mRNA translation. As a consequence, there is a decrease in both insulin-containing granules and glucose-stimulated insulin release. This supports the hypothesis that eIF2α phosphorylation inhibits mRNA translation in β-cells. It is possible that phosphorylation of eIF2α is a major mechanism whereby glucose regulates mRNA translation in the β-cell. Further studies in isolated murine islets and in MIN6 cells demonstrated that glucose stimulates mRNA translation through protein phosphatase 1-dependent dephosphorylation of eIF2α (107).
Figure 5.
UPR signaling preserves ER function and glucose-stimulated insulin secretion (GSIS). Nutrient stimuli and insulin resistance combine to increase the transcription and translation of proinsulin mRNA. The increased biosynthetic load of proinsulin requires UPR signaling for cellular adaptation and maintenance of polypeptide folding within the ER. Excessive biosynthetic load on the ER or genetic defects in UPR signaling, for example Perk-null or eIF2α Ser51Ala mutations, cause ER stress, increase interaction of misfolded proinsulin with BiP, and reduce secretory granule biogenesis. As a consequence, the pool of secretory granules is depleted, and the capacity for GSIS is lost. Humans with polymorphisms that reduce the capacity for protein folding or ER stress signaling may be predisposed to β-cell failure and development of diabetes.
Elouil et al. (108) analyzed the effect of glucose on eIF2α phosphorylation and IRE1α-mediated splicing of Xbp1 mRNA in cultured rat islets. The studies revealed that low glucose concentrations (<5 mm) increase eIF2α phosphorylation to a greater extent than IRE1α-dependent splicing of Xbp1 mRNA. Energy depletion and suboptimal conditions for protein folding may require translational attenuation to conserve cellular resources and prevent accumulation of unfolded protein before activation of other UPR-sensing pathways. Thus, eIF2α phosphorylation may be the first response to a decrease in extracellular glucose. In contrast, hyperglycemia rapidly induced splicing of Xbp1 mRNA, which was reversed by incubation of islets under normoglycemic conditions (5 mm glucose). The hyperglycemic induction of UPR gene expression through eIF2α phosphorylation and IRE1α-mediated Xbp1 mRNA splicing was prevented by inhibition of protein synthesis, suggesting that mRNA translation and the protein-folding load within the ER is a driving force in glucose-regulated UPR signaling. These data are in contrast to reported observations that glucose challenge in vivo or high glucose stimulation of cultured cells activates IRE1α autophosphorylation independent of Xbp1 mRNA splicing in freshly isolated islets and in INS-1 insulinoma cells, respectively (109). Thus, further studies of primary cultured islets are required to resolve these apparent discrepancies regarding hyperglycemic activation of IRE1α and catalysis of Xpb1 mRNA splicing.
2. Glucotoxicity causes oxidative stress and unfolded protein accumulation.
Oxidative stress is a key mediator of glucotoxicity in the β-cell (110,111). The intracellular concentration of glucose in the β-cell is coupled with extracellular variations. Glucose is required to maintain glycolytic flux and regulate the ATP/ADP ratio for signaling stimulus-induced secretion of insulin. However, chronic or excessive hyperglycemia also results in the production of damaging ROS. Glucose can generate ROS through numerous mechanisms including oxidative phosphorylation, glyceraldehyde autoxidation, the hexosamine pathway, and generation of advanced glycation end products (112). Oxidative stress leads to a loss of insulin gene expression and glucose-stimulated insulin secretion through 1) JNK activation and FOXO-1 translocation into the nucleus, and 2) posttranscriptional reduction in the levels of nuclear PDX-1 and MafA (113,114,115,116). Hyperglycemia increases proinsulin biosynthesis and thereby activates the UPR. The accumulation of unfolded protein in the ER lumen can generate ROS and contribute to the total amount of ROS produced during hyperglycemia (Fig. 4). In this manner, glucotoxicity could cause ER stress leading to diminished insulin gene expression, β-cell failure, and apoptosis. Thus, intervention to decrease ER stress, as described below in Section VII, has potential to reduce glucotoxicity in the β-cell.
Furthermore, accumulation of unfolded protein within the ER lumen may increase the amount of oxidized cytosolic protein through disruption of the glutaredoxin system (Fig. 4) (117). Oxidation of cytosolic proteins is limited by glutaredoxin-catalyzed reduction of protein disulfides to thiols. This process utilizes glutathione to regenerate reduced glutaredoxin, and glutathione is replenished by glutathione reductase in a reaction that consumes nicotinamide adenine dinucleotide phosphate (NADPH). β-Cells express high levels of glutaredoxin, suggesting the glutaredoxin cycle is a key metabolic pathway in the β-cell (118). Consumption of excessive cellular glutathione due to remodeling of disulfide bonds within misfolded proteins could diminish the production of reduced glutaredoxin and thereby cause accumulation of oxidized cytosolic proteins. In addition, the increased utilization of NADPH to regenerate glutathione could impair stimulus secretion coupling through depletion of NADPH, a glucose-regulated signal of secretory granule exocytosis (118).
D. Fatty acids and cytokines activate UPR signaling
It was reported that fatty acid and cytokine exposure cause UPR sensor activation and induction of ER stress markers in cultured clonal β-cells (119,120). Oleate strongly increases mRNA levels for several UPR-induced genes and can activate transcription from an ATF6 transcriptional reporter construct. The induction of UPR-induced genes by palmitate was also reported (121). Cytokine exposure decreased SERCA2b expression, decreased ER calcium storage, and activated Xbp1 mRNA splicing. Interferon-γ treatment alone was sufficient to potentiate ER stress (120,122). In clonal β-cells, IL-1 exposure promoted nitric oxide production, leading to phosphorylation of eIF2α, Xbp1 mRNA splicing, and activation of UPR gene expression (123). These data suggest that proinflammatory cytokine signaling may sensitize β-cells to ER stress. Further studies are required to elucidate the role of different UPR subpathways in response to proinflammatory cytokines in β-cells.
In conclusion, studies in this newly emerging field directed to define the role of ER stress in β-cell biology provide evidence that IAPP and proinsulin may attain misfolded conformations that activate the UPR in β-cells. The UPR is likely an integral signal required for the β-cell to adapt and survive conditions of misfolding in the ER lumen. Glucose, as the key regulator of insulin secretion and β-cell function, acutely enhances translation of proinsulin in the β-cell and coordinately activates the UPR. Although the mechanism is poorly understood, fatty acid exposure was associated with activation of UPR-regulated gene expression that could be a determinant of fatty acid-induced apoptosis. Because nutrient excess, high-fat diet, and hyperglycemia are parameters often associated with type 2 diabetes, the UPR may be constitutively activated in the β-cells of these patients. Furthermore, inflammation may further exacerbate ER stress in β-cells because cytokine exposure may generate ROS, compromise calcium storage within the ER, and render β-cells susceptible to accumulation of misfolded protein.
VI. The UPR and Diabetes—A Causal Role of ER Stress in Diabetes of Men and Mice
To determine the relevance of a signaling pathway to the development of a disease, it is essential to measure markers of the signaling pathway in samples from diseased individuals. If an animal model of the disease exists, markers of the candidate signaling pathway can also be evaluated in afflicted animals. In rare instances, there may be human subjects with genetic mutations that provide definitive evidence that a signal transduction pathway is causative in the development of disease. Manipulations to either activate or inhibit the pathway are often evaluated in animals to provide further insight. Applying this logic to the relationship between ER stress and diabetes predicts that 1) UPR gene induction should be detectable in the islets of human diabetes patients and diabetic animal models; 2) accumulation of unfolded protein in β-cells should cause β-cell failure and diabetes; and 3) genetic deletion of critical UPR signal transduction components should cause overwhelming ER stress and diabetes. Direct experimental evidence now exists that supports each of these predictions and together strongly implicate a causative role for ER stress in β-cell failure and diabetes, both in men and mice. The UPR-related genetic mutations that are known to cause diabetes are discussed below and described collectively in Table 1.
Table 1.
Diabetes phenotypes due to genetic alteration of ER homeostasis
| Gene | Mutation | Genotype | Species | Effect | Phenotype | Ref. |
|---|---|---|---|---|---|---|
| Client protein | ||||||
| Ins2 | Akita, Munich | Het | M | Misfolded proins. | Young adult onset diabetes | 18,64 |
| Ins | Missense | Het | H | Misfolded proins. | Neonatal diabetes | 126 |
| Iapp | hIAPP | Transgene | M | Xs hIAPP | Young adult onset diabetes | 88,89 |
| UPR signaling | ||||||
| Perk | Null | Hom | M | Deletion | Young adult onset diabetes | 15,90,91 |
| Missense/deletion | Hom | H | Loss function | Wolcott-Rallison syndrome | 131,132 | |
| SNPs | Polymorphism | H | ? | Type 1 diabetes | 133 | |
| eIF2α | Ser51Ala | Hom | M | No phos. | β-Cell deficiency | 54 |
| eIF2α | Ser51Ala | Het | M | Reduced phos. | High fat diet-diabetes | 16 |
| Ire1α | Null | Hom | M | Deletion | Embryonic lethal, ∼E10.5 | 146 |
| Xbp1 | Null | Hom | M | Deletion | Embryonic lethal, ∼E12.5 | 147 |
| Xbp1 | Null | Het | M | Deletion | High fat diet-ins. resistance | 148 |
| Atf6α | Null | Hom | M | Deletion | Normal | 47,48 |
| Atf6β | Null | Hom | M | Deletion | Normal | 47,48 |
| Atf6α/β | Null | Dual hom | M | Deletion | Embryonic lethal∼E8 | 48 |
| Atf6 | SNPs | Polymorphism | H | ? | Type 2 diabetes | 151,152 |
| ER resident/chaperone | ||||||
| P58IPK | Null | Hom | M | Deletion | Adult diabetes | 138 |
| Wfs1 | Null | Hom | M | Deletion | Adult diabetes | 142,143,144 |
| Missense | Hom | H | Loss function | Wolfram syndrome | 140,141 | |
| Orp150/Grp170 | Null | Het | M | Deletion | Insulin resistance | 172 |
| mOrp150 | Transgene | M | Xs ORP150 | Insulin sensitivity | 172 | |
| SNPs | Polymorphism | H | ? | Insulin sensitivity | 173 |
Het, Heterozygote; Hom, homozygote; M, Mus musculus; H, Homo sapien; Xs, excess; proins., proinsulin; Phos, phophorylation; ?, unknown effect.
A. UPR markers are detectable in islets from diabetic men and mice
Perhaps the most difficult test of UPR association with diabetes is the detection of ER stress or defective ER stress signaling in samples from human patients. However, CHOP nuclear localization was recently reported in pancreata of human obese diabetic individuals, but it was rarely found in perinuclear or nuclear localization in pancreata from control or type 1 diabetic patients (89). The classical UPR-induced proteins p58IPK, BiP, and CHOP were significantly elevated in islets in tissue sections from type 2 diabetes patients (121). Increased levels of eIF2α phosphorylation, increased splicing of Xbp1 mRNA, and increased CHOP and BiP protein were detected in the islets of db/db mice, a common model of insulin resistance and β-cell failure (121,124). The detection of UPR-induced signals in these samples does not prove that ER stress was a causative event in the disease process; however, it does provide the first evidence that persistence of markers of the UPR is elevated specifically in the islets of diabetic men and mice.
Substantiation that excessive ER stress or defective stress signaling are pathogenic determinants of human diabetes will require more advanced knowledge of the stimuli and function of the UPR in β-cells and development of sensitive methods to detect markers of the UPR in human samples. One outstanding question is whether proinsulin synthesis is increased and associated with increased proinsulin misfolding in β-cells of individuals with insulin resistance. The discovery of drugs that modulate ER stress signaling that can be evaluated in animal models of ER stress and diabetes and in human clinical trials will greatly advance our understanding of the importance of ER stress in development and progression of diabetes.
B. Mutant proinsulin is sufficient to induce β-cell failure and diabetes
Studies of Akita and Munich mice reveal that mutations at cysteine residues that interfere with proper disulfide bond formation within proinsulin induce ER stress and severe β-cell destruction (18,64). Deletion of the UPR-induced proapoptotic gene Chop delayed onset of hyperglycemia and β-cell failure in the Akita mouse (64). Human proinsulin with the analogous Akita C96Y mutation was analyzed and compared with wild-type proinsulin through the development of expression constructs that fuse green fluorescence protein (GFP) with the C peptide (125). In these studies it was possible to elucidate that processing of hProC96YCpepGFP to insulin was completely impaired in INS-1 cells and expression was “proteotoxic” in comparison to control hProCpepGFP.
In humans, it was recently discovered that permanent neonatal dominantly inherited diabetes in 16 families was associated with missense mutations in the Ins gene (126). The mutations were predicted to impair proinsulin disulfide bond formation and activate ER stress. The missense mutations affected residues directly involved in disulfide bond formation, crucial residues adjacent to disulfide bridges, and also presented new cysteine residues that could interfere with correct bond pairing as nascent proinsulin molecules undergo oxidative folding. One of the human mutations was an analogous mutation to the Akita Ins2C96Y mutation in mice. Thus, disruption of disulfide bond pairing in proinsulin, a crucial determinant of secondary structure and protein folding, is sufficient to induce diabetes in both humans and mice.
C. Gene mutations in the PERK/eIF2α pathway demonstrate that reduced UPR signaling is sufficient to cause diabetes in humans and mice
Wolcott-Rallison syndrome was first reported in the early 1970s as a human disease characterized by infantile diabetes, multiple epiphyseal dysplasia, and growth retardation (127,128). Pancreas atrophy and endocrine and exocrine insufficiency were observed (129,130). Wolcott-Rallison syndrome has been associated with multiple other pathologies including osteopenia, hepatic and renal complications, cardiovascular disease, and mental retardation. Remarkably, it was learned nearly 30 yr later that this syndrome results from loss of protein kinase function mutations in the eIF2α kinase PERK (EIF2AK3) (131,132). Furthermore, linkage of the Perk gene to diabetes was found in analysis of polymorphisms at this locus in South Indian populations of type 1 diabetes (133).
Null mutation of the Perk gene in the mouse recapitulates many of the defects of the human syndrome including diabetes due to degeneration of β-cell mass after birth and failure of the exocrine pancreas (15,50,90). In these studies, ER distention, a characteristic of ER dysfunction, was observed in pancreatic β-cells. In addition, the rate of glucose-stimulated proinsulin synthesis was enhanced, consistent with a defect in the ability to properly attenuate proinsulin mRNA translation. The findings suggested that the β-cells of these mice were susceptible to ER overload and unresolvable ER stress leading to apoptosis (Fig. 5). Conditional deletion of Perk at varying times in development suggests that development of β-cell mass, but not maintenance of a population of adult β-cells, is dependent upon this kinase (91). It is possible that one or more of the other eIF2α kinases, protein kinase general amino acid control 2 (GCN2), protein kinase heme-regulated inhibitor (HRI), and ds-RNA-activated protein kinase (PKR)-like ER kinase, are capable of supporting the minimal requirement for eIF2α phosphorylation and translational control in response to in vivo stimuli.
Concurrently, mice that harbor a homozygous knock-in mutation at the PERK phosphorylation site in eIF2α (Ser51Ala) were shown to have defects in embryonic β-cell survival, liver glycogen storage, postnatal induction of gluconeogenesis, inhibition of translation under conditions of ER stress, and transcriptional induction of UPR genes (54). This Ser51Ala mutation very effectively blocks ER stress-induced translation attenuation and transcriptional induction. It also prevents any compensatory phosphorylation due to activation of other eIF2α kinases. Because the homozygous eIF2α Ser51Ala mutation was a neonatal lethal phenotype with a severe β-cell deficiency, further studies were performed by analysis of β-cell function and diabetes in heterozygous eIF2α Ser51Ala mice (16). The heterozygous animals did not spontaneously manifest β-cell failure due to reduced ER stress signaling. However, upon feeding a 45% high-fat diet, these mice developed elevated fasting blood glucose, glucose intolerance, and a β-cell secretory failure. It was demonstrated that the insulin secretion defect was due to an increased rate of glucose-stimulated translation that overwhelmed the protein folding machinery of the ER and led to 1) distention of the ER compartment, 2) prolonged association of proinsulin with the ER chaperone BiP, and 3) reduced secretory granule content (Fig. 5). Thus, regulation of translational initiation through eIF2α phosphorylation is required for ER stress signaling to prevent β-cell dysfunction when insulin demand is increased due to a high-fat diet and insulin resistance.
Because distention of the ER and β-cell death in homozygous eIF2α Ser51Ala islets was apparent embryonically in the absence of any exogenous pressure for β-cell failure, it is likely that there are physiological stimuli that invoke the UPR in β-cells early in development and that responsiveness to these stimuli through eIF2α phosphorylation is crucial for β-cell survival (16,54). In addition, these findings demonstrated that translational control through eIF2α phosphorylation is essential to maintain the functional integrity of the ER. These observations are unlikely to result from a defect in transcriptional control through ATF4, because Atf4−/− mice do not display β-cell defects. The sum of these findings and others indicates that genetic defects in the PERK/eIF2α signal transduction pathway are sufficient to disrupt regulated mRNA translation and interfere with ER function in the β-cell, thereby causing reduced insulin secretion, β-cell death, and diabetes in mice and humans.
D. Deletion of the putative ER co-chaperone gene p58IPK causes destruction of islet mass and diabetes
The protein p58IPK was first described as an inhibitor of the double-stranded RNA-activated eIF2α protein kinase PKR. It was subsequently shown to inhibit activation of the eIF2α kinase PERK (134,135). The subcellular localization and function of this protein have been a subject of debate; however, there is evidence that this protein is imported into the ER lumen (136). p58IPK is a member of the DnaJ family that functions to stimulate the ATPase activity of members of the Hsp70 family. Therefore, it was proposed that p58IPK may act in the ER lumen as a co-chaperone for the Hsp70 family member BiP (136). Mice with null mutation of p58IPK develop spontaneous diabetes due to destruction of the islet mass, and p58IPK-null mutation worsens the outcome of diabetes due to the Akita Ins2 C96Y mutation (137,138). These intriguing findings merit further study on the role of p58IPK co-chaperone function in proinsulin folding and maturation and in diabetes. The observations suggest that there may be a number of protein-folding chaperones that play highly significant roles in preservation of ER function in the β-cell to prevent diabetes.
E. Wolfram syndrome is a genetic disease that results in ER dysfunction and β-cell failure
Wolfram syndrome is a rare autosomal-recessive neurodegenerative disorder that is characterized by juvenile-onset diabetes mellitus, optic atrophy, and hearing impairment (139). This syndrome is caused by loss-of-function mutations in the Wfs1 gene that encodes the protein Wolframin (140,141). Although WFS1 is not a direct sensor of the UPR, analysis of Wfs1−/− mice indicates that WFS1 function is closely linked with ER homeostasis. Wfs1-null mutation reduces intracellular calcium signaling upon glucose stimulation, induces UPR-regulated genes, and disrupts cell cycle control, leading to apoptosis (142,143,144). Recently, a physical interaction between WFS1 and the Na(+)/K(+)ATPase β1 subunit was discovered, and it was discerned that WFS1 was required for trafficking of the subunit to the cell surface. Reduced levels of this ATPase subunit were detected in the plasma membrane fraction of Wfs1 mutant fibroblasts and of Wfs1 knockdown MIN6 β-cells (145). Wolframin may serve a general function to assist in the assembly of subunits of oligomeric proteins before exit from the ER. Consistent with these observations, loss of function in the chaperone WFS1 causes ER stress and diabetes.
F. What will we learn from deletion of the IRE1α and ATF6 UPR signaling pathways in mice?
A fundamental question regarding β-cell function and survival is which UPR subpathways are required for β-cell function and what elements of these responses are protective or detrimental to β-cell survival upon acute or unresolvable ER stress. Future studies should investigate this question through the analysis of mice with conditional alleles for β-cell null mutation of Ire1α and Atf6. Evidence that defects in the IRE1α and ATF6 subpathways of the UPR are akin to null mutation of PERK signaling and are causative of human diabetes has yet to be presented; however, such an observation would solidify the concept that the UPR sensors act in concert with each sensor supporting a unique and indispensable role in preservation of β-cell function and/or survival.
1. Is IRE1α required for β-cell function?
Previous null mutation of Ire1α in all tissues of the mouse produced a severe phenotype of embryonic lethality at approximately embryonic day (E) 10.5 (40,146). This is the most developmentally severe phenotype obtained upon deletion of a UPR sensor and perhaps reflects the role of IRE1α as the most fundamental and evolutionarily conserved ER stress sensor. The early lethality of Ire1α deletion requires a conditional-null allele of Ire1α in pancreatic β-cells for the study of the role of IREα in function and survival. IRE1α is essential to up-regulate ERAD and protein secretory capacity in response to unfolded protein accumulation. Thus, it is possible that Ire1−/−-null β-cells will have a defect in ERAD and, therefore, accumulate misfolded proinsulin leading to β-cell failure. Future studies to evaluate the degradation of proinsulin in the absence and presence of ER stress in Ire1α-null β-cells should yield important insight into the role of IRE1α in β-cell function. To date, no genetic causes of altered IRE1α function have been reported in humans. It is possible that the severity of such mutations is lethal before birth, and that there may also be selective pressure against heterozygous null mutations in Ire1α in the human genome.
Null mutation in all tissues of the mouse of the downstream splicing target of IRE1α, Xbp1, is also an embryonic lethal phenotype at approximately E12.5 (147). In addition, Xbp1 deletion, like Perk deletion, produces a defect in pancreatic acinar cells (11). However, these mice do not have a significant β-cell defect. Heterozygous Xbp1−/+ mice fed a high-fat diet develop insulin resistance in liver and muscle due to JNK activation and inactivating phosphorylation of IRS-1 and IRS-2 (148). Because IRE1α not only mediates splicing of Xbp1 mRNA but also couples to TRAF2 and JNK activation, IRE1α activation and signaling to JNK may be enhanced when the capacity for Xbp1 mRNA splicing is reduced (75). Subsequent studies revealed that insulin resistance, hyperglycemia, and glucose intolerance in ob/ob mice could be reversed by small chemical chaperones that may improve protein folding in the ER lumen (149,150). However, this hypothesis requires further experimental support by demonstrating that the small molecules actually improve protein folding in the ER. These observations support the notion that ER stress leads to insulin resistance, possibly through IRE1α-mediated JNK activation. The insulin resistance may further compromise ER function in the β-cell by increasing the demand for insulin production.
2. How does ATF6 affect β-cell function in humans and mice?
There is evidence that missense mutations in the UPR sensor ATF6α in humans within Dutch and Pima Indian cohorts are linked to type 2 diabetes (151,152). Combined null mutation of α and β isoforms of ATF6 in mice was recently reported to cause an early developmental lethal phenotype (48). However, single null mutation of either isoform produces a viable animal that does not develop overt diabetes in the absence of any exogenous stimuli or other genetic predisposition. Studies using Atf6α−/− MEFs suggest that adaptation to chronic stress requires ATF6α (47); therefore, it remains to be determined whether physiologically relevant perturbation of β-cell function in these mice will reveal a role for ATF6 in controlling ER stress and β-cell failure.
The completed analysis of the physiological function of each UPR subpathway in β-cell function will provide a solid foundation for future studies of the role of ER stress in β-cell failure and disease. Likewise, future studies are required to elucidate the roles of these pathways in human neonatal, type 1, and type 2 diabetes.
VII. How Can the Accumulation of Misfolded Protein and ER Stress Be Managed to Prevent β-Cell Failure?
The UPR is a complex system designed to maintain ER homeostasis under basal conditions and to adapt to fluctuations in nutrient availability, ROS, and demand for increased protein synthesis, folding, and secretion. Will it be possible to intervene to preserve β-cell function and survival when ER homeostasis is perturbed? The transcriptional changes of the collective UPR are vast, including approximately 20% of known genes, and this response includes regulation of gene expression that is a direct consequence of the primary UPR transcription factors, XBP1, ATF6, ATF4, and CHOP, and also secondary regulation of gene expression due to altered signaling in pathways that are closely linked to the UPR. For example, JNK activation, cell death signaling, and oxidative stress can occur in response to ER stress. Constitutive activation of any one UPR sensor may not be sufficient to improve β-cell function in the context of diabetes. However, analysis of expression of a mutant IRE1α that can be activated by an ATP analog in other cell types suggests that a portion of ER stress-induced cell death can be prevented by pharmacological activation of IRE1α (153,154). Recent x-ray crystal structures for the lumenal and cytosolic domains of IRE1α could facilitate development of ligands that regulate activation of wild-type IRE1α (155,156,157). In summary, the potential for therapeutic intervention will require the identification of molecular targets based on both the upstream UPR activators and the downstream UPR responses specific to each UPR subpathway.
Therapeutically, for diseases related to ER stress, the primary objective is the development of strategies to prevent newly synthesized protein from accumulating as misfolded protein. However, it is important to classify diseases of protein misfolding in the ER into two groups. For mutant misfolded proteins that cause recessive loss-of-function diseases, it would be beneficial to improve folding efficiency. For those misfolded proteins that cause ER stress and cell death, i.e., genetically dominant-inherited defects such as mutations in proinsulin (126), it may be beneficial to intervene to reduce ER stress and/or cell death signals. The management of each of these types of disease may require disease-specific therapeutic approaches (Fig. 6).
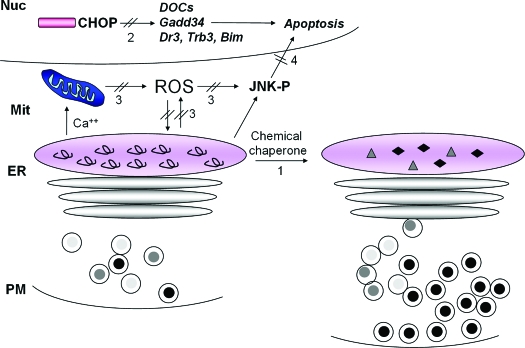
Figure 6.
Therapeutic interventions to improve ER function and/or prevent cell death. Potential strategies to improve protein folding in the ER and/or prevent cell death are depicted. Small molecule chemical chaperones may improve polypeptide folding and prevent chronic, irresolvable ER stress (1). As the proapoptotic transcription factor CHOP plays a causal role in ER stress-induced death, strategies to interfere with CHOP induction or function may prevent β-cell failure (2). Because there is a close relationship between oxidative stress and ER stress, antioxidant therapy (3) may increase insulin mRNA expression and translation, as well as improve proinsulin folding to restore GSIS. Finally, JNK activation is a component of the cell death response to both oxidative, and ER stress and inhibition of this death signal may prevent β-cell death (4). It is possible that the reduction of one or more death signals would be sufficient to halt progression to cell death and allow the adaptive functions of the UPR and antioxidant protective mechanisms to preserve productive protein folding within the ER.
A. Intervention to improve protein folding
Development of small molecules to improve folding of nascent proinsulin or IAPP by stabilizing appropriate secondary structures specific to these polypeptides may improve β-cell function. Small molecules specifically designed to interact with polypeptide-folding intermediates can stabilize the proper three-dimensional conformation. An example of this approach is the development of sugar analogs of the substrate for α-glucocerebrosidase to improve its folding in patients with Gaucher’s disease that results from folding-defective mutations (158). It is also possible that more general chemical chaperones could be an effective treatment (159). Both proinsulin and IAPP are small polypeptides that may be readily “escorted” into their native conformation. It is encouraging that chemical chaperone therapy with 4-phenylbutryic acid (PBA) and tauroursodeoxycholic acid (TUDCA) was effective in reversing ER stress-related insulin resistance in ob/ob mice (149). PBA was initially investigated as a chemical chaperone successful for the treatment of diseases associated with protein-misfolding such as α1-antitrypsin deficiency and cystic fibrosis due to ΔF508 mutation in the cystic fibrosis transmembrane regulator protein. It remains to be determined whether this strategy could directly promote proinsulin-folding and improve β-cell function. It is possible that proinsulin and IAPP are not the only pathologically misfolded proteins that cause β-cell failure and death in diabetes. ER stress generated in response to inefficient assembly of multimeric protein complexes or misfolding of other difficult-to-fold proteins may contribute to β-cell failure and may be corrected by a general chemical chaperone therapy.
B. Intervention to inhibit cell death signals
If the primary stimulus of unfolded protein cannot be halted, it may be beneficial to block proapoptotic signals associated with prolonged UPR signaling. Cell death upon ER stress can be dependent upon the intrinsic pathway of apoptosis initiated by outer mitochondrial membrane permeability transition. Alternatively, ER stress-dependent caspase activation or induction of BH3-only containing BCL2 family members, such as BCL2 interacting mediator of cell death (BIM), p53 up-regulated modulator of apoptosis (PUMA), and NADPH oxidative activator (NOXA), may signal apoptotic events. Certainly, the UPR-induced gene product CHOP plays a fundamental role in the apoptotic response (61,71,160). The Akita Ins2C96Y mutation creates very severe ER stress because the proinsulin produced from this allele cannot form a critical disulfide bond and is inherently misfolded. Chop deletion significantly preserved β-cell function in heterozygous, but not homozygous, Akita mice and delayed development of hyperglycemia (64). As Chop-null mice are phenotypically normal with a normal lifespan and no spontaneous development of tumors, development of specific CHOP inhibitors may have promise to prevent β-cell death that results from ER stress.
Intriguingly, the glucagon-like peptide 1 agonist exendin-4 improves survival of purified rat β-cells and INS-1 cells upon ER stress. It was proposed that exendin-4 enhances recovery of translation upon ER stress through PKA-dependent induction of ATF4, CHOP, and GADD34 (124). The effect of exendin-4 to enhance proinsulin translation was also observed in studies of islets acutely exposed to this drug (161). Because glucagon-like peptide 1 receptor agonists are a promising class of therapeutics that improve β-cell function in type 2 diabetic patients, it is tempting to speculate that at least a portion of this improvement is due to improved recovery of β-cells from periodic physiological ER stress.
In type 2 diabetes, insulin resistance and hyperglycemia are stimuli for both ER stress and oxidative stress. Although the relationship between ER stress and oxidative stress is not well understood, it is possible that antioxidants may improve ER function in β-cells. ER stress and oxidative stress are closely linked pathways that may be coordinately relieved by antioxidant therapy. There exists evidence that indicates antioxidants may improve diabetic complications in mice and humans. Vitamin C was shown to improve insulin sensitivity and glucose homeostasis in ob/ob mice (162). Although not characterized, it is possible that these agents act to improve ER protein folding, similar to that observed in ob/ob mice treated with PBA and TUDCA. Alternatively, PBA and TUDCA may improve insulin sensitivity through their antioxidant activity.
Antioxidants were shown to be highly effective in increasing insulin mRNA content and β-cell mass, while decreasing β-cell apoptosis in db/db mice (163). Furthermore, glucose homeostasis was improved in Zucker diabetic fatty rats administered a triad of N-acetyl cysteine, vitamin C, and vitamin E. This combination improved arginine-stimulated insulin secretion in type 2 diabetic patients (110). Because polypeptide misfolding elevates ROS production and ROS production in turn interferes with productive protein folding, intervention to reduce ROS may reduce the direct pathology of ROS and also improve polypeptide and proinsulin folding in the β-cell.
Although the mechanism for antioxidant improvement of β-cell function is not known, a key effect may be prevention of JNK activation. An oxidizing environment causes oxidation and inhibition of JNK-inactivating phosphatases by converting their catalytic cysteine to sulfenic acid (164). As a consequence, activated JNK accumulates, FOXO-1 nuclear localization increases, and PDX1 is translocated from the nucleus to the cytoplasm (111). Significantly, JNK inhibition protected β-cells from oxidative stress, prevented apoptosis, improved islet graft function (165), and also improved systemic insulin responsiveness. For this reason, there should be further analysis of the impact of JNK inhibitors on β-cell function and survival. The sum of these findings supports the notion that oxidative stress and ER stress play central roles in the pathogenesis of type 2 diabetes and that targeted therapy to intervene to prevent JNK activation may reduce progression of insulin resistance to diabetes.
Finally, oxidative stress that occurs during isolation and engraftment of islets and that caused by inflammatory cytokines associated with immune rejection will negatively impact upon the success of islet transplantation. Aside from activation of JNK, oxidative stress also reduced mRNA levels for MafA and PDX-1, which leads to reduced insulin gene expression (116). Indeed, antioxidant treatment and expression of ROS detoxifying enzymes both improved transplantation success (166,167,168). It is probable that overwhelming ER stress is coordinately invoked with oxidative stress under these conditions and plays a major role in β-cell failure upon transplantation. The investigation of ER stress under conditions of transplantation and inhibition of ER stress toward improved β-cell function in transplantation will be an exciting new area of research.
VIII. Conclusion
In conclusion, a steady stream of high-quality research has established that ER stress plays a pivotal role in failure of β-cells, both in humans and in rodent models. Continued efforts to discern the specific roles of each of the UPR sensors IRE1α, ATF6, and PERK in β-cell function and to dissect the UPR-related pathways that may predispose to β-cell failure upon irresolvable ER stress will direct future research investigations. Optimistically, these studies will identify novel therapeutic modalities that may have impact in the treatment of diabetes.
Supplementary Material
Acknowledgments
The authors thank Janet Mitchell for assistance in the preparation of this review.
Footnotes
R.J.K. is supported as an Investigator of the Howard Hughes Medical Institute and by National Institutes of Health Grants DK42395 and HL052173.
Disclosure Statement: The authors have nothing to disclose.
First Published Online April 24, 2008
Abbreviations: ASK1, Apoptosis signal-regulating kinase 1; ATF6, activating transcription factor 6; BCL2, B cell lymphoma 2; BiP, binding Ig protein or glucose regulated protein 78 (GRP78); CHOP, C/EBP homologous protein; E, embryonic day; eIF2α, eukaryotic initiation factor 2 α-subunit; ER, endoplasmic reticulum; ERAD, ER-associated protein degradation; GFP, green fluorescence protein; hIAPP, human IAPP; IAPP, islet amyloid protein; IRE1α, inositol requiring protein 1α; JNK, c-Jun amino-terminal kinase; MEFs, mouse embryonic fibroblasts; NADPH, nicotinamide adenine dinucleotide phosphate; PBA, 4-phenylbutryic acid; PDI, protein disulfide isomerase; PERK, dsRNA-activated protein kinase (PKR)-like ER kinase; ROS, reactive oxygen species; TDX, thioredoxin; TNFR1, TNF receptor 1; TRAF, TNF receptor-associated factor; TUDCA, tauroursodeoxycholic acid; UPR, unfolded protein response; XBP1, X-box binding protein 1.
References
- Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS 2003 Global analysis of protein expression in yeast. Nature 425:737–741 [DOI] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM 2004 ER chaperone functions during normal and stress conditions. J Chem Neuroanat 28:51–65 [DOI] [PubMed] [Google Scholar]
- Kleizen B, Braakman I 2004 Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol 16:343–349 [DOI] [PubMed] [Google Scholar]
- Ellgaard L 2004 Catalysis of disulphide bond formation in the endoplasmic reticulum. Biochem Soc Trans 32:663–667 [DOI] [PubMed] [Google Scholar]
- Sevier CS, Kaiser CA 2006 Conservation and diversity of the cellular disulfide bond formation pathways. Antioxid Redox Signal 8:797–811 [DOI] [PubMed] [Google Scholar]
- Barlowe C 2003 Signals for COPII-dependent export from the ER: what’s the ticket out? Trends Cell Biol 13:295–300 [DOI] [PubMed] [Google Scholar]
- Lee MC, Miller EA, Goldberg J, Orci L, Schekman R 2004 Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol 20:87–123 [DOI] [PubMed] [Google Scholar]
- Molinari M 2007 N-Glycan structure dictates extension of protein folding or onset of disposal. Nat Chem Biol 3:313–320 [DOI] [PubMed] [Google Scholar]
- Kincaid MM, Cooper AA 2007 Misfolded proteins traffic from the endoplasmic reticulum (ER) due to ER export signals. Mol Biol Cell 18:455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JN, Gunn KE, Sriburi R, Brewer JW 2004 Stressed-out B cells? Plasma-cell differentiation and the unfolded protein response. Trends Immunol 25:17–24 [DOI] [PubMed] [Google Scholar]
- Lee AH, Chu GC, Iwakoshi NN, Glimcher LH 2005 XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J 24:4368–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner AJ, Wasley LC, Kaufman RJ 1992 Overexpression of GRP78 mitigates stress induction of glucose regulated proteins and blocks secretion of selective proteins in Chinese hamster ovary cells. EMBO J 11:1563–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ 2006 Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol 4:e374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermonval M, Cacan R, Gorgas K, Haas IG, Verbert A, Buttin G 1997 Differential fate of glycoproteins carrying a monoglucosylated form of truncated N-glycan in a new CHO line, MadIA214214, selected for a thermosensitive secretory defect. J Cell Sci 110 (Pt 3):323–336 [DOI] [PubMed] [Google Scholar]
- Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D 2001 Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell 7:1153–1163 [DOI] [PubMed] [Google Scholar]
- Scheuner D, Mierde DV, Song B, Flamez D, Creemers JW, Tsukamoto K, Ribick M, Schuit FC, Kaufman RJ 2005 Control of mRNA translation preserves endoplasmic reticulum function in β cells and maintains glucose homeostasis. Nat Med 11:757–764 [DOI] [PubMed] [Google Scholar]
- Umebayashi K, Hirata A, Fukuda R, Horiuchi H, Ohta A, Takagi M 1997 Accumulation of misfolded protein aggregates leads to the formation of Russell body-like dilated endoplasmic reticulum in yeast. Yeast 13:1009–1020 [DOI] [PubMed] [Google Scholar]
- Herbach N, Rathkolb B, Kemter E, Pichl L, Klaften M, de Angelis MH, Halban PA, Wolf E, Aigner B, Wanke R 2007 Dominant-negative effects of a novel mutated Ins2 allele causes early-onset diabetes and severe β-cell loss in Munich Ins2C95S mutant mice. Diabetes 56:1268–1276 [DOI] [PubMed] [Google Scholar]
- McCracken AA, Brodsky JL 2003 Evolving questions and paradigm shifts in endoplasmic-reticulum-associated degradation (ERAD). Bioessays 25:868–877 [DOI] [PubMed] [Google Scholar]
- Kincaid MM, Cooper AA 2007 ERADicate ER stress or die trying. Antioxid Redox Signal 9:2373–2387 [DOI] [PubMed] [Google Scholar]
- Fujita E, Kouroku Y, Isoai A, Kumagai H, Misutani A, Matsuda C, Hayashi YK, Momoi T 2007 Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II). Hum Mol Genet 16:618–629 [DOI] [PubMed] [Google Scholar]
- Kaniuk NA, Kiraly M, Bates H, Vranic M, Volchuk A, Brumell JH 2007 Ubiquitinated-protein aggregates form in pancreatic β-cells during diabetes-induced oxidative stress and are regulated by autophagy. Diabetes 56:930–939 [DOI] [PubMed] [Google Scholar]
- Kaufman RJ, Scheuner D, Schroder M, Shen X, Lee K, Liu CY, Arnold SM 2002 The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol 3:411–421 [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ 2006 Divergent roles of IRE1α and PERK in the unfolded protein response. Curr Mol Med 6:5–36 [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P 2007 Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529 [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D 2000 Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2:326–332 [DOI] [PubMed] [Google Scholar]
- Liu CY, Schroder M, Kaufman RJ 2000 Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J Biol Chem 275:24881–24885 [DOI] [PubMed] [Google Scholar]
- Shen J, Chen X, Hendershot L, Prywes R 2002 ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell 3:99–111 [DOI] [PubMed] [Google Scholar]
- Mimura N, Hamada H, Kashio M, Jin H, Toyama Y, Kimura K, Iida M, Goto S, Saisho H, Toshimori K, Koseki H, Aoe T 2007 Aberrant quality control in the endoplasmic reticulum impairs the biosynthesis of pulmonary surfactant in mice expressing mutant BiP. Cell Death Differ 14:1475–1485 [DOI] [PubMed] [Google Scholar]
- Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ 2006 Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124:587–599 [DOI] [PubMed] [Google Scholar]
- Kondo S, Saito A, Hino S, Murakami T, Ogata M, Kanemoto S, Nara S, Yamashita A, Yoshinaga K, Hara H, Imaizumi K 2007 BBF2H7, a novel transmembrane bZIP transcription factor, is a new type of endoplasmic reticulum stress transducer. Mol Cell Biol 27:1716–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuRose JB, Tam AB, Niwa M 2006 Intrinsic capacities of molecular sensors of the unfolded protein response to sense alternate forms of endoplasmic reticulum stress. Mol Biol Cell 17:3095–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Bailly-Maitre B, Reed JC 2005 Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115:2656–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JS, Shamu CE, Walter P 1993 Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73:1197–1206 [DOI] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D 2002 IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92–96 [DOI] [PubMed] [Google Scholar]
- Shen X, Ellis RE, Lee K, Liu CY, Yang K, Solomon A, Yoshida H, Morimoto R, Kurnit DM, Mori K, Kaufman RJ 2001 Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 107:893–903 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K 2001 XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K 2003 A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell 4:265–271 [DOI] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH 2003 XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23:7448–7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ 2002 IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev 16:452–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Okada T, Yoshida H, Kaufman RJ, Nagata K, Mori K 2006 Derlin-2 and Derlin-3 are regulated by the mammalian unfolded protein response and are required for ER-associated degradation. J Cell Biol 172:383–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M, Sitia R 2005 The secretory capacity of a cell depends on the efficiency of endoplasmic reticulum-associated degradation. Curr Top Microbiol Immunol 300:1–15 [DOI] [PubMed] [Google Scholar]
- Hollien J, Weissman JS 2006 Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313:104–107 [DOI] [PubMed] [Google Scholar]
- Pirot P, Naamane N, Libert F, Magnusson NE, Orntoft TF, Cardozo AK, Eizirik DL 2007 Global profiling of genes modified by endoplasmic reticulum stress in pancreatic β cells reveals the early degradation of insulin mRNAs. Diabetologia 50:1006–1014 [DOI] [PubMed] [Google Scholar]
- Haze K, Yoshida H, Yanagi H, Yura T, Mori K 1999 Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell 10:3787–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadanaka S, Okada T, Yoshida H, Mori K 2007 Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol Cell Biol 27:1027–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ 2007 ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell 13:351–364 [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K 2007 Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev Cell 13:365–376 [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K 2004 Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem (Tokyo) 136:343–350 [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D 1999 Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271–274 [DOI] [PubMed] [Google Scholar]
- Kimball SR 1999 Eukaryotic initiation factor eIF2. Int J Biochem Cell Biol 31:25–29 [DOI] [PubMed] [Google Scholar]
- Wek RC, Cavener DR 2007 Translational control and the unfolded protein response. Antioxid Redox Signal 9:2357–2371 [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D 2000 Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6:1099–1108 [DOI] [PubMed] [Google Scholar]
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ 2001 Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 7:1165–1176 [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D 2003 An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11:619–633 [DOI] [PubMed] [Google Scholar]
- Vattem KM, Wek RC 2004 Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA 101:11269–11274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Yoshida H, Akazawa R, Negishi M, Mori K 2002 Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J 366:585–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ1999 Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J 339 (Pt 1):135–141 [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Brewer JW, Diehl JA, Hendershot LM 2002 Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol 318:1351–1365 [DOI] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D 1998 CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12:982–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D 2004 CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18:3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri S, Oyadomari S, Yano S, Morioka M, Gotoh T, Hamada JI, Ushio Y, Mori M 2004 Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ 11:403–415 [DOI] [PubMed] [Google Scholar]
- Silva RM, Ries V, Oo TF, Yarygina O, Jackson-Lewis V, Ryu EJ, Lu PD, Marciniak SJ, Ron D, Przedborski S, Kholodilov N, Greene LA, Burke RE 2005 CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of Parkinsonism. J Neurochem 95:974–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M 2002 Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest 109:525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, Mori M 2001 Nitric oxide-induced apoptosis in pancreatic β cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci USA 98:10845–10850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa I, Zeng H, Harding HP, Ron D 2001 Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J Cell Biol 153:1011–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa I, Zhang Y, Zeng H, Jungreis R, Harding HP, Ron D 2003 Stress-induced gene expression requires programmed recovery from translational repression. EMBO J 22:1180–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima E, Takeuchi A, Haneda M, Yagi A, Hasegawa T, Yamaki K, Takeda K, Akira S, Shimokata K, Isobe K 2003 The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress: elucidation by GADD34-deficient mice. FASEB J 17:1573–1575 [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Wang HG 2004 CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem 279:45495–45502 [DOI] [PubMed] [Google Scholar]
- Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H 2005 TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J 24:1243–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A 2007 ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129:1337–1349 [DOI] [PubMed] [Google Scholar]
- Wang XZ, Kuroda M, Sok J, Batchvarova N, Kimmel R, Chung P, Zinszner H, Ron D 1998 Identification of novel stress-induced genes downstream of chop. EMBO J 17:3619–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sok J, Wang XZ, Batchvarova N, Kuroda M, Harding H, Ron D 1999 CHOP-dependent stress-inducible expression of a novel form of carbonic anhydrase VI. Mol Cell Biol 19:495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ 2001 Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21:1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D 2000 Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287:664–666 [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H 2002 ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 16:1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H 1998 Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17:2596–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Nishitoh H, Ichijo H, Kyriakis JM 2000 Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol 20:2198–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiume K, Saitoh M, Ichijo H 2002 Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J Cell Physiol 191:95–104 [DOI] [PubMed] [Google Scholar]
- Yang Q, Kim YS, Lin Y, Lewis J, Neckers L, Liu ZG 2006 Tumour necrosis factor receptor 1 mediates endoplasmic reticulum stress-induced activation of the MAP kinase JNK. EMBO Rep 7:622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH 2006 Autocrine tumor necrosis factor α links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol Cell Biol 26:3071–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H, Nakano H 2005 Tumor necrosis factor α (TNFα) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFα. J Biol Chem 280:33917–33925 [DOI] [PubMed] [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A 2006 Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7:880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MR, Tyagi SC, Kerklo MM, Nicolls MR 2005 Type 2 diabetes mellitus as a conformational disease. Jop 6:287–302 [PubMed] [Google Scholar]
- D’Alessio DA, Verchere CB, Kahn SE, Hoagland V, Baskin DG, Palmiter RD, Ensinck JW 1994 Pancreatic expression and secretion of human islet amyloid polypeptide in a transgenic mouse. Diabetes 43:1457–1461 [DOI] [PubMed] [Google Scholar]
- Butler AE, Jang J, Gurlo T, Carty MD, Soeller WC, Butler PC 2004 Diabetes due to a progressive defect in β-cell mass in rats transgenic for human islet amyloid polypeptide (HIP Rat): a new model for type 2 diabetes. Diabetes 53:1509–1516 [DOI] [PubMed] [Google Scholar]
- Matveyenko AV, Butler PC 2006 Islet amyloid polypeptide (IAPP) transgenic rodents as models for type 2 diabetes. ILAR J 47:225–233 [DOI] [PubMed] [Google Scholar]
- Huang CJ, Haataja L, Gurlo T, Butler AE, Wu X, Soeller W, Butler PC 2007 Induction of endoplasmic reticulum stress induced β cell apoptosis and accumulation of polyubiquitinated proteins by human islet amyloid polypeptide. Am J Physiol Endocrinol Metab 293:E1656–E1662 [DOI] [PubMed] [Google Scholar]
- Huang CJ, Lin CY, Haataja L, Gurlo T, Butler AE, Rizza RA, Butler PC 2007 High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated β-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes 56:2016–2027 [DOI] [PubMed] [Google Scholar]
- Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR 2002 The PERK eukaryotic initiation factor 2 α kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 22:3864–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR 2006 PERK EIF2AK3 control of pancreatic β cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab 4:491–497 [DOI] [PubMed] [Google Scholar]
- Tu BP, Weissman JS 2004 Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol 164:341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra J, Kaufman R 2007 Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword. Antioxid Redox Signal 9:2277–2293 [DOI] [PubMed] [Google Scholar]
- Deniaud A, Sharaf El Dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C 2007 Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene 27:285–299 [DOI] [PubMed] [Google Scholar]
- Walter L, Hajnoczky G 2005 Mitochondria and endoplasmic reticulum: the lethal interorganelle cross-talk. J Bioenerg Biomembr 37:191–206 [DOI] [PubMed] [Google Scholar]
- Lee GH, Kim HK, Chae SW, Kim DS, Ha KC, Cuddy M, Kress C, Reed JC, Kim HR, Chae HJ 2007 Bax inhibitor-1 regulates endoplasmic reticulum stress-associated reactive oxygen species and heme oxygenase-1 expression. J Biol Chem 282:21618–21628 [DOI] [PubMed] [Google Scholar]
- Lenzen S, Drinkgern J, Tiedge M 1996 Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 20:463–466 [DOI] [PubMed] [Google Scholar]
- Itoh N, Okamoto H 1980 Translational control of proinsulin synthesis by glucose. Nature 283:100–102 [DOI] [PubMed] [Google Scholar]
- Schuit FC, In’t Veld PA, Pipeleers DG 1988 Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic β cells. Proc Natl Acad Sci USA 85:3865–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicksteed B, Alarcon C, Briaud I, Lingohr MK, Rhodes CJ 2003 Glucose-induced translational control of proinsulin biosynthesis is proportional to preproinsulin mRNA levels in islet β-cells but not regulated via a positive feedback of secreted insulin. J Biol Chem 278:42080–42090 [DOI] [PubMed] [Google Scholar]
- Wicksteed B, Uchizono Y, Alarcon C, McCuaig JF, Shalev A, Rhodes CJ 2007 A cis-element in the 5′ untranslated region of the preproinsulin mRNA (ppIGE) is required for glucose regulation of proinsulin translation. Cell Metab 5:221–227 [DOI] [PubMed] [Google Scholar]
- Newgard CB, McGarry JD 1995 Metabolic coupling factors in pancreatic β-cell signal transduction. Annu Rev Biochem 64:689–719 [DOI] [PubMed] [Google Scholar]
- Schuit FC, Kiekens R, Pipeleers DG 1991 Measuring the balance between insulin synthesis and insulin release. Biochem Biophys Res Commun 178:1182–1187 [DOI] [PubMed] [Google Scholar]
- Van Lommel L, Janssens K, Quintens R, Tsukamoto K, Vander Mierde D, Lemaire K, Denef C, Jonas JC, Martens G, Pipeleers D, Schuit FC 2006 Probe-independent and direct quantification of insulin mRNA and growth hormone mRNA in enriched cell preparations. Diabetes 55:3214–3220 [DOI] [PubMed] [Google Scholar]
- Xu G, Marshall CA, Lin TA, Kwon G, Munivenkatappa RB, Hill JR, Lawrence Jr JC, McDaniel ML 1998 Insulin mediates glucose-stimulated phosphorylation of PHAS-I by pancreatic β cells. An insulin-receptor mechanism for autoregulation of protein synthesis by translation. J Biol Chem 273:4485–4491 [DOI] [PubMed] [Google Scholar]
- Gomez E, Powell ML, Greenman IC, Herbert TP 2004 Glucose-stimulated protein synthesis in pancreatic β-cells parallels an increase in the availability of the translational ternary complex (eIF2-GTP.Met-tRNAi) and the dephosphorylation of eIF2 α. J Biol Chem 279:53937–53946 [DOI] [PubMed] [Google Scholar]
- Vander Mierde D, Scheuner D, Quintens R, Patel R, Song B, Tsukamoto K, Beullens M, Kaufman RJ, Bollen M, Schuit FC 2007 Glucose activates a protein phosphatase-1-mediated signaling pathway to enhance overall translation in pancreatic β-cells. Endocrinology 148:609–617 [DOI] [PubMed] [Google Scholar]
- Elouil H, Bensellam M, Guiot Y, Vander Mierde D, Pascal SM, Schuit FC, Jonas JC 2007 Acute nutrient regulation of the unfolded protein response and integrated stress response in cultured rat pancreatic islets. Diabetologia 50:1442–1452 [DOI] [PubMed] [Google Scholar]
- Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F 2006 Regulation of insulin biosynthesis in pancreatic β cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab 4:245–254 [DOI] [PubMed] [Google Scholar]
- Robertson R, Zhou H, Zhang T, Harmon JS 2007 Chronic oxidative stress as a mechanism for glucose toxicity of the β cell in type 2 diabetes. Cell Biochem Biophys 48:139–146 [DOI] [PubMed] [Google Scholar]
- Kaneto H, Katakami N, Kawamori D, Miyatsuka T, Sakamoto K, Matsuoka TA, Matsuhisa M, Yamasaki Y 2007 Involvement of oxidative stress in the pathogenesis of diabetes. Antioxid Redox Signal 9:355–366 [DOI] [PubMed] [Google Scholar]
- Robertson RP 2004 Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet β cells in diabetes. J Biol Chem 279:42351–42354 [DOI] [PubMed] [Google Scholar]
- Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM 2004 FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J 23:4802–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon JS, Tanaka Y, Olson LK, Robertson RP 1998 Reconstitution of glucotoxic HIT-T15 cells with somatostatin transcription factor-1 partially restores insulin promoter activity. Diabetes 47:900–904 [DOI] [PubMed] [Google Scholar]
- Kawamori D, Kajimoto Y, Kaneto H, Umayahara Y, Fujitani Y, Miyatsuka T, Watada H, Leibiger IB, Yamasaki Y, Hori M 2003 Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH(2)-terminal kinase. Diabetes 52:2896–2904 [DOI] [PubMed] [Google Scholar]
- Harmon JS, Stein R, Robertson RP 2005 Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic β cells. J Biol Chem 280:11107–11113 [DOI] [PubMed] [Google Scholar]
- Fernandes AP, Holmgren A 2004 Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal 6:63–74 [DOI] [PubMed] [Google Scholar]
- Ivarsson R, Quintens R, Dejonghe S, Tsukamoto K, in’t Veld P, Renstrom E, Schuit FC 2005 Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes 54:2132–2142 [DOI] [PubMed] [Google Scholar]
- Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL 2004 Free fatty acids and cytokines induce pancreatic β-cell apoptosis by different mechanisms: role of nuclear factor-κB and endoplasmic reticulum stress. Endocrinology 145:5087–5096 [DOI] [PubMed] [Google Scholar]
- Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL 2005 Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic β-cells. Diabetes 54:452–461 [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ 2007 Endoplasmic reticulum stress contributes to β cell apoptosis in type 2 diabetes. Diabetologia 50:752–763 [DOI] [PubMed] [Google Scholar]
- Pirot P, Eizirik DL, Cardozo AK 2006 Interferon-γ potentiates endoplasmic reticulum stress-induced death by reducing pancreatic β cell defense mechanisms. Diabetologia 49:1229–1236 [DOI] [PubMed] [Google Scholar]
- Chambers KT, Unverferth JA, Weber SM, Wek RC, Urano F, Corbett JA 2008 The role of nitric oxide and the unfolded protein response in cytokine induced β-cell death. Diabetes 57:124–132 [DOI] [PubMed] [Google Scholar]
- Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, Pipeleers D, Ling Z, Drucker DJ 2006 GLP-1 receptor activation improves β cell function and survival following induction of endoplasmic reticulum stress. Cell Metab 4:391–406 [DOI] [PubMed] [Google Scholar]
- Liu M, Hodish I, Rhodes CJ, Arvan P 2007 Proinsulin maturation, misfolding, and proteotoxicity. Proc Natl Acad Sci USA 104:15841–15846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, Lipton RB, Greeley SA, Patch AM, Ellard S, Steiner DF, Hattersley AT, Philipson LH, Bell GI 2007 Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci USA 104:15040–15044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolcott CD, Rallison ML 1972 Infancy-onset diabetes mellitus and multiple epiphyseal dysplasia. J Pediatr 80:292–297 [DOI] [PubMed] [Google Scholar]
- Stoss H, Pesch HJ, Pontz B, Otten A, Spranger J 1982 Wolcott-Rallison syndrome: diabetes mellitus and spondyloepiphyseal dysplasia. Eur J Pediatr 138:120–129 [DOI] [PubMed] [Google Scholar]
- Thornton CM, Carson DJ, Stewart FJ 1997 Autopsy findings in the Wolcott-Rallison syndrome. Pediatr Pathol Lab Med 17:487–496 [PubMed] [Google Scholar]
- Castelnau P, Le Merrer M, Diatloff-Zito C, Marquis E, Tete MJ, Robert JJ 2000 Wolcott-Rallison syndrome: a case with endocrine and exocrine pancreatic deficiency and pancreatic hypotrophy. Eur J Pediatr 159:631–633 [DOI] [PubMed] [Google Scholar]
- Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C 2000 EIF2AK3, encoding translation initiation factor 2-α kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet 25:406–409 [DOI] [PubMed] [Google Scholar]
- Senee V, Vattem KM, Delepine M, Rainbow LA, Haton C, Lecoq A, Shaw NJ, Robert JJ, Rooman R, Diatloff-Zito C, Michaud JL, Bin-Abbas B, Taha D, Zabel B, Franceschini P, Topaloglu AK, Lathrop GM, Barrett TG, Nicolino M, Wek RC, Julier C 2004 Wolcott-Rallison syndrome: clinical, genetic, and functional study of EIF2AK3 mutations and suggestion of genetic heterogeneity. Diabetes 53:1876–1883 [DOI] [PubMed] [Google Scholar]
- Allotey RA, Mohan V, McDermott MF, Deepa R, Premalatha G, Hassan Z, Cassell PG, North BV, Vaxillaire M, Mein CA, Swan DC, O’Grady E, Ramachandran A, Snehalatha C, Sinnot PJ, Hemmatpour SK, Froguel P, Hitman GA 2004 The EIF2AK3 gene region and type I diabetes in subjects from South India. Genes Immun 5:648–652 [DOI] [PubMed] [Google Scholar]
- Lee TG, Tang N, Thompson S, Miller J, Katze MG 1994 The 58,000-dalton cellular inhibitor of the interferon-induced double-stranded RNA-activated protein kinase (PKR) is a member of the tetratricopeptide repeat family of proteins. Mol Cell Biol 14:2331–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Frank CL, Korth MJ, Sopher BL, Novoa I, Ron D, Katze MG 2002 Control of PERK eIF2α kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc Natl Acad Sci USA 99:15920–15925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski DT, Kang SW, Goodman AG, Garrison JL, Taunton J, Katze MG, Kaufman RJ, Hegde RS 2007 The role of p58IPK in protecting the stressed endoplasmic reticulum. Mol Biol Cell 18:3681–3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S, Yun C, Fisher EA, Kreglinger N, Kreibich G, Oyadomari M, Harding HP, Goodman AG, Harant H, Garrison JL, Taunton J, Katze MG, Ron D 2006 Cotranslocational degradation protects the stressed endoplasmic reticulum from protein overload. Cell 126:727–739 [DOI] [PubMed] [Google Scholar]
- Ladiges WC, Knoblaugh SE, Morton JF, Korth MJ, Sopher BL, Baskin CR, MacAuley A, Goodman AG, LeBoeuf RC, Katze MG 2005 Pancreatic β-cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum molecular chaperone gene P58IPK. Diabetes 54:1074–1081 [DOI] [PubMed] [Google Scholar]
- Barrett TG, Bundey SE 1997 Wolfram (DIDMOAD) syndrome. J Med Genet 34:838–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanim F, Kirk J, Latif F, Barrett TG 2001 WFS1/wolframin mutations, Wolfram syndrome, and associated diseases. Hum Mutat 17:357–367 [DOI] [PubMed] [Google Scholar]
- Cryns K, Sivakumaran TA, Van den Ouweland JM, Pennings RJ, Cremers CW, Flothmann K, Young TL, Smith RJ, Lesperance MM, Van Camp G 2003 Mutational spectrum of the WFS1 gene in Wolfram syndrome, nonsyndromic hearing impairment, diabetes mellitus, and psychiatric disease. Hum Mutat 22:275–287 [DOI] [PubMed] [Google Scholar]
- Ishihara H, Takeda S, Tamura A, Takahashi R, Yamaguchi S, Takei D, Yamada T, Inoue H, Soga H, Katagiri H, Tanizawa Y, Oka Y 2004 Disruption of the WFS1 gene in mice causes progressive β-cell loss and impaired stimulus-secretion coupling in insulin secretion. Hum Mol Genet 13:1159–1170 [DOI] [PubMed] [Google Scholar]
- Riggs AC, Bernal-Mizrachi E, Ohsugi M, Wasson J, Fatrai S, Welling C, Murray J, Schmidt RE, Herrera PL, Permutt MA 2005 Mice conditionally lacking the Wolfram gene in pancreatic islet β cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia 48:2313–2321 [DOI] [PubMed] [Google Scholar]
- Yamada T, Ishihara H, Tamura A, Takahashi R, Yamaguchi S, Takei D, Tokita A, Satake C, Tashiro F, Katagiri H, Aburatani H, Miyazaki J, Oka Y 2006 WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic β-cells. Hum Mol Genet 15:1600–1609 [DOI] [PubMed] [Google Scholar]
- Zatyka M, Ricketts C, da Silva Xavier G, Minton J, Fenton S, Hofmann-Thiel S, Rutter GA, Barrett T 2008 Sodium-potassium ATPase β 1 subunit is a molecular partner of Wolframin, an endoplasmic reticulum (ER) protein involved in ER stress. Hum Mol Genet 17:190–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Wong HN, Song B, Miller CN, Scheuner D, Kaufman RJ 2005 The unfolded protein response sensor IRE1α is required at two distinct steps in B cell lymphopoiesis. J Clin Invest 115:268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimold AM, Etkin A, Clauss I, Perkins A, Friend DS, Zhang J, Horton HF, Scott A, Orkin SH, Byrne MC, Grusby MJ, Glimcher LH 2000 An essential role in liver development for transcription factor XBP-1. Genes Dev 14:152–157 [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS 2004 Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306:457–461 [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS 2006 Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313:1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligi GS 2007 Endoplasmic reticulum stress and inflammation in obesity and type 2 diabetes. Novartis Foundation Symp. 286:86–94; discussion 94–98, 162–163, 196–203 [DOI] [PubMed] [Google Scholar]
- Thameem F, Farook VS, Bogardus C, Prochazka M 2006 Association of amino acid variants in the activating transcription factor 6 gene (ATF6) on 1q21–q23 with type 2 diabetes in Pima Indians. Diabetes 55:839–842 [DOI] [PubMed] [Google Scholar]
- Meex SJ, van Greevenbroek MM, Ayoubi TA, Vlietinck R, van Vliet-Ostaptchouk JV, Hofker MH, Vermeulen VM, Schalkwijk CG, Feskens EJ, Boer JM, Stehouwer CD, van der Kallen CJ, de Bruin TW 2007 Activating transcription factor 6 polymorphisms and haplotypes are associated with impaired glucose homeostasis and type 2 diabetes in Dutch Caucasians. J Clin Endocrinol Metab 92:2720–2725 [DOI] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P 2007 IRE1 signaling affects cell fate during the unfolded protein response. Science 318:944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Upton JP, Hagen A, Callahan J, Oakes SA, Papa FR 2008 A kinase inhibitor activates the IRE1α RNase to confer cytoprotection against ER stress. Biochem Biophys Res Commun 365:777–783 [DOI] [PubMed] [Google Scholar]
- Credle JJ, Finer-Moore JS, Papa FR, Stroud RM, Walter P 2005 On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc Natl Acad Sci USA 102:18773–18784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Liu CY, Back SH, Clark RL, Peisach D, Xu Z, Kaufman RJ 2006 The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc Natl Acad Sci USA 103:14343–14348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Dey M, Neculai D, Cao C, Dever TE, Sicheri F 2008 Structure of the dual enzyme ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell 132:89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Sawkar AR, Whalen LJ, Wong CH, Kelly JW 2007 Isofagomine- and 2,5-anhydro-2,5-imino-D-glucitol-based glucocerebrosidase pharmacological chaperones for Gaucher disease intervention. J Med Chem 50:94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri TK, Paul S 2006 Protein-misfolding diseases and chaperone-based therapeutic approaches. FEBS J 273:1331–1349 [DOI] [PubMed] [Google Scholar]
- Masud A, Mohapatra A, Lakhani SA, Ferrandino A, Hakem R, Flavell RA 2007 Endoplasmic reticulum stress-induced death of mouse embryonic fibroblasts requires the intrinsic pathway of apoptosis. J Biol Chem 282:14132–14139 [DOI] [PubMed] [Google Scholar]
- Alarcon C, Wicksteed B, Rhodes CJ 2006 Exendin 4 controls insulin production in rat islet β cells predominantly by potentiation of glucose-stimulated proinsulin biosynthesis at the translational level. Diabetologia 49:2920–2929 [DOI] [PubMed] [Google Scholar]
- Abdel-Wahab YH, O’Harte FP, Mooney MH, Barnett CR, Flatt PR 2002 Vitamin C supplementation decreases insulin glycation and improves glucose homeostasis in obese hyperglycemic (ob/ob) mice. Metabolism 51:514–517 [DOI] [PubMed] [Google Scholar]
- Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki Y, Hori M 1999 Beneficial effects of antioxidants in diabetes: possible protection of pancreatic β-cells against glucose toxicity. Diabetes 48:2398–2406 [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M 2005 Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120:649–661 [DOI] [PubMed] [Google Scholar]
- Noguchi H, Nakai Y, Matsumoto S, Kawaguchi M, Ueda M, Okitsu T, Iwanaga Y, Yonekawa Y, Nagata H, Minami K, Masui Y, Futaki S, Tanaka K 2005 Cell permeable peptide of JNK inhibitor prevents islet apoptosis immediately after isolation and improves islet graft function. Am J Transplant 5:1848–1855 [DOI] [PubMed] [Google Scholar]
- Li X, Chen H, Epstein PN 2004 Metallothionein protects islets from hypoxia and extends islet graft survival by scavenging most kinds of reactive oxygen species. J Biol Chem 279:765–771 [DOI] [PubMed] [Google Scholar]
- Mysore TB, Shinkel TA, Collins J, Salvaris EJ, Fisicaro N, Murray-Segal LJ, Johnson LE, Lepore DA, Walters SN, Stokes R, Chandra AP, O’Connell PJ, d’Apice AJ, Cowan PJ 2005 Overexpression of glutathione peroxidase with two isoforms of superoxide dismutase protects mouse islets from oxidative injury and improves islet graft function. Diabetes 54:2109–2116 [DOI] [PubMed] [Google Scholar]
- Bertera S, Crawford ML, Alexander AM, Papworth GD, Watkins SC, Robbins PD, Trucco M 2003 Gene transfer of manganese superoxide dismutase extends islet graft function in a mouse model of autoimmune diabetes. Diabetes 52:387–393 [DOI] [PubMed] [Google Scholar]
- Dickhout JG, Sood SK, Austin RC 2007 Role of Endoplasmic reticulum calcium disequilibria in the mechanism of homocysteine-induced ER stress. Antioxid Redox Signal 9:1863–1874 [DOI] [PubMed] [Google Scholar]
- Tsutsumi S, Gotoh T, Tomisato W, Mima S, Hoshino T, Hwang HJ, Takenaka H, Tsuchiya T, Mori M, Mizushima T 2004 Endoplasmic reticulum stress response is involved in nonsteroidal anti-inflammatory drug-induced apoptosis. Cell Death Differ 11:1009–1016 [DOI] [PubMed] [Google Scholar]
- Chen G, Ma C, Bower KA, Shi X, Ke Z, Luo J 2008 Ethanol promotes endoplasmic reticulum stress-induced neuronal death: Involvement of oxidative stress. J Neurosci Res 86:937–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Miyazaki M, Matsuhisa M, Takano K, Nakatani Y, Hatazaki M, Tamatani T, Yamagata K, Miyagawa J, Kitao Y, Hori O, Yamasaki Y, Ogawa S 2005 The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes 54:657–663 [DOI] [PubMed] [Google Scholar]
- Kovacs P, Yang X, Permana PA, Bogardus C, Baier LJ 2002 Polymorphisms in the oxygen-regulated protein 150 gene (ORP150) are associated with insulin resistance in Pima Indians. Diabetes 51:1618–1621 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.