Abstract
Clinical Relevance
Glaucoma is a leading cause of blindness in the world, and the identification of genes that contribute to this disease is essential. Elevated intraocular pressure (IOP) is a principal risk factor for primary open-angle glaucoma (POAG), and an intriguing quantitative trait that may strongly influence the development of disease.
Objectives
The objective of this study was to identify genetic loci that control IOP.
Methods
We performed a genomewide scan of IOP, using 486 pedigrees ascertained through a population based cohort, the Beaver Dam Eye Study. Linkage analysis was performed using the modified Haseman-Elston regression models and variance components linkage analysis.
Results
Seven regions of interest were identified on chromosomes 2, 5, 6, 7, 12, 15 and 19. The novel linkage region on chromosome 19p had an empirical multipoint pvalue of 6.1×10−5. Two of the regions (2 and 19) are especially interesting since each has been identified as potential linkage regions for blood pressure.
Conclusions
The results of this genomewide scan provide evidence that a quantitative trait locus (QTL) may influence elevated IOP and may co-localize with blood pressure loci. These loci may control systemic pressure reflected in the eye and vascular system.
Keywords: Intraocular Pressure, Hypertension, Linkage Analysis, Glaucoma
Introduction
Primary open-angle glaucoma (POAG) is a progressive eye disease that often results in blindness. Worldwide, an estimated 37 million people are blind, 12% of which are attributable to glaucoma 1. Intraocular pressure, (IOP) is a physiologic characteristic that is present in every eye and is essential to maintain the structural and functional integrity of the eye. Higher intraocular pressure is associated with higher risk of damage to the optic nerve and can result in irreversible vision loss or blindness. Elevated IOP is a primary risk factor for the development of glaucoma
Several family studies have shown that genetic variation contributes to the development of POAG 2-12. Genetic regions that influence glaucoma have also been identified in large population based studies 3;10. To fully understand the genetic basis of POAG we focused on the principal risk factor, IOP. The goal of this study was to identify quantitative trait loci (QTL) controlling intraocular pressure across its range of values and thereby influencing the development of glaucoma. The identification of genes that contribute to variation in IOP may help to elucidate the pathology and mechanisms that result in vision loss due to glaucoma.
Subjects and Methods
Population
The Beaver Dam Eye Study (BDES) is a population based cohort study established in 1988 and a detailed description is available 13. Eligibility requirements for inclusion in the study were individuals between the ages of 43−84 years and residence in Beaver Dam, Wisconsin. In 1988−1990, a total of 5924 eligible persons were identified by a private census of the community, of whom 4926 (83.1%) participated fully in the baseline evaluation of the BDES. Family relationships and pedigrees were constructed from participant information and later confirmed at the first follow up visit (1993−1995). Of the 4926 individuals who enrolled at baseline, 2,336 individuals had known familial relationships in the cachement area of the study. For 2044 individuals, we have complete IOP measurements at baseline; DNA for genotypic analysis was available on 1979 individuals. Since the pedigrees were derived from an entire township, and eligibility for entry into the study was restricted to generally older individuals, most of the pedigrees are not “deep” or multi-generational. Instead, these pedigrees are “hedges” with multiple siblings, cousins and spouses but limited parental or grandparental phenotypic information. There are 486 pedigrees (of 602 original pedigrees) used for analysis after excluding families uninformative for linkage (i.e. parent-offspring trios). The mean size of all the pedigrees is 10.5 ± 12.5, and for individuals with both IOP phenotype information and genotype information is 4 ± 5. All individuals gave informed consent and the Institutional Review Board at the University of Wisconsin approved all protocols. The NHGRI Institutional Review Board also approved the statistical analyses of these existing data.
Clinical Evaluation
Detailed medical histories and eye examinations were performed on all participants including assessment of IOP and glaucoma. Intraocular pressure was measured using a Goldmann applanation tonometer after instilling a drop of fluorescein combined with a topical anesthetic (Flouress Barnes-Hind Armour Pharmaceutical Co., Kankakee, IL) in each eye. The examinations were carried out by trained observers who participated in quality control programs to maintain consistency and validity of measurements 14. Blood pressure was measured according to the Hypertension Detection and Follow-up Program protocol 15.This method entails measuring the blood pressure three times, the second and third with a random zero sphygmomanometer. The mean of these latter two blood pressures was used.
DNA extraction and Genotyping
For 76% of individuals, DNA was extracted from buffy coat separated at the time of the blood draw from the second or third Beaver Dam visit and stored at −80 centigrade. For 24% of individuals, DNA was extracted from frozen whole blood cells from the first exam. A genomewide scan was performed at the Center for Inherited Disease Research (CIDR) using automated fluorescent microsatellite analysis. PCR products were sized using a capillary sequencing platform. The marker set consisted of 404 short tandem repeat markers with an average spacing of 9 cM throughout the genome. The marker set is a modified version of the Marshfield Genetics version 8 screening set with an average heterozygosity of 0.76. The overall missing data rate was 3.5%.
Statistical Methods
Error testing
The data were deposited into a web-based secure database, GENELINK.16 CIDR released the data after running GAS to check for Mendelian inconsistencies and to identify any systematic lab or binning problems. Relationship errors were further identified using PREST17 and RELCHECK18 which both examine extended pedigrees. The reclassifications of errors resulted in a reduction of 2435 sib pairs to 2427 genotype-verified sibpairs in the full dataset. We also identified any residual Mendelian errors using MARKERINFO in S.A.G.E.v4.6 19 All errors were corrected prior to any analyses.
Linkage analyses
For quantitative analyses, the higher IOP measurement from either eye was used. IOP was normally distributed and did not need additional transformations. We adjusted for covariates (age, sex, systolic blood pressure and treatment for IOP) outside of the linkage analysis programs. Using linear regression, we modeled variation in IOP due to these covariates. Then, for each individual, we calculated the predicted deviation from the mean due to that individual's specific values of the covariates and subtracted this from the mean, thus creating a mean-adjusted residual deviation. This adjusted value was then used in our linkage analysis. IOP was assessed for deviations from normality using normal quantile plots, and measures of skewness and kurtosis. Allele frequencies at marker loci were estimated from founders using the maximum likelihood option in the program FREQ (S.A.G.E.v 4.6). Non-parametric linkage analysis was conducted using the Haseman-Elston regression as implemented in SIBPAL (S.A.G.E. v. 4.6). A weighted combination of squared trait difference and squared mean-corrected trait sum (option W4) was used. This method adjusts for the non-independence of sib-pairs and the non-independence of squared trait sums and differences. We performed up to 1 million Monte Carlo permutations using SIBPAL to determine the empirical p values.
In addition, we performed variance components analysis using nuclear families in MERLIN (MERLIN v0.10.2).20 Prior to running variance components in MERLIN, our pedigrees were reduced to nuclear families using an option in the statistical program, MEGA2.21 MERLIN performs multipoint variance components linkage analysis under the assumption of no dominance and calculates the locus specific heritability for each trait. No ascertainment correction was necessary since the Beaver Dam Eye Study is a population based cohort. Allele frequencies were calculated in MERLIN for founders only. We also performed multipoint variance components using the entire pedigrees in SOLAR (SOLAR v2.1.4)22 since it is not limited by larger pedigree size and included covariates in the analysis. Allele frequencies were estimated in FREQ (SAGEv4.6), and IBD calculations were done within SOLAR for these analyses.
Results and Discussion
Table 1 provides clinical characteristics for the BDES participants with available IOP measurements and genotype information. Forty-five percent of the individuals were male, and the median age was 62 years for men and 65 years for women. The median IOP measurement was 16 mm Hg and did not differ between genders. The mean and median IOP measurements (adjusted and unadjusted) were very similar supporting a normal distribution for this quantitative trait. In addition, we saw no deviation from normality using quantile plots or measures of skewness and kurtosis. 1041 individuals were classified as hypertensive (SBP>139 and/or DBP >89 and/or on blood pressure lowering medication) The mean systolic blood pressure was 133 mm Hg and the mean diastolic blood pressure was 77 mm Hg. Our previous segregation analysis (Duggal et al, 2005) showed that age, sex, treatment for IOP and systolic blood pressure were important covariates in our models, and they were included in all analyses. In total, we had 1979 individuals with both genotype and IOP phenotype information. Of these 1979 participants, there were 1059 full sib pairs, 59 half-sib pairs, 1567 cousin pairs, 686 avuncular pairs and 1 grandparent-grandchild pair. Among the 1524 sibships with genotype data, 1293 had 0 parents with phenotype data, 181 had 1 parent with phenotype data and 50 had 2 parents with phenotype data.
Table 1.
Characteristics of the Beaver Dam Eye Study participants with IOP measurements and DNA available for genotyping (n=1979)
| Male (%) | 45 (n=896) | |
| Female (%) | 55 (n=1083) | |
| Glaucoma (%) | 4.6 (n=92) | |
| Treatment for IOP (%) | 2.4 (n=48) | |
| Age (years) | Median (Range) | 64 (43−86) |
| IOP (mmHg) | Median (Range) | 16.0 (6−36) |
| IOP (mmHg) adjusted | Median (Range) | 11.9 (2−32) |
| SBP (mmHg) | Median (Range) | 131 (81−221) |
| DBP (mmHg) | Median (Range) | 78 (42−127) |
| Normotensive (%) | 47 (n=936) | |
| Hypertensive (%) | 53 (n=1041) | |
| (SBP>139 or DBP >89 or on BP lowering meds) |
IOP Linkage Results
The genome wide scan using Haseman-Elston regression of sibpairs identified 7 regions with an empirical multipoint linkage signal of less than 0.01 (chromosomes 2, 5, 6, 7, 12, 15 and 19). Figure 1 graphically displays the multipoint linkage regression results for all chromosomes, and Table 2 details the empirical multipoint p values and variance components p values of significance. The strongest evidence for linkage was identified on chromosome 19 near marker D19S586, with singlepoint and multipoint empirical p values of 2.0 × 10−4 and 6.1 × 10−5, respectively. This novel linkage region reached suggestive evidence for linkage according to the highly conservative Lander and Krugylak thresholds 23. The chromosome 19 region spans approximately 20cM with a one lod drop physical interval from 15cM to 35cM.
Fig 1.
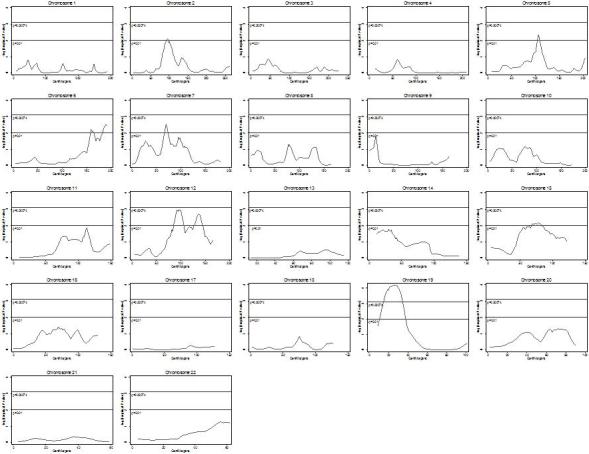
Table 2.
Genome Wide Linkage Scan Regions of Interest
| Chromosome | Nearest Marker | Physical Location | Method | Locus Specific Heritability | Pvalue/LOD |
|---|---|---|---|---|---|
| 2 | D2S1777 | 99cM | Haseman-Elston | 0.007 | |
| |
|
--- |
VC nuclear families |
13% |
0.10 |
| 5 | D5S1725 | 98 cM | Haseman-Elston | 0.004 | |
| |
|
--- |
VC nuclear families |
24% |
0.009 |
| 6 | D6S1027 | 187 cM | Haseman-Elston | 0.003 | |
| |
|
--- |
VC nuclear families |
22% |
0.02 |
| 7 | D7S1818 | 70 cM | Haseman-Elston | 0.003 | |
| --- | VC nuclear families | 28% | 0.0015 | ||
| |
|
64 cM |
VC full pedigrees |
20% |
1.43 |
| 12 | D12S1064 | 95 cM | Haseman-Elston | 0.0012 | |
| VC nuclear families | 23% | 0.01 | |||
| |
|
88 cM |
VC full pedigrees |
20% |
1.28 |
| 15 | D15S131 | 71 cM | Haseman-Elston | 0.007 | |
| --- | VC nuclear families | 21% | 0.03 | ||
| |
|
--- |
VC full pedigrees |
22% |
1.59 |
| 19 | D19S586 | 33 cM | Haseman-Elston | 0.00006 | |
| --- | VC nuclear families | 28% | 0.005 |
VC: Variance components Linkage Analysis; Haseman-Elston: Haseman-Elston Regression Analysis. Only LOD scores >1.0 are reported for VC full pedigrees.
Empirical p values are reported for Haseman-Elston regression analysis and nominal p values for variance components analysis.
This same region, near marker D19S586, was also identified in variance components linkage analysis of the nuclear family data (p value, 0.005, locus-specific h2= 28.4%). Of the 6 additional regions identified by Haseman-Elston regression, 5 were also supported with variance components analysis of the nuclear family data (Table 2) with locus specific heritability ranging from 20−28%. Additionally, we performed variance component linkage analysis using extended pedigree data; however, we did not identify any regions that reached a lod score of 2.0 or greater. Using the entire pedigree we identified 3 regions with lod score >1.0: chromosome 15 (71cM) with a lod score of 1.59 and a locus specific heritability of 22%, chromosome 12 (88cM) with a lod of 1.28 and a locus specific heritability of 20% and chromosome 7 (64cM) with a lod of 1.43 and a locus specific heritability of 20%.
Discussion
We performed a genomewide scan of IOP in families from a population-based cohort, the Beaver Dam Eye Study. The region providing the strongest evidence of linkage was on Chromosome 19p near marker D19S586. Analysis using both Haseman-Elston regression of sibling data (p value = 0.00006) and variance components analysis of nuclear family data (p value = 0.005) supported linkage of IOP to this same region on Chromosome 19. Intriguingly, this telomeric region of 19p also yielded suggestive evidence for linkage (LOD = 2.3) in a genomewide scan for systolic blood pressure in 18 Dutch dyslipidemic families 24. This region has also been identified as highly suggestive for systolic blood pressure (LOD =2.1, p value = 0.00094) in a study of 206 families in the Quebec Family Heart Study25. In a study of hypertensive sibpairs (black and white) from the HyperGEN network, a small peak (LOD = 1.31) was also reported within this region (24cM) among white sibpairs 26 These are sibling pairs with hypertension diagnosed before 60 years of age and without Type 1 diabetes. More recently, a study of pulse pressure (the difference between systolic and diastolic blood pressure) in the Family Blood Pressure Program study which includes black, Asian, Hispanic and white families found evidence for linkage (LOD = 3.1) to this same locus among blacks and whites 27. Our evidence for a QTL for intraocular pressure coupled with these studies of hypertension, systolic or pulse pressure all suggest that this region shows strong evidence of harboring a quantitative trait gene related to both IOP and systemic blood pressure.
Although the linkage peak on chromosome 2 was not substantiated using variance components, this region is still of strong interest since it also appears to co-localize with a blood pressure locus. In the Quebec Family Heart Study25, Rice and colleagues found suggestive evidence for linkage of systolic blood pressure ( LOD = 2.26, p value = 0.00062) within the same 20 cM region on chromosome 2. Additionally, in a study of pulse pressure in Mexican-Americans from the San Antonio Family Heart Study, a linkage peak of 1.28 was found at marker D2S1790 which is within 4 cM of our locus28. This region on chromosome 2 has also been identified as a glaucoma locus, GLC1B. In an initial study of 6 glaucoma families, Stoilova and colleagues found significant linkage with a maximum parametric lod score of 6.48 7. Recently, a study of a single 4 generation Tasmanian glaucoma family which included 15 living descendants, identified a 9 cM region on chromosome 2 (exact NPL = 0.005) that overlaps with GLC1B confirming this as a glaucoma locus 29. Our findings suggest that this region on chromosome 2 may harbor a single gene that influences systemic blood pressure with strong effects on glaucoma, or possibly 2 or more genes in close proximity that influence pressure and glaucoma independently.
It is not surprising that 2 of our linkage regions appear to co-localize with blood pressure loci. A previous analysis in the Beaver Dam Eye Study demonstrated a significant correlation of intraocular pressure with systolic and diastolic blood pressures at baseline and follow-up {Klein, 2005 12 /id}. For a 10 mm Hg increase in systolic blood pressure or diastolic blood pressure there was a 0.21 mm Hg increase ( 95% CI: 0.16, 0.27) and 0.43 mm Hg increase (95% CI: 0.35, 0.52) in IOP, respectively. This study showed that systemic blood pressure and intraocular pressure may be intertwined and intimates that a common mechanism or gene may be controlling pressure in the eye and vascular system.
Interestingly, we did not replicate our own pilot linkage analysis of IOP in the Beaver Dam Eye Study 30. In our previous study we performed linkage analysis using 263 sibling pairs from 102 pedigrees previously selected for age related maculopathy and identified two interesting potential linkage regions on chromosomes 6 ( p value = 0.008) and 13 (p value = 0.0007). However, after expanding our study to include more individuals (n=1979) and pedigrees (n=486) and utilizing a population based sample not selected for any other traits, these previous linkage regions were no longer significant. This is not particularly surprising since the initial pilot study used a minimal sample of individuals compared to our more complete current study, and neither region on chromosome 6 or 13 was significant using Lander and Krugylak thresholds of significance. However, this further highlights the importance of replication or confirmation studies for any important epidemiologic or genetic findings.
It was revealing that for this quantitative trait the most powerful method of analysis was Haseman-Elston regression using sibling pairs. Analysis of complete pedigrees using variance components found limited evidence for linkage. This is most likely due to the type of pedigrees in the Beaver Dam Eye Study. Unlike more traditional linkage studies that aim to maximize information using multigenerational pedigrees with affected individuals across generations, the Beaver Dam Eye Study pedigrees were derived from a population based cohort study, and age at enrollment was 43−86 years. Although these pedigrees are large, they most often lack a parental generation for both phenotype and genotype information and are comprised predominantly of sibships and cousin pairs. In this case, the major contributors to linkage information were sibpairs or individuals within a nuclear family. The use of the entire “hedge” pedigree may have introduced heterogeneity for this complex trait from extreme branches of the pedigrees where variation was controlled by different IOP genes. We know from studies of glaucoma and blood pressure, that it is unlikely that only one gene controls IOP and the use of the larger pedigrees may have actually diluted our findings. However, we are reassured that both nuclear family methods, the Haseman-Elston regression and Merlin variance components, identified the same regions. Additionally, for 3 of our loci variance components using the entire pedigrees also highlighted the same regions. For these population-based pedigrees, using nuclear families appears to be the most powerful method for the identification of linkage peaks for traits hypothesized to be genetically heterogenous (both locus and allelic).
In summary, we report the results of our initial genomewide scan for IOP, and identify 7 loci of interest. Each of these loci deserves further attention and they will hopefully be replicated in additional populations or further refined with dense SNP fine mapping to identify the genes controlling IOP. Two of our regions co-localize with blood pressure loci and suggest a gene or genes at chromosomes 19p (genome wide suggestive linkage) and 2p may control pressure in the eye and blood. Understanding the mechanism of elevated IOP may help elucidate our understanding of both hypertension and glaucoma.
Acknowledgements
We are grateful to participants of the Beaver Dam Eye Study. This research was supported by the National Eye Institutes R01 EY015286 (BEKK), U10 EY06594 (RK and BEKK) and in part by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health. Drs. Barbara and Ronald Klein are recipients of Senior Investigator Awards from Research to Prevent Blindness, New York, NY. The results of this paper were obtained by using the program package S.A.G.E. which is supported by a U.S. Public Health Service Resource Grant (RR03665) from the National Center for Research Resources, Bethesda, Maryland. Dr. Duggal has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2−3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
Reference List
- 1.Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull.World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Fingert JH, Stone EM, Sheffield VC, Alward WL. Myocilin glaucoma. Surv.Ophthalmol. 2002;47:547–561. doi: 10.1016/s0039-6257(02)00353-3. [DOI] [PubMed] [Google Scholar]
- 3.Nemesure B, Jiao X, He Q, et al. A genome-wide scan for primary open-angle glaucoma (POAG): the Barbados Family Study of Open-Angle Glaucoma. Hum.Genet. 2003;112:600–609. doi: 10.1007/s00439-003-0910-z. [DOI] [PubMed] [Google Scholar]
- 4.Rezaie T, Child A, Hitchings R, et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- 5.Sarfarazi M, Child A, Stoilova D, et al. Localization of the fourth locus (GLC1E) for adult-onset primary open-angle glaucoma to the 10p15-p14 region. Am.J.Hum.Genet. 1998;62:641–652. doi: 10.1086/301767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheffield VC, Stone EM, Alward WL, et al. Genetic linkage of familial open angle glaucoma to chromosome 1q21-q31. Nat.Genet. 1993;4:47–50. doi: 10.1038/ng0593-47. [DOI] [PubMed] [Google Scholar]
- 7.Stoilova D, Child A, Trifan OC, Crick RP, Coakes RL, Sarfarazi M. Localization of a locus (GLC1B) for adult-onset primary open angle glaucoma to the 2cen-q13 region. Genomics. 1996;36:142–150. doi: 10.1006/geno.1996.0434. [DOI] [PubMed] [Google Scholar]
- 8.Stone EM, Fingert JH, Alward WL, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 9.Trifan OC, Traboulsi EI, Stoilova D, et al. A third locus (GLC1D) for adult-onset primary open-angle glaucoma maps to the 8q23 region. Am.J.Ophthalmol. 1998;126:17–28. doi: 10.1016/s0002-9394(98)00073-7. [DOI] [PubMed] [Google Scholar]
- 10.Wiggs JL, Allingham RR, Hossain A, et al. Genome-wide scan for adult onset primary open angle glaucoma. Hum.Mol.Genet. 2000;9:1109–1117. doi: 10.1093/hmg/9.7.1109. [DOI] [PubMed] [Google Scholar]
- 11.Wirtz MK, Samples JR, Kramer PL, et al. Mapping a gene for adult-onset primary open-angle glaucoma to chromosome 3q. Am.J.Hum.Genet. 1997;60:296–304. [PMC free article] [PubMed] [Google Scholar]
- 12.Wirtz MK, Samples JR, Rust K, et al. GLC1F, a new primary open-angle glaucoma locus, maps to 7q35-q36. Arch.Ophthalmol. 1999;117:237–241. doi: 10.1001/archopht.117.2.237. [DOI] [PubMed] [Google Scholar]
- 13.Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98:1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 14.Klein BE, Klein R, Linton KL. Intraocular pressure in an American community. The Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1992;33:2224–2228. [PubMed] [Google Scholar]
- 15.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group Prev.Medicine. 1976;2:207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 16.Gillanders EM, Masiello A, Gildea D, et al. GeneLink: a database to facilitate genetic studies of complex traits. BMC Genomics. 2004;5:81. doi: 10.1186/1471-2164-5-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun L, Wilder K, McPeek MS. Enhanced pedigree error detection. Hum Hered. 2002;54:99–110. doi: 10.1159/000067666. [DOI] [PubMed] [Google Scholar]
- 18.Broman KW, Weber JL. Estimation of pairwise relationships in the presence of genotyping errors. Am.J.Hum.Genet. 1998;63:1563–1564. doi: 10.1086/302112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.S.A.G.E. Statistical analysis for genetic epidemiology Cork, Ireland, Computer program package available from Statistical Solutions Ltd. 2002.
- 20.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin--rapid analysis of dense genetic maps using sparse gene flow trees. Nat.Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- 21.Mukhopadhyay N, Almasy L, Schroeder M, Mulvihill WP, Weeks DE. Mega2: data-handling for facilitating genetic linkage and association analyses. Bioinformatics. 2005;21:2556–2557. doi: 10.1093/bioinformatics/bti364. [DOI] [PubMed] [Google Scholar]
- 22.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am.J.Hum.Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat.Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 24.Allayee H, de Bruin TW, Michelle DK, et al. Genome scan for blood pressure in Dutch dyslipidemic families reveals linkage to a locus on chromosome 4p. Hypertension. 2001;38:773–778. doi: 10.1161/hy1001.092617. [DOI] [PubMed] [Google Scholar]
- 25.Rice T, Rankinen T, Province MA, et al. Genome-wide linkage analysis of systolic and diastolic blood pressure: the Quebec Family Study. Circulation. 2000;102:1956–1963. doi: 10.1161/01.cir.102.16.1956. [DOI] [PubMed] [Google Scholar]
- 26.Rao DC, Province MA, Leppert MF, et al. A genome-wide affected sibpair linkage analysis of hypertension: the HyperGEN network. Am.J.Hypertens. 2003;16:148–150. doi: 10.1016/s0895-7061(02)03247-8. [DOI] [PubMed] [Google Scholar]
- 27.Bielinski SJ, Lynch AI, Miller MB, et al. Genome-wide linkage analysis for loci affecting pulse pressure: the Family Blood Pressure Program. Hypertension. 2005;46:1286–1293. doi: 10.1161/01.HYP.0000191706.41980.29. [DOI] [PubMed] [Google Scholar]
- 28.Atwood LD, Samollow PB, Hixson JE, Stern MP, MacCluer JW. Genome-wide linkage analysis of pulse pressure in Mexican Americans. Hypertension. 2001;37:425–428. doi: 10.1161/01.hyp.37.2.425. [DOI] [PubMed] [Google Scholar]
- 29.Charlesworth JC, Stankovich JM, Mackey DA, et al. Confirmation of the adult-onset primary open angle glaucoma locus GLC1B at 2cen-q13 in an Australian family. Ophthalmologica. 2006;220:23–30. doi: 10.1159/000089271. [DOI] [PubMed] [Google Scholar]
- 30.Duggal P, Klein AP, Lee KE, et al. A genetic contribution to intraocular pressure: the beaver dam eye study. Invest Ophthalmol.Vis.Sci. 2005;46:555–560. doi: 10.1167/iovs.04-0729. [DOI] [PubMed] [Google Scholar]


