Abstract
Acute exacerbations are the major cause of asthma morbidity, mortality, and health-care costs and are difficult to treat and prevent. The majority of asthma exacerbations are associated with rhinovirus (RV) infection, but evidence supporting a causal relationship is weak and mechanisms are poorly understood. We hypothesized that in asthmatic, but not normal, subjects RV infection would induce clinical, physiologic, and pathologic lower airway responses typical of an asthma exacerbation and that these changes would be related to virus replication and impaired T helper 1 (Th1)/IL-10 or augmented Th2 immune responses. We investigated physiologic, virologic, and immunopathologic responses to experimental RV infection in blood, induced sputum, and bronchial lavage in 10 asthmatic and 15 normal volunteers. RV infection induced significantly greater lower respiratory symptoms and lung function impairment and increases in bronchial hyperreactivity and eosinophilic lower airway inflammation in asthmatic compared with normal subjects. In asthmatic, but not normal, subjects virus load was significantly related to lower respiratory symptoms, bronchial hyperreactivity, and reductions in blood total and CD8+ lymphocytes; lung function impairment was significantly related to neutrophilic and eosinophilic lower airway inflammation. The same virologic and clinical outcomes were strongly related to deficient IFN-γ and IL-10 responses and to augmented IL-4, IL-5, and IL-13 responses. This study demonstrates increased RV-induced clinical illness severity in asthmatic compared with normal subjects, provides evidence of strong relationships between virus load, lower airway virus-induced inflammation and asthma exacerbation severity, and indicates augmented Th2 or impaired Th1 or IL-10 immunity are likely important mechanisms.
Keywords: exacerbation, respiratory viruses, immunology, human experimental virus infection
Acute exacerbations are the major cause of asthma morbidity, mortality (1), and health-care costs (2). Inhaled steroids are associated with reduced risk of exacerbation (3); however, optimal therapy in adults reduces exacerbation frequency by only ≈40–50% (4, 5). In school-age children inhaled steroids were ineffective at reducing exacerbation frequency, duration, or severity (6), and in preschool children oral steroids are also reported ineffective (7). Current therapy is therefore of limited efficacy and new more effective therapies are urgently required. To identify targets for development of new treatments, better understanding of mechanisms of virus-induced asthma exacerbations is required.
Virus infections are associated with 80–85% of pediatric (8, 9) and ≈75% of adult (10, 11) asthma exacerbations, and rhinoviruses (RVs) account for two-thirds of virus detections. Asthmatic individuals are more susceptible to naturally occurring RV infection than normal individuals in that lower respiratory tract symptoms and changes in peak expiratory flow (PEF) are more severe and of longer duration (12). We recently found increased RV replication and impaired IFN responses to ex vivo RV infection in primary bronchial epithelial cells from asthmatic compared with normal subjects (13, 14). However, relationships between viral load and asthma exacerbation severity have not been established.
Increased sputum neutrophils are observed in virus-induced asthma exacerbations (10, 15); however, studying lower respiratory inflammatory mechanisms during naturally occurring asthma exacerbations is extremely difficult. Therefore relationships between virus-induced airway inflammation and asthma exacerbation severity have not been established.
Human experimental RV infection in mild asthmatic volunteers report RV infection is associated with augmented physiological and inflammatory responses to allergen challenge (16, 17) and reductions in PEF (18), forced expiratory volume in 1 s (FEV1) (19), and increases in bronchial hyperreactivity (20). However, studies that have investigated both asthmatic and normal subjects have not demonstrated differences between subject groups in reductions in lung function or lower airway inflammatory responses (21, 22). Finally, asthma is associated with augmented T helper 2 (Th2) immunity, whereas weaker Th1 responses have been associated with impaired viral clearance in allergic subjects (23). However, the importance of Th1/2 immunity in virus-induced asthma exacerbation is unknown. Similarly, IL-10 expression is down-regulated in stable asthma (24), and its role in acute exacerbations is poorly understood (25).
We investigated the hypotheses that RV infection would induce significantly greater lower respiratory symptoms, reductions in lung function, and increases in bronchial hyperreactivity and lower airway inflammation in asthmatic compared with normal subjects. We also considered that virus load and virus-induced airway inflammation would be related to severity of clinical illness, and Th1/2 and IL-10 immune responses would be related to virus load and clinical outcomes during infection.
Results
Recruitment and Baseline Characteristics of Subjects.
Eleven atopic asthmatic and 17 nonatopic normal volunteers were recruited into the study. One asthmatic and two normal volunteers did not continue after the baseline phase. There were no serious adverse events and no subjects required inhaled or oral steroids for their asthma. Baseline characteristics of the recruited subjects are in supporting information (SI) Table S1.
Confirmation of RV16 Infection.
Successful experimental RV16 infection was confirmed in all subjects (SI Text and Table S2). The time course of virus loads in nasal lavage for both subject groups are shown in Fig. S1 a and b. These were similar in both groups and characteristic of an acute infection, with undetectable virus in most volunteers on day 1 after inoculation, followed by a rapid increase in virus loads on day 2 after inoculation with a peak around days 2–4. Virus was also detected in the lower airway in both groups of subjects. Virus loads in both upper and lower respiratory tracts were between 0.5 and 2 Logs greater in the asthmatic compared with the normal subjects; however, these differences were not statistically significant (Fig. S1c).
RV Induction of Cold Symptoms.
Both groups had significantly increased cold symptoms during infection. Daily cold scores peaked for both groups on day 3. Relative to the mean score on days −4 to 0 there were significant increases in daily cold score for asthmatic subjects on days 1–4 and 8–10 and for normal subjects on days 3–7. Differences in peak [asthmatic 9 (6,10.5) vs. normal 6 (3, 9) P = 0.24] and total [25 (24,36.5) vs. 13 (3.5,26.5) P = 0.11] cold scores between the asthmatic and normal groups were not statistically significant.
RV Induction of Chest Symptoms.
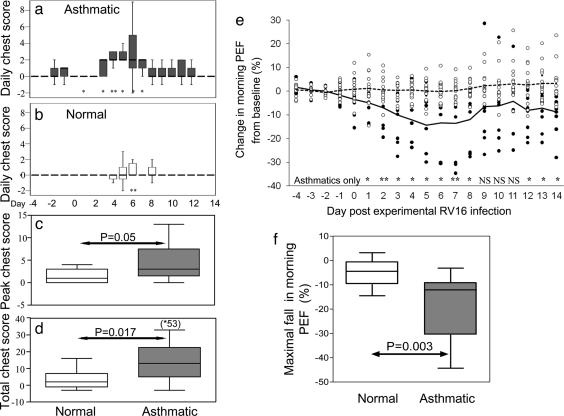
Both groups also had significantly increased chest symptoms (Fig. 1 a and b) during infection. Daily chest scores peaked later than cold symptoms for both groups at day 6. Relative to the mean score on days −4 to 0 there were significant increases in daily chest scores for asthmatic subjects on days 1 and 3–7 but for normal subjects only on day 6. Daily chest scores were significantly greater in the asthmatic group than the normal group on day 3 (P = 0.018), day 4 (P = 0.002), and day 7 (P = 0.021). Peak chest score (Fig. 1c) was higher in the asthmatic group [asthmatic 3 (1.5, 7.5) vs. normal 1 (0, 3) P = 0.05] as were total chest symptoms (Fig. 1d) in the asthmatic subjects [13 (5, 25)] compared with normal subjects [2 (−1, 7)], P = 0.017.
Fig. 1.
Lower respiratory symptom scores and lung function (PEF) during RV16 infection. Lower respiratory (chest) symptom scores were determined after RV16 inoculation in the asthmatic and normal subject groups. Chest scores are displayed in a–d and PEF are shown in e and f. Chest symptoms were more severe in the asthmatic group. Daily chest scores were significantly increased above baseline for the asthmatics on days 1 and 3–7 (a) and for the normals only on day 6 (b). *, P < 0.05 and **, P < 0.01 compared with the corresponding baseline day. The peak chest score (c; P = 0.05) and the 2-week total chest score (d ; P = 0.017) were both significantly greater in the asthmatic group compared with those recorded in the normal group. Daily PEF is expressed as the percentage change in morning PEF from baseline. In e, individual 3-day rolling mean data are plotted for days −4 to 14 (asthmatics, ●; normals, ○) as is the group median percentage change (asthmatics, solid line; normals, dotted line). In the normal group no significant change in PEF was seen on any of the days after inoculation. In contrast in the asthmatic group there were significant falls in PEF on days 1–8 and 12–14 (**, P < 0.01; *, P < 0.05 compared with baseline). In addition, the maximum percentage fall in PEF (f) was greater in the asthmatics (P = 0.003).
RV Induced Reductions in Lung Function.
RV infection induced no significant changes in PEF or FEV1 in the normal group on any study day (P = not significant on all days), in the asthmatic group there were significant falls in FEV1 on day 3 and 5–14 (data not shown) and in PEF on days 1–8 and 12–14 (Fig. 1e). In addition, the median maximum percentage fall was significantly greater in the asthmatic group for both FEV1 [12.5 (5.1, 17.9) vs. 3.8 (0.3, 6.8), P = 0.027] and PEF [Fig. 1f; 10.8 (9.2, 30.6) vs. 4.5 (0.8, 9.6) P = 0.003].
RV Induction of Bronchial Hyperreactivity.
For the asthmatic group there was a significant reduction in both the provocative concentration (PC)20 [Fig. S2a; − 0.6 doubling dilutions (−0.6, −1.3) P = 0.038] and the PC10 histamine [−0.9 (0.1, −1.5) P = 0.05] at day 6 relative to baseline. In contrast, there was no significant change in PC10 [Fig. S2b; 0.2 (−0.9, 0.7) P = 0.9] for the normal group. As a consequence, at infection, the asthmatics had significantly greater bronchial hyperreactivity [day 6 PC10 for asthmatic subjects 0.3 mg/ml vs. 6.4 mg/ml for normal subjects P < 0.001].
Relationship Between Clinical Illness Severity and Virus Load.
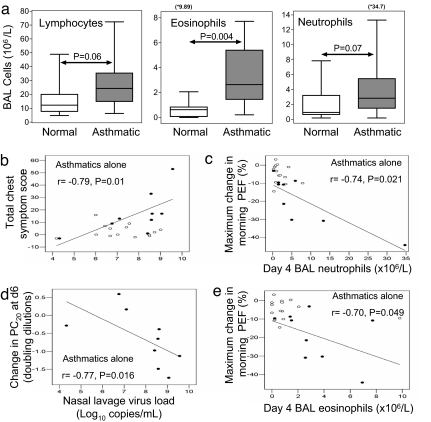
There was a strong significant correlation between total lower respiratory symptoms and nasal virus load in the asthmatic group (Fig. 2b; r = 0.79, P = 0.01), but none in normal subjects. There was also a strong significant inverse correlation between the change in PC20 and virus load in the asthmatic group (Fig. 2d; r = −0.77, P = 0.016).
Fig. 2.
Differences in BAL leukocyte concentrations during RV infection and relationships between virus load and airway inflammation and clinical illness severity are shown. (a) At day 4 BAL leukocyte concentrations were greater in the asthmatic than in the normal group. This difference was significant for eosinophils (Middle) and approached statistical significance for lymphocytes (Left) and neutrophils (Right). (b–e) The relationships between RV virus load and BAL inflammatory cells numbers and clinical illness severity were investigated in the two subject groups. In the asthmatic group (●) there was a significant correlation between peak virus load after inoculation and total chest symptom score (b) and the fall in histamine PC20 at day 6 (d) and between the maximum fall in PEF and the day 4 BAL neutrophil concentration (c) and the BAL day 4 eosinophil concentration (e). There were no significant correlations in the normal group (○).
Changes in Peripheral Blood Lymphocyte Subtypes During Infection.
In asthmatic, but not in normal, subjects there were significant falls at day 4 vs. baseline in blood CD4+ T cells (×109/liter) [0.8 (0.5, 0.9) vs. 0.8 (0.8, 1.0) P = 0.028]; CD8+ T cells [0.3 (0.2, 0.5) vs. 0.5 (0.4, 0.7) P = 0.028] and B cells [0.2 (0.1, 0.2) vs. 0.2 (0.1, 0.4) P = 0.028] with a trend for reduction in NK cells [0.1 (0, 0.2) vs. 0.2 (0.1, 0.5) P = 0.116].
Bronchoalveolar Lavage (BAL) Leukocyte Concentrations During Infection in Asthmatic and Normal Subjects.
Reductions in blood leukocytes during infection were consistent with their recruitment to the lung. We therefore next determined whether lower airway inflammatory responses were increased in asthmatic subjects and related to clinical illness. Lower airway inflammation was increased during infection in the asthmatic group relative to normal volunteers, although it was only statistically significant for eosinophils: lymphocytes (all ×106/liter BAL; Fig. 2a) [asthmatics 24.3 (14.9, 35.4) vs. normal 18.2 (7.7, 19.6) P = 0.06], eosinophils [asthmatics 2.6 (1.4, 5.4) vs. normals 0.8 (0.1, 0.9) P = 0.004], and neutrophils [asthmatics 2.9 (1.5, 9.6) vs. normals 1.5 (0.7, 3.2) P = 0.07].
Induced Sputum Cell Counts During Infection in Asthmatic and Normal Subjects.
Infection induced increased numbers (106/g sputum) of total nonsquamous cells for all subjects combined at day 7 [2.1 (1.1, 3.0) vs. baseline 1.0 (0.8, 2.1) P = 0.019] and total leukocytes [1.9 (0.9, 2.7) vs. 0.8 (0.6, 1.9) P = 0.011]. Among leukocytes, percent neutrophils increased during infection for all subjects from baseline [20.5 (11.1, 34.3)] both at day 3 [29.8 (17.3, 47.2), P = 0.044] and day 7 [46.6 (21.5, 58.7), P = 0.001], as did absolute neutrophil counts on day 7 [0.5 (0.3, 1.4) vs. baseline 0.2 (0.1, 0.4) P = 0.002]. There were no significant increases in eosinophil numbers during infection in sputum (data not shown).
Neutrophil counts were three to four times higher at days 3 and 7 in the asthmatic relative to the normal group, but these differences were not significant (P = not significant for both]. Sputum percentage of eosinophils were higher in the asthmatic group at day 3, asthmatics 2.0 (0.6, 3.2) vs. normals 0 (0,0.1), P = 0.003.
Relationship Between Immunopathology and Clinical Illness Severity and Virus Load.
For blood lymphocytes there were strong significant relationships between virus load and both the percentage fall in lymphocytes at day 4 (r = −0.9; P = 0.037) and the percentage fall in CD8+ T cells (r = −1.0; P < 0.001) in asthmatic, but not normal, subjects (P = not significant).
Lower airway inflammation was related to clinical illness severity in asthmatic subjects as both BAL neutrophils (r = −0.74; P = 0.021) and eosinophils (r = −0.7; P = 0.049) were significantly related to maximal fall in PEF (Fig. 2 c and d, respectively), and sputum eosinophil counts on day 3 correlated with total lower respiratory symptoms (r = 0.83; P = 0.042) and on day 7 with fall in PC10 histamine (r = −0.87; P = 0.05), there were no such relationships in normal subjects (P = not significant).
Relationship Between Th1/2 Immune Responses and Clinical Illness Severity and Virus Load.
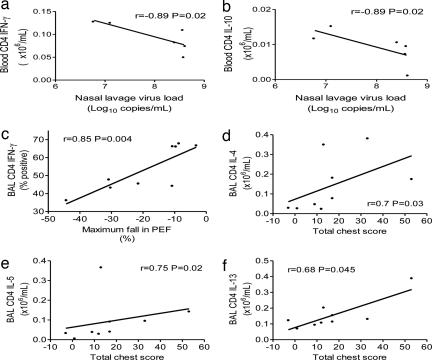
To investigate whether Th1 or Th2 immune responses were associated with severity of RV asthma exacerbations, we related Th1/2 responses determined at the baseline studies before infection to virus load and clinical outcomes in response to RV infection in the asthmatic subjects. We observed that both IFN-γ (Fig. 3a) and IL-10 (Fig. 3b) production by peripheral blood CD4+ T cells were associated with protection, in that stronger responses were associated with lower virus loads and less severe common cold symptoms [blood CD4 IFN-γ+ cells vs. nasal lavage virus load (r = −0.89, P = 0.02); blood CD4 IL-10+ cells vs. nasal lavage virus load (r = −0.89, P = 0.02) vs. total cold score (r = −0.97, P < 0.001])]. These data are based on small subject numbers, as only a few subjects had blood cells available for these studies. The data thus must be interpreted with caution and require confirmation in larger subject numbers.
Fig. 3.
Relationships between blood CD4+ IFN-γ and IL-10 production and nasal lavage virus load and between BAL CD4+ IFN-γ and IL-4, IL-5, and IL-13 production and reductions in lung function and lower respiratory symptoms in asthmatic subjects. (a and b) Peripheral blood CD4+ IFN-γ and IL-10 production were assessed at baseline before experimental RV infection by intracellular cytokine staining. In the asthmatic group there was a significant inverse correlation between peak virus load and both CD4+ IFN-γ (a) and IL-10 (b) production and between total cold symptom score and IL-10 production (data not shown; P < 0.001). (c–f) BAL CD4+ IFN-γ and IL-4, IL-5, and IL-13 production were assessed at baseline before experimental RV infection by intracellular cytokine staining. In the asthmatic group there was a significant inverse correlation between the maximum fall in PEF and CD4+ IFN-γ production (c) and significant positive correlations between CD4+ IL-4 (d), IL-5 (e), and IL-13 (f) production and total chest symptom score.
We next investigated responses in the lower airway and their relation to lower respiratory outcomes and found that IFN-γ production by BAL CD4+ T cells was also associated with protection, as stronger responses were strongly related to less severe falls in PEF (Fig. 3c; r = 0.85, P = 0.004), whereas in contrast, BAL CD4+ T cell production of the Th2 cytokines IL-4, IL-5, and IL-13 were all associated with adverse outcomes, as stronger responses were related to more severe lower respiratory symptom scores (Fig. 3 d–f; IL-4, r = 0.7, P = 0.03; IL-5, r = 0.75, P = 0.02; IL-13, r = 0.68, P = 0.045).
Lower Airway Cells from Asthmatics Are Deficient in Th1 and Have Augmented Th2 Cytokine Production.
Finally, having observed that IL-10 and/or IFN-γ were associated with lower virus loads and less severe symptoms and lung function reductions, whereas the Th2 cytokines IL-4, IL-5 and IL-13 were associated with more severe asthma symptoms in this experimental model of RV-induced asthma exacerbations, we next wanted to determine whether production of possibly protective cytokines was deficient and/or Th2 cytokines was augmented in asthma.
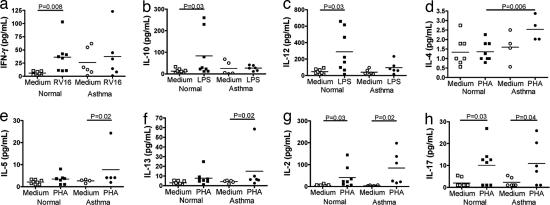
We therefore measured levels of these cytokines in supernatants of BAL cells from the baseline bronchoscopy in same asthmatic and normal subjects, after they had been stimulated for 48 h ex vivo with RV, phytohemagglutinin (PHA), or LPS.
We observed that levels of IFN-γ (P = 0.008), IL-10 (P = 0.03), and the Th1-promoting cytokine IL-12 (P = 0.03) were all induced significantly by virus or LPS in cells from normal subjects, but no significant induction was observed in those from asthmatic subjects (Fig. 4 a–c). In contrast, levels of the Th2 cytokine IL-4 induced by PHA were significantly higher (P = 0.006) in the asthmatic subjects than those observed in the normal subjects (Fig. 4d). The other Th2 cytokines, IL-5 and IL-13, were induced by PHA only in the cells from asthmatic subjects (both P = 0.02), whereas no significant induction was observed in those from normal subjects (Fig. 4 e and f). We also measured levels of the T cell cytokines IL-2 and IL-17 and found that they were induced by PHA to similar levels in both asthmatic (P = 0.03 and 0.02, respectively) and normal subjects (P = 0.03 and 0.04, respectively) (Fig. 4 g and h).
Fig. 4.
Deficient induction of Th1 cytokines and IL-10 and augmented induction of Th2 cytokines from BAL cells from asthmatic compared with normal subjects. BAL cells from the baseline bronchoscopy before experimental RV infection were incubated for 48 h with medium alone (open symbols) or RV-16, LPS, or PHA (closed symbols). Cytokine release into supernatants of cells from normal (squares) or asthmatic subjects (circles) was assessed by ELISA. (a–c) Significant induction of the Th1 cytokines IFN-γ (a), IL-10 (b), and IL-12 (c) was restricted to normal subjects. (d–f) Levels of the Th2 cytokine IL-4 were significantly greater in the stimulated asthmatic cells than in stimulated normal cells (d), and significant induction of the Th2 cytokines IL-5 (e) and IL-13 (f) was restricted to cells from asthmatic subjects. (g and h) In contrast, the T cell cytokine IL-2 (g) and the Th17 cytokine IL-17 (h) were induced normally in both subject groups.
Discussion
We have used experimental RV infection in asthmatic and normal volunteers to investigate the pathogenesis of virus-induced asthma exacerbations. We found that asthmatic subjects had markedly increased lower respiratory symptoms compared with normal subjects who developed only minimal lower respiratory symptoms. The total lower respiratory tract symptom score in asthmatics was >6 times greater than that recorded by the normal volunteers. In addition, only asthmatic subjects had reductions in lung function and increases in bronchial hyperreactivity in response to infection. These observations confirm that RV infection in asthma is associated with greatly increased lower respiratory clinical illness severity.
The mechanisms by which RV infections induce lower respiratory symptoms and alterations in lung physiology in asthma are not well understood (26). However, given that primary bronchial epithelial cells from asthmatic subjects replicate RV efficiently in vitro, whereas those from normal individuals are much more resistant to RV replication (13), a likely explanation is that increased virus load in asthma leads to increased virus-induced inflammation. We observed that virus load was strongly correlated with severity of both lower respiratory symptoms and increases in bronchial hyperreactivity, but these relationships were exclusive to asthmatic subjects (Fig. 2 d and f), suggesting an important role for virus load in determining the severity of clinical illness in asthma exacerbations.
To determine whether virus load was also associated with increased inflammation, we next investigated inflammatory cells in blood, sputum, and BAL. In blood, RV infection resulted in reductions in circulating lymphocytes. This observation would be consistent with recruitment of lymphocytes to an inflamed airway. This interpretation is supported by the strong correlation between the fall in blood lymphocytes during infection and virus load and the fact that it was observed only in asthmatic subjects.
In the lower respiratory tract, RV infection was associated with significant increases in eosinophils in BAL, and in total cells, total leukocytes and neutrophils in sputum. Involvement of these cells in the pathogenesis of virus-induced asthma exacerbations was supported by the strong relationships observed between reductions in lung function and BAL neutrophil and eosinophil counts (Fig. 2 e and g) and in sputum between eosinophils and both lower respiratory symptoms and bronchial hyperreactivity. Again, all of these relationships were exclusive to asthmatic subjects.
Previous seminal work reported RV infection augmented responses to inhaled allergen in allergic subjects (16, 17); however, no previous study to our knowledge has demonstrated differences in response to experimental RV infection between asthmatic and normal subjects in terms of symptom severity, changes in lung function, or induction of nonspecific bronchial hyperreactivity. Similarly, no previous study to our knowledge has demonstrated differences between asthmatic and normal subjects in terms of their lower airway inflammatory response to RV infection. We also have related virus load to measures of clinical illness severity and airway inflammation. Our data support virus load in asthmatic subjects being related to increased lower airway inflammation, and in turn increased lower airway inflammation being related to increased symptoms, reductions in lung function, and increases in bronchial hyperreactivity. They therefore provide strong support for an important role for RV infection in the pathogenesis of asthma exacerbations.
Increases in CD4 and CD8 T cells were observed in bronchial mucosa during experimental RV infection (21); however, little else is known regarding the role of lymphocyte subtypes in virus-induced asthma. We therefore investigated B cell, CD4 and CD8 T cell, and NK cell populations in peripheral blood and BAL. All blood lymphocyte subtypes decreased during infection, and these reductions were again restricted to asthmatic subjects. In BAL, all lymphocyte subtypes were more numerous in the asthmatic group than normal subjects during infection, although this finding was not statistically significant. These data are consistent with the reductions in blood lymphocyte subsets being a consequence of recruitment to the airway and being related to the severity of the viral infection, as reductions in lymphocyte numbers were strongly related to virus load in asthmatic subjects.
The mechanisms of increased RV-induced lower respiratory illness in asthma are very poorly understood. We have recently reported deficient IFN-β and IFN-λ production in asthma may be important (13, 14); however, asthma is associated with augmented Th2 immune responses and possibly impaired IL-10 regulatory responses. We therefore investigated the role of Th1 and Th2 cytokines and IL-10 in this RV-induced asthma exacerbation model. We found blood and BAL IFN-γ and IL-10 responses were associated with protection from RV-induced illness (lower virus loads, reduced symptoms, and less severe reduction in lung function), whereas the Th2 cytokines IL-4, IL-5, and IL-13 all were associated with more severe virus-induced asthma symptoms. These data strongly implicate impaired Th1 or impaired IL-10 responses and augmented Th2 responses in the pathogenesis of RV-induced asthma exacerbations.
In view of these findings we next determined whether production of possibly protective Th1 cytokines or IL-10 was impaired and production of the possibly harmful Th2 cytokines increased in airway cells from the asthmatic subjects. We found IFN-γ and IL-12 (both Th1 cytokines) and IL-10 all were significantly induced in cells from normal subjects, but not in cells from asthmatic subjects. The induction of IL-10 and IL-12 by LPS appeared most strikingly different between groups, suggesting differences in macrophage biology may be important, whereas the lack of induction of IFN-γ could have been a consequence of higher production in unstimulated cells in asthmatic subjects. In contrast, induction of the Th2 cytokines, although modest, was seen only in asthmatic subjects. These data are based on small subject numbers, as only a few subjects had sufficient cells remaining for these studies. The data should be interpreted with caution and require confirmation in larger subject numbers. However, these data showing impaired or augmented production, along with the relationships with disease severity observed above, suggest that novel approaches that inhibit Th2 cytokines or that augment Th1 cytokine or IL-10 production might have therapeutic potential in the treatment of virus-induced asthma exacerbations.
There are significant difficulties in studying natural virus-induced asthma exacerbations including heterogeneity in exacerbation aetiology, asthma severity, and treatment, timing of clinical sampling in relation to onset of infection, and levels of baseline antiviral immunity, in addition to difficulty with invasive sampling in patients with acute illness. Experimental RV infection offers an attractive experimental model of asthma exacerbations. Subjects are given a standard dose of a single virus serotype, and clinical data collection and invasive sampling can be carried out under controlled conditions repeatedly and at accurately defined time points. These advantages permit conclusions to be drawn with very much smaller numbers of subjects than would be needed for studies in naturally occurring exacerbations.
This human model of RV-induced asthma exacerbation reproduced many aspects of naturally occurring exacerbations and provides a number of relevant clinical, physiologic, virologic, and pathologic responses suitable for use as outcomes in intervention studies. We believe a less intensive version of this model could be useful in testing future possible treatments for RV-induced asthma exacerbations, for example, Th1-promoting or Th2-inhibiting approaches, IL-10, IFN-β (13), or IFN-λ (14).
In conclusion, we have demonstrated clear differences between the responses of asthmatic and normal subjects to RV infection in terms of clinical symptoms and airway physiology. We have related these changes to virus load and both clinical outcomes and virus load with airways inflammation. Further, we identify deficient induction of Th1 cytokines and IL-10 and augmented induction of Th2 cytokines in asthma and demonstrate that Th1 cytokines and IL-10 are associated with protection from exacerbation, whereas Th2 cytokines were associated with increased disease severity. These observations provide compelling evidence supporting an important role for RV-induced lower airway inflammation in precipitating asthma exacerbations, perhaps through impaired Th1/IL-10 and augmented Th2 responses. Our recent development of a mouse model of RV-induced exacerbation of allergic airway inflammation (27) should allow the importance of Th1 and Th2 cytokines and IL-10 to be tested in vivo. We anticipate that these models will be valuable in future studies investigating the immunopathology of virus-induced asthma and studies testing candidate new treatments.
Methods
Study Design.
RV16 experimental infections were induced in RV16 seronegative atopic asthmatic and normal nonatopic adult subjects (Table S1). Clinical and atopic status were defined by questionnaire, skin prick testing, serum IgE and lung function testing including PEF, FEV1, forced vital capacity (FVC), and histamine challenge (SI Text). Normal subjects were taking no medication, and asthmatics inhaled short-acting β2 agonists only. Subjects were free of common cold symptoms for 6 weeks before starting the study. All were nonsmokers.
Baseline, acute infection, and convalescent samples of blood, nasal lavage, induced sputum, and BAL were taken (Fig. S3a). Baseline samples were taken 14 days before infection, and diaries were kept to record symptoms and home lung function throughout the study (SI Text). All subjects gave informed consent and the study was approved by St Mary's National Health Service Trust Research Ethics Committee.
Experimental Infection with RV16.
Infection was induced by using 10,000 tissue culture 50% infective dose RV16 on day 0 by nasal spray as described (28) (SI Text). After inoculation, subjects returned home.
Assessment of Blood and Airway Inflammation and T Cell Cytokine Production.
Blood Coulter counts were performed. Absolute and differential induced sputum and BAL cell counts were determined on cytospins (SI Text). Fresh peripheral blood mononuclear cells and BAL cells were stained for CD4 and CD8 T cells and NK and B cells and intracellular type 1 (IFN-γ) and type 2 (IL-4, IL-5, ans IL-13) cytokines and IL-10 were determined in CD4+ T cells by three- and four-color flow cytometry (BD LSR flow cytometer) as described (29) (SI Text).
BAL Cell Cultures.
Cells from the BAL obtained at baseline bronchoscopy were incubated with medium alone, RV16, LPS, or PHA. After 48 h supernatants were harvested and stored at −80°C for cytokine analysis by Luminex.
Supplementary Material
Acknowledgments.
This work was supported by a Medical Research Council Clinical Research Fellowship (to S.D.M.), a British Medical Association H.C. Roscoe Fellowship (to S.D.M.), British Lung Foundation/Severin Wunderman Family Foundation Lung Research Program Grant P00/2, Asthma UK Grants 02/027 and 05/067, Wellcome Trust Grants 063717 and 083567/Z/07/Z for the Centre for Respiratory Infection, Imperial College, and the National Institute for Health Research Biomedical Research Center funding scheme.
Footnotes
Conflict of interest statement: S.L.J. has received consulting /lecturing fees and/or research grants from AstraZeneca, Centocor, GlaxoSmithKline, Merck, Pfizer, Sanofi-Pasteur, and Synairgen.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804181105/DCSupplemental.
References
- 1.Campbell MJ, Holgate ST, Johnston SL. Trends in asthma mortality. Br Med J. 1997;315:1012. [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss KB, Sullivan SD. The health economics of asthma and rhinitis. I. Assessing the economic impact. J Allergy Clin Immunol. 2001;107:3–8. doi: 10.1067/mai.2001.112262. [DOI] [PubMed] [Google Scholar]
- 3.Johnston NW, et al. The September epidemic of asthma exacerbations in children: A search for etiology. J Allergy Clin Immunol. 2005;115:132–138. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pauwels RA, et al. Early intervention with budesonide in mild persistent asthma: A randomized, double-blind trial. Lancet. 2003;361:1071–1076. doi: 10.1016/S0140-6736(03)12891-7. [DOI] [PubMed] [Google Scholar]
- 5.Pauwels RA, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;337:1405–1411. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 6.Doull IJ, et al. Effect of inhaled corticosteroids on episodes of wheezing associated with viral infection in school-age children: Randomized double-blind placebo controlled trial. Br Med J. 1997;315:858–862. doi: 10.1136/bmj.315.7112.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oommen A, et al. Efficacy of a short course of parent-initiated oral prednisolone for viral wheeze in children aged 1–5 years: Randomized controlled trial. Lancet. 2003;362:1433–1438. doi: 10.1016/S0140-6736(03)14685-5. [DOI] [PubMed] [Google Scholar]
- 8.Johnston SL, et al. Community study of role of viral infections in exacerbations of asthma in 9- to 11-year-old children. Br Med J. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chauhan AJ, et al. Personal exposure to nitrogen dioxide (NO2) and the severity of virus-induced asthma in children. Lancet. 2003;361:1939–1944. doi: 10.1016/S0140-6736(03)13582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wark PA, et al. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J. 2002;19:68–75. doi: 10.1183/09031936.02.00226302. [DOI] [PubMed] [Google Scholar]
- 11.Grissell TV, et al. Interleukin-10 gene expression in acute virus-induced asthma. Am J Respir Crit Care Med. 2005;172:433–439. doi: 10.1164/rccm.200412-1621OC. [DOI] [PubMed] [Google Scholar]
- 12.Corne JM, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and nonasthmatic individuals: A longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 13.Wark PA, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contoli M, et al. Role of deficient type III interferon-λ production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 15.Pizzichini MM, et al. Asthma and natural colds. Inflammatory indices in induced sputum: A feasibility study. Am J Respir Crit Care Med. 1998;158:1178–1184. doi: 10.1164/ajrccm.158.4.9712082. [DOI] [PubMed] [Google Scholar]
- 16.Lemanske RFJ, et al. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest. 1989;83:1–10. doi: 10.1172/JCI113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calhoun WJ, et al. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest. 1994;94:2200–2208. doi: 10.1172/JCI117581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bardin PG, et al. Peak expiratory flow changes during experimental rhinovirus infection. Eur Respir J. 2000;16:980–985. doi: 10.1183/09031936.00.16598000. [DOI] [PubMed] [Google Scholar]
- 19.Grunberg K, et al. Experimental rhinovirus 16 infection causes variable airway obstruction in subjects with atopic asthma. Am J Respir Crit Care Med. 1999;160:1375–1380. doi: 10.1164/ajrccm.160.4.9810083. [DOI] [PubMed] [Google Scholar]
- 20.Cheung D, et al. Rhinovirus inhalation causes long-lasting excessive airway narrowing in response to methacholine in asthmatic subjects in vivo. Am J Respir Crit Care Med. 1995;152:1490–1496. doi: 10.1164/ajrccm.152.5.7582282. [DOI] [PubMed] [Google Scholar]
- 21.Fraenkel DJ, et al. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med. 1995;151:879–886. doi: 10.1164/ajrccm/151.3_Pt_1.879. [DOI] [PubMed] [Google Scholar]
- 22.Fleming HE, et al. Rhinovirus-16 colds in healthy and in asthmatic subjects: Similar changes in upper and lower airways. Am J Respir Crit Care Med. 1999;160:100–108. doi: 10.1164/ajrccm.160.1.9808074. [DOI] [PubMed] [Google Scholar]
- 23.Gern JE, et al. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 24.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 25.Busse WW, Gern JE. Is interleukin-10 a “10” in virus-provoked asthma? Am J Respir Crit Care Med. 2005;172:405–406. doi: 10.1164/rccm.2506005. [DOI] [PubMed] [Google Scholar]
- 26.Message SD, Johnston SL. Host defense function of the airway epithelium in health and disease: Clinical background. J Leukocyte Biol. 2004;75:5–17. doi: 10.1189/jlb.0703315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartlett NW, et al. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat Med. 2008;14:199–204. doi: 10.1038/nm1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bardin PG, et al. Experimental rhinovirus infection in volunteers. Eur Respir J. 1996;9:2250–2255. doi: 10.1183/09031936.96.09112250. [DOI] [PubMed] [Google Scholar]
- 29.Cho SH, et al. Peripheral blood CD4+ and CD8+ T cell type 1 and type 2 cytokine production in atopic asthmatic and normal subjects. Clin Exp All. 2002;32:427–433. doi: 10.1046/j.1365-2222.2002.01281.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.