Abstract
The Caenorhabditis elegans (C. elegans) dopamine (DA) transporter (DAT-1) regulates DA signaling through efficient DA reuptake following synaptic release. In addition to its DA transport function, DAT-1 generates detectible DA-gated currents that may influence neuronal excitability. Previously, we provided evidence that single Cl-channel events underlie DAT-1 currents. In these studies, we identified a distinct population of altered DAT-1 currents arising from DAT-1 transgenic constructs bearing an N-terminal GFP fusion. The presence of these channels suggested disruption of an endogenous regulatory mechanism that modulates occupancy of DAT-1 channel states. A leading candidate for such a regulator is the SNARE protein syntaxin 1A (Syn1A), previously found to interact with homologous transporters through N-terminal interactions. Here we establish that UNC-64 (C. elegans Syn1A homologue) associates with DAT-1 and suppresses transporter channel properties. In contrast, GFP::DAT-1 is unable to form stable transporter/UNC-64 complexes that limit channel states. Although DAT-1 and GFP::DAT-1 expressing DA neurons exhibit comparable DA uptake, GFP::DAT-1 animals exhibit swimming-induced paralysis (SWIP), a phenotype associated with excess synaptic DA release and spillover. We propose that loss of UNC-64/DAT-1 interactions leads to enhanced synaptic DA release, providing a novel mechanism for DA neuron sensitization that may be relevant to mechanisms of DA-associated disorders.
Keywords: Caenorhabditis elegans, channels and transporters, dopaminergic neurons
The DA transporter (DAT) terminates postsynaptic DA receptor activation by removing DA from the synaptic cleft, thus regulating dopaminergic synaptic transmission. DAT also exhibits a channel mode of conduction that depolarizes DA neurons constitutively (1) as well as during DA uptake (2). DAT belongs to a family (SLC6) of transporters whose members translocate neurotransmitters and other solutes by coupling transport to the transmembrane Na+ gradient. In addition to DAT, serotonin, norepinephrine, glutamate, and GABA transporters (SERT, NET, GLT-1 and GAT1) have been shown to exhibit channel states (3–6). Transporter-mediated channel events are difficult to observe in native cells due to their small amplitude and low open probability. Neurotransmitter transporters also have relatively slow average permeation rates for substrates (7), suggesting that channel opening is not an obligatory event but rather represents conductance states open only under special circumstances, as a possible component of regulated neural signaling or as a maladaptive feature supporting drug action or neural disorders (8, 9).
In our previous investigation establishing the biophysical properties of the Caenorhabditis elegans (C. elegans) DAT-1 protein (2), we estimated that only about one-tenth of DAT-1 molecules exhibit channel activity, raising the possibility that an endogenous regulatory mechanism normally suppresses DAT-1 channel activity. One potential regulatory molecule is the presynaptic protein syntaxin 1A (Syn1A), a key element of the molecular machinery involved in transmitter release (10). Several studies have drawn explicit attention to the physical and functional interaction of Na/Cl-depended transporters with Syn1A (11–14). Moreover, Syn1A has been shown to regulate gating and permeation of other channels including CFTR Cl− channels (15) and voltage-gated Ca2+ channels (16). Two groups of investigators have shown that Syn1A supports surface trafficking of NET and GAT1, presumably through its conventional role in SNARE-mediated vesicle fusion. In addition, a direct interaction of Syn1A with these transporters through their cytoplasmic N-termini appears to both limit transport activity and channel properties (12, 14). One hypothesis to integrate this dual regulation of neurotransmitter transport by Syn1A is that Syn1A can facilitate delivery to the surface of transporters restricted in their ability to transport substrates until other regulatory signals that disrupt interactions are received. Alternatively, Syn1A may constrain generation of currents that could amplify synaptic depolarization and trigger excessive neurotransmitter release. In this regard, Quick demonstrated that Syn1A suppresses SERT-mediated macroscopic currents in mammalian 5-HT neurons (13). To date, however, no data as to how Syn1A changes the unitary conductance of neurotransmitter transporter channels have been advanced in any preparation, nor have functional consequences on synaptic transmission been elaborated. Here we establish the impact of an N-terminal modification of DAT-1 protein on single-transporter-channel currents in C. elegans DA neurons. We provide evidence that the C. elegans Syn1A (UNC-64) physically associates with DAT-1 and constrains DAT-1 channel activity. Disruption of nematode thrashing behavior in animals expressing GFP::DAT-1 that fail to support UNC-64 interactions reveals a vital role for UNC-64/DAT-1 interactions in sustaining normal DA signaling in vivo.
Results
GFP Tag on the DAT N Terminus Modifies DAT-Mediated Channel Properties.
In our previous report (2), we showed that the C. elegans line BY314, which expresses an N-terminal GFP-tagged DAT-1 in the context of endogenous DAT-1, exhibited 40% more channel-events in cultured DA neurons than WT animals. Expression of GFP::DAT-1 did not alter the unit conductance of recorded channels, although both DA accumulation and channel frequency increased (2). The observed increase in DAT-1 channel events in BY314 neurons could simply reflect a higher number of transporters expressed in this line. However, our open-time histograms also revealed that 28% of DA elicited channels in these neurons exhibited much longer open times, 19 ± 3 ms, than WT neurons, 6.9 ± 3 ms (2). This phenomenon encouraged us to more closely inspect the determinants of DAT-1 channel properties.
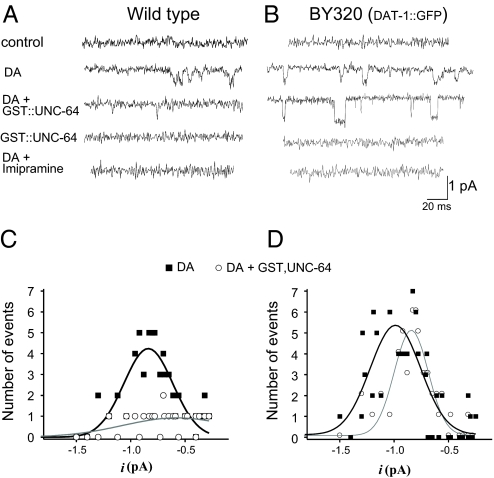
To test whether the presence of the N-terminal GFP tag we placed on DAT-1 was responsible for the altered DAT-1 channel openings in the BY314 line, we created a transgenic line that expresses the GFP::DAT-1 translational fusion in DA neurons on a background deficient in endogenous DAT-1 (BY320). DA neurons cultured from BY320 worms were patched and single channel recordings were performed in the outside-out configuration (Fig. 1). As in the WT and BY314 neurons (2), 1 μM DA perfusion-activated, single-channel events that were selectively inactivated when the DAT-1 blocker imipramine (IMP, 100 nM) was co-perfused with DA or when extracellular Na+ was replaced with NMDG+ (Fig. 1 A). The channel amplitude (−0.8 ± 0.2 pA at −120 mV) was not changed by these manipulations, although the frequency of channels from BY320 neurons was 66% higher from WT animals and 28% higher than those recorded in BY314 neurons (data not shown). Furthermore, the average channel open time was increased in BY320 DA neurons relative to WT, with a mean open time of 17 ± 5 ms (Figs. 1 C and 1 D. These experiments indicate that N-terminal GFP tag that we added to DAT-1 has a stimulatory action on channel open time and frequency but not on channel amplitude.
Fig. 1.
Channel events are increased in GFP-tagged DAT-1. (A and B) Transporter channels from (A) WT and (B) BY320 C. elegans DA neurons in outside-out patches recorded at −120 mV. Single channel analysis revealed an open probability value of P = 0.002, and 0.0072 for channels recorded in WT and BY320 neurons, respectively. Data derive from n ≥ 8 neurons for each genotype. (C and D) Representative open-time histograms from DA-induced single-channel events shown in A and B, respectively. Channels with open time less than 4 ms were excluded to simplify the histograms.
GFP Attached to DAT-1 N Terminus Prevents Interaction with UNC-64.
The higher channel activity measured in DA neurons expressing the N-terminal, GFP::DAT-1 translational fusion could reflect an intrinsic perturbation of channel kinetics of the transporter or could arise from disrupted interactions of an ancillary protein that normally interacts with DAT-1 through the N terminus of the transporter. Syn1A represents the best candidate for such a regulator as the SNARE protein has been found to interact with mammalian DAT through N-terminal contacts (17) and has also been observed to modulate the transport and macroscopic currents of NET, SERT, glutamate and GABA transporters (11–14). To pursue this idea, COS-7 cells were transiently transfected with HA-tagged UNC-64 (HA::UNC-64) alone or with WT DAT-1 and GFP::DAT-1 cDNAs. Three days after transfection, whole-cell detergent extracts were immunoprecipitated using DAT-1 and HA antibodies, and HA::UNC-64 assessed by Western blotting (Fig. 2 A). DAT-1 antibodies failed to immunoprecipitate HA::UNC-64 from cells expressing UNC-64 alone, although UNC-64 expression is evident after immunoprecipitation with HA antibodies. Immunoprecipitations of DAT-1/HA::UNC-64 co-transfected cells revealed the presence of HA::UNC-64 in DAT-1 immunoprecipitates (Fig. 2 B). In contrast, HA::UNC-64 was not immunoreciptated from cells co-transfected with GFP::DAT-1/UNC-64 (Fig. 2 C). In the same samples, we successfully immunoprecipitated GFP::DAT-1 with GFP antibodies (Fig. 2 C). We repeated our analysis by probing anti-GFP immunoprecipitates of GFP::DAT-1/HA::UNC-64 cell extracts antibodies with anti-HA antibodies (Fig. 2 D), but again observed no coimmunoprecipitated HA::UNC-64, whereas the SNARE protein could be identified in anti-HA immunoprecipitates.
Fig. 2.
GFP::DAT-1 lacks UNC-64 interactions. (A, B, and D) Representative blots of COS-7 cells expressing HA::UNC-64 (A), HA::UNC-64/DAT-1 (B), or GFP::DAT-1/HA::UNC-64 or HA::UNC-64 alone (D), probed with anti-HA after immunoprecipitation. (C) Representative blots of cells transfected with GFP::DAT-1/HA::UNC-64 or GFP::DAT-1 alone, immunoprecipitated with anti-GFP or HA antibodies and probed with anti-GFP.
Perfused UNC-64 Regulates DAT-1 Channels.
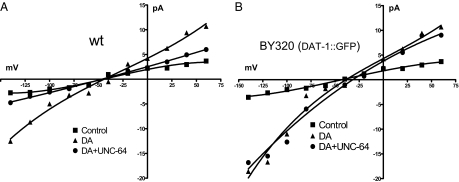
Our results of monitoring whole cell currents (see Fig. 4) and biochemical studies indicate that the presence of GFP at the DAT-1 N terminus precludes interaction of tagged transporters with UNC-64. If this is true, UNC-64 applied directly to DAT-1 channels should regulate transporter channels, whereas no effect would be expected on GFP::DAT-1 channels. We therefore recorded DAT-1 channels after intracellular perfusion of the cytoplasmic tail of UNC-64, isolated as a GST-fusion protein (GST::UNC-64). Control experiments demonstrated that GST alone gave no response (data not shown) nor did perfusion of GST::UNC-64 in the absence of DA produce effects on membrane currents with either WT or GFP::DAT-1 cells (Figs. 3 A and B). When DA was bath-applied to activate DAT-1 channels, WT DAT-1 expressing cells that were perfused with 3 μM GST::UNC-64 displayed an 83% decrease in DAT-1 channel activity (Figs. 3 A and C). Interestingly, the same perfusion failed to alter the frequency of GFP::DAT-1 derived channels (Figs. 3 B and D). Amplitude histograms revealed no significant difference in the channel amplitudes of GFP::DAT-1 channels after GST::UNC-64 perfusion. Inclusion of GST::UNC64 in the recording pipette of WT DA neurons was found to reduce DA-induced whole-cell currents essentially to levels with DAT-1 antagonist (DA + IMP = Control) −5.1 ± 0.7 pA vs. −3.3 ± 0.8pA, respectively (Fig. 4 A). In contrast, no significant change in GST::UNC-64 perfusions of GFP::DAT-1 neurons, −23 ± 3 and −19 ± 4 pA respectively (Fig. 4 B). Figures. 4A and 4B also show that the BY320 neurons exhibited 30% larger DA-induced whole-cell currents than the WT neurons. In current-clamp experiments, we tested whether UNC-64/DAT-1 interactions limit the ability of DA to elicit changes in membrane potential following DAT-1 activation. DA neurons from WT cells, recorded with 3 μM GST::UNC-64 in the pipette changed their resting potentials from −35 ± 3 to −46 ± 5 mV (P < 0.05, unpaired t test) after application of 1 μM DA. BY320 DA neurons exhibit a more negative resting potential and fail to register a DA-elicited depolarization (−45 ± 4 to −42 ± 5 mV).
Fig. 4.
UNC-64 does not inhibit DAT-1 whole-cell currents in GFP::DAT-1 neurons. Representative recordings of the current–voltage relationship elicited in presence of 1 μM DA + 10 μM imipramine (filled squares), 1 μM DA (filled triangles) and 1 μM DA + 3 μM GST,UNC-64 (filled circles), in WT (A) and BY320 (B) neurons. Membrane potential was held at –40 mV and stepped from −140 to +60 mV.
Fig. 3.
UNC-64 inhibition of DAT-1 channel activity is absent in GFP::DAT-1 neurons. (A and B) Channel events were recorded from cells cultured from WT (A) and BY320 (B) lines. Membrane potential was held at −120 mV. Single-channel recordings were performed in outside-out configuration, placing the intracellular face of the membrane in contact with the pipette solution containing GST::UNC-64. (C and D) Amplitude histograms of WT (C) and BY320 (D) patches in presence of DA (■) and DA+GST::UNC-64 (○) derive from analyses of 8 neurons for each genotype.
DA Neuron-Selective Expression of GFP::DAT-1 Fusion Triggers Swimming-Induced Paralysis.
In nematodes, vigorous swimming or “thrashing” behavior can be readily recorded in individual animals and is negatively regulated by DA. Thus, McDonald et al. defined a behavioral phenotype, swimming-induced paralysis (SWIP), that emerges in DAT-1 deficient animals as a consequence of DA spillover to extrasynaptic, inhibitory DA receptors on motorneurons (18). This paradigm offers an opportunity to explore the physiologic impact of loss of UNC-64 interactions on DAT-1 in vivo. Young adult WT, DAT-1 KO or BY320 worms were placed in 40 μl water in a single well of a Pyrex Spot Plate with or without IMP. After 10 min in water, only 16 ± 4% of WT worms were paralyzed versus 73 ± 9% of DAT-1 KO animals (Fig. 5 A). BY320 transgenic animals displayed a surprisingly high degree of SWIP, as 70 ± 5% of these animals were immobile after 10 min (Fig. 5 A). In the presence of 100 μM IMP, 92 ± 4% of WT worms paralyzed within 10 min, whereas IMP did not significantly increase the number of paralyzed DAT-1 KO animals (73 ± 9% vs. 85 ± 7%, respectively; data not shown). Although BY320 animals have a similarly elevated basal rate of SWIP as DAT-1 KO animals, IMP significantly increased the number of BY320 paralyzed worms (70 ± 5% vs. 90 ± 4%, respectively, P < 0.01 Student's t test). To exclude the possibility that the greater extent of paralysis in BY320 worms was related to reduced expression of the GFP::DAT-1 in these animals, we measured [3H]DA accumulation in cultured DA neurons from BY320 and WT worms (Fig. 5 B). As previously described (18), the GFP::DAT-1 transgene expressed in the DAT-1 KO background completely restores normal DA uptake. We also found that BY320 animals exhibit comparable DA uptake as cells from WT cultures. Kinetic studies demonstrates that no change in DA transport Vmax was evident comparing WT and BY320 neurons (77 ± 14 vs. 61 ± 2 nM respectively, data not shown). We also found that IMP blocked [3H]DA accumulation with the same potency in both WT and BY320 neurons (Ki = 20 ± 4 and 18 ± 4 nM respectively, data not shown).
Fig. 5.
GFP-DAT-1 expression causes swimming-induced paralysis. (A) From 10 to 20 animals of each worm genotype were analyzed per each single SWIP assay, as described in Methods. The total number of each strain was 120 of WT (filled squares, 99 of WT+IMP (open sqaures), 73 of KO (triangles), 81 of BY320 (filled circles) and 78 of BY320 (open circles) worms. (B) DA transport activity in cultured embryonic cells. Values shown represent specific [3H]-DA uptake calculated by subtracting total DA uptake values form parallel samples incubated in presence of 100 nM imipramine. Data are derived from 3 or more independent experiments. Means were compared using Paired t test and with P < 0.01.
Discussion
Previously, we identified DAT-1 mediated channel events in cultured C. elegans DA neurons. In primary cultures derived from transgenic strain BY314, which express both endogenous DAT-1 and an N-terminal GFP-tagged DAT-1 in all DA neurons, we measured an increase in [3H]-DA accumulation and DAT-1 channel frequency relative to WT DA neurons (2). The increase in both DA uptake and DAT-1 channel-events was initially suspected to reflect simply a higher number of transporters expressed in the BY314 line given the presence of the wild-type DAT-1 gene. However, open-time histograms revealed that although 72% of transporter channels in BY314 neurons exhibit the same open time as WT neurons, 6.9 ± 3 ms, the remainder exhibited ≈3-fold longer open times (19 ± 3 ms). In this report, we provide evidence that UNC-64 normally provides constitutive repression of the channel mode of DAT-1. In DA neurons isolated from BY320 transgenic worms that express the GFP::DAT-1 fusion protein in a DAT-1 KO background, we again measured higher DAT-1 associated channel activity than in WT cultures, and even more than in BY314 neurons. Interestingly, the channel open time measured in BY320 neurons was comparable to that recorded in the subpopulation of BY314 neurons (17 ± 5 vs. 19 ± 3 ms, respectively). This implies that the GFP::DAT-1 fusion protein expressed in the BY314 neurons accounts for the subpopulation of channels with a longer open time. Increased channel activity in BY320 cells over the BY314 neurons may reflect the fact that in the BY320 all transporters are derived from the fusion protein, whereas a proportion of transporters expressed in the BY314 are expected to exhibit WT properties.
Beside a demonstration that GFP attached to the N-terminal domain of DAT alters its channel kinetics, we also present 2 important results that support our contention that UNC-64 normally suppresses DAT-1 channel activity. First, DAT-1, but not GFP::DAT-1, co-immunoprecipitated with UNC-64. Second, DAT-1 channels, but not those established by GFP::DAT-1 expression, were suppressed by perfused GST::UNC-64. In heterologous expression systems, preliminary results indicate that Syn1A binding to DAT N terminus may be increased by exposure to substrate (Aurelio Galli, personal communication). These data together with our present results strongly suggest that DA stimulates DAT-1 channel activity which is subsequently blocked by Syn1A binding to DAT. This hypothesis would explain the low frequency of DAT-mediated channels in WT neurons; if Syn1A cannot bind to DAT-1 due to the N-terminal GFP tag, no subsequent suppression of channels occurs. Interestingly, the frequency of channels increases most when GFP::DAT-1 is expressed in a DAT-1 KO background (BY320). The lower frequency of channels recorded when GFP::DAT-1 is expressed in a WT background (BY314) may be due to multimeric interaction between GFP::DAT-1 and WT DAT-1. DAT proteins form higher order multimers and DAT mutants exhibit dominant-negative suppression of WT DAT trafficking and function (19, 20). Moreover, because UNC-64 blocks both the DA-induced single-channel and whole-cell currents in DA neurons expressing WT DAT-1 but not in cells expressing GFP::DAT-1, Syn1A/DAT-1 interactions appear to be a key player controlling the ability of DAT to contribute DA neuron excitability (1). Quick first advanced an important role for Syn1A in control of SERT-dependent currents in 5-HT neurons as cleavage of Syn1A with botulinum toxin C uncovers SERT currents absent from untreated 5-HT neurons (13). Our findings concur with this idea and extend them to the single channel level in identified neurons.
Finally, because of our ability to express mutant DAT-1 proteins selectively in DA neurons of living animals and subsequently monitor a DA-dependent behavior (SWIP), Syn1A/DAT interactions are revealed to dictate critical aspects of DA signaling in vivo. The SWIP behavior evident in DAT-1 transgenic animals is known to be dependent on presynaptic DA synthesis, vesicular DA release, efficient DA clearance and the DA receptor DOP-3 (18). Our experiments show that IMP, a DAT-1 inhibitor, increases SWIP rate in WT but not DAT-1 KO animals. Although the GFP::DAT-1–expressing animals of the BY320 line display comparable SWIP rate as DAT-1 KO animals, suggesting elevated synaptic DA, IMP further increases their paralysis. Because loss of UNC-64 interactions depolarizes DA neurons via increased channel activity, we speculate that BY320 animals exhibit more DA release due to the increased excitability of DA neurons. The additional IMP-induced increase in BY320 animals represents an in vivo report of functional DA re-uptake at DA synapses in these animals, paralleling our in vitro measurement of [3H]DA uptake. Because WT animals treated with IMP paralyze more robustly than DAT-1 KO in water, this may indicate that DAT-1 KOs have inherently reduced SWIP capacity. We could imagine that the loss of DAT-1 leads to a compensatory change in presynaptic DA homeostasis, a phenomenon that has been already reported for DAT KO mice (21).
Whether the enhanced DAT-1 channel activity in GFP::DAT-1 animals directly drives SWIP versus contributions from other manifestations of perturbed DAT-1/UNC-64 interactions cannot be discerned at present. We did monitor WT and BY320 levels of DA uptake; however, these measures are derived from embryonic cultures, and thus other factors may come into play in adult, wired DA synapses. One possibility that should be explored further is whether DAT-1 serves as a reservoir for an important pool of SYN1A that can support enhanced DA release when liberated. Regardless, our data reveal that unsuspected behavioral consequences can arise from dysregulated Syn1A/DAT interactions. Our findings suggest therefore that altered Syn1A/DAT interactions in humans could support disorders known to feature abnormal stimulation of DA receptors (e.g., psychostimulant addiction) or that are treatable with DA receptor blockers (e.g., psychotic disorders). Interestingly, variations in expression of the Syn1A gene have been associated with schizophrenia (22), suggesting that abnormal DAT/Syn1A association may also contribute to the cognitive and psychologic impairments seen in schizophrenia. Because our system permits relatively straightforward genetic manipulations and knock out animals, we are eager to investigate to what extent DAT/Syn1A association controls the DA receptor activation. We are thus presently characterizing mutants expressing both GFP::DAT-1 and DA receptor deletions to extend the physiologic impact of disrupted DAT-1/syntaxin 1A interactions.
Methods
C. elegans Strains and Husbandry, and Antibodies.
C. elegans strains were cultured on bacterial lawns of NA-22 and maintained at 14 to 20°C using standard methods (23). The wild-type strain is N2 Bristol. The BY320 strain was created by crossing the DAT-1 deficient line dat-1(ok157) with BY314 worms that express GFP::DAT-1 in an N2 background (2). The dat-1(ok157) strain was a gift of J. Duerr and J. Rand (Oklahoma Medical Research Foundation) Mutant lines were initially screened using single worm PCR (24, 25) with oligos directed to the gene of interest. C. elegans genomic DNA was also used for PCR genotyping at a concentration of 1 ng/μl. The DAT-1 antibody was a rabbit polyclonal produced by ProSci Inc. (Poway, CA) using a GST:C-terminal DAT-1 fusion peptide (18).
Creation of Plasmids, Cell Transfection, and Western Blot Analysis.
Plasmids: Pdat-1::GFP:DAT-1 (2) and Pdat-1::GFP (26) were previously described. To examine the functional necessity of the N-terminal domain in DAT-1, we cloned DAT-1 into the mammalian vector pEGFP-C3 (Clontech Laboratories). COS-7 cells were maintained in DMEM (GIBCO) supplemented with 10% FBS, 2 mM L-glutamine and 100 units/ml penicillin, and 100 mg/ml streptomycin. Cells (1 × 105) plated in 24-well plates were transfected with 1 μg cDNAs, using FuGENE 6 (Roche). Three days later, cells were washed twice with PBS/Ca2+/Mg2+ and then incubated in Lysis buffer containing 20 mM Tris-HCl, pH 7.5, 1 mM EDTA, 0.5 mM PMSF, 1% SDS, 1 mM sodium orthovanadate, 1 mM sodium pyrophosphate, 1 μM microcystin-LR, and 1 μM aprotinin and leupeptin for 1 h at 4°C. Cell lysates were recovered by centrifugation at 13,000g for 30 min at 4°C. Samples were then immunoprecipiated with DAT-1, GFP (Santa Cruz Biotechnology) or HA (Roche) antibodies and then analyzed by SDS/PAGE (10% gel) followed by immunoblotting.
Unc-64::GST-fusion cDNA was graciously provided by Michael Nonet (Washington University School of Medicine St. Louis, MO) and then subcloned into the pcDNA3 mammalian expression. UNC-6::GST-fusion protein was expressed in BL21DE3 Escherichia coli bacteria and purified using glutathione-agarose beads (Sigma) with elution by glutathione. Eluates were analyzed by SDS/PAGE and Coomassie-blue staining, revealing a single band at ≈55 kDa, the expected size for the fusion of UNC-64 (32 kDa) and GST (24 kDa) (data not shown).
Electrophysiologic and Transport Assays.
C. elegans DA neurons, cultured as previously described (2) were evaluated for DAT-1 dependent whole-cell currents using electrodes pulled to 8 to 12 MΩ resistance and internal solution consisting of 125 mM K gluconate, 18 mM KCl, 4 mM NaCl, 0.6 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, and 10 mM EGTA (pH 7.2 and 325 osmolarity, adjusted with sucrose). External solution contained 145 mM NaCl or NMDG+, 5 mM KCl, 1 mM CaCl2, 5 mM MgCl2, 10 mM Hepes, and 20 mM d-glucose (pH 7.2 and 350 osmolarity). Butaclamol and spiperone (both at 1 μM) were included in the external solution to limit DA receptor activation. Data for single-channel analyses, acquired using Fetchan and Pstat software (Axon Instruments), were imported in Origin 7.0 to construct channel-amplitude and open-time histograms. To facilitate analyses, recordings were binned into 5 second segments. Channel opening frequency was obtained by dividing the area used for the amplitude histograms by the time of the trace (5 sec). Traces that exhibited a number of channel events insufficient to perform the amplitude histograms were excluded (≈40–60% of all recordings). Uptake assays were prepared as described previously (2).
C. elegans Assay for Swimming-Induced Paralysis (SWIP).
The SWIP phenotype of DAT-1 deficient lines has been recently reported (18). All worms were grown on NGM plates spread with NA22 bacteria. Young adult hermaphrodites from each line were placed in 40 μl water with or without 100 μM IMP in a single well of a Pyrex Spot Plate (Fisher catalog number 13–748B) and the number of paralyzed worms was tabulated each minute.
Acknowledgments.
We thank Jane Wright and Paul McDonald for assistance with molecular biology techniques, Tammy Jessen and Shannon Hardie for C. elegans husbandry and SWIP behavior, respectively, and Dawn S. Matthies and Michael L. Nonet for helpful discussion. This work was supported by National Institutes of Health Grants NS34075 and MH58921.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Ingram SL, Prasad BM, Amara SG. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat Neurosci. 2002;5:971–978. doi: 10.1038/nn920. [DOI] [PubMed] [Google Scholar]
- 2.Carvelli L, McDonald PW, Blakely RB, DeFelice LJ. Dopamine transporters depolarize neurons by a channel mechanism. Proc Natl Acad Sci USA. 2004;101:16046–16051. doi: 10.1073/pnas.0403299101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin F, Lester HA, Mager S. Single-channel currents produced by the serotonin transporter and analysis of a mutation affecting ion permeation. Biophys J. 1996;71:3126–3135. doi: 10.1016/S0006-3495(96)79506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galli A, Blakely RD, DeFelice LJ. Patch-clamp and amperometric recordings from norepinephrine transporters: Channel activity and voltage-dependent uptake [see Comments] Proc Natl Acad Sci USA. 1998;95:13260–13265. doi: 10.1073/pnas.95.22.13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wadiche JI, Amara SG, Kavanaugh MP. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 6.Cammack JN, Schwartz EA. Channel behavior in a gamma-aminobutyrate transporter. Proc Natl Acad Sci USA. 1996;93:723–727. doi: 10.1073/pnas.93.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad BM, Amara SG. The dopamine transporter in mesencephalic cultures is refractory to physiological changes in membrane voltage. J Neurosci. 2001;21:7561–7567. doi: 10.1523/JNEUROSCI.21-19-07561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahlig KM, et al. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc Natl Acad Sci USA. 2005;102:3495–3500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagley E, Gerke M, Vaughan C, Hack S, Christie M. GABA transporter currents activated by protein kinase A excite midbrain neurons during opioid withdrawal. Neuron. 2005;45:433–445. doi: 10.1016/j.neuron.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 10.Sudhof TC. The synaptic vesicle cycle, a cascade of protein–protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 11.Quick MW. The role of SNARE proteins in trafficking and function of neurotransmitter transporters. Handb Exp Pharmacol. 2006;175:181–196. doi: 10.1007/3-540-29784-7_9. [DOI] [PubMed] [Google Scholar]
- 12.Sung U. et al. A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity. J Neurosci. 2003;23:1697–1709. doi: 10.1523/JNEUROSCI.23-05-01697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quick MW. Regulating the conducting states of a mammalian serotonin transporter. Neuron. 2003;40:537–549. doi: 10.1016/s0896-6273(03)00605-6. [DOI] [PubMed] [Google Scholar]
- 14.Deken SL, Beckman ML, Boos L, Quick MW. Transport rates of GABA transporters: Regulation by the N-terminal domain and syntaxin 1A. Nat Neurosci. 2000;3:998–1003. doi: 10.1038/79939. [DOI] [PubMed] [Google Scholar]
- 15.Naren AP, et al. Regulation of CFTR chloride channels by syntaxin and Munc18 isoforms. Nature. 1997;390:302–305. doi: 10.1038/36882. [DOI] [PubMed] [Google Scholar]
- 16.Bezprozvanny I, Scheller RH, Tsien RW. Functional impact of syntaxin on gating of N-type and Q-type calcium channels. Nature. 1995;378:623–626. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- 17.Lee KH, Kim MY, Kim DH, Lee YS. Syntaxin 1A and receptor for activated C kinase interact with the N-terminal region of human dopamine transporter. Neurochem Res. 2004;29:1405–1409. doi: 10.1023/b:nere.0000026404.08779.43. [DOI] [PubMed] [Google Scholar]
- 18.McDonald PW, et al. Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J Neurosci. 2007;27:14216–14227. doi: 10.1523/JNEUROSCI.2992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hastrup H, Sen N, Javitch JA. The human dopamine transporter forms a tetramer in the plasma membrane: Cross-linking of a cysteine in the fourth transmembrane segment is sensitive to cocaine analogs. J Biol Chem. 2003;278:45045–45048. doi: 10.1074/jbc.C300349200. [DOI] [PubMed] [Google Scholar]
- 20.Torres GE, et al. Oligomerization and trafficking of the human dopamine transporter mutational analysis identifies critical domains important for the functional expression of the transporter. J Biol Chem. 2003;278:2731–2739. doi: 10.1074/jbc.M201926200. [DOI] [PubMed] [Google Scholar]
- 21.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 22.Albert HC, et al. Association between schizophrenia and the syntaxin 1A gene. Biol Psychiatry. 2004;56:24–29. doi: 10.1016/j.biopsych.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barstead RJ, Kleiman L, Waterston RH. Cloning, sequencing, and mapping of an alpha-actinin gene from the nematode Caenorhabditis elegans. Cell Motil Cytoskeleton. 1991;20:69–78. doi: 10.1002/cm.970200108. [DOI] [PubMed] [Google Scholar]
- 25.Williams BD, Schrank B, Huynh C, Shownkeen R, Waterston RH. A genetic mapping system in Caenorhabditis elegans based on polymorphic sequence-tagged sites. Genetics. 1992;131:609–624. doi: 10.1093/genetics/131.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nass R, Hall DH, Miller DM, III, Blakely RD. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99:3264–3269. doi: 10.1073/pnas.042497999. [DOI] [PMC free article] [PubMed] [Google Scholar]