Abstract
In recent years, China has become an increasingly important and the largest chestnut producer in the world. This study aimed to evaluate the nutritional value and microbiological quality of the roasted freeze-dried Chinese chestnut (Castanea mollissima) (RFDC) coated with dark chocolate (DCC) and milk chocolate (MCC) for industrial use and commercial consumption. Chocolate coating significantly improved the nutritional value of chestnut. RFDC had high levels of starch (66.23%) and fibers (3.85%) while DCC and MCC contained significantly high amounts of sucrose, protein, fat and minerals. Furthermore, the protein content doubled in MCC rather than in DCC. This could be attributed to the different formulations in the two products. Milk powder and whey protein constituted the source of protein in MCC while cocoa powder added to MCC formulation constituted an additional source of minerals. The amino acid profile showed differences in amino acid composition related to the sample’s protein content, indicating their good nutritional quality. The moisture contents in all RFDC, DCC and MCC were suitable for industrial processing. These results provide information about the additional nutrients of chocolate-coated chestnut and confirm that the product is an interesting nutritional food. The combination of freeze-drying and chocolate-coating generally results in greater reductions on microbiological loads, extending shelf life of harvested chestnut for commercial application. This is an alternative strategy to add value to chestnut, minimizing the significant losses in harvested fruits and providing a wider range of choices of new products to the consumer disposal.
Keywords: Chestnut, Coating, Dark chocolate, Milk chocolate, Nutritional qualities, Microbiological analysis
INTRODUCTION
Chestnut (Castanea sp.) is an important nut crop in the world. China is the main producer of chestnut with 825 000 million tons in 2005 (FAOSTAT, 2005), which accounted for 73% of the world’s total production, yet, in 2002, Asia ranked highest (44.3%) in the world production of chestnut, of which China produced 49.4% (Bounous, 2002). Chestnut has a short shelf life due to its high moisture and sugar content (Attanasio et al., 2004). In China, 1/3 of the annual chestnut harvest is exported to Japan (Vossen, 2000), and the remaining proportion is mainly roasted and consumed locally, during which a large part is damaged, causing a great economic loss. Therefore, designing and application of an edible coating suitable for processed chestnuts are an effective and promising way to improve the shelf life of product and to add the nutritional value for commercial production purposes.
Our previous investigations revealed that the use of whey protein isolate-pullulan (WPI-Pul) coating was effective in the control of overgrowth of spoilage organisms and surface discoloration of roasted chestnuts (Gounga et al., 2008). Besides, WPI-Pul coating improved greatly the sensory attributes of fresh and dried chestnuts (Gounga et al., 2007a). Furthermore, the double coating with chocolate was shown to be satisfactory in improving the quality and increasing the shelf life of chestnut, hence providing a wider range of choices of new products at the consumer’s disposal (Gounga et al., 2007b). However, consumers expect food to be safe and more nutritious.
Chocolate is a highly nutritious energy source, with a fast metabolism and good digestibility. The presence of cocoa, milk and sugar in its composition can be the warrant for an appropriate ingestion of proteins, carbohydrates, fats, minerals and vitamins (Campos and Benedet, 1994).
The composition of nutrients in food in general and new processed food in particular may change for many different reasons. Some of the sources of variation are biological, such as variety of plant, pre-harvest cultural practices, as well as seasonal and annual factors. Other factors are related to differences from post-harvest, storage and processing (Torelm and Danielsson, 1998). It has been so far reported that chestnuts are an interesting healthy food due to their biochemical and nutrient composition (Borges et al., 2008), and the addition of new components considerably modifies the nutritional value of their initial raw material and microbiological quality. This work aimed, therefore, to evaluate the nutritional change of the roasted freeze-dried Chinese chestnut (RFDC) after chocolate-coating and its microbiological quality during storage.
MATERIALS AND METHODS
Sample preparation
Freshly roasted Chinese chestnuts were purchased from a local chestnut shop (Jinliwang, Wuxi, China), peeled at room temperature and frozen at −20 °C in a Haier freezer (Haier Co., Ltd., Shanghai, China) for 72 h. The fruits were then dried for 72 h using a freeze-dryer (Labconco Corporation, Kansas, USA), sealed up in high density polyethylene bag and kept in a desiccator containing silica gel until further use.
Whey protein isolate (WPI) (New Zealand Milk Products, Fonterra Ltd., Auckland, New Zealand) and pullulan (Pul) (Food Ingredients Hayashibara Shoji, Inc., Okayama, Japan) were dissolved in distilled water at a 10:1 (w:w) ratio of WPI:Pul to prepare a 7% (w/v) WPI-Pul coating solution. The solution was heated at 90 °C for 30 min in a water bath with stirring and pH was adjusted to 7.0 using 1 mol/L NaOH. The denatured solution was then cooled down to ambient temperature and glycerol (Gly) added as plasticizer to avoid coating brittleness at an optimum WPI:Gly ratio of 3.6:1 (w:w) (Gounga et al., 2007c). The solution was degassed using an ultrasonic oscillator.
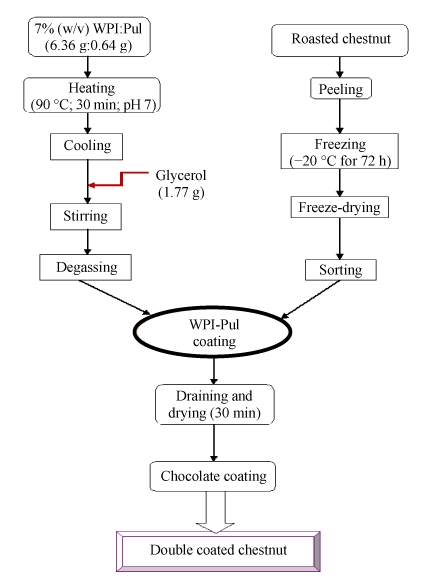
The prepared freeze-dried chestnuts were dipped into coating solution for 30 s. Residual solutions were drained off and the coated fruits were dried at room temperature using a fan for 30 min. The WPI-Pul coated chestnuts were further coated separately with dark chocolate and milk chocolate. Dark chocolate and milk chocolate were prepared according to the formulations in Table 1. The schematic flow diagram of the formulation preparation of the chocolate-coated chestnut is summarized in Fig.1.
Table 1.
Recipes used for the formulations of the dark chocolate and milk chocolate
| Chocolate | Ingredients (%) |
|||||||
| Cocoa mass | Cocoa butter | Cocoa powder | Sucrose | Milk powder | Whey powder | Soy lecithin | Vanillin | |
| Dark chocolate | 45.00 | 13.50 | − | 41.05 | − | − | 0.40 | 0.05 |
| Milk chocolate | 10.00 | 29.55 | 8.00 | 33.50 | 14.00 | 4.50 | 0.40 | 0.05 |
Fig. 1.
Flow chart for the production of chocolate-coated chestnut
Nutritional evaluation
Freeze-dried chestnuts and chocolate-coated chestnuts were ground separately in a grinder and homogenized. Samples were tested in triplicates for all measurements.
Moisture. Moisture content was gravimetrically determined by drying a sample in an oven at 105 °C until constant weight was obtained (Chinese Standard GB/T 5009.3-2003).
Protein. The Kjeldahl method was used to determine total nitrogen content (Chinese Standard GB/T 5009.5-2003). The percentages of nitrogen were converted to protein content in percent by multiplying a conversion factor of 4.86, as reported by Lamelas (2000) for Galician chestnuts.
Fat. Fat was analyzed by the Soxhlet extraction method (Chinese Standard GB/T 5009.6-2003).
Crude fiber. Crude fiber is the acid and alkali-insoluble residue that was analyzed according to the Chinese Standard GB/T 5009.10-2003.
Ash. Ash content was determined by gravimetric measurement of the sample residue after ignition in an oven at 512 °C to constant weight (Chinese Standard GB/T 5009.4-2003).
Starch. The quantitative analyses of starch were assessed by an enzymatic procedure according to the AOAC (1997) method. One gram of milled sample was washed with aqueous ethanol and pre-treated with dimethyl-sulphoxide at 100 °C to disperse starch before analysis. Then the samples were incubated with 1.0 ml thermostable α-amylase and amyloglucosidase to hydrolyse the starch at 50 °C for 30 min using sodium acetate buffer at pH 4.5. The solution was heated at 100 °C for 20 min to deactivate the enzymes. Glucose was determined by spectrophotometric measurement at 540 nm (Model UV-1100, Rui Li Analytical Instrument Co., Beijing, China).
Sucrose, glucose, fructose and galactose. Glucose, sucrose, fructose and galactose contents were determined using high performance liquid chromatography (HPLC), after drying and hydroalcoholic extraction (70/30, v/v). A dry sample (0.3 g) was introduced in an Erlenmeyer flask of 10 ml, and then a hydroalcoholic solution at 70% (v/v) added, before putting on the top and introducing it in an ultrasound bath for 30 min at 60 °C. Then it was centrifuged at 5000 r/min for 20 min. The supernatant, which contains the sugars of this first extraction, was filtered trough a 0.45-µm filter before analysis by HPLC. The analytical column was Sugarpack-1 (6.5 mm×300 mm i.d.). The mobile phase was water with 0.4 ml/min flow rate. The column oven was kept at 85 °C. The volume of the injection was 10 µl.
Amino acid composition. A modified method of AOAC 982.30a (AOAC, 1990) was used for amino acid analysis. The 0.5 g of dried sample was hydrolyzed with 8 ml of 6 mol/L HCl under vacuum at 110 °C for 22 h. After cooling, the hydrolysate was washed with distilled water, filtered (Whatman No. 2) and dried at 60 °C (also under vacuum) in a rotary evaporator. The dried sample was then dissolved in 0.01 mol/L HCl. The amino acids in the hydrolysate were separated and quantified by injecting 1 μl into an Agilent 1100 HPLC equipped with HP Hypersil ODS column (125 mm×4.6 mm×5 μm) and fluorometric detector. The flow rate was 1.0 ml/min and the column temperature was 40 °C.
Microbiological analysis
Microbiological analysis was conducted on dark chocolate-coated chestnut (DCC) and milk chocolate-coated chestnut (MCC) stored at two different conditions [7 °C, (82±5)%RH (relative humidity) and (25±2) °C, (30±2)%RH]. A commercialized product of chocolate-coated peanut was provided by Liangfeng Food Group Import and Export Co., Ltd. (Zhangjiagang, Jiangsu, China) to be used as reference sample (REF). Samples were analyzed for coliform count, Escherichia coli, Salmonella spp. and yeasts and molds at Days 0 and 180 storage according to the methods described by Manafi (2003). The growth media used for the microbiological enumeration of the different microorganisms were: Plate Count Agar (PCA), MacConkey Agar (for Escherichia coli), Salmonella-Shigella Agar, Baird-parker Agar (for Staphylococcus aureus) and Czapek Agar (for yeasts and molds). For each test, 3 g of ground sample was homogenized in 30 ml of corresponding broth in sterile flask and incubated for 24 h at 37 °C. Samples (1 ml) were aseptically removed and progressive decimal dilutions (101~108) were prepared in water peptone, mixed using a vortex for a few seconds and plated onto appropriate media. The inoculated plates were incubated for 48 h at 37 °C except for yeasts and molds determination for 5 d at 25 °C (Koburger, 1968) and the colonies were counted manually using an impermanent marker.
Statistical analysis
In order to statistically analyze the results, all the experiments were done in triplicates except for microbiological determination (duplicates) and all measurements were carried out at least twice. Analysis of variance (ANOVA) was performed using SAS Software (SAS 8.1 for Window, SAS Inc., Cary, NC, USA) and the difference between treatment means was determined by the multiple range test (Duncan, 1955). Differences were considered at 95% significance level.
RESULTS AND DISCUSSION
Nutritional evaluation
Table 2 shows the results of the nutrient compositions of the two different chocolate-coated chestnuts as compared to the uncoated freeze-dried chestnut. The biochemical composition of RFDC did not show any significant difference (P>0.05) when compared to fresh roasted chestnut (data not shown), which is in accordance with the findings of Míguelez et al.(2004) who reported that freeze drying does not alter the sugar contents of fresh chestnut unlike vacuum drying. Besides, the respective values obtained for sugar contents, proteins, fat, fibers and minerals were similar to the ranges reported for French (Bergougnoux, 1978; Desmaison and Adrian, 1986), Italian (Bassi and Marangoni, 1984), Portuguese (Torres-Pereira Gaspar et al., 1992) chestnuts. However, this result was not sufficient to confirm any similarity/dissimilarity between Chinese and overseas chestnuts due to the fact that only one variety was investigated in this study.
Table 2.
The nutrient compositions of chocolate-coated and uncoated chestnuts
| Samples | Moisture (%) | Starch (%) | Glucose (%) | Sucrose (%) | Fructose (%) | Galactose (%) | Proteins (%) | Lipids (%) | Fibers (%) | Minerals (%) |
| RFDC | 7.31±0.16a | 66.23±4.63a | 0.07±0.01a | 9.17±1.00a | 0.13±0.11a | ND | 8.15±0.70a | 2.53±0.06a | 3.85±0.01a | 2.12±0.03a |
| DCC | 7.09±0.44a | 33.27±5.56b | 0.04±0.01a | 26.48±2.69b | 0.04±0.00b | 0.05±0.01 | 9.54±2.07b | 18.33±0.27b | 2.53±0.02b | 2.27±0.16ab |
| MCC | 4.70±0.52b | 23.23±6.07c | 0.004±0.001a | 30.63±5.68c | 0.04±0.01b | 0.40±0.02 | 15.37±1.80c | 19.70±0.18c | 2.52±0.12b | 2.95±0.01b |
All compositions, except moisture, were measured in dry matter; Means followed by different superscript letters in the same column are significant different (P<0.05). RFDC: Roasted freeze-dried chestnut; DCC: Dark chocolate-coated chestnut; MCC: Milk chocolate-coated chestnut; ND: Not determined
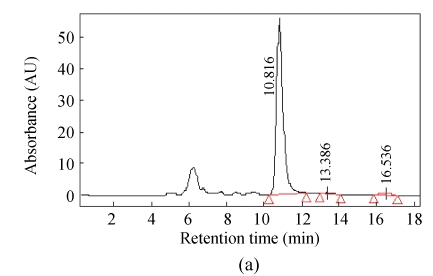
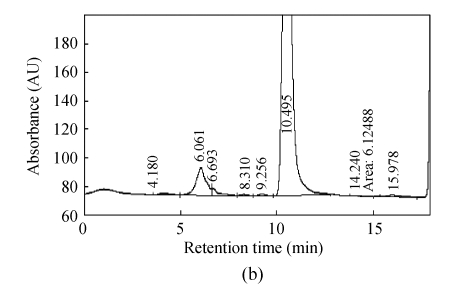
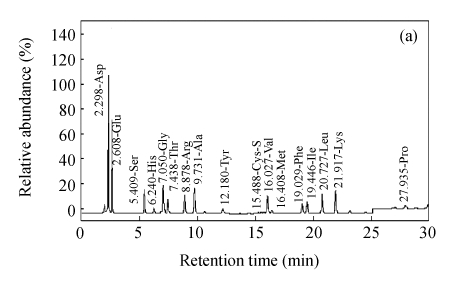
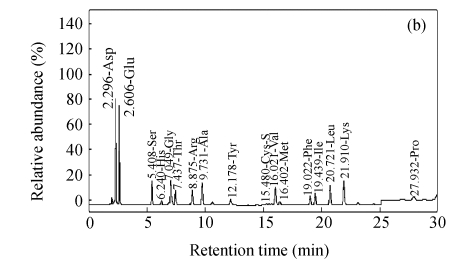
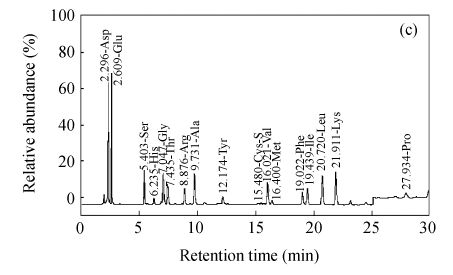
Fig.2 shows representative HPLC chromatogram of sugars in RFDC, DCC and MCC.
Fig. 2.
HPLC chromatograms of sugar profiles in (a) RFDC, (b) DCC and (c) MCC
Chocolate coating significantly affected the nutrient composition of chestnut. There was a significant decrease in starch, while sucrose content trebled in both chocolate-coated chestnuts and protein content was increased from 8.15% (RFDC) to 9.54% and 15.37% for DCC and MCC respectively. Values with a slight, yet significant difference were observed regarding the fibers and minerals. The moisture content was relatively high assuming that processed chocolate contains 2.78% moisture (data provided by the manufacturer). The reason for high moisture content in the doubly coated chestnut could be attributed to the moisture picked up from the dipping into the first coating solution. However, the moisture content of freeze-dried coated chestnut is suitable for industrial processing (Gounga et al., 2008).
Comparing the nutrient changes in DCC and MCC, sucrose content was 15% higher in MCC than in DCC. The milk powder used probably contains sugar (sucrose) incorporated during its processing. This shows a resultant increase in the sucrose content of MCC (Table 2). This result is in contradiction with sensory evaluation which revealed that DCC appeared to be sweeter than MCC (Gounga et al., 2007b). However, the total carbohydrate remained dominant in DCC (62.23%) than in MCC (56.82%). The protein content doubled in MCC when compared with DCC. This could be explained by the different formulations in the two chocolates. Milk powder and whey protein (Table 1) constituted the source of protein in MCC. Cocoa powder, present only in milk chocolate formulation and known as an effective source of potassium, sodium, magnesium and phosphorus (Pedro et al., 2006), could explain the slight increase of mineral content in MCC. Pertwee (1992) stated that 100 g of cocoa powder contains 18.5 g of protein, 950 mg of Na, 1500 mg of K, 520 mg of Mg and 660 mg of P as reported by Bruinsma and Taren (1999). For both DCC and MCC, it can be seen that there were significant increases in lipids. Due to the high saturated fat content in chocolate (Mursu et al., 2004), the lipid content in chocolate-coated chestnuts was 7 times higher than that in uncoated chestnut.
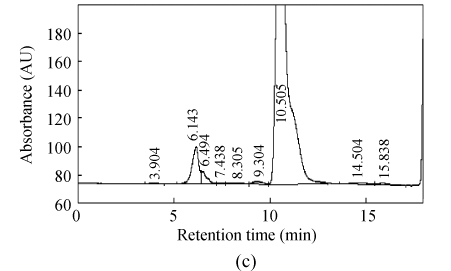
The amino acid profiles (total and free amino acids) of RFDC, DCC and MCC are given in Table 3. Amino acid profiles were dominated by aspartic acid, followed by glutamic acid, arginine, leucine, valine and glycine. Similar results were reported by Borges et al.(2008) who investigated chestnut cultivars from Portugal. The amino acid composition differed only slightly between the raw material and the processed chocolate-coated chestnuts. The results suggest that the differences in amino acid were related to their nutritional quality and protein content. The results also show that except for methionine+cysteine, the amino acid profiles of all samples were generally higher in essential amino acid (EAA) profiles compared with the suggested pattern of requirement by FAO/WHO (1990) for adult humans. This indicates their good nutritional quality and confirms that both uncoated and chocolate-coated chestnuts are interesting healthy food. Fig.3 shows representative HPLC chromatogram of amino acid in RFDC, DCC and MCC.
Table 3.
Amino acid patterns of RFDC, DCC and MCC (mg/g sample)
| Amino acid | RFDC |
DCC |
MCC |
EAAa (g/100 g protein) | |||
| FAA | TAA | FAA | TAA | FAA | TAA | ||
| Aspartic acid | 3.480 | 13.02 | 1.570 | 8.56 | 1.170 | 8.13 | |
| Glutamic acid | 1.870 | 9.33 | 1.060 | 8.65 | 1.380 | 9.28 | |
| Serine | 0.100 | 2.74 | 0.060 | 2.49 | 0.044 | 2.79 | |
| Histidine | 0.098 | 1.57 | 0.050 | 1.16 | 0.043 | 1.26 | 1.6 |
| Glycine | 0.104 | 3.26 | 0.099 | 2.62 | 0.101 | 2.62 | |
| Threonine | 0.250 | 2.36 | 0.110 | 2.11 | 0.089 | 2.41 | 0.9 |
| Arginine | 1.840 | 4.33 | 0.740 | 3.04 | 0.053 | 2.66 | |
| Alanine | 0.630 | 3.39 | 0.390 | 2.66 | 0.037 | 2.84 | |
| Tyrosine | 0.110 | 1.49 | 0.150 | 1.43 | 0.130 | 1.63 | |
| Cysteine-S | 0.004 | 0.36 | 0.0004 | 0.39 | 0.00007 | 0.42 | |
| Valine | 0.150 | 3.35 | 0.130 | 2.95 | 0.066 | 3.05 | 1.3 |
| Methionine | 0.014 | 0.77 | 0.007 | 0.71 | 0.007 | 0.83 | 1.7b |
| Phenylalanine | 0.180 | 2.65 | 0.150 | 2.31 | 0.066 | 2.35 | |
| Isoleucine | 0.079 | 2.59 | 0.075 | 2.24 | 0.037 | 2.45 | 1.3 |
| Leucine | 0.089 | 4.04 | 0.160 | 3.62 | 0.059 | 4.22 | 1.9 |
| Lysine | 0.088 | 2.84 | 0.083 | 2.58 | 0.045 | 2.82 | 1.6 |
| Proline | 0.210 | 1.26 | 0.069 | 1.34 | 0.035 | 1.40 | |
| Total | 9.310 | 59.35 | 4.900 | 48.86 | 4.170 | 51.16 | |
RFDC: Roasted freeze-dried chestnut; DDC: Dark chocolate-coated chestnut; MCC: Milk chocolate-coated chestnut; TAA: Total amino acid; FAA: Free amino acid.
Suggested profile of essential amino acid (EAA) requirements for adults in (FAO/WHO, 1990);
Methionine+cysteine
Fig. 3.
HPLC chromatograms of amino acids in (a) RFDC, (b) DCC and (c) MCC
All in all, the chocolate coating improved significantly the nutritional value of chestnut as seen in this investigation. According to CMANCA (1996), chocolate provides a number of nutrients the body requires daily. A milk chocolate bar weighing 39.689 g contains about 3 g of protein, and 15% riboflavin, 9% calcium, and 7% iron of the daily values. Although no information related to chocolate-coated chestnut has been reported, many kinds of similar products are commercially available. Almonds and peanuts added to chocolate increase the nutrients in a bar. This is particularly true for protein as reported by CMANCA (1996). The same source reported that milk chocolate bars with almonds also have increased amounts of calcium, iron and riboflavin. Moreover, chocolate, having some other potentialities, could be assimilated to drug, as reported in (Bruinsma and Taren, 1999). Therefore, chocolate may be used as a form of self-medication for dietary deficiencies (Rodin et al., 1991) or to balance low levels of neurotransmitters involved in the regulation of mood, food intake and compulsive behaviors (Macdiarmid and Hetherington, 1995). Bruinsma and Taren (1999) reviewed that chocolate contains several biologically active constituents which potentially cause abnormal behaviors and psychological sensations that parallel those of other additive substances.
Microbiological analysis
The microbiological evaluation of the chocolate-coated chestnuts (tested samples) and chocolate-coated peanut (reference sample) stored at both 7 °C and (25±2) °C was based on the specifications for chocolate and chocolate products (Chinese National Standard GB 9678.2, 2003). The results are shown in Table 4. It can be observed that there were no remarkably levels of yeasts and molds in the tested samples, nor in the reference samples at both storage temperatures, yet yeasts and molds are the major cause of deterioration or spoilage of most fruits and vegetables (Goepfert, 1980). This is in agreement with our previous findings that reported the decay incidence of the same products stored at 4 °C and 23 °C (Gounga et al., 2007b).
Table 4.
Enumeration of microorganisms in DDC and MCC as compared to the REF used at different storage conditions
| Conditions | Storage time | TPC (aerobic) (CFU/g) | Escherishia coli (CFU/g) | Salmonella spp. | Shigella spp. | Staphylococcus aureus | Yeasts and molds (CFU/g) | |
| DCC | 7 °C; (82±5)%RH | Day 0 | 1.2×102 | <20 | ND | ND | ND | <20 |
| Day 180 | 1.2×102 | <20 | ND | ND | ND | <20 | ||
| (25±2) °C; (30±2)%RH | Day 0 | 1.2×102 | <30 | ND | ND | ND | <30 | |
| Day 180 | 1.2×102 | <30 | ND | ND | ND | <30 | ||
| MCC | 7 °C; (82±5)%RH | Day 0 | 1.2×102 | <20 | ND | ND | ND | <20 |
| Day 180 | 1.2×102 | <20 | ND | ND | ND | <20 | ||
| (25±2) °C; (30±2)%RH | Day 0 | 1.2×102 | <30 | ND | ND | ND | <30 | |
| Day 180 | 1.2×102 | <30 | ND | ND | ND | <30 | ||
| REF | 7 °C; (82±5)%RH | Day 0 | 1.2×102 | <20 | ND | ND | ND | <20 |
| Day 180 | 1.2×102 | <20 | ND | ND | ND | <20 | ||
| (25±2) °C; (30±2)%RH | Day 0 | 1.2×102 | <30 | ND | ND | ND | <30 | |
| Day 180 | 1.2×102 | <30 | ND | ND | ND | <30 | ||
RH: Relative humidity; TPC: Total plate count; DCC: Dark chocolate-coated chestnut; MCC: Milk chocolate-coated chestnut; REF: Reference sample (chocolate-coated peanut); ND: Not detected. The values are average observations for duplicates plates; Specifications: Hygienic standard for chocolate and chocolate products (Chinese National Standard GB 9678.2-2003)
In all the samples tested, a low level of total plate count (TPC) was observed. The TPC was lower than the recommended safety limit proposed by Chinese Hygienic Standard for chocolate and chocolate products (Chinese National Standard GB 9678.2-2003). More so, no observable colonies of Salmonella ssp., Shigella ssp. or Staphylococcus aureus were found (Table 4).
The considerably low levels of yeasts and molds and TPC are indicative of proper handling of the raw material (chestnut) and satisfactory processing conditions of both WPI-Pul and chocolate coatings at all processing stages.
Traditionally, chocolate and other confectionery products are regarded as being microbiologically stable and safe to eat. Owing to the inherent low water activity of chocolate, it is unlikely to support the growth and proliferation of bacterial pathogens. The moisture content of the chocolate used was 2.78% as earlier stated. However, Tamminga et al.(1977) reported that although Salmonellae are unable to grow in chocolate with the low water activity, they are able to survive and persist for long period of time during storage. Moreover, the low water activity of chocolate has been also reported to be a contributing factor towards the increased thermal resistance of Salmonella in chocolate, which enables the organism to survive processing conditions that would normally be expected to destroy this pathogen (D′Aoust, 1977). If such situation occurs, and in order to prevent any eventual accidental contamination, the use of tested plant extracts or essential oils after prolonged storage is worth doing (Baylis et al., 2004; Kotzekidou et al., 2008).
CONCLUSION
Chocolate-coated chestnut has been successfully processed and the determination of the proximate analysis provides information about nutritional supply in what is considered as new product. The results show a wide range of values for all the nutrients studied as compared to the raw chestnut. This is a pioneer study in China, which has shown an alternative strategy to add value to chestnut, minimizing the significant losses in harvested fruits and providing a wider range of choices of new products to the consumer’s disposal. The microbiological investigations suggest that chocolate coating led to a marked reduction in the population of spoilage micro-organisms during storage and the product was of satisfactory bacteriological quality. The product storability is going on and would be carried out up to 12 months. Therefore, the microbial indices could be proposed to estimate the shelf life of the product.
Acknowledgments
The authors are grateful to the State Key Laboratory of Food Microbiology of Jiangnan University, China and Shanghai Shengan Food Plant, China for the microbiological analysis, and Mrs. Wan-lan Ji of Liangfeng Food Co., China for allowing us to investigate their product as control sample.
References
- 1.AOAC (Association of Official Analytical Chemists) Official Methods of Analysis. 15th Ed. Arlington: Association of Official Analytical Chemists Inc; 1990. pp. 1096–1097. [Google Scholar]
- 2.AOAC (Association of Official Analytical Chemists) Official Methods of Analysis. Washington, DC: Association of Official Analytical Chemists Inc; 1997. [Google Scholar]
- 3.Attanasio G, Cinquanta L, Albanese D, Matteo MD. Effects of drying temperatures on physico-chemical properties of dried and rehydrated chestnuts (Castanea sativa) Food Chemistry. 2004;88(4):583–590. doi: 10.1016/j.foodchem.2004.01.071. [DOI] [Google Scholar]
- 4.Bassi D, Marangoni B. Contributo allo studio varietale del castagno da fruto (Castanea sativa Mill). caratteri biometrici e analisi chimico-fisiche dei frutti. Rivista di Frutticoltura. 1984;6:43–46. [Google Scholar]
- 5.Baylis CL, MacPhee S, Robinson AJ, Griffiths R, Lilley K, Betts RP. Survival of Escherichia coli O157:H7, O111:H- and O26:H11 in artificially contaminated chocolate and confectionery products. International Journal of Food Microbiology. 2004;96(1):35–48. doi: 10.1016/j.ijfoodmicro.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Bergougnoux F. Conservation, Transformation et Utilisation des Châtaignes et Marrons. Paris: Institut National de Vulgarisation pour les Fruits, Légumes et Champignons; 1978. [Google Scholar]
- 7.Borges O, Gonçalves B, Carvalho JL, Correia P, Silva AP. Nutritional quality of chestnut (Castanea sativa Mill.) cultivars from Portugal. Food Chemistry. 2008;106(3):976–984. doi: 10.1016/j.foodchem.2007.07.011. [DOI] [Google Scholar]
- 8.Bounous G. Inventory of Chestnut Research, Germplasm and References. Rome, Italy: FAO; 2002. [Google Scholar]
- 9.Bruinsma K, Taren DL. Chocolate: food or drug? Journal of the American Dietetic Association. 1999;99(10):1249–1256. doi: 10.1016/S0002-8223(99)00307-7. [DOI] [PubMed] [Google Scholar]
- 10.Campos CM, Benedet THD. Aceitabilidade de bombons (sabor passas ao rum)—recheio adicionado de proteínas de soja. Boletim da Sociedade Brasileira Ciência e Tecnologia de Alimentos. 1994;28:113–117. [Google Scholar]
- 11.Chinese Standard GB/T 5009.3. Beijing, China: Standards Press of China; Inspection of Grain and Oilseeds: Methods for Determination of Moisture in Foods. 2003
- 12.Chinese Standard GB/T 5009.4. Beijing, China: Standards Press of China; Inspection of Grain and Oilseeds: Methods for Determination of Ash in Foods. 2003
- 13.Chinese Standard GB/T 5009.5. Beijing, China: Standards Press of China; Inspection of Grain and Oilseeds: Methods for Determination of Crude Protein in Foods. 2003
- 14.Chinese Standard GB/T 5009.6. Beijing, China: Standards Press of China; Inspection of Grain and Oilseeds: Methods for Determination of Crude Fat in Foods. 2003
- 15.Chinese Standard GB/T 5009.10. Beijing, China: Standards Press of China; Inspection of Grain and Oilseeds: Methods for Determination of Crude Fiber in Foods. 2003
- 16.Chinese National Standard GB 9678.2. Beijing, China: Standards Press of China; Specifications for Hygienic Standard for Chocolate and Chocolate Products. 2003
- 17.CMANCA (Chocolate Manufactures Association National Confectioners Association) US Statistics. 1996 (Available at: http://www.candyusa.org)
- 18.D′Aoust JY. Salmonella and the chocolate industry: a review. Journal of Food Protection. 1977;40:718–727. doi: 10.4315/0362-028X-40.10.718. [DOI] [PubMed] [Google Scholar]
- 19.Desmaison AM, Adrian J. La place de la châtaigne en alimentation. Medecine et Nutrition. 1986;22(3):174–180. [Google Scholar]
- 20.Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11(1):1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- 21.FAOSTAT (Food and Agriculture Organization of the United Nations Statistical Databases) Rome, Italy: FAO; Food and Agriculture Organization of the United Nations Statistical Databases—Agriculture Data, Agricultural Production, Major Food And Agricultural Commodities and Producers. 2005 (Available at: http://www.fao.org/es/ess/top/commodity.html?lang=en&item=220&year=2005)
- 22.FAO/WHO (Food and Agriculture Organization/World Health Organization) Rome: Food and Agriculture Organization of the United Nations; Protein Quality Evaluation. Report of the Joint FAO/WHO Expert Consultation. 1990
- 23.Goepfert JM. Microbial Ecology of Foods. New York, USA: Academic Press; 1980. p. 624. [Google Scholar]
- 24.Gounga ME, Xu SY, Wang Z. Sensory attributes of freshly roasted and roasted freeze-dried Chinese chestnut (Castanea mollissima) coated with whey protein isolated-pullulan edible coating. International Journal of Agriculture Research. 2007;2(11):959–964. [Google Scholar]
- 25.Gounga ME, Xu SY, Wang Z. Application of a Double Edible Coating on Chinese Chestnut (Castanea mollissima). The 7th International Conference of Food Science and Technology; Nov. 12-15; Wuxi, China. 2007. [Google Scholar]
- 26.Gounga ME, Xu SY, Wang Z. Whey protein isolate-based edible films as affected by protein concentration, glycerol ratio and pullulan addition in film formation. Journal of Food Engineering. 2007;83(4):521–530. doi: 10.1016/j.jfoodeng.2007.04.008. [DOI] [Google Scholar]
- 27.Gounga ME, Xu SY, Wang Z, Yang WG. Effect of whey protein isolate-pullulan edible coatings on the quality and shelf-life of freshly roasted and freeze-dried Chinese chestnut. Journal of Food Science. 2008;73(4):151–161. doi: 10.1111/j.1750-3841.2008.00694.x. [DOI] [PubMed] [Google Scholar]
- 28.Koburger IA. An antibiotic-containing medium for the enumeration of fungi in foods. Bacteriology Proceedings. 1968;13:A73. [Google Scholar]
- 29.Kotzekidou P, Giannakidis P, Boulamatsis A. Antimicrobial activity of some plant extracts and essential oils against food borne pathogens in vitro and on the fate of inoculated pathogens in chocolate. Lebensmittel Wissenschaft und Technologie, Food Science and Technology. 2008;41(1):119–127. [Google Scholar]
- 30.Lamelas E. Optimizacion do Proceso de Hidrolise de Amidon de Castana Mediante un Proceso Simultaneo de Lucuefacion-Sacarificacion. Memoria Mecanografiada del Proyecto Fin de Carrera de Ingeniera Tecnica Agricola, Especialidad Industrias Agroalimentarias. Ourense: Facultad de Ciencias; 2000. [Google Scholar]
- 31.Macdiarmid JI, Hetherington MM. Mood modulation by food: an exploration of affected and cravings in “chocolate addicts”. Brazilian Journal of Clinical Psychology. 1995;34:129–138. doi: 10.1111/j.2044-8260.1995.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 32.Manafi M. Media for Detection and Enumeration of Total Enterobacteriaceae, Coliform and Escherichia coli from Water and Foods. In: Corry JEL, Curtis GDW, Baird RM, editors. Handbook of Culture Media for Food Microbiology-Progress in Industrial Microbiology. Vol. 37. Amsterdam, the Netherlands: Elsevier Science; 2003. pp. 167–192. [DOI] [Google Scholar]
- 33.Míguelez JDLM, Bernárdez MM, Queijeiro JMG. Composition of varieties of chestnuts from Galicia (Spain) Food Chemistry. 2004;84(3):401–404. doi: 10.1016/S0308-8146(03)00249-8. [DOI] [Google Scholar]
- 34.Mursu J, Voutilainen S, Nurmi T, Rissanen HT, Virtanen KJ, Kaikkonen J, Nyyssönen K, Salonen TJ. Dark chocolate consumption increases HDL cholesterol concentration and chocolate fatty acids may inhibit lipid peroxidation in healthy humans. Free Radical Biology & Medicine. 2004;37(9):1351–1359. doi: 10.1016/j.freeradbiomed.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Pedro NAR, de Oliveira E, Cadore S. Study of the mineral content of chocolate flavoured beverages. Food Chemistry. 2006;95(1):94–100. doi: 10.1016/j.foodchem.2004.12.021. [DOI] [Google Scholar]
- 36.Pertwee RG. In Vivo Interactions between Psychotropic Cannabinoids and Other Drugs Involving Central and Peripheral Neurochemical Mediators. In: Murphy L, Bartke A, editors. Marijuana/Cannabinoids: Neurobiology and Neurophysiology. Boca Raton, Fla: CRC Press; 1992. pp. 165–218. [Google Scholar]
- 37.Rodin J, Mancuso J, Granger J, Nelbach E. Food cravings in relation to body mass index, restraint and estradiol levels: a repeated measures study in healthy women. Appetite. 1991;17(3):177–185. doi: 10.1016/0195-6663(91)90020-S. [DOI] [PubMed] [Google Scholar]
- 38.Tamminga SK, Beumer RR, Kampelmacher EH, van Leusden FM. Survival of Salmonella eastbourne and Salmonella typhimurium in milk chocolate prepared with artificially contaminated milk powder. Journal of Hygiene Cambridge. 1977;79:333–337. doi: 10.1017/s002217240005316x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torelm I, Danielsson R. Variations in major nutrients and minerals in Swedish foods: a multivariate multifactorial approach to the effects of season, region and chain. Journal of Food Composition and Analysis. 1998;11(1):11–31. doi: 10.1006/jfca.1998.0561. [DOI] [Google Scholar]
- 40.Torres-Pereira Gaspar JM, Sequeira CA, Torres de Castro L. Estudo Sobre a Composiçao Química e Valor Nutritivo da Castanha, Visando a Sua Transformaçao Agro-industrial. Vila Real: Universidade de Trâs-os-montes e Alto Douro; 1992. [Google Scholar]
- 41.Vossen P. Chestnut Culture in California. Soroma Country: University of California Cooperative Extension Farm Advisor; 2000. [Google Scholar]