Abstract
We investigated the effects of fish protein hydrolysate (FPH) on growth performance and humoral immune response of the large yellow croaker (Pseudosciaena crocea R.). One thousand and two hundred large yellow croakers [initial average weight: (162.75±23.85) g] were divided into four groups and reared in floating sea cages (3 m×3 m×3 m). The animals were fed with 4 diets: basal diet only (control) or diets supplemented with 5%, 10% and 15% (w/w) FPH. The results show that dietary FPH levels significantly influenced the growth and immunity of the large yellow croaker. Compared with the control group, total weight gain (TWG) in all treatment groups, relative weight gain (RWG) and specific growth rate (SGR) in fish fed with diets supplemented with 10% and 15% FPH were significantly increased (P<0.05). Similar results were observed in immune parameters [lysozyme activity, serum complements, immunoglobulin M (IgM)]. Lysozyme activity, complement C4 and IgM were also significantly increased (P<0.05) in fish fed with diets supplemented with 10% and 15% FPH, while complement C3 level was significantly increased (P<0.05) in all treatment groups. In general, with the supplementation of FPH, particularly at dose of 10%, the growth performance and immunity of the large yellow croaker can be improved effectively.
Keywords: Fish protein hydrolysate (FPH), Growth performance, Humoral immune response, Large yellow croaker
INTRODUCTION
As one kind of important nutrients, the utilization of protein in humans and animals is widely studied. Protein is digested into amino acids and peptides by digestive enzymes and then assimilated in the intestine. Absorption of peptides is considered as a major route of transport in the intestine of vertebrates, and small peptides are absorbed faster than free amino acids (Adibi, 1997; Tesser et al., 2005). In aquaculture, immunostimulants were considered as a promising tool to enhance the resistance of cultured fish to disease and stress (Bagni et al., 2000). And some commercial feed supplements with immunostimulating capacities have already been applied (Cook et al., 2003).
Fish protein hydrolysate (FPH) has been used as an ingredient for diets of marine fish (Ouellet et al., 1997; Aguila et al., 2007). FPH contains antioxidative activity and some functional properties (Klompong et al., 2007; Thiansilakul et al., 2007; Liaset and Espe, 2008). While some studies have investigated the effects of FPH on growth performance and nonspecific immunity in several fish species (Berge and Storebakken, 1996; Bøgwald et al., 1996; Carvalho et al., 1997; Liang et al., 2006), no similar studies have been done on the large yellow croaker.
The large yellow croaker (Pseudosciaena crocea R.) belongs to predatory fish species, and is one kind of economically important marine fish species in China. With high nutritive value and better flavor, it is popularly consumed by people in Zhejiang Province, China. Due to excessive harvest, the wild large yellow croaker has nearly been depleted prior to the 1980s, and been widely cultured in China since the success of artificial hatchery (Duan et al., 2001; Mai et al., 2006). The purpose of the present study was to evaluate the effects of dietary supplementation with FPH on growth performance and humoral immune response in the large yellow croaker.
MATERIALS AND METHODS
Diets preparation
FPH was produced by hydrolyzing tissues of pollock (Theragra chalcogramma) with Flavourzyme and Alcalase. By the method of Adler-Nissen (1986), degree of hydrolysis (DH) was calculated to be 45.5%. The water-soluble protein fraction was spray-dried and stored at 4 °C for further use. FPH contained 65.61% protein, 2.06% fat and 3.99% ashes (on dry matter basis).
The experimental diets were formulated to be equal in protein, lipid and ash. White fishmeal, soybean meal and protein powder of blood corpuscles were used for sources of protein, and fish oil and α-starch were used for non-protein energy. After mixed homogeneously, the ingredients were extruded to be dry pellets (6 mm in diameter). The components and proximate analysis of the diets are presented in Table 1.
Table 1.
Formulation and proximate composition of experimental diets
| Formulation and composition | Content (%, w/w) |
|||
| Diet 1 | Diet 2 | Diet 3 | Diet 4 | |
| Ingredients | ||||
| White fish meal | 65.0 | 60.0 | 55.0 | 50.0 |
| Soybean meal | 4.5 | 4.5 | 4.5 | 4.5 |
| α-starch | 17.0 | 17.0 | 17.0 | 17.0 |
| Protein of blood powder | 1.8 | 1.8 | 1.8 | 1.8 |
| Vitamin pre-mixture | 1.0 | 1.0 | 1.0 | 1.0 |
| Mineral pre-mixture | 2.0 | 2.0 | 2.0 | 2.0 |
| Cellulose | 0.2 | 0.2 | 0.2 | 0.2 |
| Fish oil | 8.5 | 8.5 | 8.5 | 8.5 |
| Fish protein hydrolysate | 0 | 5.0 | 10.0 | 15.0 |
| Proximate analysis (on dry matter basis) | ||||
| Protein | 41.36 | 41.78 | 42.05 | 42.24 |
| Lipid | 11.69 | 11.47 | 11.56 | 11.81 |
| Fiber | 2.45 | 2.38 | 2.64 | 2.57 |
| Ash | 11.14 | 11.53 | 11.28 | 11.15 |
Note: Vitamin premix (mg/kg diet): Thiamin 25, riboflavin 45, pyridoxine HCl 20, vitamin B12 0.1, vitamin K3 10, inositol 800, pantothenic acid 60, niacin acid 200, folic acid 20, biotin 1120, retinol acetate 32, cholecalciferol 5, α-2-tocopherol 120, ascorbic acid 2000; Mineral premix (mg/kg diet): NaF 2, KI 0.8, CoCl2·6H2O 50, CuSO4·5H2O 10, FeSO4·H2O 80, ZnSO4·H2O 50, MnSO4·H2O 60, MgSO4·7H2O 1200, NaCl 100
Animals and rearing condition
Large yellow croakers weighing (162.75±23.85) g were purchased from net-cage culture field in Ningbo, Zhejiang Province, China. They were acclimated for 2 weeks before the trial started. Then 1200 healthy, energetic fish were put into 12 cages (3 m×3 m×3 m) and divided into 4 groups. Each group was repeated in triplicates and each cage was stocked with 100 fish. Fish were fed twice daily (5:00 a.m. and 17:30 p.m.) to apparent satiation. The trial lasted for 8 weeks [water temperature (26.1±2.2) °C; salinity 33‰ to 35‰; dissolved oxygen content 6.6 mg/L].
Growth performance
At the beginning and end of the trial, the animals were fasted for 24 h and their weights were determined. Growth performance was expressed as the total weight gain (TWG), relative weight gain (RWG) and specific growth rate (SGR). The calculation formulas were as follows:
| TWG (g)=Wt−Wi, |
| RWG (%)=(Wt−Wi)×100/Wi, |
| SGR (%)=(lnWt−lnWi)×100/d, |
where W i and W t are the initial and final mean weights (g), respectively, and d represents the number of feeding days.
Blood sampling
At the termination of the trial, nine fish per group (three fish randomly captured from each cage) were sampled. Blood was sampled from the caudal vein of the individual fish after anaesthetization. The whole blood was collected in a syringe, allowed to clot for 1 h in microtubes at room temperature and followed by 5 h at 4 °C, and then serum was harvested by centrifuging at 1500×g for 5 min at 4 °C. All serum samples were preserved at −20 °C prior to analysis.
Detection of lysozyme
Lysozyme activity in the serum was measured with spectrophotometry based on lysis of freezer-dried particles of Micrococcus lysodeikticus (Sigma Chemical Co., USA) (Ellis, 1990; Alcorn et al., 2002). One unit of enzyme activity was defined as the amount of enzyme causing a decrease in absorbance of 0.001 per min per ml serum.
Detection of total immunoglobulin M (IgM)
Total IgM was determined following the method of Siwicki and Anderson (1993). The assay was based on the measurement of total protein contents in plasma using a micro protein determination method (C-690; Sigma) prior to and after precipitating down the IgM molecules employing a 12% (w/v) solution of polyethyleneglycol (Sigma). The difference in the protein contents was considered as the IgM content.
Detection of serum complements (C3 and C4)
The method of immunoturbidimetry (Jiancheng Institute of Biotechnology, Nanjing, China) was adopted in the assay for serum complement level. C3 and C4 in serum samples were mixed with the antibody afforded by the kits, and then an antigen-antibody complex was produced. The optical density (OD) value was measured at 340 nm. Compared with the values of the standards from the kits, C3 and C4 contents were calculated in μg/ml.
Statistical analysis
All data were analyzed by one-way analysis of variance (ANOVA) using the software of the SPSS 11.0 for Windows. When ANOVA identified differences among groups, multiple comparisons among means were made using Duncan’s new multiple-range test. The results are presented as mean±SD, and probabilities of P<0.05 were considered significant.
RESULTS
Growth performance
As shown in Table 2, FPH supplemented to the diet could increase the TWG, RWG and SGR of large yellow croakers. Fish fed with Diet 1 (basal diet) showed the lowest TWG [(83.55±15.18) g], RWG [(51.63±8.63)%] and SGR [(0.74±0.1)%]. The highest TWG [(101.9±11.89) g], RWG [(62.41±7.62)%] and SGR [(0.86±0.08)%] were recorded in the fish fed with Diet 3 containing 10% (w/w) FPH.
Table 2.
Effects of FPH on TWG, RWG and SGR in large yellow croakers
| Group | TWG (g) | RWG (%) | SGR (%) |
| Diet 1 | 83.55±15.18a | 51.63±8.63a | 0.74±0.10a |
| Diet 2 | 91.05±5.61b | 55.84±5.41ac | 0.79±0.06ac |
| Diet 3 | 101.90±11.89c | 62.41±7.62b | 0.86±0.08b |
| Diet 4 | 93.24±13.40b | 58.06±9.00bc | 0.81±0.10bc |
Note: FPH: Fish protein hydrolysate; TWG: Total weight gain; RWG: Relative weight gain; SGR: Specific growth rate; Values (mean±SD) in the same column sharing the same superscript letter are not significantly different (P>0.05)
Lysozyme activity
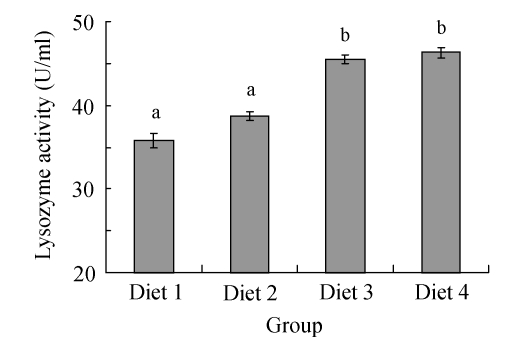
The effects of FPH on the lysozyme activity of serum in large yellow croakers were shown in Fig.1. No significant difference was observed between fish fed with Diets 1 and 2. The lysozyme activities in fish fed with Diets 3 and 4 were significantly higher than those in fish fed with Diets 1 and 2 (P<0.05).
Fig. 1.
Effects of fish protein hydrolysate (FPH) on the lysozyme activity of serum in large yellow croakers. Each bar represents mean±SD. Different letters stand for statistically significant differences at P<0.05
Complement level
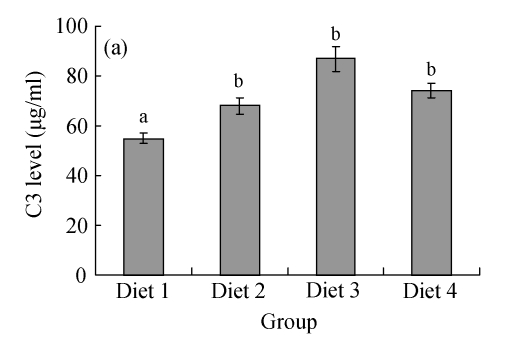
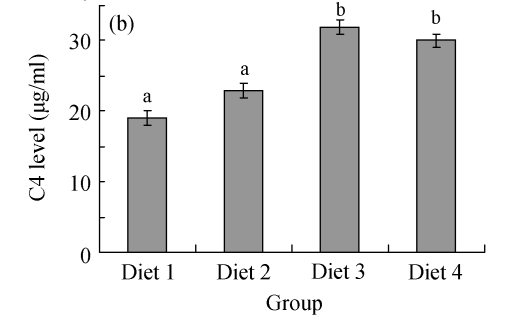
It was found that the levels of serum complements in large yellow croakers were significantly influenced by FPH. As shown in Fig.2a, C3 levels in Diets 2~4 were all significantly higher than that in Diet 1 (P<0.05). As shown in Fig.2b, C4 levels in Diets 3 and 4 were significantly higher than those in Diets 1 and 2 (P<0.05).
Fig. 2.
Effects of fish protein hydrolysate (FPH) on the serum complements of large yellow croakers. Each bar represents mean±SD. Different letters stand for statistically significant differences at P<0.05
Total serum IgM
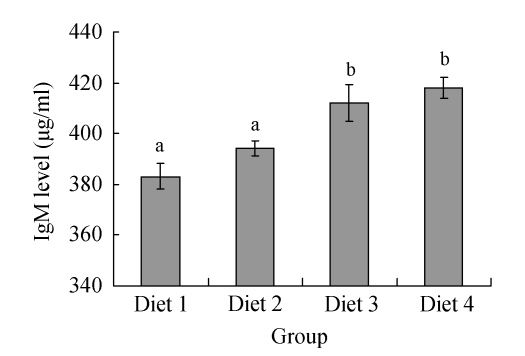
FPH had a notable influence on total serum IgM level of large yellow croakers (Fig.3). Total serum IgM levels were significantly higher in Diets 3 and 4 and peaked at (418±4) μg/ml for Diet 4, while there were no significant differences observed between Diets 1 and 2 treatments and between Diets 3 and 4 treatments.
Fig. 3.
Effects of fish protein hydrolysate (FPH) on total serum IgM level of large yellow croakers. Each bar represents mean±SD. Different letters stand for statistically significant differences at P<0.05
DISCUSSION AND CONCLUSION
Previous studies have shown that diets supplemented with FPH can improve growth performance of fish species (Berge and Storebakken, 1996; Carvalho et al., 1997; Refstie et al., 2004). In contrast, other study reported that protein hydrolysate cannot serve as an efficient dietary component (Kolkovski and Tandler, 2000). Positive results were observed in this study, growth performance of the large yellow croaker was improved by supplement with different doses of FPH to basal diets. Bitter flavor in FPH, caused by soluble peptides with a high content of amino acids with hydrophobic functional groups, may be controlled by the hydrolytic enzymes and separation methods (Refstie et al., 2004). The major causes for positive effects of FPH on fish are high palatability stimulating digestive enzymes and channeling more biomass production (Cahu et al., 1999; Oliva-Teles et al., 1999; Aguila et al., 2007).
In seawater as well as in fresh water species, the best results were obtained by using a moderate level of hydrolysate, while high levels depressed fish development (Cahu et al., 1999). In this study, growth performance of fish fed with Diet 3 was better than that of fish fed with Diet 4. It might have been caused by the bitterness of FPH, so moderate level of supplement is necessary.
Lysozyme and complements are important indices of nonspecific immunity in fish (Murray et al., 2003). Lysozyme is one of the major components in the immune defense system in both invertebrates and vertebrates (Song et al., 2006). It is known to act as a nonspecific immune mediator against parasitic, bacterial and viral infections, and in response to infection its activity is found to increase in fish blood (Puangkaew et al., 2004). In the present study, 10% and 15% (w/w) FPH in the diet could enhance the lysozyme activity of the large yellow croaker effectively. The results are in agreement with the previous study in sea bass (Liang et al., 2006).
Complement, an important component of the innate immune system, plays important roles in immune surveillance and clearance of invading pathogens. It has been demonstrated that fish complement can lyse foreign cells and opsonize foreign organisms for destruction by phagocytes (Gasque, 2004). There are also indications that complement fragments participate in inflammatory reactions (Holland and Lambris, 2002). The present results show that three doses of FPH in the diet could increase serum C3 level significantly. The complement C4 level in serum of fish fed with diets supplemented with 10% and 15% (w/w) FPH was observed to be significantly higher.
Immunoglobulins are well recognized to provide protection in animals and humans against various diseases. IgM is a major component of the teleost humoral immune system (Watts et al., 2001; Cuesta et al., 2004). IgM is present in blood and other body fluids, and plays a role as an immune effector molecule (Ross et al., 1998). Total serum IgM levels of fish fed with the assayed immunostimulant-supplemented diets were statistically higher than those of fish fed with a non-supplemented diet (Cuesta et al., 2004). In the present study, compared with fish fed with basal diet, the large yellow croaker fed with diets supplemented with 10% and 15% FPH contained significantly higher levels of total serum IgM.
It is reported that dietary protein can influence immune system of crustacea (Bachère, 2000; Pascual et al., 2004). The inclusion of FPH in diet may afford more sufficient digestible protein which could be impacted better into the immune system of fish. Furthermore, fish proteins are likely to contain a large amount of bioactive sequences and constitute a potential reservoir for potent immunomodulators (Duarte et al., 2006). Biologically active peptides with immunostimulating and antibacterial properties can be produced during the hydrolyzing procedure (Bøgwald et al., 1996; Kotzamanis et al., 2007). We assume that FPH used in this study contains such bioactive peptides. In the current work, no significant effects were observed in large yellow croakers fed with Diet 2. It may be due to the deficient contents of digestible protein and bioactive peptides.
Peptides with different molecular weight were mixed together in FPH. Further research is required to determine the components and their concentrations. And it is also necessary to evaluate the effects of size-fractionated FPH on large yellow croakers. From the results of this feeding trial, it is concluded that growth performance and immune parameters of the large yellow croaker can be improved by supplementing FPH to basal diets, and that 10% (w/w) FPH gives the best results.
Footnotes
Project (No. 2006C12098) supported by the Science and Technology Department of Zhejiang Province, China
The papers for the Postdoctoral Fellows Association of Zhejiang Province, China
References
- 1.Adibi SA. The oligopeptide transporter (Pept-1) in human intestine: biology and function. Gastroenterology. 1997;113(1):332–340. doi: 10.1016/S0016-5085(97)70112-4. [DOI] [PubMed] [Google Scholar]
- 2.Adler-Nissen J. Enzymatic Hydrolysis of Food Proteins. New York: Elsevier Applied Science Publishers; 1986. [Google Scholar]
- 3.Aguila J, Cuzon G, Pascual C, Dominguesd PM, Gaxiolac G, Sánchezc A, Maldonadoe T, Rosas C. The effects of fish hydrolysate (CPSP) level on Octopus maya (Voss and Solis) diet: digestive enzyme activity, blood metabolites, and energy balance. Aquaculture. 2007;273(4):641–655. doi: 10.1016/j.aquaculture.2007.07.010. [DOI] [Google Scholar]
- 4.Alcorn SW, Murray AL, Pascho RJ. Effects of rearing temperature on immune functions in sockeye salmon (Oncorhynchus nerka) Fish Shellfish Immunol. 2002;12(4):303–334. doi: 10.1006/fsim.2001.0373. [DOI] [PubMed] [Google Scholar]
- 5.Bachère E. Shrimp immunity and disease control. Aquaculture. 2000;191(1-3):3–11. doi: 10.1016/S0044-8486(00)00413-0. [DOI] [Google Scholar]
- 6.Bagni M, Archetti L, Amadori M, Marino G. Effect of long-term administration of an immunostimulant diet on innate immunity in sea bass (Dicentrarchus labrax) J Vet Med B. 2000;47(10):745–751. doi: 10.1046/j.1439-0450.2000.00412.x. [DOI] [PubMed] [Google Scholar]
- 7.Berge GM, Storebakken T. Fish protein hydrolyzate in starter diets for Atlantic salmon (Salmo salar) fry. Aquaculture. 1996;145(1-4):205–212. doi: 10.1016/S0044-8486(96)01355-5. [DOI] [Google Scholar]
- 8.Bøgwald J, Dalmo R, Leifson RM, Stenbern E, GildBerg A. The stimulatory effect of a muscle protein hydrolysate from Atlantic cod, Gadus morhua L. on Atlantic salmon, Salmo salar L., head kidney leucocytes. Fish Shellfish Immunol. 1996;6(1):3–16. doi: 10.1006/fsim.1996.0002. [DOI] [Google Scholar]
- 9.Cahu CL, Zambonino Infante JL, Quazuguel P, Le Gall MM. Protein hydrolysate vs. fish meal in compound diets for 10-day old sea bass Dicentrarchus labrax larvae. Aquaculture. 1999;171(1-2):109–119. doi: 10.1016/S0044-8486(98)00428-1. [DOI] [Google Scholar]
- 10.Carvalho AP, Escaffre AM, Oliva Teles A, Bergot P. First feeding of common carp larvae on diets with high levels of protein hydrolysates. Aquac Int. 1997;5(4):361–367. doi: 10.1023/A:1018368208323. [DOI] [Google Scholar]
- 11.Cook MT, Hayball PJ, Hutchinson W, Nowak BF, Hayball JD. Administration of a commercial immunostimulant preparation, EcoActivaTM as a feed supplement enhances macrophage respiratory burst and the growth rate of snapper (Pagrus auratus, Sparidae (Bloch and Schneider)) in winter. Fish Shellfish Immunol. 2003;14(4):333–345. doi: 10.1006/fsim.2002.0441. [DOI] [PubMed] [Google Scholar]
- 12.Cuesta A, Meseguer J, Esteban MA. Total serum immunoglobulin M levels are affected by immunomodulators in seabream (Sparus aurata L.) Vet Immunol Immunopathol. 2004;101(3-4):203–210. doi: 10.1016/j.vetimm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Duan Q, Mai K, Zhong H, Si L, Wang X. Studies on the nutrition of the large yellow croaker, Pseudosciaena crocea R. I: growth response to graded levels of dietary protein and lipid. Aquac Res. 2001;32(Suppl. 1):46–52. doi: 10.1046/j.1355-557x.2001.00048.x. [DOI] [Google Scholar]
- 14.Duarte J, Vinderola G, Ritz B, Perdigón G, Matar C. Immunomodulating capacity of commercial fish protein hydrolysate for diet supplementation. Immunobiology. 2006;211(5):341–350. doi: 10.1016/j.imbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Ellis AE. Lysozyme Assays. In: Stolen JS, Fletcher DP, Anderson BS, et al., editors. Techniques in Fish Immunology. Fair Haven, USA: SOS Publications; 1990. pp. 101–103. [Google Scholar]
- 16.Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41(11):1089–1098. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Holland MCH, Lambris JD. The complement system in teleosts. Fish Shellfish Immunol. 2002;12(5):399–420. doi: 10.1006/fsim.2001.0408. [DOI] [PubMed] [Google Scholar]
- 18.Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102(4):1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- 19.Kolkovski S, Tandler A. The use of squid protein hydrolysate as a protein source in microdiets for gilthead seabream Sparus aurata larvae. Aquac Nutr. 2000;6(1):11–15. doi: 10.1046/j.1365-2095.2000.00125.x. [DOI] [Google Scholar]
- 20.Kotzamanis YP, Gisbert E, Gatesoupe FJ, Zambonino Infante J, Cahu C. Effects of different dietary levels of fish protein hydrolysates on growth, digestive enzymes, gut microbiota, and resistance to Vibrio anguillarum in European sea bass (Dicentrarchus labrax) larvae. Comp Biochem Physiol, Part A Mol Integr Physiol. 2007;147(1):205–214. doi: 10.1016/j.cbpa.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 21.Liang M, Wang J, Chang Q, Mai K. Effects of different levels of fish protein hydrolysate in the diet on the nonspecific immunity of Japanese sea bass, Lateolabrax japonicus (Cuvieret Valenciennes, 1928) Aquac Res. 2006;37(1):102–106. doi: 10.1111/j.1365-2109.2005.01392.x. [DOI] [Google Scholar]
- 22.Liaset B, Espe M. Nutritional composition of soluble and insoluble fractions obtained by enzymatic hydrolysis of fish-raw materials. Process Biochem. 2008;43(1):42–48. doi: 10.1016/j.procbio.2007.10.007. [DOI] [Google Scholar]
- 23.Mai K, Wan J, Ai Q, Xu W, Liufu Z, Zhang L, Zhang C, Li H. Dietary methionine requirement of large yellow croaker, Pseudosciaena crocea R. Aquaculture. 2006;253(1-4):564–572. doi: 10.1016/j.aquaculture.2005.08.010. [DOI] [Google Scholar]
- 24.Murray AL, Ponald JP, Alcorn SW, Fairgrieve WT, Shearer KD, Roley D. Effects of various feed supplements containing fish protein hydrolysate or fish processing by products on the innate immune functions of juvenile coho salmon (Oncorhynchus kisutch) Aquaculture. 2003;220(1-4):643–653. doi: 10.1016/S0044-8486(02)00426-X. [DOI] [Google Scholar]
- 25.Oliva-Teles A, Cerqueira AL, Goncalves P. The utilization of diets containing high levels of fish protein hydrolysate by turbot (Scophthalamus maximus) juveniles. Aquaculture. 1999;179(1-4):195–201. doi: 10.1016/S0044-8486(99)00162-3. [DOI] [Google Scholar]
- 26.Ouellet DR, Seoane JR, Veira DM, Proulx JG. Effects of supplementation with fish meal or fish protein hydrolysate on growth, nutrient digestibility and rumen fermentation of growing cattle fed grass silage. Anim Feed Sci Technol. 1997;68(3-4):307–326. doi: 10.1016/S0377-8401(97)00035-7. [DOI] [Google Scholar]
- 27.Pascual C, Zenteno E, Cuzon G, Sánchez A, Gaxiola G, Taboada G, Suárez J, Maldonado T, Rosas C. Litopenaeus vannamei juveniles energetic balance and immunological response to dietary protein. Aquaculture. 2004;236(1-4):431–450. doi: 10.1016/j.aquaculture.2004.01.015. [DOI] [Google Scholar]
- 28.Puangkaew J, Kiron V, Somamoto T, Okamoto N, Satoh S, Takeuchi T, Watanabe T. Nonspecific immune response of rainbow trout (Oncorhynchus mykiss Walbaum) in relation to different status of vitamin E and highly unsaturated fatty acids. Fish Shellfish Immunol. 2004;16(1):25–39. doi: 10.1016/S1050-4648(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 29.Refstie S, Olli JJ, Standal H. Feed intake, growth, and protein utilisation by post-smolt Atlantic salmon (Salmo salar) in response to graded levels of fish protein hydrolysate in the diet. Aquaculture. 2004;239(1-4):331–349. doi: 10.1016/j.aquaculture.2004.06.015. [DOI] [Google Scholar]
- 30.Ross DA, Wilson MR, Miller NW, Clem LW, Warr GW. Evolutionary variation of immunoglobulin μ-heavy chain RNA processing pathways: origins, effects, and implications. Immunol Rev. 1998;166(1):143–151. doi: 10.1111/j.1600-065X.1998.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 31.Siwicki AK, Anderson DP. Nonspecific Defense Mechanisms Assay in Fish. II. Potential Killing Activity of Neutrophils and Macrophages, Lysozyme Activity in Serum and Organs and Total Immunoglobulin Level in Serum. In: Siwicki AK, Anderson DP, Waluga J, editors. Fish Disease Diagnosis and Prevention Methods. Poland: Olsztyn; 1993. pp. 105–112. [Google Scholar]
- 32.Song Z, Wu T, Cai L, Zhang L, Zheng X. Effects of dietary supplementation with Clostridium butyricum on the growth performance and humoral immune response in Miichthys miiuy . J Zhejiang Univ Sci B. 2006;7(7):596–602. doi: 10.1631/jzus.2006.B0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tesser MB, Terjesen BF, Zhang Y, Portella MC, Dabrowski K. Free- and peptide-based dietary arginine supplementation for the South American fish pacu (Piaractus mesopotamicus) Aquac Nutr. 2005;11(6):443–453. doi: 10.1111/j.1365-2095.2005.00373.x. [DOI] [Google Scholar]
- 34.Thiansilakul Y, Benjakul S, Shahidi F. Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decap-terus maruadsi) Food Chem. 2007;103(4):1385–1394. doi: 10.1016/j.foodchem.2006.10.055. [DOI] [Google Scholar]
- 35.Watts M, Munday BL, Burke CM. Isolation and partial characterisation of immunoglobulin from southern bluefin tuna Thunnus maccoyii Castelnau. Fish Shellfish Immunol. 2001;11(6):491–503. doi: 10.1006/fsim.2000.0329. [DOI] [PubMed] [Google Scholar]