Abstract
The objective of the present study was to compare the toxicity and availability of Fe(II) and Fe(III) to Caco-2 cells. Cellular damage was studied by measuring cell proliferation and lactate dehydrogenase (LDH) release. The activities of two major antioxidative enzymes [superoxide dismutase (SOD) and glutathione peroxidase (GPx)] and differentiation marker (alkaline phosphatase) were determined after the cells were exposed to different levels of iron salts. The cellular iron concentration was investigated to evaluate iron bioavailability. The results show that iron uptake of the cells treated with Fe(II) is significantly higher than that of the cells treated with Fe(III) (P<0.05). Fe(II) at a concentration >1.5 mmol/L was found to be more effective in reducing cellular viability than Fe(III). LDH release investigation suggests that Fe(II) can reduce stability of the cell membrane. The activities of SOD and GPx of the cells treated with Fe(II) were higher than those of the cells treated with Fe(III), although both of them increased with raising iron supply levels. The results indicate that both Fe(II) and Fe(III) could reduce the cellular antioxidase gene expression at high levels.
Keywords: Iron availability, Caco-2 cells, Fe(II), Fe(III), Toxicity
INTRODUCTION
Iron is an essential micronutrient involved in flesh oxygen transport and energy metabolism. Investigation of iron deficiency is carried out widely in the world (Glahn et al., 2002; Kloots et al., 2004). However, in the dietary sources, it may cause iron toxicity when adding iron excessively or choosing iron supplement unsuitably (Núñez et al., 2001). At present, the species of iron supplements are diverse, but mainly are Fe(II) and Fe(III). Except injection, most iron supplements must pass through the intestine. Therefore, the intestine is the major site of a barrier to large quantities of iron (Rossi et al., 1996), and its conditions are a direct basis to study how to choose the form of the iron supplement. Iron’s cellular toxicity is dependent on, besides its valency, solubility and linkage, the intestine conditions and response time. Therefore, it is important to introduce an intestine model to study physiology and toxicology of iron in the intestine. In vitro digestion/Caco-2 cell model was established by Glahn et al.(1996; 1998), and was confirmed to be a useful tool of simulating the flesh intestinal tract iron absorption with nimble and highly effective characters. The Caco-2 cells are derived from a human colon carcinoma, and have similar characteristics of structure and biochemical functions to those of the intestinal epithelium (Walgren et al., 2000). The Caco-2 cells grown on porous polycarbonate membrane spontaneously differentiate into the intestinal epitheliums after confluence to express the characteristic of the continual monolayer (Glahn et al., 1998; Walgren et al., 2000). They are widely used for pharmaceutical, biochemical and toxicological studies as well as for investigation of crossing membrane transportation (Núñez et al., 2001; Zödl et al., 2004; Gan and Dhiren, 1997).
The accumulative toxicity of metals in cells may be produced via the cell transportation, which may cause reduction in cell viability and damage to DNA (Srigiridhar et al., 2001). In an investigation of the Caco-2 cell line, Núñez et al.(2001) discovered that increasing iron concentrations resulted in increased damage to DNA. Certain metals are observed on the specialized interference function of these cell monolayers. Shielding effect may be one of the main functions on the tight junctions of epithelial cells in human intestine (Watzl et al., 1999; Okada et al., 2000), and cadmium could reduce the tight junctions of Caco-2 cells in monolayer (Rossi et al., 1996). Furthermore, it was demonstrated that iron altered tight junction permeability of Caco-2 cells in monolayer cultures (Glahn et al., 1996), and it was believed that the iron toxicity displayed mainly the cell antioxidant function (Zödl et al., 2004; Zager et al., 1993). The iron could initiate lipid peroxidation of membranes, and produced malondial dehyde (MDA) to inactivate many kinds of enzymes and lead to cell injury. The Fe(II) is a main actor in the Fenton response, producing excessive free radical to attack cell membrane and depress stability or increase the penetrability of the membrane (Zhao, 1998; Xing et al., 2008). In addition, iron toxicity to cells is related to its valency and supply levels. The objective of the present study is to investigate toxicological effects of various concentrations of Fe(II) and Fe(III) on the Caco-2 cells.
MATERIALS AND METHODS
Cell culture
Caco-2 cells were obtained from the Institute of Biochemistry and Cell Biology (SIBS, CAS, Shanghai, China) and were used at Passages 20~43. The cells were maintained in 25-cm2 flasks in Dulbecco’s minimal essential medium (DMEM) supplemented with 10% (v/v) fetal bovine serum, 1% (w/v) non-essential amino acids, 20 mmol/L 2-[4-(2-hydroxyethyl)-1-piperazine]-ethanesulfonic acid (HEPES), 2 mmol/L L-glutamine, 100 U/ml penicillin G, and 100 µg/ml streptomycin. The culture medium was replaced with a fresh medium every 2~3 d (Peng et al., 2008). After being nearly confluent, the cells were washed with phosphate-buffered saline (PBS) to remove any unattached cells. The attached cells were harvested using ethylenediaminetetraacetic acid (EDTA) solution. The cells were seeded in 24-well plates at a density of approximately 250 000 cells/ml. The cells were used for further studies and were exposed to known concentrations of iron in the culture medium and incubated for 22~24 h. Cytological and biochemical assessments were carried out on the cell lysate and culture medium for determination of markers of cell damage.
Preparation of iron solutions
Ferric chloride was dissolved in deionized water and sterilized by using a 0.22-µm filter. The solutions were diluted with the medium (1:20, v/v) to give the concentrations of 0.25, 0.5, 1.0, 1.5, 3.0 and 5.0 mmol/L in the culture medium.
Proliferation and viability index
After collection of cells with trypsin/EDTA, cell proliferation was measured using a hemocytometer according to the trypan blue method. Viability index was calculated as the ratio of the number of living cells to the total number of cells. Dead cells were detected by a blue staining counted in a microscope.
MTT (3-[4,5-dimethyl-2-thiazol-2-yl]-2,5-diphenyl-tetrazolium bromid) uptake
After the cells were exposed to iron, the medium was aspirated and 1 ml phenolred-free medium with 0.4 mg/ml MTT (Sigma, USA) was added to each well and incubated for a period of 4 h. The medium was aspirated and the blue formation was dissolved in 1 ml dimethyl sulphoxide (DMSO) (Sigma, USA). Finally, the absorption was measured at 552 nm using a spectrophotometer (Bio-Red-680, Bio-Red, USA) (Okada et al., 2000).
LDH (lactate dehydrogenase) release
LDH activity was measured in an aliquot of culture medium according to the method of Guzzie and Gad (Zhang, 2004). The LDH Testing Kit was bought from Jiancheng Biochemical Co., Ltd. (Nanjing, China). The change in absorbency was measured by the spectrophotometer at 540 nm.
Enzyme assays
The alkalinity phosphatase activity was measured by the alkalinity phosphatase Testing Kit, and the superoxide dismutase (SOD) activity by the SOD Testing Kit. Glutathione peroxidase (GPx) activity was determined spectrophotometrically. Oxidation of NADPH was continuously monitored using GSH-GSSG glutathione reductase model at 340 nm. All Testing Kits were bought from Nanjing Jiancheng Biochemical Co., Ltd. (Nanjing, China).
Intracellular iron concentration
The cells were treated with different levels of iron and washed twice with 2 ml D-Hank after being carried out from nutrient liquid. Then the cells in various wells were harvested using trypsin-EDTA solution, and the cell lysate was removed into the 5 ml epoxy epoxide tube (EP). The cells were then dried at 100 °C for 24 h. Prior to analysis, wet digestions were carried out with 5 ml concentrated HNO3 and approximately 5 drops of 30% (v/v) H2O2 on a heating plate at 80 °C (Zödl et al., 2003). The digestion residual was dissolved with 1.4 mol/L nitric acid solution and added with the deionized water to 5-ml flasks. Intracellular iron uptake was measured by inductively coupled argon plasma-mass spectrometer (ICP-MS) (Aglient 7500a, America). The average relative standard deviation (RSD) for sample analysis was below 5%.
RESULTS
Effects on cell viability
The MTT assay value, cell number and viability index decreased with the increase of iron treatment levels, except that the MTT value increased slightly when the iron supply level increased from 0.25 to 0.5 mmol/L (Table 1). And the MTT assay values of all Fe(II) treatments were lower than those of all Fe(III) treatments, especially at iron supply level >1.5 mmol/L (Table 1). These results indicate that Fe(II) is much more powerful to decrease cell viability and proliferation than Fe(III) (P<0.05).
Table 1.
Toxicological parameters in Caco-2 cells after iron exposure#
| Iron concentrations (mmol/L) | MTT assay value |
Proliferation (×105) |
Viability index |
|||
| FeSO4 | FeCl3 | FeSO4 | FeCl3 | FeSO4 | FeCl3 | |
| 0.25 | 1.13±0.11 | 1.10±0.17 | 2.98±0.07 | 3.06±0.13 | 96.2±0.5 | 97.8±1.0 |
| 0.5 | 1.27±0.07 | 1.34±0.09 | 3.06±0.06 | 3.04±0.16 | 95.2±0.3 | 95.2±0.5 |
| 1.0 | 1.23±0.05 | 1.27±0.18 | 2.87±0.06 | 3.09±0.09 | 94.3±0.2 | 94.1±1.0 |
| 1.5 | 0.94±0.04 | 1.23±0.14* | 2.46±0.12 | 2.83±0.21 | 90.3±2.3 | 93.8±3.8 |
| 3.0 | 0.88±0.08 | 1.19±0.07* | 2.13±0.10 | 2.67±0.10* | 82.1±0.5 | 90.1±2.2* |
| 5.0 | 0.72±0.02 | 1.14±0.08* | 2.00±0.06 | 2.68±0.14* | 77.1±2.5 | 88.2±2.5* |
Values represent mean±SEM, n=6;
P<0.05, Fe(III) vs Fe(II) at the same concentration according to the Duncan’s test
Effects on cell membrane stability
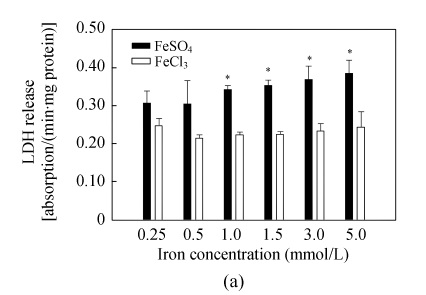
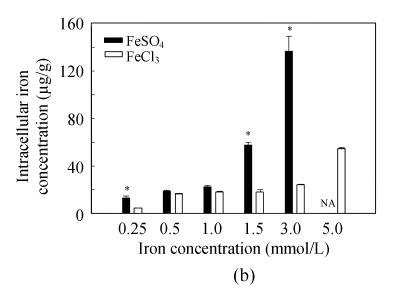
LDH release assay is an important method to assess cell membrane stability. The values of LDH release increased significantly with rising iron supply level, and Fe(II) had much stronger effects on membrane stability than Fe(III) at iron level >1.0 mmol/L (Fig.1a), implying that high level of Fe(II) is more harmful to cell membrane than that of Fe(III). The intracellular iron concentration of Caco-2 cells was found to increase with elevation of iron supply levels, and at the same iron level, more iron was accumulated in the cells with Fe(II) treatment than in those with Fe(III) treatment (Fig.1b). Significant differences were observed at iron supply levels of 0.25, 1.5 and 3.0 mmol/L.
Fig. 1.
(a) Membrane stability (LDH release) and (b) intracellular iron concentration (cells were dissolved in HNO3 after broken up by ultrasonic, and then intracellular iron uptake was measured by ICP-MS) of Caco-2 cells after incubation with 0.25, 0.5, 1.0, 1.5, 3.0 and 5.0 mmol/L of FeSO4 and FeCl3. Values represent mean±SEM, n=6; *Significant difference between the same concentration of Fe(II) and Fe(III) at P<0.05; NA: Not analyze
Effects on cell enzyme activities
At all treated levels, alkaline phosphate and GPx activities of cells treated with Fe(II) were considerably higher than those of cells treated with Fe(III), although there were no significant differences observed among different Fe(II) levels (Table 2). At low iron levels (<1.5 mmol/L), SOD activities of cells treated with Fe(II) were over 5 times higher than those of cells treated with Fe(III), whereas at iron supply levels >1.5 mmol/L, slight higher SOD activities were noted in the Fe(III) treated cells than in the Fe(II) treated ones. Interestingly, SOD activities of cells treated with Fe(III) increased gradually when iron level increased from 1.5 to 3 mmol/L.
Table 2.
Effects of increasing iron levels on enzyme activities (U/grot)#
| Iron concentrations (mmol/L) | Alkaline phosphatase activity |
SOD activity |
GPx activity |
|||
| FeSO4 | FeCl3 | FeSO4 | FeCl3 | FeSO4 | FeCl3 | |
| 0.25 | 89.0±37.8* | 22.2±1.5 | 14.9±7.3* | 2.4±1.5 | 193±110 | 149±172 |
| 0.5 | 66.9±6.4* | 15.2±7.2 | 62.8±13.6* | 5.5±0.7 | 382±116 | 192±105 |
| 1.0 | 87.2±15.2 | 49.9±21.2 | 48.7±15.2* | 4.0±5.1 | 991±255* | 108±25 |
| 1.5 | 96.9±24.3* | 40.3±15.4 | 57.4±12.2* | 8.9±3.2 | 492±264 | 469±183 |
| 3.0 | 77.4±10.3 | 55.5±12.8 | 61.3±19.3 | 83.6±13.8 | 855±146* | 520±15 |
| 5.0 | 85.5±12.2 | 111.2±39.2 | 83.2±10.0 | 101.6±33.7 | 1118±32* | 606±25 |
Values represent mean±SEM, n=6;
P<0.05, Fe(II) vs Fe(III) at the same concentration according to the Duncan’s test
DISCUSSION AND CONCLUSION
MTT assay is an important measuring of cellular toxicity in immunology, and was firstly used in cellular immunology by Mosmann (Zödl et al., 2005). MTT can reflect the sensitivity of live cells to extrinsic stimulation; therefore, it also has an important value to assess cell viability. Here, the result of MTT assay indicates that at high iron levels, Fe(II) has stronger effects on decreasing Caco-2 cell viability than Fe(III), which is similar to previous reports (Walgren et al., 2000). When pre-confluent and post-confluent Caco-2 cells were treated with 100~3000 µmol/L Fe(II), the cell viability index and proliferation were decreased with increasing iron concentration (Zager et al., 1993). The study of Vero monkey kidney cells treated with different forms of iron [the half maximal effective concentration (EC50): 5.5 and 22 mmol/L, respectively] also found that the proliferation of cells treated with Fe(II) decreased about fourfold as that of cells treated with Fe(III) (García-Alfonso et al., 1996).
Both Fe(III) and Fe(II) can initiate lipid peroxidation and increase of MDA, but they had different effects (Glahn et al., 1996). Here, we found that the damage of membrane stability was affected by Fe(II) more than by Fe(III). The reason might be that Fe(II) can perform the Fenton reaction directly, and produce a great number of free radicals, subsequently inducing lipid peroxidation (Glahn et al., 1996); however, the reaction speed caused by Fe(III) was much slower (Glahn et al., 1998; Chamnongpol et al., 2002). The study of the Fe(III) toxicity shows that Fe(III) bound to nitrilotriacetate (at molar ratios of 1:1) could decrease membrane stability of Caco-2 cells, whereas Fe(III)/citric had no obvious effects (Walgren et al., 2000). And Fe(III)/NTA caused more oxidative damage than Fe(III) salts (Cai et al., 1998). Fe(III)/NTA was even more effective in inducing lipid peroxidation in rabbit small intestinal microvillus membrane vesicles than Fe(II) ascorbate (Walgren et al., 2000), which might be due to action of NTA. However, NTA alone did not decrease viability (via MTT assay) in E9 and MV5 cells (Zödl et al., 2005). Those results indicate that toxicological effects of Fe(III) to cells may be related to other specific participants. Lipid peroxidation caused by both Fe(III) and Fe(II) was complex (Zhao, 1998).
A higher intracellular iron concentration in Caco-2 cells was found when incubated with Fe(II) than that with Fe(III), which was one of major causes for membrane damage. In this work, we demonstrated that the intracellular iron concentration in Caco-2 cells incubated with Fe(II) was higher than that with Fe(III) at all iron treated levels (Fig.1b). Intracellular free iron can initiate oxidative stress leading to cellular injury (Mccord, 1996). The results of enzyme activity assay also indicate that with increasing iron level, both iron forms could elevate activities of antioxidative enzymes, e.g., SOD and GPx. Nevertheless, other studies suggested that the SOD activities of Caco-2 cells were not affected by iron supplementation (Zödl et al., 2005). It was reported that oral iron supplementation decreased the activity of MnSOD in rat colon mucosa (Kuratko, 1998). In the intestinal epithelial cell line IEC-6 which had been exposed to iron (FeSO4) up to 2 mmol/L for 48 h, an increase in MnSOD activity was observed (Kuratko, 1999). The higher activities of SOD and GPx in Fe(II) treated cells might be resulted from the expression of Fe(II) induced oxidative stress genes (Zödl et al., 2003). Compared to Fe(II), the ability of Fe(III) induced oxidative stress was relatively low, especially at low concentrations. Greater activities of SOD and GPx at higher iron levels suggest that the oxidative stress in cells was increased due to the increasing iron levels (Baker and Baker, 1992). The cells could consume antioxidative enzymes to resist oxidative stress at low iron levels. Different from Fe(II), Fe(III) can damage cells by producing free superoxide negative ion (Chamnongpol et al., 2002). In this study, we found that the Fe(III) treated cells used intrinsic SOD to rid free superoxide negative ion as iron concentration increased from low levels to 1.5 mmol/L, which might cause the increasing catalase level with iron concentration in the medium decreased (Okada et al., 2000), thereby depleting GPx. In high iron levels, both Fe(II) and Fe(III) may induce the expression of antioxidative genes. Although it seems that the high concentration of iron (>1.5 mmol/L) rarely occurs in vivo, in some situations, it is still impossible to avoid, for instance, in high iron contamination mining area or adding too much iron to the fortification food. Furthermore, in the animal feed, it is true that high concentration as much as 200×10−6 iron is always supplemented, especially in China.
In conclusion, we found that iron form and concentration have different effects on iron uptake and toxicity to Caco-2 cells, and that the toxicological effects of Fe(II) to Caco-2 cells are higher than those of Fe(III). Fe(II) can alter membrane stability and penetrability, resulting in higher LDH release values at the same treat level as Fe(III). At low iron levels, cells can use intrinsic antioxidative enzymes to resist iron oxidative damage. At high levels, iron can induce expression of cell antioxidative genes. However, in this work, the Caco-2 monolayer was exposed to “pure” solution of iron salts, which may not be comparable to the real intestinal environment. Therefore, further studies in conditions closer to a physiological environment are needed.
Footnotes
Project supported by the International Cooperative Project from the Ministry of Science and Technology of China (No. 2006DFA31030), the Bureau of Science and Technology of Zhejiang Province (No. 2006C32019) and HarvestPlus-China (No. 8022), and the Program for Changjiang Scholars and Innovative Research Team in University of China (No. IRT0536)
References
- 1.Baker SS, Baker RDJr. Antioxidant enzymes in the differentiated Caco-2 cell line. In Vitro Cellular & Developmental Biology-Animal. 1992;28(9-10):643–647. doi: 10.1007/BF02631040. [DOI] [PubMed] [Google Scholar]
- 2.Cai L, Tsiapalis G, Cherian MG. Protective role of zinc-metallothionein on DNA damage in vitro by ferric nitrilotriacetate (Fe-NTA) and ferric salts. Chemico-Biological Interactions. 1998;115(2):141–151. doi: 10.1016/S0009-2797(98)00069-6. [DOI] [PubMed] [Google Scholar]
- 3.Chamnongpol S, Dodson W, Cromie MJ, Harris ZL, Groisman EA. Fe(III)-mediated cellular toxicity. Molecular Microbiology. 2002;45(3):711–719. doi: 10.1046/j.1365-2958.2002.03041.x. [DOI] [PubMed] [Google Scholar]
- 4.Gan LL, Dhiren RT. Applications of the Caco-2 model in the design and development of orally active drugs: elucidation of biochemical and physical barriers posed by the intestinal epithelium. Advanced Drug Delivery Reviews. 1997;23(1-3):77–81. doi: 10.1016/S0169-409X(96)00427-9. [DOI] [Google Scholar]
- 5.García-Alfonso C, Lopez-barea J, Sanz P, Repetto G, Repetto M. Changes in antioxidative activities induced by Fe(II) and Fe(III) in cultured vero cells. Archives of Environmental Contamination and Toxicology. 1996;30(4):431–436. doi: 10.1007/BF00213392. [DOI] [PubMed] [Google Scholar]
- 6.Glahn RP, Wien EM, van Campen DR, Miller DD. Caco-2 cell iron uptake from meat and casein digests parallels in vivo studies: use of a novel in vitro method for rapid estimation of iron bioavailability. Journal of Nutrition. 1996;126(1):332–339. doi: 10.1093/jn/126.1.332. [DOI] [PubMed] [Google Scholar]
- 7.Glahn RP, Lee OA, Yeung A, Goldman MI, Miller DD. Caco-2 cell ferritin formation predicts nonradiolabeled food iron availability in an in vitro digestion/Caco-2 cell culture model. Journal of Nutrition. 1998;128(9):257–264. doi: 10.1093/jn/128.9.1555. [DOI] [PubMed] [Google Scholar]
- 8.Glahn RP, Cheng ZQ, Welch MR. Comparison of iron bioavailability from 15 rice genotypes: studies using an in vitro digestion/Caco-2 cell culture model. Journal of Agricultural and Food Chemistry. 2002;50(12):3586–3591. doi: 10.1021/jf0116496. [DOI] [PubMed] [Google Scholar]
- 9.Kloots W, Op den Kamp D, Abrahamse L. In vitro iron availability from iron-fortified whole-grain wheat flour. Journal of Agricultural and Food Chemistry. 2004;52(26):8132–8136. doi: 10.1021/jf040010+. [DOI] [PubMed] [Google Scholar]
- 10.Kuratko CN. Decrease of manganese superoxide dismutase activity in rats fed high levels of iron during colon carcinogenesis. Food and Chemical Toxicology. 1998;36(9-10):819–824. doi: 10.1016/S0278-6915(98)00029-5. [DOI] [PubMed] [Google Scholar]
- 11.Kuratko CN. Iron increases manganese superoxide dismutase activity in intestinal epithelial cells. Toxicology Letters. 1999;104(1-2):151–158. doi: 10.1016/S0378-4274(98)00359-2. [DOI] [PubMed] [Google Scholar]
- 12.Mccord JM. Effects of positive iron status at a cellular level. Nutrition Reviews. 1996;54(3):85–88. doi: 10.1111/j.1753-4887.1996.tb03876.x. [DOI] [PubMed] [Google Scholar]
- 13.Núñez MT, Tapia V, Toyokuni S, Okada S. Iron-induced oxidative damage in colon carcinoma (Caco-2) cells. Free Radical Research. 2001;34(1):57–68. doi: 10.1080/10715760100300061. [DOI] [PubMed] [Google Scholar]
- 14.Okada T, Narai A, Matsunaga S. Assessment of the marine toxins by monitoring the integrity of human intestinal Caco-2 cell monolayers. Toxicology in Vitro. 2000;14(3):219–226. doi: 10.1016/S0887-2333(00)00014-X. [DOI] [PubMed] [Google Scholar]
- 15.Peng ZF, Lan LX, Zhao F, Li J, Tan Q, Yin HW, Zeng HH. A novel thioredoxin inhibitor inhibits cell growth and induces apoptosis in HL-60 and K562 cells. Journal of Zhejiang University SCIENCE B. 2008;9(1):16–21. doi: 10.1631/jzus.B071605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi A, Poverini R, Lullo GD, Modesti A, Modica A, Scarino ML. Heavy metal toxicity following apical and basolateral exposure in the human intestinal cell line Caco-2. Toxicology in Vitro. 1996;10(1):27–36. doi: 10.1016/0887-2333(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 17.Srigiridhar K, Nair KM, Subramanian R, Singotamu L. Oral repletion of iron induces free radical mediated alterations in the gastrointestinal tract of rat. Molecular and Cellular Biochemistry. 2001;219(1-2):91–98. doi: 10.1023/A:1011023111048. [DOI] [PubMed] [Google Scholar]
- 18.Walgren RA, Karnaky KJ, Lindenmayer GE. Efflux of dietary flavonoid quercetin 4′-β-glucoside across human intestinal Caco-2 cell monolayers by apical multidrug resistance-associated protein-2. Journal of Pharmacology and Experimental Therapeutics. 2000;294(3):830–835. [PubMed] [Google Scholar]
- 19.Watzl B, Abrahamse SL, Lishaut STV, Neudecker C, Hänsch GM, Rechkemmer G, Pool-Zobel BL. Enhancement of ovalbumin-induced antibody production and mucosal mast cell response by mercury. Food and Chemical Toxicology. 1999;37(6):627–637. doi: 10.1016/S0278-6915(99)00035-6. [DOI] [PubMed] [Google Scholar]
- 20.Xing CH, Zhu MH, Cai MZ, Liu P, Xu GD, Wu SH. Developmental characteristics and response to iron toxicity of root border cells in rice seeding. Journal of Zhejiang University SCIENCE B. 2008;9(3):261–264. doi: 10.1631/jzus.B0710627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zager RA, Schimpf BA, Bredl CR, Gmur DJ. Inorganic iron effects on in vitro hypoxic proximal renal tubular cell injury. Journal of Clinical Inestigation. 1993;91(2):702–708. doi: 10.1172/JCI116251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang ZR. Cultural Cytology and Cell Cultural Technology. Shanghai: Shanghai Scientific and Technical Publishers; 2004. pp. 427–432. (in Chinese) [Google Scholar]
- 23.Zhao BL. Free Oxygenic Radicals and Savageness Antioxidant. Beijing: Publishing Company of Science; 1998. pp. 81–85. (in Chinese) [Google Scholar]
- 24.Zödl B, Zeiner M, Sargazi M, Roberts NB, Marktl W, Steffan I, Cem Ekmekcioglu C. Toxic and biochemical effects of zinc in Caco-2 cells. Journal of Inorganic Biochemistry. 2003;97(4):324–330. doi: 10.1016/S0162-0134(03)00312-X. [DOI] [PubMed] [Google Scholar]
- 25.Zödl B, Sargazi M, Zeiner M, Roberts NB, Steffan I, Marktl W, Ekmekcioglu C. Toxicological effects of iron on intestinal cells. Cell Biochemistry and Function. 2004;22(3):143–148. doi: 10.1002/cbf.1065. [DOI] [PubMed] [Google Scholar]
- 26.Zödl B, Zeiner M, Paukovits P, Steffan I, Marktl W, Ekmekcioglu C. Iron uptake and toxicity in Caco-2 cells. Microchemical Journal. 2005;79(1-2):393–397. doi: 10.1016/j.microc.2004.10.019. [DOI] [Google Scholar]