Abstract
Transcriptional induction of many stress-response genes is dependent on stress-induced nuclear accumulation of stress-activated protein kinases (SAPKs). In the fission yeast Schizosaccharomyces pombe, nuclear accumulation of the SAPK Spc1 (also known as StyI) requires activating phosphorylation catalyzed by the SAPK kinase Wis1; however, it is unknown whether the localization of Spc1 is regulated by nuclear transport factors. Herein are reported studies that show that Spc1 localization is regulated by active transport mechanisms during osmotic stress. Nuclear import of Spc1 requires Pim1, a homologue of the guanine nucleotide exchange factor RCC1 that is essential for nucleocytoplasmic shuttling of proteins. Nuclear export of Spc1 is regulated by the export factor Crm1. An Spc1–Crm1 complex forms as Spc1 is exported from the nucleus. Wis1 and the tyrosine phosphatases Pyp1 and Pyp2 that inactivate Spc1 are excluded from the nucleus by a Crm1-independent mechanism; hence the nuclear import of Spc1 leads to transient isolation from its regulatory proteins. Thus, active nucleocytoplasmic shuttling is required for both the function and regulation of Spc1 during the osmotic shock response.
INTRODUCTION
Stress-activated protein kinases (SAPKs) regulate the transcriptional response to osmotic shock and other forms of stress in all eukaryotic species, including yeasts and humans (Karin et al., 1997; Ip and Davis, 1998; Wilkinson and Millar, 1998). During activation by SAPK kinases (SAPKs), SAPKs translocate into the nucleus where they phosphorylate transcription factors (Gaits et al., 1998). Thus, the physical movement of SAPKs between the nucleus and cytoplasm is a defining feature of stress signal transduction, and yet relatively little is known about how it is accomplished or regulated. Uncovering the mechanisms that control the localization of SAPKs and their regulators is therefore essential for understanding the transcriptional response to stress.
Spc1 (also known as StyI), a Schizosaccharomyces pombe homologue of the SAPKs human p38 and budding yeast Hog1p, responds to osmotic shock and many other types of stress (Millar et al. 1995; Shiozaki and Russell, 1995, 1996; Degols and Russell, 1997). Spc1 is activated by the SAPKK Wis1, which phosphorylates Spc1 at threonine-171 and tyrosine-173. These sites of phosphorylation are conserved among all SAPKs and mitogen-activated protein kinases (MAPKs). After phosphorylation by Wis1, Spc1 translocates to the nucleus where it phosphorylates Atf1, a transcription factor that is structurally similar to mammalian ATF-2, a substrate of p38 (Shiozaki and Russell, 1996; Wilkinson et al., 1996).
Fission yeast has recently emerged as an important model system for studying regulated localization of SAPKs (Gaits et al., 1998). These studies established that nuclear import of Spc1 requires activating phosphorylation catalyzed by Wis1. Interestingly, activating phosphorylation appears to play a structural role in the import of Spc1. The nuclear import defect of Spc1 mutant protein that cannot be phosphorylated on threonine-171 or tyrosine-173 is not rescued by coexpression of active Spc1 (Gaits et al., 1998). Experiments with fission yeast also showed that nuclear accumulation of Spc1 depends on nuclear localization of Atf1, indicating that Spc1 undergoes a sustained interaction with Atf1 in the nucleus (Gaits et al., 1998).
Many fundamental questions remain to be answered about the regulated localization of SAPKs. Paramount among these questions is whether SAPKs are transported in and out of the nucleus by active processes (Nigg, 1997; Ullman et al., 1997; Melchior and Gerace, 1998; Weis, 1998). SAPKs are relatively small proteins (∼40 kDa); thus in theory they might passively diffuse into the nucleus without active transport. This idea is consistent with the observation that SAPKs and MAPKs, including Spc1, apparently lack typical NLS sequences; however, a recent study suggested that nuclear import of a mammalian MAPK is dependent on dimerization induced by activating phosphorylation (Khokhlatchev et al., 1998). A MAPK dimer is above the size exclusion limit for passive diffusion between the nucleus and cytoplasm. This result suggests that nuclear import of a MAPK dimer must depend on an active process. Likewise, it appears that localization of MAPKs or SAPKs might also be regulated by an active nuclear export mechanism.
Precise regulation of SAPKs is essential for the appropriate response to stress and for long-term survival. This notion is evident from the fact that constitutive activation of Spc1 is lethal in fission yeast (Millar et al., 1995; Shiozaki and Russell, 1995; Shiozaki and Russell, 1996). Moreover, the time course of activation and inactivation of Spc1 in response to osmotic shock can be quite rapid: ∼20 min in the case of exposure to 0.6 M KCl. Thus, it is important that Spc1 not only be rapidly activated in response to stress but also rapidly inactivated as cells adapt to adverse environmental conditions. Attenuation of the osmotic shock response is largely regulated by Pyp1 and Pyp2, two tyrosine phosphatases that dephosphorylate Spc1 on tyrosine-173 (Millar et al., 1995; Shiozaki and Russell, 1995). Interestingly, expression of pyp2+ mRNA is regulated by Spc1 and Atf1, thus forming a negative feedback loop (Degols et al., 1996; Shiozaki and Russell, 1996; Wilkinson et al., 1996).
In this report, we describe studies that explore mechanisms controlling the localization of Spc1 and its regulators Wis1, Pyp1, and Pyp2. We establish that nucleocytoplasmic translocation of Spc1 requires the nuclear import and export machinery. Moreover, Spc1 interacts with a component of the nuclear export machinery. We also show that proper regulation of Spc1 is dependent on nuclear export processes, because Wis1, Pyp1, and Pyp2 are cytoplasmic proteins. Thus, our studies demonstrate that the function and regulation of an SAPK is strongly influenced by active nuclear import and export mechanisms. Nucleocytoplasmic shuttling mechanisms are conserved between yeast and mammals; thus the principles of SAPK nucleocytoplasmic transport discerned in fission yeast likely apply to human SAPKs.
MATERIALS AND METHODS
Media, Strains, and General Techniques
S. pombe strains used in this study are listed in Table 1. They are derivatives of 972h− and 975h+ (Mitchison, 1970). Growth media and basic genetic and biochemical techniques for fission yeast have been described (Alfa et al., 1993). Yeast extract medium YES and synthetic minimal medium EMM2 were used in growing S. pombe cells.
Table 1.
S. pombe strains used in this study
| Strains | Genotype | Source or reference |
|---|---|---|
| PR109 | h− | Lab stock |
| KS1366 | h− spc1::ura4+ | Shiozaki and Russell, 1995 |
| GD1892 | h− wis1-12myc (ura4+) | Gaits et al., 1998 |
| GD1942 | h− spc1-12myc(ura4+) | Gaits et al., 1998 |
| GD1953 | h− pyp1-12myc(ura4+) | This study |
| GD1955 | h− pyp2-12myc(ura4+) | This study |
| FG2153 | h+ spc1AY-12myc(ura4+) | Gaits et al., 1998 |
| FG2154 | h+ spc1TF-12myc(ura4+) | Gaits et al., 1998 |
| FG2155 | h+ spc1AF-12myc(ura4+) | Gaits et al., 1998 |
| FG2235 | h− pim1-d1 spc1-12myc(ura4+) | This study |
| FG2260 | h− crm1-809 pyp1-12myc(ura4+) | This study |
| FG2262 | h− crm1-809 pyp2-12myc(ura4+) | This study |
| FG2264 | h− crm1-809 spc1-12myc(ura4+) | This study |
| FG2265 | h− crm1-809 wis1-12myc(ura4+) | This study |
| FG2159 | h+ spc1-12myc(ura4+) pyp1::leu2+ | This study |
| FG2160 | h− spc1-12myc(ura4+) pyp2::leu2+ | This study |
All strains are leu1-32 ura4-D18, except FG2264 and FG2265, which are only leu1-32.
Plasmid Construction
Sequences encoding the different proteins of the Spc1 pathway were amplified by PCR and cloned in the pRIP-12myc vector as BamHI–KpnI fragments as described previously (Gaits et al., 1998). The resultant plasmids were integrated into PR109 by homologous recombination. The construction of the Spc1 mutants was described previously (Gaits et al., 1998). The pREP41-spc1+-myc, pREP41-spc1-AY-myc, pREP41-spc1-TF-myc, and pREP41-spc1-AF-myc plasmids were constructed by replacing the BglII–SstI cassette of a pREP41-spc1+-HAhis vector by the equivalent cassette from the pRIP-spc1+-12myc. These plasmids were linearized at the NdeI site to introduce an NLS from the SV40 large T antigen at the N terminus of Spc1. The NLS was introduced as a DNA linker derived from the amino acid sequence PKKKRK. The crm1+ sequence was introduced after PCR amplification in a pREP41-HAhis vector or the pGEX-KG as a NdeI–NotI fragment.
Stress Treatment of Cells
Cells were grown to early logarithmic phase (OD600 = ∼0.5) in YES or EMM2 medium at 30°C as described (Alfa et al., 1993). The high-osmolarity stress was achieved by adding one-third of the volume of prewarmed YES medium containing 2.4 M KCl to the culture to obtain a final KCl concentration of 0.6 M. For the immunoblotting analysis, cells were harvested by filtration and immediately frozen in liquid nitrogen.
Immunoblotting
Cells were lysed in 0.2 ml of lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 5 mM EDTA, 10% glycerol, 50 mM NaF, 1 mM Na3VO4, 1 μg/ml each of leupeptin, aprotinin, and pepstatin, 1 mM PMSF). Total proteins were resolved by SDS-PAGE. The phosphorylation status of Spc1 WT, Spc1myc, or the Spc1AY, TF, and AF mutants was evaluated by immunoblotting with the anti-phospho p38 (tyr 182) MAPK antibody (New England Biolabs, Beverly, MA). The amount of Spc1myc loaded was evaluated with the anti-myc 9E10 (Covance, Richmond, CA) antibody. GST-Crm1 was detected with a rabbit polyclonal antibody (generous gift of L. Hengst, Scripps Research Institute, La Jolla, CA), and the Crm1-HAhis was detected with the anti-HA 12CA5 antibody (generous gift of I. Wilson, Scripps Research Institute). Immunoreactive bands were revealed with horseradish peroxidase-conjugated secondary antibodies and the ECL Western blotting detection system (Pierce, Rockford, IL).
Affinity Purification
Cells expressing Crm1 tagged with two hemagglutinin (HA) epitopes and six histidine residues were harvested and lysed in buffer L. This buffer contains 50 mM Tris, pH 8.0, 150 mM NaCl, 5 mM EGTA, 10 mM imidazole, 0.2% NP40, 10% glycerol, 50 mM NaF, 1 mM Na3VO4, 1 μg/ml each of leupeptin, aprotinin, and pepstatin, and 1 mM PMSF. After centrifugation, the supernatant was incubated with Ni2+-nitrilotriacetic acid (NTA)-agarose beads and analyzed by SDS-PAGE, and the purified proteins were detected by immunoblotting. GST-Crm1 produced in bacteria was purified on glutathione (GSH)–Sepharose beads after lysis in buffer A. This buffer contains PBS, pH 8.0, 0.25% Sarkosyl, 1 μg/ml each of leupeptin, aprotinin, and pepstatin, and 1 mM PMSF. The beads were then washed five times in buffer A and three times in buffer L. Total extracts prepared in buffer L were added to the GST-Crm1-GSH–Sepharose beads. The purified complexes were analyzed by SDS-PAGE followed by immunoblotting.
Indirect Immunofluorescence Microscopy
Cells were harvested and fixed as described (Gaits et al., 1998). The myc-tagged proteins were stained with the anti-myc 9E10 antibody (BAbCo) and revealed with a CY3-conjugated anti-mouse immunoglobulin G. Cells were examined using a Nikon Eclipse E800 microscope (Nikon, Melville, NY).
RESULTS
Myc-tagged Forms of Spc1, Wis1, Pyp1, and Pyp2 Are Functional
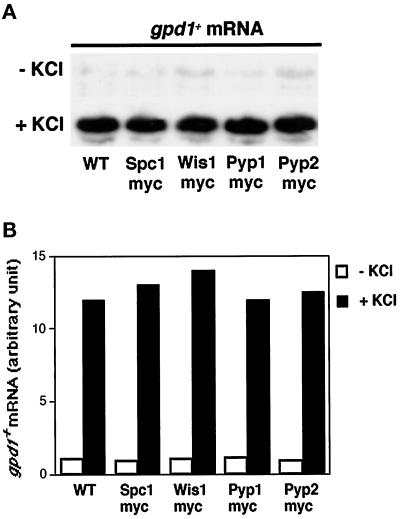
Several strains were constructed to study the subcellular distribution and nucleocytoplasmic transport of proteins involved in the regulation of the Wis1-Spc1 kinase cascade (Table 1). The chromosomal copies of the genes encoding the kinases Wis1 and Spc1 and the phosphatases Pyp1 and Pyp2 were replaced by copies of each gene encoding a protein that contains 12 tandem copies of the myc epitope at the C terminus. This allowed the proteins to be detected with antibodies to the myc epitope (see MATERIALS AND METHODS). When cells are challenged with stresses such as osmotic stress, heat shock, UV treatment, or nutrient starvation, Spc1 directs the expression of a subset of stress-response genes by regulating the transcription factor Atf1 (Shiozaki and Russell, 1996; Wilkinson et al., 1996). One of those genes, gpd1+, encodes glycerol-3-phosphate dehydrogenase (Pidoux et al., 1990). To confirm that the tagged versions of the proteins functioned normally, we evaluated the osmotic stress response of the various strains. The strains expressing Spc-12myc, Wis1–12myc, Pyp 1–12myc, or Pyp2–12myc were all able to induce gpd1+ transcription in a manner that was indistinguishable from wild type (Figure 1). These strains were no more sensitive to killing by osmotic stress than wild-type (our unpublished data). Moreover, Δpyp1 pyp2–12myc and pyp1–12myc Δpyp2 strains were viable (our unpublished data), whereas Δpyp1 Δpyp2 strains are inviable (Millar et al., 1992; Ottilie et al., 1992) Thus, the myc-tagged versions of Spc1, Wis1, Pyp1, and Pyp2 appeared to be fully functional.
Figure 1.
Strains bearing chromosomal replacements of the stress-response genes by myc-tagged copies respond as wild type. (A) PR109 (wild type), GD1892 (wis1–12myc), GD1942 (spc1–12myc), GD1953 (pyp1–12myc), and GD1955 (pyp2–12myc) were challenged with 0.6 M KCl for 15 min and harvested, and total RNA was extracted for Northern analysis of gpd1+ mRNA. (B) Quantification of gpd1+ expression is provided for each strain. Signals were normalized relative to the leu1+ control.
Nuclear Translocation of Spc1 Requires Functional Pim1
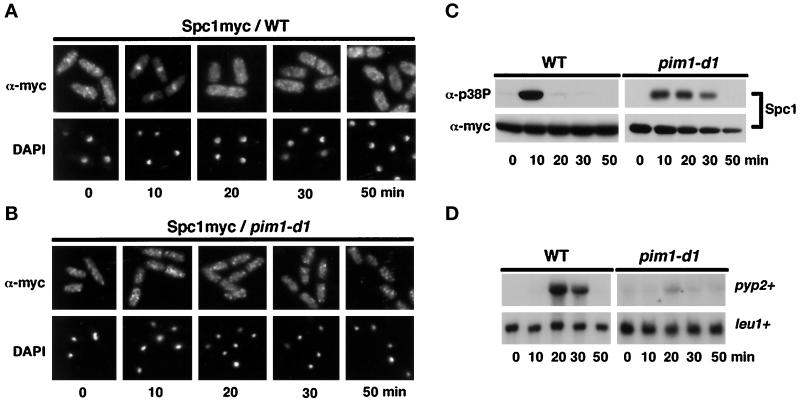
Active nucleocytoplasmic trafficking requires RCC1, a guanine nucleotide exchange factor for Ran, a small GTPase protein (Nigg, 1997; Melchior and Gerace, 1998). RCC1 is known as Pim1 in fission yeast (Matsumoto and Beach, 1991). Spc1 localization was monitored in a pim1 mutant to determine whether the nuclear import machinery is required for the nucleocytoplasmic relocalization of Spc1 in response to osmotic stress. Pim1 is essential for viability; therefore, this experiment used the temperature-sensitive pim1-d1 allele (Sazer and Nurse, 1994; Demeter et al., 1995). On osmotic shock (0.6 M KCl) in wild-type cells, Spc1 became concentrated in the nucleus (Figure 2A). Nuclear accumulation of Spc1 was coincident with phosphorylation on tyrosine-173 (Figure 2C). In pim1-d1 cells incubated at the restrictive temperature of 36°C, Spc1 was also rapidly phosphorylated on tyrosine-173 in response to osmotic shock (Figure 2C); however, no nuclear accumulation of Spc1 was observed in the pim1-d1 cells (Figure 2B). In wild-type cells, dephosphorylation of Spc1 on tyrosine-173 was completed within 20 min of osmotic shock, whereas in the pim1-d1 cells, tyrosine-173 dephosphorylation was sustained for at least 30 min (Figure 2C). Activation of the Spc1 pathway induces transcription of pyp2+. Northern analysis confirmed that Spc1-dependent transcriptional induction of pyp2+ mRNA was defective in pim1-d1 cells (Figure 2D). These findings showed that stress-induced nuclear accumulation of Spc1 is dependent on an active process catalyzed by Pim1.
Figure 2.
Nuclear accumulation of Spc1 requires Pim1 function. (A) GD1942 (spc1–12myc) cells were exposed to osmotic stress (0.6 M KCl in YES medium). Immunolocalization with antibodies to myc revealed that Spc1 became concentrated in the nucleus at the 10-min time point. Nuclei were visualized with the DNA stain DAPI. (B) A pim1-d1 spc1–12myc strain was incubated at the restrictive temperature of 36°C for 3 h and stressed with 0.6 M KCl. Spc1 failed to accumulate in the nucleus in response to stress. (C) Phosphorylation of Spc1 on tyrosine-173 was detected by immunoblot analysis using antibodies to the phosphorylated tyrosine-182 epitope of human p38 (α-p38P). Osmotic stress caused tyrosine phosphorylation of Spc1 in wild-type (WT) and pim1-d1 cells. Total Spc1–12myc protein was detected with antibodies to myc. (D) Exponentially growing wild-type cells (PR109) and pim1-d1 mutant were stressed with KCl and harvested at the indicated time, and total RNA was extracted for Northern analysis of pyp2+ or leu1+ mRNA (loading control).
The Negative Regulators of Spc1 Are Cytoplasmic
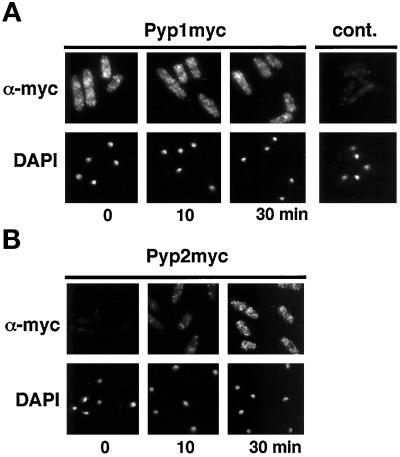
Tyrosine-173 dephosphorylation of Spc1 can occur within a few minutes after Spc1 is imported into the nucleus (Figure 2). Pyp1 and Pyp2, two tyrosine-specific phosphatases, catalyze this reaction (Millar et al., 1995; Shiozaki and Russell, 1995). Dephosphorylation of Spc1 on tyrosine-173 is essential for viability, because the lethality of a pyp1 pyp2 double mutant is completely suppressed by spc1 or wis1 mutations (Millar et al., 1995; Shiozaki and Russell, 1995). These findings underscore the importance of the negative regulation of Spc1; thus, investigations were performed to determine which nucleocytoplasmic translocation events were required to attenuate the stress signal. One possibility was that Pyp1 or Pyp2 must translocate into the nucleus to dephosphorylate Spc1. In this model, dephosphorylation of Spc1 by Pyp1 or Pyp2 might be required to export Spc1 from the nucleus. An alternative model proposed that Spc1 must be exported from the nucleus to be dephosphorylated by Pyp1 or Pyp2 in the cytoplasm. Determining the localization of Pyp1 and Pyp2 in strains that expressed 12 myc-tagged forms of these proteins, under the regulation of their own promoters, after chromosomal replacement of pyp1+ or pyp2+, tested these hypotheses. Immunofluorescence microscopy revealed that Pyp1 was localized in the cytoplasm (Figure 3A). Nuclear exclusion of Pyp1 was unaffected by osmotic stress (Figure 3A). Pyp2 was only very faintly detected in unstressed cells (Figure 3B), because expression of pyp2 mRNA is very low in the absence of stress (Shiozaki and Russell, 1996; Wilkinson et al., 1996). After osmotic stress, Pyp2 was readily detected as a cytoplasmic protein that appeared to be excluded from the nucleus (Figure 3B).
Figure 3.
Pyp1 and Pyp2 are cytoplasmic proteins. Cells that had the pyp1–12myc or pyp2–12myc alleles were exposed to osmotic stress (0.6 M KCl in YES medium) for 0, 10, or 30 min. Pyp1 and Pyp2 were detected with antibodies to myc. Nuclei were visualized with DAPI. (A) Pyp1 appeared to be excluded from the nucleus. Pyp1 abundance appeared unchanged in response to stress. (B) The abundance of Pyp2 increased in response to stress. Pyp2 also appeared to be excluded from the nucleus.
Pyp1 and Pyp2 Phosphatase Activity Impacts Spc1 Localization
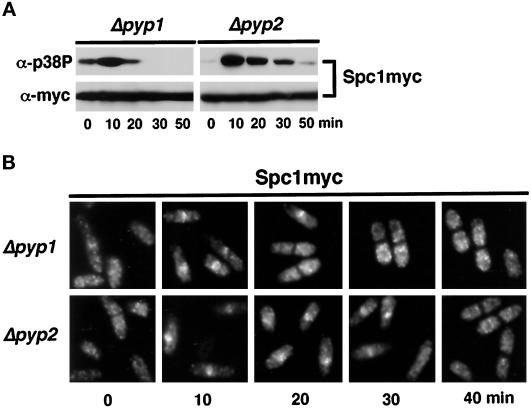
These data strongly indicated that Pyp1 and Pyp2 were excluded from the nucleus, suggesting that activated Spc1 must be exported from the nucleus to be dephosphorylated on tyrosine-173. This hypothesis was explored further through examination of Spc1 localization and tyrosine phosphorylation in pyp1 or pyp2 mutants. In pyp1 mutants, Spc1 has a higher basal level of tyrosine phosphorylation, which is further increased in response to osmotic stress (Figure 4A). Spc1 was largely dephosphorylated and excluded from the nucleus at the 20-min time point, although some cells (∼25%) exhibited a nuclear stain for Spc1. In contrast, Spc1 was highly phosphorylated and abundant in the nucleus of pyp2 cells in the 20-min sample (Figure 4). Substantial Spc1 tyrosine phosphorylation and nuclear localization was also detected in the 30-min sample, whereas by 40 min Spc1 was largely dephosphorylated and cytoplasmic (Figure 4). Furthermore, Spc1 nuclear accumulation was abolished in cells that overexpressed either of the phosphatases (our unpublished observations). These findings indicate that timely dephosphorylation and relocalization of Spc1 are dependent on Pyp1 and Pyp2. Pyp2 appears to have a greater role, although it should be noted that phenotype of pyp1 mutants is partly ameliorated by the higher level of basal expression of Pyp2 in these cells. These studies further suggest that in wild-type cells, Spc1 is rapidly dephosphorylated on translocation from the nucleus to the cytoplasm. Thus, the sustained nuclear localization of Spc1 in pyp2 mutants is presumably due to rapid relocalization of Spc1 back into the nucleus.
Figure 4.
Deletion of pyp1+ or pyp2+ affects Spc1 localization. (A) FG2159 (Δpyp1 spc1–12myc) and FG2160 (Δpyp2 spc1–12myc) cells were stressed with 0.6 M KCl in YES medium. Aliquots were harvested for immunoblotting with p38P and myc antibodies. (B) Immunofluorescence analysis of Spc1 cellular distribution in Δpyp1 and Δpyp2 cells.
Spc1 Nucleocytoplasmic Transport Is Dependent on the Nuclear Export Machinery
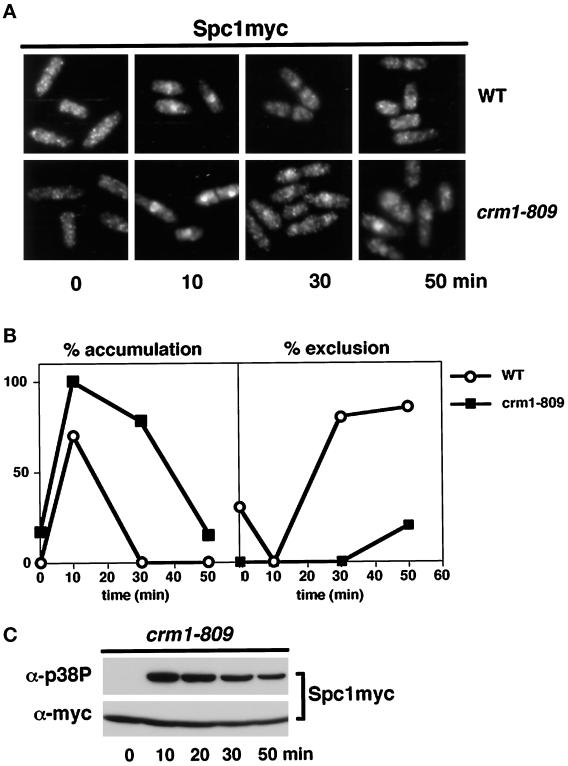
These findings suggested that active translocation of Spc1 from the nucleus to the cytoplasm is required to inactivate Spc1. This model made two important predictions: 1) nuclear export of Spc1 should be an active process dependent on nuclear export factors, and 2) impairment of Spc1 nuclear export should reduce the rate at which Spc1 is dephosphorylated. Fission yeast Crm1 is a nuclear receptor required to export proteins bearing the NES sequence (Fornerod et al., 1997; Fukuda et al., 1997b; Ossareh-Nazari et al., 1997; Stade et al., 1997). Accordingly, the localization of Spc1 was examined in a crm1 mutant. Crm1 is essential for viability; therefore, this experiment was carried out with the cold-sensitive crm1–809 allele that exhibits a partial nuclear export defect at 30°C, a temperature at which crm1–809 cells are viable (Yanagida, 1989; Fukuda et al., 1997a). Immunolocalization studies revealed that nuclear accumulation of Spc1 induced by osmotic stress was substantially increased and sustained in crm1–809 cells relative to wild type (Figure 5, A and B). At the 10-min time point, the Spc1 nuclear signal in crm1–809 cells was consistently more intense than observed in wild-type cells. At the 30-min time point after the initial exposure to stress, Spc1 was excluded from the nucleus of wild type, whereas in the crm1–809 cells the intensity of the nuclear Spc1 signal was greater than the cytoplasmic signal (Figure 5A). As predicted by the hypothesis, tyrosine phosphorylation of Spc1 was sustained for a greater period in the crm1–809 cells relative to wild-type cells (compare Figures 5C and 2C).
Figure 5.
Spc1 nuclear localization and tyrosine phosphorylation are enhanced in crm1–809 cells. (A) A culture of spc1–12myc (GD1942) or crm1–809 spc1–12myc (FG2264) cells grown at 30°C was exposed to osmotic stress (0.6 M KCl in YES medium) for the indicated time periods. The cells were fixed in cold methanol and stained for Spc1 localization. (B) Quantification of the nuclear accumulation or exclusion of Spc1 in wild-type (○) and crm1–809 cells (▪). Data represent the average of four experiments. (C) Samples were also taken to evaluate Spc1 phosphorylation.
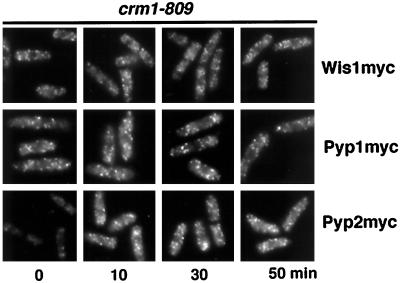
Our findings suggested that Crm1 mediates nuclear export of Spc1, delivering Spc1 to Pyp1 and Pyp2 phosphatases located in the cytoplasm; however, a process in which Wis1 shuttled between the nucleus and cytoplasm by a Crm1-dependent process might also explain these findings. A failure of Wis1 nuclear export in a crm1–809 mutant might allow Wis1 to phosphorylate Spc1 in the nucleus, leading to sustained tyrosine phosphorylation and nuclear localization of Spc1. Alternatively, Wis1 might shuttle into the nucleus, bind to Spc1, and escort it from the nucleus; however, Wis1 localization was unaffected in crm1–809 cells (Figure 6). As was observed with wild-type cells, Wis1 was excluded from the nucleus in stressed and unstressed crm1–809 cells (Figure 6). Thus, the sustained nuclear localization and tyrosine phosphorylation of Spc1 in crm1–809 cells was not caused by a change in Wis1 localization.
Figure 6.
Localization of Wis1, Pyp1, and Pyp2 are unaffected by crm1–809. Cells with the wis1–12myc, pyp1–12myc, or pyp2–12myc genes in a crm1–809 mutant background were grown at 30°C and challenged with 0.6 M KCl, harvested, and fixed in cold methanol. The localization of the myc-tagged proteins was followed with myc antibody.
An alternative explanation for these findings was that Pyp1 or Pyp2 were required to escort Spc1 from the nucleus. This model would not explain why the tyrosine phosphorylation of Spc1 should be sustained in crm1–809 cells, unless the tyrosine phosphatases were inactive while in the nucleus. This possibility would be consistent with the observation that catalytically inactive forms of the phosphatases associate tightly with Spc1 in vivo (Millar et al., 1995; Shiozaki and Russell, 1995; Degols et al., 1996); however, both Pyp1 and Pyp2 were excluded from the nucleus in crm1–809 cells (Figure 6). The immunofluorescence analysis of Pyp2 in the crm1–809 mutant revealed that Pyp2 was expressed as early as 10 min after exposure to stress, and it strengthens the notion that an increased amount of active Spc1 is present in the nucleus (Figure 6). These findings demonstrate that effects on Spc1 activity and localization in crm1–809 cells are not due to changes in the localization of the kinase or phosphatases that regulate Spc1. Moreover, these findings emphasize the conclusion that the activated Spc1 that has translocated into the nucleus is isolated from the kinase and phosphatases that regulate its activity.
Spc1 Associates with the Exportin Crm1
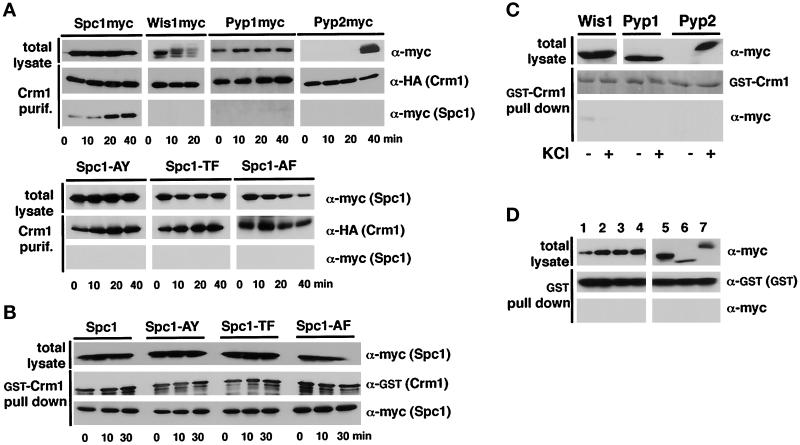
Our findings showed that nuclear export of Spc1 was regulated by the exportin Crm1. This conclusion prompted studies to determine whether Spc1 interacts with the Crm1 nuclear export machinery. The crm1+ gene was fused to an HAHIS sequence tag encoding the HA epitope and hexahistidine. This construct, placed under the control of thiamine-repressible nmt41 promoter, was cloned into a plasmid and transformed into a fission yeast strain that expressed myc-tagged Spc1. Cells were exposed to a 40-min time course of osmotic stress. Crm1 was purified with Ni2+-NTA, and the precipitate was analyzed by immunoblotting with HA and myc antibodies. This analysis revealed a small amount of association between Crm1 and Spc1 before stress and for 10 min thereafter (Figure 7A). The association between Crm1 and Spc1 substantially increased at the 20- and 40-min time points, coincident with the export of Spc1 from the nucleus (Figure 7A). Similar experiments were carried out with strains that expressed myc-tagged forms of Wis1, Pyp1, or Pyp2. Analysis of total lysates revealed that Wis1 migrated with reduced electrophoretic mobility in response to stress (Figure 7A). This effect was due to phosphorylation of Wis1 catalyzed by upstream-activating protein kinases (Samejima et al., 1997; Shieh et al., 1998; Shiozaki et al., 1998); however, there was no detectable association between Crm1 and Wis1 (Figure 7A). This result supports data that showed that nuclear exclusion of Wis1 does not require Crm1. Pyp1 and Pyp2 did not associate with Crm1 (Figure 7A), which agrees with the finding that Crm1 is not required for the nuclear exclusion of these phosphatases.
Figure 7.
Spc1 associates with Crm1. (A) A plasmid that expressed Crm1–HAHIS was introduced into strains that expressed 12myc-tagged forms of Spc1, Wis1, Pyp1, or Pyp2 (top panel) or 12myc-tagged forms of mutant Spc1 proteins (bottom panel). The mutant Spc1 proteins were Spc1-AY (spc1-T171A), Spc1-TF (spc1-Y173F), or Spc1-AF (spc1-T171A, Y173F). Cells were stressed with 0.6 M KCl and harvested at the indicated time points. Total lysates were incubated in native conditions with NTA-agarose beads to purify Crm1–HAHIS and associated proteins. The purified proteins were resolved by SDS-PAGE and detected by immunoblotting. Crm1–HAHIS was detected with antibodies to HA, whereas the myc-tagged proteins were detected with antibodies to myc. The 40-min total lysate sample of Spc1myc is apparently underloaded. (B) Mutant forms of Spc1 interact with Crm1 in vitro. GST-Crm1 produced in bacteria was bound to GSH–Sepharose and added to homogenates prepared from cells that expressed wild-type or mutant forms of Spc1 that were 12myc-tagged. These cells were exposed to osmotic stress before harvest. The GSH–Sepharose was washed and analyzed by immunoblotting. (C) Wis1, Pyp1, and Pyp2 do not interact with Crm1 in vitro. GD1892 (wis1–12myc), GD1953 (pyp1–12myc), or GD1955 (pyp2–12myc) were harvested before (−) or after osmotic stress (+), and total lysate was prepared. The homogenates were incubated with GST-Crm1 absorbed on GSH–Sepharose beads, washed, and analyzed by immunoblotting. The quantity of GST-crm1 was assessed by amido-black staining. (D) None of the myc-tagged protein copurifies in vitro with GST alone. Homogenates from spc1–12myc (lane 1), spc1-AY-12myc (lane 2), spc1-TF-12myc (lane 3), spc1-AF-12myc (lane 4), wis1–12myc (lane 5), pyp1–12myc (lane 6), or pyp2–12myc (lane 7) were prepared after KCl treatment. Total lysates were incubated with GST absorbed on GSH–Sepharose beads, and the results of the pull-down experiment were analyzed by immunoblotting.
These findings suggested that Crm1 escorts Spc1 from the nucleus. If this hypothesis were correct, then mutant Spc1 proteins that cannot accumulate in the nucleus would not associate with Crm1. Phosphorylation of threonine-171 and tyrosine-173 are required for nuclear localization of Spc1 (Gaits et al., 1998). Thus, spc1 mutations that replaced threonine-171 with alanine (T171A) or tyrosine-173 with phenylalanine (Y173F) were constructed in a Crm1–HAHIS strain background. Crm1 failed to associate with Spc1-AY (T171A), Spc1-TF (Y173F), or Spc1-AF (T171A-Y173F) (Figure 7A).
These findings suggested that Spc1 must translocate to the nucleus to interact with Crm1; however, an alternative explanation was that Spc1 must be phosphorylated to associate with Crm1. Therefore, an in vitro assay was developed to determine whether phosphorylation of Spc1 was required for association with Crm1 (see MATERIALS AND METHODS). GST-Crm1 fusion protein was produced in bacteria and purified with GSH–Sepharose. GST-Crm1 was produced in bacteria to reduce the possibility that it might complex with a protein that might affect association with Spc1. The purified GST-Crm1, bound to GSH–Sepharose, was incubated with lysates from cells that expressed wild-type Spc1 or the phosphorylation-site mutant forms of Spc1. The GSH–Sepharose was extensively washed and analyzed by immunoblotting. The wild-type and mutant forms of Spc1 all bound equally well to GST-Crm1 (Figure 7B). On the other hand, Wis1, Pyp1, and Pyp2 bound GST-Crm1 very poorly or not at all (Figure 7C). A weak Wis1 signal was detected when GST-Crm1 was incubated with a lysate from nonstressed cells. This binding might be due to an association between Wis1 and Spc1 (Gaits et al., 1998). None of the myc-tagged proteins bound to the unfused GST control (Figure 7D). These findings suggest that Spc1 does not need to be phosphorylated to bind Crm1. Thus, it appears that the in vivo association between Spc1 and Crm1 is driven by the nuclear localization of Spc1.
Addition of an NLS to the Spc1 Phosphorylation Mutants Rescues Their Nuclear Import Defect and Enables Normal Nuclear Export to Occur after Stress
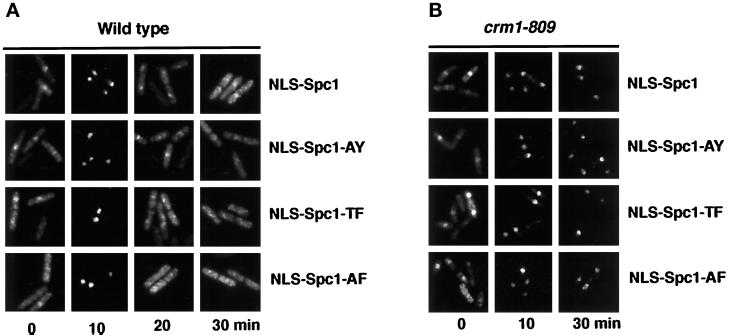
Our data strongly suggested that it is the availability of Spc1 in the nucleus, rather than its phosphorylation status, that drives the association with Crm1. To investigate the importance of the threonine-171 and tyrosine-173 phosphorylation in the export of Spc1, we constructed chimeras bearing a classical NLS from the SV40 large T antigen (Wu et al., 1996). The peptide PKKKRK was fused at the N terminus of Spc1, Spc1-AY, Spc1-TF, and Spc1-AF. The constructs were cloned into a vector allowing the expression of myc-tagged protein under the control of the thiamine-repressible nmt41 promoter. The resulting plasmids were transformed in wild-type cells, and the localization of the NLS-Spc1, NLS-Spc1-AY, NLS-Spc1-TF, and NLS-Spc1-AF was determined. Before stress, ∼40% of the cells in all the strains exhibited moderate nuclear staining for NLS-Spc1 (Figure 8). The variation in nuclear staining appears to be caused by differences in plasmid copy number. Indeed, ∼5% of cells exhibited very low or no NLS-Spc1 staining, which was presumably due to plasmid loss. At the 10-min time point after exposure to osmotic stress, both wild-type and mutant NLS-Spc1 appeared to be very highly concentrated in the nucleus of all strains (Figure 8). In fact, the cytoplasmic signal of NLS-Spc1 at this time point was very low. This amount of nuclear accumulation of NLS-Spc1 appeared substantially greater than that observed for endogenous Spc1 (for comparison see Figure 2A). Importantly, we observed that the wild-type and mutant NLS-Spc1 proteins were rapidly relocalized back into the cytoplasm at the 20- and 30-min time points after stress (Figure 8). These findings indicated that the nuclear export machinery is able to export phosphorylated, partially phosphorylated, and unphosphorylated forms of Spc1.
Figure 8.
NLS-Spc1myc, NLS-Spc1-AYmyc, NLS-Spc1-TFmyc, and NLS-Spc1-AFmyc are imported and exported from the nucleus in the same way. (A) Wild-type cells were transformed with the pREP41-NLS-spc1+-myc, pREP41-NLS-spc1+-AY-myc, pREP41-spc1+-TF-myc, or pREP41-spc1+-AF-myc. Cells were grown in EMM2 without thiamine to allow expression of the constructs from the nmt41 promoter. After incubation in YES medium for 4 h, the cells were stressed with 0.6 M KCl, harvested at the indicated time, and processed for immunofluorescence analysis. The localization of the proteins was assessed with antibodies to myc. (B) crm1–809 cells were transformed with the same vectors and treated as described in A.
We then tested whether the export of the fusion proteins is dependent on Crm1 by following the localization of NLS-Spc1 in a crm1–809 background (Figure 8B). At the 10-min time point of stimulation, essentially all of the NLS-Spc1 protein, both wild type and mutant, appeared to concentrate in the nucleus. As expected, NLS-Spc1 remained nuclear at the 30-min time point, because of the exportin system failure. The same pattern of nuclear accumulation was observed for all of the phosphorylation mutants (Figure 8B). Taken together, these data support the conclusion that activating phosphorylation of Spc1 is not intrinsically required for Crm1-mediated nuclear export.
DISCUSSION
In this study, we have investigated the regulation of nucleocytoplasmic shuttling of the SAPK Spc1 during osmotic stress. We reported previously that Spc1 nuclear translocation is dependent on activating phosphorylation catalyzed by the MAPKK Wis1 and that nuclear retention of Spc1 requires Atf1, the transcription factor substrate of Spc1 (Gaits et al., 1998). Evidence for the importance of activating phosphorylation for the nuclear accumulation of ERK2, a mammalian MAPK, was also reported recently (Khokhlatchev et al., 1998). These studies indicated that activating phosphorylation induces ERK2 dimerization that is required for nuclear import; however, an important question remained to be answered. Is the nuclear translocation of a MAPK or SAPK an active event involving the nuclear import machinery or is it the result of free diffusion through the nuclear pore? To answer this question, we took advantage of mutations that impair nucleocytoplasmic transport in fission yeast. We investigated the relocalization of Spc1 in a strain defective for Pim1, the fission yeast equivalent of RCC1. RCC1, which is required for nucleocytoplasmic trafficking, is the nucleotide exchange factor for the small GTPase Ran. In the pim1-d1 mutant, nuclear translocation of Spc1 was profoundly impaired, although stressed-induced phosphorylation of Spc1 was intact. These findings strongly suggest that stress-induced nuclear accumulation of Spc1 is dependent on an active nuclear import process.
As expected, the pim1-d1 mutation also greatly reduced the stress-induced transcription of genes regulated by the Spc1 pathway. This result can be understood in two ways. First, it reflects the requirement of a functional nuclear import system for Spc1 to be transported into the nucleus; however, Spc1 does not appear to have a classic NLS sequence. This observation suggests that Spc1 might interact with other proteins that interface with the nuclear import machinery. Indeed, gel filtration experiments have indicated that both inactive and active MAPKs and SAPKs are components of large protein complexes (Khokhlatchev et al., 1998; Schaeffer et al., 1998; Whitmarsh et al., 1998; our unpublished data). The second possibility is that failure to accumulate Spc1 in the nucleus of pim1-d1 cells is due to a problem with the nuclear localization of Atf1, the nuclear substrate of Spc1; however, Atf1, which is normally nuclear with or without stress, was detected as a nuclear protein in ∼90% of the pim1-d1 cells exposed to the same experimental protocol that was used for the Spc1 localization experiments (our unpublished data). Thus, the deficiency of Spc1 nuclear accumulation in pim1-d1 cells appears to be due to a specific defect in the nuclear import of Spc1.
Having found that nuclear accumulation of Spc1 requires active nuclear import machinery, we then asked a second important question. What is the relationship between dephosphorylation and relocalization of Spc1 into the cytoplasm during stress adaptation? In response to osmotic shock, the nuclear accumulation of Spc1 is a transient phenomenon that peaks at 10 min and returns to the preshock state within 20 min. This relocalization correlates perfectly with changes in tyrosine phosphorylation of Spc1. These data suggest that there is a connection between export and dephosphorylation of Spc1. Dephosphorylation of Spc1 by nuclear phosphatases might induce its export into the cytoplasm. Alternatively, nuclear export of Spc1 might be required for its dephosphorylation by cytoplasmic phosphatases. To discriminate between the two possibilities, we investigated the localization of Pyp1 and Pyp2, the two phosphatases that are required to inactivate Spc1. Immunofluorescence analysis revealed that both phosphatases are cytoplasmic, and their localization remained unchanged on osmotic stress. Moreover, Spc1 phosphorylation status and localization were both altered in Δpyp1 and Δpyp2 mutant cells. In Δpyp1 and Δpyp2 mutants, because of lower cytoplasmic phosphatase activity, Spc1 remains phosphorylated and presumably shuttles between the nucleus and cytoplasm until it is dephosphorylated in the cytoplasm. Taken together, these data strongly suggest that inactivation of Spc1 is a cytoplasmic event that is dependent on previous nuclear export of Spc1.
These observations suggested that relocalization and dephosphorylation of Spc1 should be impaired by a mutation that causes a defect in active nuclear export, such as the crm1–809 mutation. When the function of the exportin Crm1 was impaired, the nuclear accumulation of Spc1 was increased, and it remained nuclear at times when it is normally completely excluded from the nucleus in wild-type cells. In parallel, the level of Spc1 tyrosine-173 phosphorylation remained high for an extended period in crm1–809 cells. This finding reinforces the idea that nuclear export is required to deliver nuclear Spc1 to phosphatases located in the cytoplasm. Thus, adaptation of the stress response, as reflected by inactivation of Spc1, is strongly influenced by the nuclear export machinery.
These observations led to a complementary question. Is there an inactive nuclear fraction of Spc1 that is activated by transiently nuclear Wis1? Studies by Fukuda et al. (1997b) identified an NES sequence at the N-terminal extremity of a MAPKK. Deletion of these sequences caused overexpressed protein to be distributed equally in the nucleus and the cytoplasm, whereas the wild-type MAPKK is completely excluded from the nucleus (Fukuda et al., 1997b). These studies suggested that MAPKK might transiently enter the nucleus and phosphorylate an inactive fraction of nuclear MAPK. The N-terminal region of the SAPKK Wis1 has two putative NES sequences, which suggests that NES-dependent nuclear export might be important for nuclear exclusion of Wis1; however, Wis1 remained cytoplasmic in crm1–809 cells. This result suggests that Wis1 does not shuttle between the nucleus and cytoplasm. Perhaps Wis1 has functional NES sequences that are required for nuclear export in rare instances in which Wis1 monomers diffuse into the nucleus, but Wis1 nuclear exclusion might normally be mediated by another mechanism. It is likely that Wis1 has stable interactions with other proteins of the Spc1 pathway, such as the SAPKKKs, and these interactions might be sufficient to exclude Wis1 from the nucleus. These interactions might occur through the N termini of MAPKKs and SAPKKs; thus, deletion of the N-terminal domain eliminates not only NES sequences but also sequences required for stable interactions with other proteins. In this regard it will be interesting to determine whether nuclear exclusion of MAPKKs is diminished by leptomycin B, an inhibitor of mammalian Crm1 homologues (Fornerod et al., 1997; Fukuda et al., 1997a; Ossareh-Nazari et al., 1997). In the case of stress pathways in fission yeast, our findings apparently exclude mechanisms in which nucleocytoplasmic shuttling of Wis1 is involved in regulation of Spc1 activation or localization. Our studies have also shown that nuclear exclusion of Pyp1 and Pyp2 are not dependent on Crm1 activity. These findings argue against a model in which transient nuclear entry of the tyrosine phosphatases leads to the dephosphorylation of nuclear Spc1.
Experiments described in this report suggest that Crm1 has a direct role in catalyzing the nuclear export of Spc1. In fact, a protein complex involving Spc1 and Crm1 was detected with timing that was coincident with osmotic stress adaptation and nuclear export of Spc1. Among all the tested proteins of the Spc1 cascade, Spc1 was the only protein that copurified with Crm1 in a stress-dependent manner. Wis1, Pyp1, and Pyp2 did not associate with Crm1, confirming the localization data of these proteins in wild-type and crm1–809 cells. The phosphorylation status of Spc1 does not seem to trigger the association with Crm1. There is a lag between the peak of Spc1 phosphorylation and its association with Crm1. Furthermore, the peak of association with Crm1 correlates with the exclusion of Spc1 from the nucleus. The phosphorylation mutants of Spc1 failed to associate with Crm1. These mutant proteins also fail to undergo nuclear import, which is consistent with the idea that Crm1 only associates with proteins available in the nucleus. An alternative possibility is that activating phosphorylation of Spc1 is directly required for interaction with Crm1; however, this model is inconsistent with the observation that Spc1 phosphorylation-site mutant proteins associated with bacterially produced GST-Crm1 in an in vitro experiment. To test this hypothesis further, we expressed phosphorylation-site mutant proteins that had an NLS. This fusion provides an alternative nuclear import system that functions independently of Spc1-specific regulation. All of the mutants were able to accumulate in the nucleus with the same time course and rate as a wild-type NLS-Spc1 fusion. All of the NLS-containing chimeras were then exported from the nucleus after stress, suggesting that phosphorylation status per se is not required for the association with the export machinery. Moreover, this process is dependent on Crm1 activity because it was diminished by the crm1–809 mutation. It is important to note that the addition of an NLS was insufficient to cause unregulated nuclear accumulation of Spc1. This result suggests that relief from cytoplasmic retention is also important for stress-induced nuclear localization of Spc1; however, elimination of Wis1, the protein that is the most obvious candidate to be a cytoplasmic anchor for Spc1, is insufficient to cause nuclear accumulation of Spc1 (Gaits et al., 1998). Perhaps activating phosphorylation of Spc1 has the dual effects of releasing Spc1 from cytoplasmic anchors and promoting association with the nuclear import machinery.
Crm1 and Spc1 coprecipitate from cell lysates, but this finding does not necessarily demonstrate that the two proteins interact directly. Exportin proteins such as Crm1 interact directly with NES sequences (Fornerod et al., 1997; Fukuda et al., 1997b; Ullman et al., 1997); therefore we initiated studies to determine whether Spc1 has an NES sequence. We focused our attention on the 122–132 region of Spc1, LYQILRGLKFV, which has a close match to known NES sequences, although in fact there are several leucine- and isoleucine-rich regions of Spc1 that resemble NES sequences. The 122–132 region, which is highly conserved between Spc1, budding yeast Hog1, and mammalian p38, was very effective at causing the nuclear exclusion of GST (our unpublished data). These observations show that Spc1 has at least one NES-like sequence and support the hypothesis of a direct association between Crm1 and Spc1.
The findings described in this report complement a recent study of the Saccharomyces cerevisiae Hog1p SAPK from Silver and colleagues (Ferrigno et al., 1998). These studies showed that osmotic stress induces the transient nuclear import of Hog1p by a process that requires dual activating phosphorylation of Hog1p. These findings are consistent with our analysis of Spc1 (Gaits et al., 1998). Silver and colleagues (Ferrigno et al., 1998) also showed that nuclear import of Hog1p requires Ran-GSP1, a homologue of Ran in fission yeast. These findings are compatible with our studies showing that Pim1 is required for nuclear import of Spc1. Hog1p import also requires Nmd5p, a novel importin β homologue (Ferrigno et al., 1998). In agreement with the studies described herein, Silver and colleagues (Ferrigno et al., 1998) showed that Hog1p export requires the function of Xpo1, the homologue of Crm1. These investigators also mentioned that tyrosine dephosphorylation of Hog1p is unaffected by an xpo1 mutation. This finding is clearly different from the Spc1 data shown in this report. This result suggests that one or both of the tyrosine phosphatases that dephosphorylate Hog1p might be localized in the nucleus in budding yeast.
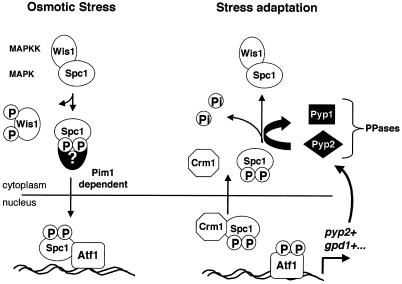
As illustrated in Figure 9, we have shown that Spc1 translocates in and out of the nucleus in a period of <20 min after the initial exposure to osmotic stress. Active import and export of Spc1 drive these movements. While in the nucleus, Spc1 is apparently unresponsive to changes in stress conditions because it is isolated from Wis1, Pyp1, and Pyp2. Thus, it is perhaps not surprising that rapid nucleocytoplasmic shuttling of Spc1 should occur and be dependent on active processes. Nuclear export of Spc1 appears to involve a direct interaction with Crm1. The detection of an NES motif in Spc1, as mentioned above, supports this model. Nuclear import of Spc1 requires phosphorylation that is catalyzed by Wis1, but Spc1 does not have a classic NLS. Spc1 might have an untypical NLS, or perhaps association with other proteins is required for nuclear import of Spc1. The understanding of the nuclear import machinery in fission yeast is rather limited; however, we recently identified an S. pombe gene that has sequence homology to the RanBP7 protein, which binds Ran complexes and promotes nuclear import of ribosomal proteins (Görlich et al., 1997; Jäkel and Görlich, 1998). Work is under way to determine whether this protein is involved in nuclear import of Spc1.
Figure 9.
Model for the nucleocytoplasmic transport of the SAPK Spc1/StyI in fission yeast. Osmotic stress results in the phosphorylation of the MAPKK Wis1, which in turn phosphorylates Spc1, releasing it from the cytoplasmic anchoring complex. Phosphorylated Spc1 is translocated into the nucleus via association with an unknown nuclear import protein. The import is dependent on the Ran exchange factor Pim1. Activated Spc1 released in the nucleus displays strong affinity for its substrate and nuclear anchor, the transcription factor Atf1 (Gaits et al., 1998). Spc1 activates Atf1 to induce the transcription of a subset of genes required for stress adaptation, such as gpd1+, which encodes an enzyme involved in the regulation of the intracellular glycerol concentration, or pyp2+, which encodes the phosphatase Pyp2. Dissociation from phosphorylated Atf1 allows Spc1 to form a complex with the exportin Crm1 in order to be translocated to the cytoplasm. Atf1-dependent transcription of pyp2+ leads to an increase in the cytoplasmic phosphatase activity against Spc1. After export, Spc1 is dephosphorylated in the cytoplasm and resumes association with Wis1.
Human SAPKs p38 and JNK (Jun NH2-terminal kinase) phosphorylate the nuclear transcription factors ATF-2 and Jun, respectively (Karin et al., 1997; Ip and Davis, 1998). Thus, it is likely that human SAPKs translocate into the nucleus in response to stress, a prediction confirmed in the case of activation of JNK by UV irradiation (Cavigelli et al., 1995). Mechanisms that regulate the nuclear import and export of human SAPKs are unknown. There is substantial structural and functional similarity between fission yeast Spc1 and human SAPKs. Likewise, active nucleocytoplasmic protein shuttling mechanisms appear to be highly conserved between yeasts and mammals. Thus, it is likely that the mechanisms regulating nuclear import and export of Spc1 will apply to human SAPKs.
ACKNOWLEDGMENTS
We thank M. Yanagida, S. Sazer, and S. Forsburg for providing yeast strains. S. Reed and L. Hengst provided the antibody to GST. We thank K. Shiozaki, G. Degols, and G. Ammerer for advice and helpful discussions. Members of the Scripps Cell Cycle Groups provided support and encouragement. F.G. is a recipient of a fellowship from the Leukemia Society of America. This work was supported by National Institutes of Health.
REFERENCES
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with Fission Yeast. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1993. [Google Scholar]
- Cavigelli M, Dolfi F, Claret FX, Karin M. Induction of c-fos expression through JNK-mediated TCF/Elk-1 phosphorylation. EMBO J. 1995;14:5957–5964. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degols G, Russell P. Discrete roles of Spc1 kinase and Atf1 transcription factor in the UV response of Schizosaccharomyces pombe. Mol Cell Biol. 1997;17:3356–3363. doi: 10.1128/mcb.17.6.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degols G, Shiozaki K, Russell P. Activation and regulation of Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:2870–2877. doi: 10.1128/mcb.16.6.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter J, Morphew M, Sazer S. A mutation in the RCC1-related protein pim1 results in nuclear envelope fragmentation in fission yeast. Proc Natl Acad Sci USA. 1995;92:1436–1440. doi: 10.1073/pnas.92.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrigno P, Posas F, Koepp D, Saito H, Silver PA. Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin beta homologs NMD5 and XPO1. EMBO J. 1998;17:5606–5614. doi: 10.1093/emboj/17.19.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997a;390:308–310. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Gotoh Y, Nishida E. Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 1997b;16:1901–1908. doi: 10.1093/emboj/16.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaits F, Degols G, Shiozaki K, Russell P. Phosphorylation and association with the transcription factor Atf1 regulate localization of Spc1/StyI stress-activated kinase in fission yeast. Genes Dev. 1998;12:1464–1473. doi: 10.1101/gad.12.10.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D, Dabrowski M, Bischoff FR, Kutay U, Bork P, Hartmann E, Prehn S, Izaurralde E. A novel class of RanGTP binding proteins. J Cell Biol. 1997;138:65–80. doi: 10.1083/jcb.138.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)–from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Jäkel S, Görlich D. Importin beta, transportin, RanBP5 and RanBP7 mediate nuclear import of ribosomal proteins in mammalian cells. EMBO J. 1998;17:4491–4502. doi: 10.1093/emboj/17.15.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb MH. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Beach D. Premature initiation of mitosis in yeast lacking RCC1 or an interacting GTPase. Cell. 1991;66:347–360. doi: 10.1016/0092-8674(91)90624-8. [DOI] [PubMed] [Google Scholar]
- Melchior F, Gerace L. Two-way trafficking with Ran. Trends Cell Biol. 1998;8:175–179. doi: 10.1016/s0962-8924(98)01252-5. [DOI] [PubMed] [Google Scholar]
- Millar JBA, Buck V, Wilkinson MG. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 1995;9:2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- Millar JBA, Russell P, Dixon JE, Guan KL. Negative regulation of mitosis by two functionally overlapping PTPases in fission yeast. EMBO J. 1992;11:4943–4952. doi: 10.1002/j.1460-2075.1992.tb05601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison JM. Physiological and cytological methods for Schizosaccharomyces pombe. Methods Cell Physiol. 1970;4:131–146. [Google Scholar]
- Nigg EA. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for the role of Crm1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- Ottilie S, Chernoff J, Hannig G, Hoffman CS, Erikson RL. The fission yeast genes pyp1+ and pyp2+ encode protein tyrosine phosphatases that negatively regulate mitosis. Mol Cell Biol. 1992;12:5571–5580. doi: 10.1128/mcb.12.12.5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux AL, Fawell EH, Armstrong J. Glycerol-3-phosphate dehydrogenase homologue from Schizosaccharomyces pombe. Nucleic Acids Res. 1990;18:7145. doi: 10.1093/nar/18.23.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima I, Mackie S, Fantes PA. Multiple modes of activation of the stress-responsive MAP kinase pathway in fission yeast. EMBO J. 1997;16:6162–6170. doi: 10.1093/emboj/16.20.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazer S, Nurse P. A fission yeast RCC1-related protein is required for the mitosis to interphase transition. EMBO J. 1994;13:606–615. doi: 10.1002/j.1460-2075.1994.tb06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer HJ, Catling AD, Eblen ST, Collier LS, Krauss A, Weber MJ. MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science. 1998;281:1668–1671. doi: 10.1126/science.281.5383.1668. [DOI] [PubMed] [Google Scholar]
- Shieh JC, Martin H, Millar JB. Evidence for a novel MAPKKK-independent pathway controlling the stress activated StyI/Spc1 MAP kinase in fission yeast. J Cell Sci. 1998;111:2799–2807. doi: 10.1242/jcs.111.18.2799. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Cell-cycle control linked to the extracellular environment by MAP kinase pathway in fission yeast. Nature. 1995;378:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Conjugation, meiosis and the osmotic stress response are regulated by Spc1 kinase via Atf1 transcription factor in fission yeast. Genes Dev. 1996;10:2276–2288. doi: 10.1101/gad.10.18.2276. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Shiozaki M, Russell P. Mcs4 mitotic catastrophe suppressor regulates the fission yeast cell cycle through the Wik1-Wis1-Spc1 kinase cascade. Mol Biol Cell. 1998;8:409–419. doi: 10.1091/mbc.8.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stade K, Ford C, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Ullman KS, Powers MA, Forbes DJ. Nuclear export receptors: from importin to exportin. Cell. 1997;90:967–970. doi: 10.1016/s0092-8674(00)80361-x. [DOI] [PubMed] [Google Scholar]
- Weis K. Importins and exportins: how to get in and out of the nucleus. Trends Biochem Sci. 1998;23:185–189. doi: 10.1016/s0968-0004(98)01204-3. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Cavanagh J, Tournier C, Yasuda J, Davis RJ. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science. 1998;281:1671–1674. doi: 10.1126/science.281.5383.1671. [DOI] [PubMed] [Google Scholar]
- Wilkinson MG, Millar JB. SAPKs and transcription factors do the nucleocytoplasmic tango. Genes Dev. 1998;12:1391–1397. doi: 10.1101/gad.12.10.1391. [DOI] [PubMed] [Google Scholar]
- Wilkinson MG, Samuels M, Takeda T, Toone WM, Shieh J-C, Toda T, Millar JBA, Jones N. The Atf1 transcription factor is a target for the StyI stress-activated MAP kinase pathway in fission yeast. Genes Dev. 1996;18:2289–2301. doi: 10.1101/gad.10.18.2289. [DOI] [PubMed] [Google Scholar]
- Wu L, Shiozaki K, Aligue R, Russell P. Spatial organization of the Nim1-Wee1-Cdc2 mitotic control network in Schizosaccharomyces pombe. Mol Biol Cell. 1996;7:1749–1758. doi: 10.1091/mbc.7.11.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida M. Gene products required for chromosome separation. J Cell Sci Suppl. 1989;12:213–229. doi: 10.1242/jcs.1989.supplement_12.18. [DOI] [PubMed] [Google Scholar]