Abstract
Genital coinfections increase an individual’s risk of becoming infected with HIV-1 by sexual contact. Several mechanisms have been proposed to explain this, such as the presence of ulceration and bleeding caused by the coinfecting pathogen. Here we demonstrate that Langerhans cells (LCs) are involved in the increased susceptibility to HIV-1 in the presence of genital coinfections. Although LCs are a target for HIV-1 infection in genital tissues, we found that immature LCs did not efficiently mediate HIV-1 transmission in an ex vivo human skin explant model. However, the inflammatory stimuli TNF-α and Pam3CysSerLys4 (Pam3CSK4), the ligand for the TLR1/TLR2 heterodimer, strongly increased HIV-1 transmission by LCs through distinct mechanisms. TNF-α enhanced transmission by increasing HIV-1 replication in LCs, whereas Pam3CSK4 acted by increasing LC capture of HIV-1 and subsequent trans-infection of T cells. Genital infections such as Candida albicans and Neisseria gonorrhea not only triggered TLRs but also induced TNF-α production in vaginal and skin explants. Thus, during coinfection, LCs could be directly activated by pathogenic structures and indirectly activated by inflammatory factors, thereby increasing the risk of acquiring HIV-1. Our data demonstrate a decisive role for LCs in HIV-1 transmission during genital coinfections and suggest antiinflammatory therapies as potential strategies to prevent HIV-1 transmission.
Introduction
HIV-1 infection, the causative agent of AIDS, is still on the rise. It was estimated that 2 to 4 million people acquired HIV-1 in 2007, while already 31 to 36 million people are HIV-1 infected worldwide (1). Thus, the HIV-1 pandemic, for which there is currently no cure or vaccine available, is still growing. Heterosexual transmission of HIV-1 across genital epithelial tissue is the primary route of HIV-1 dissemination worldwide (1, 2). Therefore, increased knowledge about how sexual transmission of HIV-1 occurs and the identification of factors facilitating or enhancing this process are essential for the development of effective strategies to reduce new infections.
Many biological factors are involved in sexual transmission of HIV-1, and the risk of HIV-1 acquisition varies depending on these factors, including viral loads (3), viral variants, and host susceptibility, which may include contraception, male circumcision, and genital coinfection (2). Moreover, different soluble factors in body fluids might influence transmission such as factors in breast milk (4) and semen (5). Furthermore, genital coinfections have been linked to increased susceptibility to HIV-1 and include ulcerative sexually transmitted diseases (STDs) such as genital herpes, gonorrhea, syphilis, and chlamydial infections (6) as well as yeast and bacterial vaginal infections (7–9). However, the mechanisms accounting for increased HIV-1 susceptibility in the presence of genital coinfections are unclear. It is argued that these infections increase susceptibility by recruiting HIV-1 target cells into the site of infection (10) or by causing ulceration and subsequent bleeding (6). In addition, we hypothesize that pathogenic structures or inflammatory cytokines that are induced upon infection change the function of key players in the transmission of HIV-1 (11).
Langerhans cells (LCs) are a subset of DCs that reside in the epidermis of skin and in mucosal epithelia such as ectocervix, vagina, and foreskin (12, 13). LCs are therefore likely to be the first cells that encounter HIV-1 upon sexual transmission. However, there is debate about whether LCs are also the first cells infected by HIV-1 and whether they are involved in the initiation of systemic disease (12, 14–16). Several ex vivo skin explant studies have shown that LCs are susceptible to HIV-1 and transmit HIV-1 to T cells (12, 17, 18). We have recently demonstrated that HIV-1 infection of LCs and subsequent transmission to T cells is an inefficient process. Langerin, a C-type lectin specifically expressed by LCs, captures HIV-1 and acts as a protective barrier for HIV-1 infection by targeting HIV-1 to Birbeck granules for degradation. However, when the Langerin function is blocked or saturated using high virus concentration this barrier can be overcome. These conditions allow LC infection and subsequent HIV-1 transmission to occur (16). Thus, LCs are an essential checkpoint where it is decided whether the virus is degraded or transmitted, and we hypothesize that its activation state and the encountered viral loads are a decisive factor.
Both inflammatory cytokines and pathogen-associated molecular patterns (PAMPs) induce LC activation (19–21), and these factors, which are present during coinfections, might breach the protective function of LCs to allow HIV-1 transmission. PAMPs are recognized by TLRs, and TLR triggering on LCs results in LC maturation (21). Here we set out to investigate the effect of bacterial and fungal coinfections on HIV-1 transmission by LCs. To mimic the epithelial environment, we developed an ex vivo skin explant transmission model to investigate the effect of coinfection on HIV-1 transmission. We demonstrate that TLR agonists, bacterial and fungal pathogens, induce the production of the proinflammatory cytokine TNF-α. Strikingly, both TNF-α and the TLR1/TLR2 ligand Pam3CysSerLys4 (Pam3CSK4) strongly enhance HIV-1 transmission by LCs using distinct mechanisms. TNF-α increases HIV-1 replication in LCs, whereas Pam3CSK4 enhances HIV-1 capture. Our data demonstrate that in response to inflammatory cytokines and pathogenic structures present during genital coinfection, LCs mediate HIV-1 transmission. Identification of these risk factors that increase HIV-1 susceptibility forces a reevaluation of LC function in HIV-1 transmission and might help toward the development of strategies to prevent HIV-1 transmission.
Results
An ex vivo model to determine the effect of coinfection on HIV-1 transmission.
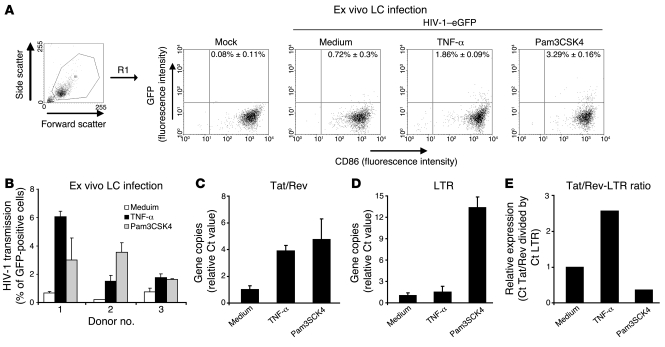
Bacterial and fungal coinfections of the genital tract result in increased risk of HIV-1 infection (6, 9, 22). To investigate the effect of different bacterial pathogens on HIV-1 transmission, we set up an ex vivo HIV-1 transmission model using human epidermal sheets (Figure 1A). The advantage of this model is that LCs reside in their physiological cellular environment, which is essential for LC function (23). Upon culture of the epidermal sheets, LCs migrated out of the epidermis, similar to LCs in the epithelial tissues that migrate toward the T cell–rich areas upon activation (12).
Figure 1. An ex vivo skin model to investigate the effect of inflammation on HIV-1 transmission is shown.
(A–C) Epidermal sheets were floated on medium in a 24-well plate and where indicated stimulated with different stimuli. After 6 hours the sheets were inoculated with HIV-1–eGFP. After 3 days, the epidermal sheets were removed and CCR5+ Jurkat T cells were added for an additional 7 days. Filled dendritic forms indicate LCs; open dendritic forms indicate GFP+-infected LCs; filled circles indicate CCR5+ Jurkat T cells; open circles indicate GFP+-infected T cells. (B) The migrated epidermal cells (day 3) were stained with antibodies against CD1a, Langerin, CD86, and CD3 and analyzed by flow cytometry to determine their phenotype and HIV-1 infection. Gates are based on isotype control (Supplemental Figure 1). R1 is gated on the larger cells in the population. Open histograms represent isotype-control; filled histogram specific antibody staining; the mean ± SD of the specific staining is depicted in the upper-right corner. The percentage of GFP+, CD3+, and CD1a+ cells ± SD is depicted in the upper-right corner of the dot plots. (C) Samples of the cocultures (day 5, 7, and 10) that were not stimulated with any stimulus were analyzed for GFP expression by flow cytometry. The percentage of GFP+ cells ± SD is depicted in the upper-right corner. As control for HIV-1 infection, the same concentration of HIV-1–eGFP was added to wells without epidermal sheets and processed similarly as the other conditions.
We have used the recombinant R5-tropic HIV-1 strain NL4.3-eGFP-BaL, expressing the eGFP upon replication (referred to as HIV-1–eGFP) (24), to investigate transmission of HIV-1 by LCs ex vivo by adding the virus to epidermal sheets. LC infection by HIV-1 is relatively inefficient and high virus concentrations are needed to infect LCs (16). HIV-1 infection of LCs ex vivo was analyzed by measuring GFP expression using flow cytometry, and indeed, infection of unstimulated emigrated CD1a+ LCs was low (<0.5%) and high virus concentrations (30 ng p24/sheet; 1.2 × 104 IU/sheet) were needed to detect infection in emigrated LCs (Figure 1B). To optimize virus entry into the sheets and subsequent interaction with immature residing LCs, we have incubated the sheets with virus in a low volume and diluted the volume 6-fold after 2 hours. LCs migrated out of the sheets after more than 12 hours (data not shown), strongly suggesting that the observed effects are due to HIV-1 infection of immature LCs in the epidermal sheets in situ. During the next 3 days, LCs, in contrast to keratinocytes, migrated out of the epidermis as determined by flow cytometry. The majority of migrated cells expressed CD1a and Langerin and therefore represent LCs (Figure 1B). These emigrated LCs have a mature phenotype as shown by the high expression levels of CD86 (Figure 1B). The emigrated LC fraction contained 4.3% ± 4.5% T cells, of which about half were present in clusters with LCs (2.6% ± 2.7% of the total amount of cells), and 37% ± 11% of the T cell population was CD8+ (Figure 1B, lower left panel; isotype control in Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI34721DS1). Although CD4+ T cells are susceptible to HIV-1 infection, we never observed more than 0.1% infection in this population (data not shown), probably due to the lack of activation.
At day 3, the epidermis was removed and CCR5+ Jurkat T cells were added to determine HIV-1 transmission to T cells by LCs. The ex vivo LC transmission of HIV-1 to T cells was followed by flow cytometry. Viral infection of T cells was low but detectable after 7 days (mean of 1.8%; Figure 1C). This is in line with previous data that HIV-1 transmission by LCs is inefficient and that high virus loads are needed to induce infection (16, 25). At later time points, infection was more clearly detectable in the T cell culture (mean of 14.4% at day 10; Figure 1C). At high virus concentrations, a small amount of residual virus remained infectious after 3 days and could infect CCR5+ Jurkat T cells independent of LCs, as demonstrated by the low infection rate of CCR5+ Jurkat T cells in absence of epidermal sheets (mean of 0.55% at day 7; Figure 1C). Thus, the ex vivo model is suitable to investigate the effect of LC activation on HIV-1 transmission in conditions of high virus concentrations, reflecting high viral loads of the infected partner in vivo.
Exposure of skin and vagina biopsies to pathogens and TLR agonists results in TNF-α production.
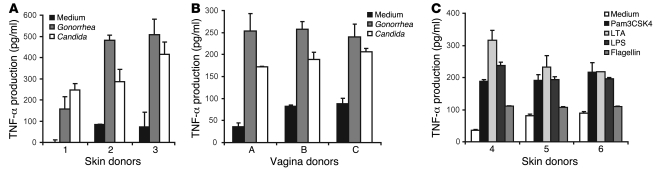
Microbes induce the production of several inflammatory cytokines and chemokines by interacting with TLRs on epithelial cells or LCs, which might affect HIV-1 transmission. Since TNF-α enhances HIV-1 replication in T cells and macrophages by activation of the NF-κB (26, 27), we investigated whether bacteria or TLR agonists induce the proinflammatory cytokine TNF-α. We incubated skin and vaginal biopsies from epithelial tissue with TLR agonists, heat-killed Candida albicans and Neisseria gonorrhea, since these pathogens have been associated with reproductive tract infections (28, 29). After 24 hours supernatant was harvested and TNF-α production was analyzed. The cytokines were measured after 24 hours when only a small number of cells had migrated out of the tissue, strongly indicating that the main effects of the pathogens are due to interactions with residing cells. Both Candida and Neisseria induced TNF-α production in epidermal and vaginal biopsies (Figure 2, A and B). Notably, TLR agonists LPS, lipoteichoic acid (LTA), Pam3CSK4, and flagellin also induced TNF-α production in the skin biopsies, indicating that TLR triggering mediates the production of TNF-α (Figure 2C).
Figure 2. Different pathogens and pathogenic ligands induce TNF-α production in skin and vaginal biopsies.
Skin or vagina epithelial biopsies were treated with Candida albicans, Neisseria gonorrhea (A and B), Pam3CSK4, LTA, LPS, or flagellin (C). After 24 hours, the supernatant was collected, and TNF-α production was measured by ELISA. Error bars represent the mean ± SD of duplicates. For each panel, the different donors were measured in 2 independent experiments.
In addition to TNF-α, we analyzed the production of the inflammatory cytokines IL-6 and IL-1β and the chemokine IL-8. IL-6 and IL-8 were both highly produced in skin biopsies. However, no differences were observed between unstimulated and stimulated tissues after 24 hours (Supplemental Figure 2, D and E) or 48 hours of culture (L. de Witte and M.A.W.P. de Jong, unpublished observations). Furthermore, the production of IL-1β in all skin biopsies was below the detection limit (<5 pg/ml; L. de Witte and M.A.W.P. de Jong, unpublished observations).
Candida albicans can switch between the yeast and hyphal form, which can differently affect immune responses and, moreover, differences between immune activation by live and heat-killed Candida have been observed (30). Therefore, we have compared the cytokine responses induced by heat-killed yeast with those of the live hyphal and yeast forms of Candida albicans. The live yeast form was obtained by treating Candida albicans yeasts with Amphotericin B, which prevents growth and thereby differentiation into the hyphal form. All Candida albicans forms resulted in TNF-α production, while IL-8 remained unchanged (Supplemental Figure 2, A and C). Live Candida albicans treated with Amphotericin B enhanced IL-6 production significantly compared with the medium control, but this increase was not significant compared with the other stages of Candida albicans (Supplemental Figure 2B).
Thus, bacterial and fungal pathogens associated with genital tract infections induce TNF-α production both in epithelial and vaginal tissues and this can affect HIV-1 transmission.
TNF-α and TLR ligands affect HIV-1 transmission ex vivo.
Next, we investigated whether TNF-α or TLR agonists influence HIV-1 transmission by LCs in the ex vivo transmission model. Extracellular bacterial and yeast microbes are mainly recognized by TLR2 (in combination with TLR1 or TLR6), TLR4, and TLR5 (28, 31). Therefore, we used the TLR agonists Pam3CSK4 (TLR1/TLR2), LTA (TLR2/TLR6), LPS (TLR4), and flagellin (TLR5). Epidermal sheets were preincubated with TLR agonists or TNF-α and subsequently infected with HIV-1–eGFP. At day 3 CCR5+ Jurkat T cells were added and infection was followed in time by fluorescent microscopy and flow cytometry. Almost no infected cells were detected in the medium control condition, confirming that HIV-1 transmission to T cells is not efficient under normal conditions, even though we used high concentrations of virus (Supplemental Figure 3 and Figure 3A). Strikingly, many syncytia were observed in the TNF-α–treated cultures at day 7, and high levels of GFP expression were detected in the T cells by fluorescent microscopy and flow cytometry (Figure 3A and Supplemental Figure 3). Further analyses by flow cytometry demonstrated that in the TNF-α–treated condition up to 50% of the T cells were infected (Figure 3, A and B). Although the variation between donors ranged from 25% to 50%, the increase in HIV-1 transmission by TNF-α was observed for all donors (Figure 3B).
Figure 3. TNF-α and Pam3CSK4 enhance HIV-1 transmission ex vivo.
(A–D) Epidermal sheets were stimulated with TNF-α, Pam3CSK4, LTA, LPS, or flagellin for 6 hours, and ex vivo transmission was determined as in Figure 1. (A) Cocultures of donors number 1–4 (day 7) were analyzed for GFP expression by flow cytometry. HIV-1 transmission is depicted as percentage of CCR5+ Jurkat T cells positive for GFP expression. Error bars represent the mean ± SD of duplicates. (B) Results obtained with donors number 1–8 are depicted, and the values were analyzed for statistical differences by ANOVA (**P < 0.01, ***P < 0.001 versus the no treatment condition). (C) Before addition of the CCR5+ Jurkat T cells the migrated cells were extensively washed. At day 7, supernatant was collected, and viral content was monitored by p24 ELISA. A representative experiment of 2 is depicted. (D) To analyze donor differences, 4 different skin donors were incubated with TNF-α, LTA, LPS, or flagellin at 2 different days, and ex vivo transmission was determined as described in Figure 1. The samples were measured for 13 days. Error bars represent the mean ± SD of duplicates.
The TLR1/TLR2 ligand Pam3CSK4 also increased HIV-1 transmission to T cells (Supplemental Figure 3 and Figure 3A) and about 5%–50% of the T cells were infected (Figure 3, A and B). Although the increased transmission was observed for all donors and did increase over time, the effect was more variable compared with the effect of TNF-α (Figure 3B). TLR agonists LTA, LPS, and flagellin marginally increased HIV-1 transmission in some donors (Figure 3, A and B). Enhancement of HIV-1 transmission by the different stimuli was dependent on interaction of HIV-1 with the epidermis, since transmission was not increased by the different stimuli in absence of epidermal sheets (Figure 3A). The increased transmission in the presence of TNF-α and Pam3CSK4 was statistically significant (P < 0.001 and P < 0.01, respectively), while overall, the effect of other TLR agonists was not significant (Figure 3B). To validate that GFP expression upon infection with HIV-1–eGFP correlates with the amount of virus produced, viral particles in the supernatant were quantified by an HIV-1 capsid ELISA (Figure 3C). The ex vivo experiment was performed as described before; however, migrated cells were washed before addition of the CCR5+ Jurkat T cells to remove dead virus particles of the initial inoculum. Only low amounts of HIV-1 p24 were detected in the untreated sheets, whereas, as expected based on the GFP expression, high amounts of viruses were detected after TNF-α and Pam3CSK4 treatment. These data demonstrate that GFP expression correlates with viral production (Figure 3C). Transmission to T cells by LCs from 4 different donors was followed over time (Figure 3D). The TLR5 agonist, flagellin, increased HIV-1 transmission in 3 donors, whereas LPS enhanced HIV-1 transmission in 1 out of 4 donors (Figure 3D). LTA did not enhance HIV-1 transmission. Thus, the effects of TLR agonists flagellin and LPS are donor dependent.
TNF-α and Pam3CSK4 enhance HIV-1 transmission by LCs ex vivo.
TNF-α and TLR ligands can induce LC migration (32, 33) and as such might contribute to enhanced HIV-1 transmission. We excluded the effect of LC migration by measuring transmission of HIV-1 by an LC-enriched single-cell suspension, consisting of 5%–10% LCs. Similar to the ex vivo model, both TNF-α and Pam3CSK4 induced HIV-1 transmission by the LCs present in the epidermal single-cell suspension (Figure 4A), demonstrating that increased transmission by these compounds is independent of LC migration.
Figure 4. TNF-α and Pam3CSK4 enhance HIV-1 transmission ex vivo.
(A) Epidermal single-cell suspensions were stimulated with TNF-α and Pam3CSK4, and after 2 hours, the cells were inoculated with HIV-1–eGFP. After 2 hours the cells were washed and CCR5+ Jurkat T cells were added for 7 days and analyzed for GFP expression by flow cytometry. A representative experiment out of 2 donors is depicted. (B) Epidermal sheets were stimulated with TNF-α, Pam3CSK4, LTA, LPS, or flagellin for 6 hours, and ex vivo transmission was determined as in Figures 1 and 3. A part of the migrated cells was extensively washed before addition of the CCR5+ Jurkat T cells (right panel). HIV-1 transmission is depicted as percentage of CCR5+ Jurkat T cells positive for GFP expression. Error bars represent the mean ± SD of duplicates. A representative experiment out of 2 donors is depicted. (C) Epidermal sheets were stimulated with TNF-α and Pam3CSK4 before incubation with HIV-1–eGFP. After 3 days, the migrated cells were washed and LCs were isolated by CD1a-selection using magnetic beads. The CD1a+ and CD1a– fraction were added to CCR5+ Jurkat T cells. The cocultures were monitored by flow cytometry at different days. Error bars represent the mean ± SD of duplicates. A representative experiment out of 2 is shown.
To further investigate a specific role for LCs during HIV-1 transmission ex vivo and to exclude direct effect of the TLR ligands, produced soluble factors, or cell-free HIV-1 on T cells, the migrated cells were washed before addition of the CCR5+ Jurkat T cells. Pam3CSK4 and TNF-α increased HIV-1 transmission with the same fold increase in this setting (Figure 4B), indicating that these compounds affect LC function in HIV-1 transmission. However, the percentage of infection was lower compared with the conditions that were not washed, indicating the presence of de novo synthesized virus in the medium or the presence of soluble factors that act on the target cells. The effect of flagellin was abrogated after washing the migrated cells (Figure 4B), indicating that flagellin directly or indirectly acts on the CCR5+ Jurkat T cells rather than on the LCs. To further confirm that Pam3CSK4 and TNF-α increase HIV-1 transmission by LCs and to exclude effects of keratinocytes or minor T cell contaminations (4.3% ± 4.5% T cells) (Figure 1), we isolated the CD1a+ LCs from the migrated cells. Only the CD1a+ LC fraction mediated HIV-1 transmission (Figure 4C), suggesting that contaminating T cells or keratinocytes are not involved in the observed effect of TNF-α and Pam3CSK4 on transmission. CD1a+ isolation resulted in a reduction of 50% ± 8.5% of the T cells (data not shown), and we observed a low percentage of LC–T cell clusters (0.47% ± 0.42%). To further investigate the contribution of the LCs, we depleted the CD3+ population, including the LC–T cell clusters. After depletion of the T cells and LC–T cell clusters, there was still a strong increase in transmission in the presence of TNF-α or Pam3CSK4 (data not shown). Thus, using the ex vivo transmission model we demonstrate that TNF-α and Pam3CSK4 increase HIV-1 transmission by LCs to T cells.
TNF-α enhances HIV-1 replication in LCs.
Next we investigated whether the TNF-α– and Pam3CSK4-mediated increase in HIV-1 transmission is the consequence of an increase in LC infection, since HIV-1 transmission by LCs under steady-state conditions depends on direct infection of LCs with HIV-1 (16, 34). Epidermal sheets were inoculated with replication-competent HIV-1–eGFP in the presence of TNF-α or Pam3CSK4, and the infection of LCs was determined by flow cytometry at day 3 (Figure 5, A and B). Low infection of LCs was detected without stimuli (Figure 5, A and B) as demonstrated previously (16). Although donor variations were observed, both TNF-α and Pam3CSK4 increased LC infection compared with the medium control (Figure 5B). Thus, both stimuli increase LC infection, which contributes to the observed increase in HIV-1 transmission by LCs (Figure 3). We have investigated the expression of CD4, CCR5, and Langerin after ex vivo stimulation of the cells with Pam3CSK4 and TNF-α. The increased infection was independent of expression of the entry receptors CD4 and CCR5 or the expression of the protective receptor Langerin, since the expression levels did not significantly change after ex vivo stimulation (Supplemental Figure 4). Langerin could also affect HIV-1 transmission after LC infection. However, no differences in Langerin expression were observed after HIV-1 infection in presence of Pam3CSK4 and TNF-α (M.A.W.P. de Jong and L. de Witte, unpublished observations). Notably, the fold increase in percentage of infected LCs after stimulation with either TNF-α or Pam3CSK4 was less pronounced than the fold increase in transmission, indicating that LCs produce more virus progeny due to increased replication or that LCs transmit initial captured virus particles. First, we investigated whether the stimuli induced HIV-1 replication in LCs by measuring the presence of the multiply spliced mRNAs encoding the HIV-1 early genes tat and rev with quantitative real-time PCR, which is a method to determine HIV-1 replication (35). Next, we investigated whether viral capture was altered by the stimuli, by measuring the amount of full-length viral RNA after 6 hours as a measure for viral particle copies. Epidermal single-cell suspensions contained 1.16% ± 1.74% CD3+ T cells (n = 8) and were incubated with HIV-1 in presence or absence of TNF-α and Pam3CSK4. After 6 hours, the cells were washed extensively and lysed for mRNA capture. Both TNF-α and Pam3CSK4 increased the production of Tat/Rev transcripts (Figure 5C), demonstrating that both stimuli induce HIV-1 production in LCs. LCs treated with Pam3CSK4 contained a high amount of full-length viral RNA (Figure 5D), strongly suggesting that Pam3CSK4 increases HIV-1 capture by LCs. We did not observe any increase in full-length viral RNA in TNF-α–treated LCs, confirming that TNF-α solely enhanced HIV-1 replication and not HIV-1 capture. Next, we determined the ratio between Tat/Rev transcripts and full-length viral RNA in the different LCs as a measure for active HIV-1 replication per viral copy. Notably, TNF-α induced a strong upregulation of HIV-1 replication per viral copy, whereas Pam3CSK4 did not increase or in some donors decreased HIV-1 replication per viral copy (Figure 5E). Similar results were obtained with emigrated LCs (M.A.W.P. de Jong and L. de Witte, unpublished observations), demonstrating that the isolation procedure does not interfere with the observed results. These data suggest that the increased Tat/Rev transcription observed in Pam3CSK4-treated LCs is due to increased HIV-1 capture and integration but not to increased rate of replication, in contrast to TNF-α treatment that solely enhances HIV-1 replication (Figure 5). Thus, TNF-α and Pam3CSK4 induce HIV-1 infection of LCs through increased replication and viral uptake, respectively.
Figure 5. TNF-α and Pam3CSK4 enhance HIV-1 infection.
(A and B) Epidermal sheets were stimulated with TNF-α or Pam3CSK4. After 6 hours, the sheets were inoculated with HIV-1–eGFP. After 3 days, the epidermal sheets were removed, the migrated cells were harvested, stained for CD86, and subsequently analyzed for GFP expression by flow cytometry. (A) Infection is depicted in dot plots. The percentage of GFP+ cells ± SD is depicted in the upper-right corner. (B) HIV-1 infection is depicted as percentage of cells positive for GFP expression. Error bars represent the mean ± SD of duplicates. The 3 depicted donors have been measured in 2 independent experiments. (C–E) Epidermal single-cells suspensions were stimulated with TNF-α or Pam3CSK4 for 30 minutes and inoculated with NL4.3-BaL. After 6 hours, the cells were washed and analyzed by quantitative real-time PCR analysis for HIV-1 replication by Tat/Rev transcripts and viral uptake by full-length viral RNAs (LTR). The Ct values were normalized for cellular GAPDH, and relative mRNA expression of HIV-1–inoculated medium control samples was set at 1. (C) Transcription per viral copy in cell was determined by the ratio of Tat/Rev mRNA and full-length viral RNA. A representative experiment out of 4 donors is depicted. Error bars represent the mean ± SD of duplicates.
Pam3CSK4 but not TNF-α increases trans-infection by LCs.
Both TNF-α and Pam3CSK4 significantly enhance HIV-1 transmission ex vivo (Figures 3 and 4). However, both stimuli induced distinct responses among the different donors, suggesting that different mechanisms are involved (Figures 3 and 5). HIV-1 transmission from monocyte-derived DCs (moDCs) to T cells can occur via de novo production of virus or via trans-infection (36), in which DCs capture HIV-1 and transfer the same virus particle to T cells. Under normal conditions, LCs do not efficiently mediate trans-infection (16), and transmission is mainly dependent on de novo virus production.
We investigated whether infection of activated LCs is indeed essential to the observed increased HIV-1 transmission using replication-defective HIV-1 (BaL-pseudotyped NL4.3-eGFPΔ envelope) in the ex vivo transmission model. Epidermal sheets were inoculated with replication-defective HIV-1 in the presence of TNF-α or Pam3CSK4, and transmission to CCR5+ Jurkat T cells was determined. Notably, Pam3CSK4 increased HIV-1 trans-infection to T cells (Figure 6, A and B). TNF-α slightly enhanced trans-infection in some donors; however, this was not significant between the different donors (Figure 6B), demonstrating that Pam3CSK4 and TNF-α increase HIV-1 transmission through different mechanisms. We measured trans-infection of single-cycle HIV-1 by LCs from an LC-enriched skin epidermal single-cell suspension. Similar results were obtained using these cell suspensions (Figure 6, C and D), demonstrating that HIV-1 trans-infection was not dependent on increased LC migration. Next, we compared transmission of single-cycle HIV-1 with replication-competent HIV-1. Pam3CSK4 increased transmission of both viruses to similar levels, suggesting that a major part of the increase of HIV-1 transmission by Pam3CSK4-stimulated LCs is mediated by trans-infection (Figure 6E). Thus, Pam3CSK4 but not TNF-α enhances trans-infection of HIV-1 by LCs.
Figure 6. Pam3CSK4 enhances trans-infection of HIV-1.
(A and B) Epidermal sheets or (C–E) epidermal single-cells suspensions were stimulated with TNF-α or Pam3CSK4 (E) after replication-competent HIV-1–eGFP to show where the virus is used. After 6 hours the sheets/cells were inoculated with replication-competent HIV-1–eGFP or single-cycle HIV-1–eGFP (HIV-1 NL4.3-eGFPΔ envelope pseudotyped with the BaL envelope). After 3 days, the epidermal sheets were removed or the cell suspensions were washed and CCR5+ Jurkat T cells were added. At day 5, the cocultures were analyzed for GFP expression by flow cytometry. (A) The response to different virus doses is depicted. (B and D) The mean of the responses of different donors are depicted, the values were analyzed by ANOVA for statistical differences. **P < 0.01. Error bars represent the mean ± SD of duplicates.
Pam3CSK4 increases HIV-1 capture by LCs.
To further investigate the mechanisms that are involved in the increased trans-infection after Pam3CSK4 stimulation, we investigated the interaction of HIV-1 and Pam3CSK4-stimulated LCs using immunofluorescence microscopy. Emigrant LCs were stimulated for 1 hour, and subsequently, inoculated with HIV-1 for 2 hours. Pam3CSK4 induced clustering of the emigrant LCs to clusters of 5–30 cells (Supplemental Figure 5A), and the cells had a more polarized morphology (Supplemental Figure 5B), indicating that the cells are activated. In unstimulated cells, single HIV-1 particles were observed in approximately 1 of 10 cells (different positive cells depicted in 7A and Supplemental Figure 6). Strikingly, multiple HIV-1 particles were observed in about 30% of the Pam3CSK4-stimulated cells (Figure 7, A and B, and Supplemental Figure 6). HIV-1 was often observed in clusters both on the cell membrane as well as in intracellular vesicles (Figure 7B and Supplemental Figure 6B). These data further confirm that Pam3CSK4 increases capture of HIV-1 by LCs. Exosomes of DCs have been implicated in trans-infection of HIV-1 (37). We therefore analyzed whether HIV-1 in Pam3CSK4-stimulated cells colocalized with HLA class I, molecules that are abundantly present in exosomes (37). In some cells, colocalization of HIV-1 and HLA class I was observed; however, virus was also observed in clusters on the cell membrane that did not colocalize with HLA class I (Supplemental Figure 7). Thus, in Pam3CSK4-stimulated cells HIV-1 is present both intracellularly and on the cell membrane.
Figure 7. Pam3CSK4 increases HIV-1 capture and primarily transmits cell-bound HIV-1.
(A and B) Emigrant LCs were stimulated with Pam3CSK4 for 1 hour and inoculated with NL4.3 BaL. After 2 hours, cells were extensively washed. The cells were fixed, permeabilized, and stained for the LC-marker CD1a and HIV-1 p24. The cells were counterstained with isotype-specific Alexa antibodies (red, HIV-1 p24; green, CD1a). Single HIV-1 particles are indicated with arrows. Original magnification, ×630. A representative experiment out of 2 is depicted. (C and D) Epidermal single-cells suspensions were stimulated with Pam3CSK4. After 6 hours, cells were inoculated with single-cycle HIV-1. After 2 hours, the cell suspensions were washed, and subsequently cells were treated with trypsin at 37°C to remove cell-bound HIV-1 or a PBS control (C), or with HIV-1 neutralizing antibody b12 at 4°C to neutralize cell-bound, but not internalized, HIV-1 and an isotype control (D). Cells were washed and CCR5+ Jurkat T cells were added. At day 5, the cocultures were analyzed for GFP expression by flow cytometry. A representative experiment out of 3 is depicted. Errors bars represent the SD of duplicates. (E) Epidermal single-cells suspension was stimulated with TNF-α or Pam3CSK4 before being inoculated with different concentrations of HIV-1–eGFP. After 2 hours, the cell suspensions were washed, and CCR5+ Jurkat T cells were added. HIV-1 transmission was followed by flow cytometry. A representative experiment out of 2 donors is depicted. Error bars represent the mean ± SD of duplicates. TCID, tissue-culture infectious dose.
To investigate whether intracellular or extracellular HIV-1 is transmitted to the target cells, we treated the epidermal single-cell suspension with trypsin after incubation with the single-cycle HIV-1. Strikingly, trypsin treatment abrogated the increased transmission induced by Pam3CSK4 (Figure 7C), demonstrating that primarily cell-bound HIV-1 is transmitted after Pam3CSK4 treatment. To confirm these results, we selectively neutralized cell-surface bound HIV-1 using the broadly anti–HIV-1 neutralizing antibody b12 that binds to HIV-1 gp120 and prevents binding to CD4 or chemokines receptors (38). Pam3CSK4-treated LCs were incubated with HIV-1 for 2 hours at 37°C, washed, and subsequently treated with the neutralizing antibody b12 at 4°C to neutralize cell-bound but not internalized HIV-1. Strikingly, addition of this antibody completely abolished the transmission induced by Pam3CSK4 (Figure 7D), without affecting the viability (data not shown). Therefore, these data demonstrate that Pam3CSK4 increases HIV-1 binding to LCs and transmission of these mostly cell-bound virus particles to target cells.
The high viral concentrations needed in the ex vivo model to investigate transmission have been shown to saturate the protective function of Langerin (16). Therefore, we investigated whether TNF-α and Pam3CSK4 enhance transmission at low viral titers using isolated LCs. At low viral concentrations, Pam3CSK4, in contrast to TNF-α, strongly induced HIV-1 transmission to T cells (Figure 7E), suggesting that Pam3CSK4 overcomes the protective Langerin function. In contrast, TNF-α was only able to enhance HIV-1 transmission at those virus concentrations that already allowed transmission, supporting our data that TNF-α–mediated transmission is dependent on primary LC infection.
Discussion
In order to reduce the growing HIV-1 pandemic, there is a strong pressure to understand HIV-1 transmission. Sexual transmission of HIV-1 is the major route of infection worldwide (1), and little is known about the factors governing HIV-1 transmission and susceptibility to HIV-1. Genital coinfection is a risk factor for acquiring HIV-1 (2), indicating that pathogens or host responses to the pathogens create a genital environment that favors HIV-1 transmission. Here we demonstrate a potential role for LCs in the increased susceptibility to HIV-1 during fungal and bacterial coinfections.
Due to their epithelial localization and function, LCs are the first cells to encounter HIV-1 in the genital epithelial tissues (12, 13). Several studies suggest that HIV-1 subverts the function of LCs for viral transmission to T cells, thereby infecting the host (18, 34). However, other reports argue that not LCs but T cells and subepithelial antigen-presenting cells are the first cells infected by HIV-1 (14, 15, 39). Moreover, our recent data demonstrate that LCs are not efficiently infected by HIV-1 and form a barrier to HIV-1 infection through the function of the C-type lectin Langerin (16); Langerin captures HIV-1, resulting in efficient virus degradation, which prevents infection. However, Langerin inhibitors or high virus concentrations (>100 ng p24/106 LCs) that saturate Langerin allow LC infection and subsequent HIV-1 transmission (16). These data suggest that under normal conditions LCs function as a barrier but that specific conditions might change LC function and promote transmission. Using an ex vivo transmission model, we demonstrate here that indeed HIV-1 transmission by LCs is low and inefficient; high concentrations of HIV-1 (30 ng p24 per epidermal sheets, containing approximately 105 LCs) were needed to observe transmission by LCs. Strikingly, activation of LCs by TNF-α or Pam3CSK4 induces a strong increase in transmission of HIV-1, suggesting that under conditions of high viral loads and inflammation, LCs play a pivotal role in HIV-1 transmission.
Inflammatory conditions induce LC maturation, which includes downregulation of antigen capture and processing as well as increased migration from the periphery to the lymphoid tissues. Monocyte-derived LC-like cells are more susceptible to HIV-1 infection after activation with CD40L (40). Moreover, a recent study demonstrated that CD34+-derived LC-like cells only efficiently transfer HIV-1 after activation by a combination of LPS and TNF-α (41). Although primary LCs are unresponsive to LPS (21), these studies using LC-like models suggest that LC activation is an important determinant in whether LCs protect against HIV-1 infection or transmit the virus to T cells. Moreover, the cellular environment might also be pivotal to LC function in HIV-1 transmission. Therefore, we have used an ex vivo skin model to investigate the role of primary LCs and the environment in HIV-1 transmission during coinfections and inflammatory conditions. Although the LC fractions contained a small percentage of T cells, we were able to demonstrate the specific contribution of LC by using CD1a isolation and CD3 depletion.
Coinfections with pathogens activate LCs through TLR triggering. Therefore, we investigated whether different bacterial TLR agonists influence HIV-1 transmission in the ex vivo model. We demonstrated that TLR2, TLR4, and TLR5 agonists increase HIV-1 transmission in tissues derived from some donors, whereas no increase was observed in other donors. Notably, the TLR4 agonist LPS did induce HIV-1 transmission by LCs in some donors, even though LCs do not express TLR4 (L. de Witte and M.A.W.P. de Jong, unpublished observations) (20, 21). These data suggest that the observed increase for at least the TLR4 agonist is due to an indirect activation of LCs, such as production of proinflammatory cytokines by keratinocytes, since keratinocytes and epithelial cells do express TLR4 (20). Indeed, we observed the production of TNF-α after LPS stimulation of skin biopsies. This might be variable throughout donors due to different levels of TLR expression and activity, resulting in the variation in HIV-1 susceptibility (16). The TLR5 agonist flagellin enhanced HIV-1 transmission in our ex vivo model in half of the donors. However, the effect of flagellin was indirect, since the increased transmission was abrogated after washing the emigrated LCs and flagellin did not increase HIV-1 capture or replication in LCs as determined by RT-PCR (L. de Witte and M.A.W.P. de Jong, unpublished observations). Moreover, flagellin itself did not increase susceptibility of CCR5+ Jurkat T cells (L. de Witte and M.A.W.P. de Jong, unpublished observations), suggesting that flagellin induces the production of a soluble factor by surrounding cells that enhances infection of T cells. Furthermore, TNF-α neutralization by antibodies in the ex vivo experiment did not prevent the increase of transmission by flagellin (Supplemental Figure 8), suggesting that TNF-α is not involved in the enhanced transmission by flagellin.
In contrast to the other TLR agonists, Pam3CSK4 enhanced transmission in all donors tested. Pam3CSK4 is a synthetic tripalmitoylated lipopeptide that mimics the acetylated amino terminus of bacterial lipoproteins (42, 43). Recognition of Pam3CSK4 is mediated by a dimer of TLR1 and TLR2 (44). LCs and keratinocytes both express TLR1 and TLR2 (20, 21, 45). Neutralizing antibodies against TNF-α did not abrogate the Pam3CSK4 induction of HIV-1 transmission (Supplemental Figure 8), demonstrating that Pam3CSK4 increased transmission independent of TNF-α. Using a single-cycle replication-defective HIV-1, we demonstrate that the increase in HIV-1 transmission by Pam3CSK4-stimulated LCs is independent of HIV-1 infection of LCs, demonstrating that the increase is at least partly due to trans-infection of T cells; HIV-1 capture by LCs is increased and the surface-bound viruses are efficiently transmitted to T cells. This is supported by our finding that HIV-1 capture by LCs is strongly increased after Pam3CSK4 stimulation. Single particles and clusters of HIV-1 are observed intracellularly and on the cell membrane, some colocalizing with HLA class I molecules, which might reflect exosomes. Although HIV-1 was observed in intracellular vesicles, cell-bound HIV-1 was primarily transmitted after Pam3CSK4 stimulation. Pam3CSK4 might upregulate the expression of receptors that increase HIV-1 capture, such as heparan sulfate proteoglycans (46). However, heparinase treatment did not abrogate HIV-1 transmission after Pam3CSK4 treatment (L. de Witte and M.A.W.P. de Jong, unpublished observations), indicating that heparan sulfates are not involved. Possibly, Pam3CSK4 enhances LC activation, which results in increased HIV-1 binding. Notably, enhanced HIV-1 capture by Pam3CSK4 is observed within 30 minutes (L. de Witte and M.A.W.P. de Jong, unpublished observations) and Pam3CSK4-stimulated LCs rapidly change phenotypically and form clusters. A recent report demonstrated that Pam3CSK4 stimulation in T cells results in the activation of NF-κB and upregulation of the expression of CCR5. After 24 hours of stimulation, these activated T cells are more susceptible to both X4- and R5-tropic HIV-1 (47). However, we observed increased trans-infection, which is independent of fusion of HIV-1 with LCs, strongly suggesting that increased HIV-1 capture but not infection is responsible for transmission after Pam3CSK4 stimulation.
TLR agonists as well as whole pathogens can increase HIV-1 transmission indirectly by changing the cytokine environment. Indeed, TLR agonists, Neisseria gonorrhea and the different forms of Candida albicans induced the production of proinflammatory cytokine TNF-α in vaginal and skin tissues ex vivo. Therefore, we investigated what the effect of proinflammatory cytokine TNF-α was on HIV-1 transmission. Our data demonstrate that TNF-α strongly upregulated HIV-1 transmission by LCs ex vivo, suggesting that LC activation by TNF-α leads to increased susceptibility to HIV-1 under coinfection circumstances. Further study of the molecular mechanism demonstrates that TNF-α increased the level of HIV-1 replication in infected LCs, which results in enhanced HIV-1 transmission of de novo produced viruses to T cells. This is further supported by the need for HIV-1 replication in LCs, since TNF-α– in contrast to Pam3CSK4-treated LCs do not transmit replication-defective HIV-1 to T cells ex vivo. Moreover, TNF-α did not increase HIV-1 capture by LCs but enhanced initiation of HIV-1 transcription as determined by the increase in the multiply spliced transcripts of the early HIV-1 genes tat and rev. These data are supported by previous studies demonstrating that TNF-α enhances replication of HIV-1 in T cells and macrophages (27). These data strongly suggest that coinfections abrogate the protective barrier function of LCs and allow LC infection and subsequent HIV-1 transmission. Inflammation not only affects LC function but also epithelial and sometimes subepithelial tissues, resulting in the production of inflammatory factors, influx of immune cells, ulceration, and bleeding. Different mechanisms have been proposed to explain the increased risk of HIV-1 acquisition during coinfection such as genital ulceration (6). However, we here demonstrate that the role of LCs should be taken into account.
We have previously shown that Langerin protects LCs from HIV-1 infection and thereby prevents HIV-1 transmission (16). Here we have used high viral concentrations in the ex vivo transmission model, which were previously shown to saturate the Langerin function (16). Titration of HIV-1 in the transmission assays suggests that Pam3CSK4 but not TNF-α activation overcomes the protective function of Langerin. Pam3CSK4 induced viral transmission by LCs even at low concentrations when Langerin was not saturated. In contrast, TNF-α only increased HIV-1 transmission at concentrations that already allowed HIV-1 transmission under normal conditions. Since TNF-α enhances HIV-1 transcription, low levels of infection are a prerequisite for TNF-α to enhance HIV-1 transmission and therefore dependent on the Langerin function. In contrast, Pam3CSK4 enhances capture of HIV-1 for transmission to T cells. Therefore, at low viral loads, there could be competition between (unknown) surface attachment receptors and Langerin for HIV-1. Our data suggest that LC activation by Pam3CSK4 is strong enough to negate the protection by Langerin. From the observed mechanisms, we speculate that the presence of TNF-α is extremely important when Langerin function has been compromised and initial infection has taken place. In contrast, TLR1/TLR2 activation by pathogens during coinfections will overcome the Langerin barrier and induce HIV-1 transmission by LCs through increased HIV-1 uptake and transmission to T cells, thereby strongly enhancing the susceptibility of the host to HIV-1 infection. In our model, no differences in surface Langerin expression were observed after addition of TNF-α or Pam3CSK4. However, we cannot exclude that the degradation function or clustering of Langerin is hampered in the presence of these compounds. Further studies will be necessary to investigate the effects of LC activation on Langerin function.
Since the development of an effective vaccine or a curative treatment for HIV-1 is not progressing rapidly, the WHO stated that focus should be on the development of an agent that prevents HIV-1 transmission and that can be applied topically, such as a genital cream (1). Our results indicate that coinfection results in a changed LC phenotype that can result in transmission, mediated through different mechanisms. To prevent LC infection and subsequent transmission, it is attractive to try to block the entry receptors of HIV-1 on the LCs, such as CD4 and CCR5 (48). However, TLR activation by coinfecting pathogens might increase HIV-1 transmission by yet unknown mechanisms, such as the increased capture of HIV-1 and subsequent trans-infection observed with Pam3CSK4 treatment. Since these mechanisms are potential targets for therapies these need to be further unravelled. Since TNF-α enhances LC infection and subsequent transmission, the cytokine itself or its receptor represent targets to prevent HIV-1 transmission. Moreover, these results emphasize the argument that prevention and fast intervention to treat coinfections and especially chronic coinfections should be taken as a serious point in the prevention of HIV-1 transmission.
Methods
Antibodies and reagents.
The following reagents were used: tripalmitoylated lipopeptide Pam3CSK4 (Invivogen), LTA from Staphylococcus aureus (Sigma-Aldrich), LPS from Salmonella Typhosa (Sigma-Aldrich), flagellin derived from Bacillus Subtilis (Invivogen), recombinant human TNF-α (Strathmann Biotec), DCGM4-PE (anti-Langerin; Beckman Coulter Inc.), HI149-FITC (anti-CD1a; Pharmingen), goat anti–mouse-FITC (Zymed Laboratories Inc.), HA5.2B7-PE (CD86; Immunotech), 2D7-PE (anti-CCR5; Pharmingen), A-07751-PE (anti-CD4), anti–TNF-α (Biovision), KC57-RD1-PE (anti–HIV-1 p24; Beckman Coulter Inc.), HLA-ABC-m3-FITC (anti–HLA-B27; Abcam), NA1/34 (anti-CD1a; Dako Cytomation), IgG PE isotype (BD Biosciences), IgG1 isotype control (mouse IgG1; Biolegend), and HIV-1 neutralizing antibody b12 (obtained from the NIH AIDS Research and Reference Reagent Program from Dennis Burton and Carlos Barbas [both at The Scripps Research Institute, La Jolla, California, USA]). The following plasmids were generously provided by C. Aiken (Vanderbilt University, Nashville, Tennessee, USA): BaL envelope, pNL4.3eGFPΔ envelope, and pNL4.3eGFP-BaL. The human CCR5 lentiviral vector pLOX (LV-CCR5) was generously provided by V. Piguet (University Hospital and Medical School of Geneva, Geneva, Switzerland) (49, 50).
Cell lines and viruses.
Jurkat T cells expressing CCR5 were generated by retroviral transduction as previously described (49, 50), and 293T cells were transfected with NL4.3-BaL or NL4.3-eGFP-BaL proviral plasmids (9 μg) together with VSV-G envelope plasmid (1 μg). At day 2, viruses were harvested and used to infect Jurkat T cells to generate virus stocks. For single-round infection assay, 293T cells were transfected with pNL4.3eGFPΔ envelope (4 μg) and BaL envelope plasmids (2 μg) and virus was harvested on day 3. All virus stocks were quantified by p24 ELISA (Perkin Elmer Life Sciences) and titrated using the indicator cells TZM-blue (contributed by John C. Kappes, Xiaoyun Wu [both at University of Alabama, Birmingham, Alabama, USA], and Tranzyme Inc. through the NIH AIDS Research and Reference Reagent Program) (51).
Ex vivo assays.
Human tissue was obtained from healthy donors undergoing corrective breast or abdominal surgery after informed consent in accordance with our institutional guidelines. Epidermal sheets (1 cm2) were prepared as described previously (16) and incubated with Pam3CSK4 (5 μg/ml), LPS (10 μg/ml), LTA (10 μg/ml), flagellin (1 μg/ml), or TNF-α (0.1 μg/ml). TNF-α was titrated in the ex vivo experiments for optimal HIV-1 transmission, Pam3CSK4 was titrated for optimal HIV-1 infection of Jurkat cells, and the other ligands were used at concentrations that activate DCs (20, 46). After 6 hours, the sheets were inoculated with HIV-1 NL4.3-BaL-eGFP (30 ng p24 [12 × 103 IU]/sheet) or the single-round BaL-pseudotyped NL4.3-eGFPΔ envelope (10 or 100 ng p24/sheet; 5 × 102 and 5 × 103 IU/sheet). After 3 days, the sheets were removed and CCR5+ Jurkat T cells (2 × 105 cells) were added. Infection was monitored by flow cytometry, p24 ELISA, and immunofluorescence microscopy (original magnification, ×200; Leica DMIL fluorescence microscope; Leica Microsystems).
LC transmission.
LC-enriched epidermal single-cell suspensions were generated as described (16). As determined by CD1a-staining, these single-cell suspensions generally contained 5%–10% LCs. Although trypsin digestion might result in loss of some CD4 expression (52), isolated LCs were infected by HIV-1 (16). Epidermal cells (1.5 × 105 cells) were preincubated with Pam3CSK4 (5 μg/ml) or TNF-α (0.1 μg/ml) for 4 hours and inoculated with HIV-1 NL4.3-BaL-eGFP (20 ng p24 [8 × 103 IU]/sheet) or the single-round BaL-pseudotyped NL4.3-eGFPΔ envelope (50 ng p24 [2.5 × 103 IU]/sheet). At day 3, cells were washed and CCR5+ Jurkat T cells (2 × 105 cells) were added. Infection was measured by flow cytometry.
Trypsin treatment.
LCs (5 × 104 cells) were preincubated with Pam3CSK4 (5 μg/ml) for 30 minutes and subsequently inoculated with BaL-pseudotyped NL4.3-eGFPΔ envelope (50 ng p24 [2.5 × 103 IU]/sheet) for 2 hours. To cleave off HIV-1, LCs were washed in PBS and subsequently incubated with 50 μl of a trypsin solution (0.05% trypsin in PBS) for 10 minutes. By washing 3 times in complete Iscove’s modified Dulbecco’s medium trypsin was inactivated. To neutralize cell-bound but not internalized HIV-1, neutralizing antibody b12 or an isotype control was added to the cells (20 μg/ml) for 30 minutes at 4°C to prevent internalization of the antibody. Cells were washed to remove unbound antibody and CCR5+ Jurkat T cells (2 × 104 cells) were added, and transmission was determined by flow cytometry. Viability of the cells after trypsin treatment was determined by trypan blue staining and was more than 90%.
Ex vivo stimulation.
Biopsies of fresh skin and vagina tissue were taken within 3 hours after the operation using a 6-mm biopsy punch (Microtek Medical). The biopsies were floated in a 48-well plate on 250 μl Iscove’s modified Dulbecco’s medium, 10% FCS, and 10 μg/ml gentamycine and stimulated with heat-killed or live Candida albicans (0.25 × 106/ml) in the presence or absence of Amphotericin B (25 μg/ml) (53) and paraformaldehyde-inactivated Neisseria gonorrhoea LGTD (2.5 × 106/ml), generously provided E.C. Gotschlich (Rockefeller University, New York City, New York, USA) (54), LPS (10 μg/ml), or LTA (10 μg/ml). After 24 hours, supernatant was collected and frozen. The samples were analyzed for different cytokines by ELISA (Biosource International).
Viral replication.
Epidermal single cells (5 × 104 cells) were preincubated with Pam3CSK4 (5 μg/ml) or TNF-α (0.1 μg/ml) for 30 minutes and inoculated with HIV-1 NL4.3-BaL (20 ng p24 [2 × 103 IU]/sheet). After 6 hours, the cells were washed extensively with PBS, after which host mRNA and viral RNA were specifically isolated with the mRNA capture kit (Roche). For real-time PCR analysis, PCR amplification was performed in the presence of SYBR green, as previously described (55). Multiply spliced HIV-1 transcripts were detected with primers that detect both Tat and Rev mRNAs across an intron. Full-length viral RNA was detected with primers against the long-terminal repeat (LTR) U5 sequence (forward) and the gag coding sequence (reverse). The sequences are as follows: HIV-1 LTR-gag, forward, GTGTGGAAAATCTCTAGCAGTGG, reverse, CGCTCTCGCACCCATCTC; HIV-1 Tat-Rev, forward, ATGGCAGGAAGAAGCGGAG, reverse, ATTCCTTCGGGCCTGTCG. Specific primers for HIV-1 LTR, Tat/Rev, and GAPDH (55) were designed by Primer Express 2.0 (Applied Biosystems). Transcription was adjusted for GAPDH transcription, and relative mRNA expression of HIV-1–infected control samples was set at 1.
Immunofluorescence microscopy.
LCs (2 × 105 cells) were preincubated with Pam3CSK4 (5 μg/ml) for 1 hour at 37°C. The cells were inoculated with HIV-1 NL4.3-BaL (200 ng p24 [2 × 104 IU]/sheet). The cells were washed, stained for p24, and analyzed by confocal microscopy (Leica AOBS SP2 CSLM system).
Statistics.
Values for percentage of GFP-expressing CCR5+ Jurkat T cells for 1 donor are calculated as the mean of duplicate measurements and represented as the mean ± SD of the duplicate. To determine the variation in transmission among different donors, the mean values of GFP expression of CCR5+ Jurkat T cells of different donors were analyzed with ANOVA. When the overall F test was significant, differences among the donors were further investigated with the post-hoc Bonferroni test using Graphpad Prism software. A probability of P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We are grateful to the Boerhaave Kliniek for providing us with their valuable support. We would like to thank T. van Montfort and W.A. Paxton from Academic Medical Center (Amsterdam, The Netherlands) for providing help with the neutralization assays. We are grateful to M.A.A. van Trotsenburg from the VU Medical Center for providing the tissues and his advice. We greatly thank the AIDS Research and Reference Program for various antibodies, cells, and viruses. M.A.W.P. de Jong and L. de Witte were supported by grants from the Dutch Organization for Scientific Research program (NWO) (NWO 912-04-025, to L. de Witte; NWO 917-46-367, to M.A.W.P. de Jong). S.I. Gringhuis is supported by the Dutch Asthma Foundation (3.2.03.39).
Footnotes
Nonstandard abbreviations used: LC, Langerhans cell; LTA, lipoteichoic acid; Pam3CSK4, Pam3CysSerLys4.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:3440–3452 (2008). doi:10.1172/JCI34721
Marein A.W.P. de Jong and Lot de Witte contributed equally to this work.
References
- 1. Joint United Nations Programme on HIV/AIDS and World Health Organisation 2007. 2007 AIDS epidemic update. http://www.unaids.org . [Google Scholar]
- 2.Royce R.A., Sena A., Cates W., Jr., Cohen M.S. Sexual transmission of HIV. N. Engl. J. Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 3.Ito A., et al. Monoclonal antibody (5F3) defining renal cell carcinoma-associated antigen disialosyl globopentaosylceramide (V3NeuAcIV6NeuAcGb5), and distribution pattern of the antigen in tumor and normal tissues. Glycoconj. J. 2001;18:475–485. doi: 10.1023/A:1016281002344. [DOI] [PubMed] [Google Scholar]
- 4.Naarding M.A., et al. Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes. J. Clin. Invest. 2005;115:3256–3264. doi: 10.1172/JCI25105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munch J., et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Fleming D.T., Wasserheit J.N. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm. Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen M.S. HIV and sexually transmitted diseases: lethal synergy. Top. HIV Med. 2004;12:104–107. [PubMed] [Google Scholar]
- 8.Hester R.A., Kennedy S.B. Candida infection as a risk factor for HIV transmission. . J. Womens Health (Larchmt.). 2003;12:487–494. doi: 10.1089/154099903766651612. [DOI] [PubMed] [Google Scholar]
- 9.Wasserheit J.N. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm. Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 10.Sheffield J.S., Wendel G.D., Jr., McIntire D.D., Norgard M.V. Effect of genital ulcer disease on HIV-1 coreceptor expression in the female genital tract. J. Infect. Dis. 2007;196:1509–1516. doi: 10.1086/522518. [DOI] [PubMed] [Google Scholar]
- 11.de Witte L., Nabatov A., Geijtenbeek T.B. Distinct roles for DC-SIGN+-dendritic cells and Langerhans cells in HIV-1 transmission. . Trends Mol. Med. 2008;14:12–19. doi: 10.1016/j.molmed.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Kawamura T., Kurtz S.E., Blauvelt A., Shimada S. The role of Langerhans cells in the sexual transmission of HIV. J. Dermatol. Sci. 2005;40:147–155. doi: 10.1016/j.jdermsci.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Patterson B.K., et al. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am. J. Pathol. 2002;161:867–873. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spira A.I., et al. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z., et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 16.de Witte L., et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 2007;13:367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 17.Pope M., et al. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 18.Reece J.C., et al. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J. Exp. Med. 1998;187:1623–1631. doi: 10.1084/jem.187.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berthier-Vergnes O., et al. TNF-alpha enhances phenotypic and functional maturation of human epidermal Langerhans cells and induces IL-12 p40 and IP-10/CXCL-10 production. FEBS Lett. 2005;579:3660–3668. doi: 10.1016/j.febslet.2005.04.087. [DOI] [PubMed] [Google Scholar]
- 20.van der Aar A.M., et al. Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J. Immunol. 2007;178:1986–1990. doi: 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- 21.Flacher V., et al. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. . J. Immunol. 2006;177:7959–7967. doi: 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]
- 22.Chesson H.W., Pinkerton S.D. Sexually transmitted diseases and the increased risk for HIV transmission: implications for cost-effectiveness analyses of sexually transmitted disease prevention interventions. J. Acquir. Immune Defic. Syndr. 2000;24:48–56. doi: 10.1097/00126334-200005010-00009. [DOI] [PubMed] [Google Scholar]
- 23.Sugita K., et al. Innate immunity mediated by epidermal keratinocytes promotes acquired immunity involving Langerhans cells and T cells in the skin. Clin. Exp. Immunol. 2007;147:176–183. doi: 10.1111/j.1365-2249.2006.03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatterji U., et al. Naturally occurring capsid substitutions render HIV-1 cyclophilin A independent in human cells and TRIM-cyclophilin-resistant in Owl monkey cells. J. Biol. Chem. 2005;280:40293–40300. doi: 10.1074/jbc.M506314200. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura T., et al. Candidate microbicides block HIV-1 infection of human immature Langerhans cells within epithelial tissue explants. J. Exp. Med. 2000;192:1491–1500. doi: 10.1084/jem.192.10.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osborn L., Kunkel S., Nabel G.J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc. Natl. Acad. Sci. U. S. A. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuyama T., et al. Enhancement of HIV replication and giant cell formation by tumor necrosis factor. AIDS Res. Hum. Retroviruses. 1989;5:139–146. doi: 10.1089/aid.1989.5.139. [DOI] [PubMed] [Google Scholar]
- 28.Netea M.G., Van der G.C., Van Der Meer J.W., Kullberg B.J. Recognition of fungal pathogens by Toll-like receptors. Eur. J. Clin. Microbiol. Infect. Dis. 2004;23:672–676. doi: 10.1007/s10096-004-1192-7. [DOI] [PubMed] [Google Scholar]
- 29.Fisette P.L., Ram S., Andersen J.M., Guo W., Ingalls R.R. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kappaB activation in epithelial cells in a Toll-like receptor 2-dependent manner. J. Biol. Chem. 2003;278:46252–46260. doi: 10.1074/jbc.M306587200. [DOI] [PubMed] [Google Scholar]
- 30.Romani L., Bistoni F., Puccetti P. Fungi, dendritic cells and receptors: a host perspective of fungal virulence. Trends Microbiol. 2002;10:508–514. doi: 10.1016/S0966-842X(02)02460-5. [DOI] [PubMed] [Google Scholar]
- 31.Underhill D.M. Toll-like receptors and microbes take aim at each other. Curr. Opin. Immunol. 2004;16:483–487. doi: 10.1016/j.coi.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Kimber I., Cumberbatch M. Stimulation of Langerhans cell migration by tumor necrosis factor alpha (TNF-alpha). J. Invest. Dermatol. 1992;99:48S–50S. doi: 10.1111/1523-1747.ep12668986. [DOI] [PubMed] [Google Scholar]
- 33.Roake J.A., et al. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J. Exp. Med. 1995;181:2237–2247. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawamura T., et al. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8401–8406. doi: 10.1073/pnas.1432450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cullen B.R. Human immunodeficiency virus as a prototypic complex retrovirus. J. Virol. 1991;65:1053–1056. doi: 10.1128/jvi.65.3.1053-1056.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turville S.G., et al. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 2004;103:2170–2179. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- 37.Wiley R.D., Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc. Natl. Acad. Sci. U. S. A. 2006;103:738–743. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganesh L., et al. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J. Virol. 2004;78:11980–11987. doi: 10.1128/JVI.78.21.11980-11987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta P., et al. Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J. Virol. 2002;76:9868–9876. doi: 10.1128/JVI.76.19.9868-9876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawamura T., Qualbani M., Thomas E.K., Orenstein J.M., Blauvelt A. Low levels of productive HIV infection in Langerhans cell-like dendritic cells differentiated in the presence of TGF-beta1 and increased viral replication with CD40 ligand-induced maturation. Eur. J. Immunol. 2001;31:360–368. doi: 10.1002/1521-4141(200102)31:2<360::AID-IMMU360>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 41.Fahrbach K.M., et al. Activated CD34-derived Langerhans cells mediate transinfection with human immunodeficiency virus. J. Virol. 2007;81:6858–6868. doi: 10.1128/JVI.02472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffmann P., et al. Stimulation of human and murine adherent cells by bacterial lipoprotein and synthetic lipopeptide analogues. Immunobiology. 1988;177:158–170. doi: 10.1016/S0171-2985(88)80036-6. [DOI] [PubMed] [Google Scholar]
- 43.Aliprantis A.O., et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 44.Ozinsky A., et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeda K., Kaisho T., Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 46.de Witte L., et al. Syndecan-3 is a dendritic cell-specific attachment receptor for HIV-1. Proc. Natl. Acad. Sci. U. S. A. 2007;104:19464–19469. doi: 10.1073/pnas.0703747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thibault S., Tardif M.R., Barat C., Tremblay M.J. TLR2 signaling renders quiescent naive and memory CD4+ T cells more susceptible to productive infection with X4 and R5 HIV-type 1. . J. Immunol. 2007;179:4357–4366. doi: 10.4049/jimmunol.179.7.4357. [DOI] [PubMed] [Google Scholar]
- 48.Kawamura T., et al. PSC-RANTES blocks R5 human immunodeficiency virus infection of Langerhans cells isolated from individuals with a variety of CCR5 diplotypes. J. Virol. 2004;78:7602–7609. doi: 10.1128/JVI.78.14.7602-7609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arrighi J.F., et al. Lentivirus-mediated RNA interference of DC-SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells. J. Virol. 2004;78:10848–10855. doi: 10.1128/JVI.78.20.10848-10855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arrighi J.F., et al. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J. Exp. Med. 2004;200:1279–1288. doi: 10.1084/jem.20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei X., et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 2002;46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lynch G.W., et al. CD4 is expressed by epidermal Langerhans’ cells predominantly as covalent dimers. Exp. Dermatol. 2003;12:700–711. doi: 10.1034/j.1600-0625.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 53.Ito A., Handa K., Withers D.A., Satoh M., Hakomori S. Binding specificity of siglec7 to disialogangliosides of renal cell carcinoma: possible role of disialogangliosides in tumor progression. FEBS Lett. 2001;498:116–120. doi: 10.1016/S0014-5793(01)02476-0. [DOI] [PubMed] [Google Scholar]
- 54.Gotschlich E.C. Genetic locus for the biosynthesis of the variable portion of Neisseria gonorrhoeae lipooligosaccharide. J. Exp. Med. 1994;180:2181–2190. doi: 10.1084/jem.180.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia-Vallejo J.J., et al. Approach for defining endogenous reference genes in gene expression experiments. Anal. Biochem. 2004;329:293–299. doi: 10.1016/j.ab.2004.02.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.