Abstract
PURPOSE
As the first and only manifestation of ischemic heart disease, sudden unexpected cardiac death (SUCD) is a serious clinical and epidemiological concern. Prospective population studies permit the identification of risk factors for SUCD. Knowledge of the short-and long-term risks for SUCD are key to understanding the basis of any intervention. The present paper explores the effect of time since the detection of factors on the risk for SUCD.
SUBJECTS AND METHODS
The Manitoba Follow-Up Study is a longitudinal, prospective study of 3983 originally healthy young men who have been followed with routine medical examinations since 1948. During 56 years of follow-up, SUCD occurred in 171 men. This analysis examined 21 possible risk factors for SUCD, including clinical findings, social variables and electrocardiographic abnormalities. Time-dependent covariate Cox proportional hazard models were used to estimate age-adjusted relative risks for SUCD. In multivariate models, the relative risk of SUCD was estimated as a function of time since the documentation of each risk factor.
RESULTS
Excess alcohol consumption and T wave changes were associated with a high short-term risk for SUCD. Arterial hypertension and ST/T changes had sustained excess risk over both the short and long term. Newly developed left bundle branch block was a highly significant short-term risk that diminished with time.
CONCLUSION
These findings add new information for the clinical management of risk factors. The identification of time since the detection of these risk factors is an important consideration to reduce SUCD.
Keywords: Epidemiology, Longitudinal study, Risk factors, Sudden unexpected cardiac death, Time relationship
Abstract
OBJECTIF
À titre de première et seule manifestation d’une cardiopathie ischémique, la mort cardiaque subite et inattendue (MCSI) est une préoccupation clinique et épidémiologique sérieuse. Des études de population prospectives permettent de repérer les facteurs de risque de la MCSI. Il est essentiel de connaître les risques à court et à long terme de la MCSI pour comprendre le fondement de toute intervention. Le présent article analyse l’effet du temps après la détection de facteurs affectant le risque de MCSI.
SUJETS ET MÉTHODOLOGIE
L’étude de suivi du Manitoba est une étude longitudinale prospective auprès de 3 983 jeunes hommes en santé au départ, suivis dans le cadre d’examens médicaux réguliers depuis 1948. Pendant ce suivi de 56 ans, une MCSI a tué 171 hommes. Cette analyse a permis de repérer 21 facteurs de risque possibles de MCSI, y compris les observations cliniques, les variables sociales et les anomalies électrocardiographiques. Les modèles des hasards proportionnels de Cox au moyen de covariables en fonction du temps ont été utilisés pour évaluer les risques relatifs de MCSI ajustés selon l’âge. Dans des modèles multivariés, le risque relatif de MCSI a été évalué comme fonction du temps depuis la documentation de chaque facteur de risque.
RÉSULTATS
Une consommation d’alcool excessive et des changements de l’onde T s’associent à un risque élevé de MCSI à court terme. L’hypertension artérielle et les changements ST/T s’accompagnaient d’un risque supplémentaire soutenu tant à court qu’à long terme. De nouveaux blocs de branche gauche représentaient un risque considérable à court terme, qui diminuait au fil du temps.
CONCLUSION
Ces observations s’ajoutent à l’information utile pour la prise en charge clinique des facteurs de risque. L’identification du temps après la détection de ces facteurs de risque constitue un facteur important pour réduire la MCSI.
Coronary heart disease is the leading cause of death worldwide (1). In 1997, it accounted for approximately 36% of all deaths in Canada (1). Sudden cardiac death has been defined, according to the World Health Organization, as death within 24 h of cardiac symptoms (2). Ischemic heart disease (IHD) is manifested in one out of every three Canadian men during their lifetime (3) and may be diagnosed as angina pectoris, myocardial infarction or sudden cardiac death. Sudden cardiac death accounts for 25% of all deaths due to IHD (4). Sudden unexpected cardiac death (SUCD) has been defined as death in the absence of prior clinical or electrocardiographic (ECG) evidence of IHD, and accounts for 15% of all IHD (4).
In North America, the incidence rates of angina pectoris and myocardial infarction have fallen over the past few decades, attributed mainly to primary and secondary prevention strategies, while the incidence of SUCD has remained relatively constant (4–8). Although the risk factors for SUCD closely parallel those for all IHD, the incidence of SUCD can only be reduced through primary prevention because there are no prior clinical cardiac indications (9). Hence, only prospective studies of healthy people with routinely collected data can monitor specific risk factors for SUCD. Risk factors that can be detected by routine noninvasive examinations and that allow for the determination of time effects on the risk for SUCD are especially important in designing appropriate interventions.
The Manitoba Follow-Up Study (MFUS) is an ongoing, prospective, longitudinal study of cardiovascular disease in 3983 men. The prospective design of MFUS and routine medical examination of study members makes it possible to document the onset of clinical findings or ECG abnormalities and, hence, to estimate the risk of SUCD as a function of the time since the detection of these risk factors, and to delineate short-and long-term risks.
METHODS
The MFUS has followed a cohort of 3983 men since July 1, 1948. This cohort is comprised primarily of aircrew recruits to the Royal Canadian Air Force during World War II. All cohort members were free of evidence of IHD upon entry to the study. Their mean age was 31 years, with 90% aged 20 to 39 years. Annual contact with cohort members has been maintained, and routine medical examinations, resting ECGs, blood pressure readings and body build measurements obtained. The initial aim of the MFUS was to investigate the prognostic significance of ECG abnormalities as they appeared in otherwise healthy individuals (10). By 2004, 1323 men, or 33% of the cohort, were alive, at a mean age of 84 years. The MFUS is ongoing, with other research interests extended to explore the parameters of successful aging (11).
Risk factors for SUCD
Twenty-one factors, including 10 clinical findings, three social factors and eight ECG abnormalities identified during routine contacts, surveys and medical examinations with cohort members over the 56 years of follow-up were considered as risk factors. Only risk factors noted before the diagnosis of IHD were considered.
Clinical risk factors included aspects of blood pressure, clinical vascular disease, body build and lifestyle choices. Specifically, a high resting heart rate was defined as a resting heart rate of at least 100 beats/min on two successive examinations. Hypertension was defined as a resting blood pressure of at least 160 mmHg systolic or at least 95 mmHg diastolic on two readings. Isolated systolic hypertension was defined as a systolic blood pressure of at least 160 mmHg with a diastolic blood pressure of less than 90 mmHg on two routine examinations. High pulse pressure was present if the difference between the systolic and diastolic blood pressure was at least 65 mmHg on two routine examinations. A body mass index of at least 27 kg/m2 defined overweight and at least 30 kg/m2 defined obesity. Alcohol abuse was defined as self-reported, physician-reported or family member-reported heavy consumption of alcohol. Diabetes was considered to be present when reported by a physician or when there was a fasting blood sugar over 6.1 mmol/L. Other vascular diseases included diseases of the aorta, aortic aneurysm or peripheral vascular disease. Stroke included definite stroke with neurological deficit, suspected stroke or transient ischemic attack.
Three social factors were considered as fixed-time covariates. Smoking history was based on self-reports from periodic questionnaires where study members were classified as ‘ever smoked’ or ‘never smoked’. A family history of premature cardiovascular disease was defined as present if a parent or sibling of the MFUS member died from or had documented IHD before 60 years of age. Occupation was characterized by noting whether a study member’s primary employment was as a pilot during the early post-war years of the study (between 1948 and 1968).
ECG evidence of rhythm and conduction disturbances, as well as nonspecific changes, were noted. Atrial fibrillation was defined as when an ECG showed atrial activity with fibrillatory waves replacing normal atrial activation, followed by irregular QRS activity. Ventricular premature beats were defined when present on greater than 10% of the QRS complexes on an ECG. First-degree atrioventricular block was defined as a PR interval at least 0.22 s. Right bundle branch block (RBBB) was defined as a normal early QRS but with the termination directed anterior and to the right, and with the QRS complex measuring at least 0.12 s. Left bundle branch block (LBBB) was defined as an abnormal QRS complex, absent septal Q waves, a broadened QRS of at least 0.12 s, and with the axis directed generally to the left and posterior and with secondary ST changes. Left ventricular hypertrophy (LVH) was defined as increased voltages at lead I, AVL, leads II, III or AVF, or leads V1, V2, or leads V5, V6. Further, the combination of increased QRS voltage of at least 26 mm at V5 and V6 or at least 36 mm at V1 and V2 was used. ST and T wave abnormalities were coded according to the Minnesota codes 4.1, 4.2, 4.4, 5.2, 5.3 and 5.4 (12).
Statistical methodology
The Cox proportional hazard model was the primary statistical model used to assess risk factors for SUCD. ‘Time zero’ for all models was set as the date of entry to the study (July 1, 1948). Follow-up time for each member was determined as the time from entry to the earliest of: date of diagnosis of IHD; June 30, 2004; or death.
Ten clinical findings and eight ECG abnormalities were modelled as time-dependent covariates. Smoking, family history of cardiac disease and pilot occupation were considered as fixed-time covariates. A binary indicator was defined for each of the time-dependent covariates, taking the value zero when absent and a value of one from the time when the abnormality was first detected to the end of follow-up. Modelled with age at entry, age-adjusted relative risk (RR) for SUCD and 95% confidence intervals were calculated for these risk factors. A lifetime prevalence (P) of each risk factor was defined as the proportion of the total cohort with evidence of the risk factor during follow-up. From the age-adjusted RR and P, the population attributable risk for each factor was calculated as P*(RR–1)/(1+P*[RR–1]).
Because of prospective data acquisition, the date of each finding can be used to determine the time since the detection of each risk factor at any point along the time course of the study. Cox models with age and three binary indicator variables for categories of time since detection (one day to five years, five to 10 years and longer than 10 years) were fit for each risk factor. The RR calculated from the coefficients for each of these three time intervals can be interpreted as the risk of SUCD associated with the factor during a specific interval of time since detection relative to the risk of SUCD for a man still free of the factor. These Cox models served two purposes: to assess the validity of the proportionality assumption of the model and to determine whether the risk of SUCD might increase, decrease or remain constant with time since the detection or documentation of a risk factor. All significant (p<0.05) factors identified from the age-adjusted single risk factor analyses were examined in a multivariate analysis using a backward stepwise algorithm to determine the independent effects of these time-sensitive risk factors.
RESULTS
Over the 56-year duration of the MFUS, 171 men experienced SUCD. The time between the onset of symptoms and SUCD was determined for each man from family or friends in 51% of cases, from medical doctors or coroners in 41%, and from bystanders in the remaining 8%. Death occurred within seconds in 44% of cases, within minutes in 29%, and between 1 h and 24 h in 27%. Hence, while all participants met the WHO definition (2), the majority (73%) met the revised criteria of death within 1 h of symptom onset (13).
In a separate analysis, to be reported subsequently, autopsies were performed on 58 of these participants, and coronary heart disease was confirmed as the cause of death in each case. An additional 44 men complained of chest pain, new and unusual indigestion, or new and significant dyspnea in the immediate premortem period. Thus, 60% of these men had either firm or presumptive evidence of acute IHD; of the remainder of these men, the majority were witnessed to be sudden and were unexpected in all cases. Information to suggest acute coronary disease was provided by rescue teams, physicians or coroners’ reports in most of these cases. Only 11 SUCD cases were without any clinical information.
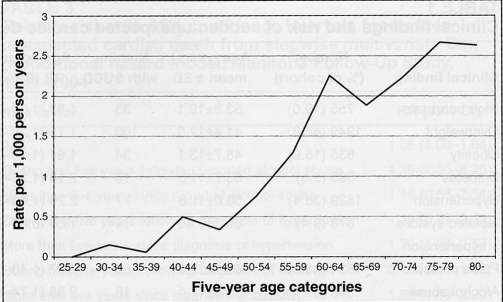
Figure 1 displays the incidence of SUCD as deaths/1000 person-years of observation within five-year age groupings. Incidence was less than 1/1000 person-years before 55 years of age and rose rapidly thereafter with age. The mean age at SUCD was 65 years, with 29% of SUCD cases occurring before 60 years of age.
Figure 1).
Incidence of sudden unexpected cardiac death in the Manitoba Follow-Up Study (1948–2004)
The first columns in Table 1 show the frequency and mean age with standard deviation of clinical findings. High heart rate, overweight, obesity, diabetes, hypertension, abnormal pulse pressure and alcohol abuse were documented at a mean age below 65 years. The prevalence of diabetes, isolated systolic hypertension, alcohol abuse and peripheral vascular disease was less than 10%. Overweight (49%) and hypertension (38%) were very common. A significant (p<0.05) age-adjusted RR for SUCD was found for all clinical factors except isolated systolic hypertension. The RR of SUCD ranged from 1.61 for obesity to a high of 2.85 for alcohol. Attributable risks were moderately high (above 0.25) for hypertension, overweight and pulse pressure. For high heart rate, diabetes, alcohol and stroke, attributable risks ranged from 0.10 to 0.15. The RR of SUCD for alcohol consumption, stroke and peripheral vascular disease declined with time since detection, and became insignificant over longer time. The RR increased with time for overweight, obesity and pulse pressure. Hypertension was significantly associated with SUCD at all time intervals and with an RR that increased over time since detection.
TABLE 1.
Clinical findings and risk of sudden unexpected cardiac death (SUCD): Manitoba Follow-up Study, 1948–2004
| Time since detection of finding (RR [95% CI])
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Clinical finding | Number (% of cohort) | Age (years), mean ± SD | Number with SUCD | Age-adjusted RR (95% CI) | Attributable risk | 1 day to 5 years | Five to 10 years | Over 10 years |
| High heart rate | 755 (19.0) | 53.8±19.1 | 33 | 1.92 (1.31–2.82) | 0.15 | 2.53 (1.24–5.19) | 2.13 (0.87–5.22) | 1.71 (1.07–2.75) |
| Overweight | 1949 (48.9) | 41.4±12.0 | 100 | 1.75 (1.29–2.38) | 0.27 | 0.51 (0.12–2.08) | 1.39 (0.64–3.04) | 1.91 (1.39–2.62) |
| Obesity | 635 (15.9) | 48.7±13.1 | 34 | 1.61 (1.10–2.36) | 0.09 | 0.79 (0.20–3.20) | 2.80 (1.36–5.72) | 1.52 (0.97–2.37) |
| Diabetes | 395 (9.9) | 63.2±11.0 | 19 | 2.14 (1.31–3.48) | 0.10 | 2.43 (1.14–5.20) | 0.86 (0.21–3.46) | 2.74 (1.42–5.28) |
| Hypertension | 1529 (38.4) | 58.0±11.8 | 77 | 2.29 (1.66–3.16) | 0.33 | 1.79 (1.02–3.16) | 1.82 (1.01–3.28) | 2.77 (1.90–4.04) |
| Isolated systolic hypertension | 373 (9.4) | 69.3±9.40 | 14 | 1.54 (0.87–2.72) | 0.05 | 1.19 (0.44–3.23) | 1.51 (0.55–4.12) | 2.00 (0.86–4.64) |
| Pulse pressure | 1824 (45.8) | 60.8±12.9 | 80 | 2.07 (1.48–2.90) | 0.29 | 1.55 (0.90–2.68) | 2.14 (1.28–3.59) | 2.40 (1.60–3.60) |
| Alcohol abuse | 346 (8.7) | 59.6±11.4 | 18 | 2.85 (1.74–4.66) | 0.10 | 5.92 (3.19–10.96) | 1.34 (0.33–5.42) | 1.67 (0.68–4.09) |
| Stroke | 431 (10.8) | 70.2±10.5 | 14 | 1.99 (1.13–3.50) | 0.10 | 2.85 (1.44–5.64) | 1.68 (0.53–5.32) | 0.95 (0.23–3.87) |
| Peripheral vascular disease | 291 (7.3) | 67.6±10.5 | 11 | 1.90 (1.02–3.54) | 0.06 | 2.47 (1.08–5.62) | 1.99 (0.63–6.30) | 1.07 (0.26–4.35) |
The prevalence of ECG abnormalities shown in Table 2 varied considerably, as did the age-adjusted RR of SUCD. The attributable risk was low, all under 0.15 for atrial fibrillation, ventricular premature beats, first-degree atrioventricular block, RBBB and LBBB. In contrast, the RR of SUCD associated with nonspecific ST or T wave changes and their combined changes, coupled with their high prevalence, resulted in a substantial attributable risk. Examination of the effects of these abnormalities with time interval since detection revealed a very high short-term significant risk that diminished with time for LVH and LBBB. The RR of SUCD associated with ST wave, T wave, and ST or T wave combined, was high initially and remained significantly increased at each time interval.
TABLE 2.
Electrocardiographic (ECG) abnormalities and risk of sudden unexpected cardiac death (SUCD): Manitoba Follow-up Study, 1948–2004
| Time since detection of finding (RR [95% CI])
|
||||||||
|---|---|---|---|---|---|---|---|---|
| ECG abnormality | Number (% of cohort) | Age (years), mean ± SD | Number with SUCD | Age-adjusted RR (95% CI) | Attributable risk | 1 day to 5 years | 5 to 10 years | Over 10 years |
| Atrial fibrillation | 273 (6.9) | 69.6±10.3 | 8 | 1.48 (0.72–3.06) | 0.03 | 1.9 (0.70–5.15) | 1.47 (0.36–5.99) | 1.03 (0.25–4.21) |
| VPB | 900 (22.6) | 59.5±13.1 | 41 | 1.59 (1.10–2.30) | 0.12 | 1.17 (0.54–2.52) | 1.57 (0.76–3.23) | 1.79 (1.14–2.81) |
| AV block | 466 (11.7) | 59.7±18.6 | 14 | 0.99 (0.57–1.72) | 0 | 1.85 (0.81–4.22) | 0.41 (0.06–2.96) | 0.82 (0.38–1.76) |
| RBBB | 196 (4.9) | 62.8±15.7 | 8 | 1.49 (0.73–3.05) | 0.02 | 0.72 (0.10–5.14) | 0.98 (0.14–7.05) | 2.03 (0.89–4.61) |
| LBBB | 85 (2.1) | 65.3±13.7 | 7 | 3.25 (1.51–7.01) | 0.05 | 7.47 (3.03–18.38) | – | 1.94 (0.48–7.89) |
| LVH | 463 (11.6) | 57.8±14.7 | 27 | 2.32 (1.53–3.51) | 0.13 | 3.93 (2.06–7.48) | 4.18 (2.12–8.23) | 1.14 (0.56–2.33) |
| ST changes | 887 (22.3) | 62.0±11.3 | 49 | 3.26 (2.29–4.66) | 0.34 | 4.5 (2.85–7.09) | 2.7 (1.40–5.21) | 2.46 (1.40–4.31) |
| T waves | 1477 (37.1) | 61.9±13.3 | 66 | 2.66 (1.91–3.72) | 0.38 | 3.63 (2.33–5.66) | 2.4 (1.37–4.22) | 2.13 (1.34–3.39) |
AV Atrioventricular; LBBB Left bundle branch block; LVH Left ventricular hypertrophy; RBBB Right bundle branch block; VPB Ventricular premature beats
Models of fixed covariates were nonsignificant, with an estimated RR of 1.54 (95% CI 0.95 to 2.50) for a family history of premature cardiovascular disease and an RR of 1.64 (95% CI 0.96 to 2.80) for smoking history. A significant age-adjusted RR of 1.47 (95% CI 1.04 to 2.07) was found for occupational history of pilot (versus not a pilot).
Table 3 presents the final multivariate model for SUCD, with age and clinical findings, as well as ECG abnormalities. After age adjustment, five significant factors were found: alcohol, hypertension, obesity, ST changes and T wave changes. In this model, alcohol was significant in the short term only. Hypertension and overweight became significant five years after detection. T wave abnormalities were significant early, and ST changes were significant over all time periods (both early and late after detection).
TABLE 3.
Relative risk with 95% confidence intervals for sudden unexpected cardiac death from stepwise multivariate Cox proportional hazard model: Manitoba Follow-Up Study, 1948–2004
| Risk factor | RR (95% CI) |
|---|---|
| Age | 1.05 (1.03–1.08) |
| During first five years following reported alcohol abuse | 4.39 (2.35–8.20) |
| More than five years since reported alcohol abuse | 1.18 (0.55–2.54) |
| During first five years following diagnosis of hypertension | 1.37 (0.77–2.43) |
| More than five years since diagnosis of hypertension | 1.79 (1.25–2.57) |
| During first five years following diagnosis of obesity | 0.46 (0.11–1.88) |
| More than five years since diagnosis of obesity | 1.63 (1.19–2.23) |
| During first five years following detection of T wave changes | 2.09 (1.24–3.52) |
| More than five years since detection of T wave changes | 1.27 (0.79–2.04) |
| During first five years following detection of ST changes | 2.54 (1.48–4.36) |
| More than five years since detection of ST changes | 1.96 (1.15–3.34) |
DISCUSSION
Although some of the clinical findings and ECG abnormalities may be transient over time, all were analyzed with consideration for the time from date of first detection. It seemed preferable to model these factors as time-dependent indicators rather than taking the last measurements before the man’s death and the last measurements at the end of follow-up for those alive. Our model provides a novel approach to understanding changes over time of risk for SUCD. Risk factors had both short- and long-term significance, as well as independent effects, in the multivariate model. High short-term risks were shown for excess alcohol consumption and newly developed LBBB. Long-term risks for obesity, and short- and long-term risks for hypertension, ST changes and T changes were thus demonstrated.
The average age at SUCD in the MFUS cohort was 65.5 years, five years younger than the average age of death of the 2660 decedents in the cohort as a whole. Many of the men with SUCD died at a relatively young age, in their 40s and 50s, which is consistent with reports from the Framingham Heart Study (9,14).
Short- and long-term analyses of men and women with sudden cardiac death from the Framingham Heart Study were presented by Cupples et al (14), but their results were presented at years 8 and 28 of the study. It is not possible to determine the number of men without prior IHD who were in each category of that report. Two reports of time-dependent risk of sudden death were found, but these dealt with patients with prior IHD (15,16). It appears that the MFUS analysis of time dependence after detection of risk factors for SUCD in men without prior IHD is unique.
A high resting heart rate was significant, with an RR of 1.92 for SUCD in univariate analysis, but dropped in multivariate analysis for unknown reasons. A high resting heart rate may reflect a high sympathetic tone compared with those individuals with lower heart rates (17) and may predict cardiovascular or hypertensive disease and sudden death (18); however, the risk for SUCD was not identified.
Overweight and obesity were themselves fairly common, and both demonstrated a long-term risk for SUCD. Moderate and severe obesity has been linked to sudden death (19), as well as to late potentials on signal-averaged ECGs, which predict a risk of ventricular arrhythmias, or sudden death (20). Lesser degrees of obesity pose an uncertain risk. Diabetes is said to be a risk for sudden death, but those data arise from cases with autonomic neuropathy (21), which was not present in MFUS participants. Diabetes was infrequent in the MFUS, appeared later in life, and although it was a significant risk for SUCD on univariate analysis, it was not a significant risk factor in the multivariate analysis.
Hypertension was highly prevalent and had a high attributable risk as a consequence. After age adjustment, the risk for SUCD was high, and the risk increased with time since detection. Systolic hypertension, diastolic hypertension and hypertension defined on the basis of both blood pressures were all considered for this analysis, and all yielded approximately the same RR. Only the combined systolic and diastolic blood pressure elevation definition is presented in the current paper. Hypertension has been identified as a risk for cardiovascular and cerebrovascular morbidity and mortality in young and old patients alike (22–25). Pulse pressure elevation occurred at a lower mean age than the average age at SUCD, was frequent, had a high attributable risk and was significant in the long term in single-variable, age-adjusted analysis. Only hypertension remained significant in the multivariate model.
Excess alcohol consumption remained a significant factor over the short term in the multivariate analysis. This short-term increased risk may be partly due to the method of identifying those with excess consumption because, in many cases, this information was provided by either family members or by physicians before the man’s imminent death, or via clinical information as part of autopsy reports. Light to moderate alcohol consumption may be protective (26–28), whereas heavy or excess alcohol consumption is a risk factor for cardiovascular disease (29,30). The relationship between alcohol dose and risk of cardiovascular death has been reported to be nonlinear or J-shaped. Alcohol in excess increases blood pressure (31) and is likely arrhythmogenic (32).
Many studies have shown stroke and peripheral vascular disease to be markers of arteriosclerotic vascular disease and, therefore, they appear as predictors of coronary disease (33,34). Both stroke and peripheral vascular disease were high short-term risks, but not long-term risks, for SUCD. Because of the high short-term risk for SUCD, it is reasonable to suppose that men with either stroke or peripheral vascular disease did not live long enough to present a long-term risk for SUCD.
Atrial fibrillation was relatively uncommon in this cohort of men without prior IHD, and occurred well beyond the average age of men with SUCD. Therefore, it had a low attributable risk and was not a significant risk factor. Ventricular premature beats were common but were not significantly associated with SUCD. This finding is supported by the Framingham Heart Study in men with SUCD (35,36) and by Rose et al (37). This finding is in contrast to prior reports from the MFUS conducted in younger men, relating ECG data to all deaths due to cardiovascular disease (38,39). Although ventricular premature beats and ventricular arrhythmias may be risk factors for sudden death, they remain unsolved therapeutic problems. Antiarrhythmic treatment can be hazardous (40), and although newer treatment with angiotensin-converting enzyme inhibitors (41), beta-blocking agents (42) or lipid-lowering agents such as statins may be of benefit, these treatments have not been tested to prevent SUCD in a disease-free population, such as the population described herein. First-degree atrioventricular block and RBBB were both uncommon in the MFUS and were not independently associated with SUCD.
New-onset LBBB is a very important high short-term risk for SUCD. In days, or even up to five years after its appearance, it carried a high relative risk 7.47 for SUCD. The prevalence of LBBB was low, occurring in only seven individuals with subsequent SUCD. Despite a low attributable risk, it must be considered to be a marker for underlying coronary disease and a warning that SUCD may ensue. MFUS has presented data on the short-term risk of LBBB and SUCD (39), and although new-onset LBBB was noted to be a harbinger of late cardiovascular disease, hypertension or sudden death, SUCD was not examined separately in the Framingham Heart Study (9,36). In another report (14), intraventricular block was a risk factor for SUCD, but the number of LBBB cases was small.
LVH was moderately common but was not significant, probably because of the concomitant effect of hypertension. LVH was a significant short- and long-term risk in the study by Cupples et al (14), but they included ST changes in the definition of LVH.
New ST changes conveyed high risks for SUCD, and T wave changes were only less so in the short term, but both remained significant over longer terms. These factors probably reflect underlying IHD, and this idea is supported by more recent studies employing newer diagnostic techniques (43). In a subsequent study of autopsy cases from MFUS, it will be possible to consider the specificity of ST and T wave changes related to the extent and area of the ischemic damage. For now, it must be said that the development of these ST and T wave changes should be considered relevant to the risk of SUCD, both in the short and long term, even if the man is asymptomatic.
Smoking is a known risk factor for IHD but was not significantly associated with SUCD in the present study. One explanation lies with our operational definition of smoking in this analysis as ever smoked versus never smoked. Many study members smoked at the end of World War II, when they were younger, but the vast majority had stopped smoking by 40 years of age. By the time of our analysis, the label of smoking may not have correctly reflected a man’s current smoking status. In that case, ex-smokers would have dominated the analysis. It is well accepted that the effect of smoking on later cardiovascular disease is nullified when the individual stops smoking (44,45).
The high risk of the occupation of pilot cannot be explained, and may be attributed to personality type or the stress of flying, but we have no data to support any explanation in this regard. The assessment of the effect of family history was not significant and may be related to the timing of the acquisition of that information in 1974. At that time, the average age of these men was 56; many had already died, which may have made the family history data inconclusive.
The MFUS can be criticized because it includes only men. Women in the Framingham Heart Study (14,36,46) had similar risk factors for SUCD compared with men, but women had a peak incidence 20 years older than men, and hemoglobin and glucose levels were of greater importance in women. Precise information concerning alcohol consumption is always difficult to obtain, even in personal interviews, but our method of collection of the data on these men’s alcohol consumption is similar to methods of other studies (26–30). Temporal changes in the treatment of primary risk factors may have confounded our data collected over a long time interval. However, Elveback et al (5) found that over 20 years, there was little change in the incidence of SUCD in either men or women. Our report does not include cholesterol or other newer biochemical markers, but the latter are unproven in long-term epidemiological trials (47). Cholesterol levels have not been found to be important risk factors for SUCD, especially in older men and women (16,46). The MFUS has the strength of a systematic, prospective collection of clinical and ECG data spanning 56 years in originally healthy young men. We present time-related risk factors that can be determined by any medical practitioner and we provide a time base to assess each relative risk for SUCD.
CONCLUSIONS
The incidence of SUCD increases with age. After age adjustment, excess alcohol consumption is a high short-term risk, an important hazard independent of other factors, and carries social and health support implications. Overweight must be recognized as a problem with long-term consequences, and poses a challenge for both clinicians and public health personnel. Hypertension is not a novel risk factor for SUCD, but the fact that the risk increases over time after detection emphasizes the need for treatment at any age or time. This information must continue to be provided for medical and lay people alike. The follow-up of hypertensive patients to assure adherence to treatment goals is of paramount importance. On the other hand, the importance of newly developed ST or T wave changes or LBBB must be recognized because of the very high short-term risk for SUCD. The risk related to ST and T wave changes persists over longer periods of time.
Our study has added important new information to the understanding of the epidemiology of SUCD. We have explored clinical, ECG and social variables, and reported that the effects of many of these factors are not constant, but rather have varying effects, with examples of both increasing and decreasing magnitude with intervals of time following first detection. These findings demonstrate early warning signs of SUCD, as well as those risks that persist over time. They must be added to the armamentarium of the practitioner at all professional levels, including the cardiologist.
ACKNOWLEDGEMENTS
A study of this scope would not be possible without the interest and unselfish contributions of the 3983 study members. The authors commend their ongoing support for the research of the Manitoba Follow-up Study. Further, the authors sincerely acknowledge Ms Priyanka Halli for her assistance during the preparation of this manuscript. The Manitoba Follow-up Study is currently funded through an operating grant from the Canadian Institutes of Health Research.
REFERENCES
- 1.Shah CP. Public Health and Preventive Medicine in Canada. 5. Toronto: Elsevier Canada; 2003. p. 212. [Google Scholar]
- 2.World Health Organization. International Statistical Classification of Diseases And Related Health Problems: Tenth Revision, 2nd edn. Geneva: World Health Organization. 1992;1:890. [PubMed] [Google Scholar]
- 3.Heart and Stroke Foundation of Canada. The Growing Burden of Heart Disease and Stroke in Canada 2003. Ottawa: Heart and Stroke Foundation of Canada; 2003. p. 61. [Google Scholar]
- 4.Tate RB, Cuddy TE, Manfreda J. Decline in incidence of ischemic heart disease over a 45-year period. The Manitoba Follow Up Study: 1948–1993. Circulation. 2000;102(Suppl):II-842, 4045. [Google Scholar]
- 5.Elveback LR, Connolly DC, Kurland LT. Coronary heart disease in residents of Rochester, Minnesota. II. Mortality, incidence, and survivorship, 1950–1975. Mayo Clin Proc. 1981;56:665–72. [PubMed] [Google Scholar]
- 6.McGovern PG, Pankow JS, Shahar E, et al. Recent trends in acute coronary heart disease – mortality, morbidity, medical care, and risk factors. The Minnesota Heart Survey Investigators. N Engl J Med. 1996;334:884–90. doi: 10.1056/NEJM199604043341403. [DOI] [PubMed] [Google Scholar]
- 7.Salomaa V, Miettinen H, Kuulasmaa K, et al. Decline of coronary heart disease mortality in Finland during 1983 to 1992: Roles of incidence, recurrence, and case-fatality. The FINMONICA MI Register Study. Circulation. 1996;94:3130–7. doi: 10.1161/01.cir.94.12.3130. [DOI] [PubMed] [Google Scholar]
- 8.Hunink MG, Goldman L, Tosteson AN, et al. The recent decline in mortality from coronary heart disease, 1980–1990. The effect of secular trends in risk factors and treatment. JAMA. 1997;277:535–42. [PubMed] [Google Scholar]
- 9.Kannel WB, Gagnon DR, Cupples LA. Epidemiology of sudden coronary death: Population at risk. Can J Cardiol. 1990;6:439–44. [PubMed] [Google Scholar]
- 10.Mathewson FA, Varnam GS. Abnormal electrocardiograms in apparently healthy people. II. The electrocardiogram in the diagnosis of subclinical myocardial disease. Serial records of 32 people. Circulation. 1960;21:204–13. doi: 10.1161/01.cir.21.2.204. [DOI] [PubMed] [Google Scholar]
- 11.Tate RB, Lah L, Cuddy TE. Definition of successful aging by elderly Canadian males: The Manitoba Follow-up Study. Gerontologist. 2003;43:735–44. doi: 10.1093/geront/43.5.735. [DOI] [PubMed] [Google Scholar]
- 12.Prineas RJ. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. Boston: J Wright; 1982. p. 225. [Google Scholar]
- 13.Goldstein S. The necessity of a uniform definition of sudden coronary death: Witnessed death within 1 hour of the onset of acute symptoms. Am Heart J. 1982;103:156–9. doi: 10.1016/0002-8703(82)90552-x. [DOI] [PubMed] [Google Scholar]
- 14.Cupples LA, Gagnon DR, Kannel WB. Long- and short-term risk of sudden coronary death. Circulation. 1992;85(Suppl 1):I11–8. [PubMed] [Google Scholar]
- 15.Benchimol D, Dubroca B, Bernard V, et al. Short- and long-term risk factors for sudden death in patients with stable angina. Int J Cardiol. 2000;76:147–56. doi: 10.1016/s0167-5273(00)00370-3. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa T, Rozanski JJ, Nogami A, Moroe K, Gosselin AJ, Lister JW. Time-dependent risk of and predictors for cardiac arrest recurrence in survivors of out-of-hospital cardiac arrest with chronic coronary artery disease. Circulation. 1989;80:599–608. doi: 10.1161/01.cir.80.3.599. [DOI] [PubMed] [Google Scholar]
- 17.Barron HV, Lesh MD. Autonomic nervous system and sudden cardiac death. J Am Coll Cardiol. 1996;27:1053–60. doi: 10.1016/0735-1097(95)00615-X. [DOI] [PubMed] [Google Scholar]
- 18.Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: The Framingham Study. Am Heart J. 1987;113:1489–94. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 19.Duflou J, Virmani R, Rabin I, Burke A, Farb A, Smialek J. Sudden death as a result of heart disease in morbid obesity. Am Heart J. 1995;130:306–13. doi: 10.1016/0002-8703(95)90445-x. [DOI] [PubMed] [Google Scholar]
- 20.Lalani AP, Kanna B, John J, Ferrick KJ, Huber MS, Shapiro LE. Abnormal signal-averaged electrocardiogram (SAECG) in obesity. Obes Res. 2000;6:20–8. doi: 10.1038/oby.2000.4. [DOI] [PubMed] [Google Scholar]
- 21.Bellavere F, Ferri M, Guarini L, et al. Prolonged QT period in diabetic autonomic neuropathy: A possible role in sudden cardiac death? Br Heart J. 1988;59:379–83. doi: 10.1136/hrt.59.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MRC Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ. 1992;304:405–12. doi: 10.1136/bmj.304.6824.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kannel WB, Gordon T, Schwartz MJ. Systolic versus diastolic blood pressure and risk of coronary heart disease. The Framingham study. Am J Cardiol. 1971;27:335–46. doi: 10.1016/0002-9149(71)90428-0. [DOI] [PubMed] [Google Scholar]
- 24.The Australian therapeutic trial in mild hypertension. Report by the Management Committee. Lancet. 1980;1:1261–7. [PubMed] [Google Scholar]
- 25.Dahlof B, Lindholm LH, Hansson L, Schersten B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension) Lancet. 1991;338:1281–5. doi: 10.1016/0140-6736(91)92589-t. [DOI] [PubMed] [Google Scholar]
- 26.Albert CM, Manson JE, Cook NR, Ajani UA, Gaziano JM, Hennekens CH. Moderate alcohol consumption and the risk of sudden cardiac death among US male physicians. Circulation. 1999;100:944–50. doi: 10.1161/01.cir.100.9.944. [DOI] [PubMed] [Google Scholar]
- 27.Mukamal KJ, Conigrave KM, Mittleman MA, et al. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N Engl J Med. 2003;348:109–18. doi: 10.1056/NEJMoa022095. [DOI] [PubMed] [Google Scholar]
- 28.Britton A, Marmot M. Different measures of alcohol consumption and risk of coronary heart disease and all-cause mortality: 11-year follow-up of the Whitehall II Cohort Study. Addiction. 2004;99:109–16. doi: 10.1111/j.1360-0443.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 29.Wannamethee G, Shaper AG. Alcohol and sudden cardiac death. Br Heart J. 1992;68:443–8. doi: 10.1136/hrt.68.11.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kauhanen J, Kaplan GA, Goldberg DE, Salonen JT. Beer binging and mortality: Results from the Kuopio ischaemic heart disease risk factor study, a prospective population based study. BMJ. 1997;315:846–51. doi: 10.1136/bmj.315.7112.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Criqui MH, Langer RD, Reed DM. Dietary alcohol, calcium, and potassium. Independent and combined effects on blood pressure. Circulation. 1989;89:609–14. doi: 10.1161/01.cir.80.3.609. [DOI] [PubMed] [Google Scholar]
- 32.Pochmalicki G, Genest M, Jibril H. Late ventricular potentials and heavy drinking. Heart. 1997;78:163–5. doi: 10.1136/hrt.78.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aronow WS, Ahn C. Prevalence of coexistence of coronary artery disease, peripheral arterial disease, and atherothrombotic brain infarction in men and women > or = 62 years of age. Am J Cardiol. 1994;74:64–5. doi: 10.1016/0002-9149(94)90493-6. [DOI] [PubMed] [Google Scholar]
- 34.Hughson WG, Mann JI, Tibbs DJ, Woods HF, Walton I. Intermittent claudication: factors determining outcome. Br Med J. 1978;1:1377–9. doi: 10.1136/bmj.1.6124.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreger BRE, Cupples AL, Kannel WB. The electrocardiogram in prediction of sudden death: Framingham Study experience. Am Heart J. 1986;113:377–82. doi: 10.1016/0002-8703(87)90281-x. [DOI] [PubMed] [Google Scholar]
- 36.Kannel WB, Schatzkin A. Sudden death: Lessons from subsets in population studies. J Am Coll Cardiol. 1985;5:141B–9B. doi: 10.1016/s0735-1097(85)80545-3. [DOI] [PubMed] [Google Scholar]
- 37.Rose G, Baxter PJ, Reid DD, McCartney P. Prevalence and prognosis of electrocardiographic findings in middle-aged men. Br Heart J. 1978;40:636–43. doi: 10.1136/hrt.40.6.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabkin SW, Mathewson FA, Tate RB. Relationship of ventricular ectopy in men without apparent heart disease to occurrence of ischemic heart disease and sudden death. Am Heart J. 1981;101:135–42. doi: 10.1016/0002-8703(81)90655-4. [DOI] [PubMed] [Google Scholar]
- 39.Rabkin SW, Mathewson FL, Tate RB. The electrocardiogram in apparently healthy men and the risk of sudden death. Br Heart J. 1982;47:546–52. doi: 10.1136/hrt.47.6.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–12. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 41.Domanski MJ, Exner DV, Borkowf CB, et al. Effect of angiotensin converting enzyme inhibition on sudden cardiac death in patients following acute myocardial infarction. A meta-analysis of randomized clinical trials. J Am Coll Cardiol. 1999;33:598–604. doi: 10.1016/s0735-1097(98)00609-3. [DOI] [PubMed] [Google Scholar]
- 42.Pitt B. The role of beta-adrenergic blocking agents in preventing sudden cardiac death. Circulation. 1992;85(Suppl I):I107–11. [PubMed] [Google Scholar]
- 43.Marwick TH, Case C, Vasey C, Allen S, Short L, Thomas JD. Prediction of mortality by exercise echocardiography: A strategy for combination with the duke treadmill score. Circulation. 2001;103:2566–71. doi: 10.1161/01.cir.103.21.2566. [DOI] [PubMed] [Google Scholar]
- 44.CDC. Maryland: US Department of Health and Human Services, Public Health Service; 1990. The health benefits of smoking cessation: A report of the Surgeon General, 1990. (DHHS publication no. [CDC] 90-8416) [Google Scholar]
- 45.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE Lung Health Study Research Group. The effects of a smoking cessation intervention on 14.5-year mortality: A randomized clinical trial. Ann Intern Med. 2005;142:233–9. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 46.Kannel WB, Wilson PW, D’Agostino RB, Cobb J. Sudden coronary death in women. Am Heart J. 1998;136:205–12. doi: 10.1053/hj.1998.v136.90226. [DOI] [PubMed] [Google Scholar]
- 47.Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105:2595–9. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]