Abstract
OBJECTIVE
To develop and validate an objective and reliable measure of acuity that will identify high-risk patients and predict length of stay following all cardiac surgery procedures.
METHODS
Logistical regression analysis of 12,683 patients undergoing cardiac surgery between 1996 and 2000 was used to identify the independent predictors of postoperative adverse events (AEs, defined as death, myocardial infarction, low cardiac output syndrome, postoperative renal failure, stroke or deep sternal wound infection). The rounded ORs for each of the 18 predictors of AEs were summed to calculate the Toronto Risk Score (TRS) for each patient. Weighted linear regression was used to determine the relationship between TRS and length of stay in the 4378 patients who underwent cardiac surgery between 2001 and 2002.
RESULTS
TRS was significantly associated with cardiovascular intensive care unit length of stay (R2=0.85, slope=0.42, intercept=0.4; P<0.001). For each unit increase in TRS, cardiovascular intensive care unit length of stay increased by 0.4±0.05 days. TRS was also significantly associated with total postoperative length of stay (R2=0.88, slope=0.71, intercept=4.9; P<0.001). TRS captured a significant increase in acuity from 1996 and 2000 (5.12±3.5) to 2001 and 2002 (5.54±3.5; P<0.001). Despite increased acuity, AEs were reduced in 2001 and 2002 (8.1%) compared with 1996 to 2000 (9.8%; P=0.012).
CONCLUSIONS
The TRS is a valid measure of acuity that can identify patients who are at high risk of experiencing an AE and having prolonged length of stay after any cardiac surgery procedure, capture changes in acuity over time and allow for continuous quality performance evaluation.
Keywords: Acuity, Cardiac surgery, Length of stay, Quality evaluation, Risk index
Abstract
OBJECTIF
Mettre au point et valider une mesure objective et fiable de l’acuité permettant d’identifier les patients à haut risque et prédire la durée de l’hospitalisation après toutes les chirurgies cardiaques.
MÉTHODES
L’analyse de régression logistique de 12 683 patients ayant subi une chirurgie cardiaque entre 1996 et 2000 a été utilisée pour identifier les facteurs de prévisibilité indépendants d’événements post-opératoires indésirables (définis comme suit : décès, infarctus du myocarde, syndrome de bas débit, insuffisance rénale post-opératoire, AVC ou infection profonde de la plaie sternale). Les RR arrondis de chacun des 18 facteurs de prévisibilité d’événements indésirables ont été additionnés afin de calculer l’indice de risque Toronto (IRT) de chaque patient. La régression linéaire pondérée a été utilisée pour déterminer le lien entre l’IRT et la durée du séjour hospitalier de 4 378 patients ayant subi une chirurgie cardiaque entre 2001 et 2002.
RÉSULTATS
Un lien important a été noté entre l’IRT et la durée du séjour aux soins intensifs cardiovasculaires (RR2 = 0,85; pente = 0,42; intersection = 0,4; p < 0,001). Pour chaque unité d’augmentation de l’IRT, la durée du séjour aux soins intensifs cardiovasculaires augmentait de 0,4 ± 0,05 jour. L’IRT a également été significativement associé à la durée du séjour post-opératoire total (RR2 = 0,88; pente = 0,71; intersection = 4,9; p < 0,001). L’IRT a permis d’identifier une augmentation significative de l’acuité entre 1996 et 2000 (5,12 ± 3,5) et 2001 et 2002 (5,54 ± 3,5; p < 0,001). Malgré l’acuité accrue, les événements indésirables ont diminué en 2001 et 2002 (8,1 %), comparativement à 1996 et 2000 (9,8 %; p = 0,012).
CONCLUSION
L’IRT est une mesure valide de l’acuité qui permet d’identifier les patients exposés à un risque élevé d’événements indésirables et de prolongation de leur hospitalisation après toute intervention de chirurgie cardiaque, d’observer les changements d’acuité dans le temps et d’assurer une évaluation continue du rendement qualitatif.
Increased acuity in patients undergoing cardiac surgery combined with constrained resources requires that both administrators and clinicians have reliable tools for quality performance evaluation, operational management and long-term planning. Acuity refers to those combinations of demographics, risk factors and pathology that are associated with poorer clinical outcomes. Over the past decade, the use of predictive rules to calculate risk-adjusted or risk-stratified operative mortality following cardiac surgery have become part of normal practice for both administrators and clinicians in many jurisdictions. Most of these algorithms have been developed for operative mortality following isolated coronary artery bypass graft (CABG) surgery (1–7), with fewer incorporating valvular and other cardiac procedures (8,9). Often, these models contain similar risk factors and risk weights (10). However, models that address hospital mortality may not reflect risk factors associated with prolonged length of stay. Adverse events (AEs) following cardiac surgery are associated with increased patient risk factors and remain the major determinants of prolonged length of stay resulting in increased costs and increased burden of illness to the patient (9).
Therefore, the present study describes the development and validation of the Toronto Risk Score (TRS) for AEs following cardiac surgery, the relationship between the risk score and length of stay, and the practical application of this scoring system.
METHODS
Patient sample
Between January 1, 1990, and December 31, 2004, 33,129 consecutive patients underwent cardiac surgery at the University Health Network – Toronto General Hospital in Toronto, Ontario. All patients had their preoperative, operative and postoperative information entered prospectively into a data registry using Microsoft Access (Microsoft Corporation, USA). The 12,683 patients undergoing cardiac surgery between January 1, 1996, and December 31, 2000, were included in the derivation data set for the construction of the risk index. All 4378 cardiac surgery patients operated on between January 1, 2001, and December 31, 2002, were included in the validation data set.
STATISTICAL ANALYSIS
General issues
SAS 8.2 (SAS Institute, USA) was used for all data analyses. Descriptive statistics for continuous variables included the mean, median, SD and standard error. Frequencies were used for categorical variables. Univariate comparisons between groups included unpaired Student’s t tests for continuous variables and contingency table analysis for categorical variables. Linear regression was used to evaluate the relationship between two continuous variables. Two-way ANOVA was used to evaluate the association of length of stay with the main effects, risk group and time, and the interaction of risk group × time.
Development of the TRS
The primary binomial outcome for this model was any postoperative AE following cardiac surgery. AE was defined as any of the following: operative death, a perioperative myocardial infarction defined by electrocardiogram and enzymatic criteria, low cardiac output syndrome (systolic blood pressure less than 90 mmHg and cardiac index less than 2.1 L/min/M2 lasting longer than 15 min despite adequate preload), a perioperative stroke, new postoperative renal failure (defined as the need for any form of dialysis) or deep sternal wound infection.
Logistic regression using backward elimination was used to develop the models and has been previously described (7). All prognostic variables were submitted to the models, and retention of a variable in the model was determined by P≤0.05. The variables submitted to the models are contained in Appendix A. Details of this database have been described at length in previous publications (7,11–16).
Logistic regression models for AEs were developed in the 1996 to 2000 data set for all open heart surgery procedures. Model discrimination was evaluated by the C statistic (analagous to the area under the receiver operator characteristic curve) (17); model precision was evaluated by the Hosmer-Lemeshow goodness-of-fit statistic (18). To construct a linear risk score, the ORs were rounded to their nearest integer to provide a risk weight. Of special note, separate logistic regression analyses were performed in procedure-specific subgroups of patients: isolated CABG procedures; valve with or without CABG; and ‘other’ procedures, such as ascending aortic surgery, left ventricular aneursysm repair and adult congenital heart surgery, etc. Risk weights for redo CABG and urgent priority were adjusted slightly to reflect the ORs in the isolated CABG surgery population which comprises the majority of patients.
The calibration of the model was evaluated in the derivation data set by weighted linear regression of the mean predicted probability of AEs versus the observed AEs for each level of the TRS (7). Weights were determined by the number of observations for each TRS unit.
Validation of the TRS
The additive TRS was applied to the 4378 patients undergoing cardiac surgery between 2001 and 2002. The association between TRS and cardiovascular intensive care unit length of stay (CVLOS) and total postoperative length of stay (PLOS) was evaluated by weighted linear regression. Length of stay variables were not normally distributed, but because of the very large sample size, the central limit theorem (19) allowed for the use of parametric analysis for continuous variables. The number of observations for each level of the score was used as weights, thereby reducing the influence of outlying scores.
Additionally, the variables contained in the TRS were tested by a forced logistic regression to determine discrimination and precision of the model in the validation data set.
Risk stratification
One of the primary purposes of developing this risk score was to identify the approximately 20% of patients who are at the highest risk of experiencing a postoperative AE. Four RR groups were constructed based roughly on quartiles of the TRS: low, moderate, high and extremely high risk. Risk-stratified comparisons were performed by contingency table analysis for categorical variables and ANOVA for length of stay.
Quality performance monitoring
The expected AE probability was calculated from the logistic regression model coefficients in the derivation data using the following formula:
where e is the exponent and β is the regression coefficient. These probabilities were used as the benchmark to evaluate quality performance for each quarter year retrospectively to 1990 and prospectively to 2004 by plotting the average expected probability of AEs minus the observed AE rate (20).
RESULTS
General issues
Between 1996 and 2000, 12,683 patients underwent cardiac surgery at the University Health Network – Toronto General Hospital. The TRS was derived in this patient sample. Only 39 (0.3%) patient records were missing information for a key variable and were dropped from the logistic regression analysis.
The validation data consisted of 4378 patients who underwent cardiac surgery between 2001 and 2002 (Table 1). Many risk factors increased significantly from the earlier time cohort, most notably age, the prevalence of patients with triple vessel disease, left main coronary artery disease, diabetes, peripheral vascular disease, hypertension and renal failure. Despite the increase in several risk factors, the prevalence of AEs decreased with time between the two patient cohorts (9.8% versus 8.6%; P=0.018). However, both CVLOS and total PLOS increased significantly from the 1996 to 2000 patient group.
TABLE 1.
Profile, procedures and outcomes
| Profile | Derivation set 1996 to 2000 | Validation set 2001 to 2002 | P |
|---|---|---|---|
| n | 12,683 | 4378 | |
| Age, years (mean ± SD) | 61±12 | 62±12 | <0.001 |
| Age ≥75 years (%) | 13 | 16 | <0.001 |
| Female sex (%) | 27 | 26 | 0.05 |
| Surgical priority (%) | |||
| Elective | 55 | 60 | |
| Urgent | 42 | 37 | |
| Emergent | 2.8 | 2.8 | <0.001 |
| Left ventricle grade (%) | |||
| 1 | 38 | 44 | |
| 2 | 41 | 25 | |
| 3 | 18 | 17 | |
| 4 | 3.4 | 4.0 | <0.001 |
| Redo CABG (%) | 4.3 | 3.2 | 0.002 |
| Any redo cardiac surgery (%) | 9.5 | 8.1 | 0.007 |
| NYHA classification 4 (%) | 48 | 43 | <0.001 |
| MI < one month preoperation (%) | 15 | 16 | 0.2 |
| Triple vessel disease (%) | 46 | 59 | <0.001 |
| Left main disease (%) | 14 | 19 | <0.001 |
| Congestive heart failure (%) | 22 | 22 | 0.9 |
| Diabetes (%) | 23 | 27 | <0.001 |
| Peripheral vascular disease (%) | 12 | 16 | <0.001 |
| Hypertension (%) | 48 | 56 | <0.001 |
| Renal dialysis (%) | 0.7 | 1.2 | <0.001 |
| Creatinine >150 μmol/L (%) | 3.9 | 5.1 | <0.001 |
| COPD (%) | 5.0 | 4.3 | 0.08 |
| Procedures (%) | |||
| Isolated CABG | 67 | 64 | |
| Isolated single valve | 12 | 13 | |
| Valve and CABG | 6.9 | 7.2 | |
| Complex valve | 15 | 17 | |
| Ascending aorta replacement | 5.3 | 6.9 | |
| Adult congenital repair | 4.2 | 3.9 | |
| Left ventricle aneurysmectomy | 1.4 | 1.6 | |
| Ischemic VSD | 0.2 | 0.1 | |
| Myxoma | 0.3 | 0.3 | |
| Myectomy | 1.0 | 1.8 | |
| Transplant | 0.1 | 0.1 | |
| Other miscellaneous | 2.2 | 2.7 | 0.9 |
| Outcomes (%) | |||
| Operative mortality | 2.4 | 2.1 | 0.3 |
| MI | 2.2 | 1.9 | 0.2 |
| Low cardiac output syndrome | 5.8 | 4.3 | <0.001 |
| Stroke | 1.5 | 1.6 | 0.8 |
| Postoperative renal failure | 1.4 | 1.7 | <0.001 |
| Sternal wound infection | 0.8 | 0.6 | 0.3 |
| Any adverse event | 9.8 | 8.6 | 0.018 |
| CVLOS, days (mean ± SD) | 2.0±4.7 | 2.6±5.4 | <0.001 |
| Median | 1.04 | 1.08 | |
| Total PLOS, days (mean ± SD) | 8.3±6.9 | 8.8±7.4 | <0.001 |
| Median | 6.0 | 7.0 | |
SD is one standard deviation. Complex valve was defined as surgery on more than one valve, concomitant coronary artery bypass graft surgery (CABG) or more than one previous redo surgery. COPD Chronic obstructive pulmonary disease; CVLOS Cardiovascular intensive care unit length of stay; MI Myocardial infarction; NYHA New York Heart Association; PLOS Postoperative length of stay; VSD Ventricular septal defect
Development and validation of the TRS
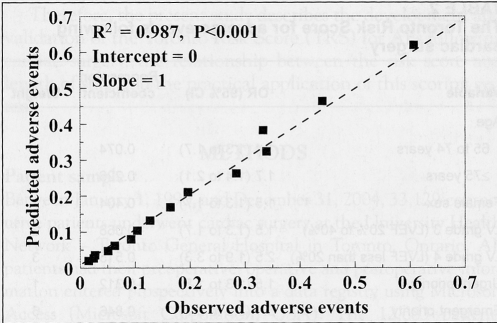
The regression coefficients, ORs and their 95% CIs, and the risk weights used to calculate the TRS are in Table 2. The model had very good discrimination (C statistic 0.744) and precision (Hosmer-Lemeshow goodness-of-fit P=0.12). To calculate the TRS, the risk weights that characterize each patient are summed. The calibration curve (Figure 1) in the derivation set demonstrated an excellent fit between predicted and observed probabilities with an R2=0.987 (P<0.001). Notably, the intercept for this regression was zero and the slope was one, indicating excellent calibration. The calibration curve in the validation set (not depicted) also demonstrated excellent fit (R2=0.94, P<0.001, slope 1.1, intercept 0.9).
TABLE 2.
The Toronto Risk Score for adverse events following cardiac surgery
| Variable | OR (95% CI) | Regression coefficient | Weight |
|---|---|---|---|
| Age | |||
| 65 to 74 years | 1.4 (1.3 to 1.7) | 0.074 | 1 |
| ≥75 years | 1.7 (1.4 to 2.1) | 0.226 | 2 |
| Female sex | 1.5 (1.3 to 1.7) | 0.404 | 2 |
| LV grade 3 (LVEF 20% to 40%) | 1.5 (1.3 to 1.7) | −0.068 | 2 |
| LV grade 4 (LVEF less than 20%) | 2.5 (1.9 to 3.3) | 0.515 | 3 |
| Urgent priority | 1.5 (1.3 to 1.8) | −0.312 | 1 |
| Emergent priority | 5.1 (3.9 to 6.7) | 0.846 | 6 |
| MI < one month preoperation | 1.4 (1.2 to 1.7) | 0.353 | 1 |
| Redo CABG | 3.1 (2.5 to 3.9) | 1.106 | 4 |
| Triple vessel disease | 1.4 (1.2 to 1.6) | 0.286 | 1 |
| Left main disease | 1.4 (1.1 to 1.6) | 0.312 | 1 |
| Congestive heart failure | 1.5 (1.3 to 1.8) | 0.326 | 2 |
| Renal insufficiency | 2.1 (1.6 to 2.6) | 0.718 | 2 |
| Diabetes | 1.2 (1.1 to 1.4) | 0.205 | 1 |
| Peripheral vascular disease | 1.4 (1.2 to 1.7) | 0.296 | 1 |
| Hypertension | 1.2 (1.0 to 1.3) | 0.142 | 1 |
| Complex valve | 1.3 (1.1 to 1.6) | 0.295 | 2 |
| Other pathology | 1.9 (1.5 to 2.2) | 0.617 | 2 |
| Constant | – | −2.595 | – |
Risk weights are summed to calculate the Toronto Risk Score. Referrent values are zero for the following: age younger than 65 years, male sex, left ventricle (LV) grades 1 or 2 (LV ejection fraction [EF] greater than 40%), elective surgery, no myocardial infarction [MI]) within the month before surgery, no previous coronary artery bypass graft surgery (CABG), less than three vessel coronary artery disease, no left main disease, no congestive heart failure, no renal insufficiency, no diabetes, no peripheral vascular disease, no hypertension, isolated CABG, simple valve surgery (only one valve, no CABG, no other procedures), no other pathology requiring surgical correction. Predicted probability of adverse events (ProbAE) can be calculated from the following formula: ProbAE = Σeβ/(1+Σeβ), where e is the exponent and β is the regression coefficient
Figure 1).
The calibration curve for predicted probability of adverse events for each unit of the Toronto Risk Score versus the observed adverse events
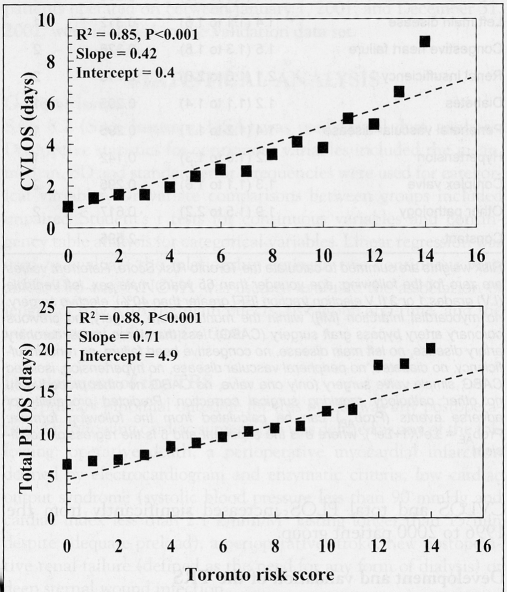
The linear regressions of CVLOS and total PLOS versus the TRS in the validation data set are depicted in Figure 2. Both regressions demonstrated excellent fit. From the linear regression equations, each unit increase in TRS was associated with an increase of 0.42±0.05 days for CVLOS (P<0.001) and 0.71±0.07 days for total PLOS (P<0.001). The TRS model had very good discrimination in the validation data set with a C statistic of 0.734 and excellent precision (Hosmer-Lemeshow goodness-of-fit P=0.9).
Figure 2).
Weighted linear regression of cardiovascular intensive care unit length of stay (CVLOS) and total postoperative length of stay (PLOS) versus the Toronto Risk Score. The dashed diagonal line represents the linear function
Tracking acuity
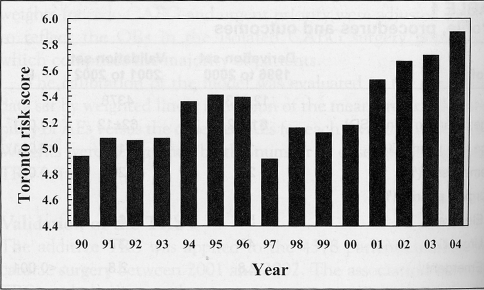
The changes in acuity over time are depicted in Figure 3. The standard errors ranged from 0.08 to 0.09 and were not plotted due to scaling. The TRS increased from 4.9±3.6 in 1997 to 5.9±3.7 in 2004.
Figure 3).
The average Toronto Risk Score each year since 1990. The standard errors ranged from 0.08 to 0.09 and were not plotted due to scale of the y-axis
Risk-stratified analysis
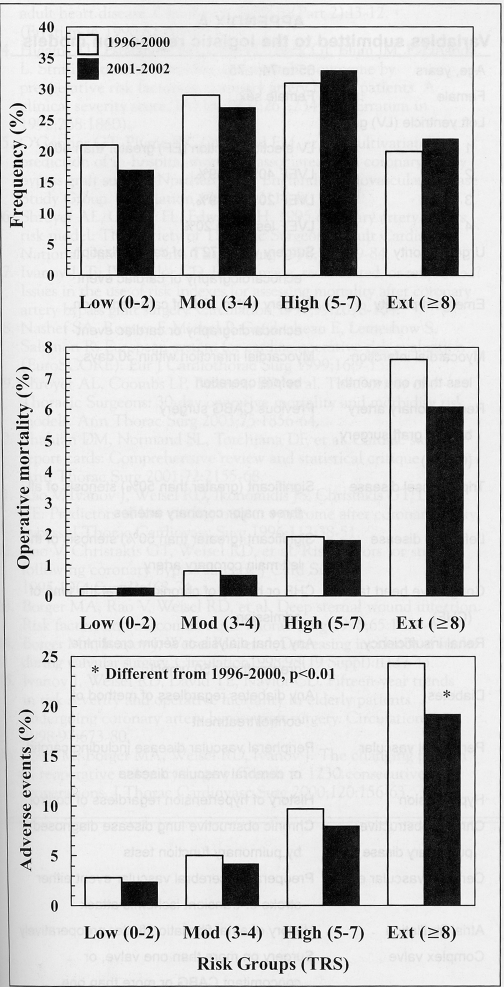
Frequency analysis of the TRS in the derivation data set guided the construction of RR groups. Low risk was defined as TRS 0 to 2 (21%), moderate risk as TRS 3 to 4 (29%), high risk as TRS 5 to 7 (31%) and extremely high risk as TRS 8 or more (20%). The change in proportion of patients in each risk subgroup (Figure 4) was significantly different between the two time periods (P<0.001).
Figure 4).
Top panel Proportion of patients in each risk stratum in the derivation data set (1996 to 2000) and validation data set (2001 to 2002). Risk-stratified operative mortality (middle panel) and adverse events (bottom panel) are also depicted. Ext Extremely high risk; High High risk; Low Low risk; Mod Moderate risk; TRS Toronto Risk Score
Also depicted in Figure 4 are the risk-stratified operative mortality and AE rates. Operative mortality and AEs increased significantly with increased risk (all P<0.001) within each time period. Despite the increase in acuity, there was a decrease in operative mortality and AE for all strata. The decrease in AE in 2001 and 2002 was significantly different from the 1996 to 2000 data for both high-risk patients (P=0.03) and extremely high-risk patients (P=0.001).
Risk-stratified length of stay is shown in Table 3. Two-way ANOVA revealed a significant (P<0.001) within time period difference between strata for both CVLOS and PLOS. There was a significant interaction between risk group and time period for CVLOS (P=0.001). Additionally, there was a significant difference between time periods for PLOS (P=0.03).
TABLE 3.
Risk-stratified length of stay
| Low risk | Moderate risk | High risk | Extremely high risk | |
|---|---|---|---|---|
| CVLOS (days ± SD) | ||||
| 1996 to 2000 | 1.3±1.0 | 1.6±2.8 | 2.2±5.4 | 3.6±7.6 |
| 2001 to 2002 | 1.5±2.3 | 1.8±2.6 | 2.6±4.5 | 4.7±9.1 |
| PLOS (days ± SD) | ||||
| 1996 to 2000 | 6.3±3.4 | 7.2±4.8 | 8.7±6.8 | 11.6±10.3 |
| 2001 to 2002 | 6.7±3.3 | 7.1±4.2 | 8.9±7.1 | 12.0±11.0 |
Results of two-way ANOVA. Cardiovascular intensive care unit length of stay (CVLOS): risk group P<0.001, time period P=0.001, risk*time interaction P=0.0012. Total postoperative length of stay (PLOS): risk group P=0.001, time period P=0.03, risk × time interaction P=0.5
Quality performance
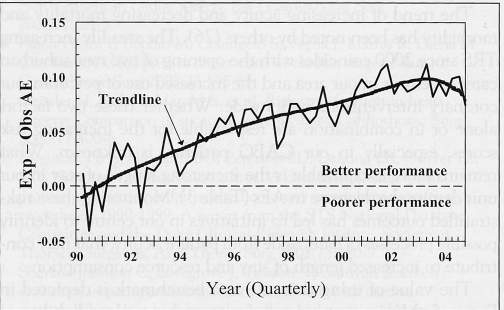
A qualitative depiction of the expected probability of AEs calculated by the logistic regression model minus observed AEs for each quarter year since 1990 is shown in Figure 5. Values above the zero line indicate performance better than expected based on case mix and severity of illness.
Figure 5).
The expected (Exp) adverse event (AE) probability minus the observed (Obs) AE rate for each quarter year since 1990. Values above the dashed zero line represent fewer AEs than expected based on case mix and illness severity
DISCUSSION
The comparison of observed patient outcomes with expected patient outcomes based on illness severity is the cornerstone of quality care evaluation, and is an essential element for any quality improvement program or operational planning. There are many risk scoring systems for operative mortality following CABG surgery (1,2,4–7) and fewer that incorporate valvular and other cardiac procedures (8,9). The use of operative mortality alone is limiting because nonfatal operative complications can have a significant effect on a patient’s functional status and quality of life and are significantly associated with prolonged length of hospital stay and increased resource consumption (9).
The TRS for AEs, which included operative mortality, following cardiac surgery, was developed to track changes in acuity (Figure 3), identify high-risk patients (Figure 4) and monitor quality performance (Figure 5) in a compulsory database containing all cardiac surgery patients, regardless of procedure. This database was very complete, with only 0.3% missing data, and has been subjected to random audits revealing an error rate of less than 1.5%. Details of this database have been previously reported (7,11,15).
The risk model in both the derivation and validation data sets had very good discrimination and precision. The calibration curves provide a benchmark for comparison of model performance in external databases. As we have shown in our previous work (7), the calibration of risk algorithms should be periodically checked and, if necessary, the model should either be recalibrated or remodelled, especially if the algorithm is being used to calculated risk-adjusted outcomes rather than evaluating temporal trends. The TRS accurately predicted length of stay in the validation data set (2001 and 2002). Few models have been developed to predict prolonged cardiovascular intensive care unit (CVICU) (21). Length of stay is not a normally distributed variable, so prolonged length of stay is often dicotomized to less than or greater than two days. These models typically have poorer discrimination possibly because a substantial number of deaths in the CVICU occur before the second day. Additionally, the definition of length of stay greater than two days in the CVICU is arbitrary and obsolete. Over the past decade, the use of short-acting anesthetics and shorter ventilation times have had a significant impact on reducing the CVICU length of stay for the majority of patients. The major determinant of prolonged length of stay in our unit is the occurrence of AEs. Therefore, as we have shown in this study, increases in patient risk captured by the TRS were significantly associated with increased length of stay.
Other scoring systems have shown similar results to ours. The European System for Cardiac Operative Risk Evaluation (EuroSCORE) (22–24) for operative mortality following all cardiac surgery has been shown to also predict direct costs. The patient population used to develop the EuroSCORE is similar to ours, although their operative morality for isolated CABG (3.4%) was somewhat higher than ours (1.5%), but similar to that seen in the Society of Thoracic Surgeons data (9). However, Geissler et al (25) evaluated six scoring systems and found that predicted values for morbidity were substantially different from predicted values for mortality. They concluded that the development of morbidity scores may improve prediction of outcomes and hospital costs. Shroyer et al (9) have recently developed a predictive risk index for operative mortality and morbidity for isolated CABG in the voluntary Society of Thoracic Surgeons database. The advantage of the TRS is that it is not limited solely to patients having isolated CABG but can be applied to all cardiac surgery procedures, and it was derived from a compulsory database containing all patients having surgery.
The current limitation of the present study is that the TRS was developed and validated in a single institution. Future studies will be conducted to externally validate this score.
The trend of increasing acuity and decreasing mortality and morbidity has been noted by others (26). The steadily increasing TRS since 2000 coincides with the opening of two new suburban cardiac centres in our area and the increased use of percutaneous coronary interventions nationwide. Whether these two factors alone or in combination are responsible for the increasing risk scores, especially in our CABG patients, is unknown. What remains more inexplicable is the increasing length of stay in our unit despite the decrease in AEs (Table 3). Monitoring these risk-stratified outcomes has led to initiatives in our centre to identify possible processes of care, aside from patient acuity, that may contribute to increased length of stay and resource consumption.
The value of using this rule as a benchmark is depicted in Figure 5. Monitoring expected-minus-observed probability of AEs offers further insights into assimilating complex risk-adjusted patterns of postoperative AEs over time. Although our method differs somewhat from the previously described variable life-adjusted display in that it does not calculate ‘lives saved’ (20,27,28), it is intuitively clear to clinicians and administrators. Lines that spike upward indicate better performance, whereas lines that spike downward indicate poorer performance. The trendline suggests that despite increasing acuity, there has been a steady increase in overall quality performance. These qualitative evaluations can alert the team to problems, which if identified early, can be corrected.
And finally, in Ontario, our funding formula for cardiac surgery is based on case mix and risk profile. By identifying the increased illness severity of our patients, we have an objective, rational argument for requesting increased funding. Whether our argument is successful remains to be seen.
In conclusion, the use of a risk scoring system for either the calculation of risk-adjusted AEs or risk-stratification can help both clinicians and administrators monitor quality performance and manage resources. The TRS is an objective and reliable measure of acuity for patients undergoing cardiac surgery.
ACKNOWLEDGEMENTS
The authors wish to express their sincere appreciation to Susan Collins, CV database manager at the Toronto General Hospital and the following cardiovascular surgeons: Dr Ronald J Baird (retired); Dr Christopher M Feindel; Dr Hugh E Scully; Dr RJ Cusimano; Dr Stephanie E Brister; Dr Lynda L Mickelborough (retired); Dr Tony Ralph-Edwards; Dr Richard D Weisel; and Dr Terry M Yau.
APPENDIX A Variables submitted to the logistic regression models
| Age, years | 65 to 74; ≥75 |
| Female | Female sex |
| Left ventricle (LV) grade | |
| 1 | LV ejection fraction (EF) greater than 60% |
| 2 | LVEF 40% to 59% |
| 3 | LVEF 20% to 39%, |
| 4 | LVEF less than 20% |
| Urgent priority | Surgery within 72 h of catheterization, echocardiography or cardiac event |
| Emergent priority | Surgery within 12 h of catheterization, echocardiography or cardiac event |
| Myocardial infarction less than one month | Myocardial infarction within 30 days before operation |
| Redo coronary artery bypass graft surgery(CABG) | Previous CABG surgery |
| Triple vessel disease | Significant (greater than 50%) stenosis of all three major coronary arteries |
| Left main disease | Significant (greater than 50%) stenosis of the left main coronary artery |
| Congestive heart failure (CHF) | CHF or history of chronic CHF at the time of admission |
| Renal insufficiency | Any renal dialysis or serum creatinine greater than 150 μmol/L |
| Diabetes | Any diabetes regardless of method of control/treatment |
| Peripheral vascular | Peripheral vascular disease including carotid or cerebral vascular disease |
| Hypertension | History of hypertension regardless of control |
| Chronic obstructive pulmonary disase | Chronic obstructive lung disease diagnosed by pulmonary function tests |
| Cerebral vascular event | Preoperative cerebral vascular event either stroke or transient ischemic attack |
| Atrial fibrillation | History of atrial fibrillation/flutter preoperatively |
| Complex valve | Surgery on more than one valve, or concomitant CABG or more than one previous valve operation |
| Other pathology | Surgery other than, or in addition to, CABG or valve or complex valve; may include: LV aneurysm resection, ascending aorta, adult congenital, maze procedure, myectomy, ventricular assist devices, septal repair, implantation of ventricular assist devices, transplants, etc |
Footnotes
DECLARATION: Presented, in part, at the Canadian Cardiovascular Congress, Edmonton, Alberta, 2002.
REFERENCES
- 1.Parsonnet V, Bernstein AD, Gera M. Clinical usefulness of risk-stratified outcome analysis in cardiac surgery in New Jersey. Ann Thorac Surg. 1996;61(2 Suppl):S8–11. doi: 10.1016/0003-4975(95)01076-9. [DOI] [PubMed] [Google Scholar]
- 2.Hannan EL, Kilburn H, Jr, O’Donnell JF, Lukacik G, Shields EP. Adult open heart surgery in New York state: An analysis of risk factors and hospital mortality rates. JAMA. 1990;264:2768–74. [PubMed] [Google Scholar]
- 3.Parsonnet V, Dean D, Bernstein AD. A method of uniform stratification of risk for evaluating the results of surgery in acquired adult heart disease. Circulation. 1989;79(Part 2):I3–12. (Erratum in 1990;82:1078) [PubMed] [Google Scholar]
- 4.Higgins TL, Estafanous FG, Loop FD, Beck GJ, Blum JM, Paranandi L. Stratification of morbidity and mortality outcome by preoperative risk factors in coronary artery bypass patients. A clinical severity score. JAMA. 1992;267:2344–8. (Erratum in 1992;268:1860) [PubMed] [Google Scholar]
- 5.O’Connor GT, Plume SK, Olmstead EM, et al. Multivariate prediction of in-hospital mortality associated with coronary artery bypass graft surgery. Northern New England Cardiovascular Disease Study Group. Circulation. 1992;85:2110–8. doi: 10.1161/01.cir.85.6.2110. [DOI] [PubMed] [Google Scholar]
- 6.Shroyer AL, Grover FL, Edwards FH. 1995 coronary artery bypass risk model: The Society of Thoracic Surgeons Adult Cardiac National Database. Ann Thorac Surg. 1998;65:879–84. doi: 10.1016/s0003-4975(98)00025-3. [DOI] [PubMed] [Google Scholar]
- 7.Ivanov J, Tu JV, Naylor CD. Ready-made, recalibrated, or remodeled? Issues in the use of risk indexes for assessing mortality after coronary artery bypass graft surgery. Circulation. 1999;99:2098–104. doi: 10.1161/01.cir.99.16.2098. [DOI] [PubMed] [Google Scholar]
- 8.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 9.Shroyer AL, Coombs LP, Peterson ED, et al. The Society of Thoracic Surgeons: 30-day operative mortality and morbidity risk models. Ann Thorac Surg. 2003;75:1856–64. doi: 10.1016/s0003-4975(03)00179-6. [DOI] [PubMed] [Google Scholar]
- 10.Shahian DM, Normand SL, Torchiana DF, et al. Cardiac surgery report cards: Comprehensive review and statistical critique. Ann Thorac Surg. 2001;72:2155–68. doi: 10.1016/s0003-4975(01)03222-2. [DOI] [PubMed] [Google Scholar]
- 11.Rao V, Ivanov J, Weisel RD, Ikonomidis JS, Christakis GT, David TE. Predictors of low cardiac output syndrome after coronary artery bypass. J Thorac Cardiovasc Surg. 1996;112:38–51. doi: 10.1016/s0022-5223(96)70176-9. [DOI] [PubMed] [Google Scholar]
- 12.Rao V, Christakis GT, Weisel RD, et al. Risk factors for stroke following coronary bypass surgery. J Card Surg. 1995;10(4 Suppl):468–74. doi: 10.1111/j.1540-8191.1995.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 13.Borger MA, Rao V, Weisel RD, et al. Deep sternal wound infection: Risk factors and outcomes. Ann Thorac Surg. 1998;65:1050–6. doi: 10.1016/s0003-4975(98)00063-0. [DOI] [PubMed] [Google Scholar]
- 14.Borger MA, Ivanov J, Weisel RD, et al. Decreasing incidence of stroke during valvular surgery. Circulation. 1998;98(19 Suppl):II137–43. [PubMed] [Google Scholar]
- 15.Ivanov J, Weisel RD, David TE, Naylor CD. Fifteen-year trends in risk severity and operative mortality in elderly patients undergoing coronary artery bypass graft surgery. Circulation. 1998;97:673–80. doi: 10.1161/01.cir.97.7.673. [DOI] [PubMed] [Google Scholar]
- 16.Yau TM, Borger MA, Weisel RD, Ivanov J. The changing pattern of reoperative coronary surgery: Trends in 1230 consecutive reoperations. J Thorac Cardiovasc Surg. 2000;120:156–63. doi: 10.1067/mtc.2000.106983. [DOI] [PubMed] [Google Scholar]
- 17.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 18.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons; 1989. [Google Scholar]
- 19.Trotter HF. An elementary proof of the central limit theorem. Arch Math. 1959;10:226–34. [Google Scholar]
- 20.Lovegrove J, Valencia O, Treasure T, Sherlaw-Johnson C, Gallivan S. Monitoring the results of cardiac surgery by variable life-adjusted display. Lancet. 1997;350:1128–30. doi: 10.1016/S0140-6736(97)06507-0. [DOI] [PubMed] [Google Scholar]
- 21.Tu JV, Mazer CD, Levinton C, Armstrong PW, Naylor CD. A predictive index for length of stay in the intensive care unit following cardiac surgery. CMAJ. 1994;151:177–85. [PMC free article] [PubMed] [Google Scholar]
- 22.Roques F, Nashef SA, Michel P, et al. Risk factors and outcome in European cardiac surgery: Analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816–22. doi: 10.1016/s1010-7940(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 23.Nashef SA, Roques F, Hammill BG, et al. Validation of European System for Cardiac Operative Risk Evaluation (EuroSCORE) in North American cardiac surgery. Eur J Cardiothorac Surg. 2002;22:101–5. doi: 10.1016/s1010-7940(02)00208-7. [DOI] [PubMed] [Google Scholar]
- 24.Pinna Pintor P, Bobbio M, Colangelo S, Veglia F, Marras R, Diena M. Can EuroSCORE predict direct costs of cardiac surgery? Eur J Cardiothorac Surg. 2003;23:595–8. doi: 10.1016/s1010-7940(02)00868-0. [DOI] [PubMed] [Google Scholar]
- 25.Geissler H, Holzl P, Marohl S, et al. Risk stratification in heart surgery: Comparison of six score systems. Eur J Cardiothorac Surg. 2000;17:400–6. doi: 10.1016/s1010-7940(00)00385-7. [DOI] [PubMed] [Google Scholar]
- 26.Ferguson TB, Jr, Hammill BG, Peterson ED, DeLong ER, Grover FL. STS National Database Committee. A decade of change – risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990–1999: A report from the STS National Database Committee and the Duke Clinical Research Insitute. Society of Thoracic Surgeons. Ann Thorac Surg. 2002;73:480–9. doi: 10.1016/s0003-4975(01)03339-2. [DOI] [PubMed] [Google Scholar]
- 27.Sherlaw-Johnson C, Lovegrove J, Treasure T, Gallivan S. Likely variations in perioperative mortality associated with cardiac surgery: When does high mortality reflect bad practice? Heart. 2000;84:79–82. doi: 10.1136/heart.84.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albert AA, Walter JA, Arnrich B, et al. On-line variable live-adjusted displays with internal and external risk-adjusted mortalities. A valuable method for benchmarking and early detection of unfavourable trends in cardiac surgery. Eur J Cardiothorac Surg. 2004;25:312–9. doi: 10.1016/j.ejcts.2003.12.009. [DOI] [PubMed] [Google Scholar]