Abstract
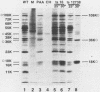
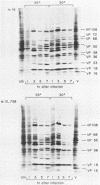
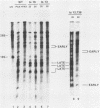
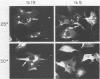
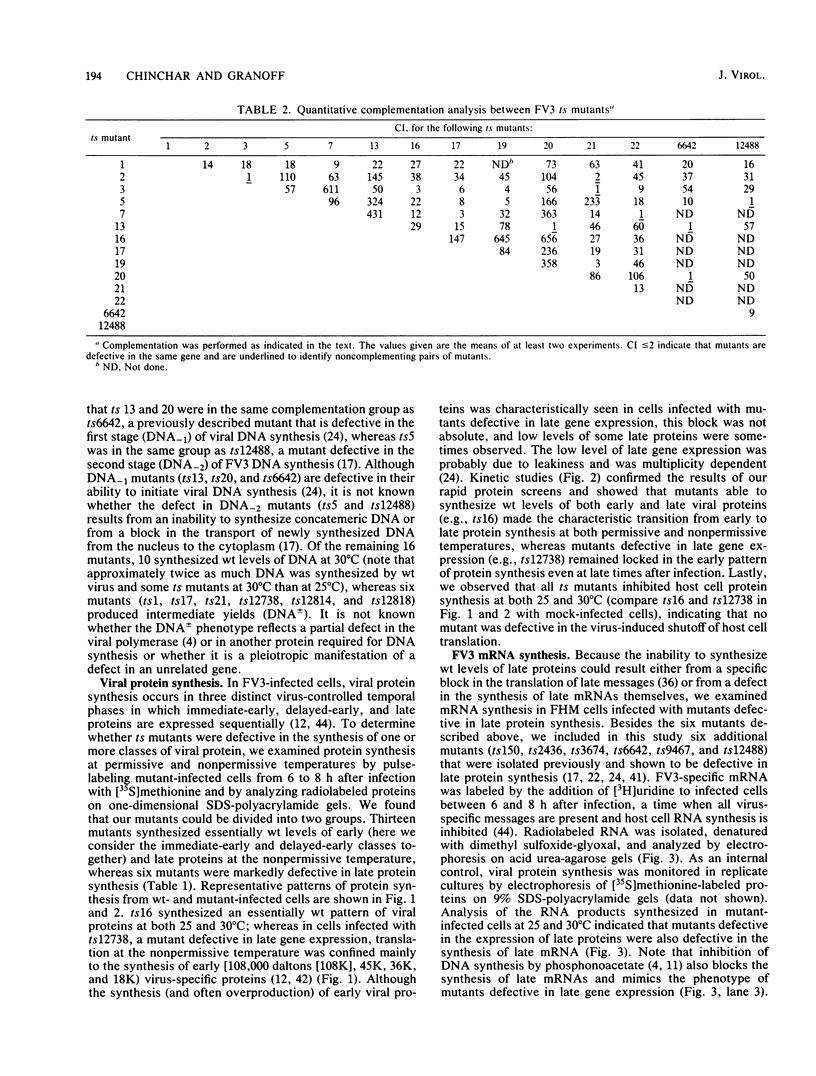
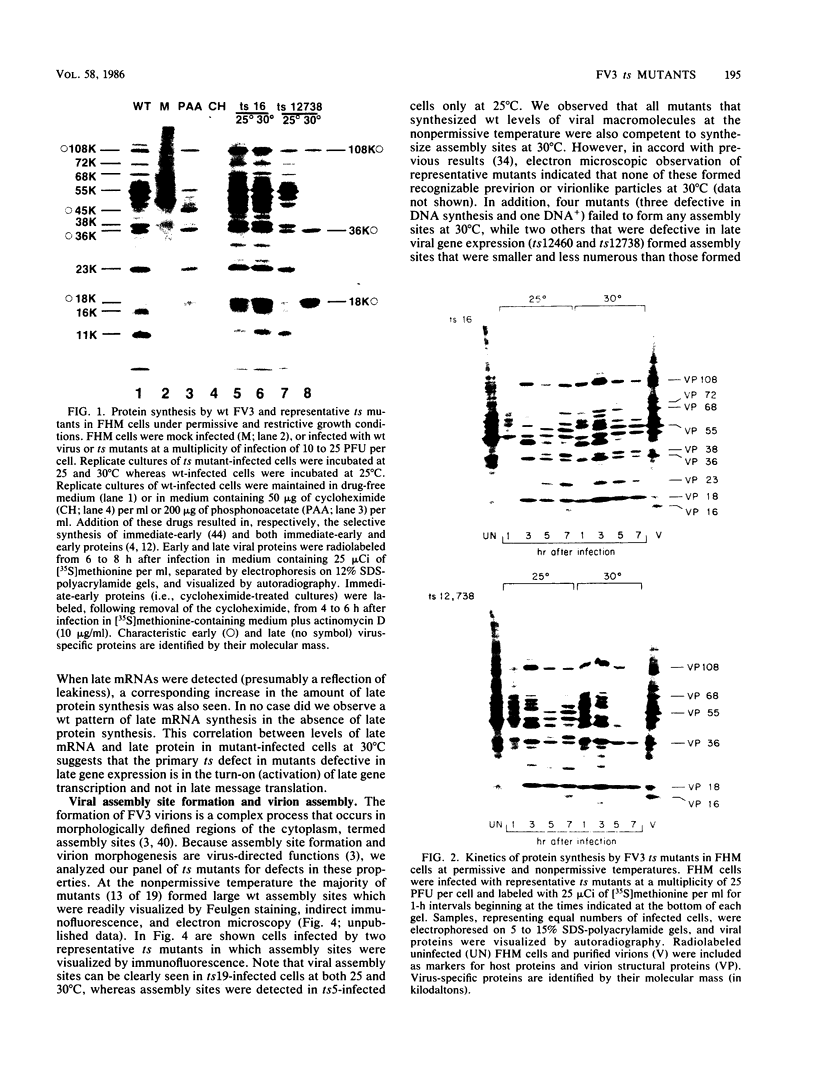
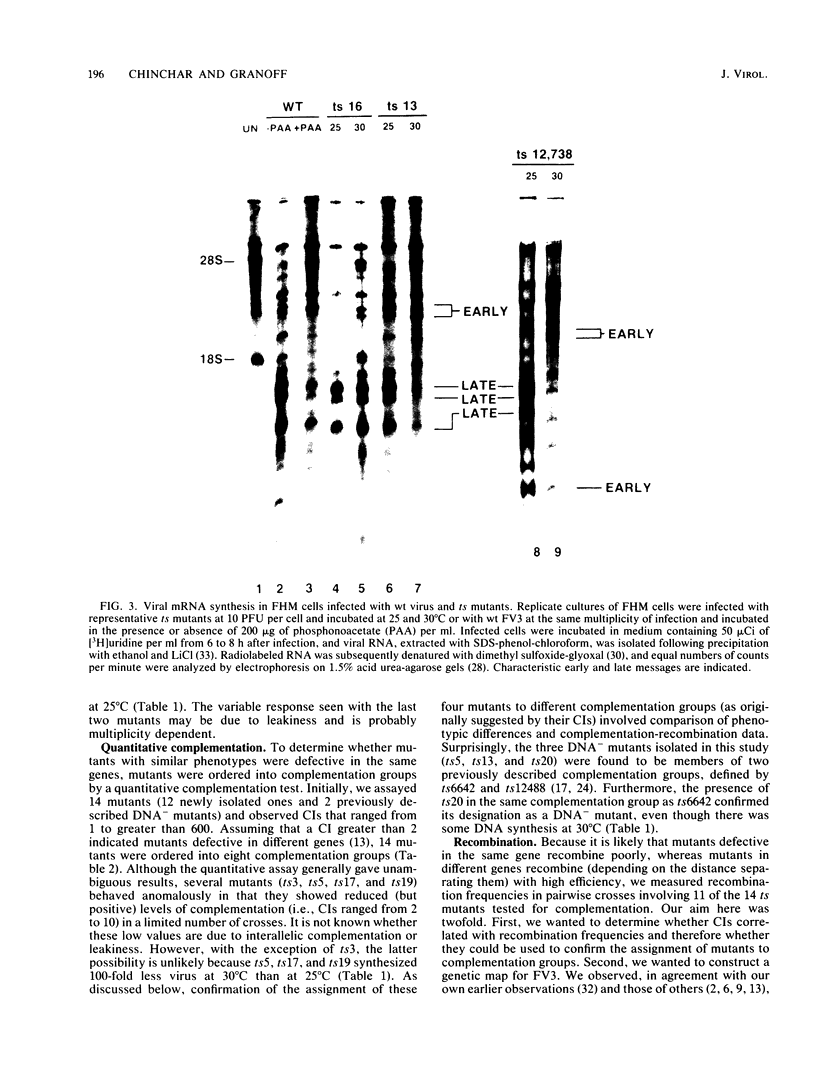
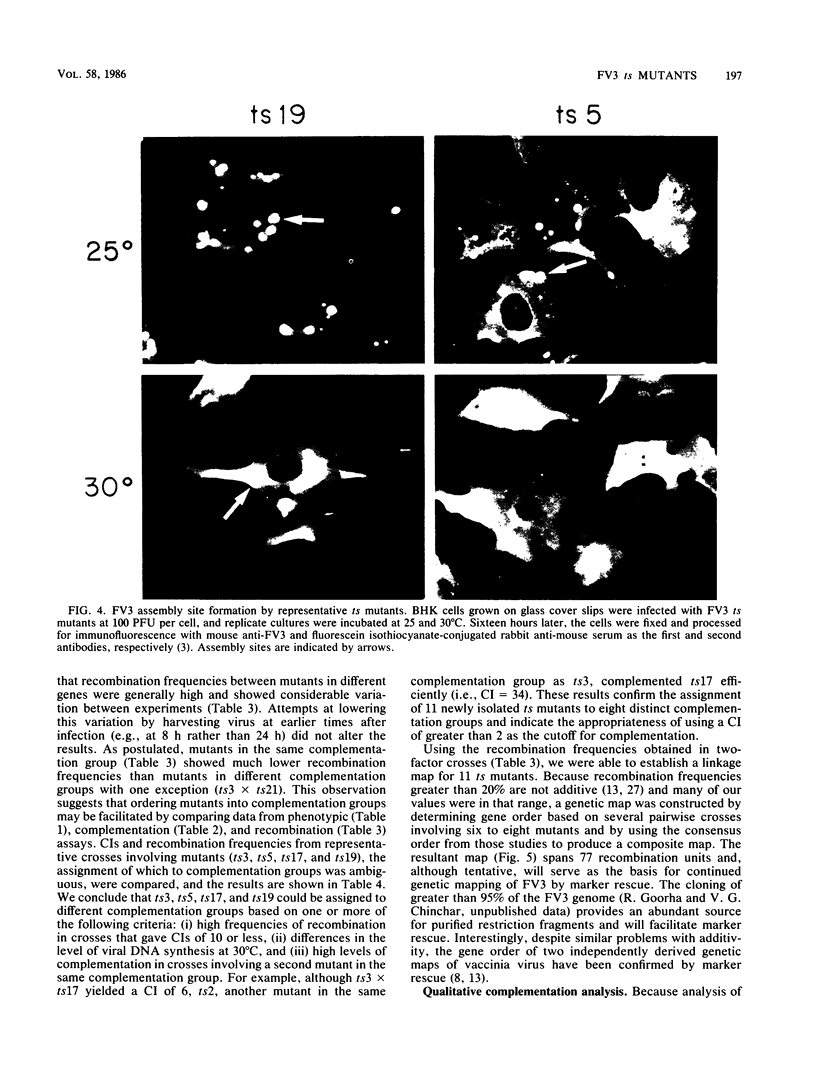
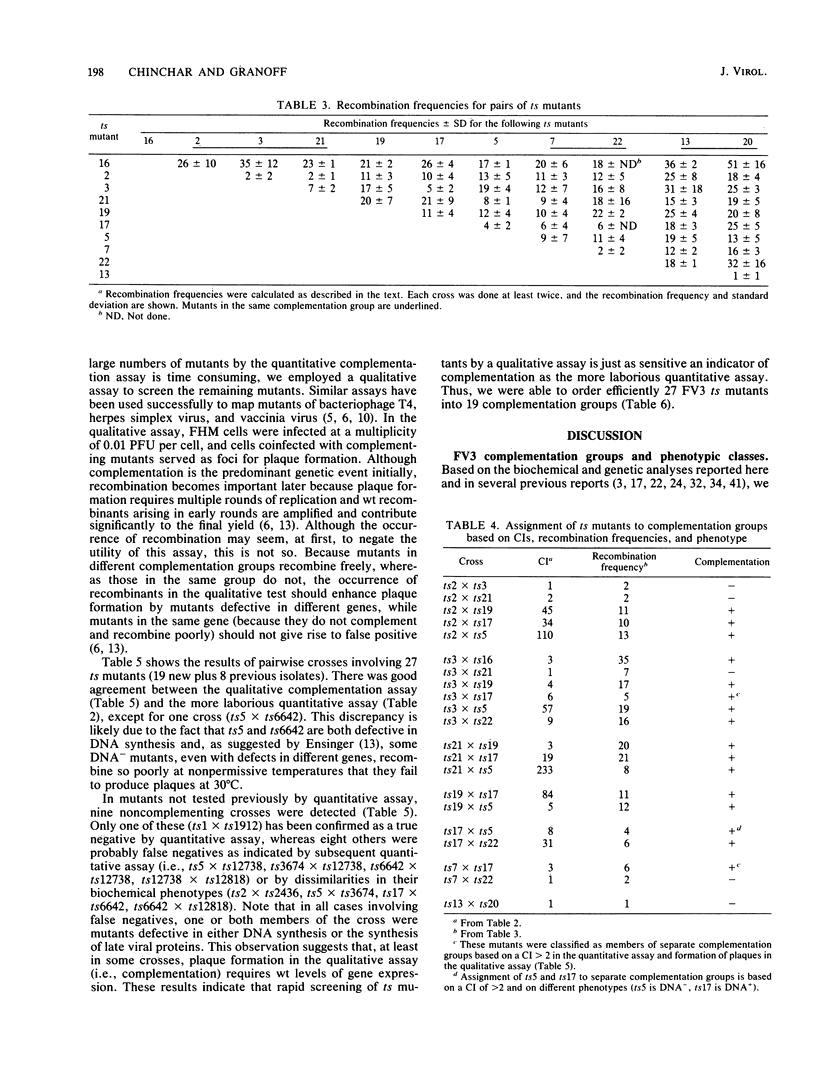
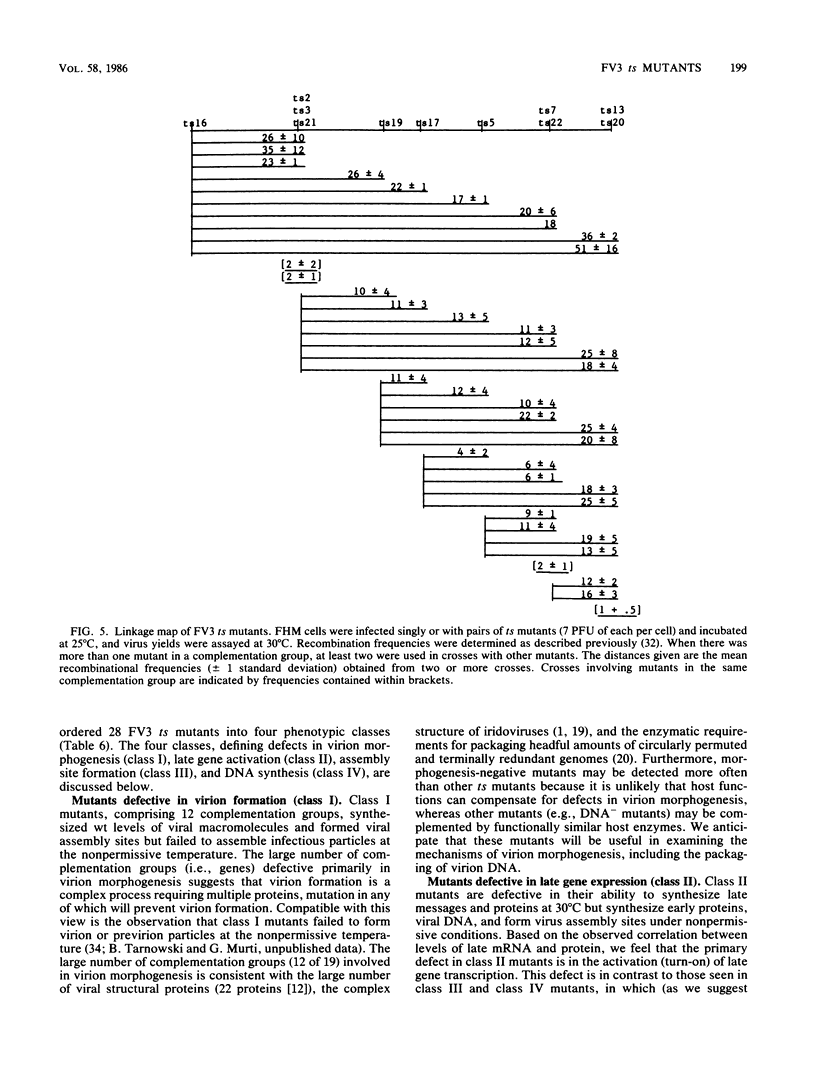
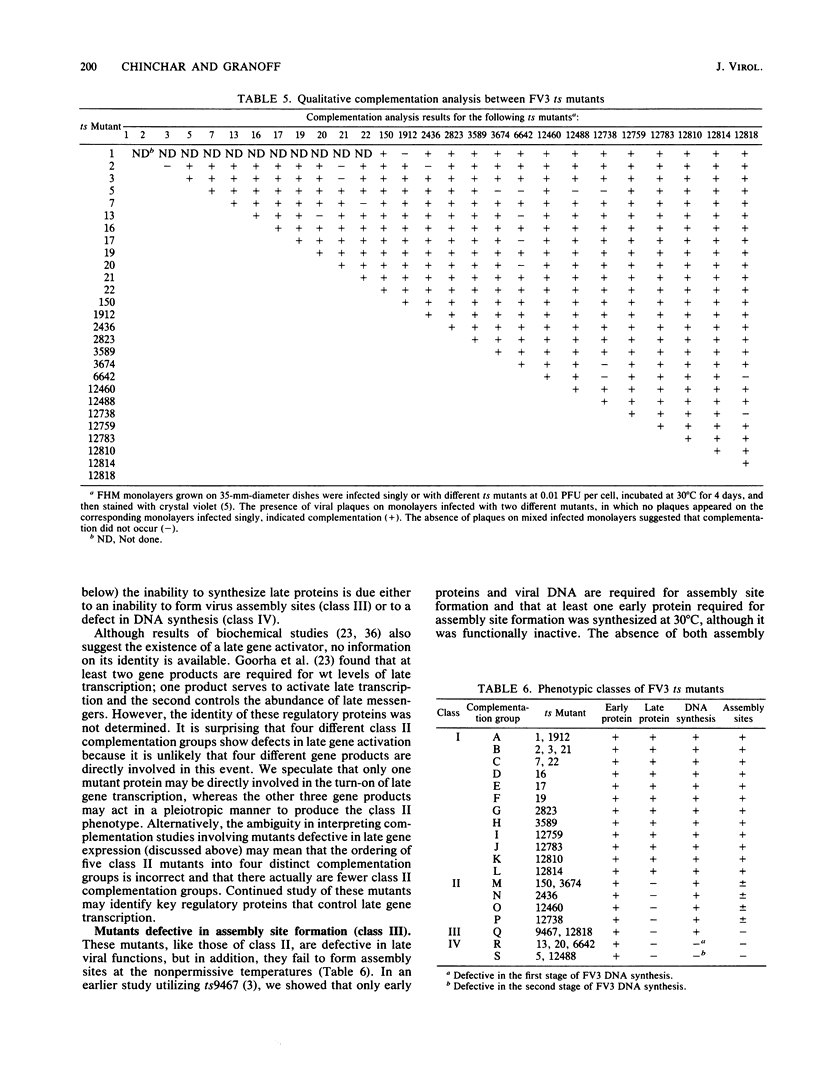
Nineteen frog virus 3 temperature-sensitive mutants were isolated after mutagenesis with nitrosoguanidine and assayed for viral DNA, RNA, and protein synthesis, as well as assembly site formation at permissive (25 degrees C) and nonpermissive (30 degrees C) temperatures. In addition, mutants were characterized for complementation by both quantitative and qualitative assays. Based on the genetic and biochemical data, the 19 mutants, along with 9 mutants isolated earlier, were ordered into four phenotypic classes which define defects in virion morphogenesis (class I), late mRNA synthesis (class II), viral assembly site formation (class III), and viral DNA synthesis (class IV). In addition, we used two-factor crosses to order 11 mutants, comprising 7 complementation groups, onto a linkage map spanning 77 recombination units.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berthiaume L., Alain R., Robin J. Morphology and ultrastructure of Lymphocystis disease virus, a fish iridovirus, grown in tissue culture. Virology. 1984 May;135(1):10–19. doi: 10.1016/0042-6822(84)90112-0. [DOI] [PubMed] [Google Scholar]
- Brown S. M., Ritchie D. A., Subak-Sharpe J. H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973 Mar;18(3):329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- Chinchar V. G., Goorha R., Granoff A. Early proteins are required for the formation of frog virus 3 assembly sites. Virology. 1984 May;135(1):148–156. doi: 10.1016/0042-6822(84)90125-9. [DOI] [PubMed] [Google Scholar]
- Chinchar V. G., Granoff A. Isolation and characterization of a frog virus 3 variant resistant to phosphonoacetate: genetic evidence for a virus-specific DNA polymerase. Virology. 1984 Oct 30;138(2):357–361. doi: 10.1016/0042-6822(84)90361-1. [DOI] [PubMed] [Google Scholar]
- Chu C. T., Schaffer P. A. Qualitative complementation test for temperature-sensitive mutants of herpes simplex virus. J Virol. 1975 Nov;16(5):1131–1136. doi: 10.1128/jvi.16.5.1131-1136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Drillien R., Spehner D., Kirn A. Complementation and genetic linkage between vaccinia virus temperature-sensitive mutants. Virology. 1982 Jun;119(2):372–381. doi: 10.1016/0042-6822(82)90096-4. [DOI] [PubMed] [Google Scholar]
- Drillien R., Spehner D. Physical mapping of vaccinia virus temperature-sensitive mutations. Virology. 1983 Dec;131(2):385–393. doi: 10.1016/0042-6822(83)90506-8. [DOI] [PubMed] [Google Scholar]
- EDGAR R. S., DENHARDT G. H., EPSTEIN R. H. A COMPARATIVE GENETIC STUDY OF CONDITIONAL LETHAL MUTATIONS OF BACTERIOPHAGE T4D. Genetics. 1964 Apr;49:635–648. doi: 10.1093/genetics/49.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. M., Bateson A., Kelly D. C. Phosphonoacetic Acid inhibition of frog virus 3 replication. J Virol. 1980 Jan;33(1):539–542. doi: 10.1128/jvi.33.1.539-542.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. M., Kelly D. C. Frog virus 3 replication: induction and intracellular distribution of polypeptides in infected cells. J Virol. 1980 Jan;33(1):28–51. doi: 10.1128/jvi.33.1.28-51.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J. Isolation and genetic characterization of temperature-sensitive mutants of vaccinia virus WR. J Virol. 1982 Sep;43(3):778–790. doi: 10.1128/jvi.43.3.778-790.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorha R., Dixit P. A temperature-sensitive (TS) mutant of frog virus 3 (FV3) is defective in second-stage DNA replication. Virology. 1984 Jul 15;136(1):186–195. doi: 10.1016/0042-6822(84)90258-7. [DOI] [PubMed] [Google Scholar]
- Goorha R. Frog virus 3 DNA replication occurs in two stages. J Virol. 1982 Aug;43(2):519–528. doi: 10.1128/jvi.43.2.519-528.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorha R. Frog virus 3 requires RNA polymerase II for its replication. J Virol. 1981 Jan;37(1):496–499. doi: 10.1128/jvi.37.1.496-499.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorha R., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. I. Virus-specific protein synthesis and its regulation. Virology. 1974 Jul;60(1):237–250. doi: 10.1016/0042-6822(74)90381-x. [DOI] [PubMed] [Google Scholar]
- Goorha R., Murti G., Granoff A., Tirey R. Macromolecular synthesis in cells infected by frog virus 3. VIII. The nucleus is a site of frog virus 3 DNA and RNA synthesis. Virology. 1978 Jan;84(1):32–50. doi: 10.1016/0042-6822(78)90216-7. [DOI] [PubMed] [Google Scholar]
- Goorha R., Murti K. G. The genome of frog virus 3, an animal DNA virus, is circularly permuted and terminally redundant. Proc Natl Acad Sci U S A. 1982 Jan;79(2):248–252. doi: 10.1073/pnas.79.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorha R., Naegele R. F., Purifoy D., Granoff A. Macromolecular synthesis in cells infected with frog virus 3. III. Virus-specific protein synthesis by temperature-sensitive mutants. Virology. 1975 Aug;66(2):428–439. doi: 10.1016/0042-6822(75)90215-9. [DOI] [PubMed] [Google Scholar]
- Goorha R., Willis D. B., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. XII. Viral regulatory proteins in transcriptional and post-transcriptional controls. J Virol. 1979 Nov;32(2):442–448. doi: 10.1128/jvi.32.2.442-448.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorha R., Willis D. B., Granoff A., Naegele R. F. Characterization of a temperature-sensitive mutant of frog virus 3 defective in DNA replication. Virology. 1981 Jul 15;112(1):40–48. doi: 10.1016/0042-6822(81)90610-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M. H., Willis D. B. Restriction endonuclease mapping of the frog virus 3 genome. Virology. 1983 Apr 15;126(1):317–327. doi: 10.1016/0042-6822(83)90481-6. [DOI] [PubMed] [Google Scholar]
- Lynch K. R., Pennica D., Ennis H. L., Cohen P. S. Separation and purification of the mRNAs for vesicular stomatitis virus NS and M proteins. Virology. 1979 Oct 15;98(1):251–254. doi: 10.1016/0042-6822(79)90543-9. [DOI] [PubMed] [Google Scholar]
- Martin J. P., Aubertin A. M., Tondre L., Kirn A. Fate of frog virus 3 DNA replicated in the nucleus of arginine-deprived CHO cells. J Gen Virol. 1984 Apr;65(Pt 4):721–732. doi: 10.1099/0022-1317-65-4-721. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murti K. G., Goorha R., Granoff A. Structure of frog virus 3 genome: size and arrangement of nucleotide sequences as determined by electron microscopy. Virology. 1982 Jan 15;116(1):275–283. doi: 10.1016/0042-6822(82)90419-6. [DOI] [PubMed] [Google Scholar]
- Naegele R. F., Granoff A. Viruses and renal carcinoma of Rana pipiens. XI. Isolation of frog virus 3 temperature-sensitive mutants; complementation and genetic recombination. Virology. 1971 May;44(2):286–295. doi: 10.1016/0042-6822(71)90260-1. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Purifoy D., Naegele R. F., Granoff A. Viruses and renal carcinoma of Rana pipiens. XIV. Temperature-sensitive mutants of frog virus 3 with defective encapsidation. Virology. 1973 Aug;54(2):525–535. doi: 10.1016/0042-6822(73)90162-1. [DOI] [PubMed] [Google Scholar]
- Raghow R., Granoff A. Cell-free translation of frog virus 3 messenger RNAs. Initiation factors from infected cells discriminate between early and late viral mRNAs. J Biol Chem. 1983 Jan 10;258(1):571–578. [PubMed] [Google Scholar]
- Raghow R., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. XIV. Characterization of the methylated nucleotide sequences in viral messenger RNAs. Virology. 1980 Nov;107(1):283–294. doi: 10.1016/0042-6822(80)90293-7. [DOI] [PubMed] [Google Scholar]
- Raghow R., Willis D. B., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. XIII. Cell-free translation of immediate early viral mRNAs. Virology. 1980 Jan 30;100(2):495–497. doi: 10.1016/0042-6822(80)90541-3. [DOI] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Timbury M. C., Calder L. Temperature-sensitive mutants of herpes simplex virus type 2: a provisional linkage map based on recombination analysis. J Gen Virol. 1976 Feb;30(2):179–186. doi: 10.1099/0022-1317-30-2-179. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Goorha R., Chinchar V. G. Macromolecular synthesis in cells infected by frog virus 3. Curr Top Microbiol Immunol. 1985;116:77–106. doi: 10.1007/978-3-642-70280-8_5. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Goorha R., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. XI. A ts mutant of frog virus 3 that is defective in late transcription. Virology. 1979 Oct 30;98(2):328–335. doi: 10.1016/0042-6822(79)90556-7. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Goorha R., Miles M., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. VII. Transcriptional and post-transcriptional regulation of virus gene expression. J Virol. 1977 Oct;24(1):326–342. doi: 10.1128/jvi.24.1.326-342.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. B., Granoff A. Frog virus 3 DNA is heavily methylated at CpG sequences. Virology. 1980 Nov;107(1):250–257. doi: 10.1016/0042-6822(80)90290-1. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. IV. Regulation of virus-specific RNA synthesis. Virology. 1976 Apr;70(2):399–410. doi: 10.1016/0042-6822(76)90281-6. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. IX. Two temporal classes of early viral RNA. Virology. 1978 May 15;86(2):443–453. doi: 10.1016/0042-6822(78)90084-3. [DOI] [PubMed] [Google Scholar]