Abstract
Background
Cathelicidins are a family of antimicrobial peptides acting as multifunctional effector molecules of innate immunity, which are firstly found in mammalians. Recently, several cathelicidins have also been found from chickens and fishes. No cathelicidins from other non-mammalian vertebrates have been reported.
Principal Findings
In this work, a cathelicidin-like antimicrobial peptide named cathelicidin-BF has been purified from the snake venoms of Bungarus fasciatus and its cDNA sequence was cloned from the cDNA library, which confirm the presence of cathelicidin in reptiles. As other cathelicidins, the precursor of cathelicidin-BF has cathelin-like domain at the N terminus and carry the mature cathelicidin-BF at the C terminus, but it has an atypical acidic fragment insertion between the cathelin-like domain and the C-terminus. The acidic fragment is similar to acidic domains of amphibian antimicrobial precursors. Phylogenetic analysis revealed that the snake cathelicidin had the nearest evolution relationship with platypus cathelicidin. The secondary structure of cathelicidin-BF investigated by CD and NMR spectroscopy in the presence of the helicogenic solvent TFE is an amphipathic α-helical conformation as many other cathelicidins. The antimicrobial activities of cathelicidin BF against forty strains of microorganisms were tested. Cathelicidin-BF efficiently killed bacteria and some fungal species including clinically isolated drug-resistance microorganisms. It was especially active against Gram-negative bacteria. Furthermore, it could exert antimicrobial activity against some saprophytic fungus. No hemolytic and cytotoxic activity was observed at the dose of up to 400 µg/ml. Cathelicidin-BF could exist stably in the mice plasma for at least 2.5 hours.
Conclusion
Discovery of snake cathelicidin with atypical structural and functional characterization offers new insights on the evolution of cathelicidins. Potent, broad spectrum, salt-independent antimicrobial activities make cathelicidin-BF an excellent candidate for clinical or agricultural antibiotics.
Introduction
Innate immunity uses gene-encoded antimicrobial peptides to form a first line of host defense against noxious microorganisms [1], [2]. A large amount of antimicrobial peptides have been identified from animals, plants and microorganisms. Several families of antimicrobial peptides including cathelicidin, liver-expressed antimicrobial peptide (LEAP) or hepcidin, histatin, and defensin have been identified from mammalians [3]–[7]. Defensins and hepcidins are characterized by the presence of multiple disulfide bridges, whereas histatins and most of cathelicidins are linear molecules without disulfide bridges.
After the first discovery of cathelicidin (Bac5) from bovine neutrophils, a large amount of cathelicidins have been identified from other mammalians [8]–[13]. As other antimicrobial peptide families, structurally divergent cathelicidins have been found, even in a single mammalian species. For example, there are at least seven cathelicidins in cattle, horse, pig, sheep, and goat [8]. Some exceptions are in human, rhesus monkey, mouse, rat, and guinea pig, only a single cathelicidin was found [8], [14]–[18].
Cathelicidin antimicrobial peptides are released from their corresponding inactive precursors by proteolytic cleavage [8]. The cathilicidin family of proteins is characterized by the presence of a highly conserved anionic cathelin domain [3], [8], [19]. Cathelin is an inhibitor of the cysteine proteinase cathepsin L [20]. In the precursors of cathelicidins, the highly conserved cathelin domains composed of about 100 amino acid residues is flanked by a signal peptide fragment (approximately 30 residues long) on its N-terminus, and by a structurally divergent cationic antimicrobial peptide region on its C-terminus [8]. Upon activation, most of cathelicidin precursors proteolytically cleaved to release the cathelin domain and the C-terminal mature antimicrobial peptides. Some intact cathelicidin precursors are also found in the biological fluids where cathelicidin expressed [3], [21]. Elastase seems to be the most common peptidase to release mature cathelicidins [22], [23]. In human hCAP18, however, protease-3 cleaves the proprotein [24]. Mature cathelicidins can be further degraded by some serine proteases because multiple cationic amino acid residues (Arg or Lys) are in the sequences of cathelicidins [25]. In addition, hCAP18 could be degraded by aspartyl protease (gastricsin) at vaginal pH. Some hydrolytic fragments of cathelicidin were found to possess increased antimicrobial abilities [26].
Recently, several cathelicidins have been identified from some non-mammalian vertebrates including hagfish [27], rainbow trout [28], [29], atlantic salmon [29], and chicken [30], [31]. As the oldest jawless craniates, hagfish lacks adaptive immunity [8], [32]. The presence of cathelicidins in hagfish may indicate that cathelicidin genes appeared early in phylogenesis [8]. Cathelicidins have been found from most of vertebrates including fish, bird, mammalian, whereas no cathelicidins have been found from amphibians and reptiles. In this wok, a cathelicidin from snake was identified and characterized.
Materials and Methods
Materials
B. fasciatus crude venom and venomous glands were collected from Guang Xi Province, China. The SMART™ PCR cDNA synthesis kit was purchased from Clontech, USA. Chromatography media Sephadex G-50 and CM-Sephadex C-25 were obtained from Amersham Bioscience, Sweden. Trifluoroacetic acid (TFA, HPLC grade) was from Perkin-Elmer. Acetonitrile (ACN, HPLC grade) was bought from Fisher Chemicals. 2,2,2-trifluroethanol-d3 98% (TFE-d3), sodium dodecyl-d25 sulfate (SDS-d25) 98.7%, trimethylsilyl-2,2,3,3-tetradeuteropropionic acid (TSP)-d4 98% and D2O 99% were purchased from Cambridge Isotope Laboratories. Reverse Phase High Performance Liquid Chromatography (RP-HPLC) C4 column (30 cm×0.46 cm) was from Agilent. The pMD18-T vector was from Takara, Dalian, China. All other reagents were of analytical or sequencing grade. The animals used for the experiments were treated according to the protocols evaluated and approved by the ethical committee of Kunming Institute of Zoology.
Isolation of cathelicidin-BF
The purification procedure was according to our previously report [33], [34]. 0.4 g B. fasciatus crude venom was first fractionated using gel filtration chromatography Sephadex G-50 column (26 cm×100 cm), equilibrated with 50 mM Tris–HCl, 50 mM NaCl (pH 7.8). The elution was performed with the same buffer and monitored at UV absorption of 280 nm. The peak having antimicrobial activity was collected and further dialyzed against PBS (pH 6.0). The dialyzed product was next subjected to the cation-exchange CM-Sephadex C-25 column (1.6 cm×30 cm). The elution was achieved with a linear NaCl gradient, at a flow rate of 1 ml/min. The peak with antimicrobial activity was collected and finally purified by reverse phase high performance liquid chromatography (C4), equilibrated with 0.1% (v/v) TFA/water. The elution was performed with a liner gradient of acetonitrile at a flow rate of 0.7 ml/min.
Primary structural analysis
The amino acid sequence of the N-terminus was determined by the automated Edman degradation using an Applied Biosystems pulsed liquid-phase sequencer, model 491. Electrospray ionization mass spectrometry (ESI-MS) was used to determine the molecular weight by a Finnigan LCQ ion trap mass spectrometer (ThermoFinnigan, San Jose, CA, USA) in positive-ion mode. The sample solutions (50%H2O/50%ACN) were infused into the mass spectrometer via a Harvard syringe pump (Holliston, MA, USA). The spray voltage was set to +4.5 kV. Spectra were acquired by summing 30 scans.
CD and NMR spectroscopy
Circular dichroism (CD) spectra were recorded at 298 K on a JASCO J-810 spectrometer (Jasco, Japan). Samples were prepared by dissolving the peptide powder to a concentration of 90 µM in TFE/H2O mixtures or in SDS micelles of different concentrations. The spectra were measured between 190 and 250 nm using 0.1 cm path-length cell with 1 nm bandwidth, 1 sec response time, and a scan speed of 100 nm/min. Three consecutive scans per sample were performed, added and averaged followed by subtraction of the signal of the solvent. The secondary structure elements of the peptides were estimated according to the Yang formula [35].
Samples for nuclear magnetic resonance (NMR) measurements contained 4 mM cantheicidin-BF in TFE-d3/H2O (9∶1, v/v) at pH 6.5, or in 300 mM SDS-d25 at pH 6.5. All NMR spectra were recorded at 298 K on a Varian Unity INOVA 600 MHz spectrometer equipped with three RF channels and a triple resonance z-axis pulsed-field gradient probe. The 2D 1H-1H TOCSY spectra were acquired with a mixing time of 75 ms, while 1H-1H NOESY spectra were acquired with mixing times of 200 and 300 ms. The watergate approach was employed for water suppression. Data were collected with 256 and 1024 complex data points in t1 and t2 dimensions, respectively. Signals were averaged over 64 transients. All NMR spectra were processed and analyzed using the NMRPipe/NMRDraw software and the Sparky program [36], [37]. Linear prediction in the t1 dimension was used before the Fourier transformation. Assignments of the proton resonances were achieved using both TOCSY and NOESY spectra. The 1H chemical shifts were referenced to TSP. The secondary structure was predicted using the Hα Chemical Shift Index approach [38].
SMART cDNA synthesis
Total RNA was extracted using TRIzol (Life Technologies, Ltd.) from the venomous glands of B. fasciatus. cDNA was synthesized by SMART™ techniques by using a SMART™ PCR cDNA synthesis kit (Clontech, Palo Alto, CA). The first strand was synthesized by using cDNA 3′ SMART CDS Primer II A, 5′-AAGCAGTGGTATCAACGCAGAGTACT (30) N-1N-3′ (N = A, C, G or T; N-1 = A, G or C), and SMART II An oligonucleotide, 5′-AAGCAGTGGTATCAACGCAGAGTACGCGGG-3′. The second strand was amplified using Advantage polymerase by 5′ PCR primer II A, 5′-AAGCAGTGGTATCAACGCAGAGT- 3′.
Screening of cDNA encoding cathelicidin-BF
The cDNA synthesized by SMART™ techniques was used as template for PCR to screen the cDNAs encoding serine protease inhibitor. Two oligonucleotide primers, BFS1 5′-AA(A/G)TT(T/C)TT(T/C)AG(A/G)AA(A/G)(C/T)T(A/T/C/G)AA(A/G)AA (A/G)-3′, in the reverse direction, a specific primer designed according to the amino acid sequence determined by Edman degradation and primer II A as mentioned in “SMART cDNA synthesis” in the sense direction were used in PCR reactions. The DNA polymerase was Advantage polymerase from Clontech (Palo Alto, CA) The PCR conditions were: 2 min at 94°C, followed by 30 cycles of 10 sec at 92°C, 30 sec at 50°C, 40 sec at 72°C. Finally, the PCR products were cloned into pGEM®-T Easy vector (Promega, Madison, WI). DNA sequencing was performed on an Applied Biosystems DNA sequencer, model ABI PRISM 377.
Expression profile of tissues
Reverse transcription-polymerase chain reaction (RT-PCR) was carried out to analyze gene expression of cathelicidin-BF in B. fasciatus. Total RNA extraction from different tissues and first-strand cDNA synthesis were the same as described above. The primers were, forward primer, 5′-cathelicidin: 5′-ATGGAAGGGTTCTTCTGGA AGACC-3′, and reverse primer, 3′-cathelicidin: 5′-CAAATTAGAAGGGGATGGAG ACC-3′. PCR conditions were: 95°C (3 min), and 30 cycles of 95°C (30 s), 56°C (30 s), 72°C (3 min) followed by a 15 min extension period at 72°C. The control PCR was performed using the specific primers (forward primer, actin-s 5′-GGGTGTGATGGT TGGCATGG-3′, and reverse primer, actin-as 5′-TGGCTGGAAGAGGGCTTCTG-3′) for snake actin, using the same conditions as above.
Alignment and phyogenetic comparison of cathelicidins
Cathelicidin sequences were obtained from the protein database at the National Center for Biotechnology Information. The phylogenetic tree is constructed by neighbor-joining analysis, using the ClustalW program (version 1.8).
Antimicrobial testing
Antimicrobial activities of cathelicidin-BF and cathelicidin-BF15 (VKRFKKFFRKLKKSV) were tested according to our previous methods [39]–[42]. Ampicillin, benzylpenicillin (Amresco) and Imipenem and Cilastatin Sodium for Injection (ICS, Merck) were used as positive controls. The details were provided in the Materials and Methods S1.
Bacteria killing kinetics
In vitro bacteria killing kinetics of cathelicidin-BF, ICS (its minimal inhibitory concentration (MIC) for Escherichia coli 08A866 is 0.15 µg/ml), and HDW (an antimicrobial peptide from the frog of Rana nigrovittata, with a amino acid sequence of FIGPVLKIATSILPTAICKIFKKC, its MIC for E. coli 08A866 is 18.7 µg/ml), respectively, were determined according to the methods described by Mygind et al [43]. The details were provided in the Materials and Methods S1.
Hemolysis, cytotoxicity, serum stability
Hemolytic activity was checked by incubating the tested samples with human red blood cells to determine hemoglobin releasing ability by measuring the absorbance at 540 nm, using 1% Triton X-100 as a positive control. Cytotoxicity and serum stability were measured according the methods described by Mygind et al [43]. The details were provided in the Materials and Methods S1.
Synthetic Peptides
All of the peptides used for the bioactivity assays and NMR analysis in this paper were synthesized by the peptide synthesizer (433A, Applied Biosystems) in AC SCIENTIFIC (Xi An) INC. (Xi An, China) and analyzed by HPLC and MALDI-TOF mass spectrometry to confirm that the purity was higher than 95%. All peptides were dissolved in water.
Results
Isolation of cathelicidin-BF from the snake venoms of B. fasciatus
The crude snake venom was separated into four fractions by Sephadex G-50 gel filtration as our previous report (Figure S1a) (Fig. S1a) [33], [34]. The fraction III, containing antimicrobial activity was further subject to CM-Sephadex C-25 cation-exchange column, and nine sub-fractions were collected (Figure S1b). The fraction VI with both trypsin-inhibitory and antimicrobial activities was further purified using RP-HPLC. The peak with antimicrobial activity is marked with an arrow in Figure S1c. The purified antimicrobial peptide was named cathelicidin-BF. The molecular mass and purity of purified cathelicidin-BF was further analyzed by a ESI mass spectrometry, giving a [M+7H]7+, [M+7H]6+, [M+7H]5+and [M+7H]4+of 521.1, 607.6, 729.1 and 991.5 (Figure S2), indicating that purified cathelicidin-BF has a molecular weight of 3637.5–3638.5.
Structure characterization of cathelicidin-BF
Purified cathelicidin-BF was subjected to amino acid sequence analysis using automated Edman degradation. Its amino acid sequence is KFFRKLKKSVKKRAKEFFKKPRVIGVSIPF. Cathelicidin-BF is composed of 30 amino acid residues including 12 basic residues (9 Lys and 2 Arg), 5 phenylalanines, and only one acidic amino acid residue (Glu). It is a lysine-rich and phenylalanine-rich peptide. Analysis using the ExPASy MW/pI tool (http://www.expasy.ch/tools/pi_tool.html) showed that cathelicidin-BF had the predicted pI (isoelectric point) of 11.79 and a predicted molecular weight of 3637.5 that matched well with the observed mass by ESI mass spectrometry (Figure S2). By BLAST search, no similar sequence was found in GenBank.
Several positive clones, which contained an insert of 750 bp were identified and isolated from B. fasciatus venomous gland cDNA library. The complete nucleotide sequence of cDNA (GenBank accession EU753183) and deduced amino acid sequence of cathelicidin-BF precursor are shown in Figure 1. Unexpectedly, the cathelicidin-BF precursor displays the maximal similarity (47%) with predicted myeloid cathelicidin 3 from Ornithorhynchus anatinus (GenBank accession XP_001512130) by BLAST search. The protein precursor is composed of 191 amino acid (aa) residues, including a predicted signal peptide, a conserved cathelin domain and a mature cathelicidin-BF. Noticeably, four cysteines that are conserved in the cathelin domain of all mammalian cathelicidins are also invariantly spaced in cathelicidin-BF precursor, suggesting that the snake cathelicidin-BF precursor is a real mammalian cathelicidin.
Figure 1. The cDNA sequence encoding cathelicidin-BF and the predicted precursor amino acid sequence.
The amino sequence of purified cathelicidin-BF is boxed. The stop codon is indicated by a star (*). The potential polyadentlation signal (AATAAA) is underlined.
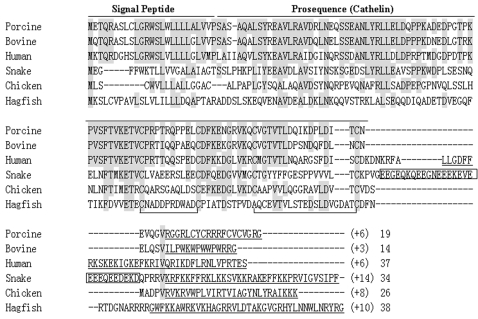
The amino acid sequence of cathelicidin-BF determined by Edman degradation is identical with the amino acid sequence deduced from the cDNA sequence. There is a possible cleavage site (Valine157) for elastase at the N-terminus of the mature cathelicidin-BF (Figure 1). Based on the possible cleavage site, a 34-aa peptide should be released from the precursor, but the purified cathelicidin-BF is only composed of 30 aa. Different from other cathelicidins, there is an acidic doman between the cathelin doman and the antimicrobial peptide in the cathelicidin-BF precursor (Figure 2).
Figure 2. Multiple sequence alignment of snake cathelicidin with other representative cathelicidins.
Cathelicidin-BF precursor is aligned with porcine, bovine, human, chicken and hagfish cathelicdins. Dashes are inserted to optimize the alignment, and conserved residues are shaded. Two intramolecular disulfide bonds in the cathelin pro-sequence are shown. Mature cathelicinds are underlined, and their net charge (in parenthesis) and length are also indicated. The acidic fragment insertion in cathelicidin-BF is boxed.
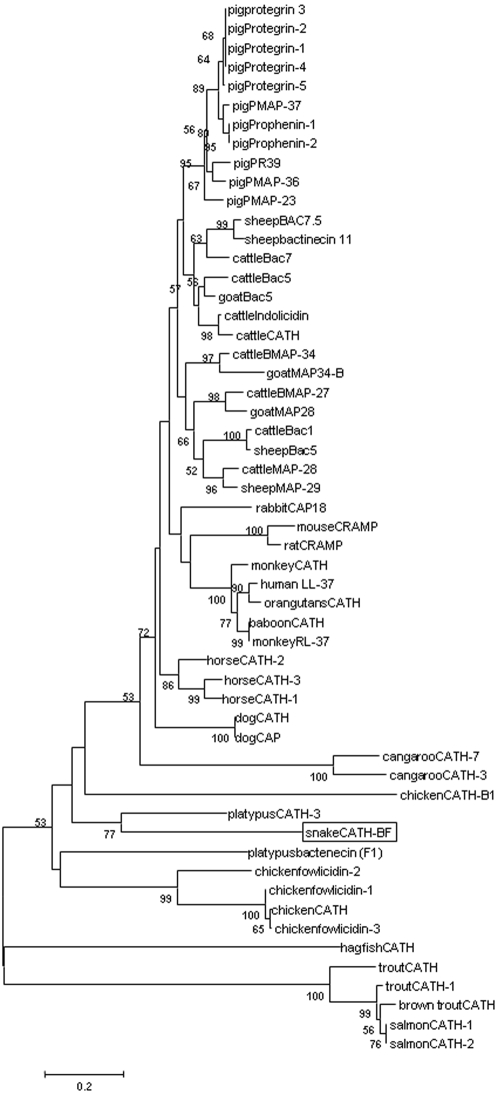
Evolution analysis revealed that all vertebrate cathelicidins formed three distinct clusters with fish cathelicidins located in a separated clade from others. Supported by a bootstrap value of 77%, cathelicidin-BF was clustered with platypus CATH-3 (Figure 3). Although platypus is a mammal, it also has reptilian features, For instance, it lays eggs and it is venomous [44]. The close evolution relationship of cathelicidin-BF found in the venoms of B. fasciatus with platypus cathelicidins may provide further proof for platypus's reptilian features.
Figure 3. Phylogenetic analysis of cathelicidins.
Phylogenetic dendrogram obtained by neighbour-joining analysis based on the proportion difference (p-distance) of aligned amino acid sites of the full-length peptide sequences. Only bootstrap values >50% (expressed as percentages of 1000 resamplings) are shown at branching points. Snake cathelicidin-BF is boxed.
Secondary structures detected by CD and NMR
The secondary structure elements in different solvent environments were detected by CD spectroscopy (Figure S3, Table S1). In H2O, the CD spectrum of cathelicidin-BF showed a strong negative band at 200 nm, indicative of a random-coil conformation. Interestingly, in TFE/H2O mixtures, the CD spectra showed double minima at 208 and 222 nm, indicating a highly α-helical conformation. The signals at 208 and 222 nm were intensified gradually by increasing concentrations of TFE, which indicated that the helicity of the peptide was increased in more hydrophobic or membrane-mimetic environments. The CD spectra of the peptide in SDS micelles also showed a typical α-helix pattern and the content of the α-helix structure increased with the increasing SDS concentration.
NMR spectra recorded on the peptide in SDS micelles were of low quality and can not be used for structural analysis, might due to aggregation of the peptide in negative charged SDS micelles. Therefore, the helical structure of the peptide in TFE/H2O mixture was investigated using NMR spectroscopy. Table S2 lists the nearly complete assignments of the proton chemical shifts of cantheicidin-BF in TFE/H2O mixture (9∶1, v/v). Comparison of the HN-HN region of NOESY spectrum recorded on cantheicidin-BF in H2O with that in TFE/H2O (9∶1, v/v) illustrates that, the peptide adopts a stable secondary structure in TFE/H2O mixture (Figure S4). The Hα CSI prediction is indicative of a helical structure in the N-terminal region comprising residues F2–F18 (Figure S5), although a well-defined three-dimensional structure of the peptide in TFE/H2O mixture has not been obtained yet, mainly due to the deficiency of enough unambiguous conformational restraints for the exact structural analysis.
The amphipathic helical conformation is well known to be a crucial factor for many antimicrobial peptides to interact with membranes [45], [46]. On basis of the rough structural analysis described above, it is indicated that the N-terminal region of cantheicidin-BF adopts a typical amphipathic α-helical conformation (Figure S6) as many other cantheicidins.
Expression profile of tissues
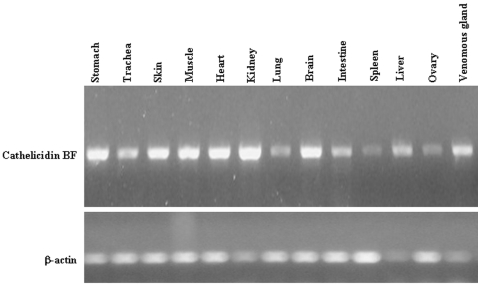
Using actin as control, expression pattern of cathelicidin-BF was investigated by RT-PCR. Tissue distribution of cathelicidin-BF expression in snake tissues were illustrated in Fig. 4. All the selected tissues including stomach, trachea, skin, muscle, heart, kidney, lung, brain, intestine, spleen, liver, ovary and venomous gland can express this protein.
Figure 4. RT-PCR analysis of cathelicidin gene expression pattern in various snake tissues using gene-specific primers with actin as a control.
Antimicrobial activities
As listed in Table 1, cathelicidin-BF and its analogue, cathelicidin-BF15 showed strong antimicrobial activities against tested microorganisms. Of the 40 tested microorganism strains, cathelicidin-BF exerted potent antimicrobial ability against most of Gram-negative bacteria (either standard strains or clinically isolated drug-resistance strains). For most of E. coli, the MICs are lower than 2.3 µg/ml, while ampicillin, benzylpenicillin and ICS are effective only to standard strain with a MIC of 18.7, 37.5 and 0.15 µg/ml respectively. The lowest MIC for K. pneumoniae is 0.3 µg/ml, while ampicillin, benzylpenicillin and ICS are effective only to standard strain with a MIC of 150, 18.7 and 9.4 µg/ml respectively. In contrast, most of S. aureus are not so sensitive for cathelicidin-BF, only one strain could be killed by cathelicidin-BF with a low MIC (4.7 µg/ml). Another Gram-positive bacteria genus, Bacillus also seems to be sensitive for cathelicidin-BF and cathelicidin-BF15. A dangerous clinically isolated strain, Salmonella typhi could also be killed by cathelicidin-BF and cathelicidin-BF15 with a low MIC (1.2 µg/ml). Cathelicidin-BF and cathelicidin-BF15 are the same effective to some fungi as bacteria, for example, C. albicans ATCC2002 (with a MIC of 4.7 µg/ml).and P. pastoris (with a MIC of 0.3 µg/ml). Cathelicidin-BF exerted obvious antimicrobial activity against some saprophytic fungus such as A. terreus GIM3.34 (with a MIC of 18.7 µg/ml), A. niculans (with a MIC of 4.7 µg/ml), and C. globosum (with a MIC of 37.5 µg/ml). All the tested classic antibiotics including Ampicillin, Benzylpenicillin and ICS had no effect on these funguses. Several other cathelicidin-BF analogues, KF1–11 (KFFRKLKKSVK), KF12–19 (KRAKEFFK) and KF20–30 (KPRVIGVSIPF) had no any antimicrobial activity.
Table 1. antimicrobial activity comparison of cathelicidin-BF with antibiotics.
| Microorganism strains | MIC(ug/ml) | |||||
| BF | BF-15 | Amp | Ben | ICS | HDW | |
| Bacillus subtilis | 9.4 | 75 | 0.02 | 0.004 | - | 2.3 |
| Bacillus pumilus | 9.4 | - | 0.15 | 0.015 | - | - |
| Bacillus cereus | 1.2 | - | 150 | 150 | - | - |
| Pseudomonas aeruginosa ATCC27853 | 1.2 | 4.7 | 150 | 18.7 | 9.4 | 37.5 |
| P. aeruginosa (IS, DR) | 2.3 | 37.5 | ND | ND | ND | - |
| P.aeruginosa 08031205(IS, DR) | 9.4 | 18.7 | ND | ND | ND | - |
| P.aeruginosa 08031014(IS, DR) | 18.7 | 75 | ND | ND | ND | - |
| Escherichia coliATCC25922 | 2.3 | 18.7 | 18.7 | 37.5 | 0.15 | 18.7 |
| E. coli 08A852 (IS, DR) | 1.2 | 18.7 | ND | ND | ND | - |
| E. coli 08A866(IS, DR) | 0.6 | 18.7 | ND | ND | ND | 18.7 |
| E. coli 08031017 (IS, DR) | 2.3 | 37.5 | ND | ND | ND | - |
| E. coli 08032813 (IS, DR) | 2.3 | >100 | ND | ND | ND | - |
| E. coli 08040726 (IS, DR) | 0.6 | >100 | ND | ND | ND | - |
| E. coli 08040722 (IS, DR) | 0.6 | 9.4 | ND | ND | ND | - |
| Staphylococcus aureus ATCC2592 | 4.7 | 75 | 0.15 | 0.03 | - | 1.2 |
| S. aureus ATCC25923 | >400 | ND | - | - | - | - |
| S. aureus 08A865 (IS, DR) | >400 | >200 | ND | ND | ND | - |
| S. aureus 08A875 (IS, DR) | 75 | >200 | ND | ND | ND | - |
| S. aureus 08031002 (IS, DR) | >100 | >200 | ND | ND | ND | - |
| S. aureus 08031013 (IS, DR) | >100 | >200 | ND | ND | ND | - |
| S. aureus 08032706 (IS, DR) | >100 | - | ND | ND | ND | - |
| S. aureus 08032712 (IS, DR) | >100 | - | ND | ND | ND | - |
| S. aureus 08032810 (IS, DR) | >100 | - | ND | ND | ND | - |
| Acinetobacter calcoaceticus | 2.3 | - | 75 | 37.5 | - | - |
| Sphingobacterium siyangense | 9.4 | >200 | - | - | - | - |
| Sacharibacillus kuerlensis | 4.7 | 4.7 | - | - | - | - |
| Serratia marcescens SA | >400 | >200 | - | - | - | - |
| Serratia marcescens MA | >400 | >200 | - | - | - | - |
| Pseudomonas luteola | 1.2 | >200 | - | - | - | - |
| Salmonella typhi (IS, DR) | 1.2 | 1.2 | - | - | - | - |
| Klebsiella pneumoniae (IS, DR) | 4.7 | - | - | - | - | - |
| K. pneumoniae 08031012 (IS, DR) | 9.4 | >200 | ND | ND | ND | - |
| K. pneumoniae 08040202 (IS, DR) | 0.6 | 75 | ND | ND | ND | - |
| K. pneumoniae 08040724 (IS, DR) | 0.3 | >100 | - | - | - | - |
| Enterococcus faecium (IS, DR) | 150 | - | - | - | - | - |
| Aspergillus terreus GIM3.34 | 18.7 | ND | ND | ND | ND | - |
| Aspergillus niculans | 18.7 | ND | ND | ND | ND | - |
| Chaetomium globosum | 37.5 | ND | ND | ND | ND | - |
| Candida albicans ATCC2002 | 4.7 | 18.7 | 0.3 | 0.03 | - | 2.3 |
| Pichia pastoris | 0.3 | 9.4 | - | - | - | - |
MIC: minimal inhibitory concentration. These concentrations represent mean values of three independent experiments performed in duplicates. BF: cathlicidin-BF, BF-15: cathlicidin-BF15, Amp: ampicillin, Ben: benzylpenicillin, ICS: Imipenem and Cilastatin Sodium for Injection, ND: no detectable activity, -: no assay, IS: clinically isolated strain, DR: drug resistance for ampicillin and benzylpenicillin.
The antimicrobial activity of cathelicidin-BF in different solutions was also investigated as listed in Table 2. In 150 mM phosphate buffer solution (PBS) and 150 mM NaCl solution, cathelicidin-BF had stronger antimicrobial activities that in water. It suggested that salts could increase cathelicidin-BF's antimicrobial ability.
Table 2. Antimicrobial activity of cathelicidin BF in different solutions.
| Microorganism | MIC | ||
| Water | 150 mM NaCl | 150 mM PBS | |
| E. coliATCC25922 | 2.3 | 2.3 | 2.3 |
| P. aeruginosa ATCC27853 | 1.2 | 0.6 | 0.6 |
| S. aureus ATCC2592 | 4.7 | 2.4 | 2.4 |
| C. albicans ATCC2002 | 4.7 | 2.4 | 2.4 |
MIC: minimal inhibitory concentration. These concentrations represent mean values of three independent experiments performed in duplicates.
Bacteria killing kinetics
Using the antibiotics ICS as a positive control, antibacterial properties of the snake cathelicidin-BF were tested by the colony counting assay. As listed in Table 3 and Table 4, cathelicidin-BF could rapidly exert its antibacterial activities. It just took less than 1 minute to kill all the E. coli at the concentration of 1, 5 or 10 times of MIC. The antibacterial activity was proved to be lethal for E. coli. E. coli was not capable of resuming growth on agar plates after a 6-h treatment with concentrations above the corresponding MICs. In contrast, the antibiotics, ICS could not clean the bacteria within 6 h at the concentration of 1 or 5 times of MIC. Only 10 times MIC of ICS could clean all the E. coli within 6 h. Furthermore, E. coli treated by 1 time MIC of ICS was capable of resuming growth during 6 h.
Table 3. Bacterial killing kinetics of cathelicidin-BF against E. coli.
| Amount of bacteria co-cultured with different samples for different time (CFU) | |||||
| Time (h) | 0 | 0.1 | 1 | 3 | 6 |
| Samples | |||||
| BFx1 | 50 | 0 | 0 | 0 | 0 |
| BFx5 | 50 | 0 | 0 | 0 | 0 |
| BFx10 | 50 | 0 | 0 | 0 | 0 |
| ICSx1 | 50 | 36 | 12 | 224 | 1082 |
| ICSx5 | 50 | 35 | 0.3 | 0.7 | 7 |
| ICSx5 | 50 | 19 | 0.3 | 0.7 | 0 |
| 0.9% salt water | 50 | 45 | 234 | 2341 | 14109 |
BF: cathlicidin-BF, CFU: colony forming unit, ICS: Imipenem and Cilastatin Sodium for Injection, ×1, ×5 and ×10: 1, 5 and 10 times
Table 4. Bacterial killing kinetics of cathelicidin-BF and HDW against E. coli.
| Amount of bacteria co-cultured with different samples for different time (CFU) | |||||
| Time (min) | 0 | 0.1 | 1 | 10 | 30 |
| Samples | |||||
| BFx1 | 50 | 32 | 0 | 0 | 0 |
| BFx5 | 50 | 20 | 0 | 0 | 0 |
| BFx10 | 50 | 10 | 0 | 0 | 0 |
| HDWx1 | 50 | 49 | 14.7 | 0 | 0 |
| HDWx10 | 50 | 38 | 3.7 | 0 | 0 |
| 0.9% salt water | 50 | 69 | 56 | 66.7 | 68.7 |
BF: cathlicidin-BF, CFU: colony forming unit, ×1, ×5 and ×10: 1, 5 and 10 times, HDW: A amphibian antimicrobial peptide from Rana nigrovittata.
In order to compare properties with other antimicrobial peptide, the frog antimicrobial peptide HDW was used as a control. Their bacteria killing kinetics during 30 min was listed in Table 4. Although HDW had a rapid bacteria killing ability, cathelicidin-BF is faster to clean E. coli than HDW. Cathelicidin-BF just took less than 1 minute to clean E. coli, while HDW took several minutes.
Hemolysis, cytotoxicity, serum stability
Cathelicidin-BF had little hemolytic activity on human red blood cells even with peptide concentrations up to 400 µg/ml. At the same concentration, cathelicidin-BF was neither cytotoxic for mouse macrophage (RAW264.7) nor for human liver tumor cell (HepG2) (data not shown). Thus, it showed considerable selectivity for microorganisms over mammalian cells in vitro.
Serum stability was checked by incubating 100 µg/ml cathelicidin-BF and cathelicidin-BF15 with 90% fresh normal human serum at 37°C for 0, 1, 2, 3, 6, 10 and 24 hours. For cathelicidin-BF, antimicrobial activities against E. coli 08A866 could not be detected after 3 h-incubation, while cathelicidin-BF15 could keep its antimicrobial activity up to 10 h in 90% fresh normal human serum. Cathelicidin-BF15 seems to be more stable than cathelicidin-BF in serum.
Discussion
Antimicrobial peptides (AMPs) and their precursor molecules form a central part of biological immunity. For the species which lack adaptive immunity, AMPs play a key role to defense microorganism infection. For their capacity to rapidly inactive infectious agents and to probably inhibit the emergence of drug resistance, AMPs have attracted considerable attention, especially for the treatment of antibiotic-resistant pathogens. The most two important AMP families, defensin and cathelicidin have been found in mammalians, birds and fish. Coincidently, both defensin and cathelicidin have not been found in both reptiles and amphibians although a beta-defensin-like protein with unusual disulfide connectivity (C1–C6/C2–C5/C3–C4), which is different from other the vertebrate beta-defensins, has been identified from a marine turtle [47], [48]. Several hundreds of gene-encoded AMPs have been found from amphibians [12], [39]–[42], [49]. Only a few peptides or proteins from reptiles have been found to exert antimicrobial activities, and most of them are phospholipases A2 or its derivatives [34], [50]–[52], and L-amino acid oxidase [53].
In the attempt to find AMPs from the snake venoms of B. fasciatus, which is a rich source of biological peptides or proteins with therapeutic potential, an AMP, cathelicidin-BF has been isolated and characterized. By screening the cDNA, cathelicidin-BF was unexpectedly found to be a C-terminus of a cathelicidin. The cathelicidin-BF precursor is composed of 191 amino acid residues with conserved cathelin domain that was flanked by signal peptide and by mature cathelicidin-BF. A conserved cleavage site (Valine157) for elastase in the processing and maturation of bovine, porcine and chicken cathelicidins [30] is also existed in the cathelicidin-BF precursor, suggesting that the snake cathelicidin is possibly processed by elastase-like proteases. Based on the hypothesis, cathelicidin-BF precursor should release a 34-aa peptide fragment (KRFKKFFRKLKKSVKKRAKEFFKKPRVIGVSIPF), which has a 4-aa (KRFK) extension at the N-terminus of the 30-aa cathelicidin-BF (KFFRKLKKSVKKRAKEFFKKPRVIGVSIPF). Two reasons may explain the length difference between the predicted 34-aa C-terminal peptide and the purified 30-aa cathelicidin-BF in this case: 1, the predicted cleavage site is right, and the purified 30-aa cathelicidin-BF is from the further processing of the 34-aa peptide; 2, Valine157 in the cathelicidin-BF precursor is not the exact protease cleavage site. In fact, some cathelicidins is not cleaved by elastase to release C-terminal active peptide fragments as mentioned in this “introduction”. An obvious feather of cathelicidin-BF is that there is a high density (40%) of basic amino acid residues in its sequence. As some other cathelicidins [30], there are multiple aromatic amino acid residues in cathelicidin-BF's sequence (5 Phenylalanines). An atypical feature of cathelicidin-BF precursor is that an acidic domain insertion (EEGEQKQEEGNEEEKEVEEEEQEED EKD) is located between the cathelin domain and the mature cathelicidin-BF. This region potentially could affect preproprotein net charge, stability, activity or processing. The similar acidic regions are also found in amphibian antimicrobial precursors, which are located between the signal peptide domains and the mature antimicrobial domains [39]–[42]. Amphibian acidic regions act as a role to neutralize the positive charge of the mature antimicrobial domains and to avoid possible toxicity of the precursor proteins.
The data of antimicrobial testing indicated that cathelicidin-BF is clearly among the most potent cathlicidins discovered to date. Among the 40 strains of tested microorganisms, 15 strains could be killed by cathelicidin-BF at <0.6 µM. For a variety of microorganisms, cathelicidin-BF had better antimicrobial ability than ampicillin, benzylpenicillin and ICS. Cathelicidin-BF's obvious ability to kill some saprophytic fungus such as A. terreus, A. niculans and C. globosum is also very interesting. It may be used as agricultural antibiotics against plant or food pathogenic microorganisms. To our knowledge, this is the first report of cathelicidin's antimicrobial activities against saprophytic fungus. In addition, cathelicidin-BF had very rapid microbe-killing efficacy. Cathelicidin-BF could kill E. coli within one minute at the dose of one time MIC. All the results suggest that cathelicidin-BF is an excellent candidate for clinical or agricultural antibiotics.
Supporting Information
Detail materials and methods
(0.03 MB DOC)
Purification of the cathelicidin from snake venom. (a) Gel filtration chromatography. Sephadex G-50 column (2.6 cm×100 cm), equilibrated and developed with 50 mM Tris-HCl plus 50 mM NaCl (pH 7.8) at a flow rate of 0.3 ml/min, fractions were collected. (b) Cation-exchange chromatography. CM-Sephadex C-25 column (16 cm×40 cm) elution was achieved with a liner NaCl gradient, at a flow rate of 1 ml/min. (c) RP-HPLC chromatography. C4 reverse phase column, equilibrated with 0.1% (v/v) TFA/water, elution was performed with an acetonitrile liner gradient at a flow rate of 0.7 ml/min. The purified peptide with antimicrobial activity is indicated by an arrow.
(0.17 MB TIF)
Electrospray ionization mass spectrometry analysis of the RP-HPLC peak containing antimicrobial activity.
(0.11 MB TIF)
Circular dichroism spectra recorded on cathelicidin-BF in different solvent environments. (A) a∼e : in SDS micelles of 0, 30, 60, 90, 120 mM; (B) a∼e: in TFE/H2O mixtures of 1∶9, 3∶7, 5∶5, 7∶3, 9∶1 (v/v).
(0.13 MB TIF)
HN-HN regions of 2D 1H-1H NOESY spectra recorded on cathelicidin-BF in H2O (left) and in TFE/H2O mixture (9∶1, v/v) (right).
(0.12 MB TIF)
Hα CSI prediction for the cathelicidin-BF peptide in TFE/H2O mixture (9∶1, v/v). h: helix; c: coil.
(0.16 MB TIF)
The opposing position of the hydrophilic and hydrophobic side chains can be seen in this end-on representation of the α-helix in the N-terminal region of cathelicidin-BF.
(0.18 MB TIF)
Contents of helical structures of cathelicidin in TFE/H2O mixtures or in SDS micelles measured by CD.
(0.03 MB DOC)
1H chemical shifts of cathelicidin-BF in TFE/H2O mixture (9∶1, v/v) at 298 K
(0.07 MB DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants of 2007AA02Z192 and 2007AA100602 from the Ministry of Science and Technology of the People's Republic of China, and KSCX2-YW-G-024 and KSCX2-YW-R-20 from Chinese Academy of Sciences.
References
- 1.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 2.Borgden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 3.Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 4.Lehrer RI. Primate defensins. Nature Rev Microbiol. 2004;2:727–738. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 5.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nature Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 6.Ganz T. Hepcidin–a peptide hormone at the interface of innate immunity and iron metabolism. Curr Top Microbiol Immunol. 2006;306:183. doi: 10.1007/3-540-29916-5_7. [DOI] [PubMed] [Google Scholar]
- 7.Kavanagh K, Dowd S. Histatins: antimicrobial peptides with therapeutic potential. J Pharm Pharmacol. 2004;56:285–289. doi: 10.1211/0022357022971. [DOI] [PubMed] [Google Scholar]
- 8.Dürr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Bals R, Wilson JM. Cathelicidins—A family of multifunctional antimicrobial peptides. Cell Mol Life Sci. 2003;60:711–720. doi: 10.1007/s00018-003-2186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gennaro R, Skerlavaj B, Romeo D. Purification, composition, and activity of two Bactenectins, antibacterial peptides of bovine neutrophils. Infect Immun. 1989;57:3142–3146. doi: 10.1128/iai.57.10.3142-3146.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zanetti M, Litteri L, Gennaro R, Horstmann H, Romeo D. Bactenecins, defense polypeptides of bovine neutrophils, are generated from precursor molecules stored in the large granules. J Cell Biol. 1990;111:1363–1371. doi: 10.1083/jcb.111.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank RW, Gennaro R, Schneider K, Przybylski M, Romeo D. Amino acid sequences of two proline-rich bactenectins. J Biol Chem. 1990;265:18871–18874. [PubMed] [Google Scholar]
- 13.Zanetti M, Del Sal G, Storici P, Schneider C, Romeo D. The cDNAof the neutrophil antibioticBac5 predicts a pro-sequence homologous to a cysteine proteinase inhibitor that is common to other neutrophil antibiotics. J Biol Chem. 1993;268:522–526. [PubMed] [Google Scholar]
- 14.Bals R, Lang C, Weiner DJ, Vogelmeier C, Welsch U, et al. Rhesus monkey (Macaca mulatta) mucosal antimicrobial peptides are close homologues of human molecules. Clin Diagn Lab Immunol. 2001;8:370–375. doi: 10.1128/CDLI.8.2.370-375.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao C, Nguyen T, Boo LM, Hong T, Espiritu C, et al. RL-37, an alpha-helical antimicrobial peptide of the rhesus monkey. Antimicrob Agents Chemother. 2001;45:2695–2702. doi: 10.1128/AAC.45.10.2695-2702.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, et al. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem. 1997;272:13088–13093. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 17.Termén S, Tollin M, Olsson B, Svenberg T, Agerberth B, et al. Phylogeny, processing and expression of the rat Cathelicidin rCRAMP: a model for innate antimicrobial peptides. Cell Mol Life Sci. 2003;60:536–549. doi: 10.1007/s000180300045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagaoka I, Tsutsumi-Ishii Y, Yomogida S, Yamashita T. Isolation of cDNA encoding guinea pig neutrophil cationic antibacterial polypeptide of 11 kDa (CAP11) and evaluation of CAP11 mRNA expression during neutrophil maturation. J Biol Chem. 1997;272:22742–22750. doi: 10.1074/jbc.272.36.22742. [DOI] [PubMed] [Google Scholar]
- 19.Gennaro R, Zanetti M. Structural features and biological activities of the cathelicidin-derived antimicrobial peptides. Biopolymers. 2000;55:31–49. doi: 10.1002/1097-0282(2000)55:1<31::AID-BIP40>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Ritonja A, Kopitar M, Jerala R, Turk V. Primary structure of a new cysteine proteinase inhibitor from pig leukocytes. FEBS Lett. 1989;255:211–214. doi: 10.1016/0014-5793(89)81093-2. [DOI] [PubMed] [Google Scholar]
- 21.Zaiou M, Gallo RL. Cathelicidins, essential gene-encoded mammalian antibiotics. J Mol Med. 2002;80:549–561. doi: 10.1007/s00109-002-0350-6. [DOI] [PubMed] [Google Scholar]
- 22.Scocchi M, Skerlavaj B, Romeo D, Gennaro R. Proteolytic cleavage by neutrophil elastase converts inactive storage proforms to antibacterial bactenecins. Eur J Biochem. 1992;209:589–595. doi: 10.1111/j.1432-1033.1992.tb17324.x. [DOI] [PubMed] [Google Scholar]
- 23.Panyutich A, Shi J, Boutz PL, Zhao C, Ganz T. Porcine polymorphonuclear leukocytes generate extracellular microbicidal activity by elastasemediated activation of secreted proprotegrins. Infect Immun. 1997;65:978–985. doi: 10.1128/iai.65.3.978-985.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen OE, Gram L, Johnsen AH, Andersson E, Bangsboll S, et al. Processing of seminal plasma hCAP-18 to ALL-38 by Gastricsin. J Biol Chem. 2003;278:28540–28546. doi: 10.1074/jbc.M301608200. [DOI] [PubMed] [Google Scholar]
- 26.Murakami M, Lopez-Garcia B, Braff MH, Dorschner RA, Gallo RL. Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J Immunol. 2004;172:070–3077. doi: 10.4049/jimmunol.172.5.3070. [DOI] [PubMed] [Google Scholar]
- 27.Uzzell T, Stolzenberg ED, Shinnar AE, Zasloff M. Hagfish intestinal antimicrobial peptides are ancient cathelicidins. Peptides. 2003;24:1655–1667. doi: 10.1016/j.peptides.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Chang CI, Pleguezuelos O, Zhang YA, Zou J, Secombes CJ. Identification of a novel cathelicidin gene in the rainbow trout, Oncorhynchus mykiss. Infect Immun. 2005;73:5053–5064. doi: 10.1128/IAI.73.8.5053-5064.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang CI, Zhang YA, Zou J, Nie P, Secombes CJ. Two cathelicidin genes are present in both rainbow trout (Oncorhynchus mykiss) and atlantic salmon (Salmo salar). Antimicrob Agents Chemother. 2006;50:185–195. doi: 10.1128/AAC.50.1.185-195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao Y, Cai Y, Bommineni YR, Fernando SC, Prakash O, et al. Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. J Biol Chem. 2006;281:2858–2867. doi: 10.1074/jbc.M507180200. [DOI] [PubMed] [Google Scholar]
- 31.Goitsuka R, Chen CL, Benyon L, Asano Y, Kitamura D, et al. Chicken cathelicidin-B1, an antimicrobial guardian at the mucosal M cell gateway. Proc Natl Acad Sci U S A. 2007;104:15063–15068. doi: 10.1073/pnas.0707037104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raison RL, dos Remedios NJ. The hagfish immune system, In: Jorgensen JM, Lomholt JP, Weber RE, Malte H, editors. The Hagfish Immune System. Chapman and Hall; 1998. pp. 334–344. [Google Scholar]
- 33.Lu J, Yang H, Yu H, Gao W, Lai R, et al. A novel serine protease inhibitor from Bungarus fasciatus venom. Peptides. 2008;29:369–374. doi: 10.1016/j.peptides.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Xu C, Ma D, Yu H, Li Z, Liang J, et al. A bactericidal homodimeric phospholipases A2 from Bungarus fasciatus venom. Peptides. 2007;28:969–973. doi: 10.1016/j.peptides.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Chang CT, Wu CS, Yang JT. Circular dichroic analysis of protein conformation: inclusion of the beta-turns. Anal Biochem. 1978;91:13–31. doi: 10.1016/0003-2697(78)90812-6. [DOI] [PubMed] [Google Scholar]
- 36.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, et al. NMRPipe: a Multidimensional Spectral Processing System Based on UNIX Pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 37.Goddard TD, Kneller DG. San Francisco: University of California; SPARKY 3, [Google Scholar]
- 38.Wishart DS, Bigam CG, Yao J, Abildgaard F, Dyson HJ, et al. 1H, 13C and 15N chemical shift referencing in biomolecular NMR. J Biomol NMR. 1995;6:135–140. doi: 10.1007/BF00211777. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Zhang C, Xu X, Wang J, Yu H, et al. Trypsin inhibitory loop is an excellent lead structure to design serine protease inhibitors and antimicrobial peptides. FASEB J. 2007;21:2466–2473. doi: 10.1096/fj.06-7966com. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Xu X, Xu C, Zhou W, Zhang K, et al. Anti-infection peptidomics of amphibian skin. Mol Cell Proteomics. 2007;6:882–894. doi: 10.1074/mcp.M600334-MCP200. [DOI] [PubMed] [Google Scholar]
- 41.Lu Y, Ma Y, Wang X, Liang J, Zhang C, et al. The first antimicrobial peptide from sea amphibian. Mol Immunol. 2008;45:678–681. doi: 10.1016/j.molimm.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Song Y, Li J, Liu H, Xu X, et al. A new family of antimicrobial peptides from skin secretions of Rana pleuraden. Peptides. 2007;28:2069–2074. doi: 10.1016/j.peptides.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 43.Mygind PH, Fischer RL, Schnorr KM, Hansen MT, et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature. 2005;437:975–980. doi: 10.1038/nature04051. [DOI] [PubMed] [Google Scholar]
- 44.Whittington CM, Papenfuss AT, Bansal P, Torres AM, Wong ES, et al. Defensins and the convergent evolution of platypus and reptile venom genes. Genome Res [Epub ahead of print] 2008 doi: 10.1101/gr.7149808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hong YH, Won HS, Ahn HC, Lee BJ. Structural elucidation of the protein- and membrane-binding properties of the N-terminal tail domain of human annexin II. J Biochem. 2003;134:427–432. doi: 10.1093/jb/mvg160. [DOI] [PubMed] [Google Scholar]
- 46.Landon C, Meudal H, Boulanger N, Bulet P, Vovelle F. Solution structures of stomoxyn and spinigerin, two insect antimicrobial peptides with an alpha-helical conformation. Biopolymers. 2006;81:92–103. doi: 10.1002/bip.20370. [DOI] [PubMed] [Google Scholar]
- 47.Lakshminarayanan R, Vivekanandan S, Samy RP, Banerjee Y, Chi-Jin EO, et al. Structure, self-assembly, and dual role of a beta-defensin-like peptide from the Chinese soft-shelled turtle eggshell matrix. J Am Chem Soc. 2008;130:4660–4668. doi: 10.1021/ja075659k. [DOI] [PubMed] [Google Scholar]
- 48.Chattopadhyay S, Sinha NK, Banerjee S, Roy D, Chattopadhyay D, et al. Small cationic protein from a marine turtle has beta-defensin-like fold and antibacterial and antiviral activity. Proteins. 2006;64:524–531. doi: 10.1002/prot.20963. [DOI] [PubMed] [Google Scholar]
- 49.Conlon JM, Kolodziejek J, Nowotny N. Antimicrobial peptides from ranid frogs: taxonomic and phylogenetic markers and a potential source of new therapeutic agents. Biochim Biophys Acta. 2004;1696:1–14. doi: 10.1016/j.bbapap.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 50.Santamaría C, Larios S, Quirós S, Pizarro-Cerda J, Gorvel JP, et al. Bactericidal and antiendotoxic properties of short cationic peptides derived from a snake venom Lys49 phospholipase A2. Antimicrob Agents Chemother. 2005;49:1340–1345. doi: 10.1128/AAC.49.4.1340-1345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santamaría C, Larios S, Angulo Y, Pizarro-Cerda J, Gorvel JP, et al. Antimicrobial activity of myotoxic phospholipases A2 from crotalid snake venoms and synthetic peptide variants derived from their C-terminal region. Toxicon. 2005;45:807–815. doi: 10.1016/j.toxicon.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 52.Nair DG, Fry BG, Alewood P, Kumar PP, Kini RM. Antimicrobial activity of omwaprin, a new member of the waprin family of snake venom proteins. Biochem J. 2007;402:93–104. doi: 10.1042/BJ20060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stiles BG, Sexton FW, Weinstein SA. Antibacterial effects of different snake venoms: purification and characterization of antibacterial proteins from Pseudechis australis (Australian king brown or mulga snake) venom. Toxicon. 1991;29:1129–1141. doi: 10.1016/0041-0101(91)90210-i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detail materials and methods
(0.03 MB DOC)
Purification of the cathelicidin from snake venom. (a) Gel filtration chromatography. Sephadex G-50 column (2.6 cm×100 cm), equilibrated and developed with 50 mM Tris-HCl plus 50 mM NaCl (pH 7.8) at a flow rate of 0.3 ml/min, fractions were collected. (b) Cation-exchange chromatography. CM-Sephadex C-25 column (16 cm×40 cm) elution was achieved with a liner NaCl gradient, at a flow rate of 1 ml/min. (c) RP-HPLC chromatography. C4 reverse phase column, equilibrated with 0.1% (v/v) TFA/water, elution was performed with an acetonitrile liner gradient at a flow rate of 0.7 ml/min. The purified peptide with antimicrobial activity is indicated by an arrow.
(0.17 MB TIF)
Electrospray ionization mass spectrometry analysis of the RP-HPLC peak containing antimicrobial activity.
(0.11 MB TIF)
Circular dichroism spectra recorded on cathelicidin-BF in different solvent environments. (A) a∼e : in SDS micelles of 0, 30, 60, 90, 120 mM; (B) a∼e: in TFE/H2O mixtures of 1∶9, 3∶7, 5∶5, 7∶3, 9∶1 (v/v).
(0.13 MB TIF)
HN-HN regions of 2D 1H-1H NOESY spectra recorded on cathelicidin-BF in H2O (left) and in TFE/H2O mixture (9∶1, v/v) (right).
(0.12 MB TIF)
Hα CSI prediction for the cathelicidin-BF peptide in TFE/H2O mixture (9∶1, v/v). h: helix; c: coil.
(0.16 MB TIF)
The opposing position of the hydrophilic and hydrophobic side chains can be seen in this end-on representation of the α-helix in the N-terminal region of cathelicidin-BF.
(0.18 MB TIF)
Contents of helical structures of cathelicidin in TFE/H2O mixtures or in SDS micelles measured by CD.
(0.03 MB DOC)
1H chemical shifts of cathelicidin-BF in TFE/H2O mixture (9∶1, v/v) at 298 K
(0.07 MB DOC)