Abstract
To investigate the ecological relevance of brain gene regulation associated with singing behavior in songbirds, we challenged freely ranging song sparrows with conspecific song playbacks within their breeding territories. Males responded by approaching the speaker, searching for an intruder and actively singing. In situ hybridization of brain sections revealed significantly higher expression of the transcriptional regulator ZENK in challenged birds than in unstimulated controls in several auditory structures and song control nuclei. We conclude that singing behavior in the context of territorial defense is associated with gene regulation in brain centers that control song perception and production, and that behaviorally regulated gene expression can be used to investigate brain areas involved in the natural behaviors of freely ranging animals.
Keywords: Avian, Immediate early gene, Learning, Neuroethology, Vocal communication
Introduction
The question of whether brain regulatory mechanisms studied in the laboratory are of ecological relevance is often neglected by investigators with a reductionistic approach to animal behavior. To address this issue, one needs to study animals in their natural setting, while they experience interactions with other individuals in the environment to which they are adapted.
The phenomenon of brain gene regulation associated with perception and production of song in songbirds has recently generated some intriguing insights into the physiology of avian vocal communication.1–6 When songbirds hear novel conspecific songs expression of the immediate early genes ZENK and c-jun is rapidly induced in their brains.1,2,7 This response is most prominent in the caudo-medial neostriatum (NCM) and hyperstriatum ventrale (CMHV),8 areas of the avian telencephalon believed to correspond to parts of the mammalian auditory cortex.9,10 In contrast, when songbirds sing, expression of ZENK is induced in nuclei of the motor pathway for song production,6 including the high vocal center (HVC) and the robust nucleus of the archistriatum (RA).11,12 This singing-induced expression is independent of auditory feedback, as it still occurs in deafened males when they sing.6
The studies described above were performed under controlled laboratory conditions with domesticated species bred in captivity. We wondered whether it was technically feasible to study behaviorally regulated changes in brain gene expression in animals in their natural habitat and if so, whether the results in the field differed from those obtained in the laboratory. To address these issues, we studied freely ranging song sparrows, a highly territorial species in which males present a robust and rather predictable behavioral response when challenged with an intruder’s song within their territories during the breeding season.13,14 We show here that challenged males displaying this territorial defense have a marked induction of ZENK in several brain regions that control song perception and production. This induction pattern is similar to that obtained with captive species, but there are also some significant differences which probably reflect the complexities of the field setting.
Materials and Methods
Animals
Freely ranging male (n = 7) and female (n = 5) song sparrows (Melospiza melodia) were captured within their natural breeding territories at the Rockefeller University Field Research Center (Millbrook, New York), between the months of April and June in 1993 and 1996.
Behavioral paradigm
Breeding territories were initially located by observing various candidate areas where song sparrows were seen flying or singing within the Field Center. After the territories were determined, the birds were observed for their preferred perching sites therein, usually a bush or small tree frequently visited by the male. A speaker and folded mist nets were then strategically positioned at these sites, and the birds’ natural behaviors were observed for periods of one to several hours, without any disturbance or stimulation. After the observation period, males were challenged (n = 4) with play-backs of tape-recorded conspecific song for 30 min, to simulate the presence of an intruder within their breeding territories. The tape consisted of a 4 s song bout, repeated every 10 s, obtained from our collection of songs recorded at the Field Center. Immediately after the playback the nets were pulled up with attached strings from a distance of about 20 m by observers hidden behind bushes or small trees. Since challenged birds tended to stay in the vicinity of the speaker (see Results), they were typically caught within the first 2–3 min after opening the nets. After capture, they were removed from the nets and killed by decapitation. Their brains were quickly dissected out while in the field, placed in a plastic mold, covered with embedding medium (TissueTek), frozen in a dry ice/ethanol bath, transported to the laboratory and kept at −70°C. Sex was confirmed by inspection of the gonads. Control males were not presented with playbacks. Those that sang less than five song bouts within the last hour of the observation period were captured (n = 3) by pulling up the nets, and their brains were processed as described above for the challenged birds.
As is commonly known for many songbirds, we noticed a high frequency of singing behavior in the early morning hours that decreased with time of day and increasing ambient temperature. The birds tended to be quieter during the hottest hours of the day (between 11.00 and 16.00 h), resuming active singing in the late afternoon. We therefore concentrated our efforts on performing the experiments during this quiet interval, to minimize brain levels of ZENK message due to this unavoidable level of natural activity.
In situ hybridization
Gene expression was analyzed by in situ hybridization, using a previously described protocol.8 Serial parasagittal brain sections (10 μm thick, up to 3.5 mm from the midline) were cut on a cryostat, mounted onto TESPA-coated (Fluka) glass slides, and stored at −70°C, 35S-labeled antisense riboprobes were synthesized from the cloned canary homologs of the ZENK8 and HAT-215 genes. The probes were then hybridized to brain sections of the captured song sparrows and of canaries (Serinus canaria) and zebra finches (Taeniopygia gutatta) from our laboratory collection after fixation of sections with 3% paraformaldehyde.5 Hybridization and washing steps were carried out at 65°C. Sections were then exposed to X-ray film for 2–3 weeks, or to a PhosphorImager screen for 2–3 days and then dipped in autoradiographic emulsion (NTB-2, Kodak) and exposed for 6 weeks. Dipped slides were then developed and counterstained with cresyl violet. As a control for background levels, some birds sections were hybridized with ZENK sense riboprobes.
Quantification
ZENK expression was quantified by counting silver grains over cells in various brain regions using NIH’s Image software, as detailed previously.6 For each area, the values obtained in the challenged group were divided by the mean value obtained in the control group to generate a normalized scale. The significance of differences between groups was determined by unpaired Student’s t-test.
Results
Behavior
Challenged males showed a characteristic response13,14 that consisted of approaching the speaker and searching actively for the intruder in the grass or behind leaves or on branches in the bushes located near the speaker. During the challenge, the birds engaged in intense singing activity (range: 140–200 bouts within 30 min), most often at two or three preferred sites around the area where the speaker was placed. The frequency of countersinging increased with time, particularly 5–10 min after the onset of the playback. Occasionally, a bird would fly away from the immediate vicinity for a few minutes, after which he would return to the area and, in some cases, change his song to later revert back to the original song. Females were more elusive: whenever they tried to approach the speaker they would be chased back into nearby bushes or out of the immediate vicinity by the defending male. Thus, we were able to capture five unstimulated females, when they happened to fly into the mist nets during control sessions, but no stimulated females.
Gene expression
All challenged birds showed increased ZENK expression in several brain regions (Fig. 1b,d) when compared with unstimulated controls (Fig. 1a,c). Prominent increases occurred in NCM and CMHV (Fig. 1b), and less pronounced induction in fields L1 and L3, the caudo-dorsal paleostriatum (Pc), the HVC shelf and the RA cup (Fig. 1d). All of the above are part of the central auditory pathways in songbirds9,10 and are known to show expression of ZENK in zebra finches and canaries exposed to novel conspecific song.8 High levels of induced ZENK expression were also found in nuclei HVC, RA, and mMAN (the medial magnocellular nucleus of the anterior neostriatum) of all challenged birds (Fig. 1b,d). These nuclei are part of the song control system11,12 and are known to express ZENK in response to singing.6 As shown in Fig. 2a, there was a 3- to 6-fold induction in challenged birds, according to the region analyzed The largest induction was seen in HVC. In contrast, ZENK induction in song nuclei of the anterior forebrain was variable: ZENK expression in area X was very high in one challenged male (Fig. 1d), low in another male, and undetectable in the other two males (not shown), whereas expression in lMAN (the lateral magno-cellular nucleus of the anterior neostriatum) was either low (Fig. 1d) or undetectable in all males.
FIG. 1.
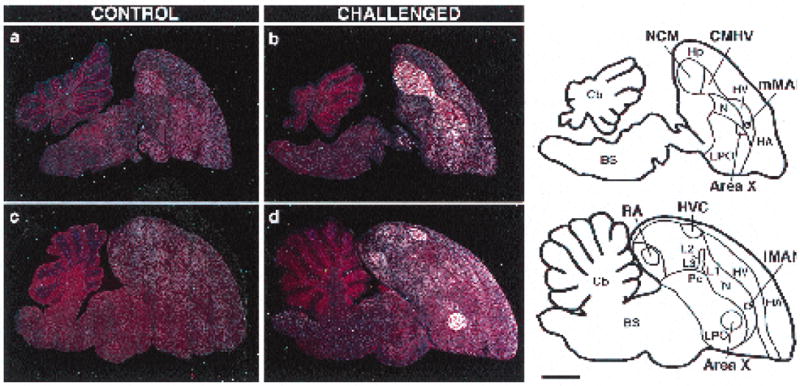
ZENK induction in the brain of male song sparrows: dark-field views of parasagittal sections at the 0.2 mm (a,b) and 2.0 mm (c,d) planes hybridized with an 35S-labeled ZENK riboprobe and counterstained with cresyl violet. Notice increased ZENK expression (white silver grains) in auditory and song control areas in challenged birds (b,d), compared with controls (a,c). The diagrams on the right represent the regions shown on the sections. Not indicated are the shelf area immediately ventral to HVC and the cup region adjacent to RA, as described elsewhere.8–10 Orientation: dorsal is up and rostral to the right. BS, brain stem; Cb, cerebellum; CMHV, caudo-medial hyperstriatum ventrale; Hp, hippocampus; HA, hyperstriatum accessorium; HV, hyperstriatum ventrale; HVC, high vocal center; L1, L2 and L3, subfields of auditory field L; LPO, lobus paraolfactorius; LMAN and mMAN. lateral and medial magnocellular nuclei of the anterior neostriatum; N, neostriatum; NCM, caudo-medial neostriatum; Pc, caudo-dorsal paleostriatum; RA, robust nucleus of the archistriatum. Bar = 2 mm.
FIG. 2.
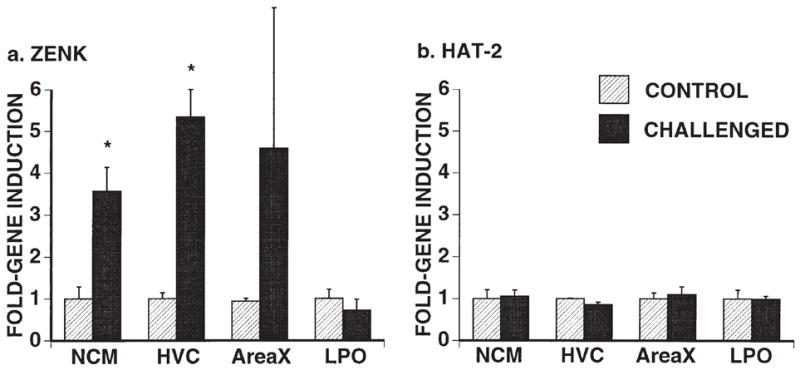
Quantification of ZENK and HAT-2 expression in song sparrow brains. Bars represent mean (± s.e.m.) of silver grain counts over representative auditory (NCM) and song control (HVC, area X) regions in challenged birds, normalized to average control values for each area. Significant differences were seen for NCM and HVC (*p < 0.008; unpaired Student’s t-test), but not for area X. Expression of ZENK in LPO (ventral to area X) and expression of HAT-2 were not modulated. HVC, high vocal center; LPO, lobus paraolfactorius; NCM, caudo-medial neostriatum; X, area X of the paleostriatum.
Lower and more variable levels of ZENK expression occurred in the rostral neostriatum and hyper-striatum, and in the cerebellum, but no consistent differences were seen between the two groups. Other brain regions such as LPO (lobus paraolfactorius) excluding area X (Fig. 2a), hippocampus and thalamus showed no differences in ZENK expression. In addition, no differences for any brain regions analyzed were seen in expression levels of HAT-2 (Fig. 2b), a forebrain-enriched mRNA15 known to be constitutively expressed.5
ZENK expression throughout the telencephalon, in particular in the neo- and hyperstriatum, was relatively higher in unstimulated controls (Fig. 1a,c) than in the laboratory control zebra finches and canaries that were hybridized in parallel (not shown). This higher basal expression in the field presumably reflects the activity levels experienced by the birds prior to their capture. ZENK expression was very low or absent in the hippocampus and parahippocampal region, telencephalic laminae, most of the brain stem, ventricular zone, meninges and choroid pleux. Expression patterns were similar in unstimulated males and females. No signal was detected in sections hybridized to the ZENK sense probe.
Discussion
The present study clearly demonstrates ZENK induction in brain areas controlling vocal communication when freely ranging male song sparrows engage in singing behavior in the context of territorial defense. This indicates that the ZENK induction previously described in laboratory animals in association with vocal communication1,6 is of relevance to the natural life of songbirds.
A comparison with laboratory experiments performed with captive canaries and zebra finches is extremely helpful in the interpretation of the present results. In those birds, ZENK induction in areas such as NCM, CMHV and fields L1 and L3 occurred in both females and males when they heard playbacks of conspecific song.8 This induction occurred in the absence of singing8 and was abolished in deafened males, whether or not they sang.6 In contrast, ZENK expression in song control nuclei such as HVC, RA, area X, lMAN and mMAN occurred only in laboratory males that sang, irrespective of hearing.6 We conclude that the expression pattern obtained in the field is explained by activation of areas controlling perceptual and motor aspects of song, largely in accordance with laboratory studies.
ZENK expression in the forebrain of control males captured in the field was generally higher than that in controls from laboratory studies,1,6,8 but still significantly lower than in challenged birds. This is an important point to stress, since the birds studied here were not placed in controlled chambers, in separation from other birds. Rather, they were experiencing interactions with other individuals and the complexities of an ever-changing environment, and could thus exhibit a full flair of natural behaviors. This fact, however, did not prevent us from observing a significant increase in ZENK expression levels in the brain.
The present field results differ from previous studies in another respect: there was considerable variation in expression levels of two nuclei of the anterior forebrain song pathway, area X and lMAN. The occurrence of ZENK expression in these areas in singing adult males6 is very intriguing, given the current understanding that these nuclei are necessary for song learning in juveniles but not for song production in adults.16–18 The variability observed in the field indicates the existence of other as yet unidentified behavioural and/or contextual variables that, in combination with singing, determine ZENK expression levels in the anterior forebrain pathway, as previously hypothesized.6
The ZENK gene encodes a transcriptional regulator19 and its activation in other systems has been linked to neuronal plasticity, in particular with induction of hippocampal long-term potentiation, or LTP.20–22 This raises the possibility that ZENK induction in the context of song perception and production is also associated with long-term neuronal modification, and could thus have a profound influence on the organization of the brain circuitry responsible for song perception and production. Interestingly, auditory responses in NCM show song-specific habituation whose maintenance requires RNA and protein synthesis at discrete time windows after song presentation.3,4 ZENK induction kinetics approximately coincides with the first such window,6,8 and ZENK is a likely candidate for an early coordinator of long-term habituation.
Irrespective of the final answer to the question above, it is clear that ZENK can be successfully used to investigate brain areas activated by natural behaviors in field experiments. This finding should be particularly useful for situations in which the behavior(s) of interest are only displayed in a natural setting, and the experimental situation cannot be reduced to a laboratory context. The use of ZENK induction for functional brain mapping provides an adequate tool for performing such experiments.
Conclusion
This study demonstrates that song production and perception in the context of songbird territorial defense are associated with gene regulation in brain centers that control vocal communication. More generally, our results show that it is possible, even simple, to study behaviorally driven gene regulation in freely ranging animals. It should thus be possible to use brain mapping with ZENK to identify regions involved in natural behaviors that cannot be studied in the laboratory.
Acknowledgments
We thank Fernando Nottebohm for his support and for making the Field Research Center available for this study. This research was supported with NIMH’s grant #2T32MH15125-18 (to E.J.), NIDCD #DC02853-01 (to C.V.M.) and #MH49877 (to H.S.), Kluge fellowships (to S.R. and E.J.) and by the Mary Cary Flagler Charitable Trust.
References
- 1.Mello CV, Vicario D, Clayton DF. Proc Natl Acad Sci USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mello CV, Nottenbohn F, Clayton DF. J Neurosci. 1995;15:6919–6925. doi: 10.1523/JNEUROSCI.15-10-06919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chew SJ, Mello CV, Vicario D, et al. Proc Natl Acad Sci USA. 1995;92:3406–3410. doi: 10.1073/pnas.92.8.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chew SJ, Vicario D, Nottebohm F. Science. 996;274:1909–1914. doi: 10.1126/science.274.5294.1909. [DOI] [PubMed] [Google Scholar]
- 5.Jarvis EJ, Mello CV, Nottebohm F. Learn Mem. 1995;2:62–80. doi: 10.1101/lm.2.2.62. [DOI] [PubMed] [Google Scholar]
- 6.Jarvis EJ, Nottebohm F. Proc Natl Acad Sci USA. 1997;94:4097–4102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nastiuk KL, Mello CV, George JM, et al. Mol Brain Res. 1994;27:299–309. doi: 10.1016/0169-328x(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 8.Mello CV, Clayton DF. J Neurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley DB, Nottebohm F. J Comp Neurol. 1979;183:455–470. doi: 10.1002/cne.901830302. [DOI] [PubMed] [Google Scholar]
- 10.Vates EG, Broome B, Mello CV, et al. J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Nottebohm F, Stokes T, Leonard CM. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 12.Nottebohm F, Kelley DB, Paton JA. J Comp Neurol. 1982;207:344–357. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- 13.Searcy WA, McArthur PD, Peters SS, et al. Behavior. 1981;177:152–163. [Google Scholar]
- 14.Wingfield JC. Horm Behav. 1985;19:174–187. doi: 10.1016/0018-506x(85)90017-0. [DOI] [PubMed] [Google Scholar]
- 15.George J, Clayton DF. Mol Brain Res. 1992;12:323–329. doi: 10.1016/0169-328x(92)90134-w. [DOI] [PubMed] [Google Scholar]
- 16.Bottjer SW, Miesner EA, Arnold AP. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- 17.Sohrabji F, Nordeen EJ, Nordeen KW. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- 18.Scharff C, Nottebohm F. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christy B, Nathans D. Proc Natl Acad Sci USA. 1989;86:8737–87. doi: 10.1073/pnas.86.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole AJ, Saffen DW, Baraban JM, et al. Nature. 1989;340:474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- 21.Wiseden W, Errington ML, Williams S, et al. Neuron. 1990;4:603–614. doi: 10.1016/0896-6273(90)90118-y. [DOI] [PubMed] [Google Scholar]
- 22.Bliss TVP, Collingridge GL. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]


